Abstract
The Coronavirus disease 19 (Covid-19) pandemic is devastating the public health: it is urgent to find a viable therapy to reduce the multiorgan damage of the disease. A validated therapeutic protocol is still missing. The most severe forms of the disease are related to an exaggerated inflammatory response. The pivotal role of reactive oxygen species (ROS) in the amplification of inflammation makes the antioxidants a potential therapy, but clinical trials are needed. The lecitinized superoxide dismutase (PC-SOD) could represent a possibility because of bioaviability, safety, and its modulatory effect on the innate immune response in reducing the harmful consequences of oxidative stress. In this review we summarize the evidence on lecitinized superoxide dismutase in animal and human studies, to highlight the rationale for using the PC-SOD to treat COVID-19.
Keywords: Lecitinized superoxide dismutase, Superoxide mimetics, Lung infections, Oxidative stress, Antioxidants, COVID-19 disease
Graphical Abstract
1. Introduction
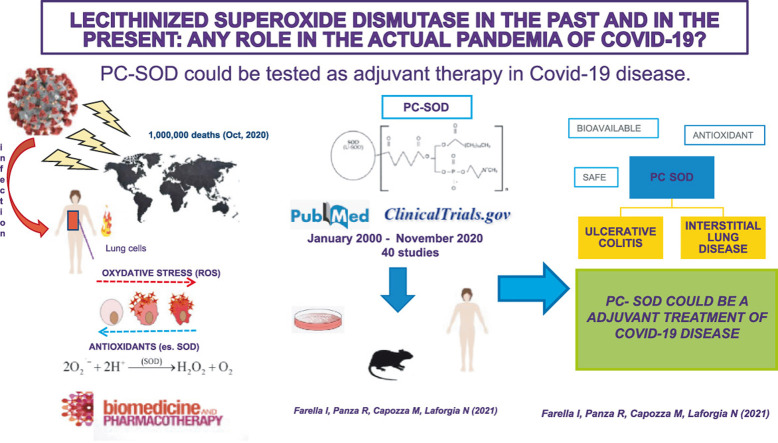
The COVID-19 is a respiratory tract infection caused by a new emerging pathogen: SARS-CoV-2, originally isolated in December 2019 in Wuhan, China. The pandemic caused by this novel coronavirus accounts for about 1000,000 deaths and 38,000,000 confirmed cases worldwide as of October 2020 [1]. To date, there is not approved treatment for this disease but various drugs are in use, from antivirals (e.g. Remdesevir) and antibiotics (azithromycin) to drugs with different anti-inflammatory action (e.g.Tocilizumab, methylprednisolone, hydroxicloroquine). Antioxidants have been proposed as adjunctive therapy, needing to be tested in clinical trials, due to their known lack of systemic toxicity [2], [3]. When the human immune system is challenged by a new pathogen, innate immunity plays a preponderant role, being the adaptive one not yet able to stimulate specific antibody production. The inflammatory cascade of innate immunity includes different mediators, such as cytokines and oxygen free radicals (ROS), responsible of oxidative stress-induced cell damage [4]. Oxidative stress is caused by an imbalance between the production of specific oxygen species and the capacity of a biological system to rapidly scavenge intermediate substances or repair the resulting damage [5]. It is well known that viral infections can alter the redox system increasing oxidant species and reducing antioxidant molecules [6], [7], [8]. Varga Z. et al. have reported that there is an association between ROS, endothelial damage and inflammation and this mechanism is also found in COVID-19 [9].
One of the most studied antioxidant metalloenzymes as a first line against ROS is the superoxide dismutase (SOD) [10]. Three isoforms of SOD exist in humans, and many other forms of this enzyme have been artificially produced. One of them, the PC-SOD, is a lecithinized SOD in which four phosphatidylcholine(PC)-derivative molecules are covalently bound to each SOD dimer. PC-SOD is effective in several experimental models such as inflammation, chemotherapy-induced cardiotoxicity, ischaemia reperfusion injury, motor dysfunction after spinal cord injury and lung fibrosis. The present research is based on studies from the past 20 years that investigated the effects of this enzyme in humans and animal models, to explore the possible role against the current pandemic.
2. Materials and methods
The present narrative review summarizes evidence regarding the role of lecitinized superoxide dismutase in animal and human studies throughout the last 20 years with the aim of determining the rationale for a possible use in the COVID-19 disease. The research was performed in the Pubmed database and Clinicaltrials.gov including studies published from January 2000 until November 2020, and it used the following keywords: lecitinized superoxide dismutase, superoxide mimetics, lung infections, oxidative stress, antioxidants and COVID-19. Search limits were set for studies exclusively published in English language.
