Abstract
Objective
To investigate the prospective associations of life-course perfluoroalkyl substances (PFASs) exposure with glucose homeostasis at adulthood.
Methods
We calculated insulin sensitivity and beta-cell function indices based on 2-h oral glucose tolerance tests at age 28 in 699 Faroese born in 1986–1987. Five major PFASs were measured in cord whole blood and in serum from ages 7, 14, 22, and 28 years. We evaluated the associations with glucose homeostasis measures by PFAS exposures at different ages using multiple informant models fitting generalized estimating equations and by life-course PFAS exposures using structural equation models.
Results
Associations were stronger for perfluorooctane sulfonate (PFOS) and suggested decreased insulin sensitivity and increased beta-cell function—for example, β (95% CI) for log-insulinogenic index per PFOS doubling = 0.12 (0.02, 0.22) for prenatal exposures, 0.04 (−0.10, 0.19) at age 7, 0.07 (−0.07, 0.21) at age 14, 0.05 (−0.04, 0.15) at age 22, and 0.04 (−0.03, 0.11) at age 28. Associations were consistent across ages (P for age interaction > 0.10 for all PFASs) and sex (P for sex interaction > 0.10 for all PFASs, except perfluorodecanoic acid). The overall life-course PFOS exposure was also associated with altered glucose homeostasis (P = 0.04). Associations for other life-course PFAS exposures were nonsignificant.
Conclusions
Life-course PFAS exposure is associated with decreased insulin sensitivity and increased pancreatic beta-cell function in young adults.
Keywords: perfluoroalkyl substances, insulin resistance, beta-cell function, type 2 diabetes, endocrine disruptors, developmental exposures
Recent biomonitoring studies show ubiquitous exposure to major perfluoroalkyl substances (PFASs), including perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), perfluorohexane sulfonic acid (PFHxS), perfluorodecanoic acid (PFDA) and perfluorononanoic acid (PFNA) (1). PFASs have been used for decades as industrial surfactants, in surface coatings and fire-fighting foams, and in a wide range of consumer products (eg, nonstick cookware, food containers, and waterproof textiles). Due to their slow degradation, PFASs persist in the environment and humans for years (1,2). Biological half-lives of the major PFASs in humans have been estimated in the range of 3 to 7 years (1). PFAS exposure starts in utero (3), with subsequent exposure via breast milk (4) and ingestion of contaminated food and water, as well as house dust (5-7). Extensive laboratory data show that PFASs act as metabolic disruptors (1) and may promote insulin resistance and impaired glucose tolerance, either through direct toxicity to the pancreas (8) or indirectly through enhanced adipogenesis and increased lipid accumulation in the adipose and liver tissues (9,10).
Previous epidemiology studies have reported associations of PFAS exposures in adulthood (11-15) or during development (16-20) with markers of insulin resistance, impaired glucose tolerance, and/or incident type 2 diabetes. However, some other cross-sectional studies have suggested no clear association (21-23), and no previous study has evaluated life-course exposure to PFAS in association to glucose homeostasis and type 2 diabetes. We therefore conducted a population-based birth cohort study to evaluate the associations of PFAS concentrations in blood samples obtained at multiple life stages (prenatal, mid-childhood, puberty, and young adulthood) with markers of glucose homeostasis at age 28 years. In agreement with the developmental origins of type 2 diabetes hypothesis (24) and previous findings in the Faroese population on the association of PFAS with metabolic hormone dysregulation and obesity outcomes (25,26), we hypothesized that the prenatal period is a critical window of heightened susceptibility to PFAS diabetogenesis. We further examined whether associations may differ according to sex, as previous studies have suggested sex-dimorphic PFAS metabolic effects (16,17,26-29).
Methods
Study Population and Design
We used data from a prospective cohort of 1022 singleton births in the Faroes Islands in 1986-1987 (30). The cohort members were invited for clinical examinations at ages 7, 14, 22, and 28 years. The participation rate in the latest examination was 70% of the eligible cohort members. Information at all visits was collected through questionnaires, medical records, blood analyses, and physical examinations. The present analysis included 699 participants who underwent a 2-h oral glucose tolerance test (OGTT) at the 28-year visit and had measured PFAS from at least 1 previous visit. None of the participants were diagnosed for type 1 or type 2 diabetes by the time of the OGTT. The ethical review boards in the Faroe Islands and at the Harvard T.H. Chan School of Public Health approved the study protocols, and mothers and/or adult cohort participants provided written informed consent at all examinations.
PFAS Exposure Assessment
Major PFASs (PFOS, PFOA, PFHxS, PFDA, and PFNA) were quantified in whole blood collected from the cord at birth and in participants’ serum at ages 7, 14, 22, and 28 years. Samples were stored at −80°C prior to analysis. PFAS analysis was conducted at the University of Southern Denmark, using online solid phase extraction and high-pressure liquid chromatography with tandem mass spectrometry as previously described (31).
