Abstract
Background
We aimed to assess the effectiveness of two doses of mRNA COVID-19 vaccines against COVID-19 with the original virus and other lineages circulating in France.
Methods
In this nationwide case-control study, cases were SARS-CoV-2 infected adults with onset of symptoms between 14 February and 3 May 2021. Controls were non-infected adults from a national representative panel matched to cases by age, sex, region, population density and calendar week. Participants completed an online questionnaire on recent activity-related exposures and vaccination history. Information about the infecting virus was based on a screening RT-PCR for either B.1.1.7 or B.1.351/P.1 variants.
Findings
Included in our analysis were 7 288 adults infected with the original SARS-CoV-2 virus, 31 313 with the B.1.1.7 lineage, 2 550 with B.1.351/P1 lineages, and 3 644 controls. In multivariable analysis, the vaccine effectiveness (95% confidence interval) seven days after the second dose of mRNA vaccine was estimated at 88% (81-92), 86% (81-90) and 77% (63-86) against COVID-19 with the original virus, the B.1.1.7 lineage, and the B.1.351/P.1 lineages, respectively. Recent (2 to 6 months) history of virologically confirmed SARS-CoV-2 infection was found to be 83% (76-88), 88% (85-91) and 83% (71-90) protective against COVID-19 with the original virus, the B.1.1.7 lineage, and the B.1.351/P.1 lineages, respectively; and more distant (> 6 months) infections were 76% (54-87), 84% (75-90), and 74% (41-89) protective against COVID-19 with the original virus, the B.1.1.7 lineage, and the B.1.351/P.1 lineages, respectively.
Interpretation
In real-life settings, two doses of mRNA vaccines proved to be effective against COVID-19 with the original virus, B.1.1.7 lineage and B.1.351/P.1 lineages.
Funding
Institut Pasteur, Research & Action Emerging Infectious Diseases (REACTing), Fondation de France (Alliance “Tous unis contre le virus”).
Keywords: COVID-19, Vaccine effectiveness, SARS-CoV-2 variants, epidemiology, case-control study
Research in context.
Evidence before this study
As of early May 2021, the World Health Organization had identified three variants of SARS-CoV-2 of concern due their increased transmissibility and/or possible immune escape: B.1.1.7, B.1.351 and P.1 lineages. COVID-19 mass vaccination efforts have begun in most countries. We conducted a systematic search of PubMed and the pre-print server MedRxiv for observational studies of COVID-19 mRNA vaccine effectiveness and these variants of concern using the terms ‘COVID-19 vaccine effect’, and ‘SARS-CoV-2 variant’ or ‘SARS-CoV-2 mutation’. This search identified two cohort studies assessing vaccine effectiveness of BNT162b2 mRNA vaccine against the original virus and B.1.1.7 lineages of SARS-CoV-2 in Israel and in the U.K., one case-control study which assessed vaccine effectiveness of BNT162b2 mRNA against the B.1.1.7 in the U.K., and one case-control study which assessed vaccine effectiveness of BNT162b2 mRNA against the B.1.1.7 and the B.1.351 lineage in Qatar.
Added value of this study
We analysed data from an ongoing nationwide case-control study to assess the effectiveness of two doses of mRNA vaccines against COVID-19 with the original SARS-CoV-2 virus and other lineages circulating in France, adjusting for a large series of potential confounders, including socio-demographic characteristics, co-morbidities, occupation, and history of past infection. We found protection against COVID-19 seven days after a second dose of mRNA vaccine to be 88% against original virus, 86% against B.1.1.7, and 77% against B.1.351/P.1 lineages.
Implications of all the available evidence
Variants of concern of SARS-CoV-2 continue to circulate as COVID-19 mass vaccination programmes are being rolled out around the world. This study indicates that two doses of mRNA vaccines are effective against COVID-19 with the original virus, B.1.1.7 and B.1.351/P.1 lineages. These are important findings for mass vaccination programmes, but further investigations are required, as well as an assessment of how these findings translate to protection against severe forms of COVID-19 that require hospitalisation.
Alt-text: Unlabelled box
1. Introduction
In late 2020, England experienced a resurgence in incidence of SARS-CoV-2 infections despite the implementation of stringent public health and social measures. This resurgence was later attributed in part to the emergence of the B.1.1.7 SARS-CoV-2 lineage – a variant which has been demonstrated to be more transmissible compared to the original virus [1]. This was followed by the emergence of the B.1.351 variant in South Africa and the P.1 variant in Brazil, both of which emerged in a context of rapid resurgence in SARS-CoV-2 incidence [2,3].
Of further concern from a public health perspective are the mutations in the SARS-CoV-2 Spike protein in B.1.351 (E484K and K417N) and P.1 (E484K and K417T) variants with potential escape to SARS-CoV-2 antibodies. The potential for immune escape has been investigated through evaluation of the neutralizing capacity of sera or plasma from individuals with past infection against B.1.351 and P.1 [4], [5], [6], [7], and sera from individuals having received mRNA COVID-19 vaccines against B.1.351 and P.1 [7], [8], [9]. Overall, the neutralizing activity seems to be similar between the original virus and B.1.1.7, much weaker for B.1.351, and intermediate for P1.
Field evaluations of COVID-19 vaccines are available against the original virus and the B.1.1.7 lineage, showing 85-94% vaccine effectiveness against COVID-19 seven days after the second dose for BNT162b2 mRNA [10,11]. For B.1.351, lower efficacy of the ChAdOx1 nCoV-19 [12], Ad26.COV2.S [13], and NVX-CoV2372 [14] vaccines have been shown during clinical trials in South Africa and breakthrough infections with B.1.1.7 and B.1.351 lineages have been documented in individuals vaccinated with BNT162b2 mRNA vaccine in Israel [15]. A recent publication estimated the effectiveness of BNT162b2 mRNA vaccine to be 75% against all clinical forms of B.1.351 infection, and 97% against severe, critical or fatal disease in Qatar [16].
Since October 2020, we have conducted an ongoing nationwide case-control study which has allowed us to investigate the places and activities associated with SARS-CoV-2 infection in France [17]. In February 2021, we modified the questionnaire to add information on history of COVID-19 vaccination, and virological information from SARS-CoV-2 testing. We have used this information to assess the effectiveness of two doses of mRNA COVID-19 vaccines against the viral lineages circulating in the country (mainly original, B.1.1.7, B.1.351 and P.1).
2. Methods
2.1. Study design and participants
The case-control study design has been previously described [17]. Briefly, cases and controls were selected from two different national databases. Cases were adults with recent SARS-CoV-2 infection diagnosed between 14 February 2021 and 3 May 2021 and were identified through the database from the Caisse Nationale d'Assurance Maladie – a national health insurance agency which receives notification of all SARS-CoV-2 infections in France. Potential cases for our study were all those diagnosed with COVID-19 and with an email address with the national health insurance agency (55% of the adult French population). Controls were adults with no documented recent SARS-CoV-2 infection selected at regular intervals from a representative panel from a database from Ipsos – a French market research and public opinion company. France is divided into 13 administrative regions. Controls were frequency-matched to cases based on age (18-29, 30-54, 55+ years), sex, region of residence, population density of residence, and calendar week. The study was conducted during a period of widespread SARS-CoV-2 transmission in France, with a nationwide 14-day notification rate of 398 cases per 100, 000 population at the start of the study period, 538 cases per 100,000 population at the end of the study period and a peak of 801 cases per 100,000 population for the week of 29 March [18].
