Abstract
Context
Isolated prolactin deficiency is a rare disorder manifesting as absence of puerperal lactation. We identified a 2-generation family with 3 women experiencing alactogenesis.
Objective
We hypothesized a heterozygous genetic mutation.
Methods
This was a family-based study. Two generations of women (proband, sister, and niece) with puerperal alactogenesis and one control were studied. Prolactin levels in the 3 women ranged from 0.618 to 1.4 ng/mL (range, 2.8-29.2 ng/mL). All the women had regular menstrual cycles during their reproductive years. The niece required fertility treatment to become pregnant and the proband and sister underwent menopause before age 45 years. Prolactin gene (PRL) exons 1 to 5 were sequenced. We sought a heterozygous, deleterious gene variant with functional consequences.
Results
We identified a heterozygous mutation (c.658C > T) changing CGA to TGA (p.Arg220Ter) in exon 5 of the prolactin gene. Transfection of PRL containing the stop gain mutation resulted in similar intracellular prolactin levels compared to PRL wild type, but little detectable immunoactive or bioactive prolactin in conditioned medium. Prolactin secretion was also impaired by a PRL stop gain mutation deleting both of the terminal cysteine amino acids (c.652A > T; p.Lys218Ter).
Conclusion
This is the first report of a PRL mutation causing familial prolactin deficiency and alactogenesis. The loss of the terminal cysteine resulted in failure of prolactin secretion. Secretion was not rescued by deleting the penultimate cysteine, with which it forms a disulfide bond. These data suggest that the PRL C terminal is critical for protein secretion.
Keywords: early menopause, lactation, prolactin deficiency
Isolated prolactin deficiency is a rare disorder manifesting as absence of lactation post partum. Although it can occur in the setting of hemorrhage and hypotension during delivery, alactogenesis in the absence of delivery complications is rare. There are 7 reports of alactogenesis in the literature, with the cause unknown in most studies (1-6). Autoimmunity in the presence of lactotroph antibodies was the only etiology identified in one previous study (1).
A genetic cause of alactogenesis is implicated based on reported families with multiple affected members (6, 7). In the only familial study with isolated prolactin deficiency, alactogenesis was reported in a mother and daughter, suggesting a dominant mode of inheritance (6). We report a family in which 3 women, 2 sisters and a daughter, experienced alactogenesis. We identified a heterozygous prolactin stop gain mutation exhibiting a dominant mode of inheritance.
Case Report
The proband (Fig. 1), currently age 66 years, had a history of alactogenesis. She had menarche at age 13 years 11 months, regular menstrual cycles during her reproductive years, and 7 pregnancies with 2 live births and 5 miscarriages. She had no breast milk production after the 2 full-term births. She underwent natural menopause before age 45 years. At age 47 years, she had a prolactin level of 1.6 ng/mL (range, 2.8-29.2 ng/mL; assay manufacturer not documented) and a normal thyrotropin.
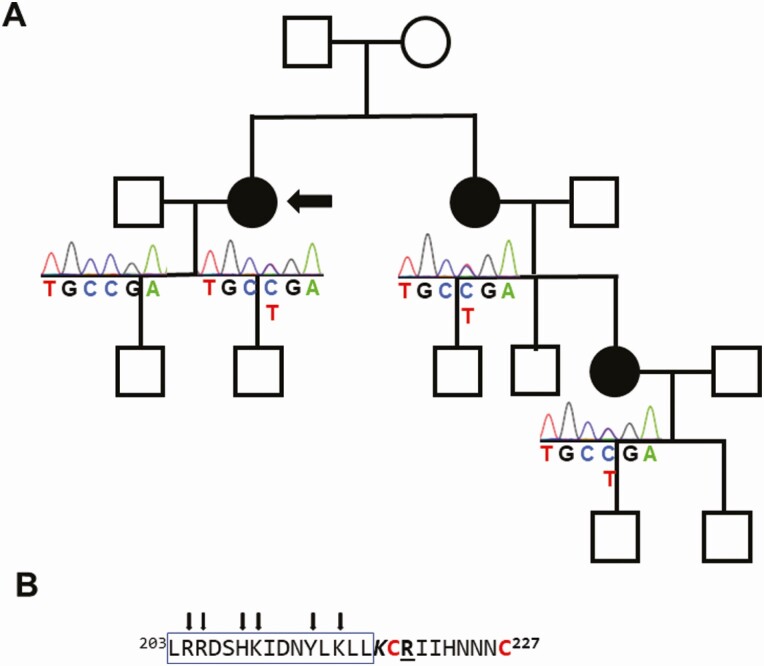
Figure 1.
