Abstract
Objectives
The aim of the study was to identify predictors determining the course of COVID-19 and antibody response in elite athletes.
Design
Observational study.
Methods
Routine medical screening with physical examination, resting ECG, and laboratory tests including antibody response was performed 12–68 days after the diagnosis of COVID-19 in 111 athletes of different sports.
Results
Clinical symptoms were observed in 84% of subjects. The severity of COVID-19 was mild in 82% of athletes and moderate in 2% of cases. Athletes aged above 26 and male were more likely to develop symptomatic COVID-19. Asymptomatic subjects were younger and predominantly female. In 18% of subjects, symptoms were still present 20 (12–68) days (median and range) since positive diagnosis. Antibody response was observed in 88% of athletes, and its magnitude correlated with time since diagnosis of COVID-19 (RT-PCR), fatigue, fever, and conjunctivitis. There were no differences in antibody response between groups distinguished by sports discipline (p = 0.50), and sex (p = 0.59), and antibody response did not correlate with BMI (p = 0.12), age (p = 0.13), the number of symptoms (p = 0.43) or their duration (p = 0.19).
Conclusions
The severity of COVID-19 in elite athletes is predominantly mild and without complications. Athletes can return to sport after two symptom-free weeks and additional heart screening is usually not required. Determination of antibodies has been shown to be a useful indicator of a previous COVID-19 disease, and some symptoms can be used as predictors of antibody response.
Keywords: Sport, COI, SARS-CoV-2, Antibody response, Return to sport, Exercise
Practical implications
-
•
The severity of COVID-19 in elite athletes is predominantly mild and without complications.
-
•
Athletes can return to sport after two symptom-free weeks and additional heart screening is usually not required.
-
•
Determination of antibodies has been shown to be a useful indicator of a previous COVID-19 disease, and some symptoms can be used as predictors of antibody response.
1. Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has introduced enormous changes in human existence, including sport. Many postponed or canceled events, limited access to training facilities, and sanitary restrictions have negatively impacted athletes' lives.1 Additionally, the threat of unpredictable health issues after the COVID-19 has emerged, and arose the question, how to return to sport safely.
The clinical course and consequences of COVID-19 in non-athletic populations have already been explored and depend mostly on the presence of comorbidities and age.2 , 3 The infection could be asymptomatic, but most frequently, in over 80% of patients, the clinical course is mild.4
There are limited data on COVID-19 clinical symptoms, complications, and antibody response in physically active people, especially elite athletes. In this group, the primary health concern is myocarditis since SARS-CoV-2 demonstrates a cardiac tropism by directly influencing cardiomyocytes and endothelial cells or indirectly by systemic inflammation via increased cytokines activity.5 The current position papers on the return to play consider those risks but recommend monitoring and returning to sports activity within two symptom-free weeks depending on disease severity.6, 7, 8, 9, 10. The guidelines mentioned above are based on experts' opinion since the published evidence on the COVID-19 clinical course, consequences, and antibody response in athletes are scanty.
The aim of the study was to identify predictors determining the course of COVID-19 and antibody response in elite athletes.
2. Methods
The study design was observational. Between July and October 2020, the routine medical screening was performed in 1425 Polish elite athletes, members of the national teams competing on the international level. Of these, 111 were tested SARS-CoV-2 positive by RT-PCR (reverse transcription polymerase chain reaction (target genes RdRp, N; Abbott m2000), and were included in the present study. All athletes were Caucasians and represented different sport categories: strength (wrestling, judo, track and field sprinting and throwing), endurance (middle distance running, cycling, speed skating, cross country skiing, swimming), team sports (handball, beach volleyball), and technical disciplines (fencing, curling). Baseline characteristics are presented in Table 1 . Medical screening was performed on a voluntary basis 20 (12–68) days (median and range) after diagnosis of COVID-19 and included detailed physical examination, resting ECG, and laboratory tests, including whole blood count, markers of inflammation (C-reactive protein — CRP) and cardiac injury (high-sensitive troponin T) using standardized equipment (Cobas 800, Roche, Rotkreuz, Switzerland). The following cut-off values were applied: leukopenia <4000/μl, neutropenia <2000/μl, leukocytosis >10,000/μl, CRP <5 mg/l, hs-troponin T < 0.014 ng/ml.