3. PC-SOD
Different endogenous defence mechanisms against the ROS damage exist such as superoxide dismutase (SOD), catalase, peroxides, vitamin A and E, all characterized by free radical scavenger activity. SOD catalyses the transformation of the superoxide anion into molecular oxygen and hydrogen peroxide by decreasing the level of superoxide anion, a known factor of cell damage [11]. Three isoforms of SOD exist in humans: cytosolic Cu,Zn SOD (SOD1), mitochondrial MnSOD (SOD2) and extracellular Cu,Zn SOD (SOD3) [12], but none of these have attractive pharmacological properties. The natural antioxidant protection is insufficient in clinical conditions in which redox system is unbalanced, consequently, a possible benefit with exogenous antioxidant drugs it has been suggested [13]. Different forms of enzyme were then synthesized but the rapid renal clearance and slow extravasation makes their use not feasible [14]. The development of PC-SOD (recombinant human SOD1 covalently coupled to four molecules of lecithin) and a chimeric recombinant superoxide dismutase consisting of SOD2 and SOD3 [13] could allow their use as drugs. PC-SOD, synthesized for the first time by Igarashi et al. in 1992, has a better affinity to the cell membrane, a significant distribution to various tissues and a longer systemic half-life compared with SOD1. In addition, it has also a 4.5-fold increase in oxygen-radical scavenger effects with a100-fold increase in protective effects against endothelial cell injuries, compared to unmodified SOD [15], [16].
PC-SOD forms a complex with serum proteins such as albumin, whereas unmodified SOD does not. The cellular content, found in lysosomes, of PC-SOD is markedly higher when compared to unmodified SOD [17]. It has a low superoxide anion-scavenging activity into the mitochondria, but it accumulates in lymphocytes with a significant effect in scavenging extracellular superoxide anions [18].
4. PC-SOD in vitro and animal models
PC-SOD has been shown to be effective in animal models of various ROS-involved diseases.
4.1. Cardiovascular system
Increased ROS have been described in myocardial ischemia-reperfusion injuries [19], [20].
Several studies have evaluated PC-SOD in ischemia-induced myocardial damage. Cultured rat cardiomyocytes exposed to hypoxia have been incubated with lecithinized SOD (100 U/ml), unmodified SOD (100 U/ml) or vehicle alone. Lecithinized SOD, but not unmodified SOD, was effectively delivered into cells, as verified by Western blot and confocal laser-scanning microscopy and cell treated with lecithinized SOD showed significant decrease of hypoxia-induced cell damage [21]. Another in vitro study was performed on large vessel endothelial cells and a human microvascular endothelial cell line after cold hypoxia/reoxygenation. PC-SOD significantly protected both cellular lines from death after 27 h of cold hypoxia. Furthermore, neutrophil adhesion to the endothelium stimulated by hypoxia and reoxygenation was significantly inhibited by treatment with PC-SOD, but not by recombined human SOD [22].
Hangaishi and coll. exposed rats to 45 min of myocardial ischemia by occluding the left coronary artery followed by 120 min of reperfusion. Rats were randomly injected intravenously, 5 min prior to reperfusion, with either lecithinized SOD, polyethylene glycol conjugated SOD (PEG-SOD), unmodified SOD, free lecithin derivative or phosphate-buffered saline. Myocardial infarction areas, verified by triphenyltetrazolium chloride staining, were smaller in lecithinized SOD-treated group compared to the others. Moreover, cardiac SOD levels were higher in lecithinized SOD group [23]. A similar study by Nakajima et al. confirmed these results [24]. These data suggested lecithinized SOD, because of its increased bioaviability, had a protective effect in tissue ischemia reperfusion injuries.
Another role of PC-SOD in the cardiovascular system was on doxorubicin-induced damage. The involvement of oxygen radicals such as superoxide anions in doxorubicin-induced cardiotoxicity is widely accepted [25]. Gertjan et al. treated with PC-SOD for six weeks BALB/c mice with doxorubicin-induced cardiotoxicity, monitoring the ECG for 8 weeks, demonstrating that PC-SOD reduces doxorubicin-induced cardiotoxicity [26].
4.2. Respiratory system
Excessive production of ROS is a significant factor in ARDS and in ventilation-induced lung damage (VILI) [27], [28], [29]. They amplify the signal mediated by transforming growth factor beta (TGF-β), a cytokine directly involved in the activation of myofibroblasts and therefore in the development of pulmonary fibrosis [30].
Matsuzawa et al. evaluated the effect, in human fetal lung fibroblast, of probucol and lovastatin (anti-hyperlipidemia drugs with anantioxidant action), together with PC-SOD, on the TGF-β-induced ROS production and also on the expression of α-SMA, a myofibroblast marker. They found that probucol and lovastatin, in addition to PC-SOD, significantly inhibited both the TGF-β-induced ROS production and α-SMA expression [31].
Kotaro et al. investigated the inhibitory effects of both PC-SOD at different doses (1 mg/kg/d, low dose and 10 mg/kg/d, high dose) and methylprednisolone (mPSL) on bleomycin (BLM)-induced pulmonary fibrosis in mice. Histopathologic evaluation revealed that the severity of fibrosis was attenuated only in mice treated with low dose PC-SOD. BLM-induced increases in total cell number, populations of lymphocytes and neutrophils, and expression of messenger RNA for interleukin-1b and platelet-derived growth factor (PDGF)-A were significantly suppressed in PC-SOD–treated mice. The suppression of PDGF-A expression was significantly greater in mice treated with low-dose PC-SOD than in mice treated with high-dose PC-SOD or mPSL [32]. The effect of PC-SOD on BLM-induced pulmonary fibrosis was confirmed in others studies: as combined therapy with catalase (to reduce the overproduction of hydrogen peroxide associated with high doses of PC-SOD, underlying the PC-SOD bell-shaped dose-response profile) [33], or with pirfenidone with a synergistic therapeutic effect [34].
