Abstract
Background
Patients often have moderate to severe pain after rotator cuff surgery, despite receiving analgesics and nerve blocks. There are many suggested ways to improve pain after rotator cuff surgery, but the effects of adopting a pathway that includes formal patient education, a long-acting nerve block, and extensive multimodal analgesia are unclear.
Questions/purposes
(1) Does adoption of a clinical pathway incorporating patient education, a long-acting nerve block, and preemptive multimodal analgesia reduce the worst pain during the first 48 hours after surgery compared with current standard institutional practices? (2) Does adoption of the pathway reduce opioid use? (3) Does adoption of the pathway reduce side effects and improve patient-oriented outcomes?
Methods
From September 2018 to January 2020, 281 patients scheduled for arthroscopic ambulatory rotator cuff surgery were identified for this paired sequential prospective cohort study. Among patients in the control group, 177 were identified, 33% (58) were not eligible, for 11% (20) staff was not available, 56% (99) were approached, 16% (29) declined, 40% (70) enrolled, and 40% (70) were analyzed (2% [4] lost to follow-up for secondary outcomes after postoperative day 2). For patients in the pathway cohort, 104 were identified, 17% (18) were not eligible, for 11% (11) staff was not available, 72% (75) were approached, 5% (5) declined, 67% (70) enrolled, and 67% (70) were analyzed (3% [3] lost to follow-up for secondary outcomes after postoperative day 2). No patients were lost to follow-up for primary outcome; for secondary outcomes, four were lost in the control group and three in the pathway group after postoperative day 2 (p = 0.70). The initial 70 patients enrolled received routine care (control group), and in a subsequent cohort, 70 patients received care guided by a pathway (pathway group). Of the 205 eligible patients, 68% (140) were included in the analysis. This was not a study comparing two tightly defined protocols but rather a study to determine whether adoption of a pathway would alter patient outcomes. For this reason, we used a pragmatic (real-world) study design that did not specify how control patients would be treated, and it did not require that all pathway patients receive all components of the pathway. We developed the pathway in coordination with a group of surgeons and anesthesiologists who agreed to apply the pathway as much as was viewed practical for each individual patient. Patients in both groups received a brachial plexus nerve block with sedation. Major differences between the pathway and control groups were: detailed patient education regarding reasonable pain expectations with a goal of reducing opioid use (no formal educational presentation was given to the control), a long-acting nerve block using bupivacaine with dexamethasone (control patients often received shorter-acting local anesthetic without perineural dexamethasone), and preemptive multimodal analgesia including intraoperative ketamine, postoperative acetaminophen, NSAIDs, and gabapentin at bedtime, with opioids as needed (control patients received postoperative opioids but most did not get postoperative NSAIDS and no controls received gabapentin or separate prescriptions for acetaminophen). The primary outcome was the numerical rating scale (NRS) worst pain with movement 0 to 48 hours after block placement. The NRS pain score ranges from 0 (no pain) to 10 (worst pain possible). The minimum clinically important difference (MCID) [12] for NRS that was used for calculation of the study sample size was 1.3 [18], although some authors suggest 1 [13] or 2 [5] are appropriate; if we had used an MCID of 2, the sample size would have been smaller. Secondary outcomes included NRS pain scores at rest, daily opioid use (postoperative day 1, 2, 7, 14), block duration, patient-oriented pain questions (postoperative day 1, 2, 7, 14), and patient and physician adherence to pathway.
Results
On postoperative day 1, pathway patients had lower worst pain with movement (3.3 ± 3.1) compared with control patients (5.6 ± 3.0, mean difference -2.7 [95% CI -3.7 to -1.7]; p < 0.001); lower scores were also seen for pain at rest (1.9 ± 2.3 versus 4.0 ± 2.9, mean difference -2.0 [95% CI -2.8 to -1.3]; p < 0.001). Cumulative postoperative opioid use (0-48 hours) was reduced (pathway oral morphine equivalent use was 23 ± 28 mg versus 44 ± 35 mg, mean difference 21 [95% CI 10 to 32]; p < 0.01). The greatest difference in opioid use was in the first 24 hours after surgery (pathway 7 ± 12 mg versus control 21 ± 21 mg, mean difference -14 [95% CI -19 to -10]; p < 0.01). On postoperative day 1, pathway patients had less interference with staying asleep compared with control patients (0.5 ± 1.6 versus 2.6 ± 3.3, mean difference -2.2 [95% CI -3.3 to -1.1]; p < 0.001); lower scores were also seen for interference with activities (0.9 ± 2.3 versus 1.9 ± 2.9, mean difference -1.1 [95% CI -2 to -0.1]; p = 0.03). Satisfaction with pain treatment on postoperative day 1 was higher among pathway patients compared with control patients (9.2 ± 1.7 versus 8.2 ± 2.5, mean difference 1.0 [95% CI 0.3 to 1.8]; p < 0.001). On postoperative day 2, pathway patients had lower nausea scores compared with control patients (0.3 ± 1.1 versus 1 ± 2.1, mean difference -0.7 [95% CI -1.2 to -0.1]; p = 0.02); lower scores were also seen for drowsiness on postoperative day 1 (1.7 ± 2.7 versus 2.6 ± 2.6, mean difference -0.9 [95% CI - 1.7 to -0.1]; p = 0.03).
Conclusion
Adoption of the pathway was associated with improvement in the primary outcome (pain with movement) that exceeded the MCID. Patients in the pathway group had improved patient-oriented outcomes and fewer side effects. This pathway uses multiple analgesic drugs, which may pose risks to elderly patients, in particular. Therefore, in evaluating whether to use this pathway, clinicians should weigh the effect sizes against the potential risks that may emerge with large scale use, consider the difficulties involved in adapting a pathway to local practice so that pathway will persist, and recognize that this study only enrolled patients among surgeons and the anesthesiologists that advocated for the pathway; results may have been different with less enthusiastic clinicians. This pathway, based on a long-lasting nerve block, multimodal analgesia, and patient education can be considered for adoption.
Level of Evidence
Level II, therapeutic study.
