Abstract
Context
Data quantifying the impact of metreleptin therapy on survival in non-human immunodeficiency virus (HIV)-related generalized lipodystrophy (GL) and partial lipodystrophy (PL) are unavailable.
Objective
This study aimed to estimate the treatment effect of metreleptin on survival in patients with GL and PL.
Design/Setting/Patients
Demographic and clinical characteristics were used to match metreleptin-treated and metreleptin-naïve patients with GL and PL. Differences in mortality risk were estimated between matched cohorts of metreleptin-treated and metreleptin-naïve patient cohorts using Cox proportional hazard models. Sensitivity analyses assessed the impact of study assumptions and the robustness of results.
Outcome Measures
This study assessed time-to-mortality and risk of mortality.
Results
The analysis evaluated 103 metreleptin-naïve patients with characteristics matched to 103 metreleptin-treated patients at treatment initiation. Even after matching, some metabolic and organ abnormalities were more prevalent in the metreleptin-treated cohort due to bias toward treating more severely affected patients. A Cox proportional hazards model associated metreleptin therapy with an estimated 65% decrease in mortality risk (hazard ratio [HR] 0.348, 95% confidence interval (CI): 0.134–0.900; P = 0.029) even though the actual number of events were relatively small. Results were robust across a broad range of alternate methodological assumptions. Kaplan–Meier estimates of time-to-mortality for the metreleptin-treated and the matched metreleptin-naïve cohorts were comparable.
Conclusions
Metreleptin therapy was associated with a reduction in mortality risk in patients with lipodystrophy syndromes despite greater disease severity in treated patients, supporting the view that metreleptin can have a positive disease-modifying impact. Confirmatory studies in additional real-world and clinical datasets are warranted.
Keywords: lipodystrophy, leptin, metreleptin, hepatic steatosis, CGL, FPLD
Lipodystrophy syndromes are a heterogeneous group of rare disorders characterized by the lack of adipose tissue and severe metabolic complications (1, 2). They are categorized into generalized and partial forms based on the extent of adipose tissue loss across the body. Generalized lipodystrophy (GL) has a more severe phenotype and is characterized by the absence or progressive loss of adipose tissue across the whole body. Adipose tissue loss in partial lipodystrophy (PL) is typically localized to select regions of the body, such as the limbs or the upper body, depending on subtype (1). The metabolic consequences of lipodystrophy syndromes, which are thought to be driven by pathological adaptations to the lack of adipose tissue and associated nutrient spillover, can increase the risk for conditions that negatively impact life expectancy, such as diabetes, hypertriglyceridemia, pancreatitis, heart disease, and kidney dysfunction (1, 3).
Leptin is a hormone involved in the regulation of energy homeostasis and is primarily produced by adipose tissue (4). Patients with GL and PL can have low leptin levels, which has been implicated as a driver of lipodystrophy-associated metabolic abnormalities (1, 5). Recombinant human methionyl leptin (metreleptin) is approved as an adjunct to diet to treat the metabolic complications of leptin deficiency in patients with GL (United States, Europe, Japan) and PL (Europe and Japan) (6, 7). Single-arm open-label studies have suggested that metreleptin can ameliorate the severity of multiple metabolic abnormalities in patients with lipodystrophy, including hyperglycemia, hypertriglyceridemia, and hepatic steatosis (8–18). However, the effect of metreleptin on mortality among patients with lipodystrophy syndromes is not yet well-established due to short follow-up periods and lack of control arms in earlier studies. Studies comparing long-term outcomes of patients with GL and PL receiving metreleptin to those who have never received the drug have not been published. The low prevalence of GL and PL and their heterogeneous natural history also make it impractical to conduct large randomized controlled trials to quantify the effect of a therapy on mortality.
In the absence of randomized controlled trials, indirect treatment comparisons may be conducted to estimate the impact of an intervention on key clinical outcomes, including in cases where the individual included studies were not designed to detect a treatment effect on a specific outcome (19, 20). We have previously characterized the natural history of lipodystrophy syndromes in a cohort of patients with GL and PL who have never received metreleptin (21). The current study draws upon data collected from this natural history study as a comparator cohort for patients who received metreleptin therapy in clinical trials. The treatment effect of metreleptin therapy on mortality among patients with GL or PL is estimated using a Cox proportional hazard model to control for differences between the natural history study and clinical trial-enrolled populations.
Methods
Study populations
Data from 2 study populations were included to estimate the treatment effect of metreleptin on mortality. Retrospective data from patients with GL and PL who received metreleptin (metreleptin-treated) were obtained from medical records of 105 patients enrolled in a single-arm clinical trial (NCT00005905) and follow-up study (NCT00025883) conducted at the US National Institutes of Health from 2000 to 2014, and from records of another 7 patients who met eligibility criteria for the 2 trials but enrolled in a nonrandomized parallel group study to evaluate the short-term effects of metreleptin initiation or withdrawal (NCT01778556). The design of these trials has been previously described (13, 17, 18). Patients enrolled in the clinical trials were required to have clinically significant non-human immunodeficiency virus (HIV)-related lipodystrophy as well as low leptin levels, diabetes, elevated insulin, and/or elevated triglycerides. National Institutes of Health investigator adjudication via in-person interviews was conducted in cases where required medical information was not readily available or interpretable in medical records. Records in which required data remained missing or uninterpretable following adjudication were excluded. Data from metreleptin-treated patients were available from the date of study enrollment until death or censoring.
Separately, retrospective data from patients with GL and PL who never received metreleptin (metreleptin-naïve) were obtained from a lipodystrophy natural history study based on medical records of 230 patients obtained from the following treatment centers: Dokuz Eylul University (Turkey), Federal University of Ceará (Brazil), National Institutes of Health (US), University of Michigan (US), and the University of São Paulo (Brazil) (21). In this study, medical records of patients with a diagnosis of non-HIV-related GL or PL prior to January 1, 2015, were eligible for inclusion to allow for sufficient observation time following diagnosis. Patients were also required to have at least 1 year of follow-up after their date of lipodystrophy diagnosis. The study excluded medical records from patients who received metreleptin at any time over the study observation period. The observation period for metreleptin-naïve patients was defined as the time period spanning from birth until the date of data abstraction, or until loss to follow-up or death.
Local institutional review board approval was obtained from all study sites prior to the initiation of data collection.
Variables and data collection
Patient data were extracted by local staff at the study sites between 2017 and 2018. Data from medical records of metreleptin-treated patients were extracted using an Excel-based data collection form, while data from medical records of metreleptin-naïve patients were extracted and entered into internet-based case report forms by local staff at each treatment center. Case report forms included drop-down lists with a prespecified list of clinical abnormalities grouped by organ systems associated with lipodystrophy. These drop-down lists also included an option to select “Other,” which then prompted data abstractors to specify the condition in an open-text field.
Data extraction focused on capturing abnormalities related to metabolic complications associated with lipodystrophy (eg, insulin resistance, diabetes, dyslipidemia, hepatic steatosis) rather than an exhaustive set of medical conditions in each patient. Patient demographics and clinical characteristics obtained from medical records included age at start of observation, age at first symptoms, sex, lipodystrophy diagnosis (GL or PL), triglyceride levels, elevated hemoglobin A1c (HbA1c; defined as ≥6.5%), episodes of pancreatitis, and the presence or absence of abnormalities in the heart, liver, and kidneys. The dates of diagnosis for elevated HbA1c, each episode of pancreatitis, each abnormality observed in the heart, liver, and kidneys, as well as the dates of triglyceride measurements and mortality events (if applicable), were also collected. Date information was imputed as the first of the month when information on the specific day of the month was missing and as January when information on the specific month was missing. Observed abnormalities in the heart, liver, and kidneys captured in the current study are described in Table 1. Patient records where data on observed abnormalities in the heart, liver and kidneys were missing (as determined by data abstractors or study investigators) were excluded.