Intravenous administration or inhalation of PC-SOD suppressed elastase-induced pulmonary emphysema and inflammation and activation of proteases, decreased the level of antiproteases, and reduced the expression of proinflammatory cytokines and chemokines. It also suppressed cigarette smoke-induced pulmonary inflammation [35]. In another study, Tanaka et al. showed that inhaled PC-SOD has a better effect on elastase-induced emphysema and alteration in lung mechanics and respiratory function in mice as compared to other drugs (ipratropium bromide, fluticasone propionate, or roflumilast) [36]. Lung damage. Intravenous administration of PC-SOD in mice with ARDS (caused by cecal ligation and puncture - CLP, administration of lipopolysaccharide toxin, and mechanical ventilation) carried out just before CLP increased the survival rate, decreased vascular permeability, tissue damage and lung inflammation [37].
4.3. Nervous system
Tsubokawa et al. tested the effects of PC-SOD in focal cerebral ischemia in rat middle cerebral artery occlusion model. The group treated with intravenously PC-SOD showed improved neurological scores (a scoring system reported by Garcia et al.based on six neurobehavioral item) [38], and a significantly reduced infarct volume. PC-SOD treatment also decreased malondialdehyde levels, cytochrome c, cleaved caspase 3 expression and increased mitochondrial Bcl-2 expression. The neuroprotective action of PC-SOD was attributed to its possible antiapoptotic effect [39].
Chikawa et al. showed a higher hindlimb motor function after spinal cord injury in rats treated with PC-SOD (40,000 units/kg), methylprednisolone (MP) (30 mg/kg), or a combination of both (PC-SOD -MP) compared to control group. PC-SOD and MP have been showed to have a down-regulatory effect on mRNA expression of pro-inflammatory substances, such as intercellular adhesion molecule–1 (ICAM-1), inducible-nitric oxide synthetase (i-NOS) andinterleukin-1b, after spinal cord compression [40].
The neuroprotective role of PC-SOD after spinal cord injury was subsequently confirmed by Takenaga et al. [41], whereas Yunoki et al. demonstrated, in rats, the effect of PC-SOD in preventing CA3 hippocampal neuronal loss after traumatic brain injury [42].
4.4. Nephro-urological system
Noiri et al. revealed the improvement of renal function in rats treated with PC-SOD after ischemic acute renal failure (after 45-min renal artery clamping) due to its activity as suppressor of peroxynitrite production or scavenging [43]. Nakagawa et al. tested the PC-SOD in ischemia/reperfusion injury in a rat model of chronic renal allograft failure, showing that inflammatory cellular response, proteinuria and apoptotic cells detected in allograft exposed to 18 h of cold ischemia were significantly reduced by pre-treatment with PC-SOD [44].
4.5. Others
In dentistry PC-SOD have been used in radiation-induced xerostomia [45], in ophthalmology to treat non-infectious corneal ulcers [46] and in emergency medicine to reduce the free radical-induced vasodilatation after severe burns [47]. In gastroenterology, it has been proposed as a treatment of ulcerative colitis Tomoaki et al. evaluated PC-SOD in an animal model of dextran sulfate sodium (DSS)-induced ulcerative colitis. DSS-induced colitis was reduced by daily intravenous administration of PC-SOD. Unmodified SOD also produced a similar effect, but only with concentration 30-times higher compared to PC-SOD. High doses of PC-SOD caused a negative effect due to the higher production of hydrogen peroxide, but this effect was suppressed by the simultaneous administration of catalase [48].
5. PC-SOD in humans
A single intravenous administrations of PC-SOD, seems to be well tolerated in respectively, healthy white volunteers, up to 80 mg, and Chinese volunteers, up to 160 mg [49], [50]. A more recent study confirmed PC-SOD safety with both single intravenous doses (40–160 mg) and repeated doses (80 mg for 7 days) in Japanese and Caucasians [51].
Two clinical trials have demonstrated the efficacy of intravenously administered PC-SOD for the treatment of rectal ulcerative colitis (RCU) and idiopathic pulmonary fibrosis (FPI) [52], [53]. In particular, the drug administered at doses of 40 mg or 80 mg once daily in patients with RCU for 4 weeks improved the disease activity score (UC-DAI), without significant side effects. In the multicentre, randomized, case-control study performed by Kamio et al., patients with stage III-IV idiopathic pulmonary fibrosis (according to the Japanese Severity Classification Scale) underwent the same treatment regimen as the previous study. Their vital capacity did not improve significantly in the PC-SOD group compared to placebo, but lactate dehydrogenase and surfactant protein A levels (two markers of interstitial pneumonia) were significantly attenuated in PC-SOD group. Neither bell-shaped dose-response profile, nor severe side effects were recorded at doses used.
A case of a 50-year-old patient with amyopathic dermatomyositis, who developed progressive interstitial pneumonia (not responding to corticosteroids and multiple immunosuppressive agents) treated with PC-SOD (40 mg/day for 4 weeks) has been reported. No adverse effects were found with positive effect on pulmonary function and exercise tolerance [54].
A clinical study to evaluate the efficacy and safety of intravenous PC-SOD in reducing myocardial reperfusion injury is ongoing (NCT03995732).
