Abstract
Wildfires are a natural disturbance in many ecosystems. However, their effect on biotic interactions has been poorly studied. Fire consumes the vegetation and the litter layer where many parasites spend part of their life cycles. We hypothesize that wildfires reduce habitat availability for parasites with consequent potential benefits for hosts. We tested this for the lizard Psammodromus algirus and its ectoparasites in a Mediterranean ecosystem. We predicted that lizards in recently burned areas would have lower parasite load (cleaning effect) than those in unburned areas and that this phenomenon implies that lizards spending their entire lives in postfire conditions experience a lower level of parasitism than those living in unburned areas. We compared the ectoparasite load of lizards between eight paired burned/unburned sites, including recent (less than 1 year postfire) and older fires (2–4 years). We found that lizards' ectoparasites prevalence was drastically reduced in recently burned areas. Likewise, lizards in older burned areas showed less evidence of past parasitic infections. Fire disrupted the host–parasite interaction, providing the opportunity for lizards to avoid the negative effects of ectoparasites. Our results suggest that wildfires probably fulfil a role in controlling vector-borne diseases and pathogens, and highlight ecological effects of wildfires that have been overlooked.
Keywords: ectoparasites, global change, mite, Ophionyssus, Psammodromus algirus, wildfire
1. Background
Fire is an intrinsic and natural process in many ecosystems, and the need to incorporate its role into the understanding of fire-prone ecosystems’ ecology has been increasingly recognized [1,2]. The knowledge of the role of fire in the ecology and evolution of plants is robust, with extensive breadth and depth [1]. Whereas this understanding for animals is quickly growing [3–5], little is known for other biodiversity components such as biotic interactions.
One of the most relevant interactions in nature is parasitism [6]. Parasites cause adverse effects on a range of behavioural, physiological, genomic and demographic factors of hosts [7,8]. Hosts have selected different antiparasitic strategies (e.g. behavioural [9]; physiological [10]) among which the immunological response is probably the most complex [11,12]. Nevertheless, developing an immune response is energetically costly and it implies trade-offs with other vital attributes [13]. Therefore, parasite prevalence is considered one of the main factors modulating the dynamics of host populations [14] (reviewed in [15]).
In fire-prone ecosystems, fire abruptly consumes most vegetation and litter, where many ectoparasites spend the independent terrestrial stages of their life cycles [16,17]. Therefore, ectoparasite populations are likely to be reduced immediately after a fire (cleaning effect). In fact, early humans and native cultures have used fire for clearing the ground from parasites and diseases [2,18], and agricultural societies use fire to reduce livestock diseases [19–21]. There are examples of livestock–parasite reduction (such as ticks and mites) after prescribed burns [22,23]. These vertebrate parasites can in turn be vectors for other parasites and pathogens such as Lyme disease [24] and hemogregarines [25,26]; thus, fire reduces the transmission of vector-borne diseases by direct and indirect effects on vectors. However, the role of fire in reducing parasites in wild populations remains unexplored.
Fire may provide a significant parasite cleaning effect from which hosts could benefit. This effect should be especially relevant for host species with the ability to survive fires and remain postfire living in burned areas. That is the case of species with traits and strategies that confer some fire survival [5,27,28] and that show limited mobility and small home ranges; hosts with large home ranges may alternate between burned and unburned patches and thus the potential cleaning effect by fire may be blurred. In this study, we explore the effect of fire on the biotic interaction of parasitism in wild conditions. We selected a lizard as model system because many lizard species survive wildfires by seeking refuge in burrows, crevices, under rocks or among roots within the burn (e.g. burrowing lizards [29–31]), and they often spend their entire lives within a burned area due to their small home ranges [32], low vagility and dispersal rates [33]. Specifically, we selected the ground-dwelling Mediterranean lizard Psammodromus algirus and its ectoparasites (mites) as a case study.
We hypothesized that wildfires reduce the habitat for ectoparasites, and thus lizards in postfire conditions benefit from this clean environment by showing a lower parasite load than those in unburned areas. To test this, we compared lizard's parasite load (number of ectoparasites) in recent burned (less than 1 year postfire) and in paired unburned sites. We also tested if this cleaning effect of fire implies that lizards spending their entire lives in postfire conditions show lower evidence of past parasitic infections than those living in unburned areas. For this, we compared an indicator of the cumulative parasitic infection (number of raised ventral scales), in lizards in older burned (more than two years from fire) and in paired unburned sites.
2. Methods
(a) . Study system
Psammodromus algirus is a medium-sized ground-dwelling lizard with a lifespan of 3–5 years [34]; it is widespread in Mediterranean landscapes of the Iberian Peninsula where high-intensity fires are common. Although it is a habitat-generalist species [35], it prefers habitats with low shrub cover [36]. Moreover, P. algirus is often found in recently burnt areas, suggesting a high ability to survive fire [37–39]. Dispersal ability of P. algirus is low (less than 20 m [40]), and its home range (usually less than 100 m2 [41]) is smaller than the typical wildfires occurring in the Mediterranean area [42,43]. Due to its relatively low mobility, individuals of this species are appropriate candidates to benefit from a postfire reduction of parasites as it is expected to fully inhabit within the burned area.
In our study areas, endoparasite prevalence in P. algirus is low, probably due to the xeric conditions of their habitat [44], whereas ectoparasites are common. Specifically, we focused on mites of the genus Ophionyssus (gamasid mites; Acari: Macronyssidae) that are ectoparasites of P. algirus [45] and act as vectors of hemoparasites [26]. Ophionyssus species have a complex life cycle that includes five developmental stages on both the host and the soil [17]. The time for completion of the development of an individual from egg to adult varies with environmental conditions, being faster in wet and warm environments [17]. According to morphological characteristics [46], we identified both larvae and nymphs in our sampled lizards (electronic supplementary material, figures S1–S3).
