Abstract
Context
Severe hypoglycemia with neuroglycopenia, termed post-bariatric hypoglycemia (PBH). typically occurs postprandially, but it is also reported after activity or mid-nocturnally.
Objective
To quantify glycemia, glycemic variability, and magnitude/duration of low sensor glucose (SG) values in patients with PBH after Roux-en-Y gastric bypass (PBH-RYGB).
Methods
This retrospective analysis of data from an academic medical center included individuals with PBH-RYGB (n = 40), reactive hypoglycemia without gastrointestinal surgery (Non-Surg Hypo, n = 20), prediabetes (Pre-DM, n = 14), newly diagnosed T2D (n = 5), and healthy controls (HC, n = 38). Masked continuous glucose monitoring (Dexcom G4) was used to assess patterns over 24 hours, daytime (6 am–midnight) and nighttime (midnight–6 am). Prespecified measures included mean and median SG, variability, and percent time at thresholds of sensor glucose.
Results
Mean and median SG were similar for PBH-RYGB and HC (mean: 99.8 ± 18.6 vs 96.9 ± 10.2 mg/dL; median: 93.0 ± 14.8 vs 94.5 ± 7.4 mg/dL). PBH-RYGB had a higher coefficient of variation (27.3 ± 6.8 vs 17.9 ± 2.4%, P < 0.0001) and range (154.5 ± 50.4 vs 112.0 ± 26.7 mg/dL, P < 0.0001). Nadir was lowest in PBH-RYGB (42.5 ± 3.7 vs HC 49.0 ± 11.9 mg/dL, P = 0.0046), with >2-fold greater time with SG < 70 mg/dL vs HC (7.7 ± 8.4 vs 3.2 ± 4.1%, P = 0.0013); these differences were greater at night (12.6 ± 16.9 vs 1.0 ± 1.5%, P < 0.0001). Non-Surg Hypo also had 4-fold greater time with SG < 70 at night vs HC (SG < 70: 4.0 ± 5.9% vs 1.0 ± 1.5%), but glycemic variability was not increased.
Conclusion
Patients with PBH-RYGB experience higher glycemic variability and frequency of SG < 70 compared to HC, especially at night. These data suggest that additional pathophysiologic mechanisms beyond prandial changes contribute to PBH.
Keywords: hypoglycemia, bariatric (metabolic) surgery, diabetes
Hypoglycemia is a challenging clinical condition that can have both immediate and long-lasting deleterious physical and psychosocial effects. While most often considered in the context of diabetes and its treatment, hypoglycemia also occurs outside of diabetes and can be further classified as fasting or reactive (after meals, activity, and other conditions). One increasingly recognized and often-debilitating cause of reactive hypoglycemia is post-bariatric hypoglycemia (PBH), which can be observed after several forms of upper gastrointestinal surgery but has been most extensively characterized after Roux-en-Y gastric bypass (RYGB) (1). In this setting, hypoglycemia can be considered a late component of the so-called dumping syndrome. Accelerated gastric emptying results in rapid increases in glucose levels, markedly increased insulin secretion, and subsequent hypoglycemia 1 to 3 hours after eating. While initial evidence suggested that this syndrome might be linked to increased islet mass (“nesidioblastosis”) (2, 3), subsequent studies demonstrated that increased insulin secretion is largely mediated by increased secretion of intestinally derived incretin hormones such as glucagon-like peptide 1. Additionally, increased insulin-independent glucose uptake (4), altered bile acid metabolism and increased fibroblast growth factor 19 (FGF19) (5-7), and reduced counterregulatory responses (8) may also contribute to altered glucose metabolism in PBH.
Continuous glucose monitoring (CGM) has been used to characterize patterns of glycemic excursion in asymptomatic patients with a history of RYGB as well as in those with symptomatic hypoglycemia after RYGB (PBH-RYGB) (9-11). RYGB is characterized by exaggerated glycemic variability, with earlier and higher postprandial peak glucose levels as compared with nonsurgical controls (2); similar patterns are also observed in pregnant women post-RYGB (12). These glycemic patterns appear to be further exaggerated in post-RYGB patients with PBH, as demonstrated by higher peak glucose, lower nadir glucose, and more daily glycemic excursions >180 mg/dL or <70 mg/dL compared with asymptomatic controls (3).
CGM is more sensitive for detection of hypoglycemia as compared with mixed meal tolerance tests (13), but specificity may be lower (3). Nielson et al found that the low blood glucose index (frequency and amplitude of hypoglycemic events) was the most reliable metric for diagnosing PBH by CGM (14). However, inaccuracy of CGM is increased within the hypoglycemic range, with discrepancies of up to 1mM in this study. As a result, CGM is often utilized for pattern recognition (together with food/activity diary) rather than for diagnosis, which requires fulfillment of Whipple’s triad using venous blood samples (1, 15)
CGM has been used as an adjunct to identify patterns of glycemic excursions in nonsurgical patients suspected of having reactive hypoglycemia; however, little information is available about patterns of nocturnal glycemia, glycemic variability, or duration of hypoglycemia in this setting (16). In one study comparing a standard diet to the macrobiotic Ma-Pi 2 diet (which is limited to whole-grains, vegetables and legumes), CGM data demonstrated the reduced frequency of hypoglycemic events during the day in patients with reactive hypoglycemia treated with the experimental diet (17).
Few data are available to compare glycemic patterns in the ambulatory setting in patients with PBH-RYGB or other forms of reactive hypoglycemia occurring in patients without a history of gastrointestinal surgery. In this study we characterized the glycemic patterns in patients with either of these forms of reactive hypoglycemia and compared them with publicly available CGM data from healthy controls and patients with newly diagnosed prediabetes or type 2 diabetes (T2D) collected in the ambulatory setting.
Methods
Research Participants and Methods
We performed retrospective analysis of existing deidentified CGM data previously obtained in the outpatient clinic and from clinical research participants at Joslin Diabetes Center to characterize the glycemic patterns of 2 experimental groups: patients who had post-bariatric hypoglycemia associated with prior Roux-en-Y gastric bypass (PBH-RYGB, n = 40) and patients with reactive hypoglycemia without a history of gastrointestinal surgery (Non-Surg Hypo, n = 20; clinical diagnoses provided in Supplementary Table 1) (18). PBH-RYGB patients included patients evaluated in the clinical setting only (n = 15) and individuals who had enrolled in prior studies (ClinicalTrials.gov NCT02733588, n = 7, and NCT03255629, n = 18) (19, 20). All participants had a complete clinical evaluation to rule out other causes of hypoglycemia, and autonomous insulin secretion was excluded by evaluation of glucose and insulin levels after fasting. We identified patients for the study based on the availability of CGM data and categorized them into their respective groups using diagnoses within their electronic health records. All CGM data were collected using masked Dexcom G4 Professional devices, which measure interstitial glucose concentrations every 5 minutes. Patients were provided with glucose meters and instructed to calibrate twice daily.