Table 1.
Definition of observed organ abnormalities reported in patient cohorts
| Cohort | Liver Abnormalities | Kidney Abnormalities | Heart Abnormalities |
|---|---|---|---|
| Metreleptin-treated | NAFLDa | Nephropathyc | Coronary artery disease |
| Hepatomegaly | Renal failure | Cardiac arrhythmiah | |
| Cirrhosis | Renal disease | Cardiomyopathyi | |
| Otherb | Otherd | Otherj | |
| Metreleptin-naïve | NAFLDa | Nephropathye | Coronary artery diseasek |
| Hepatomegaly | Chronic renal failuref | Cardiac arrhythmial | |
| Cirrhosis | ESRD | Cardiomyopathym | |
| Transplant | Heart failure | ||
| Otherg | Transplant | ||
| Othern |
Abbreviations: ESRD, end-stage renal disease; NAFLD, nonalcoholic fatty liver disease.
a Includes hepatic steatosis, nonalcoholic steatohepatitis, and steatohepatitis.
b Includes fibrosis and hepatitis.
c Includes proteinuria.
d Includes glomerulosclerosis.
e Includes albuminuria, microalbuminuria, and proteinuria.
f Includes chronic renal failure, chronic renal insufficiency, and chronic kidney disease (all recorded in open-text fields).
g Includes hematuria, kidney stones, nephromegaly, and renal hypoplasia.
h Includes atrial fibrillation, tachycardia, and irregular heart beat.
i Includes any type of hypertrophy.
j Includes any type of dilation, regurgitation, and other significant diagnoses in the heart entered by clinicians not already captured under other categories.
k Includes atherosclerosis, bypass surgery, ischemia, myocardial infarction, and probable anteroseptal infarct (recorded in open text fields).
l Includes atrial fibrillation, atrial flutter, bradycardia, and tachycardia (all but atrial fibrillation recorded in open-text fields).
m Includes ventricular hypertrophy.
n Includes aortic insufficiency, aortic outflow murmur, aortic regurgitation, aortic stenosis, ascending aorta dilated, asymmetric septal hypertrophy with a sigmoid septum, atrial-level shunt and ventricular dilation, arteriovenous (AV) malformation, AV shunt, cardiomegaly, dilated left atrium, effusion pericardial, grade II/VI midsystolic murmur at the base of the left sternal border, heart murmurs, left ventricular relaxation deficit, mild mitral insufficiency, mild mitral valve regurgitation, mild tricuspid insufficiency, mild tricuspid valve regurgitation, mitral insufficiency, mitral valve insufficiency, mitral valve prolapse, mitral valve regurgitation, moderate mitral insufficiency, pulmonary AV malformation, pulmonic valve regurgitation, subaortic stenosis, subaortic ventricular septal defect, tricuspid insufficiency, tricuspid valve regurgitation, valvular heart disease, and ventricle diastolic-systolic dysfunction.
Matching of metreleptin-treated and metreleptin-naïve patients
To match metreleptin-treated and metreleptin-naïve patients for the current analysis, it was necessary to account for the likelihood that inclusion criteria for clinical trials evaluating metreleptin led to enrollment of patients with more severe or advanced disease than those included in the lipodystrophy natural history study. Moreover, data abstracted from medical records of metreleptin-naïve patients captured relevant clinical events from birth onward, while data abstracted from records of metreleptin-treated patients primarily captured clinical events from the start of trial enrollment onward (rather than from their date of first symptoms or diagnosis). In addition, patients in the trials evaluating metreleptin therapy had undergone a series of evaluations that may be outside the routine clinical practice, such as liver biopsies or echocardiograms performed for the purpose of the clinical trial rather than a symptom-based testing strategy aligned with standard clinical practice. This difference in the data availability period was expected to accentuate perceived differences in the disease state between the 2 cohorts. Thus, patient matching required accounting for potentially significant demographic and clinical differences between cohorts as well as identifying the point of time in the life course of a metreleptin-naïve patient that represented the optimal match to each metreleptin-treated patient when they initiated metreleptin treatment.
Balanced risk set matching, previously used to compare clinical outcomes between treated and untreated patients within a registry (22–24), has been extended in the current analysis to address the need within these progressive lipodystrophy syndrome cohorts to match patients in disease progression status as well as to account for potentially significant differences in other baseline characteristics. This approach matches each metreleptin-treated patient at treatment initiation to a unique metreleptin-naïve patient at a specific index date in their observation history where the 2 patients are most similar (25). Characteristics used in the matching were age, sex, lipodystrophy diagnosis (GL or PL), the number of organs among the heart, liver, and kidneys with an observed abnormality, and elevated HbA1c levels (≥6.5%), which was based on the HbA1c measurements taken prior to treatment-initiation or the specific index date. These characteristics were selected based on their perceived relevance to mortality, the key study outcome of interest. Pancreatitis was also considered for similar reasons but was predicted to be nonoptimal for use in matching due to a large relative imbalance in observed rates of pancreatitis between the metreleptin-treated and metreleptin-naïve cohorts.
To establish optimal matches, characteristics of each metreleptin-naïve patient at all potential monthly index dates were compared with those of the candidate metreleptin-treated patient at treatment initiation (Fig. 1). A calculated Mahalanobis distance (26) was used to estimate the magnitude of differences between each metreleptin-treated patient at treatment initiation and potential metreleptin-naïve patient match across all monthly index dates, where a lower distance implies a closer match across the demographic and clinical characteristics considered. The pairing with the lowest calculated Mahalanobis distance is selected as the optimal match. Mahalanobis distance measures are commonly used in balanced risk set matching (22), have been shown to have desirable bias reduction properties (27), and facilitate matching across all covariates of interest (28). Use of Mahalanobis distance matching can yield improved balance across covariates versus propensity score matching (28). Once the match is established, each metreleptin-treated and metreleptin-naïve patient pair is observed until the date of data abstraction, or until loss to follow-up or death. As an example, in matching a male patient with GL initiating metreleptin at 11 years of age, the goal is to identify the metreleptin-naïve patient from the natural history study cohort with similar demographic characteristics (eg, also male with GL) who, at a similar age, was most comparable on other clinical manifestations reflecting disease status (eg, metabolic parameters, number of organs with observed abnormalities among the heart, liver, and kidneys) based on having the lowest calculated Mahalanobis distance. The described matching approach yields a sample of metreleptin-naïve patients with index observation dates set at a time point when their characteristics are most similar to those of the corresponding metreleptin-treated patient at treatment initiation.
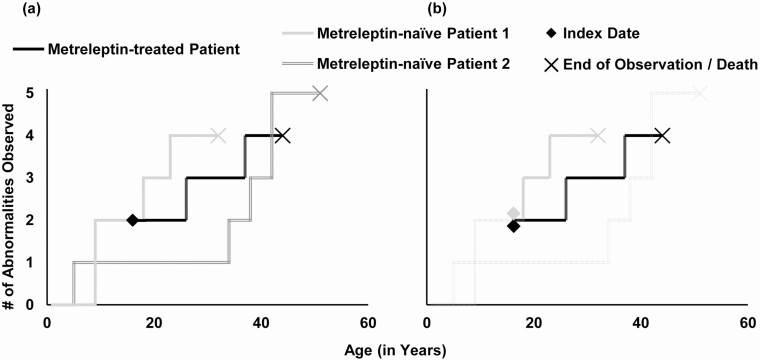
Figure 1.