2.2. Data collection
Cases and controls were invited to participate in the study by email and received information online about the study before completing a questionnaire if they agreed to participate. The questionnaire covered sociodemographic characteristics, co-morbidities, results of recent SARS-CoV-2 testing for cases (date of test, result of test, SARS-CoV-2 virus identified and categorized as original virus, B.1.1.7, B.1.351/P.1 or “other variant”), recent exposure information, and history of COVID-19 vaccination (date of vaccination, vaccine manufacturer, number of doses received). Questionnaires covered the 10 days preceding symptom onset for cases, and the 10 days preceding inclusion for controls. Participants were asked about whether they had been infected in the past, at what date, and whether diagnosis had been made by RT-PCR or rapid antigen test, serology, or by clinician suspicion without virological or serological confirmation. No sequencing data were available to confirm a true re-infection.
2.3. Vaccination
The following COVID-19 vaccines have been authorized for use in France: BNT162b2 mRNA received authorization on 27 December 2020, Moderna mRNA-1273 on 14 January 2021, ChAdOx1 nCOV-19 on 6 February 2021, and Ad26.COV2.S on 12 March 2021. mRNA vaccines were initially recommended for individuals 75 years and older, then 65 years and older with co-morbidities (2 February 2021), before being recommended for less than 55 years of age when the ChAdOx1 nCOV-19 vaccine was no longer recommended in that age group because of the risk of thrombotic thrombocytopenia [19,20] (19 March 2021). Health care workers were also among the groups prioritized for vaccination, soon after the individuals 75 years and older, and starting with health care workers older than 50 years of age. The majority of them received the ChAdOx1 nCOV-19 vaccine until mRNA vaccines were recommended for those less than 55 years of age. The recommended dose spacing was initially 3-4 weeks for the mRNA vaccines, which was extended to 6 weeks on 23 January 2021, and 9-12 weeks for the ChAdOx1 nCOV-19 vaccine, which was extended to 12 weeks on 2 March 2021. As per the vaccine recommendations in France at the time of the study, individuals with documented past infection were given one vaccine dose only [21]. By the end of the study period (1 May 2021), about 12% of the French population had been fully vaccinated. Information on the vaccination history of the participants was available for inclusion in our database from 13 January 2021, but information on the vaccine type was included only from 22 April 2021. Based on the dose spacing recommendations, no vaccinee should have received a second dose of ChAdOx1 nCOV-19 prior to 6 May 2021, one week after the end of the study period. As such, for the purposes of our analysis, we considered that all participants indicating that they had received two doses of vaccines had received two doses of mRNA vaccines. However, we could not differentiate whether participants had received BNT162b2 mRNA or Moderna mRNA-1273 based on the information available.
2.4. Identification of original virus, B.1.1.7, and B.1.351/P1 SARS-CoV-2 variants
Every specimen with a positive RT-PCR detection of SARS-CoV-2 was subsequently analyzed with a second round of RT-PCR called screening RT-PCR, with the purpose of a rapid identification of viruses belonging to the list of variants of concern. When implemented (January 2021), this screening strategy was focusing on the detection of the B.1.1.7, B.1.351 and P.1 viruses [22]. Screening relied on the detection of the N501Y mutation shared by these three VOCs and one or two additional targets specific of either the B.1.1.7 lineage (del69-70, A570D, P681H) or the B.1.351/P.1 lineages (K417N, E484K). When screening RT-PCR was negative for all targets the virus was assumed to be a “original virus” with no further testing. When discordant results were obtained, the virus was classified as “other variant”. On 30 March 2021, a random sampling of 2590 SARS-CoV-2 RT-PCR positive tests on the French territory identified 80·8% of them as B.1.1.7, 7·8% as B.1.351, and 0·4% as P.1 [23].
2.5. Statistical analyses
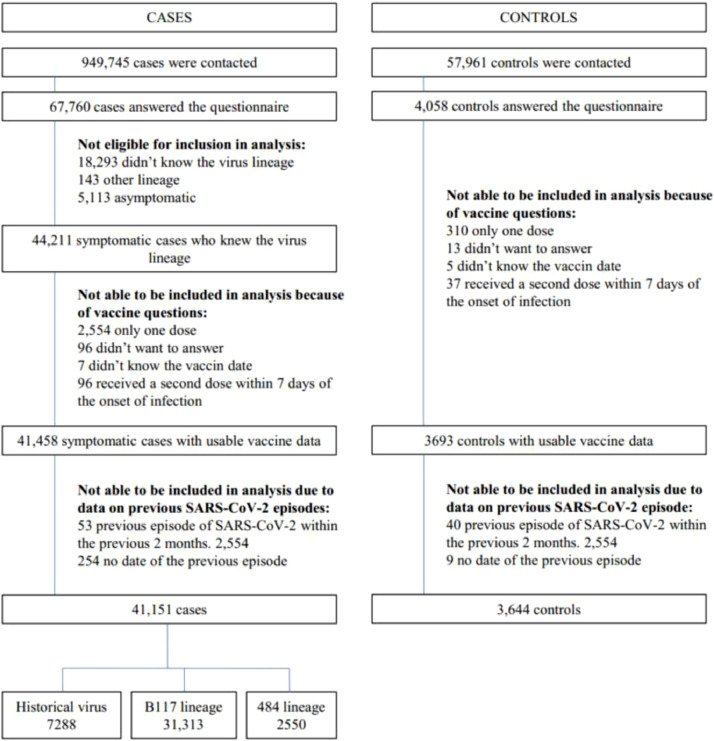
The primary objective was to determine the vaccine effectiveness seven days after the second dose of mRNA COVID-19 vaccines against COVID-19 with either original, B.1.1.7, or B.1.351/P.1 SARS-CoV-2 lineages. We chose seven days after the second dose of mRNA vaccine for comparability with recently published field mRNA vaccine effectiveness evaluation.10,11 Participants retained for the final analysis were those with COVID-19; infection with either the original virus, the B.1.1.7 lineage, or the B.1.351/P.1 lineages; who had received either no doses of COVID-19 vaccine or who had received two doses of COVID-19 vaccine at least seven days prior to symptom onset (those who had received one dose of vaccine only, or who had received their second dose within seven days prior to symptom onset were excluded from the analysis), and, for those participants who reported past SARS-CoV-2 infection, those included retained in the final analysis were those who reported a date of past SARS-CoV-2 infection of more than two months. We were not able to report the effectiveness of one dose of vaccine only, as with the data available, we were not able to distinguish one dose of mRNA vaccine from one dose of ChAdOx1 nCOV-19 vaccine. We conducted a multinomial logistic regression analysis to identify factors associated with each of the three SARS-CoV-2 virus type (original, B.1.1.7 and B.1.351/P.1) infection as the outcome. Variables introduced into the models were the vaccination status (seven days after the second dose versus not vaccinated), the matching variables (age in ten-year categories, sex, region, population density, and calendar week), and potential confounders (body mass index, history of high blood pressure, history of diabetes, history of chronic respiratory disease, history of myocardial infarction/angina pectoris, type of housing, level of education, number of persons living in the household, having children attending day-care centre or looked after by a childminder, having children attending school, being a health care worker, and history of past infection). The vaccine effectiveness was computed as one minus the adjusted odds-ratio (OR). Differences in vaccine effectiveness according to various participants characteristics (age category, sex, profession) were explored using tests for interaction. Since this analysis was not planned at the initiation of the study, we did not calculate a sample size based on an expected vaccine effectiveness before starting the study. Sample size happened to be the number of cases and controls who responded to the questionnaire during the study period, and who matched the criteria chosen for the analysis. A description of the recruitment process and numbers available is shown on the Fig. 1. All statistical analyses were performed using Stata 16·0 (StataCorp, College Station, TX, USA).
