Abstract
BNO 1095, a standardized dry extract from the fruits of Vitex agnus-castus , represents an approved herbal medicinal product for the treatment of premenstrual syndrome. Angiogenesis, the formation of new blood vessels from pre-existing capillaries, plays a major role in physiological situations, such as wound healing or tissue growth in female reproductive organs, but it is also of great importance in pathophysiological conditions such as chronic inflammatory diseases or cancer. Angiogenesis is a highly regulated multi-step process consisting of distinct key events that can be influenced pharmacologically. Few studies suggested anti-angiogenic actions of V. agnus-castus fruit extracts in in vivo and ex vivo models. Here, we provide for the first time profound in vitro data on BNO 1095-derived anti-angiogenic effects focusing on distinct angiogenesis-related endothelial cell functions that are inevitable for the process of new blood vessel formation. We found that V. agnus-castus extract significantly attenuated undirected and chemotactic migration of primary human endothelial cells. Moreover, the extract efficiently inhibited endothelial cell proliferation and reduced the formation of tube-like structures on Matrigel. Of note, the treatment of endothelial cell spheroids almost blocked endothelial sprouting in a 3D collagen gel. Our data present new and detailed insights into the anti-angiogenic actions of BNO 1095 and, therefore, suggest a novel scope of potential therapeutic applications of the extract for which these anti-angiogenic properties are required.
Key words: Vitex agnus-castus, Lamiaceae, endothelial cells, angiogenesis-related cell functions
Abbreviations
- CAM
chorioalantoic membrane
- ECM
extracellular matrix
- HMPC
Committee on Herbal Medicinal Products
- HUVECs
human umbilical vein endothelial cells
- MMPs
matrix metallopeptidases
- VEGF
vascular endothelial growth factor
- Y : FMI
forward migration index Y
Introduction
Vitex agnus-castus L., also referred to as chaste tree or monkʼs pepper, belongs to the family of Lamiaceae. The plant is originally located in the Mediterranean area, and extracts from fruits and leaves have been traditionally used to treat pre-menstrual, post-menstrual, and fertility disorders, among them amenorrhoea or dysmenorrhea, pre-menstrual syndrome, corpus luteum insufficiency, or infertility since ancient Greek and Roman times 1 , 2 , 3 , 4 . Moreover, a variety of studies suggest that V. agnus-castus shows activity against cancer, inflammation, or osteopenic syndromes but also attribute an immunomodulatory, antimicrobial, and antifungal impact 5 . According to the HMPC, a hydroethanolic dry extract (ethanol 60%, drug-extract ratio 6 – 12 : 1) from the fruits of V. agnus-castus can be applied for the treatment of premenstrual syndrome as well-established use herbal medicinal product. Other hydroethanolic dry extracts can be used for the relief of minor symptoms in the days before menstruation (premenstrual syndrome) as traditional use herbal medicinal product. [ https://www.ema.europa.eu/en/medicines/herbal/agni-casti-fructus ]. The fruit extracts are comprised of essential oils, iridoids, flavonoids, diterpenes, tannins, and phenolic compounds. While the extractsʼ mode of action is up to now widely unknown, studies suggested modulation of the delta and mu opioid receptors 6 . Moreover, in vitro studies indicate that V. agnus-castus extract inhibits prolactin secretion by binding to dopamine receptor D2 7 , 8 . Identification approaches for bioactive compounds in the plant suggested diterpenes, in particular cleroda-dienols, to be responsible for this dopaminergic actions of V. agnus-castus 3 .
However, angiogenesis–the formation of new blood vessels from pre-existing capillaries–plays a crucial role during the menstrual cycle, and potential V. agnus-castus extract-derived effects on this process are of high interest. Up to now, only few studies focused on the activity of the extract within this context. An in vivo study analyzing V. agnus-castus fruit fractions demonstrated anti-angiogenic properties in a chick CAM assay and in a zebrafish model 9 . In addition, the performance of an ex vivo rat aortic ring assay using a methanol extract of V. agnus-castus leaves revealed a marked inhibition of sprouting 10 . The identification of V. agnus-castus as being effective to suppress angiogenesis might be beneficial for opening a new perspective for additional fields of application with regard to angiogenesis-related pathophysiological conditions such a cancer or chronic inflammatory diseases.
Angiogenesis is a highly regulated multi-step process. The key events during angiogenesis include endothelial cell-driven enzymatic degradation of the ECM and endothelial cell migration and proliferation, followed by sprouting events and finally vessel maturation. In this study, we made an attempt to shed light on the potential of BNO 1095, an approved and standardized dry extract from fruits of V. agnus-castus , to affect angiogenesis-related endothelial cell functions in vitro . For the detailed investigation of crucial key steps of in vitro angiogenesis, we used primary HUVECs. Within the scope of this work, we focused on potential extract-derived effects on undirected and chemotactic endothelial cell migration and HUVEC proliferation, as well as the impact of BNO 1095 on in vitro tube formation and sprouting from HUVEC spheroids.
Results
Before actions on in vitro angiogenic features were assessed in HUVECs, potential BNO 1095-derived effects on cell viability were determined. In Fig. 1 a and b , we demonstrate that after 24 h of incubation, the extract neither impaired membrane integrity nor induced apoptosis in endothelial cells. When incubation times were extended over a period of up to 72 h, apoptosis as well as membrane integrity impairment were significantly initiated by an extract concentration of 100 µg/mL ( Fig. 1 a, b ). Due to these findings, for experimental purposes for a treatment period up to 24 h, the extract concentration of 100 µg/mL was not exceeded, while for incubation periods up to 72 h, only concentrations up to 30 µg/mL were used.