Introduction
Many patients with rotator cuff tears can be discharged home with minimal pain. However, these patients often later experience moderate to severe pain after the peripheral nerve block wears off, despite using opioids [2, 6]. Uncontrolled pain is a common cause of distress and can lead to unplanned emergency department visits [14]. Adequate analgesia can improve patient satisfaction and facilitate return to normal functioning. Use of regional anesthesia is well established, but the technique and medications used vary widely. There is considerable variation in patient education regarding expectations and recommendations for postoperative analgesia. Multimodal analgesia has been recognized as an effective way to reduce postoperative pain and opioid consumption [19] and may improve recovery after surgery [10], but it is not always comprehensively implemented. Clinical pathways can encourage adoption of best practices, improve patient education, and standardize perioperative pain therapy [19]. A multimodal pain management protocol was recently shown to improve the quality of recovery after ambulatory shoulder surgery [4]. A pathway for total shoulder arthroplasty demonstrated low pain scores and minimal intravenous opioid use when a peripheral nerve block and preemptive nonopioid analgesia were combined [7, 20].
Some relevant pathway studies investigated inpatient total shoulder arthroplasty [7, 20], which allows access to intravenous opioids not practical for outpatient use. Therefore, it is important to develop an enhanced recovery pathway for outpatient shoulder surgery that incorporates patient education regarding preemptive analgesia and appropriate opioid use [17], provides long-acting brachial plexus blockade [9, 15, 21], and uses multimodal analgesia to reduce pain as the nerve block resolves.
Recently, a protocol showed improved quality of recovery after outpatient shoulder surgery [4], but there were prominent protocol differences that may have limited the magnitude of benefit. That study did not include formal patient education, the nerve block had a likely shorter duration (ropivacaine versus bupivacaine with dexamethasone), intraoperative ketamine was not administered, and an NSAID was given for 1 day instead of 3 days. The goal of the current study was not to compare two tightly defined protocols, but rather to determine whether adoption of a pathway would alter patient outcomes. Thus, a pragmatic study design was used that did not specify how control patients would be treated and did not require that all pathway patients receive all components of the pathway. The pathway was developed by a group of surgeons and anesthesiologists who agreed to apply the pathway as much as practical for each individual patient. This would provide an evidence-based approach to postoperative pain and generate specific instructions and expectations for future patients.
Therefore, we asked: (1) Does adoption of a clinical pathway incorporating patient education, a long-acting nerve block, and preemptive multimodal analgesia reduce the worst pain during the first 48 hours after surgery compared with current standard institutional practices? (2) Does adoption of the pathway reduce opioid use? (3) Does adoption of the pathway reduce side effects and improve patient-oriented outcomes?
Patients and Methods
We recruited adult patients (American Society of Anesthesiologists physical status 1, 2, or 3) from a single center specializing in treatment of musculoskeletal disorders (Hospital for Special Surgery) into this before-and-after sequential, prospective cohort study. All patients provided written informed consent for this study. Patients were enrolled from September 2018 to January 2020. Eligible patients were between the ages of 18 and 80 years and were scheduled for elective ambulatory arthroscopic rotator cuff surgery with participating surgeons. Patients with known inoperable rotator cuff tears were not enrolled. To be eligible for the pathway group, patients also needed to be scheduled with a participating anesthesiologist.
Of the patients identified for the control group, 33% (58 of 177) were not eligible, and for 11% (20 of 177) of patients, research staff were not available. Overall, 56% (99 of 177) of patients were approached, 16% (29 of 177) declined to participate, 40% (70 of 177) were enrolled, and 40% (70 of 177) were analyzed (2% [4 of 177] lost to follow-up for secondary outcomes, after postoperative day 2) (Fig. 1). In the pathway group, 104 patients were identified for study inclusion: 17% (18 of 104) were not eligible, and for 11% (11 of 104) of patients, staff were not available. Seventy-two percent (75 of 104) of patients were approached, 5% (5 of 104) declined, 67% (70 of 104) enrolled, and 67% (70 of 104) were analyzed (3% [3 of 104] lost to follow-up for secondary outcomes, after postoperative day 2). No patients were lost to follow-up for the primary outcome; for secondary outcomes, four patients were lost in the control group and three patients in the treatment group after postoperative day 2 (p = 0.70). The trial had a pragmatic design so patients were not required to follow all pathway components.
Fig. 1.
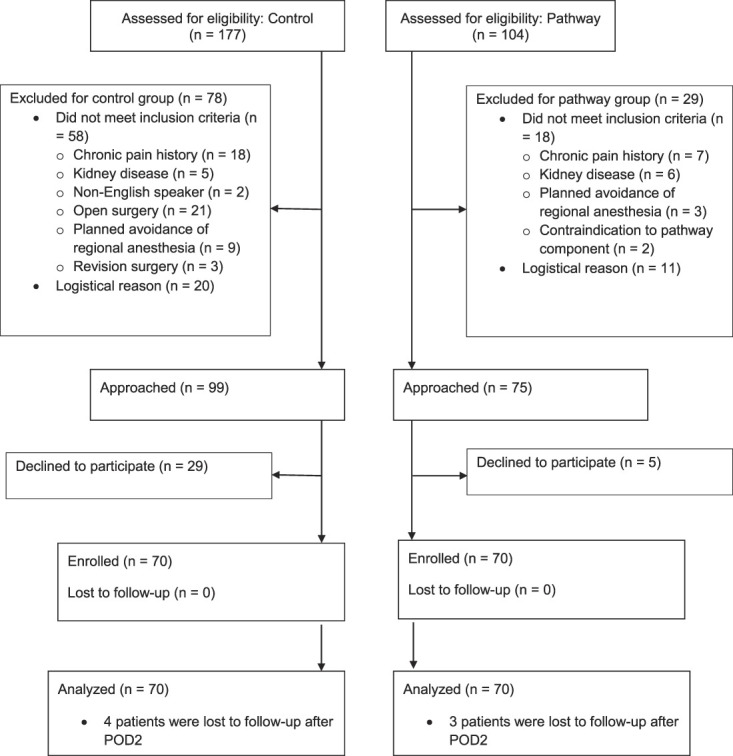
CONSORT patient flow diagram.