Fig. 1.
Flowchart of cases and controls selection for analysis.
2.6. Ethical considerations
This study received ethical approval by the Comité de Protection des Personnes Sud Ouest et Outre Mer 1 on 21 September 2020. The data protection authority Commission Nationale de l'Informatique et des Libertés (CNIL) authorized the processing of data on 21 October 2020. Informed consent was obtained from all participants. The study is registered with ClinicalTrials.gov under the identifier NCT04607941.
2.7. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
3. Results
From 18 February to 29 April 2021, 949 745 individuals with SARS-CoV-2 infection were contacted by email by the national health insurance agency, of which 67 760 (7·1%) participated in the study. When compared to the 1 720 132 adult (20 years and older) patients registered in the national COVID-19 database during the period 14 February – 4 May 2021, cases in our study were more likely to be female (66% compared to 53% in the national database), in the age group 30-59 years (72% versus 57%), and less likely to be from the Ile-De-France region (27% versus 34%), and older than 69 years (2% versus 11%) (Table S1).
At regular intervals, controls were frequency-matched to the cases, with the result that among the 57 961 adults who were contacted as controls across the study period, 4 058 (7·0%) agreed to participate in the study. Table S2 compares the professional category of the highest income in the household of controls with that of the general population in France. It shows that controls were more likely to have senior executive (30% vs 20%) positions, and less likely to have manual jobs (10% vs 19%).
The Fig. 1 describes criteria used to exclude patients and controls to reach the population included in the analysis (see Methods), which consisted of 7 288 COVID-19 cases infected with the original virus, 31 313 COVID-19 cases infected with the B.1.1.7 lineage, 2 550 COVID-19 cases infected with B.1.351/P.1 lineages, and 3 644 non-infected controls. Table 1 compares the characteristics of COVID-19 cases infected with SARS-CoV-2 original virus, B.1.1.7 lineage, and those infected with B.1.351/P.1 viruses. The circulation of the variants during the study period was not equal across France, and cases infected with the lineages B.1.1.7 or B.1.351/P.1 were more likely to be male, with higher levels of education, and not health care workers compared to those infected with the original virus. Participants infected with a B.1.351/P.1 lineage were not more likely to have prior SARS-CoV-2 infection compared to those infected with the original virus or the B.1.1.7 lineage (p=0·139) but were more likely to have become infected despite two doses of COVID-19 vaccine (P=0·003).
Table 1.
Characteristics of cases infected with SARS-CoV-2 original virus, B.1.1.7 lineage, and B.1.351/P.1 lineages and controls.
| Original virus N = 7 288 (%) | B.1.1.7 lineage N = 31 313 (%) | B.1.351/P.1 lineages N = 2 550 (%) | P value* | All cases N = 41 151 (%) | Controls N = 3 644 (%) | P value⁎⁎ | |
|---|---|---|---|---|---|---|---|
| Age (years) | <0·001 | NR | |||||
| 18-24 | 722 (9·9) | 2663 (8·5) | 188 (7·4) | 3573 (8·7) | 271 (7·4) | ||
| 25-34 | 1728 (23·7) | 7532 (24·1) | 613 (24·0) | 9873 (24·0) | 673 (18·5) | ||
| 35-44 | 1882 (25·8) | 8789 (28·1) | 715 (28·0) | 11386 (27·7) | 857 (23·5) | ||
| 45-54 | 1701 (23·3) | 7481 (23·9) | 617 (24·2) | 9799 (23·8) | 1237 (33·9) | ||
| 55-64 | 927 (12·7) | 3705 (11·8) | 328 (12·9) | 4960 (12·1) | 96 (2·6) | ||
| 65-74 | 303 (4·2) | 1068 (3·4) | 80 (3·1) | 1451 (3·5) | 340 (9·3) | ||
| 75 + | 25 (0·3) | 75 (0·2) | 9 (0·4) | 109 (0·3) | 170 (4·7) | ||
| Sex | <0·001 | NR | |||||
| Male | 2158 (29·6) | 10238 (32·7) | 891 (34·9) | 13287 (32·3) | 1225 (33·6) | ||
| Female | 5130 (70·4) | 21075 (67·3) | 1659 (65·1) | 27864 (67·7) | 2419 (66·4) | ||
| Region of residence | <0·001 | NR | |||||
| Île-de-France | 1811 (24·8) | 6674 (21·3) | 761 (29·8) | 9246 (22·5) | 1061 (29·1) | ||
| Centre - Val de Loire | 297 (4·1) | 925 (3·0) | 46 (1·8) | 1268 (3·1) | 132 (3·6) | ||
| Bourgogne – France-Comté | 314 (4·3) | 1158 (3·7) | 89 (3·5) | 1561 (3·8) | 159 (4·4) | ||
| Normandie | 355 (4·9) | 1164 (3·7) | 86 (3·4) | 1605 (3·9) | 150 (4·1) | ||
| Hauts-de-France | 857 (11·8) | 4090 (13·1) | 157 (6·2) | 5104 (12·4) | 366 (10·0) | ||
| Grand Est | 541 (7·4) | 2331 (7·4) | 593 (23·3) | 3465 (8·4) | 244 (6·7) | ||
| Pays de la Loire | 374 (5·1) | 1820 (5·8) | 173 (6·8) | 2367 (5·8) | 170 (4·7) | ||
| Bretagne | 184 (2·5) | 1123 (3·6) | 50 (2·0) | 1357 (3·3) | 122 (3·3) | ||
| Nouvelle-Aquitaine | 446 (6·1) | 1962 (6·3) | 103 (4·0) | 2511 (6·1) | 197 (5·4) | ||
| Occitanie | 506 (6·9) | 2261 (7·2) | 98 (3·8) | 2865 (7·0) | 258 (7·1) | ||
| Auvergne- Rhône-Alpes | 1007 (13·8) | 4983 (15·9) | 243 (9·5) | 6233 (15·1) | 457 (12·5) | ||
| Provence-Alpes-Côte d'Azur and Corse | 596 (8·2) | 2822 (9·0) | 151 (5·9) | 3569 (8·7) | 328 (9·0) | ||
| Population density of place of residence (inhabitants) | <0·001 | NR | |||||
| Rural or < 5,000 | 1901 (26·1) | 7966 (25·4) | 631 (24·7) | 10498 (25·5) | 843 (23·1) | ||
| 5,000 - 19,999 | 700 (9·6) | 3154 (10·1) | 227 (8·9) | 4081 (9·9) | 339 (9·3) | ||