A, Pedigree. The pedigree shows a family with dominant inheritance of alactogenesis (closed circles). The proband is indicated by the arrow. The prolactin gene (PRL) mutations (c.658C > T) in all the affected women changed arginine amino acid number 220 to a stop codon (p.Arg220Ter). The second allele was not mutated. The squares denote men and the open circles women who are unaffected or of unknown status. B, C-terminal portion of the prolactin protein. The underline indicates the location of the original p.Arg220Ter mutation. A disulfide bond normally forms between the 2 terminal cysteines, indicated in red. The italics indicates the experimental p.Lys218Ter mutation. The rectangular box indicates the α helix structure. The arrows indicate amino acids important for binding the prolactin receptor.
Her sister, currently age 71 years, had menarche 3 weeks before her 14th birthday. She gave birth to 3 children and had no breast milk production after any of the births. At age 63 years, her prolactin level was 1.4 ng/mL (range, 2.1-17.7 ng/mL; Siemens). She also had menopause before age 45 years.
Her affected sister’s daughter, currently age 40 years, had menarche at age 13 years 11 months, and infertility requiring intrauterine insemination. She had 2 pregnancies and alactogenesis post partum. At age 33 years, she had prolactin levels of 1.13 and 0.618 ng/mL and at age 36 years her prolactin level was 0.759 ng/mL (range, 2.1-17.7 ng/mL; Immulite, Siemens).
All the women had normal breast development, no hemorrhage or hypotension at the time of their deliveries, and no reported abnormalities of other hypothalamic-pituitary axes. The women were of European descent.
This study was approved by the institutional review board of the University of Utah. All participants provided written, informed consent.
Materials and Methods
DNA was extracted from whole blood using the QIAmp DNA Blood Maxi Kit (Qiagen). Genomic DNA for the 5 exons in prolactin was Sanger sequenced (Table 1).
Table 1.
Primers used to sequence prolactin gene (PRL) exons 1 to 5
| Target | Forward | Reverse |
|---|---|---|
| Exon 1 | 5′ CCGTGAGACTTCCAGATCTTC 3′ | 5′ TTAGTTAAAATTTCACATTAATCCCC 3′ |
| Exon 2 | 5′ TTCCTCGGCAGGATTACTTC 3′ | 5′ CAGAGCCCAGTAGTTCATGTG 3′ |
| Exon 3 | 5′ TTCAATGCCCAAACAACCCC 3′ | 5′ TTTGGTGCAAAGCCTGGATG 3′ |
| Exon 4 | 5′ AAATCACAAGTAACTAACCCCATTG 3′ | 5′ TTTATCATCACAGAGGTCACCG 3′ |
| Exon 5 | 5′ TCTCATTCTCAATTCTTAATTCAACAG 3′ | 5′ AAGAAGCTTGCAATGGAACG 3′ |
RNA was isolated from whole blood from the proband, her sister, and a control individual with a history of normal lactation and menopause at age 50 years using the QIAmp RNA Blood Mini Kit and subsequently treated with DNAse digestion and RNA clean-up according to the manufacturer’s instructions (Qiagen) (8). Reverse transcription was performed with SuperScript VILO MasterMix (Life Technologies) using SuperScript III RT and random primers. Quantitative real-time polymerase chain reaction was performed for the expression of prolactin gene (PRL) and β-2-microglobulin (B2M) as an endogenous control using PowerUp SYBR Green MasterMix (Applied Biosystems). Primers were designed across PRL exons 4 and 5 and B2M exons 1 and 2 to avoid amplifying genomic DNA. Primer sequences were as follows: PRL exons 4 to 5 forward 5′- ATTGAGGAGCAAACCAAACG-3′, PRL exon 4–5 reverse 5′-AGGCGAGACTCTTCATCAGC-3′, B2M forward 5′-GAGGCTATCCAGCGTACTCCA-3′, B2M reverse 5′-CGGCAGGCATACTCATCTTTT-3′. Samples were examined in triplicate and at 2 dilutions. Messenger RNA (mRNA) levels of PRL were determined using the 2–ΔΔCT method to calculate relative quantification and to correct for expression of endogenous controls and were compared between the proband, sister and control using analysis of variance (ANOVA).