Table 1.
Characteristics of the subjects and corresponding cut-off index of antibody response (COI). Mean ± SD; range.
| n (%) |
Age [yrs] | Height [cm] | Body mass [kg] | BMI [kg/m2] | COI | ||
|---|---|---|---|---|---|---|---|
| Strength | Female | 23 (20.7%) |
26 ± 6 (19–39) |
165 ± 7 (150–180) |
63 ± 11 (52–101) |
23.1 ± 3.3 (19.0–35.8) |
55.7 ± 55.5 (0.6–197.1) |
| Male | 10 (9.0%) |
25 ± 2 (22–29) |
180 ± 5 (172–187) |
89 ± 12 (73–108) |
27.5 ± 2.4 (23.9–32.5) |
6.2 ± 7.7 (0.3–25.1) |
|
| Endurance | Female | 8 (7.2%) |
26 ± 4 (21–35) |
167 ± 6 (159–177) |
65 ± 8 (56–78) |
23.1 ± 1.9 (19.8–24.9) |
15.9 ± 24.2 (0.1–71.2) |
| Male | 21 (18.9%) |
24 ± 5 (19–36) |
182 ± 7 (169–196) |
81 ± 11 (53–99) |
25.0 ± 2.9 (20.3–30.4) |
27.2 ± 30.2 (0.2–104.4) |
|
| Team sports | Female | 23 (20.7%) |
19 ± 3 (16–28) |
173 ± 7 (155–187) |
68 ± 9 (54–84) |
22.8 ± 2.1 (19–27.1) |
17.5 ± 30.3 (0.1–139.9) |
| Male | 15 (13.5%) |
18 ± 4 (16–32) |
190 ± 9 (175–213) |
88 ± 12 (68–118) |
23.9 ± 2.5 (20.1–31.3) |
25.6 ± 25.3 (0.1–74.2) |
|
| Technical disciplines | Female | 5 (4.5%) |
22 ± 6 (16–30) |
169 ± 3 (165–171) |
62 ± 8 (55–75) |
21.6 ± 2.6 (19.0–25.7) |
76.9 ± 56.0 (13.9–132.6) |
| Male | 6 (5.4%) |
27 ± 8 (17–36) |
183 ± 4 (179–189) |
78 ± 7 (74–93) |
23.2 ± 1.7 (21.0–25.9) |
32.3 ± 47.9 (2.7–124.9) |
|
| Total | 111 | 22 ± 5 (15–38) |
176 ± 11 (150–213) |
74 ± 14 (52–118) |
23.9 ± 2.9 (19.0–35.8) |
30.6 ± 40.6 (0.1–197.1) |
|
Sports disciplines apportionment: strength – wrestling (16 athletes), judo (12), track and field sprinting and throwing (5); endurance - middle distance running (2), cycling (14), speed skating (10), cross country skiing (2), swimming (1); team sports - handball (33), beach volley (5); technical disciplines - fencing (6), curling (5). The COI <1 was present in 13 subjects (12%).
For the detection of SARS-CoV-2 seropositivity, the qualitative immunoassay for the detection of antibodies (including IgG) to SARS-CoV-2 in human serum (Elecsys® Anty-SARS-CoV-2 assay), and Cobas e411 analyzer (Roche Diagnostics, Rotkreuz, Switzerland) were used.