Fig. 1.
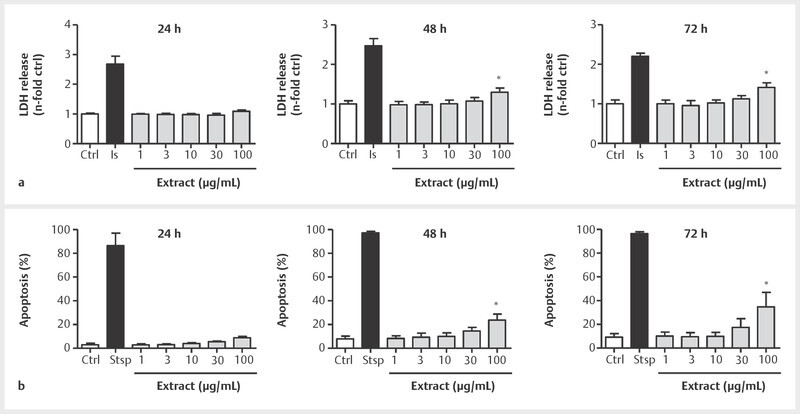
BNO 1095 does not induce cytotoxic effects in the used experimental setups. To determine potential extract-derived effects on cell viability, confluent HUVECs were treated with the indicated concentrations of BNO 1095 for 24, 48, and 72 h. a HUVECs were subsequently analyzed for membrane integrity by measuring LDH release. The LDH-induced conversion of tetrazolium salt into formazan was determined by absorbance measurement at 490 nm using a plate reader. The application of a lysis solution (ls) served as positive control. b For apoptosis measurement, HUVECs were permeabilized and simultaneously treated with propidium iodide (PI) to determine subdiploidic DNA content in cells by flow cytometry. Staurosporine served as positive control. Data are expressed as mean ± SD; A: n = 4; B: n = 3; *p ≤ 0.05 vs. ctrl.
Endothelial cell migration is one of the key features during the process of angiogenesis. To analyze potential effects of BNO 1095 on endothelial cell motility in vitro , their migratory capacity was analyzed. In a first approach, we focused on effects of undirected endothelial cell migration. Therefore, a scratch was inflicted into a HUVEC monolayer, and the cells were allowed to close the gap by undirected migration. As depicted in Fig. 2 a , we demonstrate that undirected HUVEC migration was significantly impaired upon extract treatment (30 and 100 µg/mL) as the cells were not able to close the inflicted gap. In a second approach, we determined the anti-migratory potential of the extract on directed migration of endothelial cells. In a Boyden chamber assay, HUVECs were allowed to migrate in the direction of a chemoattractant (FCS) gradient. The treatment with 30 and 100 µg/mL of BNO 1095 resulted in a markedly reduced migration towards the FCS gradient. For 100 µg/mL, this effect was significant ( Fig. 2 b ). For further insights into the effects of the extract on chemotactic migration of endothelial cells, a migration assay using chemotaxis slides and microscopic monitoring was performed. Upon extract treatment, HUVECs were allowed to migrate in the direction of an FCS gradient. Single cell tracking revealed that BNO 1095 strongly attenuated the migration capacity of HUVECs in the direction of the chemoattractant gradient, as indicated by a significantly reduced forward migration index in direction of the y-axis (FMI : Y) and velocity. Furthermore, treatment with the extract resulted in a significantly diminished Euclidean and accumulated distance covered by HUVECs, while the migration directness remained unimpaired ( Fig. 2 c ). This effect was most prominent when HUVECs were treated with 100 µg/mL of the extract. To sum up these findings, we can conclude that BNO 1095 strongly impairs migratory events and motility in HUVECs.
Fig. 2.
