Abstract
Context
Postbariatric hypoglycemia (PBH), characterized by enteroinsular axis overstimulation and hyperinsulinemic hypoglycemia, is a complication of bariatric surgery for which there is no approved therapy.
Objective
To evaluate efficacy and safety of avexitide [exendin (9-39)], a glucagon-like peptide-1 antagonist, for treatment of PBH.
Methods
A multicenter, Phase 2, randomized, placebo-controlled crossover study (PREVENT). Eighteen female patients with PBH were given placebo for 14 days followed by avexitide 30 mg twice daily and 60 mg once daily, each for 14 days in random order. The main outcome measures were glucose nadir and insulin peak during mixed-meal tolerance testing (MMTT) and hypoglycemic events captured by self-monitoring of blood glucose (SMBG), electronic diary, and blinded continuous glucose monitoring (CGM).
Results
Compared with placebo, avexitide 30 mg twice daily and 60 mg once daily raised the glucose nadir by 21% (P = .001) and 26% (P = .0002) and lowered the insulin peak by 23% (P = .029) and 21% (P = .042), corresponding to 50% and 75% fewer participants requiring rescue during MMTT, respectively. Significant reductions in rates of Levels 1 to 3 hypoglycemia were observed, defined, respectively, as SMBG <70 mg/dL, SMBG <54 mg/dL, and a severe event characterized by altered mental and/or physical function requiring assistance. CGM demonstrated reductions in hypoglycemia without induction of clinically relevant hyperglycemia. Avexitide was well tolerated, with no increase in adverse events.
Conclusion
Avexitide administered for 28 days was well tolerated and resulted in robust and consistent improvements across multiple clinical and metabolic parameters, reinforcing the targeted therapeutic approach and demonstrating durability of effect. Avexitide may represent a first promising treatment for patients with severe PBH.
Keywords: Postbariatric hypoglycemia, PBH, avexitide, exendin (939), GLP-1 antagonist, hyperinsulinemic hypoglycemia
Obesity affects over 40% of US adults. By 2030, an estimated 50% of US adults will be obese and 25% will be severely obese (1). Long-term studies in patients undergoing bariatric surgery demonstrate sustained weight loss, durable remission of type 2 diabetes, and reduction in cardiovascular events, stroke, cancer, and all-cause mortality (2-6). Due to these clinical benefits, use of surgical treatments for obesity has increased by over 50% over the past decade (7), with further increases expected due to the addition of bariatric surgery to the treatment algorithm for uncontrolled diabetes (8).
Postbariatric hypoglycemia (PBH) is a rare but growing complication of bariatric surgery. Prevalence estimates range widely due to differing diagnostic criteria used, though the occurrence of severe hypoglycemia among postbariatric patients may be as high as 29% to 39% of patients undergoing Roux-en-Y gastric bypass (RYGB) and 10% to 23% of those undergoing vertical sleeve gastrectomy (9-12). Similar clinical presentations have been described following gastrectomy (13), esophagectomy (14), and Nissen fundoplication (15). Patients present at least 6 months postoperatively with frequent postprandial episodes of hypoglycemia accompanied by neuroglycopenic signs and symptoms, including altered mental status, visual changes, motor incoordination, loss of consciousness, and seizures, putting patients at risk for injury or death from falls, motor vehicle accidents, or prolonged hypoglycemia, and rendering many unable to drive, work, live alone, or care for dependents. While the underlying physiology is incompletely understood, the presence of inappropriately high insulin secretion after oral ingestion of nutrients is well established (16-19). Hyperinsulinemia occurs in response to oral but not intravenous glucose (20), pointing to enteroinsular axis overstimulation and an exaggerated incretin effect. Plasma concentrations of glucagon-like peptide-1 (GLP-1), secreted by L-cells in response to luminal nutrient stimulation, are markedly elevated after meal intake (21-23). GLP-1 hypersecretion, along with hyperinsulinemic hypoglycemia, are fully reversible by restoring the original route of nutrient transit via gastrostomy tube feeding into the remnant stomach (24, 25), suggesting altered nutrient transit with foregut bypass and hindgut stimulation potentiates hypoglycemia via GLP-1 secretion.
At present, there are no approved pharmacotherapies for PBH. Initial management consists of dietary modification involving avoidance of simple sugars and consumption of mixed meals consisting of ample protein, healthy fats, and limited complex carbohydrates (26, 27). Second-line approaches include off-label use of acarbose, octreotide, and/or diazoxide. These medications are limited by poor tolerability and lack of efficacy, and none have been shown in controlled clinical trials to reduce hypoglycemia in the ambulatory setting. Use of calcium channel blockers or, paradoxically, GLP-1 receptor agonists has been described in case reports or small case series, though responses have not been uniform. Refractory patients have historically been offered partial pancreatectomy or, more recently, bypass reversal, though both have been associated with surgical complications and inconsistent resolution of hypoglycemia, the former with subsequent total pancreatectomy leading to insulin-dependent diabetes, and the latter with weight regain (25, 28, 29). Thus, a substantial unmet medical need remains.
Avexitide (exendin 9–39) is a 31 amino acid fragment of exenatide, a GLP-1 receptor (GLP-1r) agonist that stimulates insulin secretion and lowers plasma glucose. An entire class of drugs to treat type 2 diabetes has been developed around GLP-1r agonism. Avexitide is a GLP-1r antagonist that competes with endogenous GLP-1 for the GLP-1r, counteracting the effects of excessive GLP-1 secretion. Avexitide has been shown under experimental conditions to effectively prevent postprandial hyperinsulinemia and hypoglycemia and reduce neuroglycopenic symptoms in patients with PBH (13, 15, 30-33). PREVENT—a multicenter Phase 2 trial of avexitide conducted at 5 US academic centers—is the first randomized, placebo-controlled study to evaluate the efficacy of a pharmacologic agent for patients with PBH in the outpatient setting.