All patients underwent arthroscopic surgery for rotator cuff pathology, which included some combination of subacromial decompression, rotator cuff debridement, and/or rotator cuff repair. Concomitant procedures such as biceps tenodesis, labral repair, and acromioclavicular joint excision were performed at the discretion of the surgeon based on the patient’s pathology. All surgeries were performed in the beach chair position. The arthroscopic repair technique was individualized to best repair the patient-specific cuff configuration.
Anesthesia and Pain Management
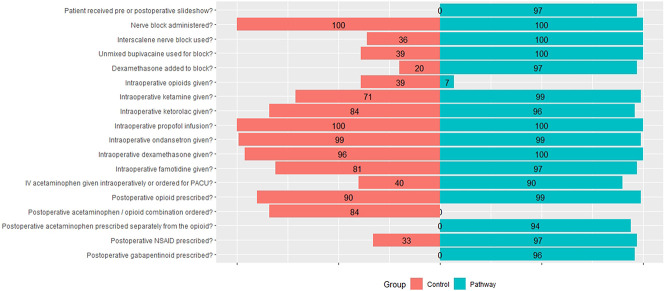
For the control group, patients received usual care for intraoperative and postoperative analgesic treatment as determined by the attending anesthesiologist and surgeon at a specialized orthopaedic hospital. Patients in the control and pathway groups all received brachial plexus nerve blocks with sedation. Supraclavicular blocks, which often cause motor blockade of the hand, were used for 50% (35 of 70) of control patients. Unmixed bupivacaine was used for only 39% [27 of 70] of the blocks, which means that most blocks (61% [43 of 70]) included mepivacaine, leading to shorter block duration (Fig. 2). Dexamethasone, which prolongs block duration, was added to 20% (14 of 70) of control blocks. Postoperative opioids were prescribed to 90% (63 of 70) of control patients; no control patients received separate prescriptions for acetaminophen. No control patients received a gabapentinoid, and 33% (23 of 70) received a postoperative NSAID.
Fig. 2.
Compliance with components of the pathway. Zero compliance is in the middle, with bar graphs representing control patients extending to the left, and bar graphs representing pathway patients extending to the right. “Unmixed bupivacaine used for block” indicates that bupivacaine (with or without an additive) was used as the sole local anesthetic for the peripheral nerve block, not mepivacaine and not a mixture of mepivacaine and bupivacaine. A color image accompanies the online version of this article.
The pathway protocol was standardized (Table 1). Meetings were conducted with post-anesthesia care unit (PACU) nurses to promote their understanding of, and adherence to, the pathway. Patient education was performed using a presentation by the anesthesiologist and/or research assistant, with instructions about peripheral nerve blockade, postoperative analgesic use, and appropriate expectations regarding postoperative pain. Patients were provided a copy of the educational presentation (see Appendix 1; Supplemental Digital Content 1, http://links.lww.com/CORR/A500). A preoperative ultrasound-guided interscalene peripheral nerve block was performed using 20 to 30 mL bupivacaine, 0.5%, with 2 mg preservative-free dexamethasone. Patients received intravenous sedation for the block of 2 to 4 mg of midazolam and 10 to 20 mg ketamine. Sedation was maintained with a propofol infusion, as needed. General anesthesia was not used. Patients received intravenous ketamine (up to 50 mg total), ondansetron (4 mg), famotidine (20 mg), dexamethasone (4 mg), and ketorolac (15 to 30 mg) during surgery. For initial postoperative analgesia, patients received 1000 mg of intravenous acetaminophen upon PACU arrival. On discharge, patients were prescribed acetaminophen 650 mg by mouth every 6 hours for 3 days, meloxicam 15 mg by mouth at bedtime for 3 days, gabapentin 300 mg by mouth at bedtime for 3 days, and oxycodone 5 to 10 mg by mouth every 4 hours, only if needed. Some aspects of the pathway (perineural dexamethasone, use of gabapentin for acute pain) are off-label uses, but these drugs are often used as we used them here. The pathway was associated with a longer duration of the effect of the nerve block (see Appendix 2; Supplemental Digital Content 2, http://links.lww.com/CORR/A501). The median (interquartile range) time until the block completely wore off was shorter for the control group (23 hours [17 to 25]) compared with pathway (28 hours [25 to 33], mean difference 7 hours [95% CI 5 to 10]; p < 0.001). Patient compliance was assessed for taking at least one dose of the three nonopioid analgesics on postoperative day 1 or postoperative day 2. Of the control patients, 87% took acetaminophen (as part of an opioid-acetaminophen combination), none took gabapentin, and 3% took meloxicam; among pathway patients, 91% took acetaminophen, 91% took gabapentin, and 84% took meloxicam.
Table 1.
Pathway protocol
| Phase | Intervention | Rationale |
| Preoperative | Computer-based presentation for patients, with a written handout provided. A uniform plan from surgery, anesthesia, and nursing. | Explain peripheral nerve block, range of block duration; set expectations regarding postoperative pain and use of multimodal analgesics; administration of nonopioids on scheduled basis regardless of pain; goal to minimize unneeded opioids. |
| Intraoperative | Ultrasound-guided interscalene nerve block (bupivacaine + dexamethasone) | Surgical anesthesia and prolonged postoperative analgesia |
| Propofol infusion | Intraoperative sedation; avoid general anesthesia | |
| Ketamine, total maximum of 50 mg IV | Reduce central sensitization of pain | |
| Dexamethasone 4 mg IV | Antiemetic and anti-inflammatory | |
| Ondansetron 4 mg IV | Antiemetic | |
| Ketorolac 15-30 mg IV | Analgesia | |
| PACU | Acetaminophen 1000 mg IV | Initial postoperative analgesic therapy |
| Postoperative | Meloxicam 15 mg at bedtime | Multimodal analgesia |
| Acetaminophen 650 mg | Multimodal analgesia | |
| Gabapentin 300 mg | Multimodal analgesia, given at bedtime only | |
| Oxycodone 5-10 mg | Used as needed to address moderate to severe pain |
IV = intravenous; PACU = postanesthesia care unit.
Primary and Secondary Study Outcomes
Our primary study goal was to determine whether adoption of the pathway was associated with reduced pain and opioid use after surgery. Research staff interviewed patients in person (while in the hospital) or by phone to collect the numerical rating scale (NRS) pain scores at rest and with movement if possible (postoperative day 0, 1, 2, 7, 14), as well as opioid intake.
