Abstract
Context
Soluble alpha klotho (sαKL) has been linked to growth hormone (GH) action, but systematic evaluation and comparisons with traditional biomarkers in acromegaly are lacking.
Objective
To evaluate the potential of sαKL to aid classification of disease activity.
Methods
This retrospective study at 2 academic centers included acromegaly patients before surgery (A, n = 29); after surgery (controlled, discordant, or uncontrolled) without (B1, B2, B3, n = 28, 11, 8); or with somatostatin analogue treatment (C1, C2, C3, n = 17, 11, 5); nonfunctioning pituitary adenomas (n = 20); and healthy controls (n = 31). sαKL was measured by immunoassay and compared with traditional biomarkers (random and nadir GH, insulin-like growth factor I [IGF-I], IGF binding protein 3). Associations with disease activity were assessed.
Results
sαKL was correlated to traditional biomarkers, particularly IGF-I (rs=0.80, P <0.0001). High concentrations before treatment (A, median, interquartile range: 4.04 × upper limit of normal [2.26-8.08]) dropped to normal after treatment in controlled and in most discordant patients. A cutoff of 1548 pg/mL for sαKL discriminated controlled (B1, C1) and uncontrolled (B3, C3) patients with 97.8% (88.4%-99.9%) sensitivity and 100% (77.1%-100%) specificity. sαKL was below the cutoff in 84% of the discordant subjects. In the remaining 16%, elevated sαKL and IGF-I persisted, despite normal random GH. Sex, age, body mass index, and markers of bone and calcium metabolism did not significantly affect sαKL concentrations.
Conclusion
Our data support sαKL as a biomarker to assess disease activity in acromegaly. sαKL exhibits close association with GH secretory status, large dynamic range, and robustness toward biological confounders. Its measurement could be helpful particularly when GH and IGF-I provide discrepant information.
Keywords: biomarkers, growth hormone, insulin-like growth factor I, insulin-like growth factor-binding protein 3, discordant
Acromegaly is characterized by excess growth hormone (GH) concentrations and, subsequently, increased insulin-like growth factor I (IGF-I). In most cases it is caused by a GH-secreting pituitary adenoma (1), and it is most often diagnosed in middle-aged adults (2). Acromegaly is associated with metabolic impairments, including insulin resistance, diabetes mellitus, and cardiovascular disease, which increase morbidity and mortality (3, 4). Alterations in bone and calcium metabolism, including hypercalcemia and hyperphosphatemia, have also been described (5).
Diagnosis of the disease, as well as monitoring of treatment, is based on clinical signs and symptoms, together with biochemical assessments. Recommendations for the use of biomarkers differ between centers and have evolved over time. However, elevated IGF-I, increased random growth hormone (GHrandom) and/or insufficient suppression of GH after oral glucose load (GHnadir) are commonly employed (1, 6, 7). Factors such as body mass index (BMI), oral estrogen intake, malnutrition, diabetes mellitus, renal failure, and liver disease can affect GH and IGF-I secretion and action (8, 9).
Therefore, discrepancies between GH and IGF-I concentrations are common, and they may lead to delays in diagnosis or difficulties in adjustment of treatment (6, 10-12). It is also known that concentrations of these biomarkers not always correlated to clinical signs and symptoms during treatment (13). Additional difficulties come from significant analytical variability among different GH and IGF-I assays, making application of uniform guidelines for diagnosis and monitoring a challenge (14). Other GH-dependent proteins like IGF binding protein 3 (IGFBP-3) or acid labile subunit have also been assessed (15, 16), but new biomarkers with the potential to contribute to earlier detection of the disease or timely adjustments of treatment are highly desirable.
Kuro et al (17) first studied the klotho gene in transgenic mice with a defect in klotho gene expression, presenting with premature aging, shortened lifespan, growth retardation, increased calcium and phosphorus, and decreased insulin concentrations. Klotho is abundantly expressed in the kidney and the choroid plexus, and to a lesser extent in the parathyroid, thyroid, pancreas, and pituitary of both rodents and humans (18). The gene encodes a 130-kDa transmembrane protein called alpha klotho, which consists of a short intracellular and an extracellular domain, the latter containing 2 internal repeats (KL1 and KL2) (17, 19, 20). Three different alpha klotho protein isoforms exist: the full-length transmembrane form (mKL), a soluble form consisting of cleaved parts of the extracellular domain (KL1 attached to KL2 (KL1-KL2) or KL1 alone), and a secreted truncated form resulting from alternative splicing and consisting of KL1 only. The full-length mKL is a co-receptor of fibroblast growth factor 23 (FGF23), which regulates calcium and phosphorus homeostasis (19, 21). Preliminary data suggest that soluble alpha klotho (sαKL) may have endocrine function, but full characterization is pending (22). While mKL acts as an FGF23 co-receptor, previous studies suggest that sαKL may have effects on insulin physiology, suppressing insulin/IGF-I receptor phosphorylation and downstream signaling events, such as tyrosine phosphorylation of insulin receptor substrates and phosphoinositide 3-kinase, thereby inhibiting insulin and IGF-I signaling (22-24).
Data regarding sαKL in pituitary diseases are rare, but a few studies, in small cohorts, have suggested a role in acromegaly. Apparently, sαKL is elevated in treatment-naïve patients compared with healthy controls, it decreases after transsphenoidal surgery, and its concentrations appear to be related to quality of life (25-30). In contrast, sαKL does not change after surgery in patients with nonfunctioning pituitary adenoma (NFPA) or prolactinoma (26). Because of the small size of the studies, a systematic evaluation of potential biological confounders is missing. Furthermore, a systematic comparison of the potential value of sαKL in diagnosis and monitoring of acromegaly to biomarkers traditionally used to define disease activity is lacking.
We therefore have measured sαKL by a KL1-KL2–specific assay in well-defined cohorts of patients with acromegaly before and after surgical and medical treatment as well as in patients with NFPA and healthy controls. We particularly were interested:
(1) to compare concentrations of sαKL at diagnosis and changes with treatment with those in the traditional biomarkers (GHrandom, IGF-I, and IGFBP-3);
(2) to assess the agreement in definition of disease activity between sαKL and established criteria (GHrandom, GHnadir, and IGF-I), with a particular focus on cases with discrepant GH and IGF-I; and
(3) to study the potential impact of biological variables and somatostatin analogues (SSA) on sαKL concentrations.
Methods
Ethics
The study protocols were approved by the Ethics Committee of the Medical Faculty of the Ludwig Maximilians Universität München (LMU), Munich, Germany, and the Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil (approval numbers: 228-16 [Munich] and CAAE: 84291917.5.0000.5149 [Belo Horizonte], respectively). All study participants signed a consent form.
Patients With Acromegaly
In this retrospective study, a total of 109 patients with acromegaly, aged 21 to 84 years (54% female), were included. The crucial criterion for inclusion was availability of serum for re-analysis of GHrandom and IGF-I at the Munich laboratory (see below for details of laboratory analyses). Patients had been referred to either the LMU (n = 44) or to the UFMG (n = 65) between 2012 and 2017, and all underwent transsphenoidal surgery. Details of the subjects are provided in Supplemental Table 1 (33). Comparison between patients from the 2 centers did not reveal significant differences in terms of anthropometric variables, disease history, or disease severity. We did not include patients with renal insufficiency, GH replacement therapy, and any medication known to interfere with the GH/IGF-I axis, apart from first-generation SSA in the subgroup investigated during medical treatment after surgery. In this group, SSA dose was escalated either until patients were controlled, or to the maximum dose (octreotide 40 mg or lanreotide 120 mg) unless patients did not tolerate the dosage.