For previous research participants, CGM data on study dates involving a mixed meal with glucagon or placebo intervention for clinical research studies were excluded from analysis. However, CGM data from all dates before and after the patient’s study visit (or intervention) were included. For these previous studies of PBH, exclusion criteria included major systemic illness, preoperative T2D, cardiac arrhythmia, hypertension, active coronary artery disease, fasting hypoglycemia, known insulinoma, pregnancy, substance or alcohol abuse, recent steroid or investigational drug exposure, and use of medications (beyond hypoglycemia treatment) known to affect insulin secretion or action (19, 20). Self-reported alcohol use for all participants is provided in Supplementary Table 2 (18). CGM data were collected on an outpatient basis, and meal timing and composition were not controlled. We provide information in Supplementary Table 3 (18) about whether participants had received prior nutritional consultation for PBH.
To compare the glycemic excursions in PBH-RYGB with glycemic excursions in prediabetes and T2D, we accessed publicly available data from a previously published study of outpatient blinded Dexcom G4 CGM data from normal weight, healthy control participants (HC, n = 38), and individuals with newly diagnosed prediabetes (Pre-DM, n = 14), and type 2 diabetes (T2D, n = 5) (21). These participants were not known to have prediabetes or T2D prior to testing but were categorized into their respective groups based on HbA1c, fasting plasma glucose, or 2-hour plasma glucose measured after oral glucose tolerance testing, according to American Diabetes Association diagnostic reference values. All patients from this published study were free of known major organ disease, chronic inflammatory conditions, malignancy, uncontrolled hypertension, eating disorder, or history of bariatric surgery, reported stable weight, and were not taking any diabetes or weight loss medications during the monitoring period.
Statistical Methods
Sensor glucose values were summarized as median and median absolute values for each group. Computed statistics included total CGM time, SG values, percent of SG values in glucose ranges, and glucose variability measurements. Additionally, we calculated J-Index (22), continuous overall net glycemic action (CONGA) (23), mean amplitude of glucose excursion (MAGE) (24), low and high blood glucose indices (LBGI and HBGI, respectively) (25), and Average Daily Risk Range (ADRR) (26).
Hypoglycemic episodes were defined as at least 3 consecutive glucose values (≥15 minutes) below 70 mg/dL (mild) or 54 mg/dL (severe), and the number of these episodes per day was calculated. In contrast, hypoglycemic events were defined as one or more consecutive sensor values (≥5minutes) below 70 mg/dL or 54 mg/dL. The duration of each hypoglycemic event (in minutes) was calculated by multiplying the consecutive number of data points below threshold by 5. We computed the mean and maximum length of these hypoglycemic events per participant. Metrics were assessed over 24 hours as well as during daytime (6 am to midnight) and nighttime (midnight to 6 am) independently.
Sensor glucose values recorded as “low” (n = 562) indicate that the interstitial glucose values were below the readable threshold of the Dexcom G4 CGM device (40 mg/dL). We converted these “low” values to 40 mg/dL in all downstream analyses. No glucose values were recorded as “high,” a metric indicating values for sensor glucose above the readable threshold of the Dexcom G4 CGM device (400 mg/dL).
The overall comparison between the 5 study groups was performed using the Kruskal-Wallis 1-way ANOVA test (non-parametric F-test). Differences between groups were evaluated using the paired Wilcoxon test (nonparametric t-test).
Results
Demographic data for the 5 study groups are provided in Table 1. Data from 38 controls, 40 individuals with PBH-RYGB, 20 individuals with reactive hypoglycemia without a history of gastrointestinal surgery (Non-Surg Hypo), 14 individuals with prediabetes, and 5 individuals with newly diagnosed T2D were available for analysis. There was a female predominance overall (χ 2 18.6, P < 0.001), with greater number of female individuals in both PBH-RYGB vs healthy controls (HC) and Hypo vs HC; there were no significant differences between PBH-RYGB and nonsurgical hypoglycemia groups. Age was similar for HC and hypoglycemia groups, but higher for prediabetes and T2D groups. Body mass index (BMI) was in the overweight range for all groups, and significantly higher for PBH-RYGB vs HC. The total and relative duration of monitoring during the day and nighttime periods was similar in all groups (Supplementary Table 4) (18).
Table 1.
Demographics and overall glucose metrics
| [A] Healthy control | [B] PBH-RYGB | [C] Non-Surg Hypo | [D] Pre-DM | [E] T2D | P value (paired)* | P value (overall)** | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 38 | 40 | 20 | 14 | 5 | [A] vs [B] | [A] vs [C] | [B] vs [C] | [B] vs [E] | |