Materials and Methods
Study Design
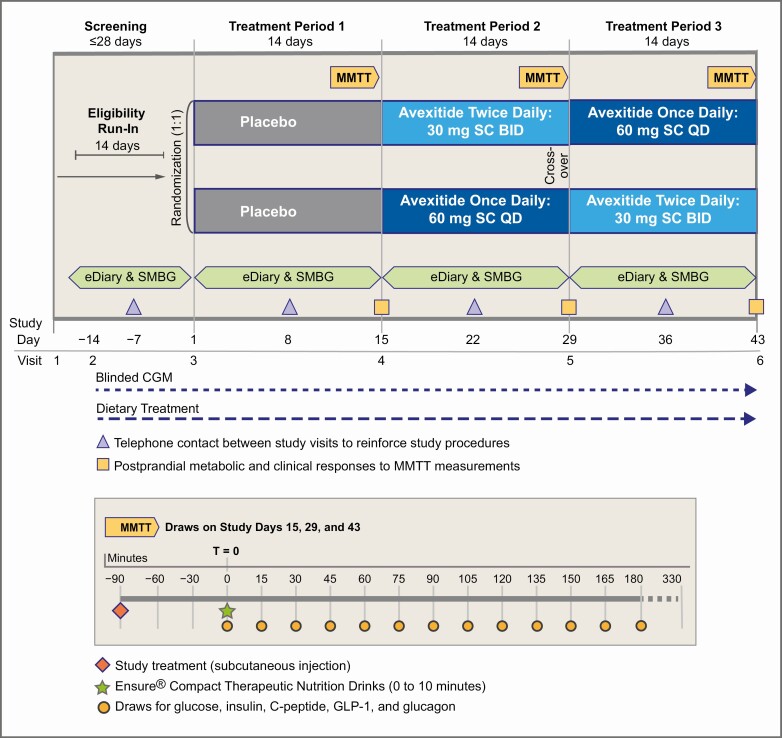
The PREVENT trial was a randomized, placebo-controlled, crossover study conducted at 5 US academic centers. The study design, consisting of 3 14-day treatment periods, is shown in Fig. 1. After a run-in period during which eligibility was confirmed, 18 participants with severe, diet-refractory PBH were randomized 1:1 to 1 of 2 arms, each differing in the order of dosing regimen. For both groups, Treatment Period 1 consisted of subcutaneous placebo injections. During Treatment Periods 2 and 3, avexitide was administered 30 mg twice daily and 60 mg once daily in crossover design and random order. At the end of each treatment period, participants underwent standardized mixed-meal tolerance testing (MMTT) in the clinical research unit (CRU) with hormonal, metabolic, and symptomatic assessments. Throughout, participants were required to adhere to PBH dietary recommendations and document all hypoglycemic events in the outpatient setting using an electronic diary (eDiary), self-monitoring of blood glucose (SMBG), and blinded continuous glucose monitoring (CGM). Avexitide dosing regimens were selected on the basis of modelling and exposure response analyses from prior investigations (31-33).
Figure 1.
Study Schematic for the PREVENT Trial (above) and mixed meal tolerance test (MMTT) sampling timepoints (below). Avexitide 30 mg twice daily, avexitide 30 mg dose every 12 hours; avexitide 60 mg once daily, avexitide 60 mg dose once each morning. CGM, continuous glucose monitoring; eDiary, electronic diary; SMBG, self-monitoring of blood glucose; SC, subcutaneous.
The study was conducted in accordance with the International Council of Harmonization Guideline for Good Clinical Practice and the Declaration of Helsinki (34) after Institutional Review Board approval at each trial site. All patients provided written informed consent before trial participation. This study was registered on ClinicalTrials.gov (NCT03373435).
Participants
Eligible participants were men or women aged 18-65 years who had undergone RYGB surgery at least 12 months before screening and had a documented history of PBH defined as Whipple’s triad, with inappropriately elevated insulin (≥3 µU/mL) or C-peptide (>0.6 ng/mL) at the time of hypoglycemia (≤54 mg/dL glucose) (17). Eligible participants were further required to exhibit at least 2 episodes of hypoglycemic symptoms confirmed by SMBG ≤54 mg/dL while following dietary guidelines during the run-in period.
Patients who had any of the following criteria were excluded from study participation: history of hypoglycemia predating RYGB surgery, history of insulinoma or other cause of endogenous hyperinsulinism; clinically significant acute medical conditions; pregnancy, lactation, and/or women of childbearing potential not using effective contraceptive methods; and use of any agents known to interfere with glucose metabolism within 5 half-lives at screening. Pregnancy status for individuals of childbearing potential was confirmed by documentation of negative plasma pregnancy test at screening and a negative urine pregnancy test on the first day of dosing. Nonchildbearing potential was defined as surgical sterility (documented hysterectomy, tubal ligation, or bilateral salpingo-oophorectomy) or postmenopausal status (defined as 12 months of spontaneous amenorrhea).
Randomization and Masking
Participants were informed that 1 treatment period would involve placebo injections and were blinded to treatment sequence and study drug composition. Participants received 2 subcutaneous injections daily throughout all 3 study periods to fulfill blinded conditions, with injections consisting of the appropriate combination of placebo and/or active avexitide 30-mg dose(s). Investigators and site staff were blinded to avexitide sequence during treatment periods 2 and 3 and to laboratory results. For safety reasons, investigators, but not participants, had access to point of care glucose results during the MMTTs to determine whether glycemic rescue was indicated.
Procedures
In-clinic MMTT procedures
At the end of each treatment period, subjects were admitted to the CRU after an overnight fast for 180-minute MMTT (Fig. 1). After a baseline blood draw, subjects consumed 2 Ensure® Compact Drinks containing 64 g of carbohydrate over 10 minutes, with labs drawn every 15 minutes (for plasma glucose, insulin, c-peptide, GLP-1, and glucagon) and bedside assessment of neuroglycopenic symptoms and point of care glucose via the HemoCue® Glucose 201 System every 30 minutes. If rescue parameters were met (the earlier of point of care glucose ≤50 mg/dL with documented neuroglycopenic symptoms or ≤40 mg/dL irrespective of symptoms), final blood samples were drawn and participants were rescued by intravenous dextrose. The primary outcome of plasma glucose nadir was based on plasma samples assayed per standard methods.
Pharmacokinetic assessments
Blood samples for the determination of plasma avexitide concentrations were collected at the end of each 14-day avexitide treatment period at –90, 0, 60, 180, and 330 minutes relative to the timing of study drug injection.
At home procedures
Throughout all treatment periods subjects used an eDiary (internet-connected web application), a CONTOUR®Next One glucometer, and blinded Dexcom Mobile G4® CGM for recording of hypoglycemic events occurring in the ambulatory setting. For each episode, patients recorded hypoglycemia symptoms/signs, the lowest SMBG reading during the episode, actions taken to treat or prevent the episode, requirement for assistance, and whether the episode was postprandial. Study drug injections were also recorded, and adherence was additionally monitored via accounting of returned study drug vials.
Outcomes
Primary and secondary endpoints were based on participant responses to MMTT in the CRU, while exploratory endpoints were based on events captured via eDiary, SMBG, and blinded CGM in the outpatient setting. Endpoint definitions are provided in Table 1. The primary outcome was postprandial plasma glucose nadir during MMTT and the main secondary outcome was postprandial insulin peak during MMTT. Exploratory outcomes captured by SMBG and eDiary were prespecified with definitions updated post hoc according to current international consensus guidelines on the reporting of hypoglycemia in clinical trials (35), as follows: Level 1 hypoglycemia: SMBG <70 mg/dL; Level 2 hypoglycemia: SMBG <54 mg/dL; Level 3 hypoglycemia: a severe event characterized by altered mental and/or physical functioning that requires assistance from another person for recovery. Exploratory outcomes captured by CGM were prespecified with definitions revised post hoc in accordance with current guidance (36) and included percent time above or below extreme glycemic thresholds (<54 mg/dL; >250 mg/dL) and number of events <54 mg/dL. Percent time <54 mg/dL and number of events <54 mg/dL were also defined temporally by fasting (12 am to 8 am) vs prandial/postprandial (8 am to 12 am) periods; the latter more broadly representing the hours during which meal-induced hypoglycemia may occur and accounting for the mean 1- to 2-hour delay from mealtime to glucose nadir observed in patients with PBH (18). The pharmacokinetic profile for each dosing regimen was evaluated on an exploratory basis. Safety assessments included adverse events, clinical laboratory results, and physical examination findings.
