Abstract
With the risks of drug development prohibitive, repurposed or repositioned medicines appear the best hope against long-COVID, a condition that still raises many unanswered questions.
Jennifer Minhas used to lead an active lifestyle, playing tennis and taking long walks around her Seattle neighborhood. But in March of 2020 she tested positive for COVID-19; she has been sick ever since. Today, walking a few hundred yards leaves her feeling exhausted, and Minhas, 54, says she still suffers from shortness of breath, migraines, cardiac arrhythmias and other debilitating symptoms. “It’s been a tough road,” she says.
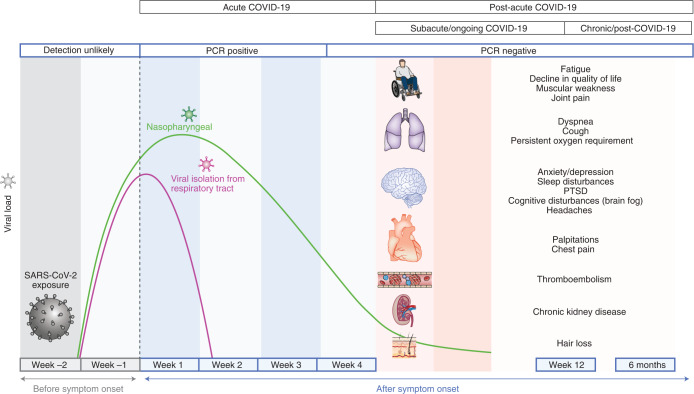
Minhas’s story is hardly unique. According to the US Centers for Disease Control and Prevention, between 10 and 30% of people infected with SARS-CoV-2 experience long-term health problems, and many of them—like Minhas—were never hospitalized or severely ill. Referred to as post-acute sequelae of SARS-CoV-2 infection (PASC), or more commonly as long-COVID, these persistent ailments can be mild or incapacitating and affect virtually any organ system in the body (Fig. 1). Affected people report extreme fatigue, body aches and pain. Many lose their sense of taste or smell, and brain fog—sluggish thinking that leaves people unable to focus—is a widespread problem. A pediatric nurse, Minhas says she has trouble remembering numbers and often feels a crushing tightness in her chest “like someone is sitting on it.” Experts worry that a subset of patients with long-COVID may never recover.
Fig. 1. Timeline of long-COVID.
Nalbandian et al. define long-COVID as persistent symptoms and/or delayed or long-term complications beyond four weeks from the onset of symptoms.
Adapted with permission from A. Nalbandian et al. Nat. Med. 27, 601–615 (2021), Springer Nature
Now long-COVID is the focus of growing attention. In February, the US National Institutes of Health announced a $1.15 billion initiative to identify the causes of long-COVID and find ways of preventing and treating the condition. With no effective treatment and a potentially large untapped market, a scattering of trials of repurposed and repositioned drugs have been initiated for long-COVID sufferers. But with the prevalence of the condition unclear—and a unifying diagnosis or even an agreed-upon definition that explains the condition’s widely varying symptoms completely lacking—drug development for long-COVID is even more of a shot in the dark than usual.
Not so, long-COVID?
Long-COVID inspires a certain amount of controversy. The degree to which it stems from the psychological shock of having had COVID-19, compounded by pandemic-related lockdowns, social isolation and fear, “is a valid question that should be debated in good faith,” says Kartik Sehgal, a medical oncologist at the Dana–Farber Cancer Institute in Boston and corresponding author of a review on long-COVID that appeared recently in Nature Medicine. Early reports of lingering symptoms coming from individuals who in many cases never were tested for SARS-CoV-2 or tested negative fuels ongoing skepticism to this day. One such report, produced in May 2020 by an online patient-centered collective called Body Politic, cited responses from 640 people, of whom just a quarter had a positive test result. Regarding the possibility of a mysterious multi-organ potentially lifelong disease in the absence of clinical evidence of organ damage (for example, a stroke), Adam Gaffney, a pulmonologist at Harvard Medical School says, “I felt that the claim was getting ahead of the evidence in part because the majority of individuals were seronegative for SARS-CoV-2 in some of the studies. But at the end of the day,” he adds, “the symptoms are real, and there may be more than one cause for symptoms in each individual patient.”
