Abstract
Context
The correlation between fibrous dysplasia/McCune–Albright syndrome (FD/MAS) skeletal disease burden on Na[18F]F positron emission tomography–computed tomography (PET-CT) and serum bone turnover markers (BTMs) was recently described. The effect of treatment on lesional fluoride burden in FD/MAS is unknown.
Objective
To investigate treatment response measurements in patients with FD/MAS who underwent Na[18F]F-PET-CT and treatment with antiresorptives.
Methods
Observational case series at an academic center of expertise for rare bone diseases. Fifteen consecutive patients were observed with FD/MAS with baseline and follow-up Na[18F]F-PET-CT parameters of healthy bone and FD lesions, BTMs, and pain scores at start of denosumab (n = 8) treatment and non-denosumab patients (n = 7). On Na[18F]F-PET-CT the volumetric measures of FD burden (fluoride tumor volume [FTV]) and “fraction affected skeleton” (FAS) represented the portion of the skeleton affected. This was correlated with BTMs and pain.
Results
Disease activity decreased significantly, with FTV 361 cm3 to 97 cm3 (P = .018) in denosumab-treated patients, but not in non-denosumab patients (P = .249). Serum P1NP and alkaline phosphatase (ALP) decreased significantly: 82 ng/mL vs 55 ng/mL (P = .023) and 119 IU/L vs 84 IU/L (P = .020), respectively. In denosumab-treated patients pain scores improved leading to pain medication reduction. This correlated with lesional uptake, but healthy bone activity did not change. BTMs and FTV correlated positively (P1NP r = 0.730, P < .001; and ALP r = 0.406, P = .006), as did change in BTMs and FTV: P1NP (P = 0.032) and ALP (P = 0.024). FAS strongly correlated with treatment-induced decrease in ALP (P = .027) and P1NP (P = .009).
Conclusion
Na[18F]F-PET-CT captured treatment-induced lesional changes which correlated with BTMs and pain reduction. Therefore Na[18F]F-PET-CT can be used as an objective local parameter of response to denosumab treatment in FD/MAS.
Keywords: Sodium fluoride PET-CT, fibrous dysplasia, follow-up, McCune–Albright, denosumab, bisphosphonates
Fibrous dysplasia/McCune–Albright Syndrome (FD/MAS) is caused by a postzygotic activating mutation of the α-subunit of the stimulatory G-protein (Gsα) (1) causing overproduction of cyclic adenosine 5′-monophosphate in affected cells of the osteogenic lineage, leading to the accelerated production of bone marrow stromal cells while inhibiting the differentiation of these progenitor cells into mature osteoblasts. Despite expressing early osteoblast biomarkers such as alkaline phosphatase (ALP), these immature cells are dysfunctional, leading to the laying down of fibro-osseous tissue that is undermineralized, of poor quality, and of disturbed microarchitecture (1). 99mTc-radiolabeled bisphosphonate bone scintigraphy (BS) is the most widely used molecular imaging method for assessing FD burden using the skeletal burden score (SBS) (2). The SBS is the weighted sum of the per-segment estimation of the percentage of affected normal bone volume on planar BS. The weighting factors used are based on average representation of that segment to a healthy adult skeleton; however, this might be an underestimation for FD-affected expansile segments, such as in the ribs (2–4). Despite BS being widely used as an adjunct for primary characterization of FD, the use in follow-up remains controversial: Collins et al. (2) reported no change in BS and SBS after bisphosphonate treatment whereas others report clinical improvement in combination with a skeletal burden response in FD (5). To date, there are no objective local parameters available to assess lesional response to treatment.
The use of positron emission tomography with integrated computed tomography using sodium fluoride-18 (Na[18F]F PET-CT) in benign disease is still scarce; the combination of substantive advantages over other techniques, improving availability and decreasing costs, has led to an increasingly renewed interest in this tracer beyond bone metastases (6–10).
We and others recently showed that Na[18F]F PET-CT provides quantitative parameters of disease burden, using the volume-based parameters fluoride tumor volume (FTV) and total lesion fluorination (TLF), that have a high correlation with serum bone turnover markers (BTMs): P1NP, ALP, N-telopeptides, and osteocalcin (4, 11). TLF is based on both the volume of increased bone activity (FTV) and its mean level of bone activity (mean standardized Na[18F]F uptake value, SUVmean). In previous observations high correlations between molecular imaging and BTMs were mainly driven by the volumetric parameter FTV, without a significant contribution of the intensity parameter SUVmean, for which the optimal normalization method is still under debate (12). Therefore FTV is to be preferred over the compound parameter TLF. Compared with standard 99mTc-radiolabeled bisphosphonate BS with hybrid single photon emission tomography and CT (SPECT-CT), Na[18F]F PET-CT has superior spatial resolution (~3.5 vs 8 mm full width at half maximum), faster pharmacokinetics of the radiopharmaceutical (1 hour or less vs 4 hours of incubation), and faster whole body 3D imaging at the cost of higher expenses and lower availability (4, 13).
We postulate that observed decreases in BTMs described in our previous study (13) reflect a local response in Na[18F]F accumulation leading to a decrease in FTV and FAS and set out to evaluate this in patients with FD/MAS treated with denosumab.
Materials and Methods
Patients were evaluated as part of an ongoing observational study. Denosumab and bisphosphonates were prescribed off-label on clinical grounds. Responses to these therapies were then analyzed retrospectively.
Population
Written informed consent was obtained from all patients and the study was approved by the medical ethics committee of the Leiden University Medical Center. All procedures in this study were in accordance with the ethical standards of the institutional and/or national regulations and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For the current retrospective analyses as part of an ongoing observational study, a written statement of consent to the use of all acquired data was obtained from each patient and all patients had the possibility to opt out. The collection of data and analyses was approved by the local ethics committee (METC Leiden-Den Haag-Delft).
Between February 2015 and April 2018 all patients with FD/MAS before the start of denosumab treatment or planned surgery were included. They all underwent at least 2 consecutive full body Na[18F]F PET-CT scans, the first performed for baseline assessment of FD disease burden at start of denosumab or under bisphosphonates (non-denosumab) and at least 1 follow-up Na[18F]F PET-CT scan, between February 2015 and August 2019 (n = 15). These patients are a subgroup of our previously reported study on Na[18F]F PET-CT in FD/MAS (4). Reasons for not having follow-up Na[18F]F PET-CT scans (n = 5) were of medically unrelated logistic nature (the scans were performed at another location), or because the second PET could not be planned within a reasonable time-window or therapy was not started at all.
Treatment Goals
The decision to start off-label denosumab was based on clinical or biochemical failure of previous bisphosphonate treatment or biochemically progressive disease despite prior treatment with bisphosphonates (mainly olpadronate). However, after full consultation, in some patients surgery for the clinically most relevant lesion was considered for a time point after the window of this study or patients refused denosumab and therefore stayed on-treatment with bisphosphonates. The goal of their treatment was decreasing pain measured by the Brief Pain Inventory (BPI), improvement of their clinical status (patients’ own report) and decrease of painkiller use, which is always the treatment goal in our outpatient clinic, also with bisphosphonates (13).