Matching Methodology: Illustrative Example. (a) Pre-match: data from treated patients span from their date of treatment initiation (ie, their index date) to the end of data availability while data from metreleptin-naïve patients span from the date of first available data to the end of data availability. An index date analogous to the treatment initiation date needs to be defined for records from metreleptin-naïve patients before they can be directly compared to a treated patient. (b) Post-match: the index date for the matched record from metreleptin-naïve patient is defined as the date where the patient was most similar to the treated patient on their date of treatment initiation. Data from this matched record now span from this index date to the end of data availability. The unmatched metreleptin-naïve patient is returned to the matching pool. Patients are generally matched at similar ages that may not be identical. The current example presents the case where two patients matched at the same index age.
The matching process also included rules to limit the impact from additional perceived sources of bias, as follows: In the baseline specification, each metreleptin-naïve patient could serve as a match to only 1 metreleptin-treated patient. This restriction was set to prevent any single patient from unduly affecting subsequent results. Patients were also required to be exactly matched on their lipodystrophy diagnosis (GL or PL) to control for differences in survival between patients with PL and GL reported in an earlier natural history study (21). To control for differences in event rates driven insufficient follow-up period, metreleptin-naïve patients were required to have at least 6 months of follow-up time after the selected index date where they were determined to be most similar to the metreleptin-treated patient.
Because the matching process is conducted sequentially without replacement, matching results can be affected by the order in which metreleptin-treated patients were selected for matching. Thus, matching was conducted across 1000 runs, with the matching order for metreleptin-treated patients randomized each time. For each resulting set of matched cohorts across the 1000 runs, the sum of the Mahalanobis distance between each metreleptin-treated and matched metreleptin-naïve patient was calculated. The set where patients were most similar (identified as the set that minimizes the sum of Mahalanobis distances over the included metreleptin-treated and matched metreleptin-naïve patient pairs across the 1000 runs) was selected for subsequent analyses.
Analyses of outcomes
The primary analysis assessed outcomes across the metreleptin-treated and the matched metreleptin-naïve cohorts. A subgroup analysis in patients with GL from each cohort was also conducted. Demographic and clinical characteristics at the treatment initiation date for patients in the metreleptin-treated cohort and at the index observation date for patients in the metreleptin-naïve cohort were reported as the means for continuous variables and as frequencies or proportions for categorical variables. The average treatment effect of metreleptin on mortality was estimated by comparing the outcome between metreleptin-treated and matched metreleptin-naïve cohorts. Kaplan–Meier survival analysis was used to estimate mean time-to-mortality from treatment initiation for metreleptin-treated patients and from the index observation date for matched metreleptin-naïve patients. A log-rank test was conducted to compare time-to-mortality between the 2 cohorts.
A Cox proportional hazards model was used to estimate the differences in risk of mortality between the 2 cohorts as a hazard ratio and to model survival probability over time. Control variables for mortality risk estimates included treatment status, lipodystrophy diagnosis (GL or PL), birth year, triglyceride levels, having an HbA1c ≥6.5%, having ≥1 episode of pancreatitis, and the presence of observed abnormalities in the heart or kidneys. The presence of liver abnormalities was not included as a control variable because it was expected to have limited predictive power (nearly every patient across both cohorts had observed liver abnormalities) and to avoid overfitting. Values for control variables were based on the treatment initiation or index observation dates. Statistical analyses were performed using R version 3.5.
Results
Data summary and matching quality
Medical records of 112 metreleptin-treated patients and 230 metreleptin-naïve patients with a diagnosis of GL or PL were obtained from study site investigators. Prior to matching, 9 patient records from the metreleptin-treated cohort were excluded due to missing data, leaving 103 evaluable records, while all records from the metreleptin-naïve cohort were retained. At the time of metreleptin-initiation, 80 patients (78%) were receiving exogenous insulin with or without oral antidiabetics, 12 patients (12%) were on oral antidiabetics without insulin, and 62 patients (60%) were on lipid-lowering therapies. As previously reported, data on medications use among metreleptin-naïve patients were available in only 89 of 230 patient records (39%) and considered incomplete for further analysis (21).
Differences in nearly every observed characteristic between the metreleptin-treated and metreleptin-naïve cohorts were statistically significant prior to matching (P < 0.05 for all). Compared with the metreleptin-treated patients, metreleptin-naïve patients were older at the time lipodystrophy symptoms were first recorded, more likely to be male, less likely to be diagnosed with GL, and less likely to have a severe metabolic phenotype as evidenced by the lower mean triglyceride levels, lower frequency of elevated HbA1c, and lower frequencies of abnormalities associated with heart, liver, and kidneys.
In both the full cohort and in the GL and PL cohorts, demographic characteristics such as age and sex were balanced after matching (Tables 2, 3, and 4). Unbalanced characteristics remaining after matching were all clinical in nature and the direction of imbalance was suggestive of greater disease severity in the metreleptin-treated cohorts (eg, greater number of patients with elevated HbA1c, greater number of patients with observed abnormalities in the heart, liver and/or kidneys).
Table 2.
Patient demographic and clinical characteristics pre- and postmatching (overall cohort)
| Treated (n = 103) | Pre-match Metreleptin-naïvea (n = 230) | Matched Metreleptin-naïve (n = 103) | |
|---|---|---|---|
| Age at first symptoms in years, mean (SD) | 13.8 (11.5) | 19.2 (16.5)** | 15.0 (14) |
| Age at start of treatment or index observation date in years, mean (SD) | 24.7 (15.7) | 21.2* (5.94) | 25.3 (17.1) |
| Male, % | 15.5 | 30.4** | 21.4 |
| Diagnosis of GL, % | 60.2 | 35.2** | 60.2 |
| GL/PL subtype,b % | |||
| AGL | 12.6 | 3.0** | 3.9* |
| CGLc | 42.7 | 31.3 | 55.3 |
| Generalized progeroid lipodystrophy | 4.9 | 0.9 | 1.0 |
| APL | 2.9 | 12.2* | 2.9 |
| FPLDd | 36.9 | 52.6* | 36.9 |
| Clinical characteristics at start of treatment or index observation date | |||
| Elevated HbA1c (≥6.5%), % | 78.6 | 24.3** | 60.2** |
| Triglyceride levels in mg/dL,b mean (SD) | 1304 (2180) | 472** (785) | 486** (592) |
| Experienced ≥1 episode of pancreatitis,b % | 40.8 | 3.91** | 10.7** |
| Number of organs among heart, liver and kidneys with observed abnormalities, mean (SD) | 2.049 (0.797) | 0.613** (0.893) | 1.650** (0.871) |
| Heart, % | 46.6 | 8.26** | 29.1** |
| Liver, % | 92.2 | 35.7** | 83.5 |
| Kidneys, % | 66.0 | 17.4** | 52.4* |
| Patients with record of triglyceride levels, n | 102 | 103e | 82e |
| Patients with record of HbA1c levels, n | 103 | 118e | 77e |
* P < 0.05.
** P < 0.01 compared with metreleptin-treated cohort.
Abbreviations: AGL, acquired generalized lipodystrophy; CGL, congenital generalized lipodystrophy; FPLD, familial partial lipodystrophy; GL, generalized lipodystrophy; HbA1c, hemoglobin A1c; NA, not applicable; SD, standard deviation.
a Index observation date for the pre-match metreleptin-naïve cohort was defined as the time at which metreleptin-naïve patients achieved the mean age at the start of treatment of the treated sample (24.7 years) or the date of their last available observation, whichever comes first.
b GL/PL subtypes, triglyceride levels and pancreatitis were not used as matching parameters for the metreleptin-naïve cohort.
c Patients with mutations in AGPAT2 were the most common (n = 26 in treated cohort; n = 22 in matched metreleptin-naïve cohort), followed by those with mutations in BSCL2 (n = 15 in treated cohort; n = 15 in matched metreleptin-naïve cohort). The treated cohort also had 2 patients with CGL who had other mutations and 1 patient with CGL where genetic testing data were either missing or a mutation could not be confirmed. The matched metreleptin-naïve cohort also had 2 patients with PTRF4 mutations, 2 patients with CGL who had other mutations, and 16 patients with CGL where genetic testing data were either missing or a mutation could not be confirmed.
d Patients with mutations in LMNA were the most common (n = 20 in treated cohort; n = 25 in matched metreleptin-naïve cohort), followed by those with mutations in PPARG (n = 8 in treated cohort; n = 3 in matched metreleptin-naïve cohort). The treated cohort also had 1 patient with FPLD who had a PCYT1A mutation and 9 patients with FPLD where genetic testing data were either missing or a mutation could not be confirmed. The matched metreleptin-naïve cohort also had 3 patients with Köbberling type FPLD; 5 patients with FPLD who had other mutations, and 2 patients with FPLD where genetic testing data were either missing or a mutation could not be confirmed.
e Counts only include patients who have lab measurements taken on or after their index observation date.
