Sleep is essential for the brain: Learning and memory benefit from sleep, whereas sleep loss causes cognitive impairment that can only be reversed by sleep (1, 2). The pressure for sleep increases with time spent awake, and sleep need also depends on the richness of the waking experience and the amount of learning (1, 3). Findings suggest that the restorative effects of sleep are linked to its ability to affect neuronal activity and synaptic plasticity, the capacity to change structure and function based on experience. Synapses are the foundation of neuronal plasticity; in an adult brain, synapses can change their strength and size within minutes or hours in response to new experience and learning (4). On pages 200 and 201 of this issue, Noya et al. (5) and Brüning et al. (6), respectively, provide evidence that sleep need and synaptic function are tightly linked.
The propensity to fall asleep does not depend only on the duration and intensity of prior waking (homeostatic regulation). Sleep is also under circadian regulation—that is, each of us has a preferred time to go to sleep according to our internal body clock. The two mechanisms are to some extent independent of each other and can be studied separately. Normally, however, they work together to consolidate sleep in one major phase of the 24-hour cycle: the night for humans and the day for nocturnal animals such as mice. The mechanisms responsible for circadian regulation are well characterized at the anatomical and molecular level (7). For example, in both humans and mice there is a master clock located in a single brain area, the suprachiasmatic nucleus of the hypothalamus. This nucleus plays a key role in the daily programming of many organismal functions, from feeding and body temperature to the sleep-wake cycle.
How circadian and homeostatic factors interact to regulate sleep, however, remains unclear. One reason is that a single brain area responsible for the homeostatic regulation of sleep has yet to be discovered. The sleep homeostat may be a distributed system composed of neurons and glial cells such as astrocytes. During waking, such a system may track the duration and intensity of brain activity by sensing cellular signals, including by-products of cellular metabolism such as adenosine. It is unknown how many homeostatic signals exist, how they interact, or how they are transmitted to the specific brain areas that are important to initiate sleep.
The studies of Noya et al. and Brüning et al. focus on the synapses of the mouse forebrain, the largest, anterior part of the brain that includes the cerebral cortex and the hippocampus. These brain regions are key areas for learning and memory and for many cognitive functions that are affected by sleep deprivation. Together, the two studies offer the first comprehensive overview of how thousands of proteins and their phosphorylation amounts, as well as the messenger RNAs (mRNAs) from which the proteins are transcribed, change across 24 hours in mice. The experiments used biochemical methods to isolate synapses, followed by deep sequencing and advanced mass spectrometry–based proteomics. The results show that synaptic mRNA expression levels undergo daily rhythms, peaking after the mice have been asleep for many hours (around dusk) or at the onset of their waking period (around dawn). A sizable minority of proteins (12%) and more than half of all phosphoproteins also cycle, showing daily rhythms in abundance and phosphorylation status, respectively, and peaking at dusk or dawn (see the figure).
figure. Synapses keep track of sleep need.
