Abstract
ARDS is a clinically heterogeneous syndrome, rather than a distinct disease. This heterogeneity at least partially explains the difficulty in studying treatments for these patients and contributes to the numerous trials of therapies for the syndrome that have not shown benefit. Recent studies have identified different subphenotypes within the heterogeneous patient population. These different subphenotypes likely have variable clinical responses to specific therapies, a concept known as heterogeneity of treatment effect. Recognizing different subphenotypes and heterogeneity of treatment effect has important implications for the clinical management of patients with ARDS. This review presents studies that have identified different subphenotypes and discusses how they can modify the effects of therapies evaluated in trials that are commonly considered to have shown no overall benefit in patients with ARDS.
Key Words: acute respiratory failure, ARDS, heterogeneity of treatment effect
Abbreviations: HFOV, high-frequency oscillatory ventilation; HTE, heterogeneity of treatment effect; LPV, lung-protective ventilation; NMBA, neuromuscular blocking agent; PEEP, positive end-expiratory pressure; VFD, ventilator-free days
ARDS is a severe, life-threatening inflammatory condition of the lung that can be caused by a wide variety of pulmonary and nonpulmonary insults, including both infectious and noninfectious causes.1 The Berlin Definition for ARDS requires the acute onset of hypoxemia, defined as a ratio of Pao2 to Fio2 ≤ 300 mm Hg with bilateral airspace disease on chest imaging not primarily due to hydrostatic edema.2 The multiple potential causes and the broad definition for ARDS lend to the clinical heterogeneity of this syndrome and contribute to the difficulty in effectively studying treatments for these patients.3,4 Table 15, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 presents a summary of randomized controlled trials of therapies for ARDS; although there are a few notable exceptions, the ARDS literature is rife with randomized trials that have not shown a mortality benefit.32 One factor that may contribute to these many indeterminate results is heterogeneity of treatment effect (HTE).
Table 1.
Summary of Randomized Controlled Trials of Therapies for ARDS and Corresponding Heterogeneity of Treatment Effects
| Therapy | Noteworthy Trial Findings | Heterogeneity of Treatment Effect |
|---|---|---|
| Lung-protective ventilation | ARMA: Lower mortality with LPV5 | None identified6,7 |
| Open lung ventilation | ALVEOLI: No difference in hospital mortality8 ExPress: No difference in 28-d mortality9 LOVS: No difference in 28-d hospital mortality10 ART: Higher 28-d mortality with open lung ventilation11 |
Open lung ventilation associated with lower mortality in: Pao2/Fio2 ratio ≤ 200 mm Hg12 PEEP responders with improved Pao2/Fio2 ratio13,14 PEEP responders with lower driving pressure15 Hyperinflammatory phenotype16 Open lung ventilation associated with higher mortality in patients with pneumonia requiring vasopressors17 |
| HFOV | OSCILLATE: Higher hospital mortality with HFOV18 OSCAR: No difference in 30-d mortality19 |
HFOV associated with lower mortality in patients with a Pao2/Fio2 ratio < 100 mm Hg (or < 64 mm Hg)20 |
| Prone positioning | PROSEVA: Lower 28-d mortality with prone positioning21 | Mortality benefit limited to a Pao2/Fio2 ratio < 150 mm Hg21 |
| NMBA | ACURASYS: Lower adjusted 90-d mortality with NMBA22 ROSE: No difference in 90-d mortality23 |
NMBA associated with lower mortality in: Pao2/Fio2 ratio <150 mm Hg22 |
| Fluid therapy | FACTT: No difference in mortality; more VFDs with conservative fluid strategy24 | Liberal fluid strategy associated with higher mortality in hyperinflammatory phenotype25 Liberal fluid strategy associated with lower mortality in less inflammatory phenotype |
| Statins | HARP-2: No difference in 28-d mortality with simvastatin26 SAILS: No difference in 60-d or hospital mortality with rosuvastatin27 |
Simvastatin associated with lower mortality in: Hyperinflammatory phenotype28 Lower APACHE II score29 Statins associated with lower mortality in sepsis-related ARDS with a Pao2/Fio2 ratio < 100 mm Hg30 Simvastatin associated with higher mortality in higher APACHE II score29 None identified for rosuvastatin31 |
ACURASYS = ARDS et Curarisation; ALVEOLI = Assessment of Low Tidal Volume and Elevated End-Expiratory Pressure to Obviate Lung Injury; APACHE II = Acute Physiology and Chronic Health Evaluation II; ARMA = Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and Acute Respiratory Distress Syndrome; ExPress = Expiratory Pressure Study; FACTT = Fluids and Catheters Treatment Trial; HARP-2 = Hydroxymethylglutaryl-CoA Reductase Inhibition in Acute Lung Injury to Reduce Pulmonary Inflammation 2; HFOV = high-frequency oscillatory ventilation; LOVS = Lung Open Ventilation Study; NMBA = neuromuscular blocking agent; OSCAR = Oscillation in ARDS; PEEP = positive end-expiratory pressure; PROSEVA = Effect of Prone Positioning on Mortality in Patients with Severe Acute Respiratory Distress Syndrome; ROSE = Reevaluation of Systemic Early Neuromuscular Blockade; SAILS = Statins for Acutely Injured Lungs from Sepsis; VFD = ventilator-free days.