| Demographics | ||||||||||
| Sex (F/M) *** | 23/15 | 37/3 | 14/6 | 8/6 | 1/4 | 0.001 | 0.048 | 0.021 | <0.0001 | 0.0009 |
| Age | 49 ± 11.0 | 51 ± 8.5 | 51 ± 11.0 | 60 ± 6.5 | 59 ± 5.0 | 0.065 | 0.249 | 0.806 | 0.245 | 0.020 |
| BMI | 25.7 ± 2.2 | 28.5 ± 3.6 | 27.1 ± 5.1 | 29.2 ± 3.4 | 26.4 ± 2.6 | <0.01 | 0.107 | 0.795 | 0.467 | 0.027 |
| Sensor glucose metrics | ||||||||||
| CGM Duration (hours) | 152 ± 6 | 88 ± 81 | 161 ± 5 | 152 ± 3 | 150 ± 3 | <0.001 | <0.01 | <0.001 | 0.094 | <0.001 |
| Mean (mg/dL) | 96.9 ± 10.2 | 99.8 ± 18.6 | 94.2 ± 9.6 | 108.8 ± 6.3 | 108.2 ± 7.6 | 0.739 | 0.727 | 0.750 | 0.042 | 0.008 |
| Median (quartiles) (mg/dL) | 95 (86, 106) | 93 (81, 111) | 92 (83, 106) | 107 (96, 122) | 102 (95, 119) | 0.412 | 0.502 | 0.938 | 0.027 | 0.002 |
| Peak (mg/dL) | 169.5 ± 34.1 | 202.0 ± 52.6 | 175.0 ± 27.4 | 190.5 ± 14.1 | 246.0 ± 62.3 | 0.001 | 0.928 | 0.010 | 0.406 | 0.001 |
| Nadir (mg/dL) | 49.0 ± 11.9 | 42.5 ± 3.7 | 47.5 ± 11.1 | 58.0 ± 6.7 | 64.0 ± 8.9 | 0.0046 | 0.283 | 0.340 | 0.002 | <0.0001 |
| Range (mg/dL) | 112.0 ± 26.7 | 154.5 ± 50.4 | 113.5 ± 37.1 | 139.0 ± 24.5 | 185.0 ± 46.0 | <0.0001 | 0.987 | 0.005 | 0.800 | <0.001 |
| Glucose variability | ||||||||||
| SD (mg/dL) | 17.6 ± 2.9 | 24.8 ± 7.3 | 18.6 ± 6.6 | 20.4 ± 4.1 | 27.3 ± 5.3 | <0.0001 | 0.460 | <0.001 | 0.930 | <0.0001 |
| Coefficient of variation (%) | 17.9 ± 2.4% | 27.3 ± 6.8% | 20.8 ± 6.2% | 19.3 ± 3.4% | 22.8 ± 3.6% | <0.0001 | 0.163 | <0.0001 | 0.264 | <0.0001 |
| Interquartile range (mg/dL) | 20.0 ± 4.4 | 28.0 ± 7.4 | 22.0 ± 7.8 | 25.0 ± 8.9 | 26.0 ± 4.4 | <0.0001 | 0.283 | 0.013 | 0.366 | <0.0001 |
| CONGA1 (mg/dL) | 17.7 ± 4.5 | 28.3 ± 9.1 | 19.5 ± 5.6 | 22.0 ± 7.0 | 26.1 ± 3.8 | <0.0001 | 0.163 | <0.0001 | 0.388 | <0.0001 |
| CONGA2 (mg/dL) | 19.9 ± 5.1 | 31.8 ± 10.5 | 22.4 ± 7.3 | 26.2 ± 7.7 | 36.0 ± 4.0 | <0.0001 | 0.251 | <0.001 | 0.713 | <0.0001 |
| CONGA4 (mg/dL) | 21.6 ± 5.5 | 32.5 ± 12.3 | 23.3 ± 6.4 | 26.8 ± 5.9 | 35.9 ± 12.8 | <0.0001 | 0.317 | 0.003 | 0.493 | <0.0001 |
| Glycemic risk | ||||||||||
| Average Daily Risk Range (ADDR) | 2.1 ± 1.0 | 3.8 ± 1.8 | 2.7 ± 1.9 | 1.4 ± 0.5 | 2.1 ± 0.2 | <0.0001 | 0.101 | 0.092 | 0.002 | <0.0001 |
| Low Blood Glucose Index (LBGI) | 2.0 ± 1.2 | 2.6 ± 2.1 | 2.6 ± 2.2 | 0.8 ± 0.5 | 0.8 ± 0.5 | 0.017 | 0.317 | 0.441 | 0.002 | <0.0001 |
| High Blood Glucose Index (HBGI) | 0.1 ± 0.1 | 0.3 ± 0.4 | 0.1 ± 0.2 | 0.4 ± 0.3 | 0.8 ± 0.9 | <0.001 | 0.450 | 0.050 | 0.314 | <0.001 |
Data are median ± median absolute deviations (MAD). *P value from paired comparison using Wilcoxon test. **P value from comparison across all groups using Kruskal-Wallis (ANOVA). ***Chi-squared test. CONGA, Continuous overlapping net glycemic action over 1 hour, over 2 hours, and over 4 hours.
Abbreviations: BMI, body mass index; CGM, continuous glucose monitoring; CONGA, continuous overall net glycemic action; PBH-RYGB, post-bariatric hypoglycemia after RYGB; Pre-DM, prediabetes; T2D, type 2 diabetes.
Glycemic Patterns in Post-Bariatric Hypoglycemia Patients
We assessed overall glycemic patterns as determined from the CGM data. As expected, mean and median glucose values and glycemic range were highest in T2D (P < 0.05 vs controls). We next focused on glycemic patterns in PBH-RYGB, comparing them with HC. Mean and median sensor glucose (SG) were similar for PBH-RYGB and HC (mean ± mean absolute deviation: 99.8 ± 18.6 vs 96.9 ± 10.2 mg/dL; median ± median absolute deviation: 93.0 ± 14.8 vs 94.5 ± 7.4 mg/dL; P > 0.05 for both) (Table 1). However, patterns of glycemia were distinct in PBH-RYGB. Peak glucose was significantly higher in PBH-RYGB than in HC (202.0 ± 52.6 vs 169.5 ± 34.1 mg/dL, P = 0.001) and nadir SG was lower in PBH-RYGB vs HC (42.5 ± 3.7 mg/dL vs 49.0 ± 11.9 mg/dL, P = 0.005).
In agreement, metrics of glucose variability were also increased in PBH-RYGB. PBH-RYGB had the highest coefficient of variation (CV) and the widest interquartile range (IQR) of SG values in comparison with all other groups (CV: 27.3 ± 6.8; IQR: 28.0 ± 7.4 mg/dL vs other groups, P < 0.001, 1-way ANOVA), with the greatest difference vs HC (CV: 17.9 ± 2.4%; IQR: 20.0 ± 4.4 mg/dL, P < 0.001 for both). Likewise, glycemic variability assessed by CONGA-4 was also increased in PBH-RYGB vs HC (33.2 ± 10.3 vs 24.1 ± 4.4 mg/dL, P < 0.001).
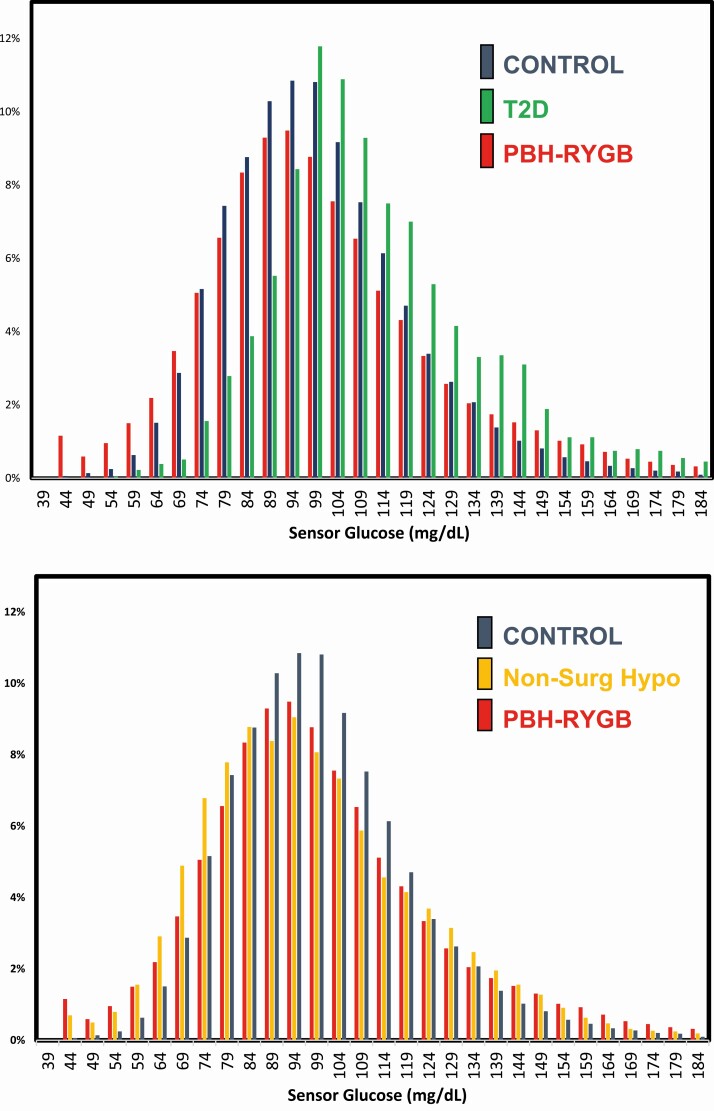
The distribution of SG values for control and PBH-RYGB patients is presented in Fig. 1A. Consistent with larger glycemic variability measures, the range of glucose values is greater in PBH-RYGB than in HC. In addition, there is a leftward shift (toward lower glucose values) in PBH-RYGB. As a control, we examined SG distribution in patients with newly diagnosed T2D on no treatment; as expected, SG values were shifted to the right, consistent with elevated glucose levels in this group.
