Abstract
Here we present the first case of newly diagnosed IgA nephropathy (IgAN) after a SARS-CoV-2 vaccination. A 30-year-old man with no known past medical history presented with gross hematuria and subnephrotic proteinuria 24 hours after the second dose of the mRNA-1273 SARS-CoV-2 vaccine. A kidney biopsy showed IgAN. He was started on an angiotensin receptor blocker, resulting in proteinuria reduction. Similar to natural infection of SARS-CoV-2, persons who receive 2 mRNA-based vaccines demonstrate robust antibodies against the receptor-binding domain (RBD) of the S1 protein. Given the uniqueness of glycosylation of RBD and potent stimulation of immune response from mRNA-based vaccine compared to other vaccines, we hypothesize that our patient developed de novo antibodies, leading to IgA-containing immune-complex deposits. This case highlights the urgency of understanding the immunological responses to novel mRNA-based SARS-CoV-2 vaccines in more diverse populations. Despite the lack of clear causality, nephrologists should be alerted if any new-onset hematuria or proteinuria is observed.
Introduction
While IgA nephropathy (IgAN) is typically triggered by upper respiratory infections, relapse of IgAN has been reported in patients receiving vaccinations. More recently, there have been 3 case reports of patients who presented with IgAN relapse following SARS-CoV-2 mRNA vaccination.1,2 Here, we present the first case of newly diagnosed IgAN after receiving the Moderna SARS-CoV-2 mRNA vaccine.
Case Report
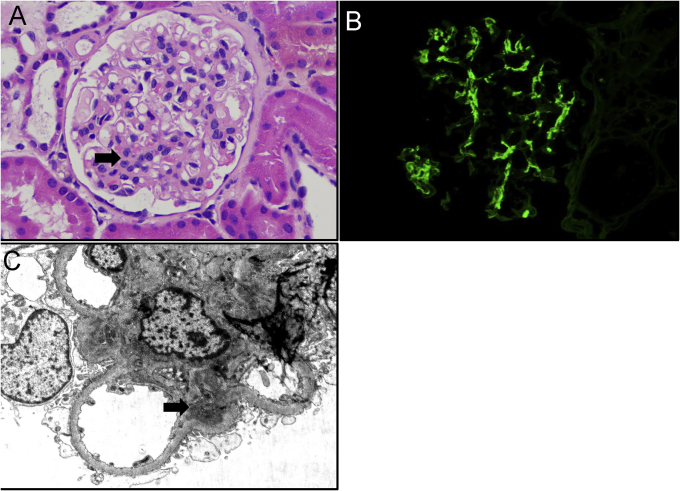
A 30-year-old-man of Western European and South American ancestry presented with new-onset hematuria and proteinuria. He had no known past medical history and had never been tested for COVID-19 infection. He did not report any known COVID-19 exposures and had not had any flu-like illness throughout the COVID-19 pandemic. He reported no family history of kidney disease, including IgAN. He received the first dose of mRNA-1273 SARS-CoV-2 vaccine, manufactured by Moderna, and remained asymptomatic during the 28-day interval between doses. However, 1 day after receiving the second vaccine, he developed fevers, chills, headache, and brown-colored urine. He presented to his primary care physician, where urinalysis showed 4+ protein (ref: negative), >30 red blood cells per high-power field (ref: 0-3), 11-30 white blood cells per high-power field (ref: 0-4), and 3+ blood (ref: negative). Creatinine was 1.02 mg/dL (Ref: 0.76-1.27 mg/dL), and estimated glomerular filtration rate was 98 cc/min/1.73 m2. Gross hematuria resolved after 48 hours, but a repeat urinalysis 10 days later showed persistent microscopic hematuria and proteinuria. He was sent to nephrology for consultation. Physical examination was normal, and blood pressure was 125/73 mm Hg. Pertinent negatives included lack of lower extremity edema, rash, lymphadenopathy, and throat erythema. Random urine protein-creatinine ratio was 0.8 g/g (ref: 0-0.2 g/g), estimating 24-hour urine protein excretion of 800 mg. Urinalysis after centrifugation revealed numerous acanthocytes, but no red blood cell casts. Kidney ultrasound showed mildly increased echogenicity of normal size and cortical thickness. Additional serological work-up for glomerulonephritis was negative, including hepatitis B and C, HIV, and antinuclear and antineutrophil cytoplasmic antibodies. Erythrocyte sedimentation rate and C-reactive protein were normal. Complements C3 (105, ref: 82-167 mg/dL) and C4 (19, ref: 12-38 mg/dL) were normal. Creatinine phosphokinase was 254 U/L (ref: 49-439 U/L). Immunoglobulin A levels were elevated at 444 mg/dL (ref: 90-386 mg/dL). Given the unclear diagnosis, a kidney biopsy was performed. Light microscopy revealed 9 glomeruli with mild mesangial expansion and hypercellularity without endocapillary hypercellularity (Fig 1A), 1 of which showed segmental adhesion of a capillary loop to the Bowman capsule. Immunofluorescence revealed 3+ diffuse granular mesangial staining for IgA (Fig 1B). Staining was weakly positive for C3 and negative for IgG and other immunoglobulins/complement antibodies. Ultrastructural examination revealed scattered immune-type electron-dense deposits in the mesangium and mild podocyte foot process effacement (Fig 1C). Pathologic features were consistent with IgAN with Oxford MEST-C classification as M1-E0-S1-T0-C0,3 and his risk of a 50% decline in estimated glomerular filtration rate or progression to kidney failure within 5 years was approximately 3.9%, as per a recent risk prediction model by the International IgA Nephropathy Network.4 He was started on losartan 25 mg daily, which was well tolerated. After 6 weeks of therapy, urine protein-creatinine ratio improved to 0.43 g/g and creatinine remained stable at 1.03 mg/dL.