6. PC-SOD in COVID-19 disease
The COVID-19 disease accounts for about 1000,000 deaths and 38,000,000 confirmed cases worldwide, as of October 2020 [1]. However, these data are likely to underestimate the overall burden of COVID-19, as only a fraction of acute infections are diagnosed and reported. Seroprevalence surveys in Europe and the United States suggest that, after adjusting for false negatives or positives, the rate of seropositivity to SARS-CoV-2 should exceed the incidence of reported cases by 10-fold or more [55], [56], [57]. Most patients affected by COVID-19 are asymptomatic or develop mild infection. However, about 14% develops severe disease requiring hospitalization and oxygen therapy and around 5% require intensive care [58]. In these cases, COVID-19 disease is often complicated by the development of acute respiratory distress syndrome (ARDS), septic shockand multi-organ failure [59]. In some cases ARDS can progress to pulmonary fibrosis [60]. Different studies have suggested that COVID-19 mortality might be due to an exaggerated virus-activated inflammatory response, the so-called “cytokine storm syndrome” in which ROS are also involved [61], [62], [63]. Cytokine storm defines an uncontrolled systemic hyper-inflammation due to cytokine overproduction, responsible of multi-organ failure and death [64]. Higher levels of IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (G-CSF), IP10, MCP1, macrophage inflammatory protein 1-α and TNF-α in COVID-19 patients admitted in intensive care units are significantly higher compared to those not requiring intensive care [65]. Several studies have evaluated, in respiratory viral infections, the interaction between ROS and pro-inflammatory cytokines production [66], [67], [68]. Infections stimulate ROS production with cell death and macrophage activation followed by cytokine production with inflammation and destruction of type II pulmonary epithelial cell. ROS stimulate cytokine production through two different pathways: NLRP3 inflammasome or redox-sensitive nuclear factor κ B (NFκB) [69] that activates the transcription of cytokines coding genes [66]. Besides, the degradation of ROS has been shown to decrease the level of circulating cytokines [70]. A role of activated vascular endothelial cells as source of proinflammatory cytokines and ROS both responsible of coagulopathy, sepsis, ARDS and cytokine storm has also been described [71]. ROS have a role in the development of ARDS [27], [28], [29] and in the pulmonary fibrosis where they amplify the signal mediated by transforming growth factor beta (TGF-β), a cytokine directly involved in myofibroblasts activation [30]. Moreover, ROS reduce pulmonary defense mechanisms during infections due to their effect of oxidation of surfactant phospholipids [72], [73], [74], [75]. Different comorbidities associated with COVID-19 severity (hypertension, chronic obstructive pulmonary disease, diabetes and cardiovascular disease) are all characterized by higher levels of ROS with impairment of endogenous antioxidant mechanisms [76], [77], [78], [79]. It is well known that mortality for COVID-19 is dramatically higher in the elderly [80] and a recent study of gene expression in type II alveolar cells have showed that SOD gene is significantly downregulated in COVID-19 patients, so that a reduction of SOD in the lungs could represent a negative prognostic factor [81].
ROS scavengers could then represent a possible adjunctive therapy for COVID-19. Superoxide dismutase (SOD) is one of the antioxidant metalloenzymes that form the first line of defense against ROS [10], directly involved in reducing the migration of inflammatory cells by regulating adhesion molecules and cytokine expression [69].
PC-SOD is a synthetic product with long-life and high bioavailability compared to other not-lecithinized forms of the enzyme [22], [23]. Its inhibitory effect on pulmonary fibrosis, lung inflammation or ARDS have been extensively studied in animal models [32], [33], [34], [35], [36], [37]. A single randomized controlled trial has been conducted in patients with stage III-IV idiopathic pulmonary fibrosis. Serum lactate dehydrogenase and surfactant protein A (both interstitial pneumonia markers) were reduced and no significant side effects were found. Even if vital capacity did not improve significantly in PC-SOD treated patients compared to placebo, this could be due to the inclusion of patients with advanced stage of the disease (III and IV) and PC-SOD would probably exert a more significant pulmonary protective effect if administered earlierduring the course of the disease [53]. The safety and tolerability of the enzyme were also confirmed byphase I and II studies, with doses up to 160 mg/day [49], [50], [51], [52], [53].
It is known that oxidative stress has a significant pathogenetic role in several diseases [82]: the use of antioxidants, i.e. PC-SOD, as a drug in COVID-19 patients could be a new potential adjuvant alterative in the treatment of the disease. The possible use of other SOD-mimetics (e.g. Mangafodipir) in severe COVID-19 patients has already been suggested [83], but a clinical trial (NCT04555096) is ongoing.
7. Conclusion
SOD-mimetics or PC- SOD could be a possible adjuvant treatment for severe forms of COVID-19 disease, due to the modulatory effect on the innate immune response and the reduction of the oxidative stress, but further randomized, controlled clinical trials are needed.
CRediT authorship contribution statement
Ilaria Farella: Conceptualization, Resources, Writing − original draft, Writing − review & editing. Raffaella Panza: Writing − review & editing, Supervision. Manuela Capozza: Writing − review & editing. Nicola Laforgia: Writing − review & editing, Supervision.
Funding statement
Dr. Raffaella Panza won a fellowship funded by Mellin S.p.A. (Milan, Italy) to attend the Doctorate (PhD) course in Biomolecular Pharmaceutical and Medical Sciences of University of Bari “Aldo Moro”.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.〈www.salute.gov.it〉.