Ticks (Acari: Ixodidae) were also present in P. algirus, but a preliminary analysis suggested a very low prevelance (only approx. 2% of sampled adult lizards infected and no presence on juveniles), and thus they were not considered in this study.
(b) . Lizard sampling and parasite quantification
The study was undertaken on the east of the Iberian Peninsula, an area that shows a typical Mediterranean climate where wildfires are common in summer [47,48]. Sampling locations were dominated by shrublands (mainly Quercus coccifera, Cistus sp. pl. Ulex parviflorus, Rhamnus alaternus, Pistacia lentiscus, Arbutus unedo, Rosmarinus oficinalis, Juniperus oxycedurs, Chamerops humilis, Brachypodium retusum), alternated with pine woodlands (Pinus halepensis) and some evergreen oak patches (Quercus ilex).
We identified eight locations for the study, where wildfires had occurred between 2012 and 2018 (electronic supplementary material, table S1). The sampling was carried out between 2016 and 2018. In each of the eight locations, we sampled lizards in the burned area and in an adjacent unburned area; both the burned and unburned areas had similar pre-fire characteristics (vegetation, topography). Lizards were collected by hand or using a pole with a slip noose, always far from the edge of the wildfire to ensure that the lizard's home range was fully inside or outside of the fire perimeter. All lizards were measured (snout–vent length, SVL; ±0.01 cm) and weighed (±0.1 g). Sex determination was carried out by observing lizards' femoral pores that are more conspicuous in adult males [49]; therefore, the sex of juvenile individuals could not be determined. All individuals were released back to the location of capture.
Mites are usually found under the ventral scales and on the dorsal scales of the tail of P. algirus (electronic supplementary material, figure S4). Ventral scales of this species are smooth and imbricate, but they raise up when an ectoparasite is present [50,51] and some remain raised up even after ectoparasites detach from the host [52] (see also electronic supplementary material, figure S5). Successive parasitic infections increase the number of raised ventral scales in lizards over time, so this measurement provides an indicator of the level of parasitic infections that individuals have experienced through their lives. Parasite load may vary due to the phenology of the parasites at the moment of sampling [53]; therefore, while the number of mites observed measures parasite load at the moment of sampling, raised ventral scales are an indicator of past infection [52].
To test the cleaning effect of fire, we used three locations with a time since fire of less than 1 year (hereafter ‘recent’ wildfires) where we performed a direct count of mites (adults and nymphs) on the lizards using a magnifying glass (10×). We carefully explored the cotton bags where lizards where kept until processed and we added any mite found there to the parasite's load of the corresponding lizard. To test if as a result of the cleaning effect of fire, lizards in postfire conditions suffer less cumulative parasitic infections than those living in unburned areas, and considering the life expectancy of P. algirus (approx. 3 years [34]), we sampled lizards at five locations in which the time since fire was 2–4 years (hereafter, ‘older’ wildfires). In such cases, we counted the number of raised ventral scales of the lizards in order to estimate the level of parasitism in lizards that have spent most of their lives under postfire conditions.
(c) . Statistical analyses
We calculated the body condition index (BCI) as the residuals of the regression of body mass on SVL (log-transformed); this was computed separately for each sex and age group [54].
Due to the large amount of zeros in parasite counts (51 and 53% for the count in recent and older wildfires, respectively), we fitted hurdle generalized linear mixed models (GLMMs) using the R package ‘glmmTMB’ v. 0.2.3 [55]. Hurdle models are partitioned in a binary process that allows us to analyse the prevalence of parasites (containing zero values), and a counting process by which we can assess the intensity of the infection when it occurred (containing the positive counts).
To analyse the number of mites on lizards (recent fires), we fitted a hurdle GLMM with a truncated negative binomial (truncated_nbinom2) error distribution, where burned versus unburned condition, sex, BCI and SVL were considered as fixed factors and location (3 levels) was included as a random factor. The use of a zero-truncated negative binomial regression allowed accounting for data overdispersion.
For the analysis of the number of raised ventral scales (older fires), we fitted a hurdle GLMM with a negative binomial (nbinom2) error distribution, where burned versus unburned condition, sex, BCI and SVL were considered as fixed factors and location (5 levels) was included as a random factor. In both regressions, interactions among fixed factors were also tested.
Models were constructed using maximum-likelihood estimation via Template Model Builder (TMB); as implemented in the R package ‘glmmTMB’ version 0.2.3 [55]. Model selection was based on the lowest Akaike's information criterion (AIC); uniformity of residuals was checked using the DHARMa package v. 0.2.4 [56]. All statistics were implemented in R v. 3.6.1 [57].
3. Results
(a) . Recent wildfires (less than 1-year postfire)
We sampled 117 lizards (32 adults, 85 juveniles) from three recently burned areas (3, 8 and 9 months postfire; electronic supplementary material, table S1) and their corresponding paired unburned areas.
The number of mites in adult lizards was independent of their sex (p = 0.086; electronic supplementary material, table S2), and therefore for subsequent analyses, we merged the data from juveniles (undetermined sex) and adults.