Figure 1.
(A) Glomerular mesangial expansion and hypercellularity (black arrow) (hematoxylin-eosin, ×200). (B) Strong glomerular mesangial deposits for IgA antisera (immunofluorescence study, ×200). (C) Ultrastructural evaluation revealed immune-type electron-dense deposits involving the mesangium (black arrow) (transmission electron microscopy, ×4,000).
Discussion
To our knowledge, this is the first reported case of newly diagnosed IgAN in a kidney biopsy related to a COVID-19 vaccine, after a very recent report of exacerbated IgAN in 2 patients who received the second dose of mRNA-1273 SARS-CoV-2 vaccine.2 Although correlation does not inherently imply causation, the timing of symptom onset shortly after the vaccine should be considered as the inciting event.
IgAN is an immune-complex disease characterized by mesangial IgA1 deposition with or without concurrent IgG and C3 deposits. Despite IgAN being the most common primary glomerulonephritis worldwide,5 its underlying pathogenesis was unclear until the recognition of circulating, poorly galactosylated IgA1 in serum and direct deposit in glomeruli by mass spectrometry analysis.6 It is noteworthy that the existence of poorly galactosylated IgA1 alone is not sufficient to trigger IgAN. Immune complexes formed by pathogenic IgA1 and glycan-specific IgG or IgA targeted at these aberrantly galactosylated areas are usually required, followed by impaired liver removal and an increased affinity for mesangial cells.7 Thus, IgAN is proposed as a multi-hit disease. It may involve genetic predispositions (gene variants encoding galactosylation) and subsequent triggering events such as infection, environmental exposure, or dietary influences that lead to anti-glycan IgG/IgA production.8 Although the occurrence of postvaccination IgAN remains scarce, the interplay between vaccination and IgA vasculitis/Henoch–Schönlein purpura has been postulated.9 Intramuscular inactivated influenza vaccine elicits hyper-responsiveness in a cohort of pre-existing IgAN patients, with excessive productions of IgA1 monomers (but not polymers).10 Recurrence of IgAN following influenza vaccine has also been reported in kidney transplant recepients.11,12
Our patient completed 2 doses of mRNA-1273 SARS-CoV-2 vaccine, a lipid nanoparticle–encapsulated mRNA vaccine.13 Once injected, mRNA encoding prefusion-stabilized spike glycoprotein is translated by host cells, and major histocompatibility complex molecules subsequently present viral protein segments to elicit immune responses with antibody production, including IgG/A/M. Importantly, the receptor-binding domain (RBD) of the S1 subunit is the immunodominant target of neutralizing antibodies in both infected patients14 and vaccinated persons.15 In contrast to other S protein areas, RBD has limited glycosylation sites, likely to allow stable and persistent entry of SARS-CoV-2 into host cells. This unique property of RBD, on the other hand, serves as the ideal epitope for vaccine development.16 Since vaccination does not elicit mucosal immune responses, one possible explanation for the IgAN seen in our patient is the production of anti-glycan antibodies that cross-react with pre-existing under-galactosylated IgA1. In addition, mRNA-based vaccine may induce stronger T follicular helper and subsequent B-cell responses in the germinal center, which may lead to more robust antibody productions. Another possibility might be an increase of pathogenic IgA production similar to the influenza vaccine, given his elevated IgA level. A recent preprint article also suggested robust spike-specific IgA responses after receiving mRNA vaccines in healthy individuals.17 Nevertheless, the diagnostic value of circulating total IgA level in IgAN remains very limited. Additionally, there is the possibility that the patient had underlying subclinical “lanthanic” IgAN that was exacerbated by the vaccine.