Plasmid Preparation
To generate a human PRL expression plasmid pcDNA3.1(-)-PRL, the full-length cDNA encoding human PRL (PlasmID Repository, Harvard Medical School; clone HsCD000000018) was polymerase chain reaction–amplified with primers 5′-CGGGCCCTCTAGACTCGA GCGGCCGCATAACTTCGTATAG-3′ (NotI site underlined) and 5′-CAGCGGTTTAAACTTAAGCTTCCCTAGCAGTTGTTGTTG-3′ (HindIII site underlined) and cloned into NotI/HindIII-digested pcDNA3.1(–) expression vector (Invitrogen) using the NEBuilder HiFi DNA Assembly Cloning Kit (New England Biolabs). The QuikChange II Site-Directed Mutagenesis kit (Agilent Technologies) was used to introduce point mutations, resulting in pcDNA3.1 (–)-c.658C > T-PRL and pcDNA3.1(–)-c.652A > T-PRL. Sequence fidelity of the resulting constructs was verified by Sanger sequencing.
MCF7 Cell Model
Human breast cancer MCF7 cells (a kind gift from Dr D. Tantin, University of Utah; ATCC HTB-22) were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Life Technologies) at 37 °C and 5% CO2. MCF7 cells were cultured at 2.5 × 105 cells/well in a 12-well plate 24 hours prior to transfection. The linearized plasmids pcDNA3.1(–)-PRL, pcDNA3.1(–)-c.658C > T-PRL, and pcDNA3.1(–)-c.652A > T-PRL were transfected into MCF7 cells using PolyJet transfection reagent (SignaGen Laboratories) as described in the manufacturer’s instructions. Stable cell lines were generated by selection of colonies resistant to 750 μg/mL G418 (Life Technologies) for approximately 6 weeks and subsequently seeded at 4.5 × 105 cells/well in duplicate in a 12-well plate. Twenty-four hours after seeding, all wells were replenished with 1-mL fresh growth media. A total of 1-mL conditioned medium was collected at 48-hour intervals and stored at –20 °C until the assays were performed.
Prolactin Immunoassay
Conditioned medium collected from transfected MCF7 cells was assayed undiluted and at 1:10 and 1:100 dilution for prolactin measurements using the Human Prolactin enzyme-linked immunosorbent assay kit (ELISA; Invitrogen). Prolactin levels were compared across treatments using one-way ANOVA on ranks with Dunn post hoc testing.
Nb2 Prolactin Bioassay
Prolactin bioassays were performed as previously described (9, 10). Nb2-11 rat pre-T lymphoma cells (Sigma-Aldrich; ACC No. 97041101) were actively grown in maintenance medium consisting of Fischer’s medium supplemented with 10% FBS, 10% gelding horse serum (HS; Atlanta Biologicals), 50 μM 2-mercaptoethanol (2-ME), 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mM ʟ-glutamine (Life Technologies), and 0.075% sodium bicarbonate (Sigma-Aldrich) at 37 °C in a humidified 5% CO2 incubator. Stationary-phase cells were obtained by incubating cells in transition medium (same as maintenance medium but with 1% FBS) for 24 hours, extensively washing the cells in Fischer’s medium with 10% HS, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid; Life Technologies), and 50 μM 2-ME and resuspending the cells in stationary medium (same as maintenance medium without FBS). To perform the proliferation bioassay, 0.2 mL of conditioned medium collected from transfected MCF-7 cell lines was added into 1.8-mL stationary phase Nb2-11 cells at 2.2 × 105 cells/mL seeded in a 12-well plate. After 48 hours of incubation, average cell density in 3 wells was determined with a hemocytometer. All experiments were performed in triplicate on separate days. Cell density was compared across treatments using ANOVA with Holm-Šidák post hoc testing.