Our secondary study goals were to determine whether adoption of the pathway was associated with improved patient-oriented outcomes and reduced side effects. A modified PainOUT questionnaire [16] was administered on postoperative day 0, 1, 2, 7, and 14. The PainOUT questionnaire includes questions rating nausea, drowsiness, dizziness, itching from 0 (none) to 10 (severe), difficulty staying and falling asleep from 0 (does not interfere) to 10 (completely interferes), satisfaction with pain treatment from 0 (extremely dissatisfied) to 10 (extremely satisfied), fraction of pain relief from 0% to 100%, fraction of time in severe pain from 0% to 100%, pain interference with activities from 0 (does not interfere) to 10 (completely interferes), and the question “Were you allowed to participate in decisions about your pain treatment as much as you wanted to?” from 0 (not at all) to 10 (very much so). The minimum clinically important difference (MCID) information is not available for the PainOUT scale. Block duration was assessed through multiple questions regarding pain and sensation in the operative arm [22]. On postoperative day 2, patients were asked about occurrence of hoarseness or difficulty breathing (potential side effects of the nerve block).
Ethical Approval
Ethical approval for this study was obtained from the institutional review board at the Hospital for Special Surgery (IRB#2018-0814). The trial was registered at https://www.clinicaltrials.gov/ (NCT03717753). The study was initially submitted to clinicaltrials.gov on September 12, 2018 before study enrollment. Due to research administration being out of office, edits to satisfy National Library of Medicine quality control review criteria were not released until a month after. No changes to planned study endpoints were made when posted on https://www.clinicaltrials.gov/.
Power Analysis and Statistical Methods
Previous evidence found the mean ± SD for NRS worst pain 24 to 48 hours after rotator cuff repair to be 7.0 ± 2.1 [9]. We determined that a sample size of 58 patients per group would provide 80% power at a two-sided alpha level of 0.05 to detect a 1.3-point difference in NRS worst pain score [18] between control and pathway groups at 0 to 48 hours postoperatively. The MCID in NRS pain has been debated; suggested values include 1 [13], 1.3 [18], and 2 [5]. For design of this study, the authors used 1.3; if we had used an MCID of 2, the sample size would have been smaller. Sample size was 140 patients to account for attrition and protocol violations (20%).
Balance on demographics, baseline measurements, and surgical procedures was compared using two-sample t-tests or Wilcoxon rank-sum tests for continuous variables, and using chi-square or Fisher exact tests for categorical variables as appropriate.
Continuous variables are summarized as means ± SDs or medians with IQR. Categorical variables are summarized as counts and percentages. All analyses were performed on an intention-to-treat basis.
We compared the primary outcome, NRS worst pain with movement at 0 to 48 hours, between control and pathway groups using the generalized estimating equations (GEE) method with an identity link, controlling for baseline NRS worst pain with movement. We conducted a sensitivity analysis using the GEE model for primary outcome, adding age and total surgery time to the control.
We compared continuous secondary outcomes measured at a single postoperative time point per patient between groups using two-sample t-tests. Categorical secondary outcomes measured at a single postoperative time point per patient were compared between groups using chi-square or Fisher exact tests, as appropriate. Secondary outcomes measured at multiple time points per patient were analyzed using the GEE method with identity link for continuous outcomes and logit link for binary outcomes. Effect sizes for continuous and binary secondary outcomes are presented as differences in means and odds ratios, respectively, with corresponding 95% confidence intervals and unadjusted p values.
All statistical hypothesis tests were two-sided. A p value less than 0.05 was determined as statistically significant. Statistical analyses were performed with SAS Version 9.4 (SAS Institute). REDCap use was supported by the National Center for Advancing Translational Science of the National Institute of Health (UL1TR000457). The manuscript was prepared in compliance with the STROBE checklist.
One hundred forty patients were enrolled (Fig. 1). Patient characteristics (Table 2) were comparable between groups. Among intraoperative characteristics, procedure time (control 47 ± 16 minutes versus pathway 57 ± 18 minutes; p < 0.001) and proportion receiving a labral repair (control 20% [14 of 70] versus pathway 3% [2 of 70]; p = 0.002) were different. We performed a sensitivity analysis to adjust for imbalances when comparing the primary outcome.
Table 2.
Patient and operation characteristics
| Control group (n = 70) | Pathway group (n = 70) | p value | |
| Women | 40 (28) | 46 (32) | 0.50 |
| ASA Level | 0.30 | ||
| I | 24 (17) | 17 (12) | |
| II | 73 (51) | 76 (53) | |
| III | 3 (2) | 7 (5) | |
| Age in years | 55 ± 12 | 59 ± 10 | 0.27 |
| BMI in kg/m2 | 28 ± 5 | 28 ± 4 | 0.07 |
| Procedure time in minutes | 47 ± 16 | 57 ± 18 | < 0.01 |
| Rotator cuff repair performed | 84 (59) | 91 (64) | 0.06 |
| Other procedures | |||
| Acromioplasty | 91 (64) | 98 (69) | 0.28 |
| AC resection | 16 (11) | 16 (11) | > 0.99 |
| Labral repair | 20 (14) | 3 (2) | < 0.01 |
| Biceps tenodesis | 34 (24) | 39 (27) | 0.60 |
Data presented as % (n) or mean ± SD; ASA = American Society of Anesthesiologists.
Results
Pain
On postoperative day 1, patients in the pathway group had lower worst pain with movement (3.3 ± 3.1) compared with patients in the control group (5.6 ± 3.0, mean difference -2.7 [95% CI -3.7 to -1.7]; p < 0.01); lower scores were also seen for pain at rest (1.9 ± 2.3 versus 4.0 ± 2.9, mean difference -2.0 [95% CI -2.8 to -1.3]; p < 0.01) (Table 3). Sensitivity analysis using the GEE model for primary outcome (adding age and total surgery time to the control) showed consistent outcomes with lower pain in the pathway group at 0 to 24 hours (difference in means -2.6 [95% CI -3.57 to -1.62]; p < 0.001).
Table 3.