SARS-CoV-2 nucleocapsid antigen is detected with an electrochemiluminescence sandwich assay (ECLIA). Results are semi-quantitative and are expressed as qualitative statements (reactive or nonreactive) and in the form of a Cut-off-Index (COI) interpreted as follows: COI ≥ 1 is considered positive, while a COI < 1 is negative.11 The COI is a numerical representation of the measured fluorescence signal, and the COI values are approximately proportional to the concentration of the IgM and IgG antibodies in the specimen. This antibody assay has also received emergency use authorization from the US Food and Drug Administration for the detection of total antibodies (IgG, IgM, and IgA) in COVID-19 infected patients.12 The concentration of 25(OH)D, a marker of vitamin D status, was determined with Liaison diagnostic system (DiaSorin, USA) by a chemiluminescent immunoassay (CLIA).
Criteria of COVID-19 severity:
Asymptomatic: SARS-CoV-2 infection without any symptoms, coincidentally confirmed with RT-PCR test.
Mild: the presence of nonspecific and self-limited fatigue, olfactory or gustatory dysfunction, or typical mild symptoms of the upper respiratory tract or gastrointestinal infection including headache, cough, sore throat, nasopharyngeal congestion, myalgia, nausea, vomiting, or diarrhea.
Moderate: symptoms persisting over seven days and including fever, chills, hypoxemia (blood oxygen saturation below 95%), pneumonia, dyspnea, chest pain or tightness at rest or during exercise.
Severe: hospitalization or oxygen therapy required (including non-invasive ventilation).
Critical: invasive ventilation required.
Each subject signed the written informed consent, and the study protocol was approved by the Ethics Committee (permission number KEBN-21-60-DK).
Unless otherwise stated, the data are presented as mean ± SD with range or as cases (by number and by percentage), the data distribution was normal (checked using Shapiro-Wilk test). Continuous results were compared with Unpaired Two-Sample Wilcoxon (COI versus sex) and Kruskal–Wallis (COI versus sports discipline) tests, and relationships were evaluated using correlation tests (COI versus BMI, COI vs age, COI vs number of symptoms or COI vs their duration). The percentage occurrence of symptoms versus age, which divides data into two subgroups, was analyzed using Pearson's chi-squared proportion tests (null hypothesis stated there are equal proportions). The aim was to assess whether there is an age threshold when the p-values of proportion tests exceeds the considered significance level. For all cases, the significance level was set at 0.05. All calculations were performed in R Software 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Of the 1425 subjects screened between July and October 2020 a total of 111 (8%) were diagnosed with COVID-19. The symptoms of COVID-19 were observed in 93 patients (84%). The severity of COVID-19 was mild in 91 (82%) athletes and moderate in 2 (2%) cases. Frequencies of symptoms were as follows: tiredness 62 (56%), olfactory or gustatory dysfunction 62 (56%), headache 55 (50%), muscle pain 42 (38%), congestion or rhinorrhea 30 (27%), cough 29 (26%), sore throat 22 (20%), fever 21 (19%), dyspnea 19 (17%), chills 19 (17%), gastrointestinal discomfort 11 (10%), conjunctivitis 10 (9%) and hemoptysis 1 (1%).
Asymptomatic subjects were younger (18.5 ± 2.1 vs. 23.2 ± 5.6 yrs., p < 0.001) and more often female (23.7% female vs. 7.7% male, p < 0.05). The relationship between the proportion of symptoms and age was shown to be significant at an age cutoff of 26 years.
At the medical screening, 20 subjects (18%) still reported abnormal symptoms, among whom in 9 (8%) additional diagnostic tests were performed: cardiac Magnetic Resonance (MR) due to exertional dyspnea, chest pain, and elevated hs-troponin T, and chest CT due to dyspnea in and hemoptysis.
The cardiac MR scans identified no cases of active myocarditis or pericarditis. There were also no signs of fibrosis suggesting prior myocarditis or structural heart diseases.
In one subject, the chest CT scans revealed pneumonia 20 days since the positive RT-PCR, whereas in others the CT scans were normal. The subjects presenting symptoms at the screening time were older (24.4 ± 5.5 vs. 22.0 ± 5.4 yrs., p < 0.05), had a longer symptom duration (7.5 ± 4.2 vs. 5.0 ± 3.9 days, p < 0.05), and were predominantly male (26.9% male vs. 10.2% female, p < 0.05).