Still, in the United States, SARS-CoV-2 testing hasn’t been widely available for most of the pandemic, Sehgal points out, particularly among “vulnerable and under-represented communities who faced the greatest risk of infection.” And now that epidemiological evidence of the existence of long-COVID is accumulating in the literature, it is increasingly recognized as a legitimate condition in its own right, according to Bryan Lau, an associate professor of epidemiology at the Johns Hopkins School of Public Health in Baltimore.
One recent study found that a third of 488 COVID-19 patients discharged from hospitals in Michigan were still reporting shortness of breath and other symptoms that for many were still worsening after two months. In April, investigators at the University of Oxford made international headlines with a report showing that one in three patients treated for COVID-19 in the United States—the authors drew from the anonymized electronic health records of >260,000 patients in total—had been diagnosed with a neurological or psychiatric disorder within six months. Anxiety and depression accounted for most of the diagnoses, but cases of stroke and dementia were also significantly higher among the COVID-19 patients than they were among matched controls. And a recent meta-analysis of 45 studies involving almost 10,000 COVID-19 patients found that 73% of people experienced at least one persistent symptom for at least 60 days after diagnosis or 30 days after recovery.
Who is likely to develop these lingering symptoms? People who were initially hospitalized or severely ill with COVID-19 face the highest risk, and women are more prone to them than men. Older people also tend to be more vulnerable, but long-COVID does show up frequently in younger people. The Center for Post-COVID Care at Mount Sinai in New York routinely treats patients between the ages of 20 and 50 for symptoms that can be incapacitating, according to Zijian Chen, an endocrinologist and the center’s director. “We have students dropping out of school,” Chen says. “They just can’t compete or get through the day because they’re so tired. It’s frustrating for patients, and also for doctors; we look and can’t see anything that explains why they’re so ill. But the first step for us is to believe what our patients are telling us.”
Zijian Chen directs the long-COVID clinic at Mt. Sinai in New York.
Mount Sinai Health System
Now, as scientists drill down into mechanisms, hypotheses that might explain long-COVID’s varied effects on the body are emerging. Many scientists view the condition as a post-viral syndrome not unlike that seen in other infectious diseases. Survivors of the first SARS epidemic in 2002–2003 had lung, cardiac and neurological problems that could last for months or years after they fell ill. Similarly, for influenza, histories of the 1918 Spanish flu pandemic are replete with references to lingering muscle wasting, tremor, depression and ‘nervous complications’. And viral triggers are also implicated in the onset of myalgic encephalomyelitis/chronic fatigue syndrome (ME-CFS), which produces symptoms, such as extreme fatigue and muscle pain, similar to those of long-COVID.
Dealing with POTS
Evidence increasingly suggests that long-COVID patients have dysautonomia—impairments in the autonomic regulation of heart rate, blood pressure, digestion and other involuntary processes. A potential consequence is a condition known as postural orthostatic tachycardia syndrome (POTS), which causes heart rates to spike to >100 beats per minute upon standing. Pam Taub, a cardiologist and associate professor at the University of California, San Diego School of Medicine, says that she’s overwhelmed by the number of long-COVID patients presenting with POTS, and for many, she adds, the associated symptoms—palpations, shortness of breath and chest pain—are worse than their initial disease. Minhas suffers from POTS and says her heart rate can spike to 140 beats per minute “just from getting up to make toast.”
But the way SARS-CoV-2 infections might contribute to POTS and other persistent problems is unclear. The virus binds angiotensin-converting enzyme 2 (ACE-2) receptors on many different cell types and disrupts inflammatory and coagulation pathways. Autopsies performed on patients who died of COVID-19 at Mount Sinai by pathologist Clare Bryce and her colleague Carlos Cordon-Cardo, a pathologist at the Icahn School of Medicine, reveal elevated inflammatory cytokines, as well as clotting disorders that affect critical organ systems throughout the body. According to Bryce, vascular damage can result from direct injury to blood vessel walls, as well as generalized inflammatory responses. Blood clots, she says, will further exacerbate vascular pathology. “If an individual survives, some of that damage may heal,” she says. “But there could be residual changes in blood vessels that do not go away, and that can produce secondary damage to the organs they supply.” Bryce says autopsied brains show evidence of clotting and oxygen deprivation, and she speculates that this sort of damage might underlie memory problems and fogginess in COVID-19 survivors reporting cognitive effects.