Pain scores
| Pain scores | Control group (n = 70) | Pathway group (n = 70) | Mean difference (95% CI)a | p value | ||
| n | NRS | n | NRS | |||
| Primary outcome, worst NRS with movement | ||||||
| Preoperative | 69 | 5.4 ± 2.6 | 70 | 6.3 ± 2.4 | ||
| POD 1 | 58 | 5.6 ± 3.0 | 57 | 3.3 ± 3.1 | -2.7 (-3.7 to -1.7) | < 0.01 |
| POD 2 | 61 | 6.1 ± 2.7 | 59 | 6.4 ± 2.5 | -0.1 (-1.0 to 0.9) | 0.88 |
| NRS at resta | ||||||
| Preoperative | 70 | 2.7 ± 2.4 | 70 | 3.2 ± 2.5 | ||
| POD 0 | 70 | 0.9 ± 2.2 | 70 | 1 ± 2.2 | 0.2 (-0.6 to 0.9) | 0.66 |
| POD 1 | 69 | 4.0 ± 2.9 | 67 | 1.9 ± 2.3 | -2.0 (-2.8 to -1.3) | < 0.01 |
| POD 2 | 65 | 4.2 ± 2.1 | 66 | 4.7 ± 2.8 | 0.4 (-0.4 to 1.2) | 0.31 |
| POD 7 | 57 | 2.3 ± 1.9 | 64 | 3 ± 2.4 | 0.6 (-0.3 to 1.4) | 0.18 |
| POD 14 | 51 | 2.2 ± 1.9 | 58 | 1.7 ± 1.4 | -0.5 (-1.3 to 0.4) | 0.31 |
Data presented as mean ± SD.
Difference in means indicates the difference between two estimated means; estimates are from a linear mixed model with group, time, group x time interaction, and baseline NRS as fixed effects and participant as a random effect; POD = postoperative day; NRS = numerical rating scale for pain.
Opioid Use
Pathway patients used less cumulative postoperative opioids (0-48 hours) (mean ± SD pathway oral morphine equivalent use was 23 ± 28 mg versus 44 ± 35 mg, mean difference 21 [95% CI 10 to 32]; p < 0.01). The greatest difference in opioid use was in the first 24 hours after surgery (pathway 7 ± 12 mg versus control 21 ± 21 mg, mean difference -14 [95% CI -19 to -10]; p < 0.01) (Table 4).
Table 4.
Opioid use
| Opioid use, mg oral morphine equivalent | Control group (n = 70) | Pathway group (n = 70) | Mean difference (95% CI)a | p value | ||
| n | Opiod use, mg | n | Opiod use, mg | |||
| Intraoperative | 70 | 11 ± 16 | 70 | 2 ± 6 | -10 (-14 to -5) | < 0.01 |
| 0-24 hours postoperative | 70 | 21 ± 21 | 63 | 7 ± 12 | -14 (-19 to -10) | < 0.01 |
| 24-48 hours postoperative | 55 | 30 ± 21 | 50 | 24 ± 20 | -7 (-12 to -1) | 0.02 |
| POD 7 | 55 | 5 ± 10 | 64 | 3 ± 7 | -1 (-6 to 4) | 0.76 |
| POD 14 | 49 | 1 ± 4 | 56 | 0 ± 1 | -2 (-7 to 3) | 0.54 |
Data presented as mean ± SD.
Difference in means indicates the difference between two estimated means; estimates are from a linear mixed model with group, time, group x time interaction, and baseline NRS or PainOUT as fixed effects and participant as a random effect; POD = postoperative day.
Side Effects and Patient-oriented Outcomes
Adoption of the pathway was associated with improvements in a number of patient-oriented outcomes (Table 5). On postoperative day 1, pathway patients had less interference with staying asleep compared with control patients (0.5 ± 1.6 versus 2.6 ± 3.3, mean difference -2.2 [95% CI -3.3 to -1.1]; p < 0.001); lower scores were also seen for interference with activities (0.9 ± 2.3 versus 1.9 ± 2.9, mean difference -1.1 [95% CI -2 to -0.1]; p = 0.03). Satisfaction with pain treatment on postoperative day 1 was higher among pathway patients compared with control patients (9.2 ± 1.7 versus 8.2 ± 2.5, mean difference 1.0 [95% CI 0.3 to 1.8]; p < 0.01). Lower scores were also seen for drowsiness on postoperative day 1 (1.7 ± 2.7 versus 2.6 ± 2.6, mean difference -0.9 [95% CI -1.7 to -0.1]; p = 0.03). On postoperative day 2, pathway patients had lower nausea scores compared with control patients (0.3 ± 1.1 versus 1 ± 2.1, mean difference -0.7 [-1.2 to -0.01]; p = 0.02).
Table 5.