The whole-blood samples were pretreated with the zinc sulfate to precipitate proteins and blood cells prior to the online solid-phase extraction. In brief, a volume of 150 μL whole blood and 200 μL 0.06 M ZnSO4 in methanol was added into a 2-mL polypropylene sample vial and instantly whirl-mixed for 30 sec. Then, 30 μL of 20 ng/mL isotope-labeled PFAS analogues (internal standard) was added, followed by whirl-mixing and centrifugation at 21 000 g for 20 min. An aliquot of 160 μL of the supernatant was transferred to a polypropylene high-pressure liquid chromatography vial with 400 μL 0.1 M formic acid and whirl-mixed prior to injection of 400 μL onto the solid-phase extraction column. The rest of the analysis was in accordance with the protocol for the serum samples. Within-batch and between-batch coefficient of variations for the serum samples were better than 8.9% and 12.9% respectively, for all analytes, whereas the whole-blood analysis varied up to 13.2%. The limit of detection (LOD) was 0.03 ng/mL for all PFAS analyses in cord whole blood and participants’ serum, except for PFHxS and PFNA analysis in cord whole blood for which the LOD was 0.05 ng/mL. Concentrations below LOD were substituted by a value equal to LOD/2. PFDA and PFNA concentrations were below the LOD in more than 35% of cord whole blood samples (see Supplementary Table 1 and Supplementary Figure 1 in (32)), and therefore prenatal exposure to these PFASs was not further considered.
Markers of Glucose Homeostasis
Participants underwent a 2-h, 75-g OGTT at the 28-year examination. Serum-insulin and plasma-glucose concentrations were measured at baseline (0 min) and at 30 and 120 min after the oral glucose intake. Plasma-glucose was determined on an ABL800 FLEX® (Radiometer Medical, Brønshøj, Denmark). Serum insulin was measured on a Cobas e411 analyzer (Roche, Hvidovre, Denmark). Insulin and glucose concentrations were then used for the calculation of fasting and OGTT-derived indices of insulin sensitivity and pancreatic beta-cell function. We calculated the glucose and insulin areas under the curve (AUC) using the trapezoidal rule:
where G0, G30, and G120 are plasma-glucose concentrations at 0, 30, and 120 min, respectively, and I0, I30, and I120 are the serum-insulin concentrations at 0, 30, and 120 min, respectively.
Other indices of insulin sensitivity and beta-cell function calculated included the Matsuda insulin sensitivity index = (10,000/√, the homeostatic model assessment of insulin resistance = /22.5, the insulinogenic index , and the corrected insulin response = × 100/( × ).
Further, we calculated the oral disposition index, an estimate of beta-cell function adjusted for insulin sensitivity, by multiplying the Matsuda ISI with the CIR (33,34). We considered the OGTT-based indices, Matsuda ISI, and IGI, as the primary outcomes of insulin sensitivity and beta-cell function evaluated in our study, as these indices have showed strong correlations with the gold standard techniques, hyperinsulinemic-euglycemic clamp and the acute insulin response to intravenous glucose, respectively, and are widely used and accepted as surrogate measures of insulin sensitivity and beta-cell function in large-scale studies (35,36). Associations of other indices provide complementary information and are also presented to facilitate comparisons with future studies.
Statistical Analysis
Log-transformations were used to normalize right-skewed distributions of the PFAS exposure variables (log base 2) and indices of insulin sensitivity and beta-cell function (ln). Out of the 699 participants included in analysis, 55% had complete PFAS data at all ages, while 78% of the participants had complete PFAS data from the prenatal, age 14 and both age 22 and age 28 adulthood examinations. Missing data on PFASs were due to incomplete follow-up or missing assent for blood draws (in particular at the age 7 childhood examination). We used multiple imputation methods to incorporate uncertainty for the missing data in the PFAS variables and secondary covariates (Table 1) and to facilitate comparisons of estimates across the examinations (37,38). Multiple imputation by chained equations was performed in subpopulations predefined by sex (39,40). Thirty multiple imputed data sets were generated, accounting for the total of 45% of children who had missing information for at least 1 PFAS measure and/or secondary covariates (41).
Table 1.