The probability of an individual being parasitized declined in burned compared to unburned areas (18% and 74% respectively; zero-inflated model: p < 0.001, table 1a), such that living in unburned environments was associated with a 4 times higher chance of carrying parasites. The probability of infection increased with lizards' size (SVL; but the interaction with fire condition was not significant; table 1a), and it was independent of their body condition (BCI; zero-inflated model: n.s., table 1a). For the parasitized lizards, the average number of mites was similar between burned (3.6 ± 3.6) and unburned (4.3 ± 4.5) areas (conditional model: n.s., table 1a), although the maximum number of mites found in burned areas was 2.3 times lower than in unburned areas (10 in burned versus 23 in unburned areas; figure 1a). The number of parasites was not related to lizard SVL and BCI (conditional model: n.s., table 1a).
Table 1.
Results of the hurdle mixed models for (a) the number of mites for recent wildfires and (b) the number of raised ventral scales for older wildfires, of lizards inhabiting burned and adjacent unburned areas. Note that in zero-inflation models, positive coefficients indicate lower parasitism and negative coefficients higher parasitism. For qualitative variables (treatment, location), the squared brackets show the factor level related to the coefficient shown. Models are displayed in figures 1a and 1b. *p < 0.05; **p < 0.01; ***p < 0.001.
| parameter | estimate | s.e. | Z | p-value |
|---|---|---|---|---|
| (a) number of mites (<1 year postfire, n = 117) | ||||
| zero-inflation model | ||||
| intercept | 1.579 | 0.731 | 2.160 | 0.031* |
| fire treatment [burned] | 3.157 | 0.590 | 5.350 | <0.001*** |
| SVL | −0.732 | 0.198 | −3.696 | <0.001*** |
| BCI | 0.256 | 1.164 | 0.220 | 0.826 |
| conditional model | ||||
| intercept | 0.243 | 0.646 | 0.376 | 0.707 |
| fire treatment [burned] | −0.212 | 0.474 | −0.448 | 0.654 |
| SVL | 0.171 | 0.122 | 1.394 | 0.163 |
| BCI | −1.064 | 1.209 | −0.88 | 0.379 |
| (b) number of raised ventral scales (2–4 year postfire, n = 241) | ||||
| zero-inflation model | ||||
| intercept | −1.884 | 0.651 | −2.895 | 0.004** |
| fire treatment [burned] | 2.290 | 0.611 | 3.751 | <0.001*** |
| BCI | 0.167 | 1.754 | 0.095 | 0.924 |
| conditional model | ||||
| intercept | −1.379 | 0.487 | −2.829 | 0.005** |
| fire treatment [burned] | −0.374 | 0.183 | −2.045 | 0.041* |
| SVL | 0.418 | 0.079 | 5.324 | <0.001*** |
| BCI | 1.031 | 0.598 | 1.725 | 0.084 |
Figure 1.
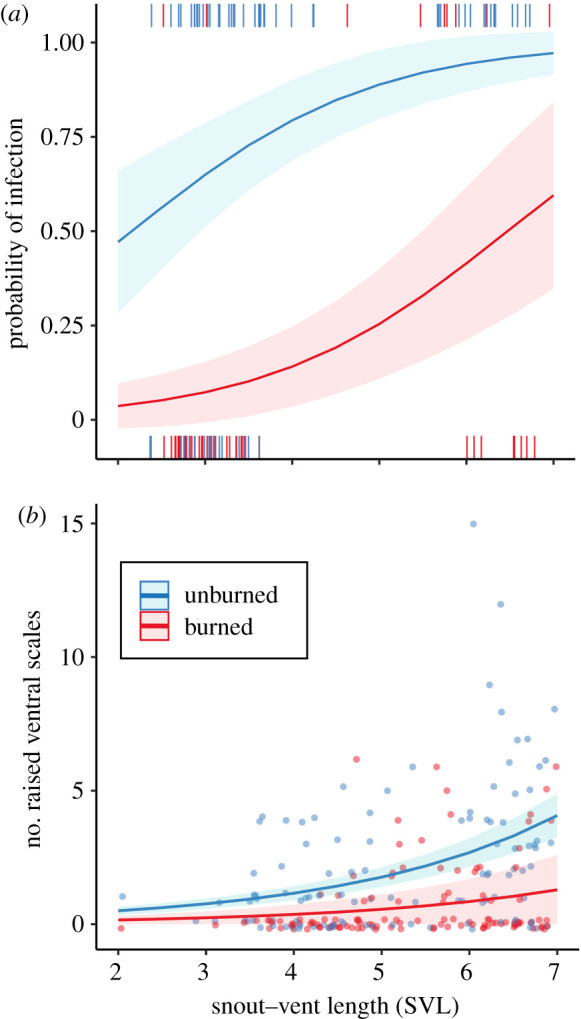
(a) Lizards' mite load in relation to lizards’ size (SVL) for recently burned areas (less than 1 year from fire; red, lower line) and the corresponding paired unburned (blue, upper line). Lines are predicted values (and confidence intervals) of conditioned on the zero-inflation component (probability of mite infection) of the hurdle mixed model. For the statistical significance, table 1a. Raw binomial data (n = 117) are represented as short vertical lines on the horizontal axes at y = 0 and 1. The data split by populations is represented in electronic supplementary material, figure S6. (b) Number of raised scales in relation to lizards' size (SVL) for burned areas (older wildfires, 2–4 years; in red, lower line) and the corresponding paired unburned (in blue, upper line). Lines are predicted values (with confidence intervals) of the zero-inflation component of the hurdle mixed model. Symbols are the raw data (n = 241). For the statistical significance, table 1b. The data split by populations is represented in electronic supplementary material, figure S7. (Online version in colour.)
(b) . Older wildfires (2–4 years postfire)
We sampled a total of 241 lizards (142 adults, 99 juveniles) from 5 different locations (5 paired burned/unburned areas; electronic supplementary material, table S1) that experienced fire 2 and 4 years ago.