Western Blot Intracellular Prolactin
Total cell lysates of transfected MCF7 cell lines were prepared by lysing cells in ice-cold radioimmunoprecipitation assay buffer consisting of 50-mM Tris HCl (pH 8.0), 150-mM NaCl, 1% NP-40, 0.5% sodium dodecyl sulfate, and protease inhibitor (Fisher Scientific) for 30 minutes at 4 °C and removing cell debris by 12 000 rpm centrifugation for 20 minutes at 4 °C. Protein concentrations of the resulting lysates were assessed using the DC protein assay (Bio-Rad). Proteins in cell lysates were heated at 95°C for 5 minutes in Laemmli samples buffer (Bio-Rad), resolved by 10% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes. The membranes were blocked in 5% nonfat milk in TBST (50-mM Tris-HCl and 150-mM NaCl with 0.1% Tween-20 adjusted at pH 7.6) for 1 hour at room temperature and incubated in a PRL primary antibody (1:1000, ab110642, Abcam) or β-actin antibody (1:15000 dilution, Novus Biologicals) diluted in 2% nonfat milk in TBST at 4 °C overnight followed by incubation with a horseradish peroxidase–conjugated antirabbit secondary antibody (Invitrogen) for 1 hour at room temperature. The membranes were washed with TBST after primary and secondary antibody incubation. The signals were developed by ECL solutions and detected by standard x-ray films (Thermo Fisher Scientific). Band density was analyzed using VisionWorks LS software (UVP). The experiment was repeated 3 times. Prolactin/β-actin levels were compared among samples using one-way ANOVA on ranks with Dunn post hoc testing.
Results
The pedigree structure suggested a dominant mode of inheritance (Fig. 1A). We identified a heterozygous mutation (c.658C > T) changing CGA (arginine) to TGA (stop codon) (p.Arg220Ter) in exon 5 of PRL. The resulting stop gain mutation truncates the final 7 amino acids from the protein (Fig. 1B).
RNA Expression
PRL mRNA expression was examined to determine whether the c.658C > T stop gain mutation resulted in nonsense-mediated decay. PRL mRNA expression was very low in whole blood and was not different among the proband, her sister, and the control (0.38 ± 0.49, 0.57 ± 0.60 vs 1.01 ± 0.17; P = .12).
Prolactin Immunoactivity
MCF7 cell lines were transfected with wild-type PRL (PRL WT) and PRL containing the stop gain mutation (PRL p.Arg220Ter) to examine the effect of the p.Arg220Ter mutation on prolactin secretion. Immunoactive prolactin levels from conditioned MCF7 medium were below the limit of the assay. Immunoactive prolactin was detectable in conditioned medium from MCF7 cells transfected with PRL WT (56.7 ± 13.8 ng/mL) and 50:50 PRL WT:PRL Arg220Ter (172.6 ± 40.8 ng/mL; P < .001), but was much lower in MCF7 cells transfected with PRL Arg220Ter (0.051 ± 0.030 ng/mL; P < .03). We hypothesized that the loss of the C terminal Cys227 resulted in the absence of a terminal disulfide bond between Cys227 and Cys219 and that the unbound Cys219 inhibited secretion (Fig. 1B). To determine whether the remaining unbound Cys219 resulted in failure of prolactin secretion, a c.652A > T (p.Lys218Ter) stop-gain mutation was created to remove Cys219 (Fig. 1B) and transfected into MCF7 cells. Prolactin levels in the conditioned medium from Lys218Ter were also very low (0.014 ± 0.018 ng/mL; P < .02).
Prolactin Bioactivity
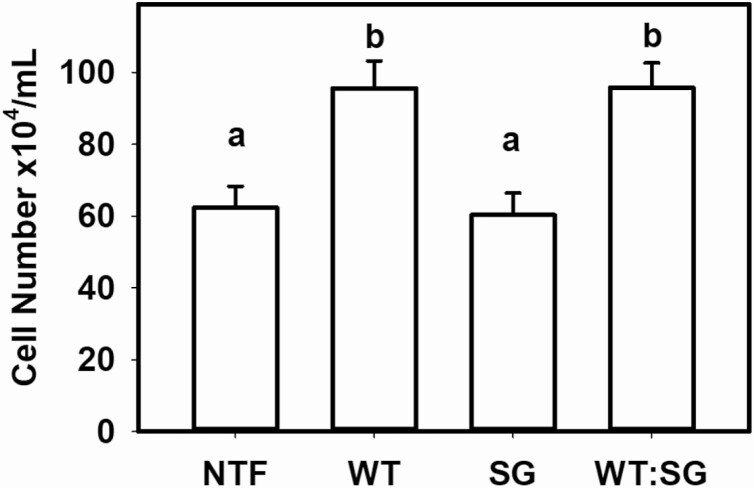
To ensure that the low prolactin levels in the PRL p.Arg220Ter mutation were not caused by failed immunoactivity, prolactin bioactivity was assessed using the Nb2 lymphoma cell assay. Nb2 cell density was higher after treatment with conditioned media derived from PRL WT–transfected MCF7 cells (95.6 ± 7.6 × 104 cells/mL) compared to media from PRL Arg220Ter transfected (60.3 ± 3.8 × 104 cells/mL) and similar to nontransfected MCF7 cell media (62.3 ± 6.2 × 104 cells/mL) (P < .001; Fig. 2). Media from MCF7 cells transfected with 50:50 PRL WT:PRL stop gain were similar to that from PRL WT (95.8 ± 6.8 × 104 cells; Fig. 2). The same relationship held constant at 1:10 and 1:50 dilutions (data not shown).