Characteristics and exposure (mean concentration) and outcome distributions in the analysis population (N = 699)
| Characteristic | % missings | n (%) or mean ± SD |
|---|---|---|
| Early life (birth in 1986-1987) | ||
| Sex, female | 0 | 355 (51) |
| Maternal prepregnancy BMI, kg/m2 | 18 | 22.3 ± 3.2 |
| Maternal age at delivery, years | 1 | 27.5 ± 5.5 |
| Smoking in pregnancy, yes | 0 | 280 (40) |
| Maternal fish intake in pregnancy, >2 dinners/week | 6 | 326 (49) |
| First-born child, yes | 0 | 231 (33) |
| Breastfeeding duration, months | 7 | 4.4 ± 3.4 |
| Cord blood PFOS, ng/mL blood | 2 | 3.2 ± 1.7 |
| Mid-childhood—age 7 years (1993-1994) | ||
| Exact age at examination, years | 6a | 6.8 ± 0.29 |
| BMI, kg/m2 | 6a | 16.2 ± 1.7 |
| Overweight, yes | 6a | 93 (14) |
| 7-year serum PFOS, ng/mL serum | 32a | 32.8 ± 10.6 |
| Puberty—age 14 years (2000-2001) | ||
| Exact age at examination, years | 7a | 13.8 ± 0.3 |
| BMI, kg/m2 | 7a | 20.5 ± 3.5 |
| Overweight, yes | 7a | 107 (16) |
| 14-year serum PFOS, ng/mL serum | 13a | 32.3 ± 9.4 |
| Adulthood—age 22 years (2008-2009) | ||
| Exact age at examination, years | 8a | 22.0 ± 0.5 |
| BMI, kg/m2 | 8a | 24.8 ± 4.6 |
| Overweight, yes | 8a | 242 (37) |
| 22-year serum PFOS, ng/mL serum | 8a | 13.4 ± 5.7 |
| Adulthood—age 28 years (20140-2015) | ||
| Exact age at examination, years | 0 | 27.7 ± 0.8 |
| BMI, kg/m2 | 0 | 26.1 ± 4.7 |
| Overweight, yes | 0 | 356 (51) |
| 28-year serum PFOS, ng/mL serum | 0 | 7.3 ± 4.0 |
| Fasting glucose (baseline), mmol/L | 0 | 5.0 ± 0.4 |
| Fasting insulin (baseline), pmol/L | 0 | 62.7 ± 36.7 |
| Matsuda insulin sensitivity index (ISI) | 1 | 14.8 ± 7.2 |
| HOMA-IR | 0 | 2.0 ± 1.2 |
| Insulinogenic index | 1 | 259 ± 406 |
| Corrected insulin response (CIR) | 1 | 2340 ± 1899 |
| Glucose AUC | 0 | 753 ± 110 |
| Insulin AUC | 1 | 47 184 ± 31 092 |
| Disposition index (ISI*CIR) | 1 | 31 096 ± 29 277 |
a Missing data due to follow-up losses or missing assent for blood draws: 6% of cohort members did not participate in the 7-year examination, 7% in the 14-year examination and 8% in the 22-year examination. A 32% of study participants did not have available serum at age 7 years, and 13% of study participants did not have available serum at age 14 years (due to follow-up loss or missing assent for blood draws).
Generalized additive models confirmed linearity for all the associations between PFASs and markers of glucose homeostasis under study (Pgain > 0.10). To evaluate differences in the associations between exposure windows, we used multiple informant models fitting generalized estimating equations (GEEs), with an unstructured covariance matrix. The multiple informant GEE model jointly estimates the exposure-outcome association for each exposure window under study and allows testing whether effect estimates are equal across all exposure windows (38). In the multiple informant GEE models, each marker of glucose homeostasis (outcome) was modeled as a function of repeated PFAS measures adjusted for an interaction term between PFASs and age at exposure assessment (0, 7, 14, 22, and 28 years) and potential confounders. Interaction cross-product terms were inserted in the multiple informant GEE models to evaluate modification also by sex. Coefficients from the multiple informant GEE models (beta, 95% CI) are interpreted as the difference in the glucose homeostasis outcome per doubling of the PFASs measured at each exposure time.
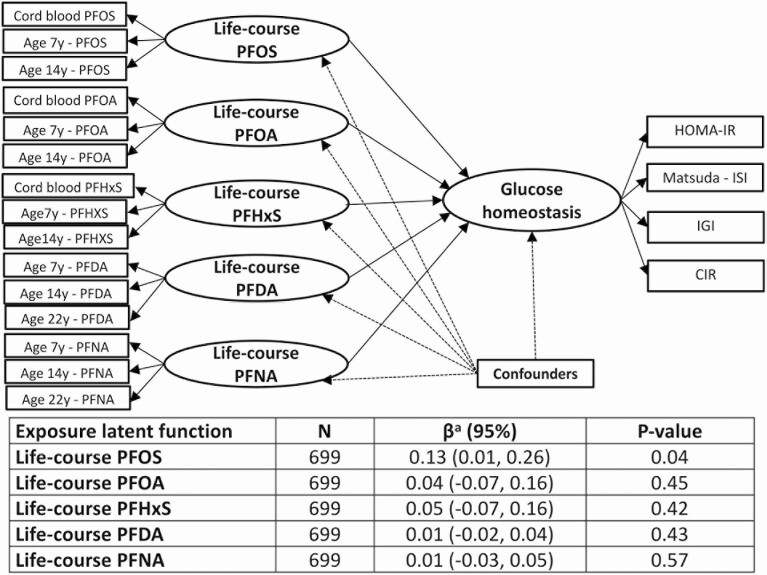
We used a structural equation modeling (SEM) approach to evaluate the associations of exposure to PFASs across the lifespan with the markers of glucose homeostasis. In this approach, we considered the measured indices of insulin sensitivity and beta-cell function as indicators of a latent function of glucose homeostasis at age 28 years and the concentrations of each PFAS at the different ages as indicators of a latent function of exposure to that PFAS over the lifespan (referred to as “life-course PFAS exposure” hereafter). To specify the best set of measured variables related to the latent functions, we used confirmatory factor analysis and evaluated the goodness of fit using the comparative fit index (acceptable if >0.90), the Tucker-Lewis index (acceptable if >0.90), the root mean square error of approximation (acceptable if <0.05), and the standardized root mean square residual (acceptable if <0.05). With this method, the best goodness of fit for the latent function of glucose homeostasis was obtained using the measured concentrations of 2 markers of insulin sensitivity (HOMA-IR and ISI) and 2 markers of beta-cell function (IGI and CIR), with the resultant factor loadings 1, −1.32, 5.08, and 4.58, respectively (see Supplementary Table 2 in (32)). In regard to the latent exposure functions, the best goodness of fit was obtained using the measured concentrations at prenatal, childhood, and puberty periods as indicators of the latent functions for PFOS, PFOA, and PFHxS and the measured concentrations at childhood, puberty, and the 22-year period as indicators of the latent functions for PFNA and PFDA (see Supplementary Table 2 in (32)). The structural part of the SEM described the association of the latent function of life-course exposure to each PFAS with the latent function of glucose homeostasis, adjusted for confounders.