For these patients a baseline scan (n = 15), first follow-up after start of treatment (n = 15), second (n = 6) and third (n = 1) follow-up were available. After baseline Na[18F]F PET-CT, denosumab 60 mg (Prolia®, Amgen Europe B.V.) trimonthly subcutaneous injections were started, in 8 of the 15 patients (13). One patient started denosumab treatment later on because of worsening of inflammatory bowel disease. All patients starting denosumab, were previously treated with bisphosphonates. Thirteen patients (87%) used bisphosphonates at baseline Na[18F]F PET-CT, primarily olpadronate ((3-dimethylamino-1-hydroxypropylidene)-1,1 bisphosphonate), as reported before (13). The non-denosumab group (n = 7) was more heterogeneous: bisphosphonates were commenced (n = 2), continued (n = 3), or ceased before the period of our investigation (n = 2). We used the 60-mg dose which is titrated in relation to complaints and biochemical response and varies per patient. The minimum maintenance dose is 60 mg twice a year (osteoporosis dose). In Tables 1 and 2, we report on the specific dosing of denosumab and bisphosphonates including the duration of treatment and the relation to the date of Na[18F]F PET-CT and BTMs. Cotreatment with calcium/D3/active vitamin D metabolites was initiated at discretion of the treating physician. Eleven patients (73%) were diagnosed with polyostotic FD of whom 2 were diagnosed with MAS, all with well-controlled endocrinopathies. The remaining 4 patients (27%) were diagnosed with monostotic FD.
Table 1.
Patient characteristics of the denosumab group
| Patient no. | Treatment, reason | Treatment dose | Interval scan 1 to Dmab (weeks) | Interval scan 1, scan 2 (weeks) | Interval scan to BTM scan 1 (weeks) | Baseline SBS | MFD/ PFD/MAS | Location FD |
|---|---|---|---|---|---|---|---|---|
| 1 | Growth + BTM progression | Dmab (60 mg every 3 months) | 2.4a | 59 | 4.6 | 3.41 | MFD | CF: zygoma/orbita |
| 2 | Growth + BTM progression | Dmab (60 mg every 6 months) | 13.4 | 90 | 0.4 | 5.60 | PFD | Spine, ribs |
| 3 | Pain and BTM progression | Dmab (60 mg every 6 months) | 2.0 | 54 | 0.4 | 0.26 | PFD | Mandibula, maxilla, ribs |
| 4 | Pain and BTM progression | Dmab (60 mg every 3 months) | 1.9 | 64 | 2.1 | 16.70 | PFD | Pelvis, left femur and tibia |
| 5 | Pain and BTM progression | Dmab (60 mg every 6 months), onwards increased up to 120 mg every 3 months | 3.0 | 61 | 14.3 | 2.05 | PFD | Ribs, thoracic spine, pelvis |
| 6 | Pain and BTMs progression | Dmab (60 mg every 3 months) | 1.7 | 65 | 2.7 | 16.68 | PFD | L4, iliac bone, femur, fibula |
| 7 | Pain and BTM progression | Dmab (60 mg every 3 months) | 1.7 | 56 | 3.3 | 37.70 | MAS | Skull, spine, pelvis |
| 8 | Pain and BTM progression | Dmab (60 mg every 3 months) | 10.0 | 78 | 4.4 | 25.28 | MAS | Spine, ribs, pelvis, femur |
a Denosumab was started before scan 1, otherwise scan 1 was performed before start of denosumab.
Table 2.
Patient characteristics of the non-denosumab group
| Patient no. | Treatment, reason | Treatment dose | Treatment active or stopped | Interval scan 1, scan 2 (weeks) | Interval scan to BTM scan 1 (weeks) | Baseline SBS | MFD/PFD/MAS | Location FD |
|---|---|---|---|---|---|---|---|---|
| 9 | Pain progression | APD (pamidronate) 1800 mg for 1 year | Stopped | 113 | 1.0 | 1.76 | MFD | Femur |
| 10 | Pain progression | Zoledronate (15 mg) | Active | 106 | 3.4 | 2.23 | MFD | Left tibia |
| 11 | Pain progression | APD (pamidronate) for 1 year | Active | 48 | 7.6 | 0.33 | MFD | Rib |
| 12 | Pain and BTM progression | Olpadronate orally and intravenously more than 4 years (formally APD, equivalent to osteoporosis dosage) | Stopped | 89 | 12.4 | 17.87 | PFD | Sternum, iliac bone, femur and tibia |
| 13 | Pain and BTMs progression | Olpadronate (50 mg) | Active | 87 | 5.4 | 15.75 | PFD | Femur and tibia |
| 14 | Pain and BTM progression | Olpadronate (56 mg) | Active | 100 | 12.6 | 16.42 | PFD | Ribs, femur and tibia |
| 15 | Pain and BTM progression | Olpadronate orally and intravenously | Active | 117 | 11.4 | 15.75 | PFD | Humerus, pelvis, femur |
Reasons for repeated scans were to observe if the improvements clinically and biochemically also reflected changes on a local level. If the activity indeed went down, we gradually increased the dosing interval of the denosumab to observe if we could even gradually taper off or treat with the “osteoporosis dose” to minimize exposure. We aimed to perform new scans at an 18-month to 24-month interval (after 4-6 injections).
The following clinical items were collected from the electronic patient files: FD localizations, demographic features, and previous and current drug use. Nonfasting morning venous blood samples were collected for evaluation of bone and mineral metabolism (ALP and P1NP) and data on change in pain complaints using the BPI were registered around the time of the Na[18F]F PET-CT (14). A follow-up Na[18F]F PET-CT was scheduled approximately 1.5 years after baseline investigation and was accompanied by an outpatient clinic visit and laboratory test. Serum ALP activity was measured using a fully automated P800 modulator system and P1NP was determined by the E-170 system (Roche BV, Woerden, The Netherlands). The upper limit of normal values for these BTMs were 98 IU/L for ALP and 59 ng/mL for P1NP in male adults and premenopausal females and 76 ng/mL for P1NP in postmenopausal females.
Quantitative Analysis of Na[18F]F PET-CT: Normal vs Pathological Bone
Acquisition and analysis of Na[18F]F PET-CT was identical to our previous baseline study (4). In short, total body PET-CT imaging (n = 37 scans) was performed approximately (median; interquartile range [IQR]) 49 minutes (44-67 minutes) after administration of 1.00 MBq/kg (0.93-1.06 MBq/kg) Na[18F]F on a Philips Gemini TF TOF 64T (Philips Healthcare; Eindhoven, The Netherlands; n = 33 scans in 11 patients) and, from July 2019 onward, on a GE Discovery MI PET-CT (GE Healthcare; Chicago, Illinois; n = 4 scans in 4 patients). All image analyses were performed independently in Osirix version 11.0 (Pixmeo SARL, Geneva, Switzerland) by a nuclear medicine physician (W.v.d.B.) with 15 years of experience in reading PET-CT, taking both PET-CT and low-dose CT into account and who was blinded for the biochemical results and treatment. PET-CT images were quantified using the standardized uptake value (SUV, in g/mL) which is the local activity-concentration of the 18F-fluoride anion (in Bq/mL) normalized for the injected Na[18F]F activity (in Bq) per unit bodyweight (in g), as bodyweight seemed the optimal measure of volume of distribution for Na[18F]F SUVs and normalizing for CT-based skeletal volume (SV) or lean body mass did not improve results (3, 4, 12, 15). The SUV cut-off to discern normal, healthy bone from pathological Na[18F]F uptake strongly varied among patients and scans and was therefore individually defined as previously published (4, 16, 17). FD burden was measured by the volumetric parameter FTV, namely the volume of FD-affected bone, excluding accumulation not attributable to FD, with SUV ≥ SUV cut-off. This parameter was found superior to FD SUV or its derivative intensity measures including peak SUV (SUVpeak) defined as the mean SUV in a 1 cm3 spherical volume with highest uptake within the FTV (12). We determined the “fraction affected skeleton” (FAS) by dividing the functional FTV, by the baseline anatomical SV (the volume of bone with a CT density between 150 and 2500 HU). This parameter thus represents the portion of the skeleton being pathologically metabolically active which is more analogous to SBS than FTV. We used baseline SV as we observed in the subset of current cohort with the largest treatment-induced change in FTV, that the SV determined on CT did not change on a per-patient analyses (mostly less than 1% change and all within 3% change, judged as within measurement error, data not shown).