Patient-oriented outcomes and side effects
| Control group (n = 70) | Pathway group (n = 70) | Mean difference (95% CI)a | p value | |||
| n | Value | n | Value | |||
| PainOUT question | ||||||
| % of pain relief, POD 0 | 64 | 80% ± 30% | 70 | 90% ± 20% | 17% (8%-26%) | 0.01 |
| % of pain relief, POD 1 | 62 | 70% ± 30% | 63 | 90% ± 20% | -3% (-12% to 6%) | < 0.001 |
| Satisfaction with pain treatment, POD 1 | 62 | 8.2 ± 2.5 | 63 | 9.2 ± 1.7 | 1.0 (0.3-1.8) | < 0.01 |
| Satisfaction with pain treatment, POD 2 | 60 | 8.6 ± 1.6 | 62 | 8.8 ± 1.8 | 0.2 (-0.6 to 1.0) | 0.61 |
| Satisfaction with pain treatment, POD 7 | 56 | 8.7 ± 1.5 | 64 | 9.1 ± 1.7 | 0.3 (-0.4 to 1.1) | 0.40 |
| Satisfaction with pain treatment, POD 14 | 51 | 9 ± 1.3 | 58 | 9.1 ± 1.7 | 0.1 (-0.7 to 0.9) | 0.83 |
| Nausea, POD 1 | 65 | 1.1 ± 2.4 | 62 | 0.6 ± 1.8 | -0.5 (-1.0 to 0.0) | 0.07 |
| Nausea, POD 2 | 62 | 1 ± 2.1 | 64 | 0.3 ± 1.1 | -0.7 (-1.2 to -0.1) | 0.02 |
| Interference falling asleep, POD 1 | 65 | 2.6 ± 3.5 | 63 | 0.5 ± 1.7 | -2.0 (-3.2 to -0.9) | 0.001 |
| Interference staying asleep, POD 1 | 65 | 2.6 ± 3.3 | 63 | 0.5 ± 1.6 | -2.2 (-3.3 to -1.1) | < 0.001 |
| % of time in severe pain, POD 1 | 64 | 20% ± 20% | 62 | 10% ± 20% | -8% (17% to 0%) | 0.046 |
| Pain interference, activities in bed, POD 1 | 62 | 3.7 ± 3.9 | 62 | 1.6 ± 3.1 | -2.1 (-3.4 to -0.9) | 0.001 |
| Pain interference, activities out of bed, POD 1 | 64 | 1.9 ± 2.9 | 64 | 0.9 ± 2.3 | -1.1 (-2 to -0.1) | 0.03 |
| Drowsiness, POD 1 | 64 | 2.6 ± 2.6 | 63 | 1.7 ± 2.7 | -0.9 (-1.7 to -0.1) | 0.03 |
| Itching, POD 1 | 64 | 0.5 ± 1.5 | 63 | 0.2 ± 0.8 | -0.3 (-0.8 to 0.3) | 0.31 |
| Itching, POD 2 | 62 | 1.2 ± 2.3 | 64 | 0.5 ± 1.6 | -0.7 (-1.3 to -0.2) | 0.007 |
| Dizziness, POD 1 | 64 | 0.9 ± 2.2 | 63 | 0.3 ± 1.1 | -0.6 (-1.1 to -0.1) | 0.02 |
| Participation in pain treatment decisions (POD 2) | 61 | 9 ± 2.8 | 63 | 9.9 ± 0.6 | 0.9 (0.2-1.6) | 0.01 |
| Side effects (POD2) | ||||||
| Hoarseness, % yes (n) | 62 | 29% (18) | 63 | 27% (17) | 0.88 (0.38-2.06)b | 0.77 |
| Breathing normally, % yes (n) | 62 | 90% (56) | 63 | 95% (60) | 0.88 (0.38-2.06)b | 0.77 |
Data presented as mean ± SD or % (n) unless otherwise indicated.
Difference in means indicates the difference between two estimated means; estimates are from a linear mixed model with group, time, group x time interaction, and baseline PainOut measurement as fixed effects and participant as a random effect.
Data presented as odds ratio (95% CI). Estimates are from a linear mixed model with group, time, and group x time interaction as fixed effects and participant as a random effect; POD = postoperative day.
Discussion
Patients undergoing outpatient rotator cuff surgery often receive some combination of a peripheral nerve block and multimodal analgesia to treat pain and reduce opioid use, but there can be a great deal of variation in the provided clinical care. Implementation of a clinical pathway can lead to greater uniformity while incorporating a larger number of recommended components. We developed an enhanced recovery pathway with patient education about analgesia and opioid use, long-acting brachial plexus blockade, and multimodal analgesia (intraoperative and postoperative). This clinical pathway improved postoperative pain, reduced opioid use after surgery, and improved patient-reported outcomes, although it is subject to limitations and effect-size issues as discussed below. The pathway was developed and implemented by surgeons and anesthesiologists, so it is recommended that clinicians who wish to apply a comparable pathway adapt the protocol to reflect local preferences and similarly recruit an enthusiastic group of colleagues to help promote pathway adoption and continued use.
Limitations
This study has several important limitations. One limitation is that it is a paired cohort study that prospectively compared outcomes before and after pathway implementation. A randomized trial would have allowed stronger assertion of causality. One advantage of conducting a paired cohort study is that the control group is more likely to reflect standard institutional practices because in a randomized trial, the large number of interventions and the involvement of numerous staff not part of the study make it difficult to prevent adoption of pathway components in the control group during the study. This study demonstrated a large number of benefits and did not document any disadvantages from the pathway, making it unlikely that the observed changes were random. Patient enrollment took about 18 months, suggesting that the observed changes were not due to gradual improvement over time. This study had a pragmatic design, with fewer exclusion criteria and higher rates of enrollment, which can make a cohort study can be more generalizable than a randomized trial.
There are organization-level limitations to use of this pathway. To be eligible for the pathway group, patients needed to be scheduled with a participating anesthesiologist. This suggests that those seeking to adopt or replicate this study would need to ensure buy-in from participating physicians. Continued effort would likely be necessary to overcome inertia and promote widespread participation, otherwise the pathway might fall into disuse as has sometimes occurred with previous innovations in practice.
An additional limitation is that not all eligible patients were enrolled due to occasional lack of availability of research staff. Research staff availability was not dictated by patient characteristics, so this factor is unlikely to change results. All patients were scheduled for rotator cuff surgery but additional procedures were allowed with heterogeneity in the surgery actually performed. This limitation probably increased the SD of the results but it is not likely that it changed the direction of the observed results. This was a single-center study conducted at a hospital with a strong institutional preference for regional anesthesia. Generalizability of the results could have been increased by a multicenter design. All study participants received regional anesthesia. It is possible that adoption of this pathway at centers that do not routinely employ nerve blocks would result in even larger effect sizes, or conversely, that inexpert attempts at nerve blocks would have fewer benefits and even potentially cause harm. The study was underpowered for evaluation of rare events. It is possible that some of the changes reflect trends over time that would have occurred regardless of the study, but study enrollment covered a relatively short period and the study demonstrated multiple differences. It is not possible to ascribe with certainty the effects of the pathway to any particular component. It is possible that some of the observed differences are statistically significant but are not large enough to be clinically important. MCIDs (the smallest change in the value of an outcome that should be considered clinically relevant) [12] have been determined for outcomes such as NRS pain but are not described for many of the secondary outcomes. Lastly, it is possible that a restricted pathway that included only patient education about opioid use as well as incorporation of a long-acting peripheral nerve block would provide similar results as this study. However, further research would be needed to answer this question.