| 20,000 - 99,999 | 854 (11·7) | 3637 (11·6) | 286 (11·2) | 4777 (11·6) | 426 (11·7) | ||
| 100,000 + | 2216 (30·4) | 10675 (34·1) | 719 (28·2) | 13610 (33·1) | 1124 (30·8) | ||
| Paris agglomeration | 1617 (22·2) | 5881 (18·8) | 687 (26·9) | 8185 (19·9) | 912 (25·0) | ||
| Calendar week | <0·001 | NR | |||||
| 7 | 1060 (14·5) | 1406 (4·5) | 154 (6·0) | 2620 (6·4) | 316 (8·7) | ||
| 8 | 1313 (18·0) | 2247 (7·2) | 240 (9·4) | 3800 (9·2) | 299 (8·2) | ||
| 9 | 902 (12·4) | 2321 (7·4) | 238 (9·3) | 3461 (8·4) | 373 (10·2) | ||
| 10 | 775 (10·6) | 2955 (9·4) | 252 (9·9) | 3982 (9·7) | 288 (7·9) | ||
| 11 | 735 (10·1) | 3700 (11·8) | 324 (12·7) | 4759 (11·6) | 342 (9·4) | ||
| 12 | 669 (9·2) | 4304 (13·7) | 301 (11·8) | 5274 (12·8) | 268 (7·4) | ||
| 13 | 622 (8·5) | 3932 (12·6) | 272 (10·7) | 4826 (11·7) | 338 (9·3) | ||
| 14 | 468 (6·4) | 3522 (11·2) | 236 (9·3) | 4226 (10·3) | 341 (9·4) | ||
| 15 | 383 (5·3) | 3347 (10·7) | 232 (9·1) | 3962 (9·6) | 280 (7·7) | ||
| 16 | 281 (3·9) | 2803 (9·0) | 219 (8·6) | 3303 (8·0) | 361 (9·9) | ||
| 17 | 80 (1·1) | 773 (2·5) | 82 (3·2) | 935 (2·3) | 241 (6·6) | ||
| 18 | 0 (0·0) | 3 (0·0) | 0 (0·0) | 3 (0·0) | 197 (5·4) | ||
| Body mass index (in kg/m2) | 0·001 | 0·034 | |||||
| < 18·5 | 3899 (53·5) | 17586 (56·2) | 1403 (55·0) | 22888 (55·6) | 1960 (53·8) | ||
| ≥ 18·5 & < 25 | 2181 (29·9) | 8917 (28·5) | 755 (29·6) | 11853 (28·8) | 1063 (29·2) | ||
| ≥ 25 | 1208 (16·6) | 4810 (15·4) | 392 (15·4) | 6410 (15·6) | 621 (17·0) | ||
| High blood pressure | 0·131 | <0·001 | |||||
| No | 6774 (92·9) | 29296 (93·6) | 2374 (93·1) | 38444 (93·4) | 3257 (89·4) | ||
| Yes | 514 (7·1) | 2017 (6·4) | 176 (6·9) | 2707 (6·6) | 387 (10·6) | ||
| Diabetes | 0·070 | <0·001 | |||||
| No | 7124 (97·7) | 30734 (98·2) | 2497 (97·9) | 40355 (98·1) | 3507 (96·2) | ||
| Yes | 164 (2·3) | 579 (1·8) | 53 (2·1) | 796 (1·9) | 137 (3·8) | ||
| Chronic respiratory disease | 0·645 | 0·042 | |||||
| No | 6726 (92·3) | 28983 (92·6) | 2352 (92·2) | 38061 (92·5) | 3404 (93·4) | ||
| Yes | 562 (7·7) | 2330 (7·4) | 198 (7·8) | 3090 (7·5) | 240 (6·6) | ||
| History of myocardial infarction / angina pectoris | 0·382 | 0·008 | |||||
| No | 7243 (99·4) | 31159 (99·5) | 2538 (99·5) | 40940 (99·5) | 3613 (99·1) | ||
| Yes | 45 (0·6) | 154 (0·5) | 12 (0·5) | 211 (0·5) | 31 (0·9) | ||
| Education | <0·001 | ||||||
| No diploma | 251 (3·4) | 670 (2·1) | 64 (2·5) | 985 (2·4) | 61 (1·7) | ||
| Pre-bachelor degree | 1467 (20·1) | 4913 (15·7) | 406 (15·9) | 6786 (16·5) | 602 (16·5) | ||
| Undergraduate degree | 1709 (23·4) | 6700 (21·4) | 519 (20·4) | 8928 (21·7) | 862 (23·7) | ||
| Graduate degree | 2545 (34·9) | 11691 (37·3) | 951 (37·3) | 15187 (36·9) | 1472 (40·4) | ||
| Post-graduate degree | 1316 (18·1) | 7339 (23·4) | 610 (23·9) | 9265 (22·5) | 647 (17·8) | ||
| Housing type | <0·001 | <0·001 | |||||
| House | 4297 (59·0) | 19423 (62·0) | 1497 (58·7) | 25217 (61·3) | 2027 (55·6) | ||
| Apartment | 2942 (40·4) | 11746 (37·5) | 1037 (40·7) | 15725 (38·2) | 1601 (43·9) | ||
| Long term care facility and social housing | 49 (0·7) | 144 (0·5) | 16 (0·6) | 209 (0·5) | 16 (0·4) | ||
| Number of persons in the household | <0·001 | <0·001 | |||||
| 1 | 1150 (15·8) | 4382 (14·0) | 390 (15·3) | 5922 (14·4) | 735 (20·2) | ||
| 2 | 1970 (27·0) | 8145 (26·0) | 682 (26·7) | 10797 (26·2) | 1176 (32·3) | ||
| 3 | 1541 (21·1) | 6802 (21·7) | 562 (22·0) | 8905 (21·6) | 728 (20·0) | ||
| 4 | 1743 (23·9) | 8269 (26·4) | 645 (25·3) | 10657 (25·9) | 711 (19·5) | ||
| 5 | 646 (8·9) | 2789 (8·9) | 207 (8·1) | 3642 (8·9) | 244 (6·7) | ||
| 6+ | 238 (3·3) | 926 (3·0) | 64 (2·5) | 1228 (3·0) | 50 (1·4) | ||
| Child in household attending daycare centre or looked after by a childminder | 0·187 | <0·001 | |||||
| No | 6627 (90·9) | 28253 (90·2) | 2305 (90·4) | 37185 (90·4) | 3488 (95·7) | ||
| Yes | 661 (9·1) | 3060 (9·8) | 245 (9·6) | 3966 (9·6) | 156 (4·3) | ||
| Child in household attending school | 0·002 | <0·001 | |||||
| No | 4039 (55·4) | 16673 (53·2) | 1402 (55·0) | 22114 (53·7) | 2348 (64·4) | ||
| Yes | 3249 (44·6) | 14640 (46·8) | 1148 (45·0) | 19037 (46·3) | 1296 (35·6) | ||
| Health care worker | <0·001 | <0·001 | |||||
| No | 6197 (85·0) | 27874 (89·0) | 2258 (88·5) | 36329 (88·3) | 3424 (94·0) | ||
| Yes | 1091 (15·0) | 3439 (11·0) | 292 (11·5) | 4822 (11·7) | 220 (6·0) | ||
| History of past SARS CoV-2 infection | 0·139 | <0 ·001 | |||||
| No | 7168 (98·4) | 30830 (98·5) | 2499 (98·0) | 40497 (98·4) | 3431 (94·2) | ||
| Indicated by virological test (>6 months) | 15 (0·2) | 55 (0·2) | 7 (0·3) | 77 (0·2) | 40 (1·1) | ||
| Indicated by virological test (between 2 and 6 months) | 51 (0·7) | 148 (0·5) | 18 (0·7) | 217 (0·5) | 127 (3·5) | ||
| Indicated by serological test | 9 (0·1) | 59 (0·2) | 7 (0·3) | 75 (0·2) | 12 (0·3) | ||
| Suspected by clinician | 45 (0·6) | 221 (0·7) | 19 (0·7) | 285 (0·7) | 34 (0·9) | ||
| History of vaccination⁎⁎⁎ | 0·003 | <0·001 | |||||
| Not vaccinated | 7259 (99·6) | 31139 (99·4) | 2525 (99·0) | 40923 (99·4) | 3451 (94·7) | ||
| Yes, two doses of COVID-19 vaccine | 29 (0·4) | 174 (0·6) | 25 (1·0) | 228 (0·6) | 193 (5·3) |
Chi-square test comparing the proportions across the three virus types.