Table 1.
Primary, secondary, and exploratory efficacy endpoints and definitions
| Efficacy endpoint | Definition |
|---|---|
| Primary efficacy endpoint | |
| Glucose nadir | The LS mean placebo-adjusted postprandial plasma glucose nadir within 3 hours of MMTT provocation. |
| Secondary and exploratory efficacy endpoints | |
| In-clinic MMTT-derived endpoints | |
| Insulin peak | The LS mean placebo-adjusted peak postprandial insulin concentration in response to meal provocation by MMTT |
| Outpatient eDiary/SMBG-derived endpoints | |
| Rate of Level 1 hypoglycemia | The LS mean placebo-adjusted number of episodes of SMBG < 70 mg/dL within each treatment period. Rate is expressed in number of distinct episodes divided by number of days for a given treatment period, then normalized to duration of 2 weeks if the treatment period was not exactly 14 days |
| Rate of Level 2 hypoglycemia (1) | The LS mean placebo-adjusted number of episodes of SMBG < 54 mg/dL within each treatment period |
| Rate of Level 3 hypoglycemia (1) | The LS mean placebo-adjusted number of severe hypoglycemia events during each treatment period characterized by altered mental and/or physical functioning that requires assistance from another person for recovery. This applies regardless of whether a patient actually receives external assistance |
| Outpatient CGM-derived endpoints | |
| Percent time (2) with glucose <54 mg/dL | The LS mean placebo-adjusted percentage of CGM values <54 mg/dL during the designated time interval (8 am to 12 am or 12 am to 8 am) during each treatment period, normalized to 14 days |
| Percent time with glucose >250 mg/dL | The LS mean placebo-adjusted percentage of CGM values >250 mg/dL during each treatment period, normalized to 14 days |
| Number of events (2) <54 mg/dL | The LS mean placebo-adjusted number of events captured by CGM with glucose measures <54 mg/dL sustained for at least 15 minutes during the designated time interval (8 am to 12 am or 12 am to 8 am) during each treatment period, normalized to 14 days |
| Number of events (2) >250 mg/dL | The LS mean placebo-adjusted number of events captured by CGM with glucose measures >250 mg/dL sustained for at least 15 minutes during each treatment period, normalized to 14 days |
Endpoint definitions include those prespecified in the study protocol and updated post hoc to comply with current international consensus guidelines on the reporting of hypoglycemia (35) and in accordance with current guidance on the use of continuous glucose monitoring (36).
Abbreviations: CGM, continuous glucose monitoring; eDiary, electronic diary; LS, least squares; MMTT, mixed meal tolerance test; SMBG, self-monitoring of blood glucose.
Statistical Analysis
A sample size of 12 completed patients was selected to provide more than 90% power to detect an increase in glucose nadir of at least 15.0 mg/dL assuming a standard deviation of 14.0 mg/dL at a significance level (α) of 0.05 using a 2-sided paired t-test. The primary efficacy endpoint was calculated and examined in a mixed-effect model, including treatment, treatment sequence, and treatment period as fixed effect, and subject within sequence as random effect. The least squares (LS) mean, SE, 95% CI, and P value were derived from the mixed-effect model for each active treatment. No multiplicity adjustment was planned, and the primary endpoint was evaluated through the nominal P values. Placebo-corrected secondary and exploratory endpoint data were analyzed in the same manner as the primary endpoint with the exception of pharmacokinetic data, which were summarized descriptively.
Results
Participants
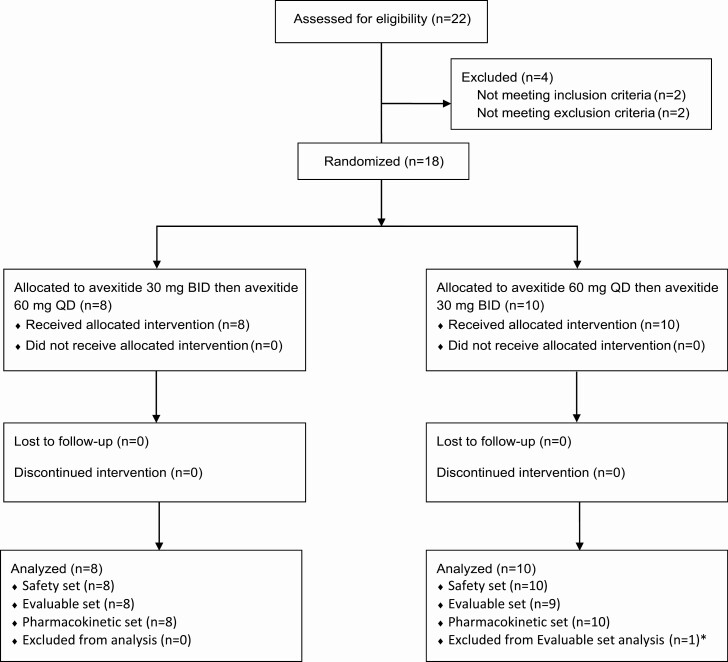
Participant baseline characteristics are shown in Table 2. Twenty-two patients were screened; 18 were randomized and completed the trial. Of the 18 participants, 2 had prior exposure to avexitide. One participant was excluded from the efficacy analysis due to major protocol deviation. The study profile is provided in Fig. 2. Participants were female, mostly white (94%) and non-Hispanic (89%), with average age 44 years, and BMI 30 kg/m2. Baseline disease characteristics reflected the severe and refractory nature of PBH: 44% reported a history of loss of consciousness, 94% reported daily or weekly hypoglycemic symptoms, 16.7% had been hospitalized due to hypoglycemia, and 11% reported history of seizure. All were refractory to dietary treatment, 83% had attempted off-label use of at least 1 medication, and 17% had undergone surgery (RYGB revision or gastrostomy tube placement) for recurrent hypoglycemia.
Table 2.