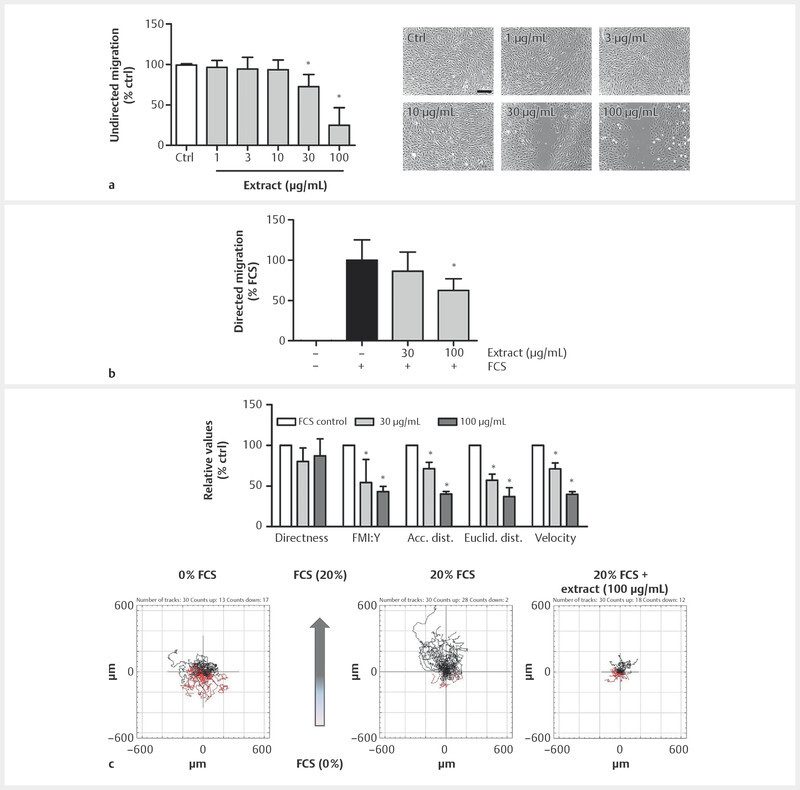
BNO 1095 attenuates endothelial cell migration. a A scratch was inflicted into a confluent HUVEC monolayer before the cells were treated with the indicated BNO 1095 concentrations. Starvation medium served as positive control (not shown) and vehicle control (DMSO 0.1%) served as control (ctrl). The cells were allowed to migrate into the gap for 12 h before image quantification was performed using ImageJ. Scale bar: 100 µm. One representative image for each condition is shown. b 100 000 HUVECs were seeded onto collagen G-coated Transwell inserts in ECGM After 4 h of incubation, the cells were treated with respective concentrations of BNO 1095 or vehicle (DMSO 0.1%) in medium 199 without serum. Subsequently, medium 199 containing 20% FCS was added to the lower compartment for the generation of a chemoattractant gradient. After 16 h, migrated HUVECs were stained with a crystal violet solution and air-dried overnight. Removing crystal violet from cells by acetic acid and absorption measurement at 590 nm allowed the quantification of migrated cells by a plate reader. FCS as chemoattractant alone served as control. c Eighteen thousand HUVECs were seeded onto chemotaxis slides. A 20% FCS gradient was added, and the cells were treated with indicated concentrations of BNO 1095 or vehicle (DMSO 0.1%). Chemotactic migration proceeded for 20 h. Tracking 30 cells per condition using ImageJ allowed determination of extract-derived effects on indicated parameters. Data are expressed as mean ± SD; A: n = 5; *p ≤ 0.05 vs. ctrl; B: n = 4; *p ≤ 0.05 vs. FCS ctrl; C: n = 3; *p ≤ 0.05 vs. FCS ctrl.
During the process of angiogenesis, endothelial cell proliferation is of high importance to induce the formation of new capillaries. Therefore, we focused in a further approach on potential extract-derived effects on HUVEC proliferation. Crystal violet staining of proliferating HUVECs revealed that the extract was able to markedly inhibit the increase of endothelial cell number with an IC 50 of 19 µg/mL ( Fig. 3 a ).
Fig. 3.
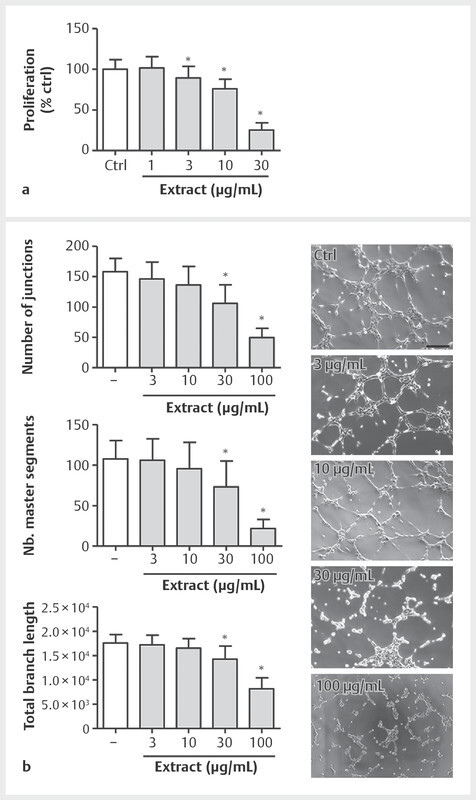
BNO 1095 inhibits endothelial cell proliferation and tube-like structure formation. a One thousand five-hundred HUVECs/well were seeded on 96-well plates. After 24 h, the cells were treated with indicated concentrations of BNO 1095 or vehicle (DMSO 0.1%). Seventy-two h later, HUVECs were fixed and stained using a crystal violet solution. After air-drying, the crystal violet was dissolved from HUVECs using acetic acid and absorbance was measured at 590 nm using a plate reader. b Ten thousand HUVECs per well were seeded on growth factor-reduced solidified Matrigel and treated with indicated concentrations of BNO 1095 or vehicle (DMSO 0.1%). The formation of capillary-like structures was allowed for 5.5 h before microscopic images were taken and quantified for the indicated parameters. Data are expressed as mean ± SD; A: n = 4; *p ≤ 0.05 vs. ctrl; B: n = 4; *p ≤ 0.05 vs. ctrl. Scale bar: 500 µm. One representative image for each condition is shown.
The formation of new blood vessels is a highly regulated multi-step process. To study extract-derived effects on angiogenesis in a well-feasible and reliable in vitro system, a tube formation assay using Matrigel was performed. This basement matrix enables HUVECs to form angiogenesis-related capillary-like structures that can be easily quantified. As depicted in Fig. 3 b , the endothelial cell network formation was successfully induced in control cells treated with DMSO (vehicle control). The application of increasing BNO 1095 concentrations strongly and significantly reduced the capacity of HUVECs to form tube-like structures, as indicated by a concentration-dependent decrease in the number of junctions and master segments as well as in the total branch length. Of note, the arrangement of cells to form master segments proceeded upon extract treatment, while the elongation for extensive branching was strongly attenuated, resulting in severe impairment of tube-like structure formation in HUVECs.