Pain
Adoption of this novel clinical pathway was associated with a decrease in early pain with movement, which was the primary outcome. Concerns have been raised that performing nerve blocks does not reduce pain but rather shifts pain to a later point because of rebound pain. There was no evidence of increased rebound pain related to prolonged duration of the nerve block, perhaps due to use of perineural dexamethasone [1]. The worst pain with movement on postoperative day 2 in the pathway group was essentially the same as the preoperative score, which further does not support increased rebound pain. A previous paper [4] demonstrated benefits from an analgesic protocol for shoulder surgery patients, but the pain scores were higher (6.5 versus 3.3 at 24 hours). Both the current study and the previous paper [4] demonstrate extensive improvements from adoption of a pathway, but the pathway components and results were different. It is difficult to make direct comparisons because of potential confounders. Our paper corroborates and confirms their findings, but there were prominent differences in the pathway and the magnitude of benefit. For instance, Elkassabany et al. [4] did not include a formal patient education component, the nerve block had a likely shorter duration (ropivacaine versus bupivacaine with dexamethasone), intraoperative ketamine was not administered, and an NSAID was given for 1 day instead of 3 days. Overall, the changes produced by adoption of the pathway primarily occurred on postoperative day 1 and were of limited magnitude. The pain difference exceeds an MCID of 2, but not by a large margin. It is, however, likely advantageous to keep patients reasonably comfortable with less opioid use. Further research is needed to determine whether the benefit in reduced pain is of sufficient magnitude and duration to merit the use of the pathway with its costs and potential risks. Larger studies could quantify and characterize complications in both control and pathway groups. The current study design and sample size do not address the question of whether the use of education, long-acting nerve blocks, and multimodal analgesia result in more or fewer complications than monotherapy with general anesthesia and opioids alone. Previous work has shown benefits for the pathway interventions considered singly, but sample-size considerations and concerns about polypharmacy remain to be addressed with larger studies. Patient-oriented research could be useful to determine whether patients wish to bear the potential costs and risks for the projected benefits.
Opioid Use
Patients in the pathway used fewer opioids during and after surgery. Previous work showed that patients given preoperative opioid education take fewer opioid medications after rotator cuff repair surgery (19% less, cumulatively at 2 weeks, with continued opioid use) [17]. In comparison, among patients in the pathway in this study, opioid use was reduced by 60% (at 0 to 48 hours), and the median opioid use was 0 mg at 7 days. This suggests that patient education, although important, does not reduce opioid use as much as the pathway, which combined patient education with a long-acting block plus multimodal analgesia. A pathway has been shown to reduce opioid use after shoulder surgery [4] but opioid use was higher (about 25 mg versus 7 mg on postoperative day 1).
Side Effects and Patient-oriented Outcomes
Differences in patient-oriented outcomes included reduced pain at rest, improved satisfaction, reduced side effects, and reduced interference by pain with activities and sleep. Most differences occurred in the first postoperative day (however, opioid sparing persisted into postoperative day 2), and some of the observed differences were likely too small to be clinically relevant. The recommended core outcome measures were assessed: pain, physical functioning, emotional functioning, satisfaction with treatment, adverse events, and participant disposition [3]. All observed differences between groups favored the pathway, which suggests that the benefits did not occur at random as a result of a multiplicity of comparisons. There was no evidence of increased adverse events associated with the pathway. This is reassuring, but given the sample size, it is likely that rare pathway-related adverse events would occur with a larger sample size. Of course, it is also likely that an examination of a larger control group sample would reveal rare control group–related side effects (potentially caused by general anesthesia, increased pain, and increased use of opioids in the control group). Recent concerns have emerged which indicate that although gabapentinoids may have opioid-sparing effects, concomitant postoperative use of gabapentinoids and opioids [8] may increase the risk of respiratory depression or confusion. As noted above, consideration should be given to effect sizes (some of which were small) and the possibility that polypharmacy may pose risks [11]. In a previous shoulder pathway study [4], pain interfered more with activities (interference with activities in bed score 4.7 versus 1.6 at 24 hours), and side effects were more prominent (drowsiness score 5.1 versus 1.7 at 24 hours).
Conclusion
Adoption of an analgesic pathway for arthroscopic rotator cuff surgery patients was associated with reductions in pain, opioid use, and side effects. Patients reported decreased interference with activities, better sleep, and higher satisfaction. There are potential risks from the nerve block as well as from multiple analgesic drugs, which may pose risks to elderly patients in particular. This pathway, based on a long-lasting nerve block, multimodal analgesia, and patient education, may be considered for adoption. In evaluating this pathway for local use, clinicians should weigh the effect sizes against the possibility of potential risks that may emerge with large-scale use, consider the difficulties involved in adapting a pathway to local practice so that pathway will persist, and recognize that this study only enrolled patients among surgeons and anesthesiologists who advocated for the pathway; results may have been different with less enthusiastic clinicians. Future studies could focus on reducing pain that occurs after 24 hours postsurgery, investigate ways to promote ongoing use of pathways, and perform ongoing surveillance of existing protocols to examine safety issues and incidence of rare side effects.
Supplementary Material
Acknowledgments
We thank Kara Fields for performing the power analysis and planning the statistical analysis, Danya DeMeo for serving as the back-up research assistant, and the perioperative nursing staff at the Hospital for Special Surgery for their excellent care and enthusiastic support. We also thank the following anesthesiologists who (in addition to the coauthors) provided clinical care for study patients: Drs. A. Lee, L. Baaklini, J Beckman, D. Bhagat, M. Brouillette, C. Wu, B. Carson, S. Cheng, D. Kim, K. Delpizzo, C. DiMeo, C. Edmonds, M. Friedman, E. Goytizolo, D. Green, J. B. Liu, M. Kalsi, V. LaSala, D. Maalouf, J. Oxendine, S. Pakala, T. Quinn, S. Kim, C. Swamidoss, L. Turteltaub, W. Urmey, and D. Wetmore.
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
The institution of one or more of the authors (JTY) has received, during the study period, funding from the Research and Education Fund of the Department of Anesthesiology, Critical Care & Pain Management at Hospital for Special Surgery. REDCap use was supported by the National Center for Advancing Translational Science of the National Institute of Health (UL1TR000457).
One of the authors certifies that he (JTY), or a member of his immediate family, has received payments or benefits, during the study period, in an amount of less than USD 10,000 from Mallinckrodt.