In randomized controlled trials, results are reported as the average of individual treatment effects for all the patients in the study population.33,34 However, some of the patients included in the study may have treatment effects that are different from this average effect.33,35 These characteristics can influence the baseline risk of developing the clinical outcome, as well as the likelihood of gaining benefit, or experiencing harm, from the treatment.36 HTE refers to a nonrandom difference in the direction or magnitude of the clinical effect of a treatment between patients that is driven by the interaction between these distinct characteristics, or subphenotypes, and the intervention being studied.35,37
The potential for HTE is an important consideration in clinical trial design and evaluation. Trials that include a more heterogeneous study population are more generalizable to clinical practice and thus have more external validity; however, they will likely have more heterogeneous treatment effects among the participants.38 By contrast, trials with a more homogeneous study population are less likely to have heterogeneous treatment effects but will have lower external validity.37 Heterogeneity in trials can be limited by enrichment of the study populations, and this can be either prognostic or predictive. Enrichment refers to the selection of patients who are more likely to respond to treatment compared with an unselected group of patients and is based on characteristics that are known prior to randomization.39, 40, 41 Prognostic enrichment is used to select patients who are more likely to develop the clinical outcome of interest, whereas predictive enrichment is used to select patients who are more likely to respond to the treatment.40,41
HTE has important implications for the management of ARDS, in which therapies evaluated in clinically heterogeneous patient populations have largely been unsuccessful.4,32 These so-called “negative” trials may have been the result of truly ineffective therapies. Alternatively, therapies may have helped some patients but harmed others, resulting in no net clinical effect in the trial. As such, there is an increasing interest in identifying different subphenotypes among patients with ARDS.42 Potential subphenotypes that have been identified as possible effect-modifiers include variables that are physiological (eg, Pao2/Fio2 ratio, respiratory system compliance), clinical (eg, underlying cause, direct vs indirect), and biological (eg, biomarkers, hyperinflammatory vs hypoinflammatory).43
There are several methods to identify HTE from trial data. The most common approach is to use the analyses of subgroups that are reported in the trial itself.44,45 Observational studies using secondary analyses of trial data can also be used. Individual patient data meta-analyses can identify important subgroup effects that could not otherwise be detected due to inadequate power.46 Latent class analysis identifies subgroups without prespecified assumptions and has been increasingly used to estimate differential treatment effects.47
The purpose of the current review was to summarize the key trials of therapies for ARDS, many of which are frequently but incorrectly referred to as negative. It is more correct to state that they did not show benefit, and in most cases they are indeterminate, as few were conducted as noninferiority trials. We present evidence for HTE according to disease severity, cause of ARDS, and inflammatory subphenotypes across different therapies and trials, and review the circumstances under which these treatments may be clinically useful.
Before discussing HTE, however, we want to highlight the notable exception to this trend, which is the limitation of intensity of mechanical ventilation by reducing tidal volume and plateau pressure. The original ARDS Network trial found a 9% absolute risk reduction in mortality.5 In secondary analyses, neither oxygenation severity6 nor cause of ARDS7 was found to interact with this treatment effect. Indeed, it seems likely that limitation of intensity of ventilation is important not just in patients with ARDS but more broadly across the spectrum of acute hypoxemic respiratory failure.48
ARDS Severity
Open Lung Ventilation
Open lung ventilation refers to a strategy of higher levels of positive end-expiratory pressure (PEEP) with or without recruitment maneuvers. This approach can increase end-expiratory lung volume, improve gas exchange, may reduce both lung stress and strain, and can minimize atelectrauma.49,50
Only two studies have shown mortality benefit associated with open lung ventilation.51,52 However, these findings were confounded by the concurrent use of higher tidal volumes in control patients. Several small trials that incorporated lung-protective ventilation (LPV) in both treatment and control groups failed to show mortality benefit from open lung ventilation.53, 54, 55, 56 Three larger trials of open lung ventilation that provided concurrent LPV to all patients were similarly unable to show a difference in mortality: the Assessment of Low Tidal Volume and Elevated End-Expiratory Pressure to Obviate Lung Injury (ALVEOLI) trial, the Expiratory Pressure (ExPress) Study, and the Lung Open Ventilation Study (LOVS).8, 9, 10 Although these trials did not show any mortality benefit from open lung ventilation, they did not reveal any signal for harm.
However, a patient-level analysis of the ALVEOLI, ExPress, and LOVS trials found that open lung ventilation was associated with lower mortality when delivered to patients with Pao2/Fio2 ratios ≤ 200 mm Hg (34.1% vs 39.1%; relative risk, 0.90; 95% CI, 0.81-1.00).12 Conversely, in the population with what we would now call mild ARDS (Pao2/Fio2 ratio, 201-300 mm Hg), there was a signal toward harm with higher PEEP, illustrating HTE with open lung ventilation according to ARDS severity.