Table 3.
Patient demographic and clinical characteristics pre- and postmatching (GL cohort)
| Treated (n = 62) | Pre-match Metreleptin-naïvea (n = 81) | Matched Metreleptin-naïve (n = 62) | |
|---|---|---|---|
| Age at first symptoms in years, mean (SD) | 9.0 (7.3) | 9.2 (11.9) | 9.8 (12.5) |
| Age at start of treatment or index observation date in years, mean (SD) | 17.7 (11.7) | 14.4* (4.9) | 16.9 (14.3) |
| Male, % | 22.6 | 40.7* | 32.3 |
| GL subtype,b % | |||
| AGL | 21.0 | 8.6 | 6.5* |
| CGL | 71.0 | 88.9* | 91.9** |
| Generalized progeroid lipodystrophy | 8.1 | 2.5 | 1.6 |
| Clinical characteristics at start of treatment or index observation date | |||
| Elevated HbA1c (≥6.5%), % | 79.0 | 34.6** | 48.4** |
| Triglyceride levels in mg/dL,b mean (SD) | 1354 (2260) | 363** (462) | 473** (687) |
| Experienced ≥1 episode of pancreatitis,b % | 33.9 | 2.47** | 6.5** |
| Number of organs among heart, liver and kidneys with observed abnormalities, mean (SD) | 2.177 (0.859) | 0.975** (0.987) | 1.516** (0.971) |
| Heart, % | 56.5 | 16** | 24.2** |
| Liver, % | 90.3 | 55.6** | 77.4 |
| Kidneys, % | 71.0 | 25.9** | 50.0* |
| Patients with record of triglyceride levels, n | 61 | 46c | 46c |
| Patients with record of HbA1c levels, n | 62 | 36c | 42c |
* P < 0.05.
** P < 0.01 compared to metreleptin-treated cohort.
Abbreviations: AGL, acquired generalized lipodystrophy; CGL, congenital generalized lipodystrophy; GL, generalized lipodystrophy; HbA1c, hemoglobin A1c; NA, not applicable; SD, standard deviation.
a Index observation date for the pre-match metreleptin-naïve cohort was defined as the time at which metreleptin-naïve patients achieved the mean age at the start of treatment of the treated sample (17.7 years) or the date of their last available observation, whichever comes first.
b GL subtype, triglyceride levels and pancreatitis were not used as matching parameters for the metreleptin-naïve cohort.
c Counts only include patients who have lab measurements taken on or after their index observation date.
Table 4:
Patient demographic and clinical characteristics pre- and postmatching (PL cohort)
| Treated (n = 41) | Pre-match Metreleptin-naïvea (n = 149) | Matched Metreleptin-naïve (n = 41) | |
|---|---|---|---|
| Age at first symptoms in years, mean (SD) | 20.7 (12.9) | 24.7 (16.1) | 22.7 (12.5) |
| Age at start of treatment or index observation date in years, mean (SD) | 35.2 (15.2) | 30.7 (7.2) | 38.0 (12.6) |
| Male, % | 4.9 | 24.8** | 4.9 |
| PL subtype,b % | |||
| APL | 7.3 | 18.8 | 7.3 |
| FPLD | 92.7 | 81.2 | 92.7 |
| Clinical characteristics at start of treatment or index observation date | |||
| Elevated HbA1c (≥6.5%), % | 78.0 | 30.9** | 78.0 |
| Triglyceride levels in mg/dL,b mean (SD) | 1230 (2090) | 483* (599) | 503* (452) |
| Experienced ≥1 episode of pancreatitis,b % | 51.2 | 6.7** | 17.1** |
| Number of organs among heart, liver and kidneys with observed abnormalities, mean (SD) | 1.854 (0.654) | 0.497** (0.794) | 1.854 (0.654) |
| Heart, % | 31.7 | 6.0** | 36.6 |
| Liver, % | 95.1 | 28.9** | 92.7 |
| Kidneys, % | 58.5 | 14.8** | 56.1 |
| Patients with record of triglyceride levels, n | 41 | 73c | 36c |
| Patients with record of HbA1c levels, n | 41 | 82c | 35c |
* P < 0.05.
** P < 0.01 compared to metreleptin-treated cohort.
Abbreviations: APL, acquired partial lipodystrophy; FPLD, familial partial lipodystrophy; PL, partial lipodystrophy; HbA1c, hemoglobin A1c; NA, not applicable; SD, standard deviation.
a Index observation date for the prematch metreleptin-naïve cohort was defined as the time at which metreleptin-naïve patients achieved the mean age at the start of treatment of the treated sample (35.2 years) or the date of their last available observation, whichever comes first.
b PL subtype, triglyceride levels and pancreatitis were not used as matching parameters for the metreleptin-naïve cohort.
c Counts only include patients who have lab measurements taken on or after their index observation date.
Impact of treatment status on mortality
In the metreleptin-treated cohort, there were 11 deaths among patients with GL (7 with congenital GL [CGL], 4 with acquired GL [AGL]), and 1 death among patients with PL [patient had familial PL (FPLD)]). In the matched metreleptin-naïve cohort, there were 9 deaths among patients with GL (all CGL) and 3 deaths among patients with PL (all FPLD). The most frequently reported causes of death as recorded in patient records were heart, liver and/or kidney disease, or infections (Table 5).
Table 5.
Causes of mortality by patient
| Patient Number | Type of Lipodystrophy | Gender | Country | Age at Death | Cause(s) of Mortalitya |
|---|---|---|---|---|---|
| Metreleptin-treated | |||||
| MT 1 | CGL | Female | US | 29 | ESRD |
| MT 2 | CGL | Female | US | 45 | ESRD |
| MT 3 | AGL | Female | US | 15 | Hepatorenal failure |
| MT 4 | AGL | Male | US | 50 | Heart failure; kidney failure |
| MT 5 | AGL | Male | US | 69 | Lymphoma |
| MT 6 | CGL | Female | US | 19 | Heart failure |
| MT 7 | CGL | Female | US | 25 | ESLD |
| MT 8 | CGL | Female | US | 18 | ESLD |
| MT 9 | AGL | Female | US | 20 | ESLD |
| MT 10 | CGL | Female | US | 20 | ESLD |
| MT 11 | CGL | Female | US | 23 | Heart failure |
| MT 12 | FPLD | Female | US | 31 | Respiratory failure |
| Metreleptin-naïve | |||||
| MN 1 | CGL | Male | US | 32 | Atypical interstitial pneumonitis; respiratory failure |
| MN 2 | CGL | Male | Turkey | 44 | Died after coronary artery bypass grafting operation |
| MN 3 | CGL | Female | Turkey | 62 | Myocardial infarction |
| MN 4 | CGL | Female | Turkey | 26 | Diabetic foot infection |
| MN 5 | CGL | Female | US | 30 | Cardiac arrest due to underlying nonischemic cardiomyopathy |
| MN 6 | CGL | Female | Brazil | 16 | Sepsis |
| MN 7 | CGL | Female | US | 18 | Heart failure related to valvular stenosis |
| MN 8 | CGL | Female | Brazil | 15 | Septic shock |
| MN 9 | CGL | Female | Turkey | 60 | Stroke |
| MN 10 | FPLD | Male | Turkey | 35 | Not documented |
| MN 11 | FPLD | Female | US | 39 | Not documented |
| MN 12 | FPLD | Female | US | 69 | Probable kidney failure |
Abbreviations: AGL, acquired generalized lipodystrophy; CGL, congenital generalized lipodystrophy; ESLD, end-stage liver disease; ESRD, end-stage renal disease; FPLD, familial partial lipodystrophy.
a As reported in patient medical records and by study investigators.