Since we demonstrated that BNO 1095 is able to substantially reduce angiogenesis-related endothelial cell functions, we used a robust 3D in vitro method to analyze extract-derived effects on endothelial cell sprouting from HUVEC spheroids embedded in collagen I. Exploiting the benefits of the ECM component collagen I and of the VEGF allows a reliable assessment of a potential impact on angiogenic cell functions in vitro . In Fig. 4 , we demonstrate that the application of VEGF to collagen I-embedded HUVEC spheroids significantly induced the formation of sprouts. Both sprout number per spheroid and total sprout number were strongly increased by the treatment with the growth factor. Pre-incubation of HUVEC spheroids with increasing concentrations of the extract significantly reduced the number of sprouts per spheroid and the total sprout length in a concentration dependent way. Interestingly, the application of 100 µg/mL of BNO 1095 decreased the total sprout length almost down to the levels of the vehicle control.
Fig. 4.
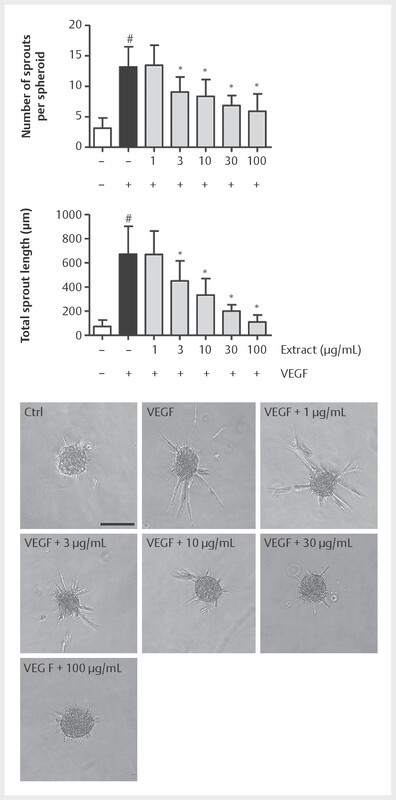
BNO 1095 reduces endothelial cell sprouting from spheroids. Four hundred HUVECs were used to form spheroids employing the hanging-drop method. The next day, HUVEC spheroids were embedded into a 3D rat tail collagen I gel. The spheroids were treated with indicated concentrations of BNO 1095 or vehicle (DMSO 0.1%) before sprout formation was induced by the application of VEGF (10 ng/mL). HUVEC spheroids were allowed to form sprouts for 20 h before they were microscopically analyzed for the indicated parameters using ImageJ. Data are expressed as mean ± SD; n = 4; *p ≤ 0.05 vs. VEGF. Scale bar: 100 µm. One representative image for each condition is shown.
Discussion
In the physiological situation, angiogenesis is a highly regulated and coordinated process. Here, the formation of new blood vessels proceeds during embryonic development, in the course of wound healing and within the menstrual cycle. Nevertheless, angiogenesis also plays a pivotal role under pathophysiological conditions. In chronic inflammatory diseases or cancer, this process is prone to be ongoing and uncontrolled. Tumor growth and metastasis are highly dependent on constant development of new blood vessels being indispensable for the supply of nutrients and oxygen and for cancer cell spreading 11 . Therefore, anti-angiogenic compounds or extracts are of utmost interest for the treatment against tumor development and might introduce a new aspect for the application of V. agnus-castus extract. Approved standardized dry extracts of V. agnus-castus fruits are therapeutically used against dysregulation of the menstrual cycle, premenstrual syndrome, infertility, or mastodynia. Beyond these applications, only few studies demonstrated V. agnus-castus -derived anti-angiogenic effects in vivo and in vitro . An ex vivo study by Sahib et al. demonstrated that a methanolic leaf extract of V. agnus-castus markedly reduced sprouting from rat aortic rings at 100 µg/mL, while sprout formation remained widely unimpaired when a chloroform or water extract was applied. In addition, in an in vitro experiment using HUVECs, they demonstrate that the methanolic leaf extract was able to reduce endothelial cell proliferation with an IC 50 of 80 µg/mL 12 . The same group demonstrated in an in vivo approach that blood vessel growth in the CAM of chicken embryos was considerably inhibited by the methanolic leaf extract 10 . Within the scope of our study, we found that the used ethanolic extract of V. agnus-castus potently reduced angiogenesis-related key features in endothelial cells in vitro . With our approach, we are the first who systematically analyzed the potential of BNO 1095, a standardized dry extract from V. agnus-castus fruits, to interfere with principal events that play a pivotal role during the process of angiogenesis, among them endothelial cell proliferation, migration (undirected and chemotactic), and sprouting in vitro .