More recently, the Alveolar Recruitment in ARDS trial (ART) found that higher PEEP paired with an aggressive lung recruitment maneuver led to increased 28-day mortality compared with a lower PEEP strategy.11 This trial, which was performed exclusively in patients with moderate to severe ARDS, did not identify any significant differences in treatment effects in either subgroup or in exploratory analyses.
Prone Positioning
In early trials of prone positioning, a shorter duration (≤ 8 h) did not decrease mortality in patients with Pao2/Fio2 ratios < 300 mm Hg or < 150 mm Hg.57,58 However, application of longer durations of prone positioning led to a nonsignificant trend toward mortality in patients with a Pao2/Fio2 ratio < 100 mm Hg and 132 ± 74 mm Hg.59,60
Based on these findings, the Effect of Prone Positioning on Mortality in Patients with Severe Acute Respiratory Distress Syndrome (PROSEVA) trial included prognostic enrichment to select patients with a Pao2/Fio2 ratio < 150 mm Hg.21 In this trial, the application of prone positioning for at least 16 h per day (mean, 17.0 ± 3 h) led to lower 28-day mortality (16.0% vs 32.8%; hazard ratio [HR], 0.39; 95% CI, 0.25-0.63) and decreased 90-day mortality (23.6% vs 41.0%; HR, 0.44; 95% CI, 0.29-0.67).
Spontaneous Breathing
Diaphragmatic contraction during spontaneous breathing can help to recruit well-perfused dependent segments of the lung that remain poorly ventilated during controlled positive pressure ventilation.61 However, spontaneous breathing also poses certain risks. It can be associated with increased tidal volumes due to increased patient effort and dyssynchrony.62,63 During spontaneous ventilation, the true transpulmonary pressure is the sum of the pressure generated by the ventilator and the respiratory muscles, potentially increasing the true driving pressure, but this increase is not apparent unless special maneuvers are performed on the ventilator.64 In addition, in heterogeneous ARDS lungs, changes in pleural pressure during spontaneous breaths are not uniformly transmitted through the airways, potentially leading to pendelluft, where air flows from one lung region to another, such that even a low tidal volume can cause regional overdistension.63,65
Observational studies suggested an association between spontaneous modes of ventilation and increased ventilator-free days (VFD) and shorter ICU lengths of stay compared with controlled ventilation but did not suggest a mortality benefit.66,67 The HTE for spontaneous breathing also seems to be driven by the severity of ARDS. Pendelluft occurs more commonly during spontaneous breathing in patients with severe ARDS, and the resulting regional overdistention and lung injury can limit potential benefits of spontaneous breathing.64,65
Neuromuscular Blocking Agents
Spontaneous breathing in ARDS can be associated with increased transpulmonary pressures, regional overdistension due to pendelluft, and large tidal volumes due to increased patient effort and ventilator dyssyncrhony.62, 63, 64, 65
The ARDS et Curarisation (ACURASYS) trial was a double-blind, placebo-controlled study that compared 48 h of neuromuscular blocking agents (NMBA) initiated within 48 h of ARDS onset vs deep sedation without NMBA in patients with a Pao2/Fio2 ratio < 150 mm Hg.22 Although there was no difference in overall 90-day mortality between the two groups (40.7% vs 48.8%; P = .08), an analysis adjusted for Pao2/Fio2 ratio, plateau pressure, and severity of illness found reduced 90-day mortality in the NMBA group (adjusted HR for death, 0.68; 95% CI, 0.48-0.98). These results, and observations of pendelluft in severe ARDS, suggest there might be HTE for NMBA based on the Pao2/Fio2 ratio; this is further supported by a subgroup analysis that showed no difference in probability of survival in patients with a baseline Pao2/Fio2 ratio ≥120 mm Hg.22,65
In the Reevaluation of Systemic Early Neuromuscular Blockade (ROSE) trial, patients with ARDS and a Pao2/Fio2 ratio < 150 mm Hg were treated with 48 h of NMBA or with usual care, including light sedation.23 Unlike the ACURASYS trial, there was no difference in 90-day mortality between the two groups (42.5% vs 42.8; between-group difference, –0.3 percentage point; 95% CI, –6.4 to 5.9).
There are important distinctions between the ACURASYS and ROSE trials that might explain these divergent outcomes. First, the PEEP strategies used in each study were different. As noted earlier, a meta-analysis of three trials of open lung ventilation suggested that higher PEEP is associated with lower mortality in patients with moderate to severe ARDS.12 The ROSE trial included ventilation with higher levels of PEEP, and this may have also decreased the risk of mortality in the patients in this trial and thus diluted any mortality benefit from NMBA, whereas the ACURASYS trial used lower PEEP. Second, the use of concomitant prone positioning was more common in ACURASYS (28% in the NMBA group, 29% in the control group) than in ROSE (15.8% of patients; between-group difference, 1.9%; 95% CI, –2.6 to 6.4). Third, patients in the control group of the ACURASYS trial were deeply sedated to facilitate blinding, and this use of deep sedation without NMBA could have contributed to worse clinical outcomes in the control group. By contrast, light sedation was used in the control arm of the open-label ROSE trial. Finally, the time between identification of ARDS and enrollment in the ACURASYS trial (median, 16 h; interquartile range, 6-29 h) was considerably longer than that in the ROSE trial (median, 7.6 h; interquartile range, 3.7-15.6 h). The earlier enrollment in the ROSE trial may have preferentially captured more patients with transient ARDS.