The diagnosis of acromegaly was harmonized between the 2 centers and was based on typical clinical findings, elevated GHrandom and IGF-I concentrations, and a pituitary tumor on sellar magnetic resonance imaging (31).
Data on GHnadir concentrations during oral glucose tolerance test (OGTT, 75 g) were available for 20 patients before surgery (Germany n = 13, Brazil n = 7), and for 26 patients after surgery (>12 weeks, without SSA; Germany n = 15, Brazil n = 11). The OGTT was not performed in patients exhibiting glycemic alteration or if the physician decided it was dispensable for diagnosis in view of other, unambiguous information.
Definition of Disease Activity and Subgroups of Patients With Acromegaly
Criteria to define disease activity based on biomarkers (GHrandom, GHnadir, IGF-I) vary between guidelines and studies (32). It was not the purpose of our study to evaluate the performance of traditional criteria or to establish a new criterion for GHnadir, but to compare sαKL in our patients to the traditional biomarkers. For this comparison, we could have used any definition of disease activity, as long as it would be uniformly applied to all patients. Among the various criteria reported in the literature, we decided to use GHrandom < 2.5 µg/L and IGF-I < 1.2 × upper limit of normal (ULN) to define “control” in our patients. Patients with GHrandom ≥ 2.5 µg/L and IGF-I ≥ 1.2 × ULN were defined as “uncontrolled,” while those with GHrandom ≥ 2.5 µg/L and IGF-I < 1.2 × ULN or GHrandom < 2.5 µg/L and IGF-I ≥ 1.2 × ULN were considered biochemically “discordant.” The rationale to use this criterion for our cohort was the observation that, in the subgroup of patients where GHnadir concentrations from OGTTs were available (n = 46), the best agreement for classification of disease activity by traditional biomarkers was found between GHnadir < 0.4 µg/L on the one hand, and GHrandom < 2.5 µg/L combined with IGF-I < 1.2 × ULN on the other.
Based on these criteria (GHrandom and IGF-I), the cross-sectional cohort of patients with acromegaly consists of the following subgroups: treatment-naïve patients with active disease before surgery (A; n = 29); patients after transsphenoidal surgery, without SSA, who were biochemically controlled (B1; n = 28), discordant (B2; n = 11), or uncontrolled (B3; n = 8); and patients after surgery on SSA treatment, who were biochemically controlled (C1; n = 17), discordant (C2, n = 11), or uncontrolled (C3; n = 5). Following surgery or initiation of SSA therapy, the assessment of the biochemical control was made after at least 6 months (median [interquartile range (IQR)]: 9 [6-12] months).
In 11 of the 28 patients who were cured by surgery alone, paired samples taken before and at least 6 months after surgery in the same subject were available for intra-individual longitudinal follow-up of sαKL concentrations.
Patients With NFPA
Twenty patients with NFPA aged 26 to 67 years (20% female) diagnosed by the presence of an adenoma-like pituitary tumor on magnetic resonance imaging without clinical or biochemical evidence of hormone hypersecretion were included in group D.
All patients with NFPA were recruited in Brazil, underwent pituitary surgery, and had immunohistochemistry results that showed follicle-stimulating hormone and/or luteinizing hormone expression (n = 15) or negativity for pituitary hormones expression (n = 5).
Healthy Subjects
Thirty-one healthy individuals with no history of pituitary disease, no evidence for acute or chronic disease, and normal medical examination (n = 18 from Germany and n = 13 from Brazil) served as controls (group E). Age and BMI were matched to the patient cohorts (details for all patient and control groups are provided in Supplemental Table 1) (33).
Laboratory Methods
In both centers, blood samples were collected from an antecubital vein, centrifuged and the serum was stored at −20 °C until analysis.
Human sαKL was measured at LMU Klinikum, Munich, Germany, or at the UFMG, Belo Horizonte, Brazil by the same solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) (Immuno Biological Laboratories, Hamburg, RRID:AB_2750859), as per manufacturer’s instructions (34, 35). The measurement range of the assay is 94 to 6000 pg/mL. If sαKL concentrations were above the limit of quantification of the assay, samples were re-assayed after 1:4 dilution (4 patients). In this study, across the 2 laboratories, the intra-assay coefficients of variation (CV) were 3.1%, 2.7%, and 3.5% at concentrations of 2969, 757, and 187 pg/mL, respectively. At the same concentrations, inter-assay CV were 2.9%, 6.5%, and 11.4%, respectively. Mean sαKL concentrations in the control subjects (n = 31; mean 773 pg/mL; range, 202-1602 pg/mL) were comparable to data previously published for this assay (mean 562 pg/mL; reference interval, 239-1266 pg/mL) (34). We therefore used the published reference interval to convert sαKL concentrations into a multiple of the ULN in addition to reporting concentrations.
For initial diagnosis, samples were analyzed at the respective local laboratories. However, for this study, all samples were sent to the same laboratory (Endocrine Laboratory at LMU Klinikum, Munich, Germany), where GHrandom, IGF-I, and IGFBP-3 were measured in 1 analytical run using the automated IDS-iSYS chemiluminescence immunoassays (CLIA, Immunodiagnostic Systems, Boldon, UK). The GH and IGF-I assays are calibrated against the most recent recombinant standards (98/574 for GH and 02/254 for IGF-I), and inter-assay CV ranged from 1.1% to 3.4% for GH and 4.0% to 8.7% for IGF-I. The limit of quantification was 0.04 µg/L for GH and 8.8 µg/L for IGF-I. Linear range for the IGFBP-3 assay is 80 to 10 000 µg/L. Extensive characterization of the analytical methods and reference intervals have been published elsewhere (9, 36-38). Results for IGF-I and IGFBP-3 are reported as concentrations and as a multiple of the ULN based on the published reference intervals.
Due to volume limitations, it was not possible to repeat measurements of GHnadir during OGTT centrally. Therefore, while GHnadir was measured by the IDS-iSYS in Germany, the Liaison (Diasorin, Sallugia, Italy) was used in Brazil. Both assays are ultrasensitive GH assays, using the latest standard, and the same cutoff of 0.4 µg/L was used to define appropriate suppression (9). However, because the GH assays were different, GHnadir concentrations were not used as the main criterion to categorize disease activity for our analysis.
Serum carboxy terminal FGF23 was measured in Munich by ELISA (Biomedica, Wien, Austria), following the manufacturer’s instructions. The intra- and inter-assay CV were <12% and <10%, respectively, and the limit of quantification was 0.08 pmol/L.
Other biochemical data, such as serum creatinine, prolactin, and calcium metabolism (calcium, phosphorus, 25-OH-vitamin D, alkaline phosphatase, parathyroid hormone) were derived from routine analytical methods of the respective central laboratories and retrospectively obtained from medical records.
Statistical Analysis
Continuous variables are presented using medians and interquartile ranges (IQR) after confirming the non-Gaussian distribution by Shapiro-Wilk and the Kolmogorov Smirnov test. For comparisons between different groups (subgroups of patients with acromegaly, NFPA, and healthy controls), we used Kruskal-Wallis test followed by Dunn’s multiple comparisons post-test, while Wilcoxon test was used to compare the same patients, before and after surgery. Differences between 2 groups (eg, micro- vs macroadenoma) were analyzed by nonparametric Mann-Whitney test. Correlations between sαKL and biological confounders were calculated by Spearman’s rank correlation. Receiver operating characteristic (ROC) curve analysis was performed to analyze performance of sαKL to discriminate between patient groups at various cutoffs. Fisher’s exact test was used to calculate positive and negative predictive values. P values of <0.05 were considered significant. All analyses were performed by GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA).