We found that BNO 1095 reduced the proliferation capacity of HUVECs with an IC 50 of 19 µg/mL, indicating an advantage over a methanolic leaf extract. In addition, we found that BNO 1095 potently inhibited undirected migration of HUVECs already at 30 µg/mL while the administration of 100 µg/mL reduced the migratory capacity of endothelial cells around by 75%. Moreover, results of a Boyden chamber assay revealed that the migration in the direction of an FCS gradient was already significantly attenuated at 30 µg/mL, and when 100 µg/mL of BNO 1095 was applied to the cells, it reduced the directed migration of HUVECs by about 40%. The performance of a 2D chemotaxis assay provided deeper insights into the impact of the extract on chemotactic migration of HUVECs. This approach not only gives novel information of endothelial cell migratory capacity per se but also sheds light on the impact of the extract on migration efficiency of the forward migration in the direction of FCS, the migrated distance, and the velocity of migration. Indeed, BNO 1095 effectively inhibited these important hallmarks of chemotactic migration that are indispensable for the process of angiogenesis. Of note, the treatment with 100 µg/mL of the extract reduced forward migration, Euclidean, and accumulated distance as well as velocity to less than 50% compared to the control. These findings indicate that important key events of angiogenic functions, endothelial proliferation, and migration are strongly and significantly inhibited by the extract. In vivo studies employing zebrafish embryos and the chick CAM assay demonstrated that chloroform and ethyl acetate fractions of a V. agnus-castus fruit extract substantially reduced microvessel formation 9 . In line with this, we show in vitro that the application of BNO 1095 reduced the formation of capillary-like structures on Matrigel and the formation of VEGF-induced sprouts from HUVEC spheroids. In the tube formation assay, this effect was detectable starting at 30 µg/mL. Interestingly, HUVECs were able to align in an assembly typical for vessel formation but were incapable of elongation, a key feature of stalk cells in the angiogenic process 13 . Importantly, in a 3D spheroid assay, BNO 1095 demonstrated a much higher inhibitory activity on HUVEC sprouting starting already at 3 µg/mL. This concentration-dependent inhibition resulted in a complete blocking of VEGF-activated total sprout length using 100 µg/mL of the extract. In the physiological situation, one of the first events in angiogenesis is the degradation of ECM components such as collagen, fibronectin, or laminin, which are enzymatically degraded by MMPs. Studies in a murine in vivo model revealed that V. agnus-castus extract significantly decreased the serum levels of MMP9 14 . In our experiments, spheroids were embedded into a collagen gel. If endothelial MMP9 levels might be down-regulated upon extract treatment, the initiation of sprouting would be impaired.
The formation of reactive oxygen species is known to promote angiogenesis 15 . Experiments using the leukemia cell line HL-60 demonstrated the down-regulation of Nox2 upon V. agnus-castus extract treatment, indicating potential antioxidant properties of the extract 16 . An in vivo study employing an aging mouse model suggested antioxidant extract activities, as catalase and superoxide dismutase activity was increased in aging mice by V. agnus-castus 17 . Therefore, it is conceivable that, at least in part, inhibitory effects of the extract on in vitro angiogenesis-related cell functions might be attributed to antioxidant actions of V. agnus-castus .
Beyond the widely known actions of V. agnus-castus for the treatment of menstrual disorders or premenstrual syndrome, by our in vitro studies, we introduced for the first time the beneficial potential of the approved ethanolic fruit extract to successfully inhibit crucial angiogenesis-related endothelial cell functions such as proliferation, migration, capillary-like structure formation, and sprouting from spheroids. These findings might disclose a new therapeutic implementation of the extract such as for the treatment of cancer or chronic inflammatory diseases that are characterized by ongoing angiogenesis. Moreover, as premenstrual disorders have often been ascribed to endometriosis, an anti-angiogenic impact of V. agnus-castus might be beneficial. The development of ectopic endometriosis is strongly dependent on angiogenesis 18 , 19 . Therefore, a variety of studies suggest anti-angiogenic therapies for the treatment of endometriosis using growth factor inhibitors, statins, endogenous angiogenesis inhibitors, dopamine agonists, phytochemicals, fumagillin, and others 20 , 21 . Although an anti-angiogenic approach might be associated with a risk of fertility impairment, a number of angiogenesis-inhibiting compounds have been identified that do not interfere with follicular development and do not induce side effects in the female reproductive organs in vivo 22 , 23 , 24 , 25 . Of note, V. agnus-castus has been described to exhibit dopaminergic actions by binding to the dopamine receptor. Other dopamine agonists such as quinagolide or cabergoline have also been shown to exert anti-angiogenic actions for the treatment of endometriosis in vivo 26 . However, clinical efficacy and success employing an anti-angiogenic strategy within this context is still unclear.
The main purpose of our in vitro study was the detailed investigation of potential effects of BNO 1095 on distinct principle endothelial cell functions that are inevitable for angiogenesis-related processes. Further studies analyzing the signaling pathways endothelial cells utilize during proliferation, migration, and sprouting will be conducted to get deeper insights into the action of BNO 1095 in the context of angiogenesis. Moreover, the identification of bioactive compounds in the extract that are responsible for the actions on distinct key events of angiogenesis-associated cell functions have to be elucidated in future studies.
Materials and Methods
BNO 1095 and compounds
BNO 1095 is an ethanolic (70% v/v) fruit extract of V. agnus-castus with a drug extract ratio of 7 – 11 : 1 and the active pharmaceutical ingredient of the herbal medicinal product Agnucaston. The extract (lot number 770 134) was kindly provided by Bionorica SE, Neumarkt, Germany. The HPLC fingerprint of the extract (UV detection at 205 nm) 27 is provided in Fig. 1S (Supporting Information). Human recombinant VEGF 165 was purchased from Peprotech. Staurosprine and methylcellulose were from Sigma-Aldrich Chemie GmbH, and Matrigel Growth Factor Reduced Basement Membrane Matrix as well as rat tail collagen I were purchased from Corning GmbH.