Figure 2.
Prolactin bioactivity. Nb2 lymphoma cell density after 48 hours of treatment with conditioned medium from nontransfected MCF7 cells (NTF) or MCF7 cells transfected with prolactin (PRL) (wild-type; WT), PRL p.Arg220Ter (stop gain; SG) or 50:50 PRL WT:PRL p.Arg220Ter (WT:SG) (analysis of variance P < .001). Triplicate wells were analyzed in each experiment and 3 experiments were performed on separate days. Differing letters indicate significant differences in cell number (P < .05; Holm-Šidák post hoc test).
Intracellular Prolactin
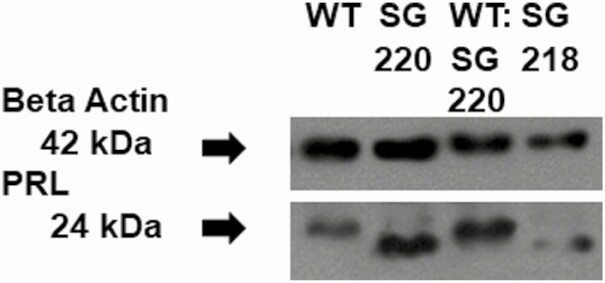
To determine whether protein production was inhibited by the p.Arg220Ter mutation, we quantified intracellular prolactin protein using a Western blot. The intracellular prolactin to β-actin protein ratio was similar in the PRL WT– (1.0 ± 0.9), 50:50 PRL WT:PRL Arg220Ter– (1.3 ± 0.3), PRL Arg220Ter– (1.3 ± 0.6), and PRL Lys218Ter– (1.2 ± 0.7) transfected MCF7 cells (P = .9, Fig. 3). The PRL Arg220Ter and PRL Lys218Ter ran at a slightly lower molecular weight as expected based on the shorter protein and loss of a negatively charged arginine. Despite confirmation that the PRL c.658C > T mutation was present in the transfected 50:50 pcDNA3.1(–)-PRL:pcDNA3.1(–)-c.658C > T-PRL MCF7 cells, there was little detectable PRL p.Arg220Ter on the Western blot.
Figure 3.
Western blot. Western immunoblot for prolactin and β-actin protein bands in MCF7 cells transfected with prolactin (PRL) (wild-type; WT), PRL p.Arg220Ter (SG220), 50:50 PRL WT:PRL p.Arg220Ter (WT:SG220), or p.Lys218Ter (SG218). p.Arg220Ter and p.Lys218Ter run at a lower molecular weight and there is little detectable PRL p.Arg220Ter in the 50:50 PRL WT:PRL p.Arg220Ter. SG, stop gain.
Discussion
We have identified a genetic cause of puerperal alactogenesis resulting from a heterozygous stop-gain mutation (p.Arg220Ter) in PRL, transcribing a truncated protein that removes the terminal 7 amino acids, leaving an unbound cysteine. Prolactin levels were very low in women carrying the p.Arg220Ter mutation. When PRL carrying the stop-gain mutation was transfected into the MCF7 cell line, there was no immunoactive or bioactive prolactin secreted. Secretion was not restored when the penultimate cysteine was also removed from prolactin. Taken together, the data suggest that the C-terminal portion of prolactin is critical for prolactin release and that the loss of the C-terminal portion of prolactin prevents release of the normal prolactin from the lactotroph.