Potential confounders adjusted for in the statistical models were selected based on directed acyclic graphs and a priori knowledge (11-23,25-29): participants’ exact age at examinations, maternal prepregnancy body mass index (BMI; in kg/m2), order of birth (first born: no/yes), maternal smoking during pregnancy (no/yes), and maternal fish intake during pregnancy (>2 dinners per week: no/yes). Because breastfeeding is a source of PFAS exposure in infancy (4) and elevated maternal PFAS exposures are associated with a shorter breastfeeding duration in the Faroese population (42), breastfeeding duration may potentially mediate associations of prenatal PFAS exposure with metabolic outcomes in the offspring. We therefore adjusted for breastfeeding in the statistical models that included postnatal only (without prenatal) PFAS concentrations. Subjects’ BMI may likewise be a mediating factor, rather than a confounder, in the association of PFAS exposure with markers of glucose homeostasis (25). Therefore, we only adjusted models for BMI at age 28 years, when the glucose homeostasis outcomes were also measured, as a sensitivity analyses. Also in sensitivity analyses with SEM, we evaluated whether association of one PFAS substantially changed after adjustment for other PFASs using PFAS latent exposure functions. Potential confounding by exposure to organochlorine compounds was assessed by including latent functions of serum concentrations of polychlorinated biphenyls and p,p’-dichlorodiphenyldichloroethylene, as these chemicals may also be diabetogenic (28). Moreover, we repeated all statistical analyses after excluding: (a) 39 participants who reported to have family history of diabetes (defined as having/had diabetes any of the biological parents and/or siblings) and (b) 1 female participant who was pregnant at the 28-year examination. Further analysis included the comparison of measured covariates between participants with complete versus those with any missing data, as well as the comparison of measured covariates between the analysis sample and the cohort participants who were excluded from this analysis due to loss of follow-up in adulthood.
Statistical analyses were performed using STATA 15 and R package version 3.6.0. Findings are interpreted based on the observed association patterns, and the magnitude and precision of estimates rather than solely relying on P-value cutoffs. For statistical interactions, significance was set at a 2-sided P-value ≤ 0.10.
Results
Almost half of the participants were males, and almost half were classified as overweight at age 28 years (ie, BMI ≥ 25 kg/m2) (Table 1). PFOS showed the highest average concentrations at all examinations, as compared to other PFASs; the highest concentrations were observed in childhood serum, compared to cord blood and adulthood serum (see Supplementary Table 1 and Supplementary Figure 1 in (32)). The lowest average concentrations were detected at all examinations for PFDA, and cord whole blood showed the lowest concentrations, compared to the serum samples. The within- and between-period correlation coefficients for all PFAS pairs ranged from nonsignificant to highly positive (−0.03 ≥ Pearson’s r ≤ 0.90) (see Supplementary Table 3 in (32)). The weakest correlations were seen for PFAS pairs between childhood and adulthood examinations (−0.03 ≥ Pearson r ≤ 0.54), and the strongest correlations for PFAS pairs between the 2 adulthood examinations (ages 22 and 28 years: 0.50 ≥ Pearson r ≤ 0.83) and between PFDA and PFNA concentrations within all examinations (0.75 ≥ Pearson r ≤ 0.90).