Results
Traditional Biomarkers and Disease Activity
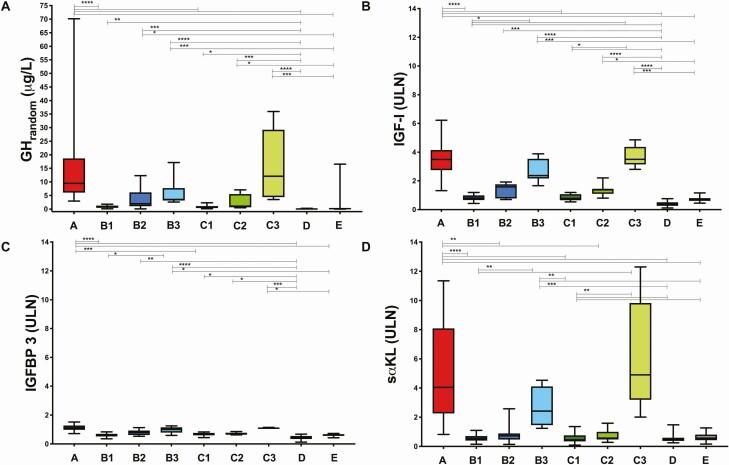
Random GH
Treatment-naïve patients (group A) had high GHrandom concentrations (median [IQR]: 9.47 µg/L [8.07-18.63]; Fig. 1A). Lower GHrandom concentrations were seen after surgical and medical disease control (B1: 0.74 µg/L [0.47-1.29] and C1: 0.84 µg/L [0.38-1.12]; A vs B1 and C1, P < 0.0001 for both comparisons). However, GHrandom did not discriminate controlled from uncontrolled and discordant patients after surgery (P > 0.05). The lowest GHrandom was seen in patients with NFPA (D: 0.05 µg/L [0.05-0.09]) and healthy subjects (E: 0.16 µg/L [0.06-0.36]). Notably, 3 healthy subjects (1 male, 2 female) had GHrandom > 2.5 µg/L, but normal IGF-I, and therefore formally would have been classified as “discordant.”
Figure 1.
A, GHrandom concentrations (µg/L); B, IGF-I (× ULN); C, IGFBP-3 (× ULN); and D, sαKL (× ULN) represented by medians and interquartile range as well as minimum-maximum ranges in treatment-naïve patients with acromegaly before (A) and after surgery controlled without medication (B1), discordant (B2) and uncontrolled (B3) or on SSA controlled (C1), discordant (C2) and uncontrolled (C3), NFPA (D) and healthy controls (E). For reasons of readability, we only indicate the significance level one time if it was the same for different comparisons (* P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001). Abbreviations: GHrandom, random growth hormone; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor-binding protein 3; sαKL, soluble alpha klotho; ULN, upper limit of normal. We intentionally use an identical range and scale for the y-axis in B, C, and D to highlight the differences in the order of magnitude of the changes in the parameters. Graphs with optimized scales for each parameter are provided in Supplemental Fig. 2.
IGF-I
Treatment-naïve patients before surgery (group A) all had elevated IGF-I concentrations: 754 µg/L (485-938), 3.48 × ULN (2.74-4.15) (Fig. 1B), which normalized after surgery in patients with controlled disease without medication (B1: 144 µg/L [126-201], 0.79 × ULN [0.66-0.99]) or on SSA (C1: 168 µg/L [128-208], 0.78 × ULN [0.65-1.07]; A vs B1 and C1, P < 0.0001 for both comparisons). There was no difference in IGF-I between patients classified as controlled by surgery alone or by surgery and SSA (B1 vs C1, P > 0.99). In contrast, in the groups classified as “uncontrolled,” IGF-I concentrations remained largely unchanged after surgery (B3: 488 µg/L [438-700], 2.36 × ULN [2.16-3.52] and C3: 717 µg/L [624-858], 3.49 × ULN [3.14-4.37]; A vs B3 and C3, P > 0.99 for both comparisons), and remained significantly higher as compared with controlled patients, patients with NFPA, and healthy controls (B3 vs B1, C1, D, and E, P = 0.02, P = 0.045, P < 0.0001, and P = 0.0005, respectively; C3 vs B1, C1, D, and E, P = 0.02, P = 0.04, P < 0.0001, and P = 0.0009, respectively). IGF-I concentrations tended to drop in the patients classified as “discordant” after surgery (B2: 323 µg/L [169-394], 1.58 × ULN [0.78-1.76], C2: 284 µg/L [249-336], 1.40 × ULN [1.10-1.42]), but the change failed to reach significance (B2 vs A, P = 0.27; C2 vs A, P = 0.86). IGF-I concentrations in discordant patients also did not differ from controlled or uncontrolled patients after surgery (C2 vs B1, P = 0.75 and P > 0.99 for all other comparisons between B2 vs C2 and B2 and C2 vs B1, C1, B3, C1). However, IGF-I in “discordant” cases remained higher than in NFPA and healthy controls (P < 0.0001). When excluding patients with GH ≥ 2.5 μg/L but IGF-I < 1.2 × ULN, IGF-I tended to be higher in discordant patients without SSA than in healthy controls (B2 vs E, P = 0.049). Moreover IGF-I remained higher in discordant patients as compared with NFPA (B2, C2 vs NFPA, P < 0.001 and P = 0.004, respectively). IGF-I had a tendency to be lower in NFPA as compared with healthy controls although it did not reach statistical significance (D: 58 µg/L [48-88] vs E: 157 µg/L [113-189], P = 0.05 and D: 0.36 × ULN [0.26-0.51] vs E: 0.69 × ULN [0.61-0.80], P = 0.34).
GHnadir During OGTT
OGTT data were available in 20 (of 29) treatment-naïve patients before surgery (group A), and all had GHnadir > 0.4 µg/L, elevated GHrandom, and elevated IGF-I. After surgery, OGTT data were available for 16 (of 28) patients classified as “biochemically controlled” without SSA (group B1), and GHnadir was <0.4 µg/L in all of them. In contrast, all 3 patients with available OGTT data from group B3 (“uncontrolled,” n = 8) had GHnadir concentrations > 0.4 µg/L. In the group classified as “discordant” (group B2, n = 11), OGTT data after surgery were available in 7 patients. In all discordant cases, a categorization based on GHnadir would have concurred with the categorization based on IGF-I: 3 of the patients had elevated IGF-I and normal GHrandom, and all 3 had elevated GHnadir (>0.4 µg/L). Four of the patients had elevated GHrandom and normal IGF-I, and all of these 4 had normal GHnadir (<0.4 µg/L).
IGFBP-3
Before surgery, IGFBP-3 concentrations in patients with acromegaly were slightly elevated (group A: 6388 µg/L [5506-7354], 1.12 × ULN [0.98-1.28]), and higher compared with patients with NFPA (group D: 2385 µg/L [1965-3180], 0.42 × ULN [0.34-0.55]), healthy controls (group E: 3692 µg/L [3389-4029], 0.63 × ULN [0.56-0.67]; A vs D and E, P < 0.0001). IGFBP-3 was also higher in group A compared with patients controlled after surgery (A vs B1, P < 0.0001; A vs C1, P = 0.004; Fig. 1C). Following surgery, IGFBP-3 concentrations turned to the normal range in all patients (B1: 3660 µg/L [2972-3986], 0.63 × ULN [0.52-0.70]; B2: 4784 µg/L [3483-5549], 0.80 × ULN [0.80-1.07]; B3: 5789 µg/L [4616-6466], 1.00 × ULN [0.78-1.15]; C1: 3918 µg/L [3468-4353], 0.69 × ULN [0.60-0.76]; C2: 4050 [3690-4691], 0.69 × ULN [0.64-0.78] and C3 (6330 µg/L [6015-6565], 1.09 × ULN [1.05-1.14]), but remained higher in uncontrolled as compared with controlled patients without medication, with NFPA, and healthy controls (B3 vs B1, D, and E, all P < 0.05).