Chi-square test comparing the proportions across cases and controls.
Participants with only one dose of vaccination, or within seven days of second dose, were excluded from this analysis
NR: not relevant (matching variables)
Table 2 describes the factors associated with recent COVID-19, by virus type. In multivariable analysis, the risk of infection increased with a history of chronic respiratory disease, lower levels of education, an increasing number of people in the household, having children in the household attend day care or a childminder, having children in the household attend school, or being a health care worker, regardless of the virus type. A past history of high blood pressure was associated with a lower risk of infection with the original virus and the B.1.1.7 lineage, and a history of diabetes with a lower risk of infection with the B.1.1.7 lineage. Previous history of virologically confirmed SARS-CoV-2 infection was found to be protective against the original virus, the B.1.1.7 lineage, and the B.1.351/P.1 lineages: 83%-88% for recent (2 to 6 months) infections, and 74%-84% for more distant (> 6 months) infections. The vaccine effectiveness (95% CI) seven days after the second dose of mRNA vaccine was estimated at 88% (81%-92%) against COVID-19 with the original virus, 86% (81%-90%) with the B.1.1.7 lineage, and 77% (63%-86%) with B.1.351/P.1 lineages. When the variable “health care worker” was dropped from the multivariable model, the OR associated with vaccine protection moved from 0·12 to 0·20 for the original virus, from 0·14 to 0·20 for the B.1.1.7 lineage, and from 0·23 to 0·34 for the B.1.351/P.1 lineages, and became very close to the OR observed for vaccine protection in univariable analysis (0·19, 0·20, and 0·35, respectively), showing that the only variable exerting an important confounding effect on the estimation of the protection conferred by vaccines was “being a health care worker”. We also wondered whether the difference in the proportion with past infection between the cases and controls may have influenced the estimates of vaccine effectiveness. While we adjusted for history of past infection in the analysis, we performed a complementary analysis by restriction in the eventuality that the adjustment would not work as expected. In this sensitivity analysis, we removed cases and controls with past infection. The OR (95% CI) for the association between complete vaccination and SARS-CoV-2 infection did not change: It was 0·11 (0·07-0·17) instead of 0·12 (0·08-0·19) for the historic virus, 0·14 (0·10-0·18) instead of 0·14 (0·10-0·19) for B.1.1.7, and 0·23 (0·14-0·37) instead of 0·23 (0·14-0·37) for B.1.351/P.1.
Table 2.
Factors (aOR and 95%CI) associated with recent COVID-19 in France (February – May 2021) by virus type among 41151 cases compared to 3644 controls.
| Original virus (n=7288) | B.1.1.7 lineage (n=31313) | B.1.351/P1 lineages (n=2550) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI)* | aOR (95%CI)⁎⁎ | OR (95% CI)* | aOR (95%CI)⁎⁎ | OR (95% CI)* | aOR (95%CI)⁎⁎ | |
| Body mass index (in kg/m2) | ||||||
| <18·5 | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) |
| ≥18·5 & <25 | 1·12 (1·02-1·24) | 1·09 (0·98-1·20) | 0·99 (0·91-1·08) | 1·00 (0·92-1·09) | 1·01 (0·90-1·15) | 1·03 (0·91-1·17) |
| ≥25 | 1·04 (0·93-1·17) | 1·05 (0·93-1·19) | 0·90 (0·81-1·00) | 0·99 (0·89-1·10) | 0·90 (0·77-1·04) | 0·98 (0·84-1·14) |
| High blood pressure | ||||||
| No | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) |
| Yes | 0·79 (0·68-0·93) | 0·80 (0·68-0·95) | 0·78 (0·69-0·90) | 0·85 (0·74-0·98) | 0·79 (0·65-0·97) | 0·86 (0·69-1·06) |
| Diabetes | ||||||
| No | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) |
| Yes | 0·79 (0·61-1·02) | 0·79 (0·61-1·03) | 0·67 (0·54-0·83) | 0·74 (0·59-0·92) | 0·73 (0·52-1·03) | 0·79 (0·56-1·13) |
| Chronic respiratory disease | ||||||
| No | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) |
| Yes | 1·21 (1·03-1·43) | 1·24 (1·05-1·47) | 1·17 (1·02-1·36) | 1·25 (1·08-1·45) | 1·24 (1·01-1·51) | 1·31 (1·07-1·61) |
| History of myocardial infarction / angina pectoris | ||||||
| No | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) |
| Yes | 1·35 (0·80-2·28) | 1·36 (0·79-2·34) | 1·13 (0·71-1·79) | 1·22 (0·75-1·97) | 1·01 (0·49-2·08) | 1·06 (0·51-2·22) |
| Education | ||||||
| No diploma | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) |
| Pre-bachelor degree | 0·52 (0·38-0·72) | 0·53 (0·38-0·73) | 0·66 (0·49-0·89) | 0·67 (0·50-0·91) | 0·58 (0·39-0·86) | 0·58 (0·39-0·86) |
| Undergraduate degree | 0·39 (0·28-0·53) | 0·39 (0·28-0·53) | 0·58 (0·43-0·78) | 0·58 (0·43-0·78) | 0·51 (0·34-0·75) | 0·50 (0·34-0·74) |
| Graduate degree | 0·34 (0·25-0·46) | 0·33 (0·24-0·46) | 0·61 (0·45-0·81) | 0·60 (0·45-0·80) | 0·54 (0·37-0·80) | 0·53 (0·36-0·77) |
| Post-graduate degree | 0·40 (0·29-0·55) | 0·42 (0·30-0·57) | 0·91 (0·68-1·23) | 0·93 (0·69-1·26) | 0·78 (0·53-1·15) | 0·78 (0·53-1·16) |
| Housing type | ||||||
| House | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) |
| Apartment | 0·88 (0·80-0·98) | 1·01 (0·91-1·12) | 0·76 (0·70-0·83) | 0·90 (0·82-0·99) | 0·77 (0·68-0·87) | 0·87 (0·76-0·99) |
| Long term care facility and social housing | 1·21 (0·67-2·20) | 1·26 (0·69-2·30) | 0·87 (0·50-1·51) | 0·95 (0·55-1·66) | 1·18 (0·57-2·44) | 1·30 (0·62-2·69) |
| Number of persons in the household | ||||||
| 1 | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) |
| 2 | 1·07 (0·95-1·21) | 1·02 (0·90-1·16) | 1·18 (1·06-1·31) | 1·10 (0·98-1·23) | 1·11 (0·94-1·30) | 1·02 (0·87-1·21) |