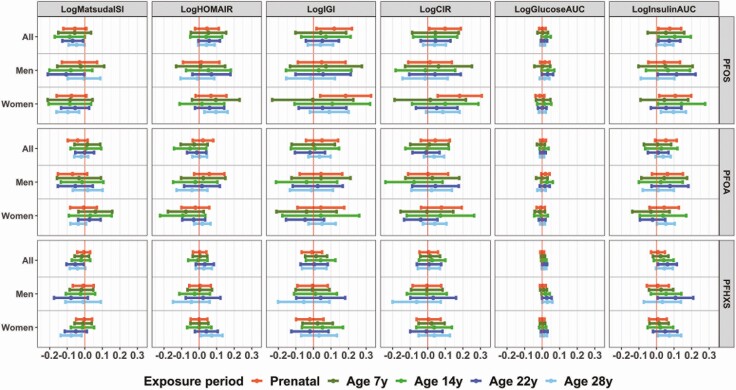
Exposure to PFOS was associated with a decrease in insulin sensitivity (as indicated by the effect estimates for Matsuda ISI and HOMA-IR) and a compensatory increase in insulin secretion (as indicated by the positive effect estimates seen for IGI, CIR, and insulin AUC) (Fig. 1). Associations between PFOS and markers of glucose homeostasis did not significantly differ according to the age at exposure assessment (P-age interaction > 0.10 in all PFOS and outcome statistical models) or sex (P-sex interaction > 0.10 in all PFOS and outcome statistical models). However, we observed associations with markers of beta-cell function (IGI, CIR, and insulin AUC) at somewhat larger magnitudes for prenatal PFOS compared to later exposure windows [eg, for log-IGI in the analysis population overall: adjusted β (95% CI) per PFOS doubling = 0.12 (0.02, 0.22) for prenatal exposure vs 0.04 (−0.10, 0.19) for exposure at age 7; 0.07 (−0.07, 0.21) for exposure at age 14; 0.05 (−0.04, 0.15) for exposure at age 22; and 0.04 (−0.03, 0.11) for exposure at age 28; P-age interaction = 0.75]. Similarly, associations between PFOS and beta-cell function markers were somewhat larger and significant only in women (Fig. 1; also see Supplementary Table 4 in (32)).
Figure 1.
Adjusted mean change (beta, 95% CI) in markers of glucose homeostasis per doubling of exposure to PFOS, PFOA, or PFHxS, in the population overall and in specific strata according to sex. Effect estimates from longitudinal Multiple informant GEE models adjusted for an interaction term between each PFAS and age at exposure assessment, exact age at examinations, sex, order of birth, maternal prepregnancy BMI, and maternal smoking and fish intake during pregnancy.
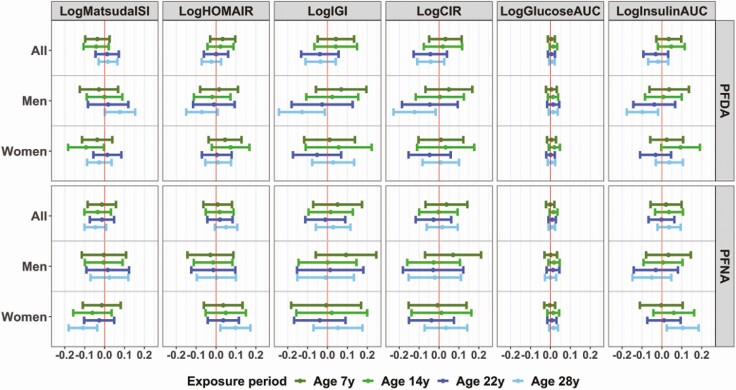
Associations for PFOA and PFHxS tended to be in the same direction as those for PFOS, but effect estimates were smaller and did not reach statistical significance (Fig. 1; also see Supplementary Table 4 in (32)). Similar to the PFOS results, interactions by age and sex were not significant for PFOA and PFHxS. In regard to postnatal exposures to PFDA and PFNA, we found few significant associations, especially for exposure at age 28 years and in one sex only, while associations in the other sex were null (Fig. 2; also see Supplementary Table 5 in (32)). More specifically, age 28 serum PFDA was associated with a significant increase in insulin sensitivity and a decrease in insulin secretion in men only (P-sex interaction = 0.128 for logMatsudaISI, 0.161 for logCIR, and 0.142 for logInsulin AUC). In contrast, age 28 PFNA was associated with a significant decrease in insulin sensitivity and an increase in insulin secretion in women only (P-sex interaction = 0.067 for logMatsudaISI, 0.062 for logHOMAIR, and 0.146 for logInsulin AUC) (Fig. 2; also see Supplementary Table 5 in (32)). No other significant associations were observed.
Figure 2.
Adjusted mean change (beta, 95% CI) in markers of glucose homeostasis per doubling of postnatal exposure to PFDA or PFNA in the population overall, and in specific strata according to sex. Effect estimates from longitudinal multiple informant GEE models including an interaction term between each PFAS and age at exposure assessment and additionally adjusted for exact age at postnatal examinations, sex, order of birth, maternal prepregnancy BMI, maternal smoking and fish intake during pregnancy, and breastfeeding duration.