- 2.Russo M., Moccia S., Spagnuolo C., Tedesco I., Russo G.L. Roles of flavonoide against coronavirus infection. Chem. -Biol. Interact. 2020;109211 doi: 10.1016/j.cbi.2020.109211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diniz L.R.L., Bezerra Filho C.d.S.M., Fielding B.C., de Sousa D.P. Natural antioxidants: a review of studies on human and animal coronavirus. Oxid. Med. Cell. Longev. 2020;2020:1–14. doi: 10.1155/2020/3173281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Penninger J.M. Identification of oxidative stress and toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean P.J. Redefining oxidative stress. Antioxid. Redox Signal. 2006;8(9–10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 6.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravier-Hernández R., Gil-del Vallea L., Valdes-Alonsoa L., Hernández-Ayala N., Bermúdez-Alfonsoa Y., Requejoa D., Rosell-Guerraa Teresa, Hernández-González-Abreua M. Oxidative stress in hepatitis C virus–human immunodeficiency virus co-infectedpatients. Ann. Hepatol. 2020;vol. 19(1):92–98. doi: 10.1016/j.aohep.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Zhang R.L., Li Y.-P. Diversified application of barcoded PLATO (PLATO-BC) platform for identification of protein interactions. Genom., Proteom. Bioinform. 2019;17:319–331. doi: 10.1016/j.gpb.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelialcellinfection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasui K., Baba A. Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm. Res. 2006;55:359–363. doi: 10.1007/s00011-006-5195-y. [DOI] [PubMed] [Google Scholar]
- 11.Miller A.F. In: Handbook of Metalloproteins. Messerschmidt A., Huber R., Poulos T., Wieghart K., editors. John Wiley&Sons; Chichester: 2001. Fe superoxidedismutase. pp. 668–682. [Google Scholar]
- 12.Marklund S. Distribution of CuZn superoxide dismutase and Mn superoxide dismutase in human tissues and extracellular fluids. Acta Physiol. Scand. Suppl. 1980;492:19–23. [PubMed] [Google Scholar]
- 13.Gao B., Flores S.C., Leff J.A., Bose S.K., McCord J.M. Synthesis and anti-inflammatory activity of a chimeric recombinant superoxide dismutase: SOD2/3. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284:L917–L925. doi: 10.1152/ajplung.00374.2002. [DOI] [PubMed] [Google Scholar]
- 14.McCord J.M., Edeas M.A. SOD, oxidative stress and human pathologies: a brief history and a future vision. BiomedPharmacother. 2005;59:139–142. doi: 10.1016/j.biopha.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi R., Hoshino J., Takenaga M., Kawai S., Morizawa Y., Yasuda A., Otani M., Mizushima Y. Lecithinization of superoxide dismutase potentiates its protective effect against Forssman antiserum-induced elevation in guinea pig airway resistance. J. Pharm. 1992;262:1214–1219. 17. [PubMed] [Google Scholar]
- 16.Igarashi R., Hoshino J., Ochiai A., Morizawa Y., Mizushima Y. Lecithinized superoxide dismutase enhances its pharmacologic potency by increasing its cell membrane affinity. J. Pharm. 1994;271:1672–1677. [PubMed] [Google Scholar]
- 17.Ishihara T., Nara N., Mizushima T. Interactions of lecithinized superoxide dismutase with serum proteins and cells. J. Pharm. Sci. 2014;103:1987–1994. doi: 10.1002/jps.24031. [DOI] [PubMed] [Google Scholar]
- 18.Ishihara T., Shibui M., Hoshi T., Mizushima T. Scavenging of superoxide anions by lecithinized superoxide dismutase in HL-60 cells. Mol. BioSyst. 2016;12(1):274–282. doi: 10.1039/c5mb00631g. [DOI] [PubMed] [Google Scholar]
- 19.Werns S.W., Shea M.J., Lucchesi B.R. Free radicals in ischemic myocardial injury. J. Free Radic. Biol. Med. 1985;1:103–110. doi: 10.1016/0748-5514(85)90013-3. [DOI] [PubMed] [Google Scholar]
- 20.Engler R., Gilpin E. Can superoxide dismutase alter myocardial infarct size? Circulation. 1989;79:1137–1142. doi: 10.1161/01.cir.79.5.1137. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima H., Ishizaka N., Hangaishi M., Taguchi J.-I., Itoh J., Igarashi, ROhno M. Lecithinizedcopper, zinc-superoxidedismutaseamelioratesprolongedhypoxia-inducedinjury of cardiomyocytes. Free Radic. Biol. Med. 2000;29(1):34–41. doi: 10.1016/s0891-5849(00)00290-2. [DOI] [PubMed] [Google Scholar]
- 22.Koo D.D.H., Welsh K.I., West N.E.J., Channon K.M., Penington A.J., Roake J.A., Fuggle S. VEndothelial cell protection against ischemia/reperfusion injury by lecithinized superoxide dismutase. Kidney Int. 2001;60(2):786–796. doi: 10.1046/j.1523-1755.2001.060002786.x. [DOI] [PubMed] [Google Scholar]
- 23.Hangaishi M., Nakajima H., Taguchi J., Igarashi R., Hoshino J., Kurokawa K., Ohno M. Lecithinized Cu, Zn-superoxide dismutase limits the infarct size following ischemia-reperfusion injury in rat hearts in vivo. Biochem. Biophys. Commun. 2001;285(5):1220–1225. doi: 10.1006/bbrc.2001.5319. [DOI] [PubMed] [Google Scholar]