Brain fog anomaly
Whether SARS-CoV-2 itself can cross into the brain has yet to be determined conclusively. Studies show high viral loads in the brains of exposed mice, and Scott Kelly, chief medical officer with CytoDyn, a biotech in Vancouver, Washington, proposes that SARS-CoV-2 could enter human brains by traveling along the olfactory nerve. Akiko Iwasaki, an immunologist at the Yale School of Medicine in New Haven, Connecticut, agrees. She led a study that included autopsies on three people who died of COVID-19, and one of them had “clear infection of neurons in the frontal cortex,” she says. Furthermore, Iwasaki’s study and research by other scientists show that SARS-CoV-2 can infect brain organoids in culture. But Avindra Nath, the intramural clinical director of the US National Institute of Neurological Disorders and Stroke (NINDS) in Bethesda, Maryland, takes a more skeptical view. Nath says, “We looked extensively for SARS-CoV-2 with a number of techniques and could not find it,” although the autopsied brains did show evidence of immune cells and vascular damage. Nath speculates that, among COVID-19 survivors, “leaky vessels might allow the entry of toxic blood compounds into the brain that lead to permanent or long-term symptoms.”
Still, extrapolating from autopsies to explain persistent cognitive declines in long-COVID is problematic because the deceased patients “are not representative of the population we want to learn something about,” says Alexander Charney, a psychiatrist at Mount Sinai. Evidence of disrupted blood vessels in the brain is specific to lethal COVID-19, he says, “but here, we’re talking about people who in many cases were diagnosed as outpatients.” Ascribing rapid cognitive changes to mild infection by SARS-CoV-2 would be an extraordinary leap, Charney ventures, adding that research is needed to ascertain how such changes might occur. One such study is ongoing at Mount Sinai. Headed by Priti Balchandani, a professor of radiology and neuroscience at the Icahn School of Medicine, it relies on ultra-high-field 7-tesla magnetic resonance imaging (MRI) to investigate cognitive symptoms in long-COVID. According to Balchandani, the technology provides high-resolution pictures of a living brain that reveal the functions and connectivity of neural circuitry “and would be most appropriate to uncover mechanisms underlying cognitive declines.”
Smoldering inflammation
Evidence implicating another symptom—persistent inflammation—in long-COVID comes from various lines of research. Liam O’Mahony, an immunologist at APC Microbiome Ireland, a research center based at University College Cork, is publishing a study showing that inflammatory markers, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and C-reactive protein, were elevated in blood plasma from 24 patients six to nine months after they were hospitalized during the pandemic’s first wave, some with COVID-19 that was initially mild. Just what drives the ongoing inflammation isn’t clear. O’Mahony suspects viral disruptions of the microbiome, which he describes as being intimately connected to host metabolism and immunity. Another possibility is that tissue injury elicits locally acting cytokines at such high levels that they spill over into the systemic circulation, causing symptoms. His research also shows that polarizing markers of adaptive immunity, including IL-17 and IL-4, are elevated in long-COVID. These cytokines ordinarily coordinate tissue defenses and repair following an infection, but may also contribute to symptoms. “Cytokines are interesting from a clinical perspective since they can be targeted with biologics,” O’Mahony says. “That’s what is exciting about this finding—we didn’t expect these particular cytokines to be elevated, and now we can try to see if changing their activity has any effects on symptomology.”
Other experts speculate the inflammation might come from low-level infections smoldering in tissue. One recent study in Nature, for instance, found that SARS-CoV-2 antigens were still detectible in gut biopsies four months after infection in people who by this time were testing negative for the virus with a nasal-swab polymerase chain reaction (PCR) assay. According to Iwasaki, who was not involved in the study, evidence for smoldering infections is further supported by anecdotal reports that some long-COVID patients feel better after vaccination. During a recent Facebook poll, nearly 40% of 962 respondents with long-COVID were claiming full or partial symptom improvements after getting vaccinated, whereas the rest reported no changes or feeling worse. Some speculate that symptom improvements might be due to a placebo effect. Still, the Nature study’s lead author, Michel Nussenzweig, an immunologist at Rockefeller University in New York, says detectable antigens don’t actually prove that SARS-CoV-2 lingers in the gut—only a culture assay can confirm that suspicion, and in lieu of that, “I don’t really know what else it can be,” he says. Moreover, none of the people who had detectible antigens in the Nature study was actually symptomatic. “So, we still need to perform these sorts of studies in long-COVID patients to determine the relevance of the findings,” says O’Mahony.