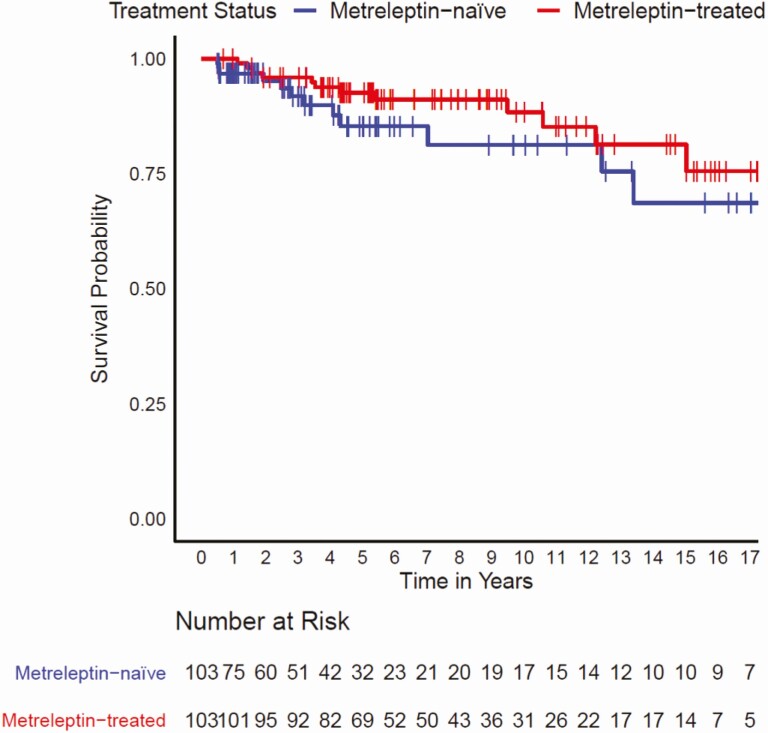
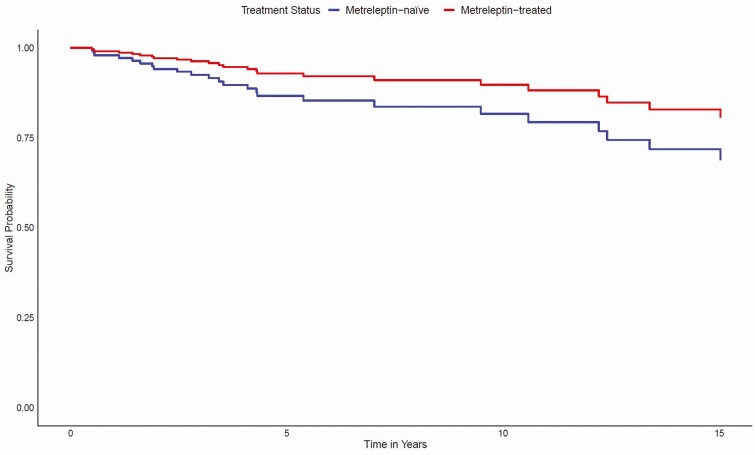
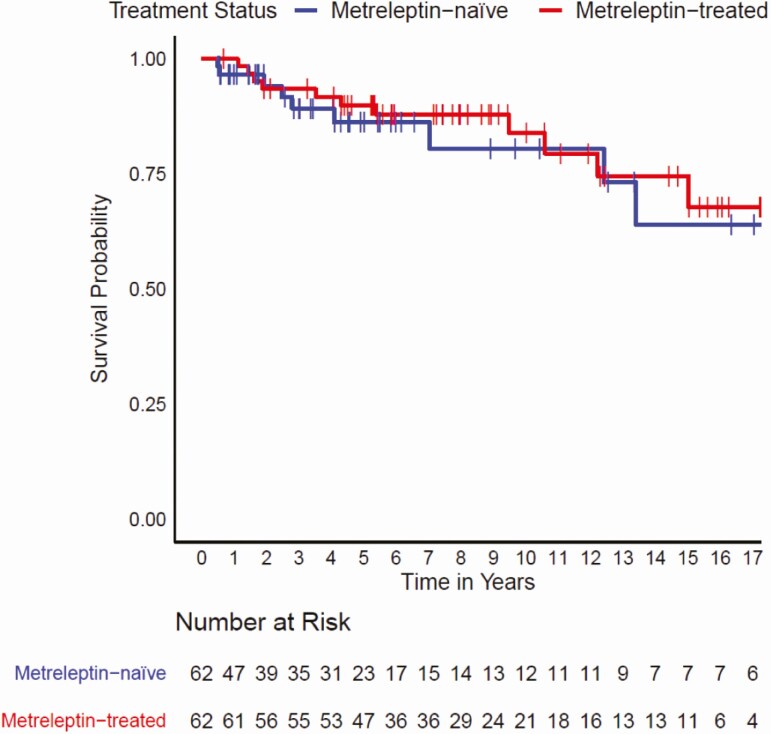
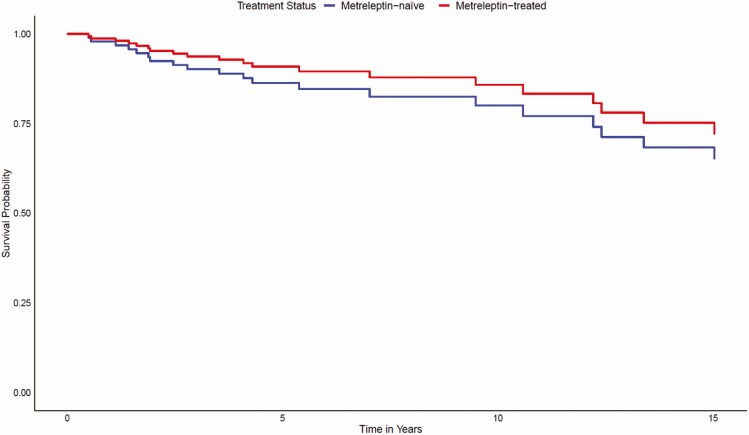
Kaplan–Meier analysis did not reveal a statistically significant difference in time-to-mortality between metreleptin-treated patients when compared with matched metreleptin-naïve patients (log-rank test P = 0.2) (Fig. 2). However, results of a Cox proportional hazards model showed that after adjusting for other covariates (ie, lipodystrophy diagnosis, birth year, triglyceride levels, elevated HbA1c, ≥1 episode of pancreatitis, and the presence of observed abnormalities in the heart or kidneys), metreleptin treatment was associated with a 65% reduction in mortality risk (hazard ratio [HR] 0.348, 95% confidence interval [CI]: 0.134–0.900; P = 0.029) (Fig. 3). Significant differences in mortality risk and time-to-mortality between metreleptin-treated and metreleptin-naïve patients in the GL subgroup were not detected from the Kaplan–Meier analysis (log-rank test P = 0.6) (Fig. 4) or Cox proportional hazards model (HR 0.455, 95% CI: 0.150–1.387; P = 0.166) (Fig. 5). The median (interquartile range [IQR]) duration of observation was 4.6 years (2.2–9.5) in the overall cohort and 5.5 years (2.5–10.5) in the GL subgroup. The Cox model was not powered to detect differences in mortality risk in subgroups of patients with AGL or CGL. Analysis of the impact of metreleptin therapy on mortality in the overall PL subgroup and in subgroups with APL or FPLD was not conducted due to the low number of mortality events.