| 3 | 1·37 (1·19-1·57) | 1·07 (0·92-1·25) | 1·56 (1·39-1·76) | 1·21 (1·05-1·38) | 1·44 (1·21-1·71) | 1·08 (0·89-1·32) |
| 4 | 1·67 (1·46-1·92) | 1·20 (1·01-1·43) | 2·03 (1·80-2·29) | 1·41 (1·21-1·64) | 1·75 (1·47-2·09) | 1·17 (0·94-1·46) |
| 5 | 1·79 (1·49-2·16) | 1·22 (0·98-1·52) | 1·97 (1·67-2·31) | 1·33 (1·10-1·61) | 1·64 (1·30-2·07) | 1·06 (0·80-1·40) |
| 6+ | 3·17 (2·28-4·41) | 2·12 (1·49-3·02) | 3·10 (2·29-4·21) | 2·19 (1·58-3·02) | 2·38 (1·59-3·54) | 1·59 (1·04-2·43) |
| Child in household attending daycare centre or looked after by a childminder | ||||||
| No | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) |
| Yes | 2·02 (1·66-2·46) | 1·89 (1·55-2·32) | 2·12 (1·77-2·54) | 1·84 (1·53-2·22) | 2·10 (1·68-2·63) | 1·89 (1·50-2·39) |
| Child in household attending school | ||||||
| No | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) |
| Yes | 1·68 (1·53-1·85) | 1·43 (1·26-1·63) | 1·74 (1·60-1·89) | 1·41 (1·26-1·58) | 1·64 (1·45-1·85) | 1·46 (1·24-1·71) |
| Health care worker | ||||||
| No | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) |
| Yes | 2·19 (1·87-2·56) | 2·80 (2·37-3·31) | 1·66 (1·44-1·92) | 2·10 (1·80-2·45) | 1·90 (1·58-2·30) | 2·33 (1·91-2·85) |
| History of past infection | ||||||
| No | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) | 1 (ref·) | 1 (ref) |
| Indicated by virological test (>6 months) | 0·25 (0·13-0·46) | 0·24 (0·13-0·46) | 0·15 (0·10-0·24) | 0·16 (0·10-0·25) | 0·26 (0·11-0·59) | 0·26 (0·11-0·59) |
| Indicated by virological test (between 2 and 6 months) | 0·18 (0·13-0·25) | 0·17 (0·12-0·24) | 0·12 (0·09-0·16) | 0·12 (0·09-0·15) | 0·18 (0·11-0·30) | 0·17 (0·10-0·29) |
| Indicated by serological test | 0·35 (0·14-0·86) | 0·31 (0·12-0·76) | 0·57 (0·30-1·11) | 0·51 (0·26-0·99) | 0·79 (0·30-2·08) | 0·69 (0·26-1·81) |
| Suspected by clinician | 0·60 (0·38-0·96) | 0·61 (0·38-0·97) | 0·70 (0·48-1·03) | 0·70 (0·48-1·03) | 0·65 (0·37-1·17) | 0·65 (0·36-1·17) |
| History of vaccination⁎⁎⁎ | ||||||
| Not vaccinated | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) | 1 (ref·) |
| Yes, completely vaccinated | 0·19 (0·12-0·29) | 0·12 (0·08-0·19) | 0·20 (0·15-0·26) | 0·14 (0·10-0·19) | 0·35 (0·22-0·55) | 0·23 (0·14-0·37) |
Adjusted for the matching variables (age, sex, region, population density and calendar week)
Adjusted for the matching and all other variables (age, sex, region and agglomeration density of residence, calendar week, body mass index, high blood pressure, diabetes, history of chronic respiratory disease, history of myocardial infarction and angina pectoris, education, housing type, number of people living in the household, number of children attending daycare or school, working as health care worker or not, and past history of infection)
Participants with only one dose of vaccine, or within seven days of second dose, were excluded for this analysis
Table 3 describes the protection associated with two doses of vaccines against different virus lineages, and by age category.
Table 3.
aOR and 95%CI for the association between two doses of COVID-19 vaccine (received >7 days prior to symptom onset) and recent symptomatic SARS-CoV-2 infection by virus type and age category.
| Controls N = 3 644 (%) | Original virus N = 7 288 (%) | aOR (95%CI)* | B.1.1.7 lineage N= 31 313 (%) | aOR (95%CI)* | B.1.351/P1 lineages N= 2 550 (%) | aOR (95%CI)* | |
|---|---|---|---|---|---|---|---|
| Age categories (in years) | |||||||
| 18-54 | 3 038 (83·4) | 6 033 (82·8) | 26 465 (84·5) | 2 133 (83·6) | |||
| Not vaccinated | 2 973 (97·9) | 6 017 (99·7) | 1 (ref·) | 26 364 (99·6) | 1 (ref·) | 2 121 (99·4) | 1 (ref·) |
| Yes, two doses of COVID-19 vaccine⁎⁎ | 65 (2·1) | 16 (0·3) | 0·10 (0·06-0·18) | 101 (0·4) | 0·12 (0·09-0·18) | 12 (0·6) | 0·17 (0·09-0·32) |
| 55+ | 606 (16·6) | 1255 (17·2) | 4848 (15·5) | 417 (16·4) | |||
| Not vaccinated | 478 (78·9) | 1242 (99·0) | 1 (ref·) | 4775 (98·5) | 1 (ref·) | 404 (96·9) | 1 (ref·) |
| Yes two doses of COVID-19 vaccine⁎⁎ | 128 (21·1) | 13 (1·0) | 0·11 (0·05-0·23) | 73 (1·5) | 0·12 (0·08-0·20) | 13 (3·1) | 0·24 (0·11-0·53) |
Adjusted for the matching variables (age, sex, region, population density, and time period in week), and potential confounders (body mass index, high blood pressure, diabetes, history of chronic respiratory disease, history of angina pectoris and myocardial infarction, housing type, level of education, number of persons living in the household, having children attending daycare centre or looked after by a childminder, having children attending school, being a health professional, and history of past infection).
Participants with only one dose of vaccine, or within seven days of second dose, were excluded from this analysis
4. Discussion
In this nationwide case-control study in France, we found protection against COVID-19 seven days after a second dose of mRNA vaccine to be 88% against original virus, 86% against B.1.1.7, and 77% against B.1.351/P.1 lineages. These findings align well with results from clinical trials against the original viruses: 95% for BNT162b2 mRNA[24], and 94% for Moderna mRNA-1273[25] vaccines; and with results from field evaluations of BNT162b2 mRNA vaccines: 94% against a mix of original viruses and B.1.1.7 in Israel[11], 85%-89% against a majority of B.1.1.7 in the United Kingdom[10,26], and 90% and 75% against B.1.1.7 and B.1.351, respectively, in Qatar[16]. We did not find difference in vaccine effectiveness by age, sex, or professional exposure through health care work.