PRL is not constrained for loss-of-function variants (11). Nevertheless, there are no stop-gain variants found in exon 5 in the gnomAD database, suggesting the importance of the C terminal. A stop-gain codon must be at least 50 nucleotides upstream from the final 3′exon-exon junction to undergo nonsense-mediated decay (12). Therefore, the mutation in this family, which causes a stop gain only 27 nucleotides upstream from the C terminus, is translated in its short form, as demonstrated herein. The prolactin C-terminal amino acid residues have restricted mobility based on a disulfide bond between the terminal 2 cysteines (Cys219 and Cys227) that packs the C-terminal tail tightly against the fourth α helix in the protein (13). The disulfide bond is disrupted by the p.Arg220Ter mutation identified in the family, and the final Cys227 is lost (14). The unbound Cys219 could then form inappropriate disulfide bonds that disrupt protein folding and secretion. However, removal of the Cys219 in addition to Cys227 does not restore protein secretion, suggesting that the tertiary structure conferred by entire C-terminal binding to the fourth α helix may be the critical interaction responsible for proper protein folding and secretion.
In lactotrophs, prolactin is concentrated in the Golgi apparatus, forming large prolactin aggregates facilitated by the acidic pH and complexing with Zn2+ (15). Prolactin is released from the Golgi in secretory granules through calcium-mediated exocytosis. The aggregated prolactin then dissolves, releasing properly folded prolactin (15). Clues to the mechanism for the failure of prolactin secretion come from a previously described autosomal dominant missense mutation in growth hormone (GH), p.Arg183His (16). GH has a highly conserved C-terminal region, which is very similar to that of prolactin (13). The p.Arg183His GH mutation also causes failure of secretion related to the increased bulk from the histidine amino acid disrupting the terminal cysteine disulfide bond that is common both to GH and prolactin, or by creating an additional Zn2+ binding site, both of which could cause abnormal aggregation and secretion blockade (15, 16). The p.Arg220Ter may similarly exert a dominant negative effect, resulting in failure of prolactin secretion.
Based on its limited critical window during pregnancy and post partum, prolactin deficiency is not clinically significant in men or in most of the lifespan in women. In the few reported cases of isolated prolactin deficiency, breast development was normal, including in the current family (1, 6). Therefore, it is not clear whether prolactin was always low in the women or if lactotrophs were destroyed over a long period time. The MCF7 cells transfected both with the p.Arg220Ter and WT preferentially selected cells with intracellular WT prolactin, as demonstrated in the Western blot. However, the small but detectable prolactin levels in these women would argue against complete lactotroph destruction.
The low circulating prolactin resulted in the inability to breastfeed. Interestingly, all 3 women experienced menarche just before their 14th birthday, which is on the later side of normal, along with either infertility or early menopause. The early age at menopause in the proband and her sister raises the possibility that prolactin plays a role in oocyte maintenance, although we did not perform whole-exome sequencing to identify other mutations that could be responsible. Rodents do require prolactin for fertility and for maintenance of the corpus luteum (17, 18). Prolactin also inhibits gonadotropin-stimulated progesterone and 17β-estradiol production in human ovaries (19). However, further studies are needed to elucidate its subtle role in humans.
We report the first genetic cause of alactogenesis by a PRL stop-gain mutation. The loss of the terminal cysteine resulted in failure of prolactin secretion. Although the mutation removed a cysteine critical for a C-terminal disulfide bond, removal of the penultimate cysteine did not rescue secretion. The mutation likely results in improper aggregation or folding. The resulting low circulating prolactin caused alactogenesis. It may also be the cause of infertility and early menopause, requiring further studies.
Acknowledgments
We thank the participants for their overwhelming interest and participation. We thank Drs Kaitlin Ditch and Satbir Singh for their work on the protocol development.