Extract preparation for in vitro characterization
The extract was dissolved in DMSO (Sigma-Aldrich Chemie GmbH) to a final concentration of 100 mg/mL by sonication in an ultrasonic water bath (35 kHz) over a period of 30 min with frequent vortexing. After centrifugation for 10 min at 3000 g , the supernatant was transferred into new tubes and stored in aliquots at − 80 °C to prevent thaw-freeze-cycles. For experimental purposes, the extract was used up to a concentration of 100 µg/mL not exceeding a final concentration of 0.1% DMSO.
Cell culture
HUVECs were isolated according to Jaffe et al. 28 . For cultivation, the cells were split in a ratio of 1 : 3 in endothelial cell growth medium (EASY ECGM; PELOBiotech) supplemented with 10% FCS (Biochrom), 100 U/mL penicillin, 100 µg/mL streptomycin (PAN-Biotech), 2.5 µg/mL amphotericin B (PAN-Biotech), and a supplement mixture (PELOBiotech) on collagen G (10 µg/mL in PBS, Biochrom)-coated plastic. The cells were cultivated under constant humidity, at 37 °C and an atmosphere with 5% CO 2 and 95% air. The cells were used for experimental purposes exclusively in passage 3.
Lactate dehydrogenase (LDH) release assay
For the determination of potential extract-derived effects on cell membrane integrity the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega GmbH) was performed according to the manufacturerʼs instructions. In brief, confluent HUVECs on 96-well plates were treated with indicated concentrations of the extract for 24, 48, and 72 h. For positive control, HUVECs were treated with a lysis solution for the last 45 min of incubation. Subsequently, 50 µL of cell culture supernatants were added to a new plate and incubated with 50 µL of substrate solution for 30 min at room temperature and protected from light. To stop the substrate converting process of the enzyme, 50 µL of stopping solution was added. The amount of LDH in the cell culture supernatant was measured at 490 nm using a plate reader (VarioskanFlash, Thermo Fisher Scientific).
Apoptosis assay
To exclude potential extract-derived effects on apoptosis induction in endothelial cells, an apoptosis assay, according to a method by Nicoletti et al. 29 , was performed. Therefore, confluent HUVECs were treated with the indicated concentrations of BNO 1095 for 24, 48, and 72 h. Staurosporine served as positive control for apoptosis induction. After each incubation period, cell culture supernatants were collected and cells were detached before they were incubated in a solution containing Triton X-100, PI, and sodium citrate at 4 °C overnight. Cells with subdiploidic DNA content were determined using flow cytometry (FACSVerse, BD Biosciences).
Proliferation assay
In this assay, 1500 HUVECs per well of a collagen G-coated 96-well plate were seeded in ECGM. Twenty-four h later, the cells were treated at indicated concentrations of the extract and were allowed to proliferate for 72 h or were fixed with a methanol-ethanol (2 : 1) solution for 10 min. After 72 h of extract incubation, HUVECs were fixed with methanol-ethanol for 10 min before they were stained using a crystal violet solution containing 20% methanol for 15 min. After drying, DNA-bound crystal violet was resolved in 20% acetic acid, and the number of cells was determined at 590 nm using a plate reader (SPECTRAFluor Plus, Tecan).
Undirected migration
For the analysis of extract-induced effects on undirected endothelial cell migration, a scratch was inflicted into a confluent HUVEC monolayer using a pipette tip. After removal of the detached cells, HUVECs were treated with indicated concentrations of the extract in ECGM. For positive control, a starvation medium (medium 199 supplemented with 1% FCS, 100 U/mL penicillin, and 100 µg/mL streptomycin) was used. Subsequently, the cells were allowed to migrate for 12 h until the gap inflicted into the control cells (ECGM containing 0.1% DMSO) was closed by endothelial cell migration. The effect of BNO 1095 on the migratory capacity of HUVECs was determined by ImageJ (software version 1.49 k).
Directed migration: Boyden chamber assay
To determine potential effects upon extract treatment on the migration of endothelial cells in the direction of a chemoattractant gradient, a Boyden chamber assay using Transwell inserts was performed. Therefore, 100 000 cells per well were seeded in ECGM on collagen G-coated Transwell inserts (Corning GmbH HQ, growth area 0.33 cm 2 , 8 µm pore size, polycarbonate). After 4 h, the cells were treated with indicated concentrations of the extract in medium 199 without FCS. In addition, a chemoattractant gradient was applied by the addition of medium 199 supplemented with 20% FCS to the lower compartment. HUVECs were allowed to migrate into the direction of the FCS gradient for 16 h. For the quantification of migrated cells, HUVECs located on the lower side of the Transwell insert were fixed with a methanol-ethanol (2 : 1) solution for 10 min before they were stained with crystal violet (in 20% methanol) for 15 min. After drying overnight, DNA-bound crystal violet was resolved in 20% acetic acid, and the amount of migrated cells was determined at 590 nm using a microplate reader (SPECTRAFluor Plus).