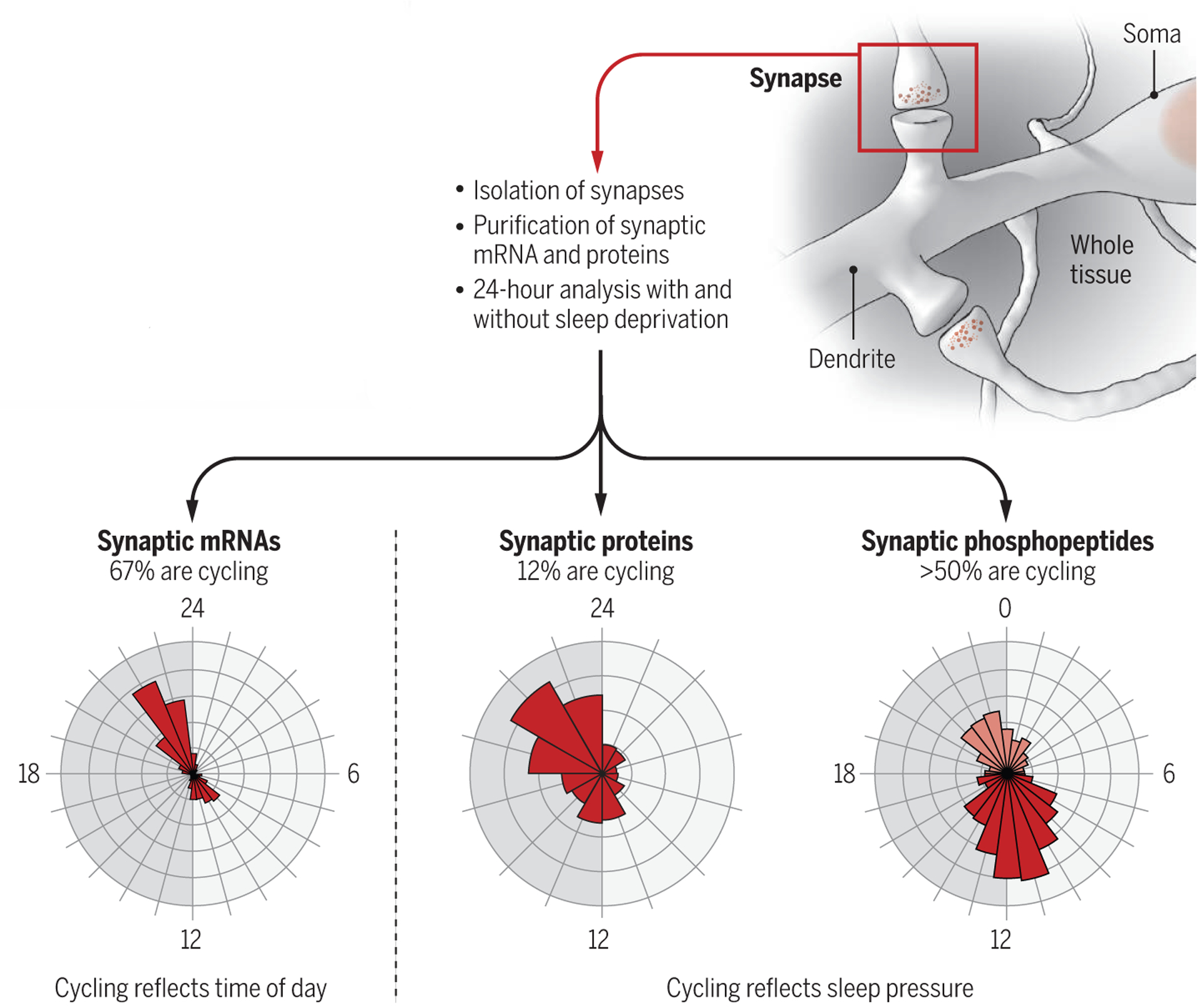
In mice, the expression of many synaptic mRNAs and proteins and protein phosphorylation oscillate across the 24-hour period in a distinct manner that is not observed in whole tissue. Dawn and dusk are peak times for both mRNA and protein expression, but their cycling reflects different processes.
Do these changes in mRNA expression, protein abundance, and protein phosphorylation reflect how long mice were asleep and awake (homeostatic factor), or do they track time across the 24 hours, independent of behavioral state (circadian factor)? Answering this question is not trivial because the propensity to fall asleep depends, at any given moment, on both time of day and duration and intensity of prior waking (8). Noya et al. and Brüning et al. deprived the mice of sleep for a few hours at different times of day and night to increase sleep pressure to a similar extent across the 24-hour cycle (9). The daily oscillations in mRNA expression were either unaffected or reduced (but not abolished) in sleep-deprived mice, indicating that a clock mechanism permits the accumulation of transcripts at specific “rush hours” around dusk and dawn, independent of whether sleep has occurred. Proteins, however, rather than DNA or RNA, carry out most cellular functions. Intriguingly, the daily rhythms of almost all cycling proteins and phosphoproteins were completely abolished when sleep need was kept high, indicating that the cycling of these proteins and phosphoproteins was driven only by sleep and waking, independent of time of day. Of note, the synaptic proteins that show daily changes in abundance differed from those that show changes in phosphorylation levels, implying that both analyses—protein abundance and phosphorylation status—are needed to fully understand how sleep pressure affects synaptic function. Moreover, there was little overlap between the changes in transcriptome, proteome, and phosphoproteome occurring in the synapses and those detected when using whole tissue. This finding reinforces the view that synapses are a special neuronal compartment, affected by circadian and homeostatic factors in distinct ways that differ from those in the cell body of neurons and glial cells.