Turning inward
Finally, scientists are investigating the possibility that long-COVID symptoms might arise in some cases from autoantibodies attacking the body’s own cells. One study found an abundance of autoantibodies up to seven months after infection even in patients who had mild initial disease. Moreover, the antibodies were detected more frequently in convalescing people who were still symptomatic than in those who were not. These results, the authors conclude, underscore the need to further investigate autoimmunity in SARS-CoV-2 infection and its role in the post-acute sequelae to COVID-19. The source of the autoantibodies is under investigation. Scientists suspect molecular mimicry (meaning that the viral antigens share similarities with self antigens) or a generalized activation of autoantibody-secreting B cells. Research by Iwasaki and her collaborator Aaron Ring, an immunologist at Yale Medical School, shows that the autoantibodies found in blood from patients acutely ill with severe COVID-19 target immune modulators, including type I interferons. “These are the same cytokines that the body uses to fight viral infections,” she says. “The virus de-weaponizes our own immune system so it can’t fight back, and the patients who have these interferon-specific antibodies are also the sickest ones, who require intensive care treatment. And patients with severe disease are also most likely to have very long-term consequences after infection.”
Under study
As research into long-COVID moves forward, investigators are tracking patients over time, gathering samples for analysis, and correlating analytical results with symptoms that worsen or improve. Mount Sinai has a number of programs in the works. The hospital’s post-COVID registry is following 800 patients with a COVID diagnosis; each gets basic lab work, an electrocardiogram and spirometry testing at periodic intervals. The aim is to “determine if symptoms are associated with diagnostics that we can match up with questionnaires and self-reported data,” says the registry’s director, Juan Wisnivesky, a pulmonologist and clinical epidemiologist at the Icahn School of Medicine.
One of the largest research efforts recently launched at the Johns Hopkins School of Public Health. Co-directed by Lau, the Johns Hopkins COVID Long Study is reaching out to 25,000 people who were either diagnosed with COVID or became symptomatic. Subjects will provide information on their demographics, health history and symptoms they had or may still be having. The top priorities, Lau says, are to assess the heterogeneity and prevalence of long-COVID in a diverse population and then determine the time to recovery. “We think most people will become fully functional, but we also worry that a subset may not,” he says. “We need to parse out the different symptoms and how they fit together with other risk factors for long-COVID, and then maybe we can begin to figure out why it takes some individuals longer to fully recover, or perhaps not recover at all.”
At the NINDS, Nath is taking a particularly intensive approach. After screening 1,300 long-COVID patients by phone, Nath’s team will select 50 individuals who will come to the hospital for a week-long evaluation. During that visit, participants will be subjected to a battery of tests, including MRI, immunological assessments and a cerebrospinal fluid analysis. Other specialists will be brought in to assess symptoms affecting the heart, lungs and different organs. “The aim is to define the clinical syndrome with all its manifestations, to understand the underlying biological mechanisms, and to identify any objective biomarkers and potential targets for treatment,” Nath wrote in an e-mail. “This will allow us to design proper clinical trials, with appropriate outcome measures and therapeutic agents that are most likely to alter the course of the illness.”
Treatments without a cause
Without a clear unifying mechanism, long-COVID treatments face an uphill battle. “It’s challenging to treat something if you don’t know what’s causing it,” says Timothy Henrich, a physician and professor at the University of California, San Francisco. Tony Butler, managing director of Roth Capital Partners in New York, says the lack of an obvious target or mechanism in long-COVID leaves investors wary, despite the growing market. Right now, he says “it’s not seen as a growth area.” Cosme Ordonez, managing director and a senior equity research analyst who covers biotech at National Securities Corporation in Boca Raton, Florida, agrees. “To develop a novel candidate, we need something more specific to the condition,” he says. “In the case of acute COVID, we know that we need to block the virus and hyperinflammatory state. But the science behind long-COVID has lots of caveats, and we don’t really know what’s driving the disease. The targets are not well defined and correlated with the syndrome, and that might explain why industry is not jumping all over this because the market is potentially huge.” As mechanistic insights into long-COVID emerge, Ordonez expects industry funding will follow, with more biotech companies jumping in “within the next two to five years,” he says.