Figure 1.
Distribution of sensor glucose values. The histograms demonstrate the % of glucose values within each sensor glucose range (5 mg/dL intervals). A, Data for controls indicated in blue bars, T2D in green, and PBH-RYGB in red. B, Data for controls (blue), Non-Surg Hypo (yellow), and PBH-RYGB (red).
Quantification of the distribution of SG values, both overall and at nighttime, is presented in Table 2. Individuals with PBH-RYGB had the lowest percentage of time-in-range (SG 70-180 mg/dL: 87.1 ± 10.3% vs other groups, P < 0.001) and a greater percentage of time with SG > 180 (0.5 ± 0.8 vs 0.0 ± 0.0, P = 0.002). Conversely, PBH-RYGB also had greater time with SG < 70, <60, and <54 mg/dL vs HC. For example, the percentage of time with SG < 70 in PBH-RYGB was 7.7 ± 8.4 vs 3.2 ± 4.1% in HC (P < 0.002), with similar increases for severe hypoglycemia (SG < 54) in PBH-RYGB (1.3 ± 1.4% vs 0.2 ± 0.2% for HC, P < 0.001).
Table 2.
Percent distribution of sensor glucose values over 24 hours and at night (12 to 6 am)
| [A] Healthy control | [B] PBH-RYGB | [C] Non-Surg Hypo | [D] Pre-DM | [E] T2D | P value (paired)* | P value (overall)** | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | 38 | 40 | 20 | 14 | 5 | [A] vs [B] | [A] vs [C] | [B] vs [C] | [B] vs [E] | |
| 24 hours | ||||||||||
| >250 mg/dL | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.001 | 0.180 | 0.055 | 0.905 | 0.002 |
| >180 mg/dL | 0.0 ± 0.0% | 0.5 ± 0.8% | 0.0 ± 0.0% | 0.3 ± 0.4% | 2.5 ± 3.7% | 0.002 | 1.000 | 0.034 | 0.408 | 0.004 |
| 70–180mg/dL | 96.3 ± 4.1% | 87.1 ± 10.3% | 91.8 ± 8.7% | 97.9 ± 2.6% | 95.1 ± 2.7% | <0.0001 | 0.084 | 0.056 | 0.028 | <0.0001 |
| <70 mg/dL | 3.2 ± 4.1% | 7.7 ± 8.4% | 4.1 ± 6.1% | 1.1 ± 1.4% | 0.5 ± 0.8% | 0.0013 | 0.205 | 0.165 | 0.003 | <0.0001 |
| <60 mg/dL | 0.5 ± 0.7% | 3.0 ± 3.2% | 1.5 ± 2.3% | 0.1 ± 0.1% | 0.0 ± 0.0% | <0.0001 | 0.105 | 0.122 | 0.002 | <0.0001 |
| <54 mg/dL | 0.2 ± 0.2% | 1.3 ± 1.4% | 0.3 ± 0.5% | 0.0 ± 0.0% | 0.0 ± 0.0% | <0.0001 | 0.110 | 0.333 | 0.003 | <0.0001 |
| Nighttime (12 – 6 am ) | ||||||||||
| >250 mg/dL | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.343 | N/A*** | 0.502 | 0.777 | 0.750 |
| >180 mg/dL | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.016 | 0.353 | 0.338 | 0.839 | 0.047 |
| 70-180 mg/dL | 98.7 ± 2.0% | 86.9 ± 16.2% | 94.0 ± 8.3% | 100.0 ± 0.0% | 100.0 ± 0.0% | <0.0001 | 0.008 | 0.239 | 0.007 | <0.0001 |
| <70 mg/dL | 1.0 ± 1.5% | 12.6 ± 16.9% | 4.0 ± 5.9% | 0.0 ± 0.0% | 0.0 ± 0.0% | <0.0001 | 0.045 | 0.184 | 0.003 | <0.0001 |
| <60 mg/dL | 0.0 ± 0.0% | 2.5 ± 3.7% | 0.5 ± 0.7% | 0.0 ± 0.0% | 0.0 ± 0.0% | <0.001 | 0.040 | 0.311 | 0.015 | <0.0001 |
| <54 mg/dL | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.0 ± 0.0% | 0.001 | 0.011 | 0.772 | 0.059 | <0.001 |
Data are median ± median absolute deviations (MAD). *P value from paired comparison using Wilcoxon test. **P value from comparison across all groups using Kruskal-Wallis (ANOVA). ***Only one participant had a non-zero percentage for this parameter, and this subject was part of the PBH-RYGB group. Therefore, any group comparisons that do not involve the PBH-RYGB group results in an N/A error, because the analysis would compare zero values in 2 groups and show no variation.
Abbreviations: PBH-RYGB, post-bariatric hypoglycemia after RYGB; Pre-DM, prediabetes; T2D, type 2 diabetes.
Patients with PBH-RYGB experienced a mean of 2.2 episodes of glucose <70 (duration ≥15 minutes) per day, with a mean duration of hypoglycemic event (≥ 5 minutes) of 39 minutes (Table 3). Both of these metrics were greater than controls (mean of 1.1 episodes per day, mean duration 27.2 minutes, P < 0.01 for both). Additionally, the number of severe hypoglycemic episodes (<54 mg/dL, duration ≥ 15 minutes) per day was higher for the PBH-RYGB group than HC (0.5 vs 0.1 episodes/ day, P < 0.002).
Table 3.