The number of raised scales on adult lizards was independent of their sex (zero-inflated model: p = 0.428; conditional model: p = 0.985; electronic supplementary material, table S2), thus for the subsequent analyses, we merged the data of juveniles (undetermined sex) and adults.
Lizards living in burned environments were less likely to show raised ventral scales (approx. 29% prevalence, zero-inflated model: p < 0.001; table 1b), in comparison with those lizards inhabiting unburned areas (approx. 68% prevalence). For the parasitized lizards, those from burned areas showed lower number of raised ventral scales (2.7 ± 1.7) than lizards from the adjacent unburned areas (3.4 ± 2.8; figure 1b; conditional model: p < 0.046; table 1b). Moreover, the maximum number of raised ventral scales found in burned areas was 2.5 times lower than in unburned areas (6 in burned versus 15 in unburned areas). The number of raised scales increased with lizard's size (SVL), but the interaction with fire treatment was not significant (table 1b).
4. Discussion
We studied the disruption of a negative biotic interaction, parasitism, by the natural perturbation of wildfires. Our results showed that fire reduces the ectoparasite load of P. algirus (the cleaning effect), suggesting that postfire environments provide a temporal window of opportunity for lizards to avoid the negative effects of ectoparasites. This finding is consistent with the decreased parasite load in livestock after prescribed fires [58]. The reduced ectoparasitism is not only observed just after the fire, but expands through the entire life of the lizards inhabiting the postfire environment (i.e. less evidence of past parasitism). Given that ectoparasites may induce costs to reptiles [8,59,60], including to P. algirus [61,62], confronting a postfire scenario with reduced parasite load is likely to be advantageous for lizards.
Parasitism increased with lizards' size (probability of carrying mites, table 1a; and raised ventral scales, table 1b). This is because size correlates with age so older individuals had more chances of getting infected [63]. We did not detect an improvement of lizard's body condition as a result of the fire-driven parasite reduction (table 1). This is because body condition is a poor indicator of fitness in relation to parasitism [64,65], and is strongly influenced by environmental resources (which are likely to change postfire). Parasitism likely affects other life attributes different from body conditions (i.e. colour ornaments [62,66]). That is, independently of their body condition, lizards in postfire environments should benefit from lower parasitism-related stress.
Evidence suggest that the disruption by fire of antagonistic interactions can be beneficial to plants (lowering seed predation and diseases [67,68]). Similarly, here, we provide evidence of the disruption of a parasite–host interaction that is likely to result in a benefit for lizards. To our knowledge, this is the first evidence of a disruption of an ectoparasite–host interaction by fire in wild populations.
The parasite reduction in the environment could be mediated by direct mortality through the burning of vegetation and soil litter. This is the most plausible mechanism considering that Ophionyssus spends part of its cycle on the ground [17], and therefore fire-driven mortality is likely to occur (e.g. for fire-driven mortality of a soil acari, see [69]). By contrast, postfire changes in environmental conditions are unlikely to explain the observed pattern; while the drier postfire conditions may limit parasite development [70], the increased postfire temperatures can also favour it [71].
The observed reduction of lizard ectoparasitism in burned areas could also be mediated by changes in the spatial habitat structure. For instance, the lower prevalence of chytrid infection in boreal toads in recently burned areas is likely due to limitations in pathogen exposure and persistence in the new postfire habitat [72]. The reduced postfire litter and plant cover may limit lizards’ exposure to mites as P. algirus actively searches for food in the litter beneath shrubs or trees [73,74]. On the other hand, lizards probably spread their parasites when sharing favourable microsites, such as shelters or suitable places for sun basking [75]. Fire, by opening the vegetation, increases the number of these favourable places [76,77], and thus reduces the lizard density in those microsites and the transmission risk among individuals [78]. Caution must be taken when extrapolating these structural effects on animals with very different ecology. For example, in animals typical of understorey (closed) environments, fire diminished the availability of microsites for the host and this led to an increase in the encounter rate, and thus in the prevalence of infection (e.g. hantavirus in rodents from boreal forests [79]).
The reported fire-driven changes in vector populations presumably occur in many other fire-prone ecosystems but remain largely unexplored. Disturbances reducing the abundance of vectors would eventually limit the parasite abundance and its persistence in the ecosystem [80]. Our findings support the possible role of wildfires in providing ecosystem services (sensu [18]) by controlling vector-borne diseases (i.e. Lyme disease) and pathogens (i.e. hemogregarines) in natural systems, thus reducing risk and exposure for humans and livestock.
This study highlights an ecological role of wildfires that has been overlooked. Understanding the role of fire in the complex networks of interactions that characterize biodiversity is essential to comprehend ecological and evolutionary processes as well as for conservation purposes in a changing world.
Supplementary Material
Acknowledgements
We thank the main field technician G. Benítez. We also thank R. Megía-Palma, S. Reguera, R. Drechsler and all CIDE members who helped with the fieldwork.
Data accessibility
Analyses reported in this article can be reproduced using the data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.g79cnp5pc [81].
The data are provided in electronic supplementary material [82].
Authors' contributions
L.Á.-R.: data curation, formal analysis, investigation, methodology, validation, visualization, writing—original draft, writing—review and editing; J.B.: conceptualization, data curation, methodology, supervision, writing—review and editing; X.S.: data curation, investigation, writing—review and editing; J.G.P.: conceptualization, funding acquisition, investigation, project administration, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Ministry of Economy and Competitiveness and Ministry of Science, Innovation and Universities from the Spanish Government (grant nos CGL2015-64086-P, PGC2018-096569-B-I00, BES-2016-078225). CIDE is a joint institute of the Spanish National Research Council (CSIC), the University of Valencia and the regional government of Valencia (Generalitat Valenciana).