The study also has an important contribution regarding history of prior infection. We found protection against COVID-19 to range from 83% to 88% for recent (2 to 6 months) virologically documented infections, and from 74% to 84% for older (>6 months) infections. Here again, the results are very similar to those obtained in several large cohort studies: 84% in the UK SIREN health care workers study[27], 89% in the UK Oxford health care workers study[28], and 81% in the Danish population-based study[29]. It should be noted that the history of past infection was based on the participant's recollection, and not, particularly when reinfection was suspected, on clear identification of two separate lineages by sequence separated by a specified gap in time.
Our results are nonetheless limited by several factors. Firstly, the low rate of participation of both cases and controls, resulting in a study population that was younger, had more females, wealthier, and likely more health-conscious compared to the intended source population. This bias may have been attenuated during multivariable analysis, and the overall consistency of the findings with those in the published literature increase our confidence in the results. Indeed, several factors associated with higher risk of infection in our study have previously been documented in other studies, such as an increasing number of household members[30,31], having children attending school[31], [32], [33], or being a health care worker[34]. Secondly, case-control studies for determining vaccine effectiveness require careful selection of controls and adjustment for potential confounders[35]. Our reliance on recruitment of controls through a representative panel from a professional market research and public opinion company, combined with fine adjustment on age, sex, region, population density, calendar week, and several other variables, seem to have adequately responded to these requirements. It is of interest that of all variables examined, many of them associated with the risk of SARS-CoV-2 infection, only being a health care worker confounded the association between vaccination and recent infection. Health care workers, being at higher risk of infection due to occupation-related exposure, and more likely to be vaccinated due to prioritisation of COVID-19 vaccine deployment, should therefore be considered either through adjustment or restriction in the analyses of vaccine effectiveness in population-based studies. The case-control design, without information on disease course, did not allow us to estimate the protection against severe forms of disease. However, a recent publication has shown similar estimates of vaccine effectiveness against SARS-CoV-2 infection (89·5% for infection with B.1.1.7 lineage and 75·0% for infection with B.1.351 lineage), which translated to very high vaccine effectiveness (97%) against severe forms of COVID-19 following 14 days or more after two doses of BNT162b2 mRNAvaccine [16]. A further limitation is the information available on the vaccine type which prevented us from assessing vaccine effectiveness by type of mRNA vaccine (BNT162b2 or mRNA-1273), vaccine effectiveness of one dose of mRNA vaccine or of one dose of ChAdOx1 nCoV-19 vaccine. More specifically, we assumed that participants who received two doses had received mRNA vaccines since the recommended schedule did not allow second doses prior to 6 May 2021, i.e., one week after the end of the study period. Looking at the national vaccine database, we observed that some individuals had received two doses of ChAdOx1 nCoV-19 vaccine before 1 May 2021 despite the recommendations. They represented only 30 077/6 502 183 (0·5%) of all second doses and must have been very rare in the Eastern part of France where the B.1.351 was first circulating and where the recommendation was made to use mRNA vaccines for immunization as early as February 2021. BNT162b2 and mRNA-1273 represented 89·8% and 9·7% of all second doses given, respectively. Although the study power was large enough to identify as statistically significant the protection conferred by past infection and vaccine against the original virus and variants of concern, it was not sufficient to document whether the protection was different across virus types. On-going recruitment of participants may eventually show these differences with time if they are of epidemiological significance. Further, the screening system initially focused on the detection of the B.1.1.7 and the B.1.351/P.1 lineages, has evolved over time with the inclusion of the E484K target in some screening tests. Therefore, depending on the combination of target mutations of the screening test used, classification of some variants could be inconsistent and occasionally appear as original virus. However, nationwide surveillance data suggest that B.1.351 was far more common in France during the study period compared to P.1 or other variants containing mutations that could contribute to reduced vaccine effectiveness [23]. Finally, the data protection authority in France does not allow the collection of data on ethnicity and for this reason, we have not been able to report on risk of infection or vaccination by ethnicity.
Overall, we have been able to show that two doses of mRNA vaccines were effective against the original virus, the B.1.1.7 lineage, and B.1.351/P.1 lineages. The results of our analysis are encouraging for the ongoing COVID-19 mass vaccination campaign in France, for which mRNA vaccines constitute the large majority of COVID-19 vaccine doses procured [36]. Despite the circulation of variants of concern, the continued rollout of mRNA COVID-19 vaccines, following the two-dose regimen, can be expected to reduce severe forms of COVID-19, as well as symptomatic infections, as per the findings of our analysis. Nonetheless, each of these variants currently circulating in France have demonstrated increased transmissibility [37], [38], [38], so it will be important that the COVID-19 mass vaccination efforts are accompanied by public health and social measures that effectively control SARS-COV-2 transmission until a substantial proportion of the population has been fully vaccinated.
Contributors
AF, SG, TC, LS, FO, CD, FC, SC, A Mailles, and DLB designed the investigation.
SG, TC, LS, AF, AS, A Mailles, and DLB developed the study questionnaire.
FO, CD, AR, and A Maurizot managed the data collection online.
OC and CVP oversaw the adherence of the study to the regulatory requirements.
LS and TC oversaw the collection of the data and maintained the database.
LS, TC, and AF performed the statistical analyses.
SW, VE, LL, AG and BL organised the screening and sequencing of SARS-CoV-2 at national level.
TC, RG and AF drafted the first versions of the manuscript.
All authors critically reviewed and approved the final version of the manuscript.
Data availability statement
The data that support the findings of this study are available from the Caisse Nationale d'Assurance Maladie, a national health insurance agency in France and from Ipsos, a French market research and public opinion specialist company. Restrictions apply to the availability of these data, which were used under authorized agreement for this study by the data protection authority Commission Nationale de l'Informatique et des Libertés (CNIL). Access to these data would therefore require prior authorization by the CNIL.
Declaration of Competing Interests
All authors have nothing to declare.
Acknowledgments
Acknowledgments
We would like to thank the Action Coordonnée transmission group of the ANRS-MIE for helpful discussions on the study design, and Violette Mouro (Conseil d'Orientation de la Stratégie Vaccinale) for providing us with data on vaccine distribution in France.
Funding
The study was funded by Institut Pasteur and Research & Action Emerging Infectious Diseases (REACTing). AF's laboratory receives support from the Labex IBEID (ANR-10-LABX-62-IBEID) and the INCEPTION project (PIA/ANR-16-CONV-0005) for studies on emerging viruses. TC is funded by the Fondation de France (Alliance “Tous unis contre le virus”). SW's laboratory receives support from Institut Pasteur, CNRS, Université de Paris, Santé publique France, Labex IBEID (ANR-10-LABX-62-IBEID), REACTing (Research & Action Emerging Infectious Diseases), and by the H2020 project 101003589 (RECOVER).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100171.