Hypoglycemic episodes and events over 24 hours and at night (12 to 6 am)
| [A] Healthy control | [B] PBH-RYGB | [C] Non-Surg Hypo | [D] Pre-DM | [E] T2D | P value (paired)* | P value (overall)** | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 38 | 40 | 20 | 14 | 5 | [A] vs [B] | [A] vs [C] | [B] vs [C] | [B] vs [E] | |
| Hypoglycemic episodes per day | ||||||||||
| 24 hours | ||||||||||
| Mild (<70 mg/dL) hypoglycemic episodes | 1.1 ± 1.4 | 2.2 ± 1.9 | 1.4 ± 1.8 | 0.3 ± 0.3 | 0.2 ± 0.2 | 0.002 | 0.355 | 0.224 | 0.002 | <0.0001 |
| Severe (<54 mg/dL) hypoglycemic episodes | 0.1 ± 0.1 | 0.5 ± 0.6 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.002 | 0.136 | 0.415 | 0.027 | <0.001 |
| Nighttime (12 – 6am) | ||||||||||
| Mild (<70 mg/dL) hypoglycemic episodes | 0.2 ± 0.2 | 0.7 ± 0.6 | 0.3 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | <0.001 | 0.064 | 0.459 | 0.003 | <0.0001 |
| Severe (<54 mg/dL) hypoglycemic episodes | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | <0.001 | 0.001 | 0.972 | 0.082 | <0.001 |
| Duration of hypoglycemic events | ||||||||||
| 24 hours | ||||||||||
| Max duration of mild hypoglycemic event (min) | 80.0 ± 89.0 | 100.0 ± 85.3 | 135.0 ± 114.9 | 42.5 ± 55.6 | 15.0 ± 22.2 | 0.072 | 0.077 | 0.505 | 0.006 | 0.003 |
| Mean duration of mild hypoglycemic event (min) | 27.2 ± 14.4 | 38.9 ± 23.7 | 39.6 ± 19.9 | 21.5 ± 19.4 | 10.0 ± 14.8 | 0.004 | 0.030 | 0.956 | 0.006 | 0.002 |
| Max duration of severe hypoglycemic event (min) | 12.5 ± 18.5 | 32.5 ± 40.8 | 32.5 ± 48.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.008 | 0.073 | 0.925 | 0.008 | <0.001 |
| Mean duration of severe hypoglycemic event (min) | 10.0 ± 14.8 | 19.5 ± 16.2 | 23.0 ± 33.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.043 | 0.084 | 0.653 | 0.009 | <0.001 |
| Nighttime (12 – 6 AM) | ||||||||||
| Max duration of mild hypoglycemic event (min) | 15.0 ± 22.2 | 65.0 ± 77.8 | 82.5 ± 89.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.001 | 0.034 | 0.975 | 0.003 | <0.001 |
| Mean duration of mild hypoglycemic event (min) | 9.2 ± 13.6 | 33.1 ± 33.4 | 33.0 ± 31.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.001 | 0.047 | 0.609 | 0.003 | <0.001 |
| Max duration of severe hypoglycemic event (min) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.002 | 0.008 | 0.966 | 0.059 | <0.001 |
| Mean duration of severe hypoglycemic event (min) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.001 | 0.009 | 0.892 | 0.059 | <0.001 |
Data are median ± median absolute deviations (MAD). *P value from paired comparison using Wilcoxon test. **P value from comparison across all groups using Kruskal-Wallis (ANOVA). A mild or severe episode is defined as ≥ 15 minutes of consecutive glucose values below 70 mg/dL or 54 mg/dL respectively. A mild or severe event is defined as ≥ 5 minutes of consecutive glucose values below 70 mg/dL or 54 mg/dL respectively.
Abbreviations: PBH-RYGB, post-bariatric hypoglycemia after RYGB; Pre-DM, prediabetes; T2D, type 2 diabetes.
Differences between PBH-RYGB and controls were even greater at night. For example, individuals with PBH-RYGB experienced further decreases in % of time-in-range during nocturnal hours (86.9 ± 16.2% vs 98.7 ± 2% for HC, P < 0.001). Strikingly, the % of time with SG < 70 was 12-fold higher for PBH-RYGB vs HC (12.6 ± 16.9% for PBH-RYGB vs 1.0 ± 1.5%, P < 0.0001), with similar increases at the <60 and <54 thresholds. Patients with PBH-RYGB experienced a mean of 0.7 episodes of SG < 70 at night, in comparison to 0.2 episodes in HC. Similarly, the mean duration of SG < 70 at night was greater in PBH-RYGB vs HC (33.1 vs 9.2 minutes, P = 0.001). No participants in any group experienced severe hypoglycemic episodes (<54 mg/dL) lasting ≥15 minutes at night.
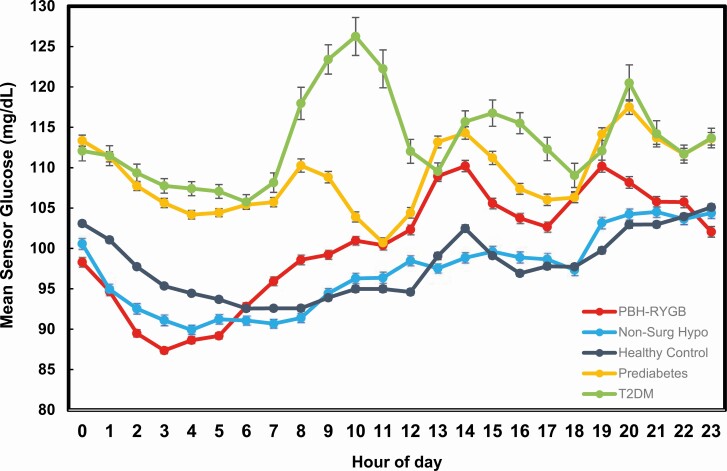
The mean glucose values by the time of day for each group are shown in Fig. 2. As expected, glucose values during both day and night are highest in those with prediabetes and T2D. In PBH-RYGB, 2 dominant patterns were observed. First, glycemic excursions were greater in PBH-RYGB vs HC during the day, likely reflecting meal-related glucose excursions in this population. Secondly, the PBH-RYGB group had the lowest mean SG values during early nocturnal hours (2-4 am).
Figure 2.
Mean hourly glucose by time of day for each of the 5 study groups.
We also evaluated relationships between the key glycemic variables altered in PBH-RYGB and BMI. There were no significant correlations between BMI and either glycemic variability, as assessed by CV (r = −0.24) or percentage of time with SG < 70 (r = −0.008).