At the time of medical screening (median 20 days since COVID-19 was diagnosed), the subjective feeling of decreased exercise tolerance was reported by three subjects (3%). Furthermore, symptoms as olfactory or gustatory dysfunction were reported in 7 subjects (6%) and headache during exercise in 1 subject (1%).
Abnormal blood test results were observed in 12 athletes (11%). In 10 cases (9%), the white blood cell count was abnormal (8 patients had leukopenia (range 2900–3900/μl) with neutropenia (range 1100–1900/μl), 1 patient had leukopenia only (3700/μl), 1 patient had leukocytosis (12,000/μl)), 1 patient had elevated CRP (7.7 mg/l), and 1 patient had elevated hs-troponin T (0.038 ng/ml).
Antibody response (COI > 1) was found in 98 athletes (88%). In the remaining 13 subjects (12%), it was below the detection threshold.
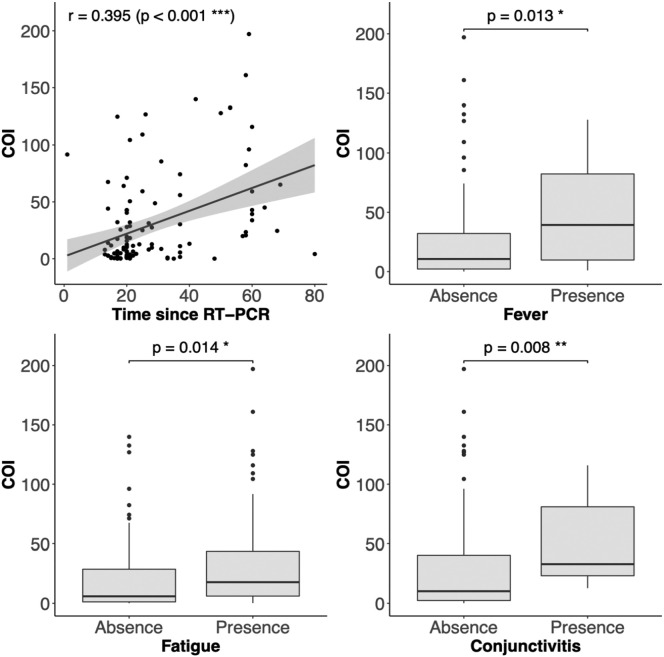
There were no differences in antibody response, expressed as the COI, between groups distinguished by the sports discipline (p = 0.50), and sex (p = 0.59), (Table 1). The COI did not correlate with BMI (p = 0.12), age (p = 0.13), the number of symptoms (p = 0.43) or their duration (p = 0.19). Positive correlations were found between COI and the time since RT-PCR test (p < 0.001), fever (p < 0.05), fatigue (p < 0.05), conjunctivitis (p < 0.01), and borderline relationship with headache (p = 0.05; Fig. 1 ). Athletes with the positive antibody response reported fatigue more frequently (18 of 98) than those with COI < 1 (1 of 13), p < 0.05.
Fig. 1.
The relationships between antibody response (COI – antibodies' cut-off index) and time since RT-PCR test and symptoms: fever, tiredness, and conjunctivitis.
The blood concentration of 25(OH)D was 32.2 ± 11.1 ng/ml. There were 14 (13%) cases of deficiency (25(OH)D < 20 ng/ml) – 6 in asymptomatic and 8 in symptomatic athletes. There was no correlation between 25(OH)D concentration and COI. It also did not influence the severity of symptoms of the COVID-19.
4. Discussion
The main finding of the present study is that the clinical presentation of COVID-19 in elite athletes in the majority of cases is mild (82%). Asymptomatic infections were recognized in 16% of cases, predominantly younger (<26 years) and female.