Results from the SEM analyses (Fig. 3) agreed with the results from the GEE analysis and suggested that PFOS exposure over the lifespan is associated with altered glucose homeostasis at age 28 years [0.13 SD units change (95% CI: 0.01, 0.26) in the latent glucose homeostasis function per SD increase in life-course PFOS exposure]. Associations for life-course exposure to other PFASs were of much smaller magnitude and did not reach statistical significance. In the SEM, all PFASs and glucose homeostasis indicators showed significant correlations with their respective latent functions (for factor loadings, see Supplementary Table 2 in (32)). Effect estimates did not change after simultaneous adjustment of the other latent life-course PFASs or organochlorine compounds functions in the SEM or after inclusion of BMI at age 28 years in the statistical models (data not shown). Excluding the 6% of cohort participants with self-reported family history of diabetes did not change the magnitude or direction of any exposure-outcome associations examined (data not shown). Moreover, excluding 1 female participant who was pregnant at the 28-year examination did not change any of the exposure-outcome associations estimated either overall or in female-specific analyses (data not shown). Further, our analysis of the disposition index, which serves as a marker of beta-cell function that accounts for insulin sensitivity, revealed null associations with all PFASs at the exposure windows examined (see Supplementary Table 6 in (32)). Comparison of measured covariates, including other PFAS concentrations and glucose homeostasis outcomes (when available), between participants with complete data (n = 382) vs those with any missing data (n = 317), as well as between the population sample (n = 699) vs cohort participants excluded from this analysis (n = 323), did not reveal any meaningful differences or a systematic pattern of missing data (see Supplementary Table 7 in (32)).
Figure 3.
Path diagram and standard error of the mean effect estimates for the association between life-course latent PFAS exposure functions and the latent glucose homeostasis function estimated based on observed markers of insulin sensitivity and beta-cell function. aChange in the standard deviation of the glucose homeostasis function per 1 SD increase in life-course PFAS exposures, adjusted for sex, order of birth, maternal prepregnancy BMI, maternal smoking and fish intake during pregnancy, and breastfeeding duration.
Discussion
This study examined multiple windows of exposure to major PFASs in regard to markers of glucose homeostasis in early adulthood. Our findings suggest that PFOS exposure is prospectively associated with decreased insulin sensitivity in early adulthood and a compensatory increase in insulin secretion by pancreatic beta cells in response to a 2-h OGTT. Associations for other PFASs, including PFOA, PFHxS, and PFNA, were in the same direction as for PFOS but were weaker and/or nonsignificant. Associations did not significantly differ between exposure windows, suggesting that both early-life and adulthood exposures may impact glucose homeostasis. We further did not find any robust evidence for sex modification, with only few effect estimates for PFOS and PFNA that were of stronger magnitude in women compared to men. The associations of PFAS exposure with decreased insulin sensitivity and a likely compensatory increase in insulin response observed in this population of young and otherwise healthy adults suggest that PFAS exposures during early life may contribute to an increased risk for type 2 diabetes development at later ages, as has been previously seen also for PFAS exposures occurring in adulthood (11,12).
Diabetogenic effects of PFAS exposure have been described in laboratory studies for both developmental (8,10,43) and adult life (44,45) exposures. For example, in line with our findings, developmental exposure of rats to PFOS induced a prediabetic state, with increased serum insulin, insulin resistance, and impaired glucose tolerance in adulthood (10) and similarly induced relevant structural and gene expression changes in the pancreas of zebrafish (8). Adult-life exposures to PFOA and PFNA also promoted increased serum insulin levels and insulin resistance in mice (44) and rats (45). Thus, it is likely that diabetogenic effects of PFAS exposure start early in life and continue as exposure persists through adulthood.
Only a few population studies so far have focused on the association of developmental PFAS exposures with markers of glucose homeostasis. Two previous Danish cohort studies reported that prenatal exposure to PFOA and less clearly to PFOS was associated with increased serum insulin concentrations in early adulthood (16) and that childhood exposure to PFOA was associated with decreased serum-insulin and beta-cell function (as indicated by a decrease in HOMA-beta) in adolescence (20). A third study in US children found no association between prenatal PFAS exposures and HOMA-IR in mid-childhood and a negative association in cross-sectional analysis of mid-childhood PFAS exposures and HOMA-IR (17). However, a recent study in US Hispanic children with overweight and obesity at age 8 to 14 years found positive prospective associations for PFOA and PFHxS with impaired glucose tolerance markers at a 2-h OGTT (18). Even though significant associations with glucose homeostasis and other metabolic markers are supported for other PFASs by previous animal (44,45) and human studies (16-18,20), in this study as in previous investigations in the Faroese population (25,28), we have observed stronger associations for PFOS compared to other major PFASs. One possible explanation for this finding may be the significantly wider exposure range seen for PFOS at all ages compared to other PFASs.
More consistent findings come from studies focused on adult exposure. For example, prospective studies have reported positive associations between PFAS exposure and type 2 diabetes risk in US female nurses (11), increased insulin resistance with or without (depending on the PFAS) an increase in beta-cell function after a 2-h OGTT in Danish pregnant women (46), and a positive association between plasma PFOA concentrations and incidence of type 2 diabetes over 15 years of follow-up in US adults at high risk for type 2 diabetes (12). In further support of a potential diabetogenic effect of adult PFAS exposures are findings from some cross-sectional studies (13,14), although some such studies showed no clear association (21-23), perhaps in part due to the absence of longitudinal exposure data and other methodological limitations linked to cross-sectional study designs. In conjunction with previous studies, findings from our prospective investigation suggest that exposure to PFASs, and especially to PFOS, is significantly associated with a decrease in insulin sensitivity that appears to be sufficiently compensated by an increase in insulin release by the pancreatic beta-cells in the young adults, as indicated by the null associations observed between PFAS exposure and the oral disposition index. To further support a link between long-term PFAS exposure and the risk of type 2 diabetes in adulthood, longitudinal study designs should integrate multiple exposure windows and long-term follow-up of participants to fully elucidate the impact of lifetime PFAS exposure on type 2 diabetes. This consideration is in line with experimental models that suggest long-term and delayed diabetogenic effects of developmental PFAS exposures that may become detectable in later adulthood (8,10,43).