High-Frequency Oscillatory Ventilation
High-frequency oscillatory ventilation (HFOV) applies a relatively high mean airway pressure that can recruit collapsed lung and prevent atelectrauma, and it delivers very small tidal volumes, which can prevent volutrauma.68 In the Oscillation for ARDS Treated Early (OSCILLATE) trial comparing HFOV vs LPV with high PEEP in patients with a Pao2/Fio2 ratio ≤ 200 mm Hg, there was increased in-hospital mortality in the HFOV group (47% vs 35%; relative risk, 1.33; 95% CI, 1.12-1.79).18 The Oscillation in ARDS (OSCAR) trial also compared HFOV vs conventional LPV in patients with ARDS and a Pao2/Fio2 ratio ≤ 200 mm Hg but found no difference in 30-day mortality between the two groups (41.7% vs 41.1%; P = .85).19 The conflicting outcomes between the trials might be due to the differences in the use of LPV in the control arms, relative imbalance between the benefits of recruitment and the harm of increased sedation, the hemodynamic effects of increased sedation, and/or higher airway pressures.18,69
In a patient-level meta-analysis of four trials of HFOV, HTE was identified for the relationship between HFOV and severity of ARDS, based on a Pao2/Fio2 ratio at the time of randomization.20 The threshold at which the effect of HFOV shifts from harm to benefit is not entirely clear, but the line of best fit occurs at a Pao2/Fio2 ratio of approximately 100 mm Hg (95% CI, 64-117).
Hydroxymethylglutaryl-CoA Reductase Inhibitors (Statins)
The Statins for Acutely Injured Lungs from Sepsis (SAILS) trial, which compared rosuvastatin vs placebo in patients with sepsis-associated ARDS, was stopped early for futility and found no difference in 60-day or in-hospital mortality (28.5% vs 24.9%; P = .21).27 Similarly, the Hydroxymethylglutaryl-CoA Reductase Inhibition in Acute Lung Injury to Reduce Pulmonary Inflammation 2 (HARP-2) trial found no difference between simvastatin and placebo on the primary outcome of VFD (12.6 ± 9.9 vs 11.5 ± 10.4; P = .21) or secondary outcome of 28-day mortality (22.0% vs 26.8%; P = .23).26 Mansur et al30 observed HTE for the effect of statins on mortality in sepsis-associated ARDS; mortality was lower in the cohort of patients with a Pao2/Fio2 ratio < 100 mm Hg treated with statins (the most common agent was simvastatin) compared with those who were not (11.5% vs 37.5%; P = .0193).
Cause of ARDS
Open Lung Ventilation
In a latent class analysis of the ART trial, Zampieri et al17 identified three distinct clusters among the patients. The association between open lung ventilation and mortality was greatest in the cluster of patients with ARDS from pneumonia who required vasopressors, and probability of harm was > 98%. In patients with miscellaneous causes of ARDS, including pneumonia but who did not require vasopressors, the probability of harm was 45%; and in patients with ARDS not due to pneumonia who required vasopressors, the probability of harm was 68%. The authors reasoned that in patients with pneumonia who have heterogeneous areas of dense consolidation, application of open lung ventilation may not be able to recruit collapsed units and could instead lead to hyperinflation and injury in other areas.70 They also suggested that the adverse hemodynamic effects and the higher fluid balance associated with recruitment maneuvers and PEEP titration might have contributed to the signal or harm, especially in patients requiring vasopressors.11 Open lung ventilation should increase recruitment and decrease driving pressure.71 However, if the lung cannot be recruited, the increased airway pressures would increase driving pressure, which is associated with higher mortality.72 The lack of meaningful improvement in lung compliance and the resulting absence of reduction in driving pressure in the ART trial might thus have been driven by inclusion of patients with non-recruitable lung.11
Inflammatory Subphenotype
Open Lung Ventilation
Using clinical and biomarker data from the original ARDS Network trial of lower tidal volumes and the ALVEOLI trial, Calfee et al16 performed a latent class analysis and identified two distinct subphenotypes that modify the effect of open lung ventilation on mortality. The hyperinflammatory subphenotype had more shock, more nonpulmonary sepsis, and higher levels of inflammatory biomarkers (IL-6 and IL-8), differentiating it from the less inflammatory subphenotype. The authors found that among hyperinflammatory patients, open lung ventilation with higher PEEP levels was associated with lower mortality and more VFD, compared with the low PEEP strategy, whereas the opposite was true in the hypoinflammatory patients.