Directed migration: 2D chemotaxis assay
To gain deeper insights into the effects of BNO 1095 on chemotactic migration of endothelial cells, 18,000 HUVECs were seeded on chemotaxis slides (ibidi GmbH) in ECGM. The cells were allowed to adhere for 4 h before ECGM was washed off and a gradient of 20% FCS in medium 199 was added to the cells. HUVECs in chemotaxis slides were allowed to migrate in the direction of the FCS gradient for 20 h in an atmosphere of 5% CO 2 and 95% air at 37 °C in a climatic chamber of a microscope (DM IL LED, Leica Microsystems). Every 10 min, a phase contrast image was captured. For quantification, 30 cells were tracked using a manual tracking tool (ImageJ, software version 1.49 k). The parameters of accumulated distance, Euclidean distance, velocity, Y : FMI, and directness were used to demonstrate extract-derived effects on endothelial chemotactic migration.
Tube formation assay
A tube formation assay using Matrigel (Corning GmbH HQ) was performed to analyze potential extract-induced inhibition of in vitro capillary-like sprout formation. Therefore, 10 µL per well of growth factor-reduced Matrigel was added to angiogenesis slides (ibidi GmbH) and allowed to solidify for 30 min at 37 °C. Subsequently, 10,000 HUVECs per well were added in ECGM medium containing the indicated concentrations of BNO 1095 on top of the solidified Matrigel matrix. HUVECs were allowed to form tube-like structures for 5.5 h before microscopic phase contrast images were captured (DM IL LED, Leica Microsystems). Image quantification (ImageJ, software version 1.49 k, angiogenesis analyzer plugin) was used for the determination of number of junctions, number of tubules, and number of master segments.
Spheroid assay
HUVEC spheroids consisting of 400 cells were formed by the hanging drop method in ECGM containing 20% methylcellulose (Sigma-Aldrich Chemie GmbH). After 24 h, spheroids were embedded into a rat tail collagen I gel. After solidification of the collagen I gel, the cells in spheroids were treated with indicated concentrations of the extract for 30 min before sprout formation was induced by VEGF. After 20 h, HUVEC spheroids were fixed with 4% formaldehyde (Roti-Histofix, Carl Roth). Potential extract-derived effects on total sprout length and mean number of sprouts were determined by microscopic analysis and image quantification using ImageJ (software version 1.49 k).
Statistical analysis
All experiments were performed independently with at least 3 different cell preparations each with at least 3 technical replicates. Statistical analysis was performed using GraphPad Prism version 5.0. For statistical evaluation, 1-way ANOVA was used followed by Tukeyʼs Post hoc test. The actual number of experiments (n) is stated in the respective figure legend. Data are expressed as mean ± standard deviation (SD). P ≤ 0.05 was considered as statistically significant.
Contributorsʼ Statement
Data collection: I. Bischoff-Kont, L. Brabenec, B. Nausch, R. Ingelfinger. Design of the study: I. Bischoff-Kont, R. Fürst, R. Ingelfinger, B. Nausch, L. Brabenec. Statistical analysis: I. Bischoff-Kont, L. Brabenec, R. Ingelfinger. Analysis and interpretation of the data: I. Bischoff-Kont, R. Fürst, L. Brabenec, B. Nausch, R. Ingelfinger. Drafting the manuscript: I. Bischoff-Kont, R. Fürst. Critical revision of the manuscript: R. Fürst, I. Bischoff-Kont, B. Nausch.
Footnotes
Conflict of Interest One of the authors (BN) is employee of the company that manufactures BNO 1095.
Supporting Information
A HPLC fingerprint of UV absorbance measurement at 205 nm of BNO 1095 (lot number 770 134) was performed.
References
- 1.Rafieian-Kopaei M, Movahedi M. Systematic review of premenstrual, postmenstrual and infertility disorders of Vitex agnus castus. Electron Physician. 2017;9:3685–3689. doi: 10.19082/3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniele C, Coon J T, Pittler M H, Ersnt E. Vitex agnus castus : a systematic review of adverse events . Drug Saf. 2005;28:319–332. doi: 10.2165/00002018-200528040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Wuttke W, Jarry H, Christoffel V, Spengler B, Seidlová-Wuttke D. Chaste tree ( Vitex agnus-castus )–pharmacology and clinical indications . Phytomedicine. 2003;10:348–357. doi: 10.1078/094471103322004866. [DOI] [PubMed] [Google Scholar]
- 4.Hobbs C. The chaste tree: Vitex agnus castus. Pharm Hist. 1991;33:19–24. [PubMed] [Google Scholar]
- 5.Souto E B, Durazzo A, Nazhand A, Lucarini M, Zaccardelli M, Souto S B, Silva A M, Severino P, Novellino E, Santini A. Vitex agnus-castus L.: main features and nutraceutical perspectives . Forests. 2020;11:761. [Google Scholar]
- 6.Chen S N, Friesen J B, Webster D, Nikolic D, van Breemen R B, Wang J, Fong H HS, Farnsworth N R, Pauli G F. Phytoconstituents from Vitex agnus-castus fruits . Fitoterapia. 2011;82:528–533. doi: 10.1016/j.fitote.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sliutz G, Speiser P, Schultz A M, Spona J, Zeillinger R. Agnus castus extracts inhibit prolactin secretion of rat pituitary cells . Horm Metab Res. 1993;25:253–255. doi: 10.1055/s-2007-1002090. [DOI] [PubMed] [Google Scholar]