Financial Support: This work was supported by the National Institute of Child Health and Human Development (grant Nos. R56HD090159 and R01HD099487 to C.K.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Glossary
Abbreviations
- 2-ME
2-mercaptoethanol
- ANOVA
analysis of variance
- B2M
β-2-microglobulin
- FBS
fetal bovine serum
- GH
growth hormone
- HS
gelding horse serum
- mRNA
messenger RNA
- PRL
prolactin gene
- WT
wild-type
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Iwama S, Welt CK, Romero CJ, Radovick S, Caturegli P. Isolated prolactin deficiency associated with serum autoantibodies against prolactin-secreting cells. J Clin Endocrinol Metab. 2013;98(10):3920-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turkington RW. Phenothiazine stimulation test for prolactin reserve: the syndrome of isolated prolactin deficiency. J Clin Endocrinol Metab. 1972;34(1):246-249. [PubMed] [Google Scholar]
- 3. Kauppila A, Chatelain P, Kirkinen P, Kivinen S, Ruokonen A. Isolated prolactin deficiency in a woman with puerperal alactogenesis. J Clin Endocrinol Metab. 1987;64(2):309-312. [DOI] [PubMed] [Google Scholar]
- 4. Falk RJ. Isolated prolactin deficiency: a case report. Fertil Steril. 1992;58(5):1060-1062. [DOI] [PubMed] [Google Scholar]
- 5. Douchi T, Nakae M, Yamamoto S, Iwamoto I, Oki T, Nagata Y. A woman with isolated prolactin deficiency. Acta Obstet Gynecol Scand. 2001;80(4):368-370. [PubMed] [Google Scholar]
- 6. Zargar AH, Masoodi SR, Laway BA, Shah NA, Salahudin M. Familial puerperal alactogenesis: possibility of a genetically transmitted isolated prolactin deficiency. Br J Obstet Gynaecol. 1997;104(5):629-631. [DOI] [PubMed] [Google Scholar]
- 7. Saito T, Tojo K, Oki Y, et al. A case of prolactin deficiency with familial puerperal alactogenesis accompanying impaired ACTH secretion. Endocr J. 2007;54(1):59-62. [DOI] [PubMed] [Google Scholar]
- 8. Kasippillai T, MacArthur DG, Kirby A, et al. Mutations in eIF4ENIF1 are associated with primary ovarian insufficiency. J Clin Endocrinol Metab. 2013;98(9):E1534-E1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gout PW, Beer CT, Noble RL. Prolactin-stimulated growth of cell cultures established from malignant Nb rat lymphomas. Cancer Res. 1980;40(7):2433-2436. [PubMed] [Google Scholar]
- 10. Clevenger CV, Chang WP, Ngo W, Pasha TL, Montone KT, Tomaszewski JE. Expression of prolactin and prolactin receptor in human breast carcinoma. Evidence for an autocrine/paracrine loop. Am J Pathol. 1995;146(3):695-705. [PMC free article] [PubMed] [Google Scholar]
- 11. Lek M, Karczewski KJ, Minikel EV, et al. ; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60 706 humans. Nature. 2016;536(7616):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagy E, Maquat LE. A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci. 1998;23(6):198-199. [DOI] [PubMed] [Google Scholar]
- 13. Teilum K, Hoch JC, Goffin V, Kinet S, Martial JA, Kragelund BB. Solution structure of human prolactin. J Mol Biol. 2005;351(4):810-823. [DOI] [PubMed] [Google Scholar]
- 14. Jomain JB, Tallet E, Broutin I, et al. Structural and thermodynamic bases for the design of pure prolactin receptor antagonists: x-ray structure of Del1-9-G129R-hPRL. J Biol Chem. 2007;282(45):33118-33131. [DOI] [PubMed] [Google Scholar]
- 15. Dannies PS. Mechanisms for storage of prolactin and growth hormone in secretory granules. Mol Genet Metab. 2002;76(1):6-13. [DOI] [PubMed] [Google Scholar]
- 16. Deladoëy J, Stocker P, Mullis PE. Autosomal dominant GH deficiency due to an Arg183His GH-1 gene mutation: clinical and molecular evidence of impaired regulated GH secretion. J Clin Endocrinol Metab. 2001;86(8):3941-3947. [DOI] [PubMed] [Google Scholar]
- 17. Horseman ND, Zhao W, Montecino-Rodriguez E, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16(23):6926-6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol. 2002;64:47-67. [DOI] [PubMed] [Google Scholar]
- 19. Demura R, Ono M, Demura H, Shizume K, Oouchi H. Prolactin directly inhibits basal as well as gonadotropin-stimulated secretion of progesterone and 17β-estradiol in the human ovary. J Clin Endocrinol Metab. 1982;54(6):1246-1250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”