Fluid Therapy
The Fluids and Catheters Treatment Trial (FACTT) was a two-by-two trial of patients with ARDS and a Pao2/Fio2 ratio < 300 mm Hg that compared a conservative fluid strategy vs a liberal fluid strategy and pulmonary artery catheters vs central venous catheters.24 There was no significant difference in mortality between the conservative and liberal strategies (25.5% vs 28.4%; 95% CI for difference, –2.6 to 8.4; P = .30) and no mortality difference between catheter type (P = .24). However, the conservative fluid strategy was associated with more VFDs (14.6 ± 0.5 vs 12.1 ± 0.5; P < .001). Using latent class analysis, patients with a hyperinflammatory subphenotype were characterized by higher levels of inflammatory markers (IL-6, IL-8, and soluble tumor necrosis factor receptor-1), lower serum bicarbonate (HCO3−) levels, and more hypotension.25 The conservative fluid strategy was associated with lower 90-day mortality in this hyperinflammatory group (40%) compared with the liberal fluid strategy (50%). The direction of treatment effect was opposite in the less inflammatory subphenotype, in which the liberal fluid strategy lowered 90-day mortality compared with the conservative fluid strategy (26% vs 18%; P value for interaction = .0039).
Hydroxymethylglutaryl-CoA Reductase Inhibitors (Statins)
A latent class analysis of the HARP-2 trial identified two subphenotypes; the hyperinflammatory subphenotype had higher levels of IL-6 and soluble tumor necrosis factor receptor-1 and more vasopressor use than the hypoinflammatory subphenotype.28 In patients with the hyperinflammatory subphenotype, simvastatin was associated with lower 28-day mortality compared with placebo (32% vs 45%; P < .0001). However, a similar analysis of the SAILS trial found no HTE for mortality according to the inflammatory subphenotype.31 The authors suggested that the absence of HTE in the analysis of the SAILS trials might have been due to the use of rosuvastatin, which has less antiinflammatory activity than simvastatin.73 Patients in the SAILS trial had higher Pao2/Fio2 ratios than those in the HARP-2 trial; as outlined earlier, the severity of ARDS can modify the interaction between statins and mortality in ARDS.30
Other Clinical Subphenotypes
PEEP Responders
Patients who respond to open lung ventilation with increased oxygenation might represent another clinical subphenotype. In a secondary analysis of the LOVS and ExPress trials, patients with > 25 mm Hg improvement in the Pao2/Fio2 ratio after application of higher PEEP had lower mortality (OR, 0.80; 95% CI, 0.72-0.89).13 A similar relationship was noted in an analysis of six trials of open lung ventilation wherein improved oxygenation in response to higher PEEP levels was associated with lower hospital and ICU mortality.14 The relationship between open lung ventilation and mortality in patients who are PEEP responsive also seems to be modified by severity of ARDS, and the relationship was greatest in those with a baseline Pao2/Fio2 ratio ≤ 150 mm Hg.13 A subsequent study found that changes in driving pressure with adjustment of PEEP were more strongly associated with mortality (adjusted HR, 1.42 per cm H2O increase; 95% CI, 1.14-1.78) than changes in Pao2/Fio2 (adjusted HR, 0.95 per 25 mm Hg increase; 95% CI, 0.90-1.00) when the variables were modeled together in the ExPress trial. When the model was applied to the AVEOLI trial cohort, changes in driving pressure were associated with mortality (adjusted HR, 1.50 per 5 cm H2O; 95% CI, 1.21-1.85), but changes in the Pao2/Fio2 ratio were not.15
Novel COVID-19 ARDS
Early reports of COVID-19-associated ARDS suggested there might be distinct subphenotypes based on recruitability and respiratory system compliance and that these could inform decisions about optimal ventilation strategies.74,75 However, several other studies have since reported compliance values among their patients with COVID-19-associated ARDS that were lower than these initial reports and more in keeping with typical ARDS.76, 77, 78, 79 Furthermore, there is currently no strong evidence to suggest that any of the proposed approaches based on these early reported potential subphenotypes improve clinical outcomes in these patients. As such, experts have largely advocated using evidence-based ARDS management strategies, including LPV, along with higher PEEP and prone positioning when indicated.80
One therapy for patients with COVID-19 that does exhibit HTE is the use of glucocorticoids. The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial found that dexamethasone reduced 28-day mortality in hospitalized patients with suspected or confirmed COVID-19 (22.9% vs 25.7%; rate ratio, 0.83; 95% CI, 0.75-0.93; P < .001).81 Among these patients, there was a differential response to this therapy based on disease severity, as shown by the level of required respiratory support. The greatest reduction in mortality was noted in the patients who received invasive mechanical ventilation (29.3% vs 41.4%; rate ratio, 0.64; 95% CI, 0.51-0.81) followed by those who required only oxygen (23.3% vs 26.2%; rate ratio, 0.82; 95% CI, 0.72-0.94). There was no benefit, and a possible trend toward harm, in patients who did not require any oxygen (17.8% vs 14.0%; rate ratio, 1.19; 95% CI, 0.91-1.55). Although this study included patients with COVID-19-associated ARDS, there is currently no further evidence to suggest additional effect modification of dexamethasone therapy among different subgroups of these patients.