Sample size restrictions may have masked potentially diverging association patterns between men and women that may require larger studies to be characterized or longer follow-up. Other potential limitations of our study include the lack of information on participants’ dietary habits and physical activity patterns as major risk factors for type 2 diabetes. However, these factors are questionable predictors of early-life PFAS exposures and are therefore unlikely to have confounded the associations observed in this study. Further, we were not able to evaluate diabetes family history as a potential effect modifier in the associations of interest as only few participants (6%) reported to have known family history of diabetes; future investigations with more optimal assessment of genetic predisposition are needed to further examine this hypothesis. The prospective and population-based study design with the extended follow-up of participants from birth to young adulthood is a major strength of the present study that has permitted the investigation of multiple time windows of PFAS exposure and lifetime exposure in association with markers of glucose homeostasis. Other important strengths include the extensive biomarker-based assessment of exposure to PFASs and other environmental contaminants that may be potentially diabetogenic, as well as the comprehensive evaluation of glucose homeostasis using 2-h OGTT. Further, the Faroese population is homogeneous in regard to socioeconomic and lifestyle factors, which can minimize unmeasured and residual confounding, although possibly limiting the generalizability of findings.
Acknowledgments
The authors are grateful to the study participants for their generous collaboration in the Faroese cohort studies. This study received funding from the National Institute of Environmental Health Sciences (NIEHS) of the NIH (grant number R01 ES021477), the Faroese Research Council and the Danish Environmental Protection Agency (EPA). DV is currently supported by NIEHS P30 ES023515 and R21 ES029328. PG is supported by P42 ES027706.
Additional Information
Disclosures: PG has provided paid expert assistance in legal cases involving PFAS exposed populations. All other authors have no competing interests to declare, financial or otherwise.
Data Availability:
The data sets generated during and/or analyzed during the current study are not publicly available. For access to individual data, inquiries should be made to the Faroese Hospital System (dfaa@health.fo), and approval must be obtained from the Faroese Ethical Review Board (vsn@vsn.fo).
References
- 1. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Perfluoroalkyls (Draft for Public Comment). US Department of Health and Human Services, Public Health Service; 2018. Accessed on February 5, 2021. doi: 10.15620/cdc:59198. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=1117&tid=237 [DOI] [Google Scholar]
- 2. Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45(19):7954-7961. [DOI] [PubMed] [Google Scholar]
- 3. Needham LL, Grandjean P, Heinzow B, et al. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45(3):1121-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jørgensen E. Breastfeeding as an exposure pathway for perfluorinated alkylates. Environ Sci Technol. 2015;49(17):10466-10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Domingo JL, Nadal M. Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: a review of the recent scientific literature. J Agric Food Chem. 2017;65(3):533-543. [DOI] [PubMed] [Google Scholar]
- 6. Hu XC, Andrews DQ, Lindstrom AB, et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett. 2016;3(10):344-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strynar MJ, Lindstrom AB. Perfluorinated compounds in house dust from Ohio and North Carolina, USA. Environ Sci Technol. 2008;42(10):3751-3756. [DOI] [PubMed] [Google Scholar]
- 8. Sant KE, Jacobs HM, Borofski KA, Moss JB, Timme-Laragy AR. Embryonic exposures to perfluorooctanesulfonic acid (PFOS) disrupt pancreatic organogenesis in the zebrafish, Danio rerio. Environ Pollut. 2017;220(Pt B):807-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu J, Shimpi P, Armstrong L, Salter D, Slitt AL. PFOS induces adipogenesis and glucose uptake in association with activation of Nrf2 signaling pathway. Toxicol Appl Pharmacol. 2016;290:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lv Z, Li G, Li Y, et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol. 2013;28(9):532-542. [DOI] [PubMed] [Google Scholar]
- 11. Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma concentrations of perfluoroalkyl substances and risk of type 2 diabetes: a prospective investigation among U.S. Women. Environ Health Perspect. 2018;126(3):037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cardenas A, Hivert MF, Gold DR, et al. Associations of perfluoroalkyl and polyfluoroalkyl substances with incident diabetes and microvascular disease. Diabetes Care. 2019;42(9):1824-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He X, Liu Y, Xu B, Gu L, Tang W. PFOA is associated with diabetes and metabolic alteration in US men: National health and nutrition examination survey 2003-2012. Sci Total Environ. 2018;625:566-574. [DOI] [PubMed] [Google Scholar]
- 14. Liu HS, Wen LL, Chu PL, Lin CY. Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013–2014. Environ Pollut. 2018;232:73-79. [DOI] [PubMed] [Google Scholar]