Figure 2.
Time to mortality for metreleptin-treated versus matched metreleptin-naïve patients (overall cohort). Vertical bars denote censoring events.
Figure 3.
Cox model-predicted mortality for metreleptin-treated versus matched metreleptin-naïve patients (overall cohort). Modeled results are after adjusting for the following covariates: lipodystrophy diagnosis (GL or PL), birth year, triglyceride levels, elevated HbA1c, having ≥1 episode of pancreatitis, and the presence of observed abnormalities in the heart or kidneys. Abbreviations: GL, generalized lipodystrophy; HbA1c, hemoglobin A1c; PL, partial lipodystrophy.
Figure 4.
Time to mortality for metreleptin-treated versus matched metreleptin-naïve Patients (GL Subgroup). Vertical bars denote censoring events. Abbreviation: GL, generalized lipodystrophy.
Figure 5.
Cox model-predicted mortality for metreleptin-treated versus matched metreleptin-naïve patients (GL subgroup). Modeled results are after adjusting for the following covariates: birth year, triglyceride levels, elevated HbA1c, having ≥1 episode of pancreatitis, and the presence of observed abnormalities in the heart or kidneys. Abbreviations: GL, generalized lipodystrophy; HbA1c, hemoglobin A1c; PL, partial lipodystrophy.
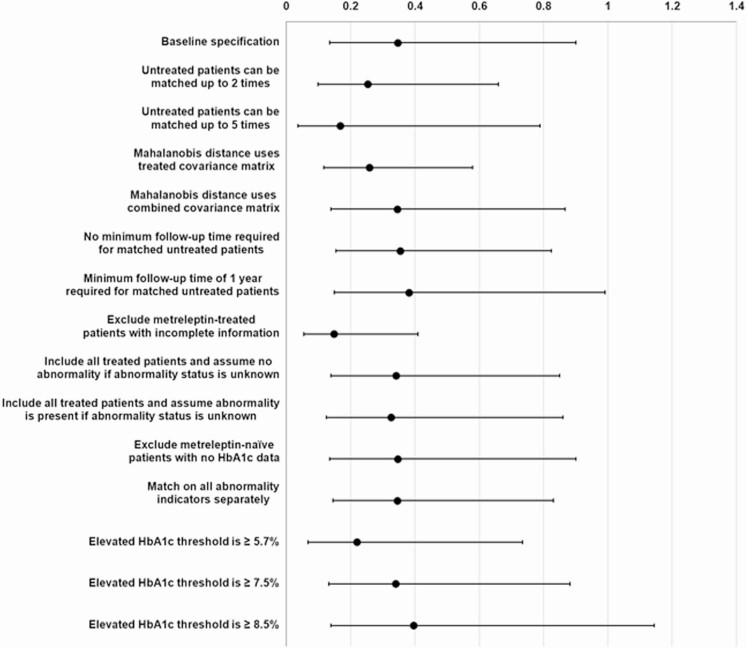
Sensitivity analysis
Sensitivity analyses were conducted to assess the effect of key assumptions underlying the methodology described in the current study (Table 6). Study results were generally not sensitive to changes in key parameters underlying the matching methodology (eg, allowing metreleptin-naïve patients to be matched to more than 1 metreleptin-treated patient, changing the minimum follow-up period required for metreleptin-naïve patients), data inclusion and exclusion criteria (eg, including patients with unknown organ abnormality status, excluding metreleptin-naïve patients with missing HbA1c data), or to alternative definitions or groupings of clinical outcomes (eg, organs with abnormalities considered individually when matching rather than as the number of organs with abnormalities).
Table 6.
Sensitivity analysis scenarios
| Group | Scenario |
|---|---|
| Matching methodology | • Number of times a metreleptin-naïve patient can be used as a match is set to (i) 2 or (ii) 5. |
| ◦ Base case: patient can only be used as a match once. | |
| • Covariance matrix used for matching is generated from (i) a combined metreleptin-treated and metreleptin-naïve cohort or (ii) from metreleptin-treated cohort alone. | |
| ◦ Base case: covariance matrix is generated from metreleptin-naïve cohort. | |
| • Minimum follow-up period required for record from a metreleptin-naïve patient to be used for matching is (i) removed entirely or (i) set to 1 year. | |
| ◦ Base case: 6 months. | |
| Data inclusion/exclusion criteria | • Records from metreleptin-treated patients without abnormality data on an organ at treatment initiation are either (i) excluded, or (ii) included but missing data are interpreted as an indication of no organ abnormalities being present, or (iii) included but missing data are interpreted as an indication of an organ abnormality being present. |
| ◦ Base case: records with missing organ abnormality data at treatment initiation are excluded unless record from a subsequent visit confirms no organ abnormality. | |
| • Records from metreleptin-naïve patients without HbA1c data are excluded. | |
| ◦ Base case: records lacking HbA1c data are included. | |
| Alternative clinical outcomes | • Matching on all organs with abnormalities is conducted separately. |
| ◦ Base case: sum of organs with observed abnormalities (among the heart, liver, and kidneys) and elevated HbA1c are used for matching. | |
| • Threshold for elevated HbA1c is set to (i) ≥5.7, or (ii) ≥7.5%, or (iii) ≥8.5%. | |
| ◦ Base case: threshold is ≥6.5%. |
Abbreviation: HbA1c, hemoglobin A1c.
Cox model estimates of the effect of metreleptin therapy on mortality were no longer statistically significant in only 1/14 tested scenarios in a sensitivity analysis (Fig. 6). This occurred when the threshold for patients classified as having elevated HbA1c was set to ≥8.5% (vs ≥6.5% in the baseline specification).
Figure 6.
Sensitivity analysis on mortality risk. Estimated treatment effect with 95% confidence intervals. Hazard ratios with confidence intervals entirely below < 1 are suggestive that the protective effect of metreleptin therapy is not significantly affected with use of the alternative specification. Abbreviations: GL, generalized lipodystrophy; HbA1c, hemoglobin A1c.
Similarly, the quality of matching remained stable when adjusting various matching parameters, including allowing metreleptin-naïve patients to be matched multiple times, removing minimum follow-up time requirements for matched patient observation histories, and choice of covariance matrix for the matching method. Notably, exploratory analyses of scenarios where metreleptin-naïve patients were allowed to be matched up to 2 or 5 times using different index observation dates yielded matched metreleptin-naïve cohorts that were more similar to the metreleptin-treated cohort. In these scenarios, only 2 observed characteristics remained significantly different between the cohorts after matching (percentage of patients with ≥1 episode of pancreatitis and mean triglyceride levels). As in the primary analysis, remaining unbalanced characteristics suggested that the metreleptin-treated cohort had more severe or more advanced disease. In both scenarios, hazard ratios for mortality risk in metreleptin-treated patients remained low and statistically significant (P < 0.01).