According to COVID Data Tracker, managed by the Centers for Disease Control and Prevention (CDC), approximately 33% of cases of COVID-19 in general US population are adults aged 18–34.13 Most elite athletes fall into this age group. It is worth noting that athletes' lifestyle, with frequent traveling and gathering in training and competition facilities, puts that group at increased risk of disease transmission.14 Additionally, exercise-induced changes in the immune system following exercise can make athletes more susceptible to infections.
Elite athletes are under regular medical supervision and monitoring during their preparation for major sports events. Due to the COVID-19 pandemic, they are obligated to do tests for SARS-CoV-2 as a preventive measure – before traveling abroad and during sports events organized in so-called sports bubbles where, for safety reasons, athletes reside and compete in isolation. Frequent testing increases the chances of detecting subjects with mild or asymptomatic infections. Patients with COVID-19 may be asymptomatic, but epidemiological studies showing the prevalence of such cases are few. The ratio of asymptomatic infections ranges from 4% to 57%.2 , 15 , 16 The systematic review of 38 studies involving 3062 COVID-19 adults revealed 11.9% of asymptomatic cases.17 In the study by Bielecki et al. in a group of 283 soldiers, 215 were infected, 113 (52,5%) were asymptomatic, 101 (47%) had a mild course, and only one (0,5%) needed oxygen and was hospitalized.18 Athletes and soldiers are similar since both are young, apparently healthy, and their daily routine and contact pattern are comparable, nevertheless the rate of asymptomatic athletes in the present study was significantly lower (16%).
The data on COVID-19 symptoms in athletes are scanty. In the study by Rajpal et al., out of 26 competitive college athletes with COVID-19, 12 (46%) reported mild symptoms, while 54% were asymptomatic.19 The similar percentage of asymptomatic athletes (58%) was observed by Schumacher et al., who studied 434 soccer players, of whom 36 had COVID-19.20 In 22 COVID-19 positive collegiate athletes examined by Clark et al., the majority of them (n = 17, 77%) experienced mild illness, while 23% were asymptomatic, in which is only slightly above the result obtained in the present study.21 The present study included 111 elite Polish athletes, most under 30, after COVID-19 infection, both symptomatic and asymptomatic. Asymptomatic infections were recognized in 16% of cases. The severity of symptoms shown in the present study (in the vast majority mild) seems to be consistent with general predictions for the same age group.2 , 3 The detailed evaluation of symptomatic and asymptomatic cases provides an interesting insight into predictors of disease progression in athletes.
The predictors for COVID-19 severity are clearly defined in the older population, namely age and comorbidities.3 , 22 In the present study, the asymptomatic subjects were significantly younger and predominantly female. Although the studied population was relatively young (mean age 22.4 ± 5.4 yrs.), the impact of age appeared to be significant with the critical value of 26 years. The asymptomatic or mild clinical course of COVID-19 in younger people can be explained by heterologous immune responses associated with routine immunizations in childhood, frequent exposure to seasonal coronaviruses, and a more diverse memory T cell repertoire.23 Moreover, an increased expression of angiotensin-converting enzyme 2 is involved in anti-inflammatory signaling and may reduce severe disease risk in children and young people.24
The better tolerance of COVID-19 in females agrees with existing data, presumably due to differences in the elicited immune responses.25 , 26 Inside the cell, the virus is sensed by the Toll-like receptor 7, subsequently inducing the type I interferon, which may influence the disease's severity. Since the Toll-like receptor 7 is expressed on the chromosome X, its dosage is sex-dependent and could cause the observed differences between men and women.27
Asymptomatic COVID-19 sports team members played a significant part in the magnitude of the outbreak's spread, similarly to asymptomatically infected young adults within general populations.28 In the present study, only 16% of the infected athletes were asymptomatic, contrary to results obtained by both Clark et al. and Rajpal et al.; nevertheless, it can be assumed that one out of six infected athletes cannot suspect himself as sick.19 , 21 Likewise, in the US, people under 30 accounted for 53% of COVID-19 cases and were suspected as more likely to transmit the infection to others.13 Given that such a high percentage of people is undiagnosed and still infectious to others, it constitutes a tremendous epidemiological threat. They also should be the target group in prevention, along with symptomatic ones.4 It is a fundamental observation that should be considered when planning the protection strategies during major sports events.
An issue vital for elite athletes is an answer to the question: when they can safely return to regular training after COVID-19? Athletes who have experienced COVID-19 may develop chronic symptoms like cough, wheeze, chest tightness, exertional breathlessness, tachycardia, and fatigue many weeks or months after the primary infection. The decisions concerning the return-to-sport of elite athletes are challenging since the SARS-CoV-2 infection may result in a significant cardiorespiratory compromise in individual subjects.6 In the present study, abnormal symptoms persisted in 20 subjects (18%). In 9 (8%) additional diagnostic tests were medically justified – the cardiac MR and chest CT – mainly due to exercise dyspnea and chest pain.
Despite initial alarming data reporting cardiac MR findings suggestive of acute myocarditis in 15% of athletes post COVID-19, as reported by Rajpal et al., subsequent studies do not seem to confirm these findings, showing only incidental frequency of cardiac injury.19 , 21 , 29, 30, 31 This undermines the suggestion of the necessity of cardiac MR testing in asymptomatic or mildly symptomatic athletes diagnosed with COVID-19. Thus, the current guidance recommends graduated return-to-sport after ten days from the cessation of symptoms without additional testing.7 The indications for cardiac imaging include usually abnormal results of initial tests – electrocardiogram, transthoracic echocardiography, cardiac troponin or the presence of cardiopulmonary symptoms such as chest pain, shortness of breath, palpitations or exercise intolerance. However, chest pain, dyspnea or fatigue after COVID-19 are usually nonspecific, while mildly elevated troponin T does not necessarily indicate cardiac injury post-COVID-19.32
The most frequent blood count abnormalities of the COVID-19 patients involve white blood cells and depend on the disease's severity and age.17 , 33 , 34 The systematic review of 38 studies involving 3062 adults with COVID-19 showed normal leukocyte count in 69.7% and lymphopenia in 56.5% of patients.17 The other literature review revealed lymphopenia as the most common finding among adults with COVID-19 infection.34 Among the 624 children and adolescents with mild COVID-19, leukocyte count was elevated in 13%, and leukopenia was found in 19% of subjects.33 Compared to adults, the difference in laboratory results observed in children and adolescents is probably due to a more active innate immune response, healthier respiratory tracts, and fewer other disorders present in younger people.35 In the present study, white blood cell count changes were observed in 10% of subjects, mostly leukopenia due to neutropenia. The changes described in the studies mentioned above were observed during the disease. In contrast, in the present study, blood samples were taken 20 (12–68) days (median and range) after a positive RT-PCR test, and since most athletes had a mild or asymptomatic COVID-19 course, it could have been assumed that athletes should not have blood count abnormalities then.
Elevated CRP during COVID-19 Henry et al. reported only in 18% of patients, whereas Zhu et al. in 74%.17 , 33 In the present study, increased CRP was found in just one case, classified as moderately severe, a cyclist with hemoptysis. He was ordered a chest CT and typical parenchymal pneumonia features present also in COVID-19 were found. Such a low number of cases with elevated CRP appears to be due to the long interval from the onset of COVID-19 and a small number of severe cases.
Elevated hs-troponin T was found in one judoka, but myocarditis and other structural heart were excluded in cardiac MR. In the aforementioned studies by both Rajpal et al. and Clark et al., no athlete had elevated troponin T serum levels.19 , 21 Recent study on soccer players demonstrated that mild elevation of cardiac troponin was occasionally observed irrespectively of positive results of SARS-CoV-2 test, as troponin has been shown to transiently raise in response to repeated, especially intensive, exercise and training.32
No diagnostic ST/T wave changes on electrocardiogram were found in the present study, which is consistent with the results of both Rajpal et al. and Clark et al.19 , 21
Vitamin D is involved in immunomodulation. Several authors have found it beneficial for the incidence and severity of COVID-19.36 That effect is usually described in older and more severely symptomatic subjects. In the present study, no relationship was found between vitamin D status and COI or severity of COVID-19, probably because athletes were young, otherwise healthy, and experienced COVID-19 mildly.
It is identified that after 14 days since the onset of COVID-19 symptoms, antibodies directed against SARS-CoV-2 can be detected in over 90% of subjects, and they are recognized as an essential predictor of population immunity.37 The antibody kinetics varied across the severity gradient with longer durations of detectable antibodies associated with more severe symptoms.38 The prevalence of the antibody positivity against SARS-CoV-2 remains unknown in the athletic population. During 9 weeks of the study by Schumacher et al. positive serology was found in 42% of 36 infected soccer players.20 In the present study, antibody response (COI > 1) was observed in 88% of athletes with COVID-19, which proves its reliability in evaluating seroprevalence. Moreover, it was confirmed that the COI is time-dependent. The COI was not different between symptomatic and asymptomatic subjects, but fatigue was reported less frequently by athletes with low antibody levels. Interestingly, fatigue, fever, and conjunctivitis correlated with COI. The next months will be critical to evaluate the robustness of the immune response to SARS-CoV-2 infection and to find clues for some open questions, such as questions about the duration of circulating antibodies and the impact of reinfection.
The study has some limitations. It is retrospective, observational, and without a non-athletic control; the time of examination since diagnosis is long and not unified (median 20, range 12–68 days). The number of athletes representing different sport disciplines is not similar.
5. Conclusion
The course of COVID-19 in elite athletes is predominantly mild and without complications. Male athletes aged above 26 are more likely to develop symptomatic COVID-19, whereas younger females are more frequently asymptomatic. Usually, additional heart screening is not required, and athletes can return to play after two asymptomatic weeks. Determination of antibodies has proved to be a useful indicator of past infection of SARS-CoV-2, and should help build the protection strategy before the major sports events during the COVID-19 pandemic.
Funding information
This research did not receive any specific funding.
Declaration of interest statement
All authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence the present work.
Confirmation of ethical compliance
The authors of this paper confirm that this research was approved by the ethical committee at the Institute of Sport—National Research Institute in Warsaw.
Acknowledgment
No financial assistance and grant support have been provided for this project.
Authors dedicate special thanks to other contributors of the study: Alicja Felkner, Agnieszka Pietrak, Ewa Stasiakowska-Grochowska, Pawel Kaliszewski and Paulina Siek.
References
- 1.Faulkner J., O’Brien W.J., McGrane B., et al. Physical activity, mental health and well-being of adults during initial COVID-19 containment strategies: a multi-country cross-sectional analysis. J Sci Med Sport. 2020 doi: 10.1016/j.jsams.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga W.J., Rhodes A., Cheng A.C., et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 5.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson M.G., Hull J.H., Rogers J., et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: a practical guide for sport and exercise medicine physicians. Br J Sports Med. 2020;54:1157–1161. doi: 10.1136/bjsports-2020-102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phelan D., Kim J.H., Chung E.H. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.2136. [DOI] [PubMed] [Google Scholar]
- 8.Hughes D., Saw R., Perera N.K.P., et al. The Australian Institute of Sport framework for rebooting sport in a COVID-19 environment. J Sci Med Sport. 2020;23(7):639–663. doi: 10.1016/j.jsams.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schellhorn P., Klingel K., Burgstahler C. Return to sports after COVID-19 infection. Eur Heart J. 2020;41(46):4382–4384. doi: 10.1093/eurheartj/ehaa448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aspetar Clinical Guidelines: Safe return to sport during the COVID-19 pandemic (2020). Available at: https://d2g8uwgn11fzhj.cloudfront.net/wp-content/uploads/2020/06/26103338/2020-June-Aspetar-Clinical-Guideline-Safe-return-to-sport-during-the-COVID-19-pandemic.pdf. (Update 31 July 2020). Accessed 5 Mai 2021.
- 11.Chan C.W., Parker K., Tesic V., et al. Analytical and clinical evaluation of the automated elecsys anti-SARS-CoV-2 antibody assay on the Roche cobas e602 analyzer. Am J Clin Pathol. 2020;154(5):620–626. doi: 10.1093/ajcp/aqaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weidner L., Gänsdorfer S., Unterweger S., et al. Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays. J Clin Virol. 2020;129:104540. doi: 10.1016/j.jcv.2020.104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID Data tracker. https://covid.cdc.gov/covid-data-tracker/index.html#datatracker-home Available at:
- 14.Walsh N.P., Gleeson M., Shephard R.J., et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 15.Mizumoto K., Kagaya K., Zarebski A., et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimball A., Hatfield K.M., Arons M., et al. Asymptomatic and presymptomatic SARSCoV- 2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J., Ji P., Pang J., et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92(10):1902–1914. doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bielecki M., Züst R., Siegrist D., et al. Social distancing alters the clinical course of COVID-19 in young adults: a comparative cohort study. Clin Infect Dis. 2021;72(4):598–603. doi: 10.1093/cid/ciaa889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajpal S., Tong M.S., Borchers J., et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–118. doi: 10.1001/jamacardio.2020.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumacher Y.O., Tabben M., Hassoun K., et al. Resuming professional football (soccer) during the COVID-19 pandemic in a country with high infection rates: a prospective cohort study. Br J Sports Med. 2021;0:1–7. doi: 10.1136/bjsports-2020-103724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark D.E., Parikh A., Dendy J.M., et al. COVID-19 myocardial pathology evaluated through screening cardiac magnetic resonance (COMPETE CMR) medRxiv. 2020 doi: 10.1101/2020.08.31.20185140. [DOI] [Google Scholar]
- 22.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi Q., Liu Y., Cheng Y., et al. Age and T-cell repertoire. Proc Natl Acad Sci. 2014;111(36):13139–13144. doi: 10.1073/pnas.1409155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felsenstein S., Hedrich C.M. SARS-CoV-2 infections in children and young people. Clin Immunol. 2020;220:108588. doi: 10.1016/j.clim.2020.108588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vahidy F.S., Pan A.P., Ahnstedt H., et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross-sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0245556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi T., Ellingson M.K., Wong P., et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27(1):28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 28.Kronbichler A., Kresse D., Yoon S., et al. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:180–186. doi: 10.1016/j.ijid.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Małek Ł.A., Marczak M., Miłosz-Wieczorek B., et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: a magnetic resonance study. J Magn Reson Imaging. 2021 doi: 10.1002/jmri.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez M.W., Tucker A.M., Bloom O.J., et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021;4 doi: 10.1001/jamacardio.2021.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moulson N., Petek B.J., Drezner J.A., et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021 doi: 10.1161/CIRCULATIONAHA.121.054824. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mascia G., Pescetelli F., Baldari A., et al. Interpretation of elevated high-sensitivity cardiac troponin I in elite soccer players previously infected by severe acute respiratory syndrome coronavirus 2. Int J Cardiol. 2021;326:248–251. doi: 10.1016/j.ijcard.2020.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry B.M., Benoit S.W., de Oliveira M.H.S., et al. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): a pooled analysis and review. Clin Biochem. 2020;81:1–8. doi: 10.1016/j.clinbiochem.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vakili S., Savardashtaki A., Jamalnia S., et al. Laboratory findings of COVID-19 infection are conflicting in different age groups and pregnant women: a literature review. Arch Med Res. 2020;51(7):603–607. doi: 10.1016/j.arcmed.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Q., Shi Y. Coronavirus disease (COVID-19) and neonate: what neonatologist need to know. J Med Virol. 2020;92(6):564–567. doi: 10.1002/jmv.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shakoor H., Feehan J., Al Dhaheri A.S., et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azkur A.K., Akdis M., Azkur D., et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(1):4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]