Soluble Alpha Klotho and Disease Activity
Treatment-naïve patients before surgery (A) had very high sαKL concentrations (5109 pg/mL [2859-10 232]; 4.04 × ULN [2.26-8.08]), which were significantly elevated compared to the published reference interval (239-1266 pg/mL) (34). They were also significantly higher compared with patients with NFPA (D: 584 pg/mL [475-756], 0.46 × ULN [0.38-0.59]) and healthy controls (E: 724 pg/mL [538-1031], 0.57 × ULN [0.43-0.81]; A vs D and E, P < 0.0001 for both comparisons; Fig. 1D). Following surgery, sαKL significantly decreased and returned to normal in all but 1 patient classified as controlled by surgery alone (B1: 666 pg/mL [510-901], 0.52 × ULN [0.40-0.71]), and all but 2 patients controlled on SSA (C1: 565 pg/mL [437-960], 0.45 × ULN [0.34-0.76]; A vs B1 and C1, P < 0.0001 for both comparisons). sαKL concentrations in controlled acromegaly were no longer different from those in patients with NFPA (D) or healthy controls (E) (B1 vs C1, D, and E; C1 vs D and E; D vs E, P > 0.99 for all comparisons). In contrast, postoperative patients who remained uncontrolled—either without medication (B3: 3063 pg/mL [1844-5199], 2.42 × ULN [1.46-4.10]) or on SSA treatment (C3: 6206 pg/mL [4043-12 455], 4.90 × ULN [3.19-9.83])—all had elevated sαKL concentrations, which were also significantly higher compared with patients who were controlled with or without SSA treatment, patients with NFPA, and healthy controls (B3 vs B1 and C1, P = 0.004; B3 vs D, P = 0.0009; B3 vs E, P = 0.009; C3 vs B1, P = 0.004; C3 vs C1, P = 0.003; C3 vs D, P = 0.001; C3 vs E, P = 0.008). In the discordant groups (B2: 920 pg/mL [593-1126], 0.73 × ULN [0.47-0.89] and C2: 723 pg/mL [610-1262], 0.57 × ULN [0.48-1.00]), sαKL concentrations were significantly lower than in patients before surgery (A vs B2 and C2, P = 0.009 and P = 0.001, respectively), but did not differ from controlled or uncontrolled patients after surgery (without medication or on SSA), patients with NFPA, or healthy controls (B2 vs B1, C1, D, and E, P > 0.99; B2 vs B3, P = 0.62; B2 vs C3, P = 0.29; C2 vs B1, C1, D, and E, P > 0.99; C2 vs B3, P = 0.20; C2 vs C3, P = 0.10). However, when comparing patients regardless of the use of SSA, and thereby increasing group sizes, discordant patients had significantly lower sαKL concentrations than uncontrolled patients (B2 + C2 vs A, P < 0.0001; B2 + C2 vs B3 + C3, P = 0.002). Accordingly, in discordant patients, sαKL concentrations were within the reference interval in 18 of the 22 patients: in 9 discordant patients with normal sαKL (mean 0.5 × ULN), GHrandom was elevated (mean 6.3 µg/L; range, 3.0-12.3 µg/L), but IGF-I was normal (mean 0.9 × ULN). In the other 9 cases with normal sαKL (mean 0.7 × ULN), GHrandom was normal (mean 1.0 µg/L; range, 0.5-2.0 µg/L), but IGF-I was elevated (mean 1.7 × ULN). Notably, all 4 cases from the discordant group where sαKL was elevated (mean 1.7 × ULN), also had elevated IGF-I (mean 1.7 × ULN), but normal GHrandom (range, 0.7-2.0 µg/L). All of the 20 patients with GHnadir < 0.4 µg/L and normal IGF-I after surgery also had normal sαKL, although 4 of them had elevated GHrandom.
Correlation of Soluble Alpha Klotho to Traditional Biomarkers of Disease Activity
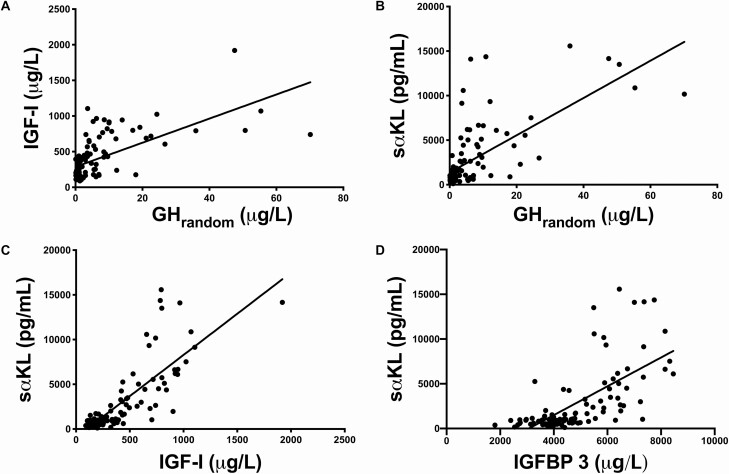
Over a wide range of concentrations, and in all groups, the strongest correlation was found between sαKL and IGF-I (rs = 0.80, P < 0.0001). GHrandom was also correlated to IGF-I (rs= 0.67, P = 0.0001) and to sαKL (rs= 0.68, P = 0.0001). sαKL was also correlated to IGFBP-3 (rs= 0.72, P < 0.0001; Fig. 2). Notably, in patients exhibiting GHrandom concentrations > 10 µg/L (n = 17), there was no more correlation between GHrandom and IGF-I (rs= 0.29, P = 0.25), while correlation between GHrandom and sαKL was maintained (rs = 0.50, P = 0.03).
Figure 2.
A, Correlation between IGF-I (µg/L) and GHrandom (µg/L) in all patients with acromegaly; rs= 0.67, P < 0.0001; B, Correlation between sαKL (pg/mL) and GHrandom (µg/L) in all patients with acromegaly; rs= 0.68, P < 0.0001; C, Correlation between sαKL (pg/mL) and IGF-I (µg/L) in all patients with acromegaly; rs= 0.80, P < 0.0001; D, Correlation between sαKL (pg/mL) and IGFBP-3 (µg/L) in all patients with acromegaly; rs = 0.72, P < 0.0001. Abbreviations: GHrandom, random growth hormone; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor-binding protein 3; sαKL, soluble alpha klotho.
In the subgroup of patients with OGTT data available (n = 46), GHnadir correlated best to sαKL and IGF-I (rs = 0.83 and P < 0.0001 for both), although correlation of GHnadir to GHrandom (rs= 0.80) and IGFBP-3 (rs= 0.74) was still significant (P < 0.0001 for both).