- 24.H. Nakajima, M. Hangaishi, N. Ishizaka, J. Taguchi, R. Igarashi, Y. Mizushima, … M. Ohno, Lecithinized copper, zinc-superoxide dismutase ameliorates ischemia-induced myocardial damage. Life Sciences, 69(8), 935–944. [DOI] [PubMed]
- 25.Keizer H.G., Pinedo H.M., Schuurhuis G.J., Joenje H. Doxorubicin (adriamycin): a critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol. Ther. 1990;47(2):219–231. doi: 10.1016/0163-7258(90)90088-j. [DOI] [PubMed] [Google Scholar]
- 26.denHartog G.J.M., Haenen G.R.M.M., Boven E., van derVijgh W.J.F., Bast A. Lecithinized copper, zinc-superoxide dismutase as a protector against doxorubicin-induced cardiotoxicity in mice. Toxicol. Appl. 2004;194:180–188. doi: 10.1016/j.taap.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Festic E., Kor D.J., Gajic O. Prevention of acute respiratory distress syndrome. Curr. Opin. Crit. Care. 2015;21:82–90. doi: 10.1097/MCC.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y.Y., Chiang C.H., Chuang C.H., Liu S.L., Jheng Y.H., Ryu J.H. Spillover of cytokines and reactive oxygen species in ventilator-induced lung injury associated with inflammation and apoptosis in distal organs. Respir. Care. 2014;59:1422–1432. doi: 10.4187/respcare.02992. [DOI] [PubMed] [Google Scholar]
- 29.Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S.M. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) Adv. Exp. Med. Biol. 2017;967:105–137. doi: 10.1007/978-3-319-63245-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Gonzalez F.J., Chandel N.S., Jain M., Budinger G.R.S. Reactive oxygen species as signaling molecules in the development of lung fibrosis. TranslationalResearch. 2017;190:61–68. doi: 10.1016/j.trsl.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuo M., Tatsuo K., Risa Y., Erika Y., Tomohiko M., Hiromichi F., Toshihiko M. Inhibitory effects of clinical reagents having anti-oxidative activity on transforming growth factor-β1-induced expression of α-smooth muscle actin in human fetal lung fibroblasts. J. Toxicol. Sci. 2011;36(6):733–740. doi: 10.2131/jts.36.733. [DOI] [PubMed] [Google Scholar]
- 32.Tamangawa K., Taooka Y., Maeda A., Hiyama K., Ishioka S., Yamakido M. Inhibitory effects of a lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis in mice. Am. J. Respir. Crit. Care Med. 2000;161(4):1279–1284. doi: 10.1164/ajrccm.161.4.9906099. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K., Ishihara T., Azuma A., Kudoh S., Ebina M., Nukiwa T., Sugiyama Y., Tasaka Y., Namba T., Ishihara T., Sato K., Mizushima Y., Mizushima T. Therapeutic effect of lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2010;298(3):L348–L360. doi: 10.1152/ajplung.00289.2009. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K.-I., Azuma A., Miyazaki Y., Sato K., Mizushima T. Effects of lecithinized superoxide dismutase and/or pirfenidone against bleomycin-induced pulmonary fibrosis. Chest. 2012;142(4):1011–1019. doi: 10.1378/chest.11-2879. 2012. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka K.-I., Tanaka Y., Miyazaki Y., Namba T., Sato K., Aoshiba K., Mizushima T. Therapeutic effect of lecithinized superoxide dismutase on pulmonary emphysema. J. Pharmacol. Exp. 2011;338(3):810–818. doi: 10.1124/jpet.111.179051. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka K.-I., Sato K., Aoshiba K., Azuma A., Mizushima T. Superiority of PC-SOD to other anti-COPD drugs for elastase-induced emphysema and alteration in lung mechanics and respiratory function in mice. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2012;302(12):L1250–L1261. doi: 10.1152/ajplung.00019.2012. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka K., Tamura F., Sugizaki T., Kawahara M., Kuba K., Imai Y., Mizushima T. Evaluation of lecithinized superoxide dismutase for prevention of acute respiratory distress syndrome in animal models. Am. J. Respir. Cell Mol. 2017;56(2):179–190. doi: 10.1165/rcmb.2016-0158OC. [DOI] [PubMed] [Google Scholar]
- 38.Garcia J.H., Wagner S., Liu K.F., Hu X.J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 39.Tsubokawa T., Jadhav V., Solaroglu I., Shiokawa Y., Konishi Y., Zhang J.H. Lecithinized superoxide dismutase improves outcomes and attenuates focal cerebral ischemic injury via antiapoptotic mechanisms in rats. Stroke. 2007;38(3):1057–1062. doi: 10.1161/01.STR.0000257978.70312.1d. [DOI] [PubMed] [Google Scholar]
- 40.Chikawa T., Ikata T., Katoh S., Hamada Y., Kogure K., Fukuzawa K. Preventive effects of lecithinized superoxide dismutase and methylprednisolone on spinal cord injury in rats: transcriptional regulation of inflammatory and neurotrophic genes. J. Neurotrauma. 2001;18(1):93–103. doi: 10.1089/089771501750055802. [DOI] [PubMed] [Google Scholar]