One of the authors certifies that he (DMD), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in an amount of USD 10,000 to USD 100,000 from Zimmer Biomet Inc.
One of the authors certifies that he (JSD), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in an amount of USD 10,000 to USD 100,000 from Zimmer Biomet; in an amount of less than USD 10,000 from Conmed.
One of the authors certifies that he (LVG), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in an amount of less than USD 10,000 from Zimmer Biomet; in an amount of less than USD 10,000 from DePuy Mitek; in an amount of USD 10,000 to USD 100,000 from Exactech Inc; in an amount of less than USD 10,000 from Smith and Nephew; in an amount of less than USD 10,000 from Imagen Technologies Inc; and in an amount of less than USD 10,000 from Responsive Arthroscopy.
Ethical approval for this study was obtained from the institutional review board at the Hospital for Special Surgery (IRB#2018-0814). The trial was registered at clinicaltrials.gov (NCT03717753).
This work was performed at Hospital for Special Surgery, New York, NY, USA.
Contributor Information
Ellen M. Soffin, Email: soffine@hss.edu.
Audrey Tseng, Email: tsenga@hss.edu.
Haoyan Zhong, Email: zhonga@hss.edu.
David M. Dines, Email: dinesd@hss.edu.
Joshua S. Dines, Email: dinesj@hss.edu.
Michael A. Gordon, Email: gordonm@hss.edu.
Bradley H. Lee, Email: leeb@hss.edu.
Kanupriya Kumar, Email: kumark@hss.edu.
Richard L. Kahn, Email: kahnr@hss.edu.
Meghan A. Kirksey, Email: kirkseym@hss.edu.
Aaron A. Schweitzer, Email: aaschweitz@gmail.com.
Lawrence V. Gulotta, Email: gulottal@hss.edu.
References
- 1.An K, Elkassabany NM, Liu J. Dexamethasone as adjuvant to bupivacaine prolongs the duration of thermal antinociception and prevents bupivacaine-induced rebound hyperalgesia via regional mechanism in a mouse sciatic nerve block model. PLoS One. 2015;10:e0123459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng J, Kahn RL, YaDeau JT, et al. The fibromyalgia survey score correlates with preoperative pain phenotypes but does not predict pain outcomes after shoulder arthroscopy. Clin J Pain. 2016;32:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9-19. [DOI] [PubMed] [Google Scholar]
- 4.Elkassabany NM, Wang A, Ochroch J, Mattera M, Liu J, Kuntz A. Improved quality of recovery from ambulatory shoulder surgery after implementation of a multimodal perioperative pain management protocol. Pain Med. 2019;20:1012-1019. [DOI] [PubMed] [Google Scholar]
- 5.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149-158. [DOI] [PubMed] [Google Scholar]
- 6.Gadsden J, Hadzic A, Gandhi K, et al. The effect of mixing 1.5% mepivacaine and 0.5% bupivacaine on duration of analgesia and latency of block onset in ultrasound-guided interscalene block. Anesth Analg. 2011;112:471-476. [DOI] [PubMed] [Google Scholar]
- 7.Goon AK, Dines DM, Craig EV, et al. A clinical pathway for total shoulder arthroplasty-a pilot study. HSS J. 2014;10:100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannon CP, Fillingham YA, Browne JA, Schemitsch EH. Gabapentinoids in total joint arthroplasty: the clinical practice guidelines of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopedic Surgeons, Hip Society, and Knee Society. J Arthroplasty. 2020;2730-2738. [DOI] [PubMed] [Google Scholar]
- 9.Kahn RL, Cheng J, Gadulov Y, Fields KG, YaDeau JT, Gulotta LV. Perineural low-dose dexamethasone prolongs interscalene block analgesia with bupivacaine compared with systemic dexamethasone: a randomized trial. Reg Anesth Pain Med. 2018;43:572-579. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606-617. [DOI] [PubMed] [Google Scholar]
- 11.Leopold SS. Editorial: When “safe and effective” becomes dangerous. Clin Orthop Relat Res . 2014;472:1999-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leopold SS, Porcher R. Editorial: The minimum clinically important difference – the least we can do. Clin Orthop Relat Res . 2017;475:929-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118:424-429. [DOI] [PubMed] [Google Scholar]
- 14.Navarro RA, Lin CC, Foroohar A, Crain SR, Hall MP. Unplanned emergency department or urgent care visits after outpatient rotator cuff repair: potential for avoidance. J Shoulder Elbow Surg. 2018;27:993-997. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld DM, Ivancic MG, Hattrup SJ, et al. Perineural versus intravenous dexamethasone as adjuncts to local anaesthetic brachial plexus block for shoulder surgery. Anaesthesia. 2016;71:380-388. [DOI] [PubMed] [Google Scholar]
- 16.Rothaug J, Zaslansky R, Schwenkglenks M, et al. Patients’ perception of postoperative pain management: validation of the International Pain Outcomes (IPO) questionnaire. J Pain. 2013;14:1361-1370. [DOI] [PubMed] [Google Scholar]
- 17.Syed UAM, Aleem AW, Wowkanech C, et al. Neer Award 2018: The effect of preoperative education on opioid consumption in patients undergoing arthroscopic rotator cuff repair: a prospective, randomized clinical trial. J Shoulder Elbow Surg. 2018;27:962-967. [DOI] [PubMed] [Google Scholar]
- 18.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27:485-489. [DOI] [PubMed] [Google Scholar]
- 19.White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg. 2005;101:S5-22. [DOI] [PubMed] [Google Scholar]
- 20.YaDeau JT, Dines DM, Liu SS, et al. What pain levels do TSA patients experience when given a long-acting nerve block and multimodal analgesia? Clin Orthop Relat Res. 2019;477:622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.YaDeau JT, Gordon MA, Goytizolo EA, et al. Buprenorphine, clonidine, dexamethasone, and ropivacaine for interscalene nerve blockade: a prospective, randomized, blinded, ropivacaine dose-response study. Pain Med. 2016;17:940-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.YaDeau JT, Paroli L, Fields KG, et al. Addition of dexamethasone and buprenorphine to bupivacaine sciatic nerve block: a randomized controlled trial. Reg Anesth Pain Med. 2015;40:321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.