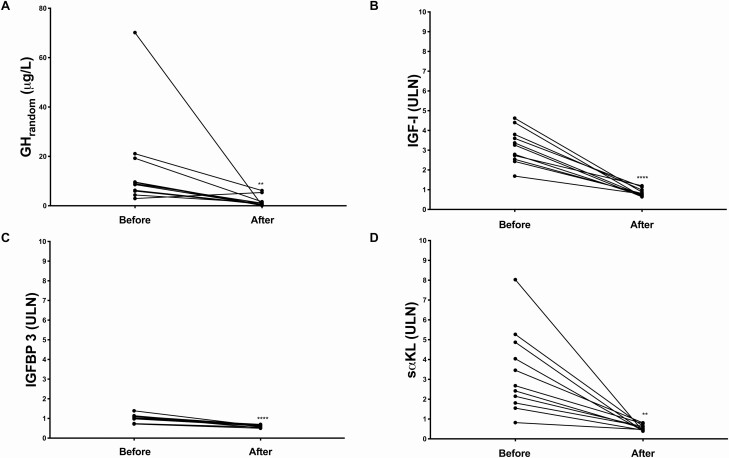
Intra-Individual Comparisons Before and After Surgery
Eleven patients were evaluated longitudinally before and after pituitary surgery (Fig. 3). In all 11 patients, surgery led to decreased clinical disease activity as perceived by patients and physicians. Biochemically, all patients were controlled after surgery, with normalization of both GHrandom (preoperative: 8.94 µg/L [5.97-19.25] vs postoperative: 0.69 µg/L [0.37-1.63], P = 0.002) and IGF-I concentrations (preoperative: 689 µg/L [467-841], 3.26 × ULN [2.54-3.8] vs postoperative: 147 µg/L [126-203], 0.78 × ULN [0.69-0.99], P < 0.0001). IGFBP-3 was marginally elevated before (5898 µg/L [5748-6499]; 1.04 × ULN [0.98-1.12]) and normalized after surgery (3483 µg/L [3244-3827], 0.62 × ULN [0.56-0.67), P < 0.0001). Concentrations of sαKL were clearly elevated before (3389 pg/mL (2295-6160), 2.68 × ULN (1.81-4.87) and normalized after surgery (667.6 pg/mL (510.4-804.1), 0.53 × ULN (0.40-0.64), P = 0.001; Fig. 3). The mean percentage decrease in GHrandom, IGF-I and sαKL after successful surgery was comparable (GHrandom: −90.1% [73-96], IGF-I: −75.2% [68.3-80.9], and sαKL: −76.7% [71.1-90.0]; GHrandom vs IGF-I, P = 0.41, GHrandom and IGF-I vs sαKL, P > 0.99 for both comparisons). The change in IGFBP-3 (−38.9% [32.1-48.5]) was significantly smaller compared with the change seen in the other biomarkers (P < 0.0001 for all comparisons).
Figure 3.
A, GHrandom (µg/L); B, IGF-I (× ULN); C, sαKL (× ULN) and D, IGFBP-3 (× ULN) in patients with acromegaly (n = 11) before and after surgery. ** P < 0.01, **** P < 0.0001. Abbreviations: GHrandom, random growth hormone; IGF-I, insulin-like growth factor I; IGFBP-3, insulin-like growth factor-binding protein 3; sαKL, soluble alpha klotho; ULN, upper limit of normal. We intentionally use an identical range and scale for the y-axis in B, C, and D to highlight the differences in the order of magnitude of the changes in the parameters.
Cutoffs for αKL Concentrations From ROC Analysis
We established ROC curves (Fig. 4) to analyze the performance of sαKL in discriminating subjects from different groups classified by the traditional biomarkers (GHrandom and IGF-I, excluding subjects with discrepant results for these biomarkers).
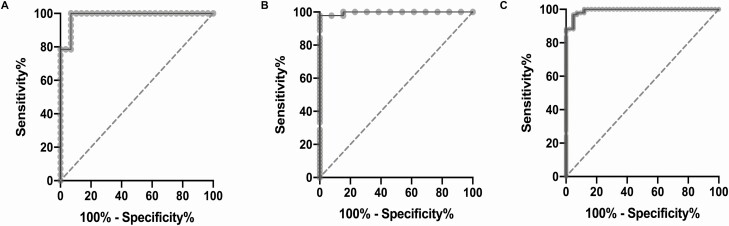
Figure 4.
A, Soluble alpha klotho (sαKL) ROC curve: before surgery, treatment-naïve (A) vs healthy controls (E). Cutoff: 1641 pg/mL, AUC 0.98 (0.96-1.00), P < 0.0001. B, Soluble alpha klotho (sαKL) ROC curve: controlled (B1, C1) vs uncontrolled (B3, C3) acromegaly after surgery. Cutoff: 1548 pg/mL, AUC 0.99 (0.98-1.00), P < 0.0001. C, Soluble alpha klotho (sαKL) ROC curve: controlled acromegaly after surgery (B1, C1), NFPA (D) and healthy controls (E) vs uncontrolled patients before or after surgery (A, B3, C3). Cutoff: 1548 pg/mL, AUC 0.99 (0.98-1.00), P < 0.0001.
The area under the curve (AUC) for sαKL in treatment-naïve patients before surgery (A) vs healthy controls (E) was 0.98 [95% CI, 0.96-1.00], P < 0.0001, and a cutoff for sαKL at 1641 pg/mL resulted in 100% sensitivity (CI, 87.9%-100.0%) with 93.1% specificity (78.0%-98.8%) (Fig. 4A). All 31 healthy subjects, but only 2/29 (6.9%) treatment-naïve patients before surgery, had sαKL concentrations below the cutoff. The best cutoff concentration (1641 pg/mL) did not change when patients with NFPA (D) and healthy controls (E) were pooled before comparing to patients (A), with 97.9% (89.1%-99.9%) sensitivity and 93.1% (78.0%-98.8%) specificity. One patient with NFPA had sαKL above the cutoff.
After surgery, the best cutoff for sαKL to discriminate between controlled (B1, C1) and uncontrolled (B3, C3) patients was in a similar concentration range (1548 pg/mL, AUC 0.99 [0.98-1.00], P < 0.0001), with 97.8% (88.4%-99.9%) sensitivity and 100% (77.1%-100%) specificity (Fig. 4B).
The same cutoff of 1548 pg/mL was best to discriminate between all controlled subjects (combining patients with controlled acromegaly [B1, C1], patients with NFPA [D], and healthy subjects [E]) and all uncontrolled subjects (combining patients with uncontrolled acromegaly before and after surgery [A, B3, C3]), with AUC of 0.99 (0.98-1.00, P < 0.0001), sensitivity of 96.8% (90.9%-99.1%), and specificity of 95.2% (84.2%-99.2%) (Fig. 4C).
For discrimination between all controlled and uncontrolled subjects, both cutoffs (1641 or 1548 pg/mL) provided very high negative predictive value (96.8% and 97.8%, respectively), while still holding high positive predictive value (91% and 93%, respectively).
The vast majority of the 25 subjects classified as discordant by the traditional biomarkers (22 patients with acromegaly, 3 healthy controls) exhibited concentrations of sαKL below the cutoffs of 1641 pg/mL (22/25) or 1548 pg/mL (21/25), respectively. All discordant subjects with sαKL above the cutoffs also had elevated IGF-I, despite normal GHrandom.
Soluble Alpha Klotho and Biological Variables
In treatment-naïve patients before surgery, sαKL concentrations were not different between micro- (7298 pg/mL [2460-11 527]) and macroadenomas (4508 pg/mL [3065-7515], P = 0.88). In line with this, concentrations of GHrandom and IGF-I also were not different between micro- and macroadenomas (GHrandom: 4.24 µg/L [3.77-25.65] and 10.00 µg/L [8.47-19.25], P = 0.15; IGF-I: 648.5 µg/L [402.8-751] and 841.4 µg/L [606-966], P = 0.08).
Concentrations of sαKL were not different between female and male in all patients with acromegaly, in the subgroups with uncontrolled, discordant, or controlled acromegaly, or when comparing subgroups before surgery, after surgery without medication, or with medical treatment (P > 0.05 for all comparisons). Concentrations of sαKL were also not different between female and male in patients with NFPA and in healthy controls (P > 0.05 for both comparisons). We also found no significant correlations of sαKL concentrations to BMI and markers of calcium or bone metabolism (25-OH-vitamin D, FGF23, total calcium, parathyroid hormone, alkaline phosphatase, and phosphorus) in any of the groups of patients with acromegaly. sαKL and BMI or FGF23 concentrations did also not correlate in patients with NFPA or healthy controls (Supplemental Tables 1-3) (33).