- 41.Mitsuko T., Yuki O., Yukie T., Akemi H., Masaya N., Hideyuki O., Rie I. Lecithinized superoxide dismutase (PC-SOD) improved spinal cord injury-induced motor dysfunction through suppression of oxidative stress and enhancement of neurotrophic factor production. J. Control Release. 2006;110(2):283–289. doi: 10.1016/j.jconrel.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Yunoki M., Kawauchi M., Ukita N., Sugiura T., Ohmoto T. Effects of lecithinized superoxide dismutase on neuronal cell loss in CA3 hippocampus after traumatic brain injury in rats. Surg. Neurol. 2003;59(3):156–160. doi: 10.1016/s0090-3019(02)01040-6. [DOI] [PubMed] [Google Scholar]
- 43.Noiri E., Nakao A., Uchida K., Tsukahara H., Ohno M., Fujita T., Brodsky S., Goligorsky M.S. Oxidative and nitrosative stress in acute renal ischemia. Am. J. Physiol. Renal. Physiol. 2001;281:F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa K., Koo D.D.H., Davies D.R., Gray D.W.R., Mclaren A.J., Welsh K.I., Fuggle S.V. Lecithinized superoxide dismutase reduces cold ischemia-induced chronic allograft dysfunction. KidneyInternational. 2002;61(3):1160–1169. doi: 10.1046/j.1523-1755.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 45.Yoshinori T., Hiroko I., Takashi S., Hiroyuki Y., Mitsuhiko M., Fumio I., Kenji M., Ichiro S. Protective effect of lecithinized SOD on reactive oxygen species-induced xerostomia. Radiat. Res. 2009;172(3):331–338. doi: 10.1667/RR1557.1. [DOI] [PubMed] [Google Scholar]
- 46.Shimmura S., Igarashi R., Yaguchi H., Ohashi Y., Shimazaki J., Tsubota K. Lecithin-bound superoxide dismutase in the treatment of noninfectious corneal ulcers. Am. J. Ophthalmol. 2003;135(5):613–619. doi: 10.1016/s0002-9394(02)02151-7. [DOI] [PubMed] [Google Scholar]
- 47.Koizumi T., Goto H., Tanaka H., Yamaguchi Y., Shimazaki S. Lecithinized superoxide dismutase suppresses free radical substrates during the early phase of burn care in rats. J. Burn. 2009;30(2):321–328. doi: 10.1097/BCR.0b013e318198e764. [DOI] [PubMed] [Google Scholar]
- 48.Ishihara T., Tanaka K.-I., Tasaka Y., Namba T., Suzuki J., Ishihara T., Mizushima T. Therapeutic effect of lecithinized superoxide dismutase against colitis. J. Pharmacol. Exp. 2008;328(1):152–164. doi: 10.1124/jpet.108.144451. [DOI] [PubMed] [Google Scholar]
- 49.Broeyer F.J.F., van Aken B.E., Suzuki J., Kemme M.J.B., Schoemaker H.C., Cohen A.F., Burggraaf J. The pharmacokinetics and effects of a long-acting preparation of superoxide dismutase (PC-SOD) in man. Br. J. Clin. 2008;65(1):22–29. doi: 10.1111/j.1365-2125.2007.02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen R., Zhao Q., Wu N., ZhongW, Jin X., Liu C., Zhu Z., Hu P. Pharmacokinetics and safety of PC-SOD, a lecithinized recombinant superoxide dismutase, in healthy Chinese subjects: a phase 1, randomized, placebo-controlled, dose-escalation study. Pharm. Dec. 2019;57(12):596–602. doi: 10.5414/CP203550. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki J., Broeyer F., Cohen A., Takebe M., Burggraaf J., Mizushima Y. Pharmacokinetics of PC-SOD, a lecithinized recombinant superoxide dismutase, after single- and multiple-dose administration to healthy japanese and caucasian volunteers. J. Clin. 2008;48(2):184–192. doi: 10.1177/0091270007309705. [DOI] [PubMed] [Google Scholar]
- 52.Okamoto S., Inoue N., Hisamatsu T., Ogata H., Suzuki Y., Matsumoto T., Hibi T. W1233 a lecithinized superoxide dismutase (PC-SOD) improves ulcerative colitis. Gastroenterology. 2008;134(4):A–660-A-661. doi: 10.1111/j.1463-1318.2008.01487.x. A–660–A–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamio K., Azuma A., Ohta K., Sugiyama Y., Nukiwa T., Kudoh S., Mizushima T. Double-blind controlled trial of lecithinized superoxide dismutase in patients with idiopathic interstitial pneumonia – short term evaluation of safety and tolerability. BMC Pulm. Med. 2014;14(1):86. doi: 10.1186/1471-2466-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawashima T., Wakabayashi T., Kuroda T., Nishide T., Matzutzawa Y., Shirai K. Lecithinizedsuperoxidedismutase treatment improvessteroid-refractoryinterstitial pneumonia. Respirology. 2010;15(8):1261–1265. doi: 10.1111/j.1440-1843.2010.01851.x. [DOI] [PubMed] [Google Scholar]
- 55.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., De Ridder D., Petrovic D., Schrempft S., Marcus K., Yerly S., Arm Vernez I., Keiser O., Hurst S., Posfay-Barbe K.M., Trono D., Pittet D., Gétaz L., Chappuis F., Eckerle I., Vuilleumier N., Meyer B., Flahault A., Kaiser L., Guessous I. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention, Commercial Laboratory Seroprevalence Survey Data. 〈https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/commercial-lab-surveys.html〉 (accessed 6 July 2020).