Although our understanding of the molecular mechanisms that track the need for sleep remains incomplete, a clear message from the studies of Noya et al. and Brüning et al. is that a good place to start looking is the synapse. This conclusion is supported by recent findings of a quantitative phosphoproteomic study performed in whole mouse brain (10). By using two different models of high sleep pressure, the average phosphorylation status of 80 proteins could track changes in sleep need; strikingly, most were synaptic proteins involved in neurotransmitter release and synaptic plasticity. Brüning et al. also found that most of these proteins had specific sites with increased phosphorylation at times of high sleep pressure. By looking across the entire 24-hour period, however, they could also identify many other sites with increased phosphorylation during sleep time.
One of the challenges of omics studies is to provide functional meaning to the long lists of genes and proteins that are identified. As a first step in this direction, Brüning et al. focused on kinases (which carry out protein phosphorylation), many of which were found to be enriched in the synapses and modulated by phosphorylation according to sleep pressure. In a few specific cases, they could infer kinase activity from the phosphorylation status of the enzyme. They were thus able to predict whether the enzyme was activated or not. The results suggest that several kinases involved in excitatory activity and synaptic potentiation become active at the transition to the waking phase (before dusk), whereas several kinases involved in inhibitory activity and synaptic depression become active at the onset of the sleep phase (dawn). This is consistent with overall higher levels of excitatory glutamatergic activity in waking relative to sleeping rodents (11). These data also agree with previous studies that isolated the whole set of synapses from rat cerebral cortex and hippocampus (12) and from mouse forebrain (13) and found a net overall increase in synaptic strength after waking relative to sleep.
The findings of Noya et al. and Brüning et al. together with previous evidence (10), leave little doubt that synaptic function reflects time spent awake and asleep in mice. One challenge will be to understand how this local signal is translated into sleep, a whole-organism phenomenon. Also, it is unknown which critical aspects of synaptic function require sleep to be restored. Two main hypotheses have been proposed to account for the beneficial effects of sleep on cognition. One idea is that sleep consolidates memories by further strengthening the synaptic connections potentiated by recent learning (1, 14). A competing hypothesis proposes that sleep leads to memory consolidation by promoting broad but selective synaptic weakening (synaptic down-selection) (15). This second mechanism could also explain why sleep can restore the capacity for new learning: by preventing synapses from becoming so strong that they cannot be further potentiated (synaptic saturation). Measuring synaptic function with single-synapse resolution—in many synapses, over many hours of sleep and waking—would be the ideal next step to test these ideas.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (P01NS083514) and the U.S. Department of Defense (W911NF1910280).
REFERENCES AND NOTES
- 1.Rasch B, Born J, Physiol. Rev 93, 681 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goel N, Basner M, Rao H, Dinges DF, Prog. Mol. Biol. Transl. Sci 119, 155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donlea JM, Shaw PJ, Adv. Genet 68, 57 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi T, Duszkiewicz AJ, Morris RG, Philos. Trans. R. Soc. Lond. B Biol. Sci 369, 20130288 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noya SB et al. , Science 366, eaav2642 (2019).31601739 [Google Scholar]
- 6.Brüning F et al. , Science 366, eaav3617 (2019).31601740 [Google Scholar]
- 7.Partch CL, Green CB, Takahashi JS, Trends Cell Biol. 24, 90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eban-Rothschild A, Appelbaum L, de Lecea L, Neuropsychopharmacology 43, 937 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maret S et al. , Proc. Natl. Acad. Sci. U.S.A 104, 20090 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z et al. , Nature 558, 435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G, J. Neurosci 29, 620 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G, Nat. Neurosci 11, 200 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Diering GH et al. , Science 355, 511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank MG, Cantera R, Trends Neurosci. 37, 491 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tononi G, Cirelli C, Neuron 81, 12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