We observed a weak negative correlation of sαKL concentrations with age when analyzing all patients with acromegaly together (rs= −0.22, P = 0.02). Among the subgroups of patients, the weak correlation was significant only in uncontrolled (rs= −0.33, P = 0.04), but not in controlled and discordant patients (P > 0.05 for both comparisons). There was no correlation of sαKL concentrations with age in patients with NFPA and in healthy controls (P > 0.05 for both). Furthermore, when patients with acromegaly, NFPA, and healthy controls were divided into age groups by decade, there was no difference in sαKL concentrations between the age groups (P > 0.05 for all [Supplemental Fig. 1]) (33).
Discussion
The aim of our study was to evaluate the potential of sαKL to assist in the assessment of disease activity in patients with acromegaly. We therefore measured concentrations of sαKL in a cohort at diagnosis and after surgical with or without medical therapy and compared them to concentrations of the traditional biomarkers GHrandom, GHnadir, IGF-I, and IGFBP-3. We were particularly interested to understand the regulation of sαKL in patients classified as controlled, uncontrolled, or discordant by traditional biochemical criteria.
As expected from previous reports (25-29), sαKL was grossly elevated at diagnosis in our patients, while it was normal in patients with NFPA and healthy subjects. On average, concentrations of sαKL in treatment-naïve patients exceeded the upper limit of the reference interval by a factor of 4.04, which was comparable, though slightly greater than the average elevation of IGF-I (3.48 × ULN), and much greater than that of IGFBP-3 (1.12 × ULN). We also noted that the highest elevation of sαKL in an individual (11.4 × ULN) clearly exceeded the highest elevation of IGF-I (6.2 × ULN), and that the upper limit of the IQR for all treatment-naïve patients was significantly higher for sαKL than IGF-I (8.08 vs 4.15 × ULN). This confirms that sαKL is a biomarker particularly sensitive to excess growth hormone. In line with this, following successful control of disease activity by surgery alone or surgery and SSA treatment, the drop in sαKL mirrored that in GHrandom and IGF-I. Patients with NFPA presented with normal IGF-I and sαKL concentrations, although IGF-I concentrations tended (P = 0.05) to be slightly lower compared to healthy subjects. We acknowledge that 9/20 patients with NFPA were in treatment for 2 or more pituitary deficiencies (such as hypogonadism, hypothyroidism, and adrenal insufficiency). Although not formally tested, this suggests they could also have GH deficiency. However, even when excluding these 9 patients from the analysis, the tendency toward lower IGF-I in NFPA remained visible.
The data on intra-individual changes in the traditional biomarkers and in sαKL associated with successful surgery (Fig. 3) further illustrate that the dynamics in changes are at least as pronounced as for IGF-I, and far greater than for IGFBP-3. Furthermore, in 94% of the patients classified as controlled by the traditional biomarkers, sαKL was also normal, and very close to the upper limit of the current reference interval in the remaining 6% (1.1, 1.2, and 1.4 × ULN, respectively).
Normalization of sαKL was seen to the same extent in patients controlled by surgery alone or by surgery and SSA treatment. This is in line with preliminary data from a smaller study reporting a decrease in sαKL concentrations in patients who were biochemically controlled with octreotide after surgery (28). In contrast to normalization in controlled patients, sαKL remained elevated in all our patients who were defined as uncontrolled by GHrandom and IGF-I after surgery with or without SSA.
Patients in whom biochemical information from GH and IGF-I does not agree represent a particular challenge in diagnosis and monitoring of acromegaly. Such “discordant” findings have raised considerable attention (10, 12). Our study included 22 patients from this category, exhibiting elevated GHrandom (≥2.5 µg/L) and normal IGF-I (<1.2 × ULN) or vice versa. All these cases were postoperative, and half of them were on SSA treatment. Notably, in the vast majority of the discrepant cases (18/22), sαKL was in the normal range, suggesting efficacy of treatment. This is supported by clinical records, which report no, or only very mild, symptoms in these patients, with headache only reported by 1/18 patients. In 50% of the discordant cases with normal sαKL, it agreed with normal GHrandom, and in 50% with normal IGF-I. Both GHrandom and IGF-I tended to be lower in patients from the discrepant groups following treatment, but due to the large overlap with concentrations seen in treatment-naïve patients, the change failed to reach statistical significance. In contrast, sαKL concentrations in the discordant group were significantly lower than in uncontrolled patients before surgery, and in the whole group of uncontrolled patients after surgery (without and with SSA). On the other hand, sαKL concentrations in the discordant group were no longer different from those in controlled patients. This adds further evidence that sαKL is particularly sensitive to changes in GH secretory status. Our data support the notion that it could be helpful in discrepant cases since normal klotho is reassuring in situations where IGF-I is normal, but GHrandom is still elevated. If this can be used to improve prediction of long-term outcome, or for early detection of recurrence, must be investigated in prospective studies. It is noteworthy that in all the remaining 4 discordant cases, where sαKL remained elevated after treatment, IGF-I also remained elevated (despite normal GHrandom), and all 4 patients reported severe symptoms, including headache, and had a worse quality of life compared with patients with normal sαKL. This is of interest since a recent study (29) had reported that changes in sαKL during successful treatment correlate with improvements particularly in quality of life and headache. The mechanisms are unclear, but it seems that sαKL could have particular relevance as a biomarker of central effects of GH excess.
We found a strong correlation between concentrations of sαKL and both IGF-I and GHrandom concentrations. It is known from previous reports that GHrandom correlates with IGF-I at concentrations up to 10 µg/L, while IGF-I tends to plateau for higher GHrandom (>10 µg/L) (39, 40), where the correlation is lost. While confirming these observations, we showed that the strong correlation with sαKL remained significant even at GHrandom concentrations above 10 µg/L. This suggests that sαKL might have a broader dynamic range compared to IGF-I, and therefore might be better suited than IGF-I to reflect grossly elevated GH concentrations. We also noted a strong correlation of sαKL to GHnadir concentrations in the patients where OGTT data were available, further supporting its close association with disease activity.
ROC analysis suggested that a cutoff of 1641 pg/ml for sαKL can discriminate treatment-naïve patients before surgery (group A) from healthy controls or all control groups (healthy controls and NFPA, groups E and D) with 100% sensitivity and 93% specificity. Notably, for these groups, the best cutoff did not change when we repeated the analysis based on the classification of patients with a stricter cutoff for GHrandom (≤1.0 µg/L instead of <2.5 µg/L). With the lower cutoff for GHrandom, however, 17 subjects after surgery would move from the “controlled group” to the “discordant group”—all because of elevated GHrandom, but normal IGF-I. Since sαKL shows strong agreement with IGF-I, discrepancies between normal sαKL and elevated GHrandom also would have become more frequent.
In contrast to a significant difference between patients with controlled and uncontrolled acromegaly, sαKL did not differ between patients with micro- and macroadenomas before surgery, and there was no association to prolactin concentrations. The source of excess sαKL in acromegaly remains to be identified (41), and our observations suggest that sαKL is only related to biochemical activity of acromegaly, and not to subtype or size of the adenoma itself. This would make it less likely that the pituitary adenoma directly secretes excess sαKL. However, it must be noted that even though we investigated the largest cohort published so far, the groups still are small, and GHrandom and IGF-I concentrations were also not different between micro- and macro-adenomas.