References
- 1.Keeley JE, Bond WJ, Bradstock RA, Pausas JG, Rundel PW. 2012. Fire in Mediterranean ecosystems: ecology, evolution and management. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Pausas JG, Keeley JE. 2009. A burning story: the role of fire in the history of life. Bioscience 59, 593-601. ( 10.1525/bio.2009.59.7.10) [DOI] [Google Scholar]
- 3.Pausas JG, Parr CL. 2018. Towards an understanding of the evolutionary role of fire in animals. Evol. Ecol. 32, 113-125. ( 10.1007/s10682-018-9927-6) [DOI] [Google Scholar]
- 4.Koltz AM, Burkle LA, Pressler Y, Dell JE, Vidal MC, Richards LA, Murphy SM, Schmitz O, Rosenblatt A. 2018. Global change and the importance of fire for the ecology and evolution of insects. Curr. Opin. Insect Sci. 29, 110-116. ( 10.1016/j.cois.2018.07.015) [DOI] [PubMed] [Google Scholar]
- 5.Nimmo DG, et al. 2019. Animal movements in fire-prone landscapes. Biol. Rev. 94, 981-998. ( 10.1111/brv.12486) [DOI] [PubMed] [Google Scholar]
- 6.Price P. 1980. Evolutionary biology of parasites. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Schmid-Hempel P. 2011. Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Bower DS, Brannelly LA, McDonald CA, Webb RJ, Greenspan SE, Vickers M, Gardner MG, Greenlees MJ. 2018. A review of the role of parasites in the ecology of reptiles and amphibians. Austral. Ecol. 44, 433-448. ( 10.1111/aec.12695) [DOI] [Google Scholar]
- 9.Loye JE, Carroll SP. 1991. Nest ectoparasite abundance and cliff swallow colony site selection, nestling development, and departure time. In Bird–parasite interactions: ecology, evolution and behaviour (eds Loye J, Zuk M), pp. 222-241. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Blatteis CM. 1986. Fever: is it beneficial? Yale J. Biol. Med. 59, 107-116. [PMC free article] [PubMed] [Google Scholar]
- 11.Owen JP, Hawley DM. 2014. Host-parasite interactions. In Eco-immunology (eds Malagoli D, Ottaviani E), pp. 73-92. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 12.Wakelin D, Apanius V. 1997. Immune defence: genetic control. In Host-parasite evolution: general principles and avian models (eds Clayton DH, Moore J), pp. 30-58. Oxford, UK: Oxford University Press. [Google Scholar]
- 13.Sheldon BC, Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317-321. ( 10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 14.Ebert D, Lipsitch M, Mangin KL. 2000. The effect of parasites on host population density and extinction: experimental epidemiology with Daphnia and six microparasites. Am. Nat. 156, 459-477. ( 10.1086/303404) [DOI] [PubMed] [Google Scholar]
- 15.Hudson PJ, Dobson AP, Lafferty KD. 2006. Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 21, 381-385. ( 10.1016/J.TREE.2006.04.007) [DOI] [PubMed] [Google Scholar]
- 16.Estrada-Peña A, De La Fuente J. 2014. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. 108, 104-128. ( 10.1016/j.antiviral.2014.05.016) [DOI] [PubMed] [Google Scholar]
- 17.Bannert B, Karaca HY, Wohltmann A. 2000. Life cycle and parasitic interaction of the lizard-parasitizing mite Ophionyssus galloticolus (Acari: Gamasida: Macronyssidae), with remarks about the evolutionary consequences of parasitism in mites. Exp. Appl. Acarol. 24, 597-613. ( 10.1023/A:1026504627926) [DOI] [PubMed] [Google Scholar]
- 18.Pausas JG, Keeley JE. 2019. Wildfires as an ecosystem service. Front. Ecol. Environ. 17, 289-295. ( 10.1002/fee.2044) [DOI] [Google Scholar]
- 19.Nyariki DM, World Agroforestry Centre, Eastern and Central Africa Regional Programme, Wasonga VO, Isae IM, Kyagaba E, Lugenja M. 2005. Indigenous techniques for assessing and monitoring range resources in East Africa. Nairobi, Kenya: World Agroforestry Centre, Eastern and Central Africa Programme. [Google Scholar]
- 20.Hepworth K, Neary M, Hutchens T. 2006. Managing internal parasitism in sheep and goats. West Lafayette, IN: Purdue University Cooperative Extension Service. [Google Scholar]
- 21.Scasta JD, Engle DM, Talley JL, Weir JR, Stansberry JC, Fuhlendorf SD, Harr RN. 2012. Pyric-herbivory to manage horn flies (Diptera: Muscidae) on cattle. Southwest. Entomol. 37, 325-334. ( 10.3958/059.037.0308) [DOI] [Google Scholar]
- 22.Davidson WR, Siefken DA, Creekmore LH. 1994. Influence of annual and biennial prescribed burning during March on the abundance of Amblyomma americanum (Acari: Ixodidae) in Central Georgia. J. Med. Entomol. 31, 72-81. ( 10.1093/jmedent/31.1.72) [DOI] [PubMed] [Google Scholar]
- 23.Gleim ER, Conner LM, Berghaus RD, Levin ML, Zemtsova GE, Yabsley MJ. 2014. The phenology of ticks and the effects of long-term prescribed burning on tick population dynamics in southwestern Georgia and northwestern Florida. PLoS ONE 9, e112174. ( 10.1371/journal.pone.0112174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgdorfer W, Barbour A, Hayes S, Benach J, Grunwaldt E, Davis J. 1982. Lyme disease-a tick-borne spirochetosis? Science 216, 1317-1319. ( 10.1126/science.7043737) [DOI] [PubMed] [Google Scholar]
- 25.Ekner A, Dudek K, Sajkowska Z, Majláthová V, Majláth I, Tryjanowski P. 2011. Anaplasmataceae and Borrelia burgdorferi sensu lato in the sand lizard Lacerta agilis and co-infection of these bacteria in hosted Ixodes ricinus ticks. Parasit. Vectors 4, 182. ( 10.1186/1756-3305-4-182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haklová-Kočíková B, et al. 2014. Morphological and molecular characterization of Karyolysus—a neglected but common parasite infecting some European lizards. Parasit. Vectors 7, 555. ( 10.1186/s13071-014-0555-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pausas JG. 2019. Generalized fire response strategies in plants and animals. Oikos 128, 147-153. ( 10.1111/oik.05907) [DOI] [Google Scholar]
- 28.Álvarez-Ruiz L, Belliure J, Pausas JG. In press. Fire-driven behavioral response to smoke in a Mediterranean lizard. Behav. Ecol. ( 10.1093/beheco/arab010) [DOI] [Google Scholar]
- 29.Floyd TM, Russell KR, Moorman CE, Lear DH van, Guynn DC, Lanham JD. 2002. Effects of prescribed fire on herpetofauna within hardwood forests of the upper Piedmont of South Carolina: a preliminary analysis. In Proc. of the 11th Biennial Southern Silvicultural Research Conf. (ed. Outcalt KW), pp. 123-127. Asheville, NC: US Department of Agricultue. [Google Scholar]
- 30.Santos X, Poquet JM. 2010. Ecological succession and habitat attributes affect the postfire response of a Mediterranean reptile community. Eur. J. Wildl. Res. 56, 895-905. ( 10.1007/s10344-010-0387-8) [DOI] [Google Scholar]
- 31.Palis JG. 1995. Post-fire herpetofauna of Morgan Ridge, Hoosier National Forest, Indiana. Bull. Chicago Herpetol. Soc. 30, 167-171. [Google Scholar]
- 32.Perry G, Garland T. 2002. Lizard home ranges revisited: Effects of sex, body size, diet habitat, and phylogeny. Ecology 83, 1870-1885. ( 10.1890/0012-9658(2002)083[1870:LHRREO]2.0.CO;2) [DOI] [Google Scholar]
- 33.Valentine LE, Schwarzkopf L. 2009. Effects of weed-management burning on reptile assemblages in Australian tropical savannas. Conserv. Biol. 23, 103-113. ( 10.1111/j.1523-1739.2008.01074.x) [DOI] [PubMed] [Google Scholar]
- 34.Comas M, Reguera S, Zamora-Camacho FJ, Moreno-Rueda G. 2019. Age structure of a lizard along an elevational gradient reveals non-linear lifespan patterns with altitude. Curr. Zool. 66, 373-382. ( 10.1093/cz/zoz063/5688747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvador A. 2015. Enciclopedia virtual de los vertebrados Españoles. Madrid, Spain: Museo Nacional de Ciencias Naturales. [Google Scholar]
- 36.Díaz JA, Carrascal LM. 1991. Regional distribution of a Mediterranean lizard: influence of habitat cues and prey abundance. J. Biogeogr. 18, 291. ( 10.2307/2845399) [DOI] [Google Scholar]
- 37.Ferreira D, Brito JC, Santos X. 2018. Long–interval monitoring reveals opposing responses of Mediterranean versus Atlantic reptile species in a biogeographic transition zone. Basic Appl. Herpetol. 32, 41-55. ( 10.11160/bah.85) [DOI] [Google Scholar]
- 38.Moreno-Rueda G, Melero E, Reguera S, Zamora-Camacho FJ, Comas M. 2019. Short-term impact of a small wildfire on the lizard Psammodromus algirus (Linnaeus, 1758): a before-after-control-impact study (Squamata: Sauria: Lacertidae). Herpetozoa 31, 173-182. [Google Scholar]
- 39.Santos X, Badiane A, Matos C. 2015. Contrasts in short- and long-term responses of Mediterranean reptile species to fire and habitat structure. Oecologia 180, 205-216. ( 10.1007/s00442-015-3453-9) [DOI] [PubMed] [Google Scholar]
- 40.Civantos E, Salvador A, Veiga JP. 1999. Body size and microhabitat affect winter survival of hatchling Psammodromus algirus lizards. Copeia 4, 1112-1117. ( 10.2307/1447988) [DOI] [Google Scholar]
- 41.Civantos E, López P, Martín J. 2010. Non-lethal effects of predators on body growth and health state of juvenile lizards, Psammdromus algirus. Physiol. Behav. 100, 332-339. ( 10.1016/J.PHYSBEH.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 42.Pausas JG, Fernández-Muñoz S. 2012. Fire regime changes in the Western Mediterranean Basin: from fuel-limited to drought-driven fire regime. Clim. Change 110, 215-226. ( 10.1007/s10584-011-0060-6) [DOI] [Google Scholar]
- 43.Chergui B, Fahd S, Santos X, Pausas JG. 2018. Socioeconomic factors drive fire-regime variability in the Mediterranean Basin. Ecosystems 21, 619-628. ( 10.1007/s10021-017-0172-6) [DOI] [Google Scholar]
- 44.Roca V, Belliure J, Santos X, Pausas JG. 2020. New reptile hosts for helminth parasites in a Mediterranean region. J. Herpetol. 54, 268. ( 10.1670/18-133) [DOI] [Google Scholar]
- 45.Álvarez-Ruiz L, Megía-Palma R, Reguera S, Ruiz S, Zamora-Camacho FJ, Figuerola J, Moreno-Rueda G. 2018. Opposed elevational variation in prevalence and intensity of endoparasites and their vectors in a lizard. Curr. Zool. 64, 197-204. ( 10.1093/cz/zoy002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moraza ML, Irwin NR, Godinho R, Baird SJE, De Bellocq JG. 2007. A new species of Ophionyssus Mégnin (Acari: Mesostigmata: Macronyssidae) parasitic on Lacerta schreiberi Bedriaga (Reptilia: Lacertidae) from the Iberian Peninsula, and a world key to species. Zootaxa 2007, 58-68. ( 10.11646/ZOOTAXA.2007.1.3) [DOI] [Google Scholar]
- 47.Pausas JG, Paula S. 2012. Fuel shapes the fire-climate relationship: evidence from Mediterranean ecosystems. Glob. Ecol. Biogeogr. 21, 1074-1082. ( 10.1111/j.1466-8238.2012.00769.x) [DOI] [Google Scholar]
- 48.Pausas JG. 2004. Changes in fire and climate in the eastern Iberian Peninsula (Mediterranean Basin). Clim. Change 63, 337-350. ( 10.1023/B:CLIM.0000018508.94901.9c) [DOI] [Google Scholar]
- 49.Iraeta P, Monasterio C, Salvador A, Díaz JA. 2011. Sexual dimorphism and interpopulation differences in lizard hind limb length: locomotor performance or chemical signalling? Biol. J. Linn. Soc. 104, 318-329. ( 10.1111/j.1095-8312.2011.01739.x) [DOI] [Google Scholar]
- 50.Gardner S, Fisher R, Barry S. 2012. Collecting and preserving parasites during reptile biodiversity surveys. Lincoln, NE: Harold W. Manter Laboratory of Parasitology.
- 51.Reardon JT, Norbury G. 2004. Ectoparasite and hemoparasite infection in a diverse temperate lizard assemblage at Macraes Flat, south Island, New Zealand. J. Parasitol. 90, 1274-1278. ( 10.1645/GE-3326) [DOI] [PubMed] [Google Scholar]
- 52.Hare K, Hare J, Cree A. 2010. Parasites, but not palpation, are associated with pregnancy failure in a captive viviparous lizard. Herpetol. Conserv. Biol. 5, 563-570. [Google Scholar]
- 53.Huyghe K, Van Oystaeyen A, Pasmans F, Tadić Z, Vanhooydonck B, Van Damme R. 2010. Seasonal changes in parasite load and a cellular immune response in a colour polymorphic lizard. Oecologia 163, 867-874. ( 10.1007/S00442-010-1646-9) [DOI] [PubMed] [Google Scholar]
- 54.Warner DA, Johnson MS, Nagy TR. 2016. Validation of body condition indices and quantitative magnetic resonance in estimating body composition in a small lizard. J. Exp. Zool. 325, 588-597. ( 10.1002/jez.2053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM. 2017. Modeling zero-inflated count data with glmmTMB. bioRxiv, 132753. (doi:10.1101/132753)
- 56.Hartig F. 2019. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.4.
- 57.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 58.Scasta JD. 2015. Fire and parasites: an under-recognized form of anthropogenic land use change and mechanism of disease exposure. Ecohealth 12, 398-403. ( 10.1007/s10393-015-1024-5) [DOI] [PubMed] [Google Scholar]
- 59.Klukowski M, Nelson CE. 2001. Ectoparasite loads in free-ranging northern fence lizards, Sceloporus undulatus hyacinthinus: effects of testosterone and sex. Behav. Ecol. Sociobiol. 49, 289-295. ( 10.1007/s002650000298) [DOI] [Google Scholar]
- 60.Sorci G, Clobert J. 1995. Effects of maternal parasite load on offspring life-history traits in the common lizard (Lacerta vivipara). J. Evol. Biol. 8, 711-723. ( 10.1046/j.1420-9101.1995.8060711.x) [DOI] [Google Scholar]
- 61.Salvador A, Veiga JP, Martin J, Lopez P, Abelenda M, Puerta M. 1996. The cost of producing a sexual signal: testosterone increases the susceptibility of male lizards to ectoparasitic infestation. Behav. Ecol. 7, 145-150. ( 10.1093/beheco/7.2.145) [DOI] [Google Scholar]
- 62.Llanos-Garrido A, Díaz JA, Pérez-Rodríguez A, Arriero E. 2017. Variation in male ornaments in two lizard populations with contrasting parasite loads. J. Zool. 303, 218-225. ( 10.1111/jzo.12478) [DOI] [Google Scholar]
- 63.Halliday WD, Paterson JE, Patterson LD, Cooke SJ, Blouin-Demers G. 2014. Testosterone, body size, and sexual signals predict parasite load in Yarrow's Spiny Lizards (Sceloporus jarrovii). Can. J. Zool. 92, 1075-1082. ( 10.1139/cjz-2014-0256) [DOI] [Google Scholar]
- 64.Sánchez CA, Becker DJ, Teitelbaum CS, Barriga P, Brown LM, Majewska AA, Hall RJ, Altizer S. 2018. On the relationship between body condition and parasite infection in wildlife: a review and meta-analysis. Ecol. Lett. 21, 1869-1884. ( 10.1111/ele.13160) [DOI] [PubMed] [Google Scholar]
- 65.Comas M. 2020. Body condition, sex and elevation in relation to mite parasitism in a high mountain gecko. J. Zool. 310, 298-305. ( 10.1111/jzo.12751) [DOI] [Google Scholar]
- 66.Megía-Palma R, Martínez J, Merino S. 2016. Structural- and carotenoid-based throat colour patches in males of Lacerta schreiberi reflect different parasitic diseases. Behav. Ecol. Sociobiol. 70, 2017-2025. ( 10.1007/s00265-016-2205-0) [DOI] [Google Scholar]
- 67.García Y, Castellanos MC, Pausas JG. 2016. Fires can benefit plants by disrupting antagonistic interactions. Oecologia 182, 1165-1173. ( 10.1007/s00442-016-3733-z) [DOI] [PubMed] [Google Scholar]
- 68.Simler-Williamson AB, Metz MR, Frangioso KM, Rizzo DM. 2021. Wildfire alters the disturbance impacts of an emerging forest disease via changes to host occurrence and demographic structure. J. Ecol. 109, 676-691. ( 10.1111/1365-2745.13495) [DOI] [Google Scholar]
- 69.Camann MA, Gillette NE, Lamoncha KL, Mori SR. 2008. Response of forest soil Acari to prescribed fire following stand structure manipulation in the southern Cascade Range. Can. J. For. Res. 38, 956-968. ( 10.1139/X07-241) [DOI] [Google Scholar]
- 70.Estrada-Peña A. 2001. Distribution, abundance, and habitat preferences of Ixodes ricinus (Acari: Ixodidae) in Northern Spain. J. Med. Entomol. 38, 361-370. ( 10.1603/0022-2585-38.3.361) [DOI] [PubMed] [Google Scholar]
- 71.Tsatsaris A, Chochlakis D, Papadopoulos B, Petsa A, Georgalis L, Angelakis E, Ioannou I, Tselentis Y, Psaroulaki A. 2016. Species composition, distribution, ecological preference and host association of ticks in Cyprus. Exp. Appl. Acarol. 70, 523-542. ( 10.1007/s10493-016-0091-9) [DOI] [PubMed] [Google Scholar]
- 72.Hossack BR, Lowe WH, Ware JL, Corn PS. 2013. Disease in a dynamic landscape: host behavior and wildfire reduce amphibian chytrid infection. Biol. Conserv. 157, 293-299. ( 10.1016/J.BIOCON.2012.09.013) [DOI] [Google Scholar]
- 73.Díaz JA, Carrascal LM. 1993. Variation in the effect of profitability on prey size selection by the lacertid lizard Psammodromus algirus. Oecologia 94, 23-29. ( 10.1007/BF00317296) [DOI] [PubMed] [Google Scholar]
- 74.Belliure J, Carrascal LM, Díaz JA. 1996. Covariation of thermal biology and foraging mode in two Mediterranean lacertid lizards. Ecology 77, 1163-1173. ( 10.2307/2265585) [DOI] [Google Scholar]
- 75.Amo L, López P, Martín J. 2004. Prevalence and intensity of haemogregarinid blood parasites in a population of the Iberian rock lizard, Lacerta monticola. Parasitol. Res. 94, 290-293. ( 10.1007/s00436-004-1212-7) [DOI] [PubMed] [Google Scholar]
- 76.Renken RB. 2006. Does fire affect amphibians and reptiles in eastern US oak forests? In Fire in eastern oak forests: delivering science to land managers (ed. Dickinson MB), pp. 158-166. Newton Square, PA: Department of Agriculture, Forest Service. [Google Scholar]
- 77.Huey RB. 1991. Physiological consequences of habitat selection. Am. Nat. 137, S91-S115. ( 10.1086/285141) [DOI] [Google Scholar]
- 78.Sorci G, De Fraipont M, Clobert J. 1997. Host density and ectoparasite avoidance in the common lizard (Lacerta vivipara). Oecologia 111, 183-188. ( 10.1007/s004420050224) [DOI] [PubMed] [Google Scholar]
- 79.Ecke F, Nematollahi Mahani SA, Evander M, Hörnfeldt B, Khalil H. 2019. Wildfire-induced short-term changes in a small mammal community increase prevalence of a zoonotic pathogen? Ecol. Evol. 9, 12 459-12 470. ( 10.1002/ece3.5688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez J, Merino S. 2011. Host-parasite interactions under extreme climatic conditions. Curr. Zool. 57, 390-405. ( 10.1093/czoolo/57.3.390) [DOI] [Google Scholar]
- 81.Álvarez-Ruiz L, Belliure J, Santos X, Pausas J. 2021. Data from: Fire reduces parasite load in a Mediterranean lizard. Dryad Digital Repository. ( 10.5061/dryad.g79cnp5pc) [DOI] [PMC free article] [PubMed]
- 82.Álvarez-Ruiz L, Belliure J, Santos X, Pausas J. 2021. Fire reduces parasite load in a Mediterranean lizard. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Álvarez-Ruiz L, Belliure J, Santos X, Pausas J. 2021. Data from: Fire reduces parasite load in a Mediterranean lizard. Dryad Digital Repository. ( 10.5061/dryad.g79cnp5pc) [DOI] [PMC free article] [PubMed]
- Álvarez-Ruiz L, Belliure J, Santos X, Pausas J. 2021. Fire reduces parasite load in a Mediterranean lizard. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Analyses reported in this article can be reproduced using the data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.g79cnp5pc [81].
The data are provided in electronic supplementary material [82].