- 57.F.P. Havers, C. Reed, T. Lim, J.M. Montgomery, J.D. Klena, A.J. Hall, N.J. Thornburg, Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Internal Medicine, 2020. [DOI] [PubMed]
- 58.Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance V 1.2 (WHO).
- 59.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;Vol 5:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lechowicz K., Drożdżal S., Machaj F., Rosik J., Szostak B., Zegan-Barańska M., Biernawska J., Dabrowski W., Rotter I., Kotfis K. COVID-19: the potential treatment of pulmonary fibrosis associated with SARS-CoV-2 infection. J. Clin. Med. 2020;9(6):1917. doi: 10.3390/jcm9061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration U. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Behrens E.M., Koretzky G.A. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69(6):1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 65.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khomich O.A., Kochetkov S.N., Bartosch B., Ivanov A.V. Redox biology of respiratory viral infections. Viruses. 2018;10(8):392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cellular & Molecular Immunology, 13, 3–10The cytokine storm of severe influenza and development of immunomodulatory therapy Qiang Liu1,3, Yuan-hong Zhou1,3 and Zhan-qiu Yang2, 2016. [DOI] [PMC free article] [PubMed]
- 68.Snelgrove R.J., Edwards L., Rae A.J., Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur. J. Immunol. 2006;36:1364–1373. doi: 10.1002/eji.200635977. [DOI] [PubMed] [Google Scholar]
- 69.Crapo J.D. Oxidative stress as an initiator of cytokine release and cell damage. Eur. Respir. J. 2003;22(Suppl. 44):4s–6s. doi: 10.1183/09031936.03.00000203a. [DOI] [PubMed] [Google Scholar]
- 70.Ishihara Y., Takemoto T., Itoh K., Ishida A., Yamazaki T. Dual role of superoxide dismutase 2 induced in activated microglia. J. Biol. 2015;290(37):22805–22817. doi: 10.1074/jbc.M115.659151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morris G., Bortolasci C.C., Puri B.K., Olive L., Marx W., O’Neil A., Athan E., Carvalho A.F., Maes M., Walder K., Berk M. The pathophysiology of SARS-CoV-2: A suggested model and therapeutic approach. Life Sci. 2020;258 doi: 10.1016/j.lfs.2020.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weismann D., Binder C.J. The innate immune response to products of phospholipid peroxidation. Biochim. Biophys. Acta. 2012;1818:2465–2475. doi: 10.1016/j.bbamem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shirey K.A., Lai W., Scott A.J., Lipsky M., Mistry P., Pletneva L.M., Vogel S.N. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497(7450):498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Lenten B.J., Wagner A.C., Navab M., Anantharamaiah G.M., Hui E.K.W., Nayak D.P., Fogelman A.M. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation. 2004;110:3252–3258. doi: 10.1161/01.CIR.0000147232.75456.B3. [DOI] [PubMed] [Google Scholar]
- 75.Immulappa R.K., Gang X., Kim J.H., Sussan T.E., Witztum J.L., Biswal S. Oxidized phospholipids impair pulmonary antibacterial defenses: evidence in mice exposed to cigarette smoke. Biochem. Biophys. Res. Commun. 2012;426:253–259. doi: 10.1016/j.bbrc.2012.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lassègue B., Griendling K. Reactive oxygen species in hypertension. AJH. 2004;17:852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 77.E. Rendra, V. Riabov, M. Mossel, T. Sevastyanova, M.C. Harmsen, J. Kzhyshkowska, Reactive oxygen species (ROS) in macrophage activation and function in diabetes Immunobiology S0171–2985(18)30213–30214. [DOI] [PubMed]
- 78.Boukhenouna S., Wilson M.A., Bahmed K., Kosmider B. Reactive oxygen species in chronic obstructive pulmonary disease. Oxid. Med. Cell. Longev. 2018;2018:1–9. doi: 10.1155/2018/5730395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He F., Zuo L. Redox roles of reactive oxygen species in cardiovascular diseases. Int. J. Mol. Sci. 2015;16(11):27770–27780. doi: 10.3390/ijms161126059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.SmritiMallapaty The coronavirus is most deadly if you are older and male - new data reveal the risks. Nature. 2020;585(7823):16–17. doi: 10.1038/d41586-020-02483-2. [DOI] [PubMed] [Google Scholar]
- 81.Abouhashem A.S., Singh K., Azzazy H.M., Sen C.K. Is low alveolar type II cell SOD3 in the lungs of elderly linked to the observed severity of COVID-19? Antioxid. Redox Signal. 2020;33:59–65. doi: 10.1089/ars.2020.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laforgia N., Di Mauro A., FaviaGuarnieri G., Varvara D., De Cosmo L., Panza R., Resta N. The role of oxidative stress in the pathomechanism of congenital malformations. Oxid. Med. Cell. Longev. 2018;2018:1–12. doi: 10.1155/2018/7404082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.KarlssonOlof Jan G., Jynge P., Ignarro L. MayMangafodipir or other SOD mimetics contribute to better care in COVID-19 patients? Antioxidants (Basel, Switzerland) 2020;9(10):971. doi: 10.3390/antiox9100971. [DOI] [PMC free article] [PubMed] [Google Scholar]