Klotho has been studied in the context of calcium metabolism, and its action is known to be related to FGF23 (23). On the other hand, acromegaly has been associated with alterations in bone and calcium metabolism (5, 42). We acknowledge our study was not designed to study bone and calcium metabolism in detail. However, the absence of any association of sαKL concentrations and its changes with 25-OH-vitamin D, FGF23, parathyroid hormone, alkaline phosphatase, total calcium, or phosphorus in our cohort makes it unlikely that the strong association of sαKL with disease activity in acromegaly is just secondary to changes in calcium metabolism associated with treatment.
Interpretation of traditional biomarkers of disease activity for acromegaly can be challenging in view of a variety of biological confounders like age, sex, BMI, and liver and kidney function (14, 37, 43-48). Our study showed negligible impact of sex, age, and BMI on sαKL concentrations. Since klotho was first described in the context of longevity (17), one might expect its concentrations would correlate with age (19, 49). However, while a weak negative correlation has been reported by 1 study (34), many others could not confirm this (50, 51) and reported similar sαKL concentrations throughout life (26, 28, 34, 41, 52-54). The few previous studies in patients with acromegaly also did not report a correlation between sαKL and age (28, 55). Therefore, in contrast to traditional biomarkers of disease activity like IGF-I (37) or GHnadir (9), there might be no need to adjust reference intervals for sαKL to age, sex, or BMI, potentially facilitating its use in a clinical setting. We acknowledge our own control group was small, and even the largest study about sαKL concentrations in healthy subjects available to date comprises of 142 subjects only (34). The upper limit of the reference interval derived from this study (1266 pg/mL) was considerably lower than the cutoff we identified by ROC analysis to best discriminate patients with active disease from healthy subjects or controlled patients (around 1600 pg/mL), suggesting that the reference interval in a healthy population needs to be defined more carefully. To explore the full potential of sαKL as a biomarker of GH action, it seems necessary to conduct larger studies to establish robust reference intervals.
In summary, our data support the notion that sαKL is a promising biomarker to assess disease activity in patients with acromegaly with high sensitivity and specificity. Its close association with GH secretory status, the large dynamic range, and the robustness toward a wide spectrum of biological confounders could make it a helpful tool, particularly in cases where measurements of GH and IGF-I provide discrepant information.
Acknowledgments
We are grateful to Mr. Mohammed Razzaque for his contribution, as related to the discussion regarding the klotho kits and a possible link between klotho and the GH axis.
Financial Support: Coordenação de Aperfeiçoamento Pessoal de Nível Superior—CAPES (JROLS), Fundação de Amparo à Pesquisa de Minas Gerais—Fapemig and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (AROJr). Part of this study was supported by a grant (120000R319) from Partnership for Clean Competition (PCC), Colorado, USA (MB).
Glossary
Abbreviation
- BMI
body mass index
- FGF23
fibroblast growth factor 23
- GH
growth hormone
- IGF-I
insulin-like growth factor I
- IGFBP-3
insulin-like growth factor–binding protein 3
- IQR
interquartile range
- LMU
Ludwig Maximilians Universität München
- NFPA
nonfunctioning pituitary adenoma
- OGTT
oral glucose tolerance test
- sαKL
soluble alpha klotho
- SSA
somatostatin analogues
- ULN
upper limit of normal
Additional Information
Disclosures: S.S. has received personal fees and grants from Novartis, Ipsen, Hexal/Sandoz, and Pfizer, has served as an advisory board member for Novartis, and has received honoraria for speaking at symposia for Novartis. A.R.O. Jr is currently an employee at Ipsen Bioscience in Cambridge/MA, USA. M.B. has received speaker and/or consultancy fees from Chiasma, Novartis, Pfizer, and Ipsen. The other authors have no conflict of interest to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Giustina A, Barkhoudarian G, Beckers A, et al. Multidisciplinary management of acromegaly: A consensus. Rev Endocr Metab Disord. 2020;21(4):667-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Petersenn S, Buchfelder M, Gerbert B, et al. ; Participants of the German Acromegaly Register . Age and sex as predictors of biochemical activity in acromegaly: analysis of 1485 patients from the German Acromegaly Register. Clin Endocrinol (Oxf). 2009;71(3):400-405. [DOI] [PubMed] [Google Scholar]
- 3. Dekkers OM, Biermasz NR, Pereira AM, Romijn JA, Vandenbroucke JP. Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2008;93(1):61-67. [DOI] [PubMed] [Google Scholar]
- 4. Gadelha MR, Kasuki L, Lim DS, Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev. 2018;40(1):258-332. [DOI] [PubMed] [Google Scholar]
- 5. Mazziotti G, Lania AGA, Canalis E. MANAGEMENT OF ENDOCRINE DISEASE: Bone disorders associated with acromegaly: mechanisms and treatment. Eur J Endocrinol. 2019;181(2):R45-R56. [DOI] [PubMed] [Google Scholar]
- 6. Fleseriu M, Biller BMK, Freda PU, et al. A Pituitary Society update to acromegaly management guidelines. Pituitary. 2021;24(1):1-13. doi: 10.1007/s11102-020-01091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melmed S, Bronstein MD, Chanson P, et al. A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schilbach K, Strasburger CJ, Bidlingmaier M. Biochemical investigations in diagnosis and follow up of acromegaly. Pituitary. 2017;20(1):33-45. [DOI] [PubMed] [Google Scholar]
- 9. Schilbach K, Gar C, Lechner A, et al. Determinants of the growth hormone nadir during oral glucose tolerance test in adults. Eur J Endocrinol. 2019;181(1):55-67. [DOI] [PubMed] [Google Scholar]
- 10. Butz LB, Sullivan SE, Chandler WF, Barkan AL. “Micromegaly”: an update on the prevalence of acromegaly with apparently normal GH secretion in the modern era. Pituitary. 2016;19(6):547-551. [DOI] [PubMed] [Google Scholar]
- 11. Ribeiro-Oliveira A Jr, Faje A, Barkan A. Postglucose growth hormone nadir and insulin-like growth factor-1 in naïve-active acromegalic patients: do these parameters always correlate? Arq Bras Endocrinol Metabol. 2011;55(7):494-497. [DOI] [PubMed] [Google Scholar]
- 12. Alexopoulou O, Bex M, Abs R, T’Sjoen G, Velkeniers B, Maiter D. Divergence between growth hormone and insulin-like growth factor-i concentrations in the follow-up of acromegaly. J Clin Endocrinol Metab. 2008;93(4):1324-1330. [DOI] [PubMed] [Google Scholar]
- 13. Geraedts VJ, Andela CD, Stalla GK, et al. Predictors of quality of life in acromegaly: no consensus on biochemical parameters. Front Endocrinol (Lausanne). 2017;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clemmons DR. Consensus statement on the standardization and evaluation of growth hormone and insulin-like growth factor assays. Clin Chem. 2011;57(4):555-559. [DOI] [PubMed] [Google Scholar]
- 15. Morrison KM, Bidlingmaier M, Stadler S, Wu Z, Skriver L, Strasburger CJ. Sample pre-treatment determines the clinical usefulness of acid-labile subunit immunoassays in the diagnosis of growth hormone deficiency and acromegaly. Eur J Endocrinol. 2007;156(3):331-339. [DOI] [PubMed] [Google Scholar]