- 8.Jarry H, Leonhardt S, Gorkow C, Wuttke W. In vitro prolactin but not LH and FSH release is inhibited by compounds in extracts of Agnus castus : direct evidence for a dopaminergic principle by the dopamine receptor assay . Exp Clin Endocrinol. 1994;102:448–454. doi: 10.1055/s-0029-1211317. [DOI] [PubMed] [Google Scholar]
- 9.Certo G, Costa R, DʼAngelo V, Russo M, Albergamo A, Dugo G, Germanò M P. Anti-angiogenic activity and phytochemical screening of fruit fractions from Vitex agnus castus. Nat Prod Res. 2017;31:2850–2856. doi: 10.1080/14786419.2017.1303696. [DOI] [PubMed] [Google Scholar]
- 10.Sahib H B, Al-Zubaidy A A, Jasim G A. Anti angiogenic activity of Vitex agnus castus methanol extract in vivo study . Iranian Journal of Pharmaceutical Sciences. 2016;12:59–68. [Google Scholar]
- 11.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahib H B, Al-Zubaidy A A, Hussain S M, Jassim G A. The anti angiogenic activity of Vitex agnus-castus leaves extracts . Int J Pharm Pharm Sci. 2014;6:863–869. [Google Scholar]
- 13.Qutub A A, Popel A S. Elongation, proliferation & migration differentiate endothelial cell phenotypes and determine capillary sprouting. BMC Syst Biol. 2009;3:13. doi: 10.1186/1752-0509-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alimohamadi R, Fatemi I, Naderi S, Hakimizadeh E, Rahmani M R, Allahtavakoli M. Protective effects of Vitex agnus-castus in ovariectomy mice following permanent middle cerebral artery occlusion . Iran J Basic Med Sci. 2019;22:1097–1101. doi: 10.22038/ijbms.2019.31692.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y W, Byzova T V. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–631. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kikuchi H, Yuan B, Yuhara E, Imai M, Furutani R, Fukushima S, Hazama S, Hirobe C, Ohyama K, Takagi N, Toyoda H. Involvement of histone H3 phosphorylation via the activation of p38 MAPK pathway and intracellular redox status in cytotoxicity of HL-60 cells induced by Vitex agnus-castus fruit extract . Int J Oncol. 2014;45:843–852. doi: 10.3892/ijo.2014.2454. [DOI] [PubMed] [Google Scholar]
- 17.Ahangarpour A, Najimi S A, Farbood Y. Effects of Vitex agnus-castus fruit on sex hormones and antioxidant indices in a d-galactose-induced aging female mouse model . J Chin Med Assoc. 2016;79:589–596. doi: 10.1016/j.jcma.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Smith S K. Regulation of angiogenesis in the endometrium. Trends Endocrinol Metab. 2001;12:147–151. doi: 10.1016/s1043-2760(01)00379-4. [DOI] [PubMed] [Google Scholar]
- 19.Kressin P, Wolber E M, Wodrich H, Meyhöfer-Malik A, Buchweitz O, Diedrich K, Malik E. Vascular endothelial growth factor mRNA in eutopic and ectopic endometrium. Fertil Steril. 2001;76:1220–1224. doi: 10.1016/s0015-0282(01)02898-9. [DOI] [PubMed] [Google Scholar]
- 20.Laschke M W, Menger M D. Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum Reprod Update. 2012;18:682–702. doi: 10.1093/humupd/dms026. [DOI] [PubMed] [Google Scholar]
- 21.Zheng W, Cao L, Xu Z, Ma Y, Liang X. Anti-Angiogenic alternative and complementary medicines for the treatment of endometriosis: a review of potential molecular mechanisms. Evid Based Complement Alternat Med. 2018;2018:4.128984E6. doi: 10.1155/2018/4128984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laschke M W, Schwender C, Scheuer C, Vollmar B, Menger M D. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum Reprod. 2008;23:2308–2318. doi: 10.1093/humrep/den245. [DOI] [PubMed] [Google Scholar]
- 23.Rudzitis-Auth J, Körbel C, Scheuer C, Menger M D, Laschke M W. Xanthohumol inhibits growth and vascularization of developing endometriotic lesions. Hum Reprod. 2012;27:1735–1744. doi: 10.1093/humrep/des095. [DOI] [PubMed] [Google Scholar]
- 24.Becker C M, Sampson D A, Rupnick M A, Rohan R M, Efstathiou J A, Short S M, Taylor G A, Folkman J, DʼAmato R J. Endostatin inhibits the growth of endometriotic lesions but does not affect fertility. Fertil Steril. 2005;84:1144–1155. doi: 10.1016/j.fertnstert.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 25.Oktem M, Esinler I, Eroglu D, Haberal N, Bayraktar N, Zeyneloglu H B. High-dose atorvastatin causes regression of endometriotic implants: a rat model. Hum Reprod. 2007;22:1474–1480. doi: 10.1093/humrep/del505. [DOI] [PubMed] [Google Scholar]
- 26.Delgado-Rosas F, Gómez R, Ferrero H, Gaytan F, Garcia-Velasco J, Simón C, Pellicer A. The effects of ergot and non-ergot-derived dopamine agonists in an experimental mouse model of endometriosis. Reproduction. 2011;142:745–775. doi: 10.1530/REP-11-0223. [DOI] [PubMed] [Google Scholar]
- 27.Nausch B, Pace S, Pein H, Koeberle A, Rossi A, Künstle G, Werz O. The standardized herbal combination BNO 2103 contained in Canephron((R)) N alleviates inflammatory pain in experimental cystitis and prostatitis. Phytomedicine. 2019;60:152987. doi: 10.1016/j.phymed.2019.152987. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A HPLC fingerprint of UV absorbance measurement at 205 nm of BNO 1095 (lot number 770 134) was performed.