Appendix. Supplementary materials
References
- 1.Volz E., Mishra S., Chand M. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021 doi: 10.1038/s41586-021-03470-x. published online March 25. [DOI] [PubMed] [Google Scholar]
- 2.Tegally H., Wilkinson E., Giovanetti M. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 3.Faria N.R., Mellan T.A., Whittaker C. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021 doi: 10.1126/science.abh2644. published online April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betton M., Livrozet M., Planas D. Sera neutralizing activities against SARS-CoV-2 and multiple variants six month after hospitalization for COVID-19. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021 doi: 10.1093/cid/ciab308. published online April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cele S., Gazy I., Jackson L. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021;593:142–146. doi: 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dejnirattisai W., Zhou D., Supasa P. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021 doi: 10.1016/j.cell.2021.03.055. published online March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Planas D., Bruel T., Grzelak L. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med. 2021 doi: 10.1038/s41591-021-01318-5. published online March 26. [DOI] [PubMed] [Google Scholar]
- 8.Lustig Y., Nemet I., Kliker L. Neutralizing Response against Variants after SARS-CoV-2 Infection and One Dose of BNT162b2. N Engl J Med. 2021 doi: 10.1056/NEJMc2104036. published online April 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu K., Werner A.P., Koch M. Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. N Engl J Med. 2021;384:1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall V.J., Foulkes S., Saei A. April 23, 2021. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study Lancet 2021 Published Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan N., Barda N., Kepten E. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhi S.A., Baillie V., Cutland C.L. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021 doi: 10.1056/NEJMoa2102214. published online March 16. DOI:10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadoff J., Gray G., Vandebosch A. Safety and efficacy of single-dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101544. published online April 21. [DOI] [PubMed] [Google Scholar]
- 14.Shinde V., Bhikha S., Hoosain Z. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021 doi: 10.1056/NEJMoa2103055. published online May 5. DOI:10.1056/NEJMoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kustin T., Harel N., Finkel U. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2 mRNA vaccinated individuals. medRxiv. 2021 doi: 10.1038/s41591-021-01413-7. 2021.04.06.21254882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Raddad L.J., Chemaitelly H., Butt A.A. National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021 doi: 10.1056/NEJMc2104974. published online May 5. DOI:10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galmiche S., Charmet T., Schaeffer L. Exposures associated with SARS-CoV-2 infection in France: a nationwide online case-control study. Lancet Regional Health Europe. 2021;7 doi: 10.1016/j.lanepe.2021.100148. (Lancet Reg Health Eur. 2021Epub 2021 Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control. Data on 14-day notification rate of new COVID-19 cases and deaths. Available from: https://www.ecdc.europa.eu/en/publications-data/data-national-14-day-notification-rate-covid-19. Accessed 2 June 2021.
- 19.Greinracher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov19 vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haute Autorité de Santé. Avis n° 2021.0018/AC/SEESP du 19 mars 2021 du collège de la Haute Autorité de santé sur la place du vaccin AstraZeneca dans la stratégie vaccinale suite à l'avis de l'agence européenne des médicaments concernant des évènements indésirables survenus dans plusieurs pays européens chez des personnes vaccinées. Available from:https://www.has-sante.fr/jcms/p_3244283/fr/avis-n-2021-0018/ac/seesp-du-19-mars-2021-du-college-de-la-haute-autorite-de-sante-sur-la-place-du-vaccin-astrazeneca-dans-la-strategie-vaccinale-suite-a-l-avis-de-l-agence-europeenne-des-medicaments-concernant-des-evenements-indesirables-survenus-dans-plusieurs-pays-europeens-chez-des-personnes-vaccinees. Accessed 2 June 2021
- 21.Haute Autorité de Santé. Stratégie de vaccination contre le SARS-CoV-2 - Vaccination des personnes ayant un antécédent de Covid-19. Available from: https://www.has-sante.fr/jcms/p_3237271/fr/strategie-de-vaccination-contre-le-sars-cov-2-vaccination-des-personnes-ayant-un-antecedent-de-covid-19. Accessed 2 June 2021.
- 22.Gaymard A., Bosetti P., Feri A. Early assessment of diffusion and possible expansion of SARS-CoV-2 Lineage 20I/501Y.V1 (B.1.1.7, variant of concern 202012/01) in France, January to March 2021. Euro Surveill. 2021 Mar;26(9) doi: 10.2807/1560-7917.ES.2021.26.9.2100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santé Publique France. Quelle est l'évolution moléculaire des virus SARS-CoV-2 circulant sur le territoire ? Résultats de l'enquête Flash#6https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/enquetes-etudes/quelle-est-l-evolution-moleculaire-des-virus-sars-cov-2-circulant-sur-le-territoire-resultats-de-l-enquete-flash-6. Published on 3 May 2021.Accessed 11 May 2021
- 24.Polack F.P., Thomas S.J., Kitchin N. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baden L.R., El Sahly H.M., Essink B. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez Bernal J., Andrews N., Gower C. Early effectiveness of COVID-19 vaccination with BNT 162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv. 2021 doi: 10.1101/2021.03.01.21252652. [DOI] [Google Scholar]
- 27.Hall V.J., Foulkes S., Charlett A. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet Lond Engl. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lumley S.F., O'Donnell D., Stoesser N.E. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen C.H., Michlmayr D., Gubbels S.M., Mølbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet Lond Engl. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward H., Atchison C., Whitaker M. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun. 2021;12:905. doi: 10.1038/s41467-021-21237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrat F., de Lamballerie X., Rahib D. Antibody status and cumulative incidence of SARS-CoV-2 infection among adults in three regions of France following the first lockdown and associated risk factors: a multicohort study. Int J Epidemiol. 2021 doi: 10.1093/ije/dyab110. (accepted) Forbes H, Morton CE, Bacon S, et al. Association between living with children and outcomes from covid-19: OpenSAFELY cohort study of 12 million adults in England. BMJ 2021; 372: n628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lessler J., Grabowski M.K., Grantz K.H. Household COVID-19 risk and in-person schooling. Science. 2021 doi: 10.1126/science.abh2939. published online April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollán M., Pérez-Gómez B., Pastor-Barriuso R. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet Lond Engl. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopalco P.L., DeStefano F. The complementary roles of Phase 3 trials and post-licensure surveillance in the evaluation of new vaccines. Vaccine. 2015;33:1541–1548. doi: 10.1016/j.vaccine.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ministère des solidarités et de la santé. Données relatives aux livraisons de vaccins contre la COVID-19. https://www.data.gouv.fr/en/datasets/donnees-relatives-aux-livraisons-de-vaccins-contre-la-covid-19. Accessed on 11 May 2021.
- 36.Davies N.G., Abbott S., Barnard R.C. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson, C.A.B. et al. Estimates of severity and transmissibility of novel South Africa
- 38.SARS-CoV-2 variant 501Y.V2. Preprint at https://cmmid.github.io/topics/covid19/sa-novel-variant.html (2021). Accessed 31 May 2021
- 38.Stefanelli P., Trentini F., Guzzetta G. Co-circulation of SARS-CoV-2 variants B.1.1.7 and P.1. medRxiv. 2021 doi: 10.1101/2021.04.06.21254923. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Caisse Nationale d'Assurance Maladie, a national health insurance agency in France and from Ipsos, a French market research and public opinion specialist company. Restrictions apply to the availability of these data, which were used under authorized agreement for this study by the data protection authority Commission Nationale de l'Informatique et des Libertés (CNIL). Access to these data would therefore require prior authorization by the CNIL.