Conclusions
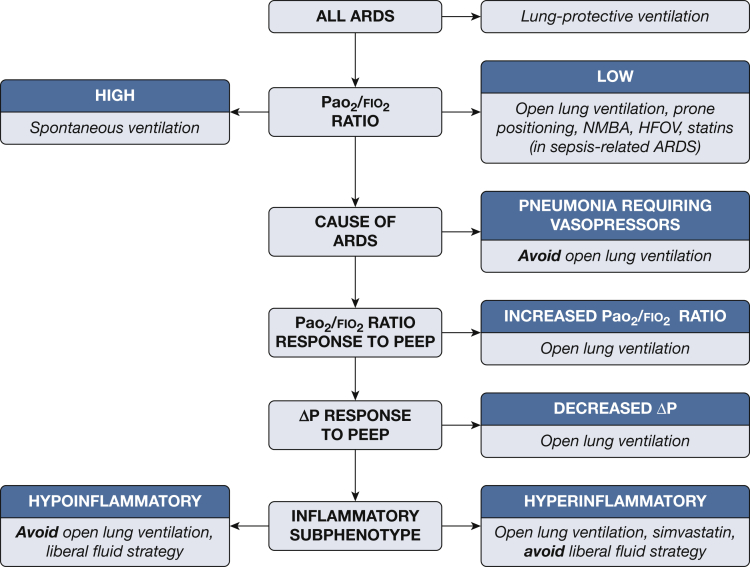
Recognizing different subphenotypes among patients with ARDS and understanding how they can modify the effects of different treatments have important implications for the study of these therapies. Furthermore, understanding the role of HTE and delivering therapies tailored to heterogeneous groups of patients with ARDS represents a major paradigm shift in the clinical management of these patients. The studies reviewed here suggest that many treatments which were previously considered to be ineffective might in fact reduce mortality under certain circumstances when they are applied to the appropriate patients. Figure 1 outlines some of these treatments and the potential conditions under which they may be useful.
Figure 1.
A potential algorithm outlining different subphenotypes of patients with ARDS and specific therapeutic considerations based on effect modification among these subphenotypes. ΔP = driving pressure; HFOV = high-frequency oscillatory ventilation; HTE = heterogeneity of treatment effect; NMBA = neuromuscular blocking agents; PEEP = positive end-expiratory pressure.
There are some important considerations about the studies that we have used to illustrate HTE for ARDS therapies that warrant additional discussion. As mentioned earlier, subgroup analysis is the most common method to identify subphenotypes in which the treatments could have differential effects. However, insufficient statistical power can lead to false-negative findings, and multiple testing can lead to false-positive findings in these analyses.82,83 Observational studies, such as individual patient data meta-analyses and latent class analyses, may have larger sample sizes that can in turn confer more power and more precise effect estimates, but these analyses are at risk for confounding.84 As such, it is possible that some of the relationships between subphenotypes and treatment modification identified through secondary analyses may be artifactual. Clinical trials performed exclusively in patients with the subphenotypes of interest would provide more definitive evidence of effect modification, but these may be difficult to perform, owing to challenges in enrolling sufficient patients to achieve adequate power.
There are also some challenges to applying these current findings in clinical practice. A variety of the effect modifiers based on some of the physiological and clinical subphenotypes that we have discussed can easily be identified at the bedside. Other effect modifiers, such as the inflammatory subphenotypes, are more difficult to apply clinically in the absence of practical and accessible methods to identify these patients. New point-of-care assays that can quantify levels of some biomarkers (eg, IL-6, soluble tumor necrosis factor receptor-1), however, have recently been used to identify inflammatory subphenotypes in patients with COVID-19-associated ARDS.85 Furthermore, even if subphenotypes can be identified in real-time, some of the threshold values that were used to classify patients in the secondary analyses may have been arbitrary and thus difficult to extrapolate to clinical use.
The studies we highlighted suggest that there are subphenotypes among patients with ARDS and that these can modify the effects of different treatments. In fact, interventions from so-called “negative trials” may ultimately confer benefit when they are delivered to some of these subphenotypes. This argues for a role of precision medicine in the management of patients with ARDS, in which therapies are delivered based on individual patient characteristics. Although the results of secondary analyses are compelling and hypothesis generating, we would hope to see trials of interventions designed to exploit HTE to better inform future clinical practice. We also look forward to the development of more tools that will allow us to detect these subphenotypes in an effort to provide tailored therapy. Furthermore, it is likely that there are other clinical, biological, or genomic subphenotypes among patients with ARDS. Thus, it remains possible, and even likely, that other therapies that were previously considered ineffective might be found to improve outcomes in selected patients with ARDS. The identification of new subphenotypes can also highlight previously unknown therapeutic targets, which may in turn suggest new treatments for certain patients with ARDS.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: E. F. reports personal fees from Abbott, ALung Technologies, Fresenius Medical Care, and MC3 Cardiopulmonary outside the submitted work. N. D. F. reports personal fees from Baxter, Getinge, and Xenios, outside the submitted work. None declared (Y. A. K.).