Glycemic Patterns in Nonsurgical Hypoglycemia Patients
We also assessed CGM patterns in patients presenting with reactive hypoglycemia, who had no prior history of gastrointestinal surgery (Non-Surg Hypo). Fasting hypoglycemia and other hormonal etiologies of hypoglycemia were excluded by history and clinical laboratory testing. Interestingly, this group had mean, median, peak, and nadir values similar to values in HC (P > 0.25 for all, Wilcoxon test). However, overall time-in-range tended to be lower (91.8 ± 8.7% vs 96.3 ± 4.1%, P = 0.084), and the overall percentage of time with SG < 70 and < 60 did not differ from HC (SG < 70: 4.1 ± 6.1% vs 3.2 ± 4.1%; SG < 60: 1.5 ± 2.3% vs 0.5 ± 0.7%, P > 0.10 for both, Wilcoxon test). However, at night, the percentage of time with SG < 70 in Non-Surg Hypo was 4-fold higher than in HC (SG < 70: 4.0 ± 5.9% vs 1.0 ± 1.5%), with similar increases for the SG < 60 threshold (0.5 ± 0.7% vs 0.0 ± 0.0%) (P < 0.05 for both, Wilcoxon test). These patterns can be seen in Fig. 2, where Non-Surg Hypo patients had values comparable to HC during the day, but lower values at night. Likewise, the frequency of mild (SG < 70) or severe hypoglycemia (<55) lasting ≥15 minutes was greater in Non-Surg Hypo than in controls, and the mean duration of both mild and severe SG events was longer in this group (39.6 ± 19.9 vs 27.2 ± 14.4 minutes for HC, P = 0.03); these values are similar to the duration in patients with PBH-RYGB (38.9 ± 23.7 minutes, P > 0.9 vs Non-Surg Hypo). As with the PBH-RYGB group, there was no significant correlation between BMI and percentage of time with SG < 70 in this population (r = −0.15).
We further analyzed patterns of glycemia in PBH-RYGB in comparison with Non-Surg Hypo. The distribution of SG values for control, PBH-RYGB, and Non-Surg Hypo patients can be visualized in Fig. 1B. Both PBH-RYGB and Non-Surg Hypo show a leftward shift in the distribution of glucose levels as compared with HC. There were no statistical differences between these groups in mean, median, and nadir glucose values, nor in % time-in-range (70-180 mg/dL) or % time below range (SG < 70, <60, and < 54 mg/dL). However, the PBH-RYGB group had a greater peak (202.0 ± 52.6 vs 175.0 ± 27.4, P = 0.01) and higher glycemic variability markers, including SD (24.8 ± 7.3 vs 18.6 ± 6.6 mg/dL), CV (27.3 ± 6.8% vs 20.8 ± 6.2%), IQR (28.0 ± 7.4 vs 22.0 ± 7.8 mg/dL), and CONGA-4 (32.5 ± 12.3 vs 23.3 ± 6.4 mg/dL), compared with Non-Surg Hypo (P < 0.005 for all, Wilcoxon test).
Discussion
We report a comparison of sensor glucose values in patients with PBH-RYGB and nonsurgical reactive hypoglycemia, in comparison with healthy controls, patients with prediabetes, and patients with newly diagnosed T2D. One of the most prominent findings from our analysis is greater glycemic variability in patients with PBH with a history of RYGB. This results from both an increased frequency of high sensor glucose values, and increased frequency of low sensor glucose values during both day and night in patients with PBH-RYGB as compared with normoglycemic controls. These patterns are consistent with the large increases in rate of glucose appearance from ingested glucose after meals in patients with PBH-RYGB (27), potentially linked to rapid gastric emptying. Subsequently, glucose drops rapidly to overtly hypoglycemic levels. This pattern is likely mediated by excessive postprandial insulin secretion, in turn related to both high glucose and glucagon-like peptide 1 levels in the early postprandial state (28). Treatment of episodes of hypoglycemia can then initiate subsequent spikes in glucose and recurrent hypoglycemia—a so-called roller coaster of hypoglycemia. (29). We do not yet understand why some patients develop PBH and others do not, but patients who develop low glucose values post-RYGB were found to have somewhat lower glucose levels and higher insulin secretion on preoperative testing, suggesting that underlying physiology in these patients may differ (30).
While glycemic variability is associated with heightened cardiovascular risk in T2D (31), the implications of increased glycemic variability in the post-RYGB population and in the subpopulation with PBH remain unknown; fortunately, overall cardiovascular disease is reduced in longitudinal cohort studies of post-bariatric patients (32, 33). Glycemic variability may also be related to postprandial symptoms, as some patients report symptoms during periods of rapid decline in glucose values, even when glucose values are within the normal range. Whether this is related to drops in glucose per se or other hormonal or vasomotor effects of rapid gastric emptying and dumping syndrome remains unknown.
Beyond variability in glucose, patients with PBH-RYGB demonstrate increased frequency and duration of SG < 70 mg/dL We acknowledge that CGM accuracy is reduced at low values, could be compromised by position-dependent compression (34), and minimum values of 40 mg/dL make assessment of true nadir sensor glucose values impossible. Nevertheless, using the same monitoring system, frequency of low sensor glucose values was greater than that for healthy controls. Moreover, the duration of events with glucose below hypoglycemia thresholds was longer for patients with PBH vs controls. Potential contributors to this pattern include reduction in self-correction of glucose potentially linked to persistent insulin-mediated glucose disposal from a prior period of increased insulin secretion (eg, post-meal), decreased counterregulatory hormone responses to hypoglycemia (8, 35), impaired sensitivity to counterregulatory hormones, or reduced glycogen reserves in the liver. Future studies should assess the glucose variability as well as the frequency and duration of hypoglycemia in patients post-RYGB without symptomatic hypoglycemia.
Differences between PBH-RYGB and controls were even more striking during nighttime hours, with increased frequency of glucose values below 70 and increased duration of low sensor glucose values. Median duration of glucose <70 or <60 was 12.6% and 2.5% in PBH-RYGB patients. Clinical history indicates that these episodes are often completely asymptomatic. In some patients, nocturnal hypoglycemia may contribute to nocturnal sweating, vivid dreams, and poor sleep quality. Physicians and other caregivers should be attuned to this possibility, and query patients carefully about nocturnal symptoms.
PBH is largely considered a “reactive” or postprandial hypoglycemia disorder; indeed, patients with PBH-RYGB have normal glucose and appropriately suppressed insulin levels after an overnight fast or a prolonged inpatient diagnostic fast. Thus, overnight hypoglycemia in this setting should not really be considered as “fasting hypoglycemia.” Rather several factors should be considered; if food was consumed shortly before bed, it is possible that nocturnal low glucose values reflect the delayed effects of postprandial insulin secretion. Alternatively, nocturnal hypoglycemia may reflect reductions in glycogenolysis due to either reduced glycogen content or reduced levels and/or resistance to the effects of counterregulatory hormones (8, 35). Mid-nocturnal drops in glucose may also reflect a failure of transition to gluconeogenesis. Finally, increases in insulin-independent glucose uptake observed in PBH-RYGB (4), independent of food intake, could contribute to lower glucose levels, potentially via glucose uptake in insulin-independent tissues such as intestine (36) or brain (37). Additional hormonal or metabolic changes, such as the 3-fold increase in basal and meal-stimulated FGF19 recently reported by our group (5), could also promote insulin-independent glucose uptake in PBH-RYGB. Clinical studies will be required to dissect these possibilities.