In the meantime, clinicians are treating symptoms as best they can and experimenting with physical rehabilitation methods, as well as breathing exercises that stimulate the vagus nerve, as a means of restoring more normal heart rates and nervous system functioning. Sehgal says the top priorities are still to define the condition and investigate underlying mechanisms. “And we should pursue clinical trials as quickly as possible,” he says.
As usual, the first place where clinicians are looking is to repurpose drugs already approved for other conditions (Table 1). Companies like Biovista are mining data from the curated literature and databases to identify 30 features (for example, genes, post-translational modifications, pathways, cell lines and diseases) that link COVID-19 symptoms with the mechanisms of approved drugs; last year, the company announced that Cablivi (caplacizumab-yhdp; two humanized variable domain (nanobody) fragments linked by a three-alanine linker and targeting von Willebrand factor) and the small-molecule combination Atozet (ezetimibe and atorvastatin) were highly suited to target specific aspects of blood clotting and inflammation in the context of COVID-19.
Table 1.
Interventions in clinical trials for long-COVID
| Company | Intervention | Target | Clinical stage |
|---|---|---|---|
| Amgen | Ivabradine (HCN channel blocker) | POTS | Off-label use |
| Ampio Pharmaceuticals | Cyclized peptide derived from aspartyl-alanyl diketopiperazine isolated from human serum | DAMPs induced by viruses | Phase 1 |
| CytoDyn | Leronlimab (monoclonal against CCR5) | Inflammation | Phase 2 |
| GioCOV, a subsidiary of Giostar | Allogeneic mesenchymal stem cells | Inflammation | Compassionate use (FDA) |
| AIM ImmunoTech | Rintatolimod (poly(I):poly(C12U)) | Virus | Phase 3 |
| PureTech | Deuterated pirfenidone | Pulmonary fibrosis | Phase 2 |
| Synairgen (Southampton, UK) | Inhaled interferon beta-1a | COPD | Phase 3 |
Elsewhere, a small-molecule drug showing promise for POTS associated with long-COVID is Amgen’s Corlanor (ivabradine), which is indicated to reduce hospitalization risk in heart failure and to treat stable symptomatic heart failure due to dilated cardiomyopathy in pediatric patients ages 6 months and older. Ivabradine is a hyperpolarization-activated cyclic-nucleotide-gated (HCN) channel blocker that lowers heart rate without dropping blood pressure. Taub and her colleagues tested the drug in a clinical study with 22 patients who had POTS and reported in February that it significantly improved quality of life and reduced heart rates upon standing. “I’ve been getting e-mails from cardiologists all over the world who say they’re now using ivabradine for COVID-related POTS,” she says.
Meanwhile, ME-CFS drug development is expanding to include patients with long-COVID. AIM ImmunoTech is the biopharmaceutical company behind Ampligen (rintatolimod, poly(I):poly(C12U)), a selective Toll-like receptor 3 (TLR3) agonist with a 20-year history of development—first with HIV and subsequently with ME-CFS. Currently approved for ME-CFS only in Argentina, the drug is given by twice weekly infusions. The Food and Drug Administration (FDA) permits these treatments on a compassionate use basis in the United States. However, the drug is available in only two locations: Incline Village, Nevada or Charlotte, South Carolina. Patients move to these towns to get the drug, and many of them are reporting symptom improvements, according to Thomas Equels, AIM ImmunoTech’s CEO. Made from double-stranded RNA, the drug modulates the innate immune system with the aim of limiting sustained infection. AIM ImmunoTech recently published data from its ongoing phase 3 clinical trial showing that patients taking rintatolimod improved on the trial’s endpoint—exercise duration on a treadmill—by ~25%. That trial has now been expanded to include patients suffering from what the company refers to as COVID-19-induced chronic fatigue. To enroll, patients need a positive COVID-19 test and long-term symptoms lasting at least three months.