- 16. Halperin I, Casamitjana R, Flores L, Fernandez-Balsells M, Vilardell E. The role of IGF binding protein-3 as a parameter of activity in acromegalic patients. Eur J Endocrinol. 1999;141(2):145-148. [DOI] [PubMed] [Google Scholar]
- 17. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45-51. [DOI] [PubMed] [Google Scholar]
- 18. Olauson H, Mencke R, Hillebrands JL, Larsson TE. Tissue expression and source of circulating αKlotho. Bone. 2017;100:19-35. [DOI] [PubMed] [Google Scholar]
- 19. Dalton GD, Xie J, An SW, Huang CL. New insights into the mechanism of action of Soluble Klotho. Front Endocrinol (Lausanne). 2017;8:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun. 1998;242(3):626-630. [DOI] [PubMed] [Google Scholar]
- 21. Razzaque MS. Bone-kidney axis in systemic phosphate turnover. Arch Biochem Biophys. 2014;561:154-158. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8(1):43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5(11):611-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sze L, Bernays RL, Zwimpfer C, Wiesli P, Brändle M, Schmid C. Excessively high soluble Klotho in patients with acromegaly. J Intern Med. 2012;272(1):93-97. [DOI] [PubMed] [Google Scholar]
- 26. Neidert MC, Sze L, Zwimpfer C, et al. Soluble α-klotho: a novel serum biomarker for the activity of GH-producing pituitary adenomas. Eur J Endocrinol. 2013;168(4):575-583. [DOI] [PubMed] [Google Scholar]
- 27. Varewijck AJ, van der Lely AJ, Neggers SJ, Lamberts SW, Hofland LJ, Janssen JA. In active acromegaly, IGF1 bioactivity is related to soluble Klotho levels and quality of life. Endocr Connect. 2014;3(2):85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jawiarczyk-Przybyłowska A, Halupczok-Żyła J, Bolanowski M. Soluble α-Klotho–a new marker of acromegaly? Endokrynol Pol. 2016;67(4):390-396. [DOI] [PubMed] [Google Scholar]
- 29. Coopmans EC, El-Sayed N, Frystyk J, et al. Soluble Klotho: a possible predictor of quality of life in acromegaly patients. Endocrine. 2020;69(1):165-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kohler S, Tschopp O, Sze L, et al. Monitoring for potential residual disease activity by serum insulin-like growth factor 1 and soluble Klotho in patients with acromegaly after pituitary surgery: is there an impact of the genomic deletion of exon 3 in the growth hormone receptor (d3-GHR) gene on “safe” GH cut-off values? Gen Comp Endocrinol. 2013;188:282-287. [DOI] [PubMed] [Google Scholar]
- 31. Radikova Z, Koska J, Huckova M, et al. Insulin sensitivity indices: a proposal of cut-off points for simple identification of insulin-resistant subjects. Exp Clin Endocrinol Diabetes. 2006;114(5):249-256. [DOI] [PubMed] [Google Scholar]
- 32. van Esdonk MJ, van Zutphen EJM, Roelfsema F, et al. How are growth hormone and insulin-like growth factor-1 reported as markers for drug effectiveness in clinical acromegaly research? A comprehensive methodologic review. Pituitary. 2018;21(3):310-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schweizer JROL, Schilbach K, Haenelt M, et al. Data from: Supplemental data_Soluble alpha klotho in acromegaly: Comparison to traditional markers of disease activity. Posted March 3, 2021. figshare. 10.6084/m9.figshare.13614083 [DOI]
- 34. Yamazaki Y, Imura A, Urakawa I, et al. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398(3):513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. RRID:AB_2750859. [Google Scholar]
- 36. Manolopoulou J, Alami Y, Petersenn S, et al. Automated 22-kD growth hormone-specific assay without interference from Pegvisomant. Clin Chem. 2012;58(10):1446-1456. [DOI] [PubMed] [Google Scholar]
- 37. Bidlingmaier M, Friedrich N, Emeny RT, et al. Reference intervals for insulin-like growth factor-1 (igf-i) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. J Clin Endocrinol Metab. 2014;99(5):1712-1721. [DOI] [PubMed] [Google Scholar]
- 38. Friedrich N, Wolthers OD, Arafat AM, et al. Age- and sex-specific reference intervals across life span for insulin-like growth factor binding protein 3 (IGFBP-3) and the IGF-I to IGFBP-3 ratio measured by new automated chemiluminescence assays. J Clin Endocrinol Metab. 2014;99(5):1675-1686. [DOI] [PubMed] [Google Scholar]
- 39. Clemmons DR, Van Wyk JJ, Ridgway EC, Kliman B, Kjellberg RN, Underwood LE. Evaluation of acromegaly by radioimmunoassay of somatomedin-C. N Engl J Med. 1979;301(21):1138-1142. [DOI] [PubMed] [Google Scholar]
- 40. Oldfield EH, Jane JA Jr, Thorner MO, Pledger CL, Sheehan JP, Vance ML. Correlation between GH and IGF-1 during treatment for acromegaly. J Neurosurg. 2017;126(6):1959-1966. [DOI] [PubMed] [Google Scholar]
- 41. Schmid C, Neidert MC, Tschopp O, Sze L, Bernays RL. Growth hormone and Klotho. J Endocrinol. 2013;219(2):R37-R57. [DOI] [PubMed] [Google Scholar]
- 42. Claessen KM, Mazziotti G, Biermasz NR, Giustina A. Bone and joint disorders in acromegaly. Neuroendocrinology. 2016;103(1):86-95. [DOI] [PubMed] [Google Scholar]
- 43. Popii V, Baumann G. Laboratory measurement of growth hormone. Clin Chim Acta. 2004;350(1-2):1-16. [DOI] [PubMed] [Google Scholar]
- 44. Pokrajac A, Wark G, Ellis AR, Wear J, Wieringa GE, Trainer PJ. Variation in GH and IGF-I assays limits the applicability of international consensus criteria to local practice. Clin Endocrinol (Oxf). 2007;67(1):65-70. [DOI] [PubMed] [Google Scholar]
- 45. Bidlingmaier M, Freda PU. Measurement of human growth hormone by immunoassays: current status, unsolved problems and clinical consequences. Growth Horm IGF Res. 2010;20(1):19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wieringa GE, Sturgeon CM, Trainer PJ. The harmonisation of growth hormone measurements: taking the next steps. Clin Chim Acta. 2014;432:68-71. [DOI] [PubMed] [Google Scholar]
- 47. Katsumata N, Shimatsu A, Tachibana K, et al. Continuing efforts to standardize measured serum growth hormone values in Japan. Endocr J. 2016;63(10):933-936. [DOI] [PubMed] [Google Scholar]
- 48. Ribeiro-Oliveira A Jr, Abrantes MM, Barkan AL. Complex rhythmicity and age dependence of growth hormone secretion are preserved in patients with acromegaly: further evidence for a present hypothalamic control of pituitary somatotropinomas. J Clin Endocrinol Metab. 2013;98(7):2959-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen G, Liu Y, Goetz R, et al. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553(7689):461-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee EY, Kim SS, Lee JS, et al. Soluble α-klotho as a novel biomarker in the early stage of nephropathy in patients with type 2 diabetes. PLoS One. 2014;9(8):e102984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM. Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem. 2013;46(12):1079-1083. [DOI] [PubMed] [Google Scholar]
- 52. Semba RD, Cappola AR, Sun K, et al. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59(9):1596-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Semba RD, Cappola AR, Sun K, et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur J Appl Physiol. 2012;112(4):1215-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Amitani M, Asakawa A, Amitani H, et al. Plasma klotho levels decrease in both anorexia nervosa and obesity. Nutrition. 2013;29(9):1106-1109. [DOI] [PubMed] [Google Scholar]
- 55. Sze L, Neidert MC, Bernays RL, et al. Gender dependence of serum soluble Klotho in acromegaly. Clin Endocrinol (Oxf). 2014;80(6):869-887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.