References
- 1.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 2.Ranieri V.M., Rubenfeld G.D., Thompson B.T., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Villar J., Kacmarek R.M., Guérin C. Clinical trials in patients with the acute respiratory distress syndrome: burn after reading. Intensive Care Med. 2014;40(6):900–902. doi: 10.1007/s00134-014-3288-6. [DOI] [PubMed] [Google Scholar]
- 4.Rubenfeld G.D. Confronting the frustrations of negative clinical trials in acute respiratory distress syndrome. Ann Am Thorac Soc. 2015;12(suppl 1):S58–S63. doi: 10.1513/AnnalsATS.201409-414MG. [DOI] [PubMed] [Google Scholar]
- 5.Brower R.G., Matthay M.A., Morris A., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Petrucci N., De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013;(2):CD003844. doi: 10.1002/14651858.CD003844.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisner M.D., Thompson T., Hudson L.D., et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(2):231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 8.Brower R.G., Lanken P.N., MacIntyre N., et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 9.Mercat A., Richard J.C., Vielle B., et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 10.Meade M.O., Cook D.J., Guyatt G.H., et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 11.Cavalcanti A.B., Suzumura É., Laranjeira L.N., et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briel M., Meade M., Mercat A., et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 13.Goligher E.C., Kavanagh B.P., Rubenfeld G.D., et al. Oxygenation response to positive end-expiratory pressure predicts mortality in acute respiratory distress syndrome. A secondary analysis of the LOVS and ExPress trials. Am J Respir Crit Care Med. 2014;190(1):70–76. doi: 10.1164/rccm.201404-0688OC. [DOI] [PubMed] [Google Scholar]
- 14.Guo L., Xie J., Huang Y., et al. Higher PEEP improves outcomes in ARDS patients with clinically objective positive oxygenation response to PEEP: a systematic review and meta-analysis. BMC Anesthesiol. 2018;18(1):172. doi: 10.1186/s12871-018-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yehya N., Hodgson C.L., Amato M.B.P., et al. Response to ventilator adjustments for predicting acute respiratory distress syndrome mortality. Driving pressure versus oxygenation. Ann Am Thorac Soc. 2021;18(5):857–864. doi: 10.1513/AnnalsATS.202007-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calfee C.S., Delucchi K., Parsons P.E., et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zampieri F.G., Costa E.L., Iwashyna T.J., et al. Heterogeneous effects of alveolar recruitment in acute respiratory distress syndrome: a machine learning reanalysis of the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial. Br J Anaesth. 2019;123(1):88–95. doi: 10.1016/j.bja.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson N.D., Cook D.J., Guyatt G.H., et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 19.Young D., Lamb S.E., Shah S., et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368(9):806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 20.Meade M.O., Young D., Hanna S., et al. Severity of hypoxemia and effect of high-frequency oscillatory ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196(6):727–733. doi: 10.1164/rccm.201609-1938OC. [DOI] [PubMed] [Google Scholar]
- 21.Guérin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 22.Papazian L., Forel J.M., Gacouin A., et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 23.Moss M., Huang D.T., Brower R.G., et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiedemann H.P., Wheeler A.P., Bernard G.R., et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 25.Famous K.R., Delucchi K., Ware L.B., et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195(3):331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAuley D.F., Laffey J.G., O’Kane C.M., et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371(18):1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 27.Truwit J.D., Bernard G.R., Steingrub J., et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calfee C.S., Delucchi K.L., Sinha P., et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santhakumaran S., Gordon A., Prevost A.T., O’Kane C., McAuley D.F., Shankar-Hari M. Heterogeneity of treatment effect by baseline risk of mortality in critically ill patients: re-analysis of three recent sepsis and ARDS randomised controlled trials. Crit Care. 2019;23(1):156. doi: 10.1186/s13054-019-2446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansur A., Steinau M., Popov A.F., et al. Impact of statin therapy on mortality in patients with sepsis-associated acute respiratory distress syndrome (ARDS) depends on ARDS severity: a prospective observational cohort study. BMC Med. 2015;13:128. doi: 10.1186/s12916-015-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha P., Delucchi K.L., Thompson B.T., et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the Statins for Acutely Injured Lungs From Sepsis (SAILS) study. Intensive Care Med. 2018;44(11):1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthay M.A., McAuley D.F., Ware L.B. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5(6):524–534. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- 33.Gabler N.B., Duan N., Liao D., Elmore J.G., Ganiats T.G., Kravitz R.L. Dealing with heterogeneity of treatment effects: is the literature up to the challenge? Trials. 2009;10:43. doi: 10.1186/1745-6215-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kent D.M., Hayward R.A. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298(10):1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 35.Iwashyna T.J., Burke J.F., Sussman J.B., Prescott H.C., Hayward R.A., Angus D.C. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192(9):1045–1051. doi: 10.1164/rccm.201411-2125CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shankar-Hari M., Rubenfeld G.D. Population enrichment for critical care trials: phenotypes and differential outcomes. Curr Opin Crit Care. 2019;25(5):489–497. doi: 10.1097/MCC.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 37.Kravitz R.L., Duan N., Braslow J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q. 2004;82(4):661–687. doi: 10.1111/j.0887-378X.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenfield S., Kravitz R., Duan N., Kaplan S.H. Heterogeneity of treatment effects: implications for guidelines, payment, and quality assessment. Am J Med. 2007;120(4 suppl 1):S3–S9. doi: 10.1016/j.amjmed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Stanski N.L., Wong H.R. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol. 2020;16(1):20–31. doi: 10.1038/s41581-019-0199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prescott H.C., Calfee C.S., Thompson B.T., Angus D.C., Liu V.X. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med. 2016;194(2):147–155. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Temple R. Enrichment of clinical study populations. Clin Pharmacol Ther. 2010;88(6):774–778. doi: 10.1038/clpt.2010.233. [DOI] [PubMed] [Google Scholar]
- 42.Bos L.D., Martin-Loeches I., Schultz M.J. ARDS: challenges in patient care and frontiers in research. Eur Respir Rev. 2018;27(147) doi: 10.1183/16000617.0107-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinha P., Calfee C.S. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12–20. doi: 10.1097/MCC.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varadhan R., Seeger J.D. In: Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Velentgas P., Dreyer N.A., Nourjah P., Smith S.R., Torchia M.M., editors. Agency for Healthcare Research and Quality; Rockville, MD: 2013. Estimation and reporting of heterogeneity of treatment effects; pp. 35–44. [PubMed] [Google Scholar]
- 45.Brookes S.T., Whitley E., Peters T.J., Mulheran P.A., Egger M., Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess. 2001;5(33):1–56. doi: 10.3310/hta5330. [DOI] [PubMed] [Google Scholar]
- 46.Varadhan R., Segal J.B., Boyd C.M., Wu A.W., Weiss C.O. A framework for the analysis of heterogeneity of treatment effect in patient-centered outcomes research. J Clin Epidemiol. 2013;66(8):818–825. doi: 10.1016/j.jclinepi.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z., Abarda A., Contractor A.A., Wang J., Dayton C.M. Exploring heterogeneity in clinical trials with latent class analysis. Ann Transl Med. 2018;6(7):119. doi: 10.21037/atm.2018.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urner M., Jüni P., Hansen B., Wettstein M.S., Ferguson N.D., Fan E. Time-varying intensity of mechanical ventilation and mortality in patients with acute respiratory failure: a registry-based, prospective cohort study. Lancet Respir Med. 2020;8(9):905–913. doi: 10.1016/S2213-2600(20)30325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suter P.M., Fairley B., Isenberg M.D. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med. 1975;292(6):284–289. doi: 10.1056/NEJM197502062920604. [DOI] [PubMed] [Google Scholar]
- 50.Lachmann B. Open up the lung and keep the lung open. Intensive Care Med. 1992;18(6):319–321. doi: 10.1007/BF01694358. [DOI] [PubMed] [Google Scholar]
- 51.Amato M.B., Barbas C.S., Medeiros D.M., et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 52.Villar J., Kacmarek R.M., Pérez-Méndez L., Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34(5):1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 53.Huh J.W., Jung H., Choi H.S., Hong S.B., Lim C.M., Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13(1):R22. doi: 10.1186/cc7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xi X.M., Jiang L., Zhu B., RM Group Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chin Med J (Engl) 2010;123(21):3100–3105. [PubMed] [Google Scholar]
- 55.Hodgson C.L., Tuxen D.V., Davies A.R., et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011;15(3):R133. doi: 10.1186/cc10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kacmarek R.M., Villar J., Sulemanji D., et al. Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Crit Care Med. 2016;44(1):32–42. doi: 10.1097/CCM.0000000000001383. [DOI] [PubMed] [Google Scholar]
- 57.Gattinoni L., Tognoni G., Pesenti A., et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345(8):568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 58.Guerin C., Gaillard S., Lemasson S., et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004;292(19):2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 59.Mancebo J., Fernández R., Blanch L., et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173(11):1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 60.Taccone P., Pesenti A., Latini R., et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 61.Neumann P., Wrigge H., Zinserling J., et al. Spontaneous breathing affects the spatial ventilation and perfusion distribution during mechanical ventilatory support. Crit Care Med. 2005;33(5):1090–1095. doi: 10.1097/01.ccm.0000163226.34868.0a. [DOI] [PubMed] [Google Scholar]
- 62.Beitler J.R., Sands S.A., Loring S.H., et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria. Intensive Care Med. 2016;42(9):1427–1436. doi: 10.1007/s00134-016-4423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida T., Nakahashi S., Nakamura M.