Participant baseline demographic and clinical characteristics
| Characteristic | Treatment Sequence | Total (N = 18) | |
|---|---|---|---|
| Placebo, avexitide 30 mg twice daily, avexitide 60 mg once dailya (N = 8) | Placebo, avexitide 60 mg once daily, avexitide 30 mg twice dailyb (N = 10) | ||
| Demographic/Anthropomorphic characteristic | |||
| Sex, female, n (%) | 8 (100) | 10 (100) | 18 (100) |
| Age, mean (SD), years | 45.5 (7.5) | 43.4 (12.0) | 44.3 (10.0) |
| Race, n (%) | |||
| Asian | 1 (12.5) | 0 | 1 (5.6) |
| White | 7 (87.5) | 10 (100) | 17 (94.4) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 0 | 2 (20.0) | 2 (11.1) |
| Not Hispanic or Latino | 8 (100) | 8 (80.0) | 16 (88.9) |
| Weight, mean (SD), kg | 81.6 (7.3) | 81.0 (16.1) | 81.23 (12.6) |
| BMI, mean (SD), kg/m2 | 30.0 (3.1) | 29.3 (4.9) | 29.6 (4.1) |
| Clinical baseline/history | |||
| Time since RYGB, mean (SD), months | 88.1 (43.3) | 97.8 (63.6) | 93.5 (54.2) |
| Pre-RYGB weight, mean (SD), kg | 124.7 (29.6) | 131.5 (14.2) | 128.5 (21.9) |
| Time to first experience of postprandial hypoglycemia, mean (SD), months | 24.1 (30.7) | 44.9 (53.4) | 35.7 (44.9) |
| History of LOC due to PBH, n (%) | 3 (37.5) | 5 (50.0) | 8 (44.4) |
| History of seizure due to PBH, n (%) | 0 | 2 (20.0) | 2 (11.1) |
| History of hospitalization due to PBH, n (%) | 1 (12.5) | 2 (20.0) | 3 (16.7) |
| Frequency of symptoms of hypoglycemia | |||
| Daily, n (%) | 3 (37.5) | 4 (40.0) | 7 (38.9) |
| Weekly, n (%) | 5 (62.5) | 5 (50.0) | 10 (55.6) |
| Monthly, n. (%) | 0 | 1 (10.0) | 1 (5.6) |
| History of type 2 DM before RYGB, n (%) | 0 | 0 | 0 |
| Following medical nutrition therapy, n (%) | 8 (100) | 10 (100) | 18 (100) |
| History of pharmacotherapy for PBH, n (%) | 5 (62.5) | 10 (100.0) | 15 (83.0) |
| History of surgery for PBH, n (%) | 1 (12.5) | 2 (20.0) | 3 (16.7) |
Data are presented as mean (standard deviation) or number (percent).
Abbreviations: Avexitide 30 twice daily, avexitide 30 mg dose every 12 h; avexitide 60 once daily, avexitide 60 mg dose once each morning; BMI, basal metabolic index; DM, diabetes mellitus; LOC, loss of consciousness; PBH, postbariatric hypoglycemia; RYGB, Roux-en-Y gastric bypass.
a Placebo (Treatment Period 1) then avexitide 30 mg twice daily (Treatment Period 2) then avexitide 60 mg once daily (Treatment Period 3).
b Placebo (Treatment Period 1) then avexitide 60 mg once daily (Treatment Period 2) then avexitide 30 mg twice daily (Treatment Period 3).
Figure 2.
Study profile (Consort). Twenty-two patients were screened and 18 were randomized and completed the study. *Evaluable set was defined as all randomized patients who received at least Treatment Period 1 placebo and Treatment Period 2 active treatment with blood glucose nadir measured during MMTT in both Treatment Periods 1 and 2, without any major protocol deviations that may confound the interpretation of efficacy. One participant was excluded from the Evaluable set analysis because glycemic rescue was not administered as indicated per protocol during the Period 1 placebo MMTT.
Efficacy Outcomes
MMTT Outcomes
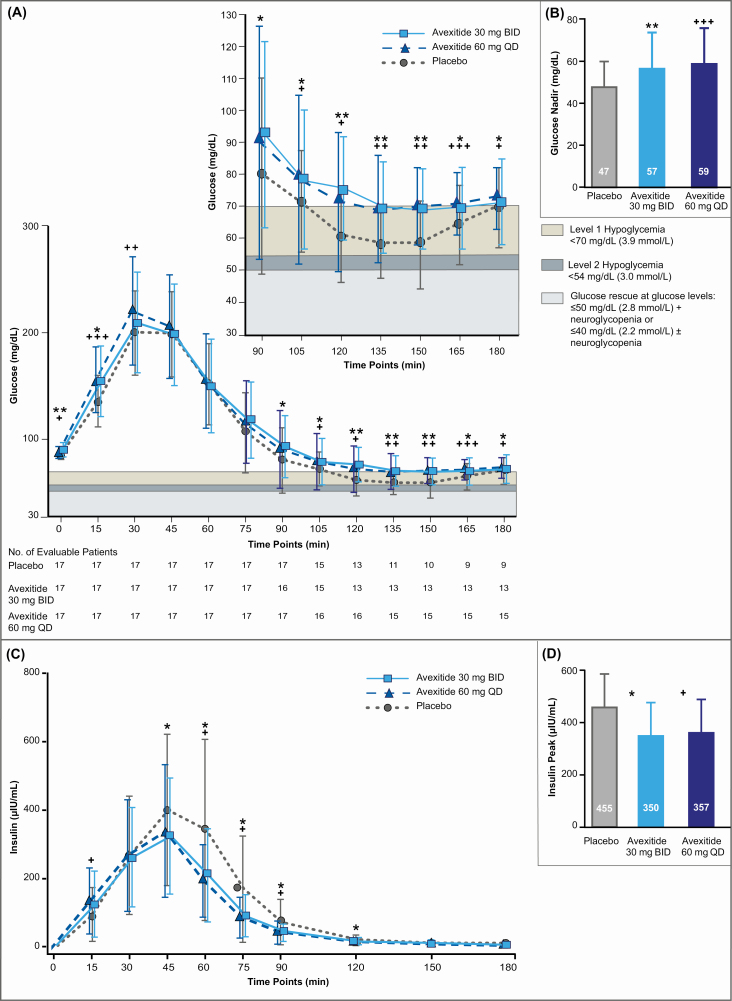
Metabolic responses to MMTT provocation at the end of each treatment period are shown in Table 3 and Fig. 3. The primary endpoint was met with statistical significance by both dosing regimens. The mean plasma glucose nadir was increased by 21% and 26% following avexitide 30 mg and 60 mg dosing, respectively, compared with placebo, corresponding to 50% and 75% fewer participants requiring rescue. Specifically, the LS mean postprandial plasma glucose nadir during MMTT provocation was 10.10 mg/dL (P = .001) and 12.19 mg/dL higher (P = .0002) following avexitide 30 mg and 60 mg dosing, respectively, than with placebo, with 24% and 12% vs 47% of participants requiring rescue following 30 mg and 60 mg vs placebo dosing, respectively. Area under the curve (AUC) glucose concentrations were 12% (P = .008) and 18% (P = .0003) higher following avexitide 30 mg and 60 mg dosing, with observed increases primarily attributable to reductions in hypoglycemia during the 90- to 180-minute period. Consistent with avexitide’s mechanism of action, peak insulin was reduced by 23% and 21% following avexitide 30 mg and 60 mg dosing, respectively. Specifically, the LS mean postprandial insulin peak was 104.53 μIU/mL (P = .029) and 96.29 μIU/mL (P = .042) lower following avexitide 30 mg and 60 mg dosing, respectively, than with placebo. Avexitide had no influence on fasting insulin, GLP-1, or glucagon but postprandial concentrations of GLP-1 and glucagon were significantly higher during both avexitide treatment regimens relative to placebo.
Table 3.