- 15. Mancini FR, Rajaobelina K, Praud D, et al. Nonlinear associations between dietary exposures to perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and type 2 diabetes risk in women: findings from the E3N cohort study. Int J Hyg Environ Health. 2018;221(7):1054-1060. [DOI] [PubMed] [Google Scholar]
- 16. Halldorsson TI, Rytter D, Haug LS, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect. 2012;120(5):668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleisch AF, Rifas-Shiman SL, Mora AM, et al. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect. 2017;125(3):481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alderete TL, Jin R, Walker DI, et al. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: a proof-of-concept analysis. Environ Int. 2019;126:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Timmermann CA, Rossing LI, Grøntved A, et al. Adiposity and glycemic control in children exposed to perfluorinated compounds. J Clin Endocrinol Metab. 2014;99(4):E608-E614. [DOI] [PubMed] [Google Scholar]
- 20. Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: the European Youth Heart Study. Diabetes Care. 2016;39(10):1745-1751. [DOI] [PubMed] [Google Scholar]
- 21. Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect. 2010;118(2):197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conway B, Innes KE, Long D. Perfluoroalkyl substances and beta cell deficient diabetes. J Diabetes Complications. 2016;30(6):993-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacNeil J, Steenland NK, Shankar A, Ducatman A. A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid (PFOA). Environ Res. 2009;109(8):997-1003. [DOI] [PubMed] [Google Scholar]
- 24. Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94(4):1027-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karlsen M, Grandjean P, Weihe P, Steuerwald U, Oulhote Y, Valvi D. Early-life exposures to persistent organic pollutants in relation to overweight in preschool children. Reprod Toxicol. 2017;68:145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shelly C, Grandjean P, Oulhote Y, et al. Early life exposures to perfluoroalkyl substances in relation to adipokine hormone levels at birth and during childhood. J Clin Endocrinol Metab. 2019;104(11):5338-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA birth cohort study. Environ Health Perspect. 2017;125:097018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Valvi D, Oulhote Y, Weihe P, et al. Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ Int. 2017;107:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Høyer BB, Ramlau-Hansen CH, Vrijheid M, et al. Anthropometry in 5- to 9-year-old greenlandic and Ukrainian children in relation to prenatal exposure to perfluorinated alkyl substances. Environ Health Perspect. 2015;123(8):841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grandjean P, Weihe P, Jørgensen PJ, Clarkson T, Cernichiari E, Viderø T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health. 1992;47(3):185-195. [DOI] [PubMed] [Google Scholar]
- 31. Vorkamp K, Nielsen F, Kyhl HB, et al. Polybrominated diphenyl ethers and perfluoroalkyl substances in serum of pregnant women: levels, correlations, and potential health implications. Arch Environ Contam Toxicol. 2014;67(1):9-20. [DOI] [PubMed] [Google Scholar]
- 32. Valvi D, Højlund K, Coull BA, et al. Valvi et al. 2021 SUPPLEMENTARY DATA_PFAS and glucose homeostasis in young adults. Figshare.com. Uploaded February 12, 2021. doi: 10.6084/m9.figshare.13967612.v1 [DOI]
- 33. Ahrén B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol. 2004;150(2):97-104. [DOI] [PubMed] [Google Scholar]
- 34. Kelstrup L, Damm P, Mathiesen ER, et al. Insulin resistance and impaired pancreatic β-cell function in adult offspring of women with diabetes in pregnancy. J Clin Endocrinol Metab. 2013;98(9):3793-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 36. Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151(2):190-198. [DOI] [PubMed] [Google Scholar]
- 37. Aloisio KM, Swanson SA, Micali N, Field A, Horton NJ. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J. 2014;14(4):863-883. [PMC free article] [PubMed] [Google Scholar]
- 38. Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119(3):409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Royston P, White IR. Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Softw. 2011;45:1-20. [Google Scholar]
- 40. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; 1987. [Google Scholar]
- 41. Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206-213. [DOI] [PubMed] [Google Scholar]
- 42. Timmermann CAG, Budtz-Jørgensen E, Petersen MS, et al. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod Toxicol. 2017;68:164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 2009;304(1-2):97-105. [DOI] [PubMed] [Google Scholar]
- 44. Du G, Sun J, Zhang Y. Perfluorooctanoic acid impaired glucose homeostasis through affecting adipose AKT pathway. Cytotechnology. 2018;70(1):479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fang X, Gao G, Xue H, Zhang X, Wang H. Exposure of perfluorononanoic acid suppresses the hepatic insulin signal pathway and increases serum glucose in rats. Toxicology. 2012;294(2-3):109-115. [DOI] [PubMed] [Google Scholar]
- 46. Jensen RC, Glintborg D, Timmermann CAG, et al. Perfluoroalkyl substances and glycemic status in pregnant Danish women: the odense child cohort. Environ Int. 2018;116:101-107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available. For access to individual data, inquiries should be made to the Faroese Hospital System (dfaa@health.fo), and approval must be obtained from the Faroese Ethical Review Board (vsn@vsn.fo).