Our study also compared glycemic patterns in patients with history of confirmed reactive hypoglycemia who had not undergone prior gastrointestinal surgery. This population was distinct from PBH-RYGB, as glycemic variability was normal, with no significant increase in glucose levels above or below the normal range overall. Strikingly, significantly lower sensor glucose values were detected only during nighttime hours. This condition has been understudied, so future analysis of meal-dependent and independent contributors to glycemic patterns in this population is needed.
Given the increased use of CGM by practicing clinicians to evaluate glucose patterns, it is important to note that healthy control individuals had a mean of 3.2% of sensor glucose values below 70 mg/dL, but only 0.2% under 54 mg/dL. This finding is consistent with the recent study demonstrating similar patterns of Dexcom G6 CGM-derived glucose values in healthy individuals, who had only 1.1% of values below 70 but only 0% to 0.2% below 54 mg/dL (38). Collectively these data underscore the need to carefully consider the “normal” range of sensor glucose values when interpreting CGM-derived sensor glucose data in an individual without diabetes. Moreover, given the nonspecific nature of symptoms associated with hypoglycemia, it is important to precisely determine the relationship of any documented symptoms to sensor glucose levels when assessing clinical significance.
We acknowledge limitations of our study. Firstly, our analysis was restricted to the subset of PBH patients who had undergone RYGB. Future studies comparing CGM patterns in individuals following sleeve gastrectomy will be important, given the increasing use of this procedure. The majority of CGM data were obtained from CGM performed for clinical evaluation, and analysis was performed retrospectively. Due to incomplete record-keeping of symptoms and timing, we are unable to determine whether episodes of low sensor glucose were symptomatic or asymptomatic. The true magnitude of low glucose values cannot be ascertained from CGM, given diagnostic inaccuracy relative to plasma sampling, and limit of reporting of sensor glucose values below 40 mg/dL. Although we utilized the same Dexcom G4 CGM, our between-group comparisons may have been influenced by demographic differences, including study site (eg, California vs Massachusetts), or small differences in age or body weight. Duration of CGM monitoring was lower in the PBH-RYGB than in the HC or Non-Surg Hypo groups, potentially influencing median glucose values and observed patterns. Longer duration of monitoring may be more likely to accurately reflect day-to-day variation in sensor glucose and capture more events of interest, but it would not directly impact the calculated percentage of time within various sensor glucose intervals. Moreover, the percentage of time captured during day and night did not differ between groups. Since this was a retrospective observational study, differences in macronutrient intake and meal timing may have differed between groups and could have contributed to altered patterns. Interindividual variation in timing of meals may have also reduced our power to detect differences in glycemic patterns during the day. Finally, we were unable to analyze the impact of activity, another important modifier of glucose metabolism, on observed patterns. Unfortunately, metrics of diet and exercise were not collected for participants in the publicly available CGM study; although we requested a diary of nutrition and activity for all other participants, most provided incomplete data.
Key findings from this study may inform clinical practice. Firstly, clinicians should be attuned to whether patients have nocturnal drops in glucose, as these may pose greater safety risk due to relative unawareness during sleep, and the possibility that unrecognized hypoglycemia at night may contribute to the development of hypoglycemia unawareness. Physicians may wish to screen patients about nocturnal sweating, poor sleep quality, vivid dreams, and morning headaches as potential symptoms linked to nocturnal hypoglycemia. If present, strategies to maintain nocturnal glucose can include bedtime snacks which do not trigger prandial insulin secretion, cornstarch administration (39), and avoidance of alcohol. Also, clinicians should be aware that nocturnal hypoglycemia may not reflect the same physiology as fasting hypoglycemia; we recommend that all patients be evaluated for insulin secretory autonomy with lab testing after an overnight fast. For patients with severe or treatment-resistant hypoglycemia, prolonged inpatient fasting testing may be required. Future studies should explore the potential of CGM technology in assisting PBH patients to recognize early hypoglycemia and prevent severe hypoglycemia.
Acknowledgments
M.E.P. gratefully acknowledges support from National Institutes of Health (NIH) R01 DK121995 (related to this manuscript), and additional support from R01 DK106193, U01 DK114156, P30 DK036836 (DRC, Joslin Diabetes Center) and the Chan Zuckerberg Foundation. D.L. was supported by the National Institutes of Diabetes, Digestive, and Kidney Disease (NIDDK) Medical Student Research Program T32 DK007260.