Experimental drugs in the pipeline
Several new agents are under development to address what could turn out to be a large new indication in medicine, given that upwards of 180 million people have tested positive for SARS-CoV-2 as of the end of June and hundreds of millions more likely have been infected.
PureTech Health, a biotherapeutics company based in Boston, is conducting phase 2 trials of LYT-100, a deuterated form of pirfenidone. Pirfenidone is approved for idiopathic pulmonary fibrosis. LYT-100 targets pro-inflammatory cytokines, including IL-6 and TNF-α, and reduces transforming growth factor (TGF)-β signaling to interrupt collagen deposition and scarring. Michael Chen, the company’s head of innovation, says the drug was already in development for lymphedema and other lung fibrotic conditions before the pandemic. “But as COVID-19 unfolded, we saw an opportunity to intervene in the inflammation and fibrosis that we thought might complicate recovery from the disease, just as it had for patients with SARS and Middle East Respiratory Syndrome,” he says. The company’s phase 2 trial aims to enroll 168 patients in the United States and Europe who initially required oxygen support during the acute phase of their illness. “The goal is to complete the trial this year,” Chen says.
On the US West Coast, CytoDyn is testing its CC-motif chemokine receptor 5 (CCR5) antagonist leronlimab, a humanized IgG4 monoclonal antibody, in a phase 2 trial with a target enrollment of 50 people. CCR5 is implicated in many disease processes, including HIV, multiple sclerosis and metastatic cancer. CytoDyn’s marketing materials claim it helps to modulate immune cell trafficking to sites of inflammation. Leronlimab has already been tested in a phase 2b/3 clinical trial as add-on therapy for respiratory illness in patients critically ill with COVID-19. Results showed a survival benefit compared with commonly used treatments, and the current phase 2 will investigate the drug as treatment for wide-ranging symptoms. In explaining the rationale for using leronlimab in long-COVID, Kelly says that CCR5 binding will help to regulate the immune response, so that macrophages and other immune cells “don’t rush in all at once and destroy tissue. We think we can coordinate immune tracking to slow inflammation.”
Ampio Pharmaceuticals in Englewood, Colorado is moving into long-COVID on the basis of positive phase 1 results in acute settings with its blood-derived cyclized peptide LMWF5A (aspartyl-alanyl-diketopiperazine), which targets hyperinflammation in the lungs. In a February press release, Ampio claimed the peptide improved all-cause mortality in patients in respiratory distress. During the new phase 1 trial, patients with respiratory symptoms lasting four weeks or more will self-administer the drug with a nebulizer at home for five days. “We hope to shorten the duration of clinical symptoms [in the chronic condition], which is what we see in acutely ill patients,” says Holli Cherevka, the company’s chief operating officer.
Taking a similar approach, Synairgen in Southampton, UK, cites improvements in critically ill patients as justification for adding long-COVID to its phase 3 trial with SNG001 (inhaled interferon beta). Phase 2 results with the drug “showed that improvement, recovery and hospital discharge were in favor of SNG001 versus placebo at day 28,” which suggests the drug “may be relevant to patients with COVID-19 who experience wide-ranging, long-term symptoms for at least a month or longer after,” according to a company spokesperson.
The long view
Minhas had luck treating her POTS with ivabradine, which was not covered by her insurance company and costs her $500 a month. “For me the drug worked wonderfully,” she says. “When taking it, my maximum heart rate while walking is no greater than 115 beats per minute. But when I’m not on the drug, it gets really high and then I feel awful.” Minhas says she’s getting stronger, and she can follow conversations and focus better than she did before. But she still suffers from multiple ailments, including chest pain, joint pain, and swelling in her hands and feet.
At Mount Sinai, Chen says patients are looking for answers about what will make them feel better. Looking out at the clinical trials, he says there’s a lot of activity, but no conclusive evidence of what may or may not work. “We’re learning how to evaluate patients in a comprehensive fashion, and break down symptoms and work to resolve them. But this is a very new disease and there’s a lot of things we don’t know. We’re writing the textbook as we go.” Some of the companies developing the first wave of repositioned drugs are hoping they will be the first to write the pharmacopeia for long-COVID.