Statistical Analysis
Percentage change in parameters (Δ) after start of denosumab or non-denosumab were quantified for SUVpeak, FTV, ALP, and P1NP according to
In 1 patient (2 scans, non-denosumab subgroup), the measured bone turnover in FD did not exceed the cut-off for healthy bone, resulting in an FTV of 0 at baseline and follow-up and therefore ΔFTV was undefined. All variables were assessed for (log)normality based on skewness and kurtosis metrics. As all main parameters showed deviation of (log)normality, defined as 0 not being within the 95%-confidence interval of both distribution metrics, these were described as median and IQR, nonparametric tests were used (Mann–Whitney U, Wilcoxon, Friedman’s analysis of variance) and the nonparametric rank correlation coefficient Spearman’s ρ was used. In order to determine the intrapatient reproducibility of SUV cut-off between baseline and any of the follow-up scans, we computed the intraclass correlation coefficient (ICC) and alpha 2-way random model on absolute agreement and interpreted this value according to Koo et al. (17). Furthermore, reproducibility was graphically described using Bland–Altman plots including systematic error (mean absolute difference) and random error (95% limits of agreement), which also visualizes heteroscedasticity.
All statistical analyses were performed using SPSS software (version 26, IBM, New York, New York, USA). A type I error (P value) below .05 was considered statistically significant.
Results
Fifteen patients met our eligibility criteria. Mean age at time of the baseline PET-CT was 47 years (range 16-68 years), 9 (60%) were women (4).The median interval between BTM measurement and Na[18F]F PET-CT was 4 weeks (IQR 1-9 weeks, n = 37 scans). This interval was significantly longer at baseline (median 24 days, biochemistry before PET-CT) than at follow-up (median 7 days, PET-CT before biochemistry) (n = 15; P = .006, Wilcoxon); however, the absolute intervals between PET and serum biomarkers of baseline, first follow-up, and second follow-up were not significantly different (n = 6 triplets, P = .568 Friedman’s analysis of variance). This is in accordance with our standardized outpatient department procedure. Specific information for each patient on reason for treatment, treatment dosing and duration of treatment, the interval between Na[18F]F PET-CT and denosumab, interval between Na[18F]F PET-CT scans, interval between Na[18F]F PET-CT and BTMs, bases SBS, and type of FD is summarized in Tables 1 and 2.
The mean interval between baseline Na[18F]F PET-CT and first follow-up Na[18F]F PET-CT after start of treatment was 18 months (range 12-26 months, n = 15 pairs) and the mean number of cycles of denosumab given was 6 at an interval of 3 months between denosumab injections (range 4-9) in the subgroup of patients that started denosumab.
Patient characteristics during baseline and first follow-up Na[18F]F PET-CT are displayed in Table 3.
Table 3.
Patient demographics
| Baseline | Follow-up | P value | |
|---|---|---|---|
| Parameter | |||
| Number of patients (n) | 15 | 15 | |
| Female (n) | 9 | 9 | |
| Age at scan (years), mean (SD) | 46.7 (14.7) | 48.3 (14.7) | |
| Biochemistry | |||
| ALP (IU/L, ULN 98); median (IQR) | 119 (83-143) | 84 (64-106) | .020 |
| P1NP (ng/mL, ULN 59); median (IQR) | 82 (51-237) | 55 (34-112) | .023 |
| Clinical parameters | |||
| BPI average; median (IQR), n = 14 (1 missing) | 5.00 (0.75-7.50) | 3.50 (0.00-7.00) | .173 |
| bisphosphonates use, n (%) | 13 (93) | 5 (36) | .001 |
| denosumab 60 mg every 3 months, n (%) | 0 (0) | 8 (57) | .002 |
| Ca/D3, n (%) | 10 (71) | 9 (64) | 1.000 |
| Imaging parameters | |||
| SBS | |||
| median (IQR) | 15.8 (2.2-17.0) | Not repeated | |
| Cut-off SUV (g/mL) | |||
| median (IQR) | 8.5 (7.2-9.1) | 7.7 (7.3-8.5) | .233 |
| SUVpeak (g/mL) | |||
| median (IQR) | 29 (18-34) | 21 (13-30) | .028 |
| FTV (cm3) | |||
| median (IQR) | 199 (77-923) | 97 (51-635) | .030 |
| FAS (%) | |||
| median (IQR) | 2.65 (1.13-18.6) | 1.45 (0.78-8.40) | n.d. |
Statistically significant values (P < .05) are presented in bold.
Abbreviations: ALP, alkaline phosphatase; BPI, structured Brief Pain Inventory; FAS, fraction affected skeleton (FTV divided by skeletal volume on CT); FTV, fluoride tumor volume; IQR, interquartile range; P1NP, total procollagen type 1 N-terminal propeptide; SBS, skeletal burden score on planar bone scintigraphy; SUV, standardized uptake value on PET-CT; n.d., could not be determined; ULN, upper limit of normal.
Quantitative Analysis of Na[18F]F PET-CT: Normal vs Pathological Bone at Baseline and Follow-up
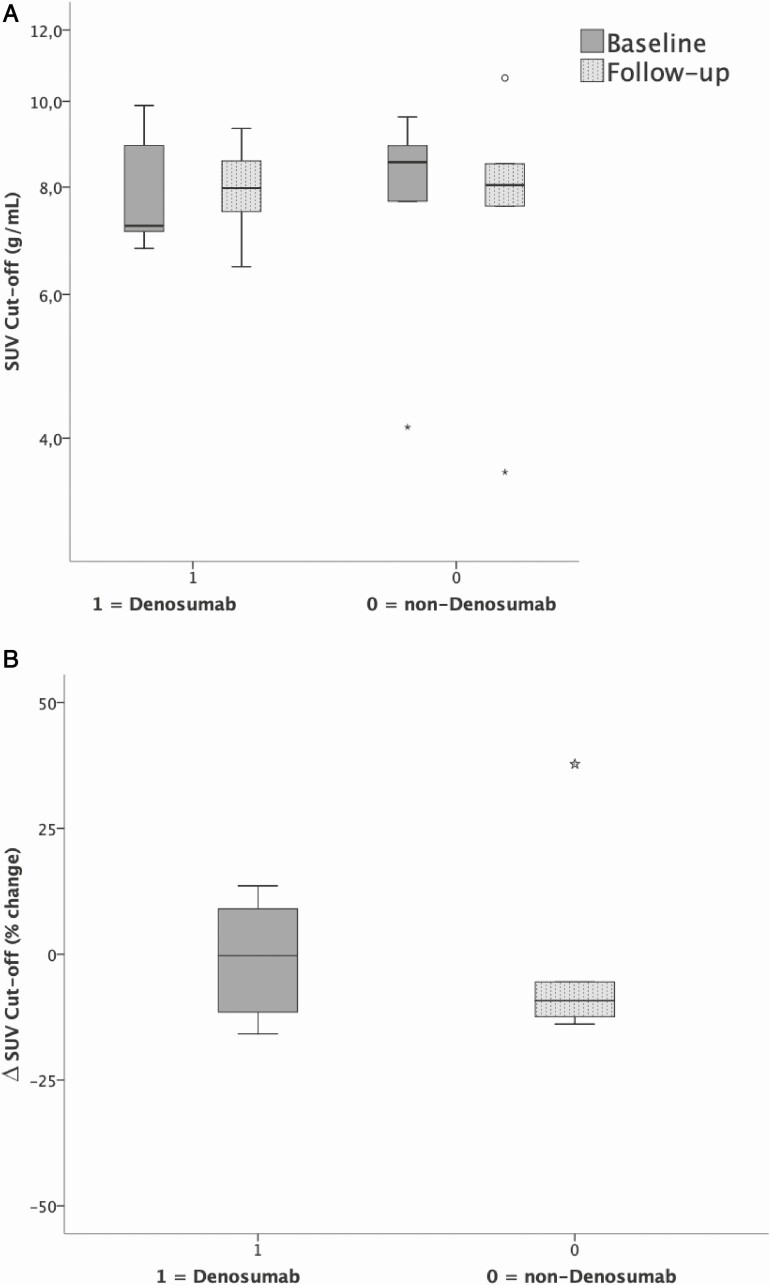
For the total of 37 PET-CT scans, the individual SUV cut-off ranged from 3.62 to 10.62 g/mL (median 7.98 g/mL), in accordance with our previous study a range larger than the predefined median ± 10%. (12). Pairwise, these individual SUV cut-offs of follow-up scans were not significantly different from baseline, as shown in Table 3. Also for the subcohorts of denosumab (n = 8) and non-denosumab (n = 7), the (distribution of) SUV cut-offs for healthy bone did not change after treatment compared with baseline, P = .237 and P = .575 (Wilcoxon), respectively.