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Disclaimer: The views expressed in this article are those of the author and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Glossary
Abbreviations
- BMI
body mass index
- CGM
continuous glucose monitoring
- CONGA
continuous overall net glycemic action
- CV
coefficient of variation
- HC
healthy controls
- IQR
interquartile range
- Non-Surg Hypo
reactive hypoglycemia without gastrointestinal surgery
- PBH
post-bariatric hypoglycemia
- PBH-RYGB
post-bariatric hypoglycemia after RYGB
- Pre-DM
prediabetes
- RYGB
Roux-en-Y gastric bypass
- SG
sensor glucose
- T2D
type 2 diabetes
Additional Information
Disclosures: M.E.P. has been a coinvestigator on an NIH R44 grant together with Xeris Pharmaceuticals. M.E.P. has consulted for Eiger Pharmaceuticals, has received investigator-initiated grant support from Janssen Pharmaceuticals, Medimmune, Sanofi, AstraZeneca, Jenesis, and Nuclea, has been a site investigator for XOMA and Xeris, and acknowledges clinical trial research trial product support from Ethicon, Covidien, NovoNordisk, Nestle, and Dexcom within the past 5 years—all unrelated to the present study. M.E.P. has submitted a patent application regarding plasma proteins contributing to hypoglycemia and pump therapies for hypoglycemia. No other relationships or activities occurred that could appear to have influenced the submitted work.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab. 2018;103(8):2815-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353(3):249-254. [DOI] [PubMed] [Google Scholar]
- 3. Patti ME, McMahon G, Mun EC, et al. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia. 2005;48(11):2236-2240. [DOI] [PubMed] [Google Scholar]
- 4. Patti ME, Li P, Goldfine AB. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity (Silver Spring). 2015;23(4):798-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulla CM, Goldfine AB, Dreyfuss JM, et al. Plasma FGF-19 levels are increased in patients with post-bariatric hypoglycemia. Obes Surg. 2019;29(7):2092-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17(9):1671-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van den Broek M, de Heide LJM, Sips FLP, et al. Altered bile acid kinetics contribute to postprandial hypoglycaemia after Roux-en-Y gastric bypass surgery. Int J Obes (Lond). 2021;45(3):619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abrahamsson N, Börjesson JL, Sundbom M, Wiklund U, Karlsson FA, Eriksson JW. Gastric bypass reduces symptoms and hormonal responses in hypoglycemia. Diabetes. 2016;65(9):2667-2675. [DOI] [PubMed] [Google Scholar]
- 9. Vidal J, Nicolau J, Romero F, et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. J Clin Endocrinol Metab. 2009;94(3):884-891. [DOI] [PubMed] [Google Scholar]
- 10. Hanaire H, Bertrand M, Guerci B, Anduze Y, Guillaume E, Ritz P. High glycemic variability assessed by continuous glucose monitoring after surgical treatment of obesity by gastric bypass. Diabetes Technol Ther. 2011;13(6):625-630. [DOI] [PubMed] [Google Scholar]
- 11. Halperin F, Patti ME, Skow M, Bajwa M, Goldfine AB. Continuous glucose monitoring for evaluation of glycemic excursions after gastric bypass. J Obes. 2011;2011:869536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonis C, Lorenzini F, Bertrand M, et al. Glucose profiles in pregnant women after a gastric bypass: findings from continuous glucose monitoring. Obes Surg. 2016;26(9):2150-2155. [DOI] [PubMed] [Google Scholar]
- 13. Kefurt R, Langer FB, Schindler K, Shakeri-Leidenmühler S, Ludvik B, Prager G. Hypoglycemia after Roux-En-Y gastric bypass: detection rates of continuous glucose monitoring (CGM) versus mixed meal test. Surg Obes Relat Dis. 2015;11(3):564-569. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen JB, Abild CB, Pedersen AM, Pedersen SB, Richelsen B. Continuous glucose monitoring after gastric bypass to evaluate the glucose variability after a low-carbohydrate diet and to determine hypoglycemia. Obes Surg. 2016;26(9):2111-2118. [DOI] [PubMed] [Google Scholar]
- 15. Cryer PE, Axelrod L, Grossman AB, et al. ; Endocrine Society . Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709-728. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen Q, Pandya S, Chin K, Parkin CG. Use of continuous glucose monitoring in detecting reactive hypoglycemia in individuals without diabetes. J Diabetes Sci Technol. 2018;12(6):1244-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soare A, Khazrai YM, Fontana L, et al. Treatment of reactive hypoglycemia with the macrobiotic Ma-pi 2 diet as assessed by continuous glucose monitoring: The MAHYP randomized crossover trial. Metabolism. 2017;69:148-156. [DOI] [PubMed] [Google Scholar]
- 18. Lee D, Dreyfuss JM, Sheehan A, Puleio A, Mulla CM, Patti ME. Data from: glycemic patterns are distinct in individuals with post-bariatric hypoglycemia after gastric bypass (PBH-RYGB). Dryad digital repository. Deposited March 23, 2021. https://datadryad.org/stash/share/-D8L55-wgNsC2_PXIxeqqbS7gWoe0YpiSGNyNK9SaDc [DOI] [PMC free article] [PubMed]
- 19. Laguna Sanz AJ, Mulla CM, Fowler KM, et al. Design and clinical evaluation of a novel low-glucose prediction algorithm with mini-dose stable glucagon delivery in post-bariatric hypoglycemia. Diabetes Technol Ther. 2018;20(2):127-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mulla CM, Zavitsanou S, Sanz AJL, et al. A Randomized, placebo-controlled double-blind trial of a closed-loop glucagon system for postbariatric hypoglycemia. J Clin Endocrinol Metab 2020;105(4):e1260-e1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hall H, Perelman D, Breschi A, et al. Glucotypes reveal new patterns of glucose dysregulation. Plos Biol. 2018;16(7):e2005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wójcicki JM. “J”-index. A new proposition of the assessment of current glucose control in diabetic patients. Horm Metab Res. 1995;27(1):41-42. [DOI] [PubMed] [Google Scholar]
- 23. McDonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7(2):253-263. [DOI] [PubMed] [Google Scholar]
- 24. Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644-655. [DOI] [PubMed] [Google Scholar]
- 25. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Clarke W. Symmetrization of the blood glucose measurement scale and its applications. Diabetes Care. 1997;20(11):1655-1658. [DOI] [PubMed] [Google Scholar]
- 26. Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433-2438. [DOI] [PubMed] [Google Scholar]
- 27. Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldfine AB, Mun EC, Devine E, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J Clin Endocrinol Metab. 2007;92(12):4678-4685. [DOI] [PubMed] [Google Scholar]
- 29. Patti ME, Goldfine AB. The rollercoaster of post-bariatric hypoglycaemia. Lancet Diabetes Endocrinol. 2016;4(2):94-96. [DOI] [PubMed] [Google Scholar]
- 30. Nannipieri M, Belligoli A, Guarino D, et al. Risk factors for spontaneously self-reported postprandial hypoglycemia after bariatric surgery. J Clin Endocrinol Metab. 2016;101(10):3600-3607. [DOI] [PubMed] [Google Scholar]
- 31. Smith-Palmer J, Brändle M, Trevisan R, Orsini Federici M, Liabat S, Valentine W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(3):273-284. [DOI] [PubMed] [Google Scholar]
- 32. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56-65. [DOI] [PubMed] [Google Scholar]
- 33. Hinerman AS, Barinas-Mitchell EJM, El Khoudary SR, Courcoulas AP, Wahed AS, King WC. Change in predicted 10-year and lifetime cardiovascular disease risk after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2020;16(8):1011-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mensh BD, Wisniewski NA, Neil BM, Burnett DR. Susceptibility of interstitial continuous glucose monitor performance to sleeping position. J Diabetes Sci Technol. 2013;7(4):863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Heide LJM, van den Broek M, van Dijk G, Emous M, van Beek AP. Diminished counterregulatory responses to meal-induced hypoglycemia 4 Years After RYGB. Obes Surg. 2021;31(2):597-602. [DOI] [PubMed] [Google Scholar]
- 36. Franquet E, Watts G, Kolodny GM, Goldfine AB, Patti ME. PET-CT reveals increased intestinal glucose uptake after gastric surgery. Surg Obes Relat Dis. 2019;15(4):643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li N, Yan QT, Jing Q, et al. Duodenal-jejunal bypass ameliorates type 2 diabetes mellitus by activating insulin signaling and improving glucose utilization in the brain. Obes Surg. 2020;30(1):279-289. [DOI] [PubMed] [Google Scholar]
- 38. Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lembo E, Lupoli R, Ciciola P, et al. Implementation of low glycemic index diet together with cornstarch in post-gastric bypass hypoglycemia: two case reports. Nutrients 2018;10(6):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.