Discussion
Although the therapeutic effects of metreleptin in lipodystrophy syndromes have been documented, whether the drug’s therapeutic effects translate into a positive effect on mortality was not assessed prior to this study. Randomized controlled trials are impractical in this setting, and while published single-arm trials may report mortality events in their respective study cohorts, they were not designed to assess the effect of metreleptin on mortality. To facilitate an analysis of metreleptin’s effect on mortality, data from metreleptin-naïve patients in our earlier natural history study were used to build a comparator cohort with characteristics matched to those of metreleptin-treated patients enrolled in clinical trials (21). The current study is the first effort to present comparative evidence suggesting that patients in a mixed GL and PL cohort treated with metreleptin have a lower risk of mortality compared with metreleptin-naïve patients. A larger sample size is needed to reliably assess the effect of metreleptin therapy on mortality risk in the GL subgroup, where a nonsignificant trend towards lower mortality was observed, as well as in subgroups of specific GL and PL subtypes. Although this retrospective analysis cannot prove causality, the findings support the view that the improvement in metabolic complications of lipodystrophy previously shown in metreleptin-treated patients may be associated with a positive impact on survival. Caution is warranted when interpreting the estimated effect of metreleptin on mortality risk reduction in PL, as 11/12 deaths in the metreleptin-treated cohort occurred in patients with GL. Furthermore, the estimated treatment effect may only be applicable to patients with characteristics similar those of the metreleptin-treated cohort described in the current study.
Major causes of mortality among patients with lipodystrophy include heart and liver disease, kidney failure, pancreatitis, and infections (1, 29). Common heart- and liver-related causes of mortality include cardiomyopathy, heart failure, myocardial infarction, cardiac arrhythmia, gastrointestinal hemorrhage, and hepatocellular carcinoma. Respiratory infections have been reported as the most common cause of mortality among patients with GL, particularly among younger patients (29, 30). The most commonly identified causes of mortality in the current analysis were consistent with those described in previous reports.
Patients with GL and PL who were recruited to clinical trials evaluating metreleptin therapy typically had more severe lipodystrophy-associated complications compared with those not referred for treatment. This was reflected in our datasets, as patients in the unmatched metreleptin-naïve cohort on average were less likely to have elevated triglycerides, elevated HbA1c > 6.5%, and observed abnormalities in the heart, liver, and/or kidneys when compared with patients in the metreleptin-treated cohort. Matching helped balance pre-existing differences in demographic and clinical characteristics between the metreleptin-treated and metreleptin-naïve cohorts. Doing so increases the confidence that observed differences in mortality risk between the study cohorts were primarily driven by treatment with metreleptin rather than pre-existing differences in demographic and clinical characteristics. Although significant differences in 7 observed cohort characteristics remained following matching, unbalanced characteristics were accounted for in the Cox regression and sensitivity analyses showed that the observed reduction in mortality risk associated with metreleptin therapy remained robust across a range of tested scenarios. The observed differences in clinical characteristics that remained after matching were not surprising, as patients with more severe symptoms, especially pancreatitis, were more likely to enroll in clinical trials evaluating metreleptin therapy. Moreover, alternative implementations of the matching method that allowed each metreleptin-naïve patient to be matched more than once yielded matched cohorts where only 2 observed cohort characteristics (pancreatitis and triglyceride levels) remained significantly different. In these alternative implementations, metreleptin therapy was associated with similarly low hazard ratios for mortality risk. The one scenario where the reported effect of metreleptin therapy on mortality was no longer statistically significant occurred when the threshold for being classified as having elevated HbA1c was set at ≥8.5%. The clinical significance of the result remains unclear. We did observe that as the threshold for being classified as having elevated HbA1c was increased, the proportion of patients who died in each group (patients without elevated HbA1c vs patients with elevated HbA1c) began to converge. We hypothesize this result may be an artifact specific to our dataset or from mapping data collected as a continuous variable (HbA1c levels) into a binary variable (elevated HbA1c status) for use in our matching methodology. Of note, prior analyses in patients with lipodystrophy have reported that the benefit of metreleptin therapy on metabolic parameters appears more pronounced in those with higher HbA1c levels (13, 15). We also note that HbA1c levels were unavailable across all the years of follow-up in a group of metreleptin-naïve patients and, as described in the methods, metabolic data were imputed by carrying forward measured parameters until a next measurement was available. Thus, a recorded value for the metabolic parameter corresponding to the years of follow-up may be an imputed value rather than an actual measurement. Longitudinal datasets with larger sample size and patient HbA1c levels recorded at each follow-up visit are needed to reliably assess the treatment effect of metreleptin therapy in patients across different HbA1c thresholds. The current study is subject to multiple limitations. The accuracy and robustness of the treatment effect estimates were dependent on the availability of patient data and the interpretation/imputation of omitted data. For example, the factors that drive participants to clinical trials evaluating metreleptin therapy likely biased the patient population towards more severe cases. This precluded our ability to conduct robust analyses to estimate treatment effects on hallmarks of disease progression, such as the transition from no diabetes to the development of diabetes. Regardless, multiple studies in patients with GL and PL have already reported data suggesting that metreleptin can decrease the severity of disease-related metabolic complications associated with low leptin levels including elevated triglyceride levels and HbA1c, diabetes, and hepatic steatosis (8–13, 15–18). Another limitation is that data abstractors in this study interpreted the absence of data in patient records describing an observed abnormality in the heart, liver, or kidney as evidence of the organ being in the normal state. For the metreleptin-naïve cohort, this would include cases where a documented abnormality was not precisely interpreted by the abstractor and therefore was not selected from the predefined drop-down lists or entered in an open-text field during data abstraction. However, our sensitivity analyses showed that excluding records from metreleptin-treated patients lacking data on observed organ abnormalities or HbA1c, or assuming an organ abnormality was present when such data were missing, did not significantly change the reported mortality results. Patients were also matched according to the number of organs with observed abnormalities among the heart, liver, and kidneys, which has potential to reduce the precision of the reported results. However, our sensitivity analysis showed that the reported mortality results did not significantly change when the abnormality status of the heart, liver, and kidneys were considered as individual parameters for matching. Differences in concomitant medication use between the metreleptin-treated and metreleptin-naïve cohorts at the index date and medication changes over the observation period are recognized as potential confounding factors. However, due to the limited availability of data on medication use among patients in the metreleptin-naïve cohort, geographical variation in the clinical management of lipodystrophy syndromes, and the challenges with accurate recording of data on concomitant medication use in clinical databases, conducting a robust analysis on medication usage patterns between the metreleptin-treated and metreleptin-naïve patients was not feasible. Of note, the medication-sparing effect of metreleptin therapy in patients with GL and PL is already discussed elsewhere in the literature (16, 18, 31). The current study was also retrospective by design and both the matching methodology and estimates of treatment effect depended on the availability of data on observable characteristics within patient records. Data outside the scope of interest at a study site may be inaccessible from patient records. The existence of unobserved differences between the metreleptin-treated and metreleptin-naïve cohorts remains a potential source of bias. Furthermore, availability of data was subject to site-specific variations in data collection practices, clinical protocols, documentation styles, and measurement methodologies, which were neither standardized nor harmonized prior to the study design. Although definitions for observed organ abnormalities were harmonized prior to matching where feasible, minor differences in these definitions remained between the metreleptin-naïve and metreleptin-treated cohorts, primarily due to variations in data collection methods across the two cohorts. Despite the remaining differences, applying the definition sets consistently within the respective cohorts could still be a useful proxy for understanding disease severity. The current study also relies on the accuracy of data recorded in patient medical charts and the procedure for data extraction at each study site. Finally, the absolute number of deaths in both the treated and untreated cohorts was small, and thus small changes in the number of mortality events in each group might have yielded different results. Hence, the statistical significance may have moved in the favorable direction by chance with the addition of the patients with PL. Further studies in larger patient cohorts are needed to validate our findings.
Results reported in the current study should be interpreted as the estimated treatment effect of metreleptin in patients with profiles comparable to those in the metreleptin-treated cohort, which may not be generalizable to broader populations of lipodystrophy syndromes, such as patients with normal or even higher leptin levels and those with less severe disease. Such patients were not well represented in the National Institutes of Health clinical trials included in the current study. Finally, although metreleptin can help manage the metabolic complications of lipodystrophy associated with low leptin levels, mechanisms unrelated to low leptin and metabolic impairment can also contribute to the broad symptomology as well as causes of mortality observed in some lipodystrophy syndromes (32).