Clinical research unit and outpatient outcomes by treatment regimen
| Parameter | Endpoint type | Mean value (SD) (N = 17) | Avexitide 30 twice daily (N = 17) | Avexitide 60 once daily (N = 17) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 17) | Avexitide 30 twice daily (N = 17) | Avexitide 60 once daily (N = 17) | Placebo-corrected valuea (SE) | 95% CI | P | Placebo-corrected valuea (SE) | 95% CI | P | ||
| Outcomes during MMTT provocation in the Clinical Research Unit | ||||||||||
| Glucose | ||||||||||
| Fasting (mg/dL) | — | 83.8 (5.3) | 87.5 (6.0) | 84.6 (4.2) | 3.8 (1.1) | 1.52, 5.99 | .003 | 0.85 (1.05) | –1.38, 3.09 | .428 |
| Peak (mg/dL) | — | 211.8 (38.5) | 218.2 (45.2) | 224.5 (47.3) | 6.3 (4.8) | –3.85, 16.49 | .205 | 11.87 (4.77) | 1.70, 22.05 | .025 |
| Nadir (mg/dL) | Primary | 47.1 (12.7) | 57.1 (16.5) | 59.2 (16.1) | 10.1 (2.5) | 4.77, 15.44 | .001 | 12.19 (2.50) | 6.85, 17.52 | .000 |
| AUC(0–180) (h × mg/dL) | — | 286.9 (70.1) | 320.0 (89.5) | 338.1 (80.7) | 33.2 (10.9) | 10.10, 56.36 | .008 | 50.09 (10.85) | 26.96, 73.23 | .000 |
| AUC(peak:nadir) (h × mg/dL) | — | 161.8 (56.5) | 192.3 (91.9) | 204.6 (86.3) | 30.4 (12.3) | 4.18, 56.7 | .026 | 41.57 (12.34) | 15.27, 67.86 | .004 |
| Insulin | ||||||||||
| Fasting (μIU/mL) | — | 4.5 (2.4) | 4.5 (2.7) | 4.1 (2.6) | 0.03 (0.35) | –0.71, 0.78 | .922 | –0.43 (0.35) | –1.18, 0.32 | .238 |
| Peak (μIU/mL) | Secondary | 454.5 (240.1) | 349.5 (156.9) | 357.2 (190.9) | –104.5 (43.2) | –196.7, –12.38 | .029 | –96.29 (43.24) | –188.5, –4.14 | .042 |
| GLP-1 | ||||||||||
| Fasting (pg/mL) | — | 12.5 (6.0) | 12.7 (3.4) | 12.7 (4.6) | 0.31 (1.23) | –2.31, 2.94 | .803 | 0.28 (1.23) | –2.35, 2.91 | .824 |
| Peak (pg/mL) | — | 326.5 (149.4) | 413.3 (193.6) | 397.1 (150.2) | 87.1 (27.6) | 28.40, 145.8 | .006 | 71.20 (27.55) | 12.48, 129.9 | .021 |
| AUC(0–180) (h×mg/dL) | — | 277.0 (117.0) | 330.8 (140.5) | 351.1 (127.5) | 53.8 (18.0) | 15.48, 92.07 | .009 | 74.74 (17.97) | 36.44, 113.0 | .001 |
| Glucagon | ||||||||||
| Fasting (pg/mL) | — | 156.3 (0) | 156.3 (0) | 156.8 (2.2) | 0.00 (0.37) | –0.79, 0.79 | 1.000 | 0.56 (0.37) | –0.23, 1.34 | .153 |
| Peak (pg/mL) | — | 178.5 (33.3) | 196.7 (49.8) | 182.9 (35.9) | 18.3 (8.4) | 0.29, 36.20 | .047 | 4.53 (8.42) | –13.43, 22.48 | .599 |
| AUC(0–180) (h × mg/dL) | — | 423.5 (108.2) | 466.7 (131.2) | 484.1 (96.7) | 43.6 (27.5) | –14.88, 102.15 | .133 | 61.21 (27.45) | 2.69, 119.7 | .042 |
| Outcomes captured by SMBG/eDiary in the outpatient setting | ||||||||||
| Rateb of Level 1c hypoglycemia | Expl. | 4.03 (3.10) | 2.81 (2.13) | 1.56 (1.27) | –1.24 (0.64) | –2.62, 0.13 | .072 | –2.51 (0.64) | –3.88, –1.14 | .001 |
| Rate of Level 2d hypoglycemia | Expl. | 2.01 (1.69) | 1.21 (1.65) | 0.81 (0.88) | –0.77 (0.34) | –1.49, –0.04 | .040 | –1.17 (0.34) | –1.90, –0.44 | .004 |
| Rate of Level 3e hypoglycemia | Expl. | 1.96 (1.94) | 1.50 (2.36) | 0.86 (1.16) | –0.49 (0.39) | –1.32, 0.34 | .224 | –1.09 (0.39) | –1.92, –0.26 | .014 |
| Outcomes captured by CGM in the outpatient setting | ||||||||||
| % Timef <54 mg/dL 8 am-12 am | Expl. | 1.97 (1.64) | 0.99 (1.12) | 1.49 (1.70) | –0.93 (0.34) | –1.65, –0.22 | .014 | –0.44 (0.34) | –1.15, 0.28 | .209 |
| % Time <54 mg/dL 12 am-8 am | — | 2.16 (2.54|) | 2.06 (2.36) | 2.95 (3.50) | –0.10 (0.79) | –1.79, 1.59 | .902 | 0.75 (0.79) | –0.94, 2.44 | .359 |
| % Time >250 mg/dL 24 hours | Expl. | 0.62 (0.82) | 0.99 (1.34) | 0.85 (0.98) | 0.34 (0.16) | 0.001, 0.680 | .049 | 0.13 (0.16) | –0.21, 0.47 | .416 |
| No. eventsg <54 mg/dL 8 am-12 am | Expl. | 4.92 (4.08) | 2.82 (3.00) | 3.46 (2.48) | –2.00 (0.67) | –3.43, –0.58 | .009 | –1.39 (0.67) | –2.82, 0.03 | .055 |
| No. events <54 mg/dL 12 am-8 am | — | 2.12 (2.30) | 2.34 (3.03) | 3.56 (4.18) | 0.23 (0.77) | –1.40, 1.86 | .768 | 1.38 (0.77) | –0.25, 3.01 | .092 |
Mean values represent pooled data by treatment. Two-sided P-value versus placebo are shown (Fisher’s exact test). P ≤ .05 shown in bold font. AUC0–180 denotes area under the concentration-time curve from 0 to 180 minutes; avexitide 30 twice daily, avexitide 30 mg dose every 12 hours; avexitide 60 once daily, avexitide 60 mg dose once each morning.
Abbreviations: CI, confidence interval; Expl., exploratory endpoint;
a Placebo-corrected values represent the least squared mean difference between the placebo and avexitide result.
b Rates are expressed as number of distinct episodes divided by number of days for a given treatment period, then normalized to duration of 14 days if the treatment period was not exactly 14 days.
c Level 1 hypoglycemia is defined as SMBG < 70 mg/dL (3.9 mmol/L) and glucose ≥54 mg/dL (3.0 mmol/L).
d Level 2 hypoglycemia is defined as SMBG < 54 mg/dL (3.0 mmol/L).
e Level 3 hypoglycemia is defined as severe hypoglycemia; a severe event characterized by altered mental and/or physical functioning that requires assistance from another person for recovery. This applies regardless of whether a patient actually receives external assistance.
f Percent time is expressed as the percentage of CGM values that are above or below the stated glycemic threshold during each treatment period.
g Number of events is defined as the number of occurrences with CGM glucose measurements <54 mg/dL sustained for at least 15 minutes during the specified time period (8 am-12 am or 12 am-8 am) during each treatment period, normalized to 14 days.
Figure 3.
Mean postprandial plasma glucose (A) and insulin (C) concentrations and mean postprandial plasma glucose nadir (B) and insulin peak (D) in response to MMTT provocation at the end of placebo (grey/dotted line), avexitide 30 mg twice daily (light blue/solid line), and avexitide 60 mg once daily (dark blue/dashed line) treatment periods. Inset within (A) shows an enlargement of axes detailing the glucose values during the 90-180 minute time period. The chart below (A) shows the number of evaluable patients at each sampling timepoint by treatment regimen, representing the number of participants not having required rescue by that timepoint. *P < .05; **P < .01; ***P < .001 for avexitide 30 mg twice daily vs placebo; +P < .05; ++P < .01, and +++P < .001 for avexitide 60 mg once daily vs placebo. Avexitide 30 mg twice daily, avexitide 30 mg dose every 12 hours; avexitide 60 mg once daily, avexitide 60 mg dose once each morning.
Outpatient Outcomes
Outpatient outcomes during each treatment period are shown in Table 3. Both avexitide treatment regimens resulted in reductions in the rates of Levels 1 to 3 hypoglycemia relative to placebo. The rate of SMBG <70 mg/dL (Level 1 hypoglycemia) was reduced by 30% (P = .072) and 61% (P = .001) and the rate of SMBG <54 mg/dL (Level 2 hypoglycemia) was reduced by 40% (P = .040) and 60% (P = .004) during avexitide 30 mg twice daily and 60 mg once daily, respectively, compared with placebo. Although severe hypoglycemia (Level 3) events occurred rarely during the short duration of this study, the rate of events was reduced by 23% (P = .224) and 56% (P = .014) during avexitide 30 mg twice daily and 60 mg once daily, respectively.
Blinded CGM demonstrated reductions in percent time with glucose <54 mg/dL without clinically relevant increases in percent time with glucose >250 mg/dL. Specifically, the mean percent time with glucose <54 mg/dL was reduced by 50% (P = .014) and 24% (P = .209) during avexitide 30 mg twice daily and 60 mg once daily treatment, respectively, while percent time with glucose >250 mg/dL remained less than 1% across treatment groups. Similarly, the mean number of hypoglycemia events with glucose <54 mg/dL as captured by CGM during daytime hours was reduced by 43% (P = .009) and 28% (P = .055) during avexitide 30 mg twice daily and 60 mg once daily, respectively.
Pharmacokinetics
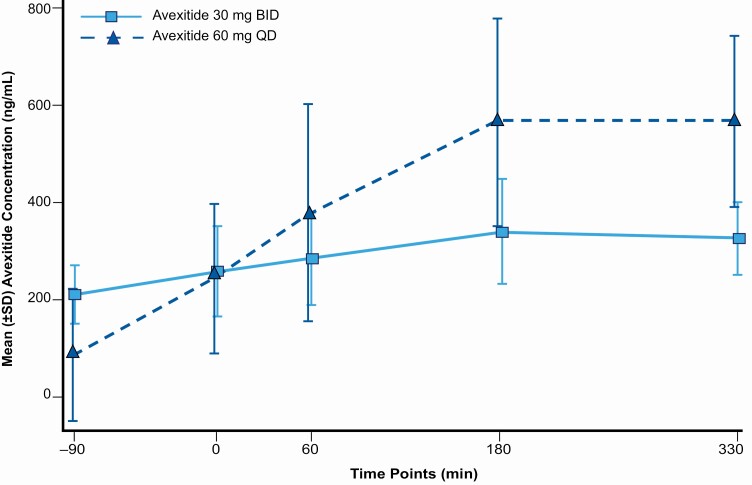
The mean pharmacokinetic profile for each dosing regimen as measured at the end of each 14-day avexitide treatment period is shown in Fig. 4. Evidence of systemic plasma exposure to avexitide was observed in all patients. Morning, predose (C0) exposure was 146% higher for the avexitide 30 mg twice daily than the 60 mg once daily dosing regimen, while peak (Cmax) and mean (AUC0-t) exposure was 72% and 40% higher for the avexitide 60 mg once daily than the 30 mg twice daily dosing regimen.
Figure 4.
Mean avexitide plasma concentration by dosing regimen: avexitide 30 mg twice daily; light blue/solid line, and avexitide 60 mg once daily; dark blue/dashed line on Days 29 and 43. On each of Days 29 and 43, a trough PK sample was collected immediately before the T = −90 minute injection of study drug. T = 0 minutes represents the start of Ensure Compact drink consumption. T = 0, 60, and 180 PK samples were collected at the same time as the plasma glucose and hormonal (insulin, C-peptide, GLP-1, glucagon) draws with PK sampling additionally obtained at T = 330 minutes.
Safety Outcomes
Treatment-emergent adverse events (TEAEs) reported in at least 10% of participants are shown in Table 4. Overall, avexitide was well-tolerated across both dosing regimens. There were no treatment-related serious adverse events and no patient withdrawals. Adverse events were generally mild to moderate and transient. Two patients experienced TEAEs deemed clinically significant and related or possibly related to study drug: 1 with mild injection site irritation and 1 with mild or moderate injection site reaction. In both instances, symptoms were focal and transient. One severe event (hypoglycemic unconsciousness during placebo) and 1 serious adverse event (presyncope during avexitide 60 mg once daily) occurred, both reported as unrelated to study drug and self-limited. The most common adverse events were injection site bruising (39%), headache (28%), and nausea (22%), each of which occurred more often during placebo than during either avexitide dosing regimen. No clinically significant changes were observed in vital signs, body weight, hematology or chemistry, and no clinically relevant increases were observed in fasting or peak postprandial plasma glucose concentrations.
Table 4.
Treatment-emergent adverse events reported in ≥10% of patients overall
| Preferred term | Number (%) of patients | |||
|---|---|---|---|---|
| Treatment | Overall (N = 18) | |||
| Placebo (N = 18) | Avexitide 30 mg twice daily (N = 18) | Avexitide 60 mg once daily (N = 18) | ||
| All TEAEs | 14 (77.8) | 7 (38.9) | 13 (72.2) | 16 (88.9) |
| Injection site bruising | 7 (38.9) | 0 | 1 (5.6) | 7 (38.9) |
| Headache | 4 (22.2) | 1 (5.6) | 1 (5.6) | 5 (27.8) |
| Nausea | 4 (22.2) | 2 (11.1) | 3 (16.7) | 4 (22.2) |
| Dizziness | 1 (5.6) | 0 | 1 (5.6) | 2 (11.1) |
| Injection site pain | 1 (5.6) | 0 | 1 (5.6) | 2 (11.1) |
| Migraine | 0 | 0 | 2 (11.1) | 2 (11.1) |
Adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 20.1. For each preferred term, each patient was counted only once during a treatment period and once in the overall total.
Abbreviations: avexitide 30 mg twice daily, avexitide 30 mg dose every 12 hours; avexitide 60 mg once daily, avexitide 60 mg dose once each morning; TEAE, treatment-emergent adverse events.
Discussion
In the present Phase 2 trial, avexitide treatment in patients with severe PBH significantly reduced the occurrence of hypoglycemia. The primary endpoint, glucose nadir during MMTT, was met for both dosing regimens evaluated in spite of the higher rate of glycemic rescue required during placebo, which had the effect of blunting the magnitude of hypoglycemia during placebo and reducing the observed treatment effect. Significant reductions in hypoglycemia were also observed in the home setting, with fewer Level 1 to 3 hypoglycemia events and less time spent with glucose <54 mg/dL as measured by eDiary, SMBG, and blinded CGM. Importantly, fasting and postprandial glucose levels remained in the normal range for each avexitide dosing regimen, both in the CRU and in the outpatient setting, suggesting that avexitide treatment does not induce hyperglycemia. Glycemic improvements were accompanied by significant reductions in postprandial insulin concentrations and significant elevations in postprandial glucagon concentrations. These hormonal changes, which likely account for the observed decrease in hypoglycemia, are consistent with avexitide’s mechanism of action: antagonism of GLP-1 activity at its receptor. This study extends the findings of prior studies involving single-does or multidose in-clinic administration of avexitide (13, 15, 30-33), confirming that hypoglycemia during MMTT is significantly improved, and demonstrating, for the first time, that avexitide administered in the ambulatory setting can prevent hypoglycemia, with benefits sustained over 28 days of consecutive treatment. Moreover, avexitide was well tolerated, with no increase in adverse events observed.
While both avexitide dosing regimens yielded significant improvements, they differed in their pharmacokinetic profile. The twice daily regimen (30 mg twice daily) provided more even and sustained pharmacokinetic exposure over the 24-hour period, while the once daily regimen (60 mg once daily) offered a more pulsatile pharmacokinetic profile, with higher daytime exposure and lower late evening/overnight coverage. Detailed examination of the 60 mg once daily CGM data revealed a greater tendency for “breakthrough” episodes of hypoglycemia in the late evening (after dinner) hours when plasma concentrations waned, which had the result of diminishing the overall treatment effect as measured by CGM and expressed as percent time and number of episodes with glucose <54 mg/dL. In contrast, the higher daytime exposure conferred by the 60 mg once daily regimen yielded more robust “daytime” effects, as measured by SMBG and eDiary, than the 30 mg twice daily regimen. Together, these results suggest that higher and more prolonged avexitide exposure may provide greater clinical improvements.
It is well known that insulin secretion is augmented by GLP-1 and the potentiation of glucose-stimulated insulin secretion by GLP-1 is dose dependent (37), supporting the hypothesis that an exaggerated GLP-1-mediated incretin effect is at root in PBH. We have previously observed a departure from the linear dose dependency of insulin secretion on glucose and GLP-1 at the highest GLP-1 concentrations (31) and a reversal of this effect during GLP-1r blockade, suggesting that the bulk of insulin secretion in patients with PBH is mediated through GLP-1r signaling and that excessive GLP-1 levels do not contribute substantially to insulin secretion via pathways that do not involve GLP-1r signaling. Although physiologic levels of GLP-1 are glucagonostatic in nonbariatric patients, glucagon is paradoxically elevated postprandially in the setting of very high GLP-1 levels in RYGB patients (21, 25). Emerging data suggest that glucagon may potentiate insulin secretion via direct GLP-1r signaling and this effect is reversable by GLP-1r antagonism (38, 39), raising the possibility that avexitide is also inhibiting glucagon-stimulated insulin secretion. Prior studies in patients with PBH (13, 21, 30, 31) have consistently shown that under conditions of extremely high GLP-1 levels, continuous intravenous infusion of avexitide significantly reduces postprandial insulin and significantly increases postprandial glucagon. The present study findings mirror prior results, providing further evidence that avexitide blunts the insulinotropic effects of GLP-1r signaling while raising glucagon levels, synergistically resulting in potent prevention of hypoglycemia.
This trial had certain limitations. While men and women were eligible for enrollment, no men enrolled. Additionally, this study enrolled patients with severe, diet-refractory disease. Thus, generalizability of study outcomes to males or those with milder disease is limited. This study was somewhat limited by the intentional placement of placebo during the first treatment period to minimize the chance of unblinding that might occur if participants took placebo after benefiting from an effective therapy and to permit evaluation of 28 consecutive days of avexitide treatment. It is likely that dietary compliance was better during the first treatment period (ie, placebo), which would have had the effect of diminishing the perceived treatment effect. Indeed, study investigators unanimously reported that their patients liberalized their diets during active treatment periods because they felt better and could tolerate a wider variety of foods. This study was also limited by no formal assessment of dietary intake and/or composition. Patients with PBH are known to experience weight regain at ≥10% higher rates than their normoglycemic postbariatric counterparts (40); a factor attributed mainly to frequent preemptive eating to avoid hypoglycemic events. Since reduction of hypoglycemia event rate may reduce pre-emptive eating and attenuate or reverse weight regain, the effects of avexitide treatment on dietary intake should be assessed in future trials. Notably, although dietary liberalization during avexitide treatment due to improved tolerability was reported by all site investigators, no weight changes were observed. Finally, it is possible that hypoglycemia unawareness may have led to underreporting of events, which if more frequent during placebo versus active treatment would have minimized the observed treatment effect.
In conclusion, avexitide administered for 28 consecutive days in patients with severe PBH was well tolerated and resulted in robust and consistent improvements across multiple clinical and metabolic parameters—both in the CRU and in the outpatient setting—reinforcing the efficacy of this targeted therapy and demonstrating durability of effect. Given the safety and tolerability profile, higher avexitide exposure may provide even greater clinical benefits and should be considered. While further evaluation of avexitide under chronic dosing conditions in larger cohorts of patients is warranted, the strength and consistency of the current study results suggest that avexitide may offer the first effective and targeted treatment for patients with severe PBH.
Acknowledgments
We thank all of the patients, investigators, study coordinators, and nursing staff who participated in the PREVENT trial for supporting its successful conduct. We thank Larry Shen, Jenny Han, and Jay Zhou for biostatistical assistance. We thank Teresa Joshi for clinical operational oversight and Caren Rickhoff for editorial assistance.
Abbreviations:
- AUC
area under the curve
- CGM
continuous glucose monitoring
- CRU
clinical research unit
- eDiary
electronic diary
- GLP-1
glucagon-like peptide-1
- GLP-1r
GLP-1 receptor
- LS
least squares
- MMTT
mixed-meal tolerance testing
- PBH
postbariatric hypoglycemia
- RYGB
Roux-en-Y gastric bypass
- SMBG
self-monitoring of blood glucose
- TEAE
treatment-emergent adverse event.
Financial Support:
This work was funded by Eiger BioPharmaceuticals, Inc.
Clinical Trial Information : ClinicalTrials.gov registration: NCT03373435 (registered December 14, 2017).
Additional Information
Disclosures: C.M.C., T.L.M, and L.P. declare being consultants to Eiger BioPharmaceuticals, Inc. H.M.L, C.J.E.L, M.J.T, D.B.D, and J.T. declare receiving research support as site investigators from Eiger BioPharmaceuticals, Inc. C.J.L is currently employed at Eli Lilly and Company and all relevant work in this manuscript was completed during her previous employment as faculty at Johns Hopkins.
Data Availability:
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Ward ZJ, Bleich SN, Cradock AL, et al. . Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-2450. [DOI] [PubMed] [Google Scholar]
- 2. Sjöström L, Lindroos AK, Peltonen M, et al. ; Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683-2693. [DOI] [PubMed] [Google Scholar]
- 3. Sjöström L, Peltonen M, Jacobson P, et al. . Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311(22):2297-2304. [DOI] [PubMed] [Google Scholar]
- 4. Arterburn DE, Johnson E, Coleman KJ, et al. . Weight outcomes of sleeve gastrectomy and gastric bypass compared to nonsurgical treatment. Ann Surg. 2020. Published online March 13, 2020. doi: 10.1097/SLA.0000000000003826 [DOI] [PubMed] [Google Scholar]
- 5. Kwok CS, Pradhan A, Khan MA, et al. . Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173(1):20-28. [DOI] [PubMed] [Google Scholar]
- 6. Adams TD, Gress RE, Smith SC, et al. . Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753-761. [DOI] [PubMed] [Google Scholar]
- 7. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. ProMED-mail website. Accessed June 26, 2018. https://asmbsorg/resources/estimate-of-bariatric-surgery-numbers
- 8. Rubino F, Nathan DM, Eckel RH, et al. ; Delegates of the 2nd Diabetes Surgery Summit . Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Diabetes Care. 2016;39(6):861-877. [DOI] [PubMed] [Google Scholar]
- 9. Lee CJ, Brown TT, Schweitzer M, Magnuson T, Clark JM. The incidence and risk factors associated with developing symptoms of hypoglycemia after bariatric surgery. Surg Obes Relat Dis. 2018;14(6):797-802. [DOI] [PubMed] [Google Scholar]
- 10. Lee CJ, Clark JM, Schweitzer M, et al. . Prevalence of and risk factors for hypoglycemic symptoms after gastric bypass and sleeve gastrectomy. Obesity (Silver Spring). 2015;23(5):1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmühler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis. 2015;11(3):564-569. [DOI] [PubMed] [Google Scholar]
- 12. Brix JM, Kopp HP, Höllerl F, Schernthaner GH, Ludvik B, Schernthaner G. Frequency of hypoglycaemia after different bariatric surgical procedures. Obes Facts. 2019;12(4):397-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larraufie P, Roberts GP, McGavigan AK, et al. . Important role of the GLP-1 axis for glucose homeostasis after bariatric surgery. Cell Rep. 2019;26(6):1399-1408.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elliott JA, Docherty NG, Murphy CF, et al. . Changes in gut hormones, glycaemic response and symptoms after oesophagectomy. Br J Surg. 2019;106(6):735-746. [DOI] [PubMed] [Google Scholar]
- 15. Calabria AC, Charles L, Givler S, De León DD. Postprandial hypoglycemia in children after gastric surgery: clinical characterization and pathophysiology. Horm Res Paediatr. 2016;85(2):140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim SH, Liu TC, Abbasi F, et al. . Plasma glucose and insulin regulation is abnormal following gastric bypass surgery with or without neuroglycopenia. Obes Surg. 2009;19(11):1550-1556. [DOI] [PubMed] [Google Scholar]
- 17. Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254. [DOI] [PubMed] [Google Scholar]
- 18. Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018;103(8):2815-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patti ME, McMahon G, Mun EC, et al. . Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48(11):2236-2240. [DOI] [PubMed] [Google Scholar]
- 20. Kim SH, Abbasi F, Lamendola C, Reaven GM, McLaughlin T. Glucose-stimulated insulin secretion in gastric bypass patients with hypoglycemic syndrome: no evidence for inappropriate pancreatic beta-cell function. Obes Surg. 2010;20(8):1110-1116. [DOI] [PubMed] [Google Scholar]
- 21. Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3(6):597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldfine AB, Mun EC, Devine E, et al. . Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678-4685. [DOI] [PubMed] [Google Scholar]
- 24. McLaughlin T, Peck M, Holst J, Deacon C. Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab. 2010;95(4):1851-1855. [DOI] [PubMed] [Google Scholar]
- 25. Davis DB, Khoraki J, Ziemelis M, Sirinvaravong S, Han JY, Campos GM. Roux en Y gastric bypass hypoglycemia resolves with gastric feeding or reversal: Confirming a non-pancreatic etiology. Mol Metab. 2018;9:15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kellogg TA, Bantle JP, Leslie DB, et al. . Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis. 2008;4(4):492-499. [DOI] [PubMed] [Google Scholar]
- 27. Suhl E, Anderson-Haynes SE, Mulla C, Patti ME. Medical nutrition therapy for post-bariatric hypoglycemia: practical insights. Surg Obes Relat Dis. 2017;13(5):888-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee CJ, Brown T, Magnuson TH, Egan JM, Carlson O, Elahi D. Hormonal response to a mixed-meal challenge after reversal of gastric bypass for hypoglycemia. J Clin Endocrinol Metab. 2013;98(7):E1208-E1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vanderveen KA, Grant CS, Thompson GB, et al. . Outcomes and quality of life after partial pancreatectomy for noninsulinoma pancreatogenous hypoglycemia from diffuse islet cell disease. Surgery. 2010;148(6):1237-45; discussion 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146(3):669-680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Craig CM, Liu LF, Deacon CF, Holst JJ, McLaughlin TL. Critical role for GLP-1 in symptomatic post-bariatric hypoglycaemia. Diabetologia. 2017;60(3):531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Craig CM, Liu LF, Nguyen T, Price C, Bingham J, McLaughlin TL. Efficacy and pharmacokinetics of subcutaneous exendin (9-39) in patients with post-bariatric hypoglycaemia. Diabetes Obes Metab. 2018;20(2):352-361. [DOI] [PubMed] [Google Scholar]
- 33. Tan M, Lamendola C, Luong R, McLaughlin T, Craig C. Safety, efficacy and pharmacokinetics of repeat subcutaneous dosing of avexitide (exendin 9-39) for treatment of post-bariatric hypoglycaemia. Diabetes Obes Metab. 2020;22(8):1406-1416. [DOI] [PubMed] [Google Scholar]
- 34. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310( 20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 35. International Hypoglycemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017;40( 1):155-157. [DOI] [PubMed] [Google Scholar]
- 36. Danne T, Nimri R, Battelino T, et al. . International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52(2):380-386. [DOI] [PubMed] [Google Scholar]
- 38. Capozzi ME, Svendsen B, Encisco SE, et al. . β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight. 2019;4(5):e126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Svendsen B, Larsen O, Gabe MBN, et al. . Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 2018;25(5):1127-1134.e2. [DOI] [PubMed] [Google Scholar]
- 40. Varma S, Clark JM, Schweitzer M, Magnuson T, Brown TT, Lee CJ. Weight regain in patients with symptoms of post-bariatric surgery hypoglycemia. Surg Obes Relat Dis. 2017;13(10):1728-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.