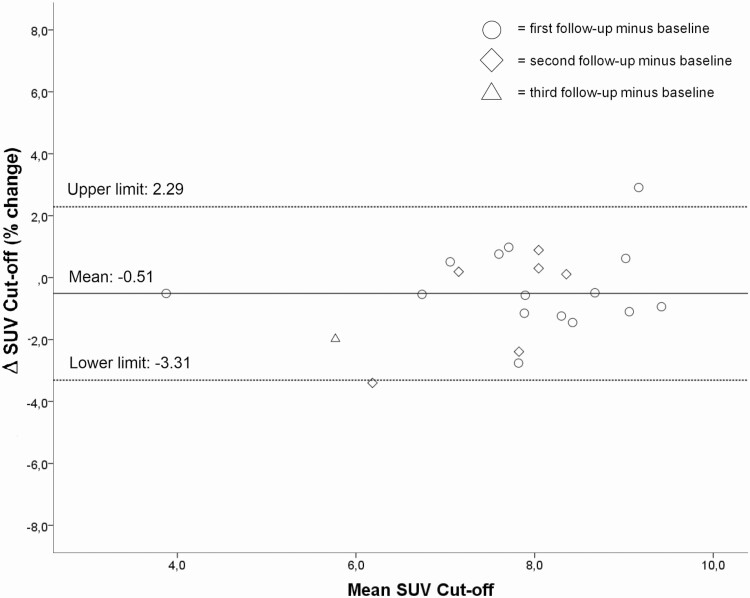
As in our previous study individual SUV cut-offs varied >10% ranging from 3.62 to 10.62 g/mL (median 7.98 g/mL), therefore individualized cut-offs were also used in this follow-up study (12). Individual SUV cut-offs of follow-up scans for healthy bone were not significantly different from baseline, Table 3, neither for denosumab (n = 8) users nor for non-denosumab (n = 7) users, P = .237 and P = .575 for FU vs BL respectively (Fig. 1). The intrapatient ICC between baseline and second Na[18F]F PET-CT SUV cut-off was 0.856 (P = 0.001). The intrapatient repeatability of SUV cut-off for healthy bone for all scans was substantial (ICC = 0.615; P = .006), Bland–Altman mean difference –0.51 g/mL, levels of agreement –3.31 to +2.29 g/mL (Fig. 2), measured in 15 patients (37 scans) between baseline and all follow-up scans.
Figure 1.
Box-and-whisker plots for the subcohorts of denosumab (n = 8) and non-denosumab (n = 7), the SUV cut-offs did not significantly change after treatment compared to baseline, P = .237 and P = .575 (Wilcoxon), respectively.
Figure 2.
Bland–Altman plot visualizing a strong relation between baseline SUV cut-off and follow-up, no heteroskedasticity.
Quantification of FD Burden on Na[18F]F PET-CT at Baseline and at First Follow-up
Median (IQR) change in FTV between baseline and at first follow-up for relevant subgroups is displayed in Table 4.
Table 4.
Median (IQR) change in FTV for relevant subgroups
| Group (n) | FTV (cm3) Median (IQR) | ∆FTV (%) Median (IQR) | P value Wilcoxon | SUVpeak (g/mL) Median (IQR) | P value Wilcoxon | ||
|---|---|---|---|---|---|---|---|
| Baseline | First follow-up | Baseline | First follow-up | ||||
| Total (15 pairs) | 199 (77-923) | 97 (51-635) | –42 (–13 to –66)a | .030 | 29 (18-34) | 21 (13-30) | .028 |
| Completed denosumab (7 pairs)b | 361 (122-1247) | 97 (56-684) | –61 (–71 to –21) | .018 | 29 (18-34) | 26 (13-30) | .176 |
| Non-denosumab (7 pairs) | 76 (13-814) | 54 (8-253) | –23 (–69 to +4)a | .249 | 26 (11-32) | 21 (11-23) | .028 |
a For 1 patient in the non-denosumab subgroup ∆FTV was undefined as FD bone turnover did not exceed the cut-off for normal bone at baseline and during follow-up (ie, FTV = 0).
b One planned denosumab patient could not complete denosumab treatment because of co-morbidity (IBD) and was removed from this analysis. This patient was included in the analysis of the group “Total” to reflect the effect in all analyzed patients.
The portion of baseline affected skeleton on Na[18F]F PET-CT (FAS) showed clearly stronger correlation with the current standard SBS (representing the segmented fraction of the affected skeleton on scintigraphy) (P = .052) than with measured pathological Na[18F]F-volume (FTV, P = .221).
Although median SUVpeak at baseline did decrease significantly in the whole group after bone remodeling therapy (P = .028), SUVpeak nonetheless did not change significantly in the subgroups of denosumab (P = .176; n = 7 pairs) and non-denosumab (P = .080; n = 7 pairs).
For the 12 patients that completed treatment (see “Materials and Methods” section), an even stronger FTV decrease was measured: median (IQR) 158 cm3 (77-754 cm3) to 82 cm3 (45-248 cm3), P = .003, median ∆FTV –59% (–21% to –69%).
In 1 patient of the denosumab group, FTV initially significantly increased from 204 cm3 to 618 cm3, which was in accordance to biochemical parameters with ALP increasing from 189 to 253 IU/L and P1NP from 396 to 473 ng/mL. This was presumably due to alternating denosumab dosing due to concomitant inflammatory bowel disease and infliximab treatment. For the total denosumab group including this patient, the median (IQR) decrease of FTV equaled 60% (–70% to –15%) as this single patient had only limited effect on median FTV decrease.
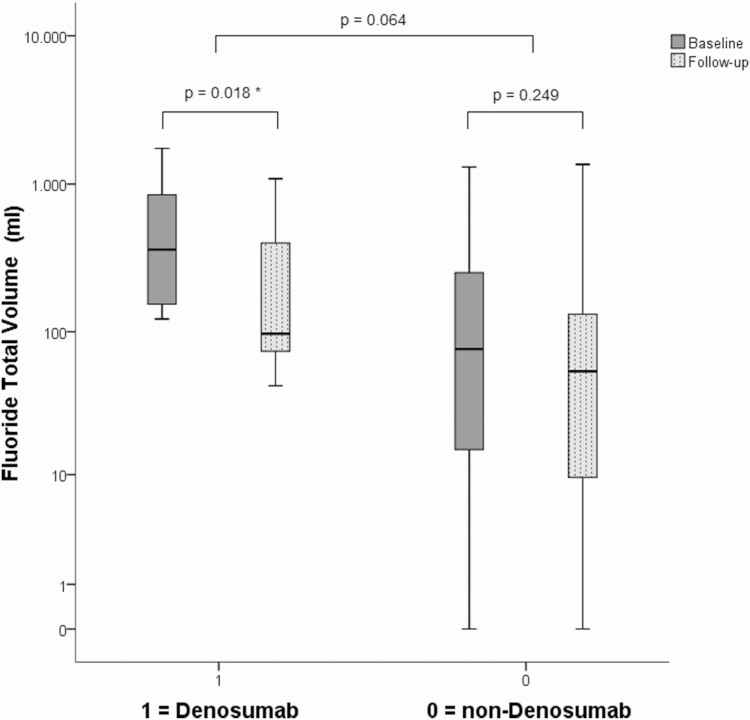
∆FTV between the denosumab group and non-denosumab group did not differ significantly, P = .064. Within the non-denosumab group, the FTV decrease did still not significantly change (P = .249), median ΔFTV of 23%, in contrast to the patients treated with denosumab median ΔFTV of 61% (P = .018).
Initial FD burden as measured by FTV on baseline Na[18F]F PET-CT in patients treated with denosumab was significantly higher (median 361 cm3 and thus indicating higher FD burden) than in non-denosumab users (median 76 cm3, P = .005), Fig. 3.
Figure 3.
FTV on Na[18F]F PET-CT scans at baseline and during follow-up in the denosumab and non-denosumab group. It is noted that initial FTV is significantly higher in patients starting denosumab in comparison to the non-denosumab group. FTV decreased significantly in the denosumab and did not decrease in the non-denosumab group.
Quantification of FD Burden Using Serum Biomarkers and Correlation With Na[18F]F PET-CT at Baseline and at First Follow-up
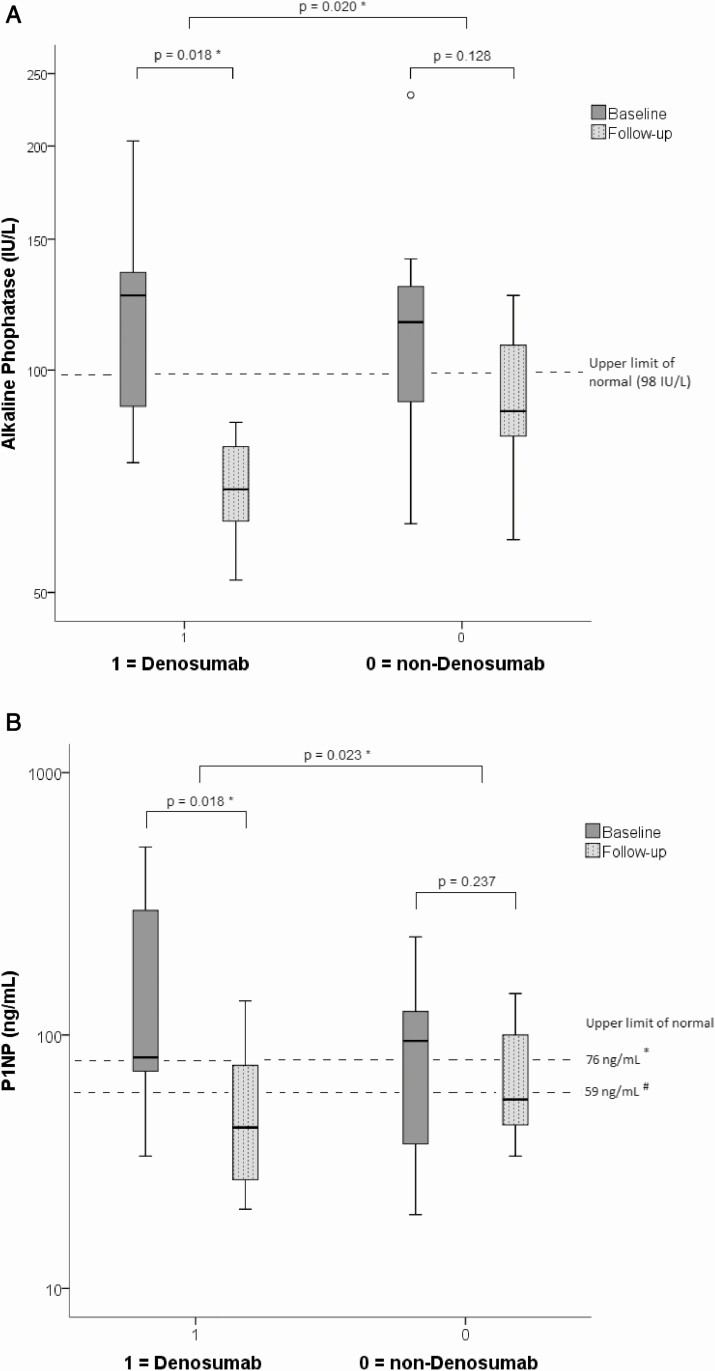
BTMs decreased significantly after start of bone remodeling therapy (n = 15 patients, 30 scans): ALP 119 IU/L (82-143 IU/L) vs 84 IU/L (64-106 IU/L), P = .020; P1NP: 82 ng/mL (51-237 ng/mL) vs 55 ng/mL (34-112 ng/mL), P = .023 Fig. 4. Baseline SBS (representing the segmented fraction of the affected skeleton on scintigraphy) showed poor correlation with BTMs (P = .671 for ALP and P = .486 for P1NP). SUVpeak on Na[18F]F PET-CT did not correlate with ALP (Spearman’s ρ = 0.216; P = .199, n = 37) or P1NP (ρ = 0.137; P = .424, n = 36) respectively and therefore further analysis on use of SUVpeak as a useful outcome measure was discontinued. FTV correlated positively with both serum biomarkers ALP (Spearman’s ρ = 0.406; P = .006, n = 37) and P1NP (Spearman’s ρ = 0.730; P < .001, n = 36). FAS strongly correlated with treatment-induced decrease in ALP (Spearman’s ρ = 0.587; P = .027) and P1NP (Spearman’s ρ = 0.640; P = .009).
Figure 4.
The BTMs ALP and P1NP (including the Upper limit of normal 98 IU/L for ALP and 59 ng/mL for P1NP in [#] in men and premenopausal women and [*] 76 ng/mL for P1NP in postmenopausal patients) in the denosumab and non-denosumab group. Both BTMs decreased significantly in the denosumab and did not in the non-denosumab group.
The baseline imaging (SUVpeak, FTV, TLF) and biochemical (ALP, P1NP) parameters did not correlate with disease response measured by either imaging (ΔFTV) or serum biomarkers (ΔALP: ρ > –0.325, P > .237 or ΔP1NP: ρ < 0.489, P > .064).
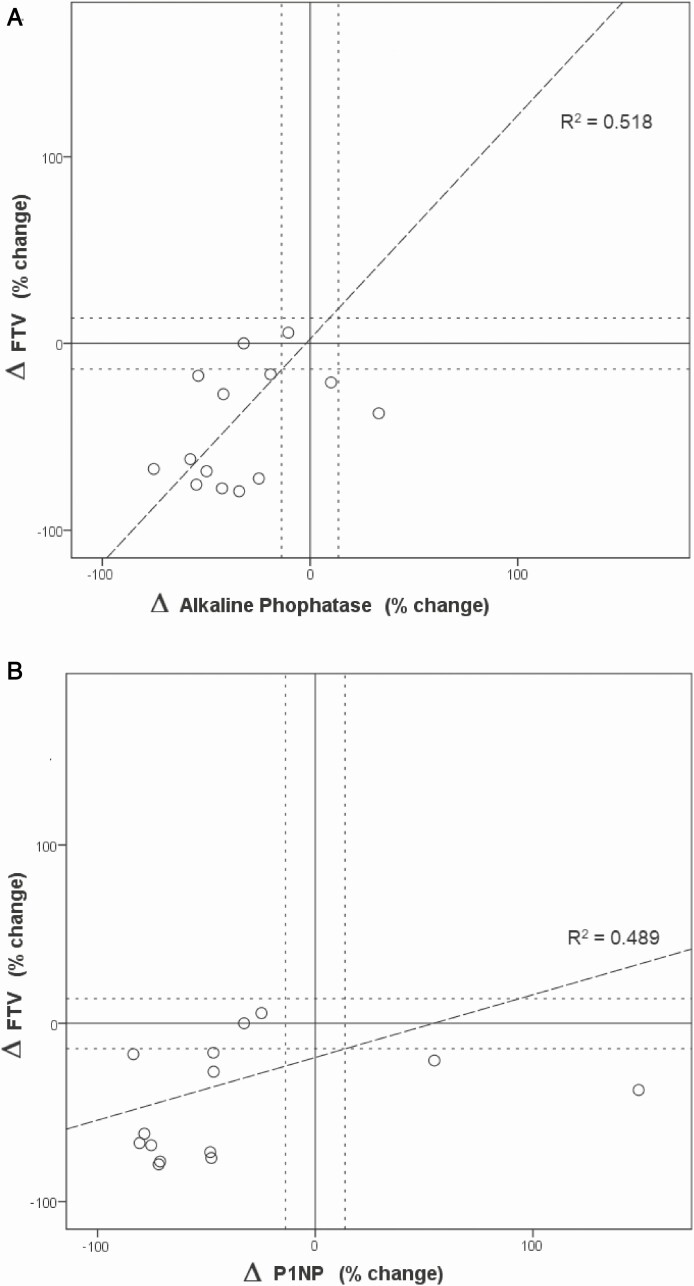
Relative change in FTV (ΔFTV) correlated positively with changes in ALP (ΔALP) (Spearman’s ρ = 0.518; P = .024) and changes in P1NP (ΔP1NP) (Spearman’s ρ = 0.489; P = .032), (see Fig. 5 and Table 4). FAS strongly correlated with ΔALP (ρ = –0.568; P = .027) and in ΔP1NP (ρ = -0.647; P = .009), see Fig. 5.
Figure 5.
The percentage change in FTV is plotted against the percentage change in ALP. The first panel shows that in 1 patient ALP increases and in contrast FTV decreases and the second panel shows that in 2 patients P1NP increases and FTV decreases. An otherwise adequate correlation was measured.
Individual Responses
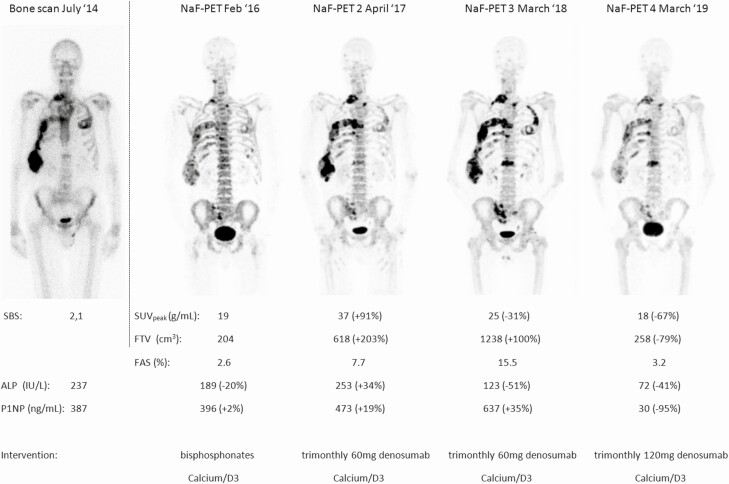
In a substantial part of our patients, either ALP or P1NP (n = 5) or both (n = 3) failed to capture increased bone turnover as serum levels were below the upper limit of normal. In these patients, lesional pathological bone turnover was increased (median FTV 76 cm3, IQR 61-289) and response to treatment could be adequately quantified (median ΔFTV-44%, IQR –67% to –19%) after either denosumab or non-denosumab and this was congruent with clinical findings (improvement of pain scores, reduced pain medication). The patients without elevated BTMs had relatively small baseline FTV (median 76 cm3), in comparison to 467 cm3 in patients with elevated BTMs. In 1 patient both Na[18F]F PET-CT quantitative parameters and P1NP were normal with solitary elevated ALP. In another patient multiple quantitative Na[18F]F PET-CT follow-up studies were performed, a male FD patient (61-year-old when commencing denosumab) who was diagnosed with inflammatory bowel disease (IBD). This patient received treatment with bisphosphonates from 2014 to 2016 and adjusted dosage of 60 mg trimonthly denosumab, because of the diagnosis of IBD in 2017. Both BTMs and Na[18F]F PET-CT showed concordant no improvement in FD burden until March 2018. From early 2019 onwards, patient was able to tolerate trimonthly 120 mg of subcutaneous denosumab. This last regimen resulted in both visual and quantitative decrease on Na[18F]F PET-CT, of which the volume based–parameter FTV and FAS correlated best with both BTMs, especially P1NP (see Fig. 6).
Figure 6.
Follow-up of FD burden in a patient with MFD (male, 59 years old at baseline BS) at start of denosumab undergoing baseline bone scintigraphy and SBS and subsequent 4 consecutive Na[18F]F PET-CT scans. This exceptional amount of scans was related to coinciding Inflammatory Bowel Disease needing treatment and subsequent suboptimal dosage of denosumab and recurring pain complaints. Percentage change in serum biomarkers is given around the date of Na[18F]F PET-CT, with change relative to the direct previous scan. After bisphosphonates treatment starting in 2013, in May 2016 patient received 60 mg of denosumab every 3 months, no other interventions. FD burden for this patient was low as measured with SBS and at baseline Na[18F]F PET-CT moderately increased when measured with FTV. On consecutive follow-up Na[18F]F PET-CT scan no. 2, a clear increase in disease activity is measured with both SUVpeak and FTV and this is reflected by simultaneous increase in both serum alkaline phosphatase and P1NP. Na[18F]F PET-CT scan No 3 interestingly shows further increase in FTV on Na[18F]F PET-CT in accordance with increased serum P1NP, but decrease of SUVpeak on Na[18F]F PET-CT and serum alkaline phosphatase. Na[18F]F PET-CT scan no. 4 is to our knowledge the first ever to visualize clear decrease in FD burden using molecular imaging and this is reflected by both Na[18F]F PET-CT-parameters and is in accordance with both serum bone formation biomarkers. Upper limit of normal 98 IU/L for ALP and 59 ng/mL for P1NP in (#) in men and premenopausal women and (*) 76 ng/mL for P1NP in postmenopausal patients.
Discussion
In this study we show that Na[18F]F PET-CT is capable of detecting therapy-induced changes in lesional bone turnover (especially FTV and FAS) in patients treated with the RANKL inhibitor denosumab. This underwrites previous findings of induced normalization of markers of bone turnover after initiation of denosumab in patients previously treated with bisphosphonates as this not only reflects a biochemical systemic response but also a local response where this could not be observed in the past with bisphosphonates (2, 13). Therefore Na[18F]F PET-CT can be used as an objective parameter of local treatment response in FD/MAS. Also, we did not observe any change in the uptake of healthy bone meaning that the decrease in activity is only lesional, not systemic. Our data also showed that Na[18F]F PET-CT observed changes in FD burden correlated with observed decreases in bone turnover measured by BTMs reflecting that the observed changes in BTMs do reflect a reduction in local FD burden activity.
In clinical practice, change of biochemical bone remodeling biomarkers together with change in FD-related skeletal pain are known outcome measures (2,13,18). An advantage of imaging over a blood test is the visualization of localization of either response or progress, as not all localizations necessarily might respond equally to therapy. In 5 out of 15 patients, serum BTMs failed to capture response of FD burden to therapy although clinically improvement was expected, especially in patients with relatively small baseline FTV, and thus low skeletal burden. In contrast, Na[18F]F PET-CT did reveal decrease in local FD activity.
Although bone scan with the SBS representing affected bone segments is widely used as an adjunct for primary characterization of FD, it might be an underestimation for FD-related disease burden to our and other’s experience (4). Moreover, the use of SBS in follow up during treatment with bisphosphonates (including pamidronate) remains controversial (2, 4, 5). A single case report showed in different disease, a giant cell tumor of bone, a significant decrease in Na[18F]F skeletal burden after initiation of denosumab (19). Use of the SBS in FD/MAS patients treated with denosumab, has not yet been reported.
Specifically in children receiving very high doses of RANKL -inhibitors concern has been raised that osteopetrosis has been reported (20). We have seen no signs of osteopetrosis in our population in our CT part of the total body Na[18F]F PET-CT scans, consisting of only adults and with dosages of denosumab as described in the methods section. These dosages might be considered a moderately increased over common dosages, but not as very high. Radiation burden for Na[18F]F PET-CT is low especially when using only 1 MBq/kg of activity (effective dose 0.024 mSv/MBq), resulting in an 80-kg adult effective dose of approximately 1.9 mSv by the radiopharmaceutical alone. In comparison, [99mTc]Tc-HDP planar scintigraphy would result in about 3 mSv, as was shown in previous studies (4, 9, 21, 22), approximately 60% higher.
As recently reported, advantages of baseline Na[18F]F PET-CT over planar BS include 3D visualization of the skeleton, as performing single-photon emission computed tomography (SPECT)/CT is more time-consuming (both incubation and acquisition) than Na[18F]F PET-CT (4). Although it is technically possible to quantify scintigraphy (both planar and SPECT-CT) by using qSPECT-CT, this is still scarcely available, thus in most hospitals BS can only be assessed either visually or semi quantitatively by the SBS (2).
Also, the spatial resolution of PET-CT surpasses both planar and SPECT-CT bone scan. Although the added value of the increased resolution has not been quantified for current clinical question, in our experience it is advantageous in discriminating increased Na[18F]F-uptake around joints caused by osteoarthritis (OA) versus primary osseous lesions in FD. This is especially the case in spinal localizations, which are frequent in both FD and (secondary) OA. In our experience uptake in OA may be equal to or even higher than in FD and therefore we advocate careful exclusion of uptake by OA and other relevant pathology (non-FD). This requires (1) sufficient spatial resolution to adequately localize the pathology, (2) additional specificity by morphological imaging (CT), and (3) a method that integrates information from both modalities and is able to discern non-FD sources of pathological Na[18F]F uptake.
After previous studies on baseline characterization of FD-burden using Na[18F]F PET-CT, our current study shows that when expanding to follow-up analysis an individual cut-off remains of importance, as interindividual variation of the SUV cut-off value for healthy bone was substantial and pairwise individual SUV cut-offs did not change significantly during treatment follow-up. Our previous studies excluded age of the patient at time of scanning, dose of Na[18F]F, weight of the patient at time of scanning, and the time of incubation of the radiopharmaceutical as well as normalization for lean body mass or skeletal volume as parameters significantly correlated with individual SUV cut-off. We can therefore only speculate on the nature of the individual variation in Na[18F]F uptake in healthy bone such as exercise shortly before scanning, although the repeatability within patients makes this somewhat less likely. Other less likely physiological factors may include renal function or other unidentified biological factors determining individual Na[18F]F uptake in healthy bone. In order to conclude that our individualized normal bone measurements are indeed determined by patient characteristics and not by the scan parameters or the scanning conditions, a more systematic (prospective) acquisition or confirmation by other groups would be necessary. As the individual SUV cut-off defining healthy bone showed a range larger than the predefined median ± 10%, individualized SUV cut-offs were also used in this follow-up study, further supporting the use of an internal reference ensuring optimal normalization of volumetric Na[18F]F PET-CT parameters. As intrapatient SUV cut-off for healthy bone decreases slightly (but insignificantly), using baseline SUV cut-off for determination of FD burden on the follow-up scan would result in an underestimation of follow-up FTV and thus an even higher than observed ΔFTV.
When comparing baseline FD with follow-up Na[18F]F PET-CT quantification, the change in FTV also correlates with change in ALP and P1NP. The strong correlation of volume of increased bone activity by FTV with ALP and P1NP confirms the specificity of our findings. SUVpeak changes did not correlate with BTMs of bone formation where FTV did, which confirms that volume-based measurements are superior over intensity-based measurements for clinical relevance. FTV is to be preferred over TLF (based on FTV and mean standardized Na[18F]F uptake value, SUVmean), based on high correlations between molecular imaging and BTMs and FTV. Secondly, the use of SUVmean is less attractive because it lacks significant contribution to the correlation with serum biomarkers and the optimal normalization method is still under debate (12). Lastly, baseline FTV divided by SV (FAS), representing the part of the skeleton being pathologically metabolically active) correlates strongly with change in BTMs representing FD burden. Doing so, high baseline FAS seems to be a strong predictor for less decrease in ALP and P1NP after denosumab. This is, as far as we know, the first study to demonstrate that molecular imaging of bone metabolism is able to measure in vivo changes of FD activity in time. Moreover, the 5 patients with relatively limited skeletal burden in which BTMs failed to capture FD burden response to therapy, Na[18F]F PET-CT did reveal significant decrease in accordance with clinical findings. For more precision, it would be worthwhile to determine the minimum change in FTV or FAS that can be observed that should be considered a true change by performing a reproducibility study.
Despite the strengths of the current study assessing guidance for treatment by using this novel technique, it is limited in cohort size (n = 15) inherent to the rarity of the studied disease combined with the need for follow-up-scanning. Other disadvantages of the current study are the retrospective analysis as part of an ongoing observational study with some variation in timing of Na[18F]F PET-CT in relation to BTMs. Specifically, the non-denosumab cohort was heterogeneous with respect to receiving or not receiving bisphosphonate treatment and for future study we do recommend to separate patients actively receiving bisphosphonates from patients not receiving active treatment (placebo). Also, the current analysis was not defined at time of the data collection. Although our current method of individualized FD measurements is somewhat laborious, we are nonetheless convinced that other methods are less effective in excluding non-FD uptake (3, 4). We aimed to perform new scans at a 18-month to 24-month interval (after 4-6 injections) but due to logistic reasons (no PET tracer available, inability for patients to go to the location of the scanner) the interval unfortunately varied. Strengths of the study include the intrapatient normalization for normal versus pathological bone, with the patient acting as its designated control. Secondly, during follow-up no other relevant intervention than mentioned bone remodeling therapy did occur and therefore no other contributing factor had to be ruled out than bisphosphonates or denosumab and possibly natural development of the disease contributing to change in Na[18F]F PET-CT-parameters. Therefore, we were able to compare consecutive patients without denosumab to those using denosumab.
In conclusion, we were able to quantify normal bone activity and FD disease activity on Na[18F]F PET-CT for baseline and follow-up using a personalized approach, in a reliable and clinically feasible manner. Furthermore, when compared to SBS (the current standard), this resulted in 3D measured FD burden change with Na[18F]F PET-CT. Na[18F]F PET-CT particularly using FTV and FAS potentially provides new clinical insight in follow-up FD disease burden, as these parameters showed high correlation with biomarkers of bone formation, especially P1NP. Therefore, Na[18F]F PET-CT could be a meaningful tool to evaluate treatment efficacy during long-term follow-up in patients with FD. Na[18F]F PET-CT might be of further clinical value in those patients with normal(ized) BTMs and clinical complaints as an alternative to objectify response to therapy. Considering the limitations of 1 observational study, we do encourage confirmation by future studies in an expanded study with structured timing of therapy, imaging and BTMs, like the study currently registered under NCT03571191, to further confirm the relations found in our real world clinical setting.
Acknowledgments
Author Contributions: Study design: N.M.A.D., W.v.d.B., and D.V. Data collection and image acquisition: N.M.A.D., F.S., D.V., E.M.W. Image analysis: W.v.d.B. and D.V. Statistical analysis: W.v.d.B., N.M.A.D., and D.V. Manuscript writing: W.v.d.B., D.V., and N.M.A.D. Manuscript revision: E.M.W., F.S., L.F.d.G.O. All authors read and approved the final version of the manuscript.
Glossary
Abbreviations
- ALP
alkaline phosphatase
- BPI
Brief Pain Inventory
- BS
bone scintigraphy
- BTM
bone turnover marker
- FAS
fraction affected skeleton
- FTV
fluoride tumor volume
- FD/MAS
fibrous dysplasia/McCune–Albright
- Gsα
α-subunit of the stimulatory G-protein
- IBD
inflammatory bowel disease
- ICC
intraclass correlation coefficient
- IQR
interquartile range
- OA
osteoarthritis
- PET-CT
positron emission tomography–computed tomography
- SBS
skeletal burden score
- SPECT
single photon emission tomography
- SUV
standardized uptake value
- SV
skeletal volume
- TLF
total lesion fluorination
Additional Information
Disclosures: The authors have no (potential) conflicts of interest.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325(24):1688-1695. [DOI] [PubMed] [Google Scholar]
- 2. Collins MT, Kushner H, Reynolds JC, et al. An instrument to measure skeletal burden and predict functional outcome in fibrous dysplasia of bone. J Bone Miner Res. 2005;20(2):219-226. [DOI] [PubMed] [Google Scholar]
- 3. Papadakis G, Manikis G, Karantanas A, et al. 18F-NaF PET/CT imaging in fibrous dysplasia of bone. J Bone Miner Res. 2019;34(9):1619-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Bruggen W, Hagelstein-Rotman M, de Geus-Oei LF, et al. Quantifying skeletal burden in fibrous dysplasia using sodium fluoride PET/CT. Eur J Nucl Med Mol Imaging. 2020;47(6):1527-1537. [DOI] [PubMed] [Google Scholar]
- 5. Wei WJ, Sun ZK, Shen CT, et al. Value of (99m)Tc-MDP SPECT/CT and (18)F-FDG PET/CT scanning in the evaluation of malignantly transformed fibrous dysplasia. Am J Nucl Med Mol Imaging. 2017;7(3):92-104. [PMC free article] [PubMed] [Google Scholar]
- 6. Rohren EM, Macapinlac HA. Spectrum of benign bone conditions on NaF-PET. Semin Nucl Med. 2017;47(4):392-396. [DOI] [PubMed] [Google Scholar]
- 7. Usmani S, Gnanasegaran G, Marafi F, Esmail A, Ahmed N, Van den Wyngaert T. The clinical significance of incidental soft tissue uptake on whole body 18F-sodium fluoride bone PET-CT. Clin Radiol. 2019;74(2):95-110. [DOI] [PubMed] [Google Scholar]
- 8. Beheshti M. 18F-sodium fluoride PET/CT and PET/MR imaging of bone and joint disorders. PET Clin. 2018;13(4):477-490. [DOI] [PubMed] [Google Scholar]
- 9. Wondergem M, van der Zant FM, Knol RJJ, et al. 99mTc-HDP bone scintigraphy and 18F-sodiumfluoride PET/CT in primary staging of patients with prostate cancer. World J Urol. 2018;36(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langsteger W, Balogova S, Huchet V, et al. Fluorocholine (18F) and sodium fluoride (18F) PET/CT in the detection of prostate cancer: prospective comparison of diagnostic performance determined by masked reading. Q J Nucl Med Mol Imaging. 2011;55(4):448-457. [PubMed] [Google Scholar]
- 11. Duarte PS, Sapienza MT. Normalization by bone volume instead of body weight or lean body mass may be better for quantifying skeletal burden in fibrous dysplasia using sodium fluoride PET/CT. Eur J Nucl Med Mol Imaging. 2020;47(6):1349–1350. [DOI] [PubMed] [Google Scholar]
- 12. van der Bruggen W, de Geus-Oei LF, Appelman-Dijkstra NM, Vriens D. Considerations on bone volume normalization in quantifying skeletal burden in fibrous dysplasia using sodium fluoride PET/CT. Eur J Nucl Med Mol Imaging. 2020;47(6):1351–1352. [DOI] [PubMed] [Google Scholar]
- 13. Majoor BCJ, Papapoulos SE, Dijkstra PDS, Fiocco M, Hamdy NAT, Appelman-Dijkstra NM. Denosumab in patients with fibrous dysplasia previously treated with bisphosphonates. J Clin Endocrinol Metab. 2019;104(12):6069–6078. [DOI] [PubMed] [Google Scholar]
- 14. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oueriagli SN, Ghfir I, Guerrouj HE, Rais NB. What role for radiobiphosphonates bone scintigraphy in the monitoring of an unusual bone giant cell tumor: a case report and literature review. Am J Nucl Med Mol Imaging. 2016;6(2):128–134. [PMC free article] [PubMed] [Google Scholar]
- 16. Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349(5):457–463. [DOI] [PubMed] [Google Scholar]
- 17. Leide-Svegborn S. Radiation exposure of patients and personnel from a PET/CT procedure with 18F-FDG. Radiat Prot Dosimetry. 2010;139(1–3):208–213. [DOI] [PubMed] [Google Scholar]
- 18. Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91(8):1882–1889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.