The current study is the first to present comparative evidence suggesting that patients with GL or PL treated with metreleptin experience can potentially reduce the risk of mortality compared with metreleptin-naïve patients from a disease natural history cohort, who are likely receiving background therapy to manage metabolic disease and comorbidities (eg, diabetes). When taken together with the earlier clinical studies describing the therapeutic effects of metreleptin on metabolic complications of lipodystrophy, the findings support the view that metreleptin therapy can have a positive disease-modifying impact in patients with lipodystrophy syndromes despite the numerous stated limitations. The low number of mortality events still warrant ample precaution in interpretation of the findings. Confirmatory studies in larger real-world and clinical trial datasets are warranted.
Acknowledgments
We would like to thank Melda Sonmez, Huseyin Onay, Samim Ozen, Tahir Atik, Tevfik Demir, Beyhan Tuysuz, Hulya Kayserili, Mehmet Nuri Ozbek, Gulcin Akinci, Ilgin Yildirim Simsir, Ulku Aybuke, Ela Temeloglu, Banu Sarer Yurekli, Nilufer Ozdemir Kutbay, Tugba Arkan, Ramazan Gen, Fatos Dilan Koseoglu, Guzin Fidan Yaylali, Mehmet Sercan Erturk, Habib Bilen, and all other collaborating physicians who referred patients to the treatment centers. We would also like to thank Diana Rus, Mario Swaidan, Adam Neidert, and Elaine Cochrane for their support in data abstraction; Courtney Ng, Dan Holmqvist, Don Lee, Reed Kamsler, and Samantha Kaufhold for their data analysis support; and Pamela Bradt for her clinical expertise and editorial support.
Financial Support: This study was supported by Aegerion Pharmaceuticals (now part of Amryt Pharma), the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases and the Lipodystrophy Fund at the University of Michigan (generously provided by the Sopha family and the White Point Foundation).
Author Contributions: EAO and RJB contributed equally to the study.
Additional Information
Disclosures: KC, DG, KJL, and ET are employees of Analysis Group, which has received consulting fees from Aegerion to conduct this study. OA is a former employee of Analysis Group. BA is/has been on the advisory board of Aegerion Pharmaceuticals, and a speaker for AstraZeneca, Eli Lilly, Novartis, Novo Nordisk, Boehringer Ingelheim, Servier, and Sanofi-Aventis. RM serves as consultant and speaker, and received a research grant from Aegerion. MCF, VOF, and RJB have writing support relationships with Aegerion Pharmaceuticals. EAO has received consulting fees indirectly through fees paid to the University of Michigan from Aegerion Pharmaceuticals, Akcea Therapeutics, Regeneron Pharmaceuticals, Thera Therapeutics, and AstraZeneca; has received grant(s) from Akcea Therapeutics, Ionis Pharmaceuticals Inc, Aegerion Pharmaceuticals, Gemphire Therapeutics, GIDynamics, and Regeneron; and had writing support relationships with Aegerion Pharmaceuticals and Boehringer Ingelheim.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of some data generated or analyzed during this study to preserve patient confidentiality and because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Brown RJ, Araujo-Vilar D, Cheung PT, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garg A. Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akinci B, Oral E, Neidert A, et al. Burden of illness associated with generalized lipodystrophy in leptin replacement therapy-naïve patients: a longitudinal medical chart review study. Endocr Rev. 2018;39(2 Suppl):S149-S150. [Google Scholar]
- 4. William WN Jr, Ceddia RB, Curi R. Leptin controls the fate of fatty acids in isolated rat white adipocytes. J Endocrinol. 2002;175(3):735-744. [DOI] [PubMed] [Google Scholar]
- 5. Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7(3):137-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Myalepta [Summary of Product Characteristics]. Accessed December 20, 2018. https://www.ema.europa.eu/documents/product-information/myalepta-epar-product-information_en.pdf. [Google Scholar]
- 7. Myalept (metreleptin) for injection for subcutaneous use [prescribing information]. Accessed . Accessed December 20, 2018. http://www.myaleptpro.com/sites/default/files/myalept_pi_sept2015_final.pdf. [Google Scholar]
- 8. Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570-578. [DOI] [PubMed] [Google Scholar]
- 9. Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54(7):1994-2002. [DOI] [PubMed] [Google Scholar]
- 10. Javor ED, Ghany MG, Cochran EK, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41(4):753-760. [DOI] [PubMed] [Google Scholar]
- 11. Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17(6):922-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Safar Zadeh E, Lungu AO, Cochran EK, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013;59(1):131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lebastchi J, Ajluni N, Neidert A, Oral EA. A report of three cases with acquired generalized lipodystrophy with distinct autoimmune conditions treated with metreleptin. J Clin Endocrinol Metab. 2015;100(11):3967-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ajluni N, Dar M, Xu J, Neidert AH, Oral EA. Efficacy and safety of metreleptin in patients with partial lipodystrophy: lessons from an expanded access program. J Diabetes Metab. 2016;7(3):659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown RJ, Meehan CA, Cochran E, et al. Effects of metreleptin in pediatric patients with lipodystrophy. J Clin Endocrinol Metab. 2017;102(5):1511-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown RJ, Valencia A, Startzell M, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown RJ, Oral EA, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine. 2018;60(3):479-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kiefer C, Sturtz S, Bender R. Indirect comparisons and network meta-analyses. Dtsch Arztebl Int. 2015;112(47):803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Falissard B, Zylberman M, Cucherat M, Izard V, Meyer F. Real medical benefit assessed by indirect comparison. Therapie. 2009;64(3):225-232. [DOI] [PubMed] [Google Scholar]
- 21. Akinci B, Oral EA, Neidert A, et al. Comorbidities and survival in patients with lipodystrophy: an international chart review study. J Clin Endocrinol Metab. 2019;104(11):5120-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li YP, Propert KJ, Rosenbaum PR. Balanced risk set matching. J Am Stat Assoc. 2001;96(455):870-882. [Google Scholar]
- 23. Li Y, Schaubel DE, He K. Matching methods for obtaining survival functions to estimate the effect of a time-dependent treatment. Stat Biosci. 2014;6(1):105-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu B. Propensity score matching with time-dependent covariates. Biometrics. 2005;61(3):721-728. [DOI] [PubMed] [Google Scholar]
- 25. Cook K, Ali O, Gupta D, et al. 2018 Longitudinal matching: A method for generating comparable samples of treated and treatment-naïve patients with progressive conditions. In: Poster presented at ISPOR Europe; November 10-14, 2018, Barcelona, Spain.
- 26. Rubin DB. Bias reduction using Mahalanobis-metric matching. Biometrics. 1980;36(2):293-298. [Google Scholar]
- 27. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. King G, Nielsen R. Why propensity scores should not be used for matching. Polit Anal. 2019;27( 4):1-20. [Google Scholar]
- 29. Lima JG, Nobrega LHC, Lima NN, et al. Causes of death in patients with Berardinelli-Seip congenital generalized lipodystrophy. PLoS One. 2018;13(6):e0199052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gupta N, Asi N, Farah W, et al. Clinical features and management of non-HIV-related lipodystrophy in children: a systematic review. J Clin Endocrinol Metab. 2017;102(2):363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oral EA, Gorden P, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with partial lipodystrophy. Endocrine. 2019;64(3):500-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vigouroux C, Guénantin AC, Vatier C, et al. Lipodystrophic syndromes due to LMNA mutations: recent developments on biomolecular aspects, pathophysiological hypotheses and therapeutic perspectives. Nucleus. 2018;9(1):235-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Restrictions apply to the availability of some data generated or analyzed during this study to preserve patient confidentiality and because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.