According to Centers for Disease Control and Prevention data, there was no reported hematuria/proteinuria except for 1 patient who developed acute kidney injury owing to obstructive nephrolithiasis, resulting in death.18 It is beyond the scope of the discussion on the causality of IgAN in our patient. Still, emerging data suggest the importance of analyzing pre-existing antibodies to self-carbohydrates in either infected or vaccinated persons.19 Another intranasal vaccine candidate against SARS-CoV-2 is currently under investigation,20 which might directly perturb dysregulated mucosal immune system in genetically predisposed persons. Of note, local mucosal responses to natural SARS-CoV-2 in the nasopharynx are shown to be dominated by early IgA-neutralizing antibodies compared to IgG and IgM and were associated with a peripheral expansion of IgA-secreting plasmablasts.21 It is therefore essential to monitor any de novo IgAN or exacerbations of pre-existing IgAN following intranasal vaccinations. Lastly, in the first published mRNA-1273 trial data, there were only 4.6% Asians among all participants. Since IgAN is more common in the Asian population, larger clinical trial data, particularly with more diverse participants, are urgently needed to fully assess the possible correlation between IgAN and vaccination against SARS-CoV-2. While providers and patients should be aware of this adverse effect, it should be emphasized that this is likely a rare occurrence and should not deter others from seeking SARS-CoV-2 vaccination. Furthermore, SARS-CoV-2 infection confers a much higher risk than IgAN, and our finding is unlikely to change the risk-benefit ratio of vaccination.
Article Information
Authors’ Full Names and Academic Degrees
Matthew Abramson, MD, Samuel Mon-Wei Yu, MD, Kirk N. Campbell, MD, Miriam Chung, MD, and Fadi Salem, MD.
Support
None.
Financial Disclosure
Kirk Campbell is a consultant for Calliditas, Travere, and Mallinckrodt. The remaining authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that they have obtained consent from the patient reported in this article for publication of the information about him that appears within this Case Report and any associated supplementary material.
Peer Review
Received April 12, 2021. Evaluated by 1 external peer reviewer with direct editorial input by the Editor-in-Chief. Accepted in revised form May 31, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Gul Rahim S.E., Lin J., Wang J.C. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int. 2021;100(1):238. doi: 10.1016/j.kint.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negrea L., Rovin B.H. Gross hematuria following SARS-CoV-2 vaccination in patients with IgA nephropathy. Kidney Int. 2021;100(2):466–468. doi: 10.1016/j.kint.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trimarchi H., Barratt J., Cattran D.C. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91(5):1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Barbour S.J., Coppo R., Zhang H. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179(7):942–952. doi: 10.1001/jamainternmed.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schena F.P., Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol. 2018;38(5):435–442. doi: 10.1016/j.semnephrol.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Hiki Y., Odani H., Takahashi M. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59(3):1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 7.Novak J., Julian B.A., Tomana M., Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol. 2008;28(1):78–87. doi: 10.1016/j.semnephrol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai K.N. Pathogenesis of IgA nephropathy. Nat Rev Nephrol. 2012;8(5):275–283. doi: 10.1038/nrneph.2012.58. [DOI] [PubMed] [Google Scholar]
- 9.Patel C., Shah H.H. Vaccine-associated kidney diseases: a narrative review of the literature. Saudi J Kidney Dis Transpl. 2019;30(5):1002–1009. doi: 10.4103/1319-2442.270254. [DOI] [PubMed] [Google Scholar]
- 10.van den Wall Bake A.W., Beyer W.E., Evers-Schouten J.H. Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J Clin Invest. 1989;84(4):1070–1075. doi: 10.1172/JCI114269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNally A., McGregor D., Searle M., Irvine J., Cross N. Henoch-Schonlein purpura in a renal transplant recipient with prior IgA nephropathy following influenza vaccination. Clin Kidney J. 2013;6(3):313–315. doi: 10.1093/ckj/sft029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer A.S., Moller B.K., Krag S., Jespersen B. Influenza virus vaccination and kidney graft rejection: causality or coincidence. Clin Kidney J. 2015;8(3):325–328. doi: 10.1093/ckj/sfv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Premkumar L., Segovia-Chumbez B., Jadi R. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5(48) doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamatatos L., Czartoski J., Wan Y.H. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021 doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez A. Glycosylation of SARS-CoV-2 steers evolutionary outcomes in the postvaccination phase. ACS Pharmacol Transl Sci. 2021;4(1):410–412. doi: 10.1021/acsptsci.1c00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campillo-Luna J., Wisnewski A.V., Redlich C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. medRxiv. 2021:2021. doi: 10.1371/journal.pone.0249499. 2003.2023.21254060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccines and Related Biological Products Advisory Committee Meeting. December 17, 2020. Vol 2021. https://www.fda.gov/media/144434/download
- 19.Butler D.L., Gildersleeve J.C. Abnormal antibodies to self-carbohydrates in SARS-CoV-2 infected patients. bioRxiv. 2020;2020 doi: 10.1093/pnasnexus/pgac062. 10.15.341479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan A.O., Kafai N.M., Dmitriev I.P. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183(1):169–184 e113. doi: 10.1016/j.cell.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterlin D., Mathian A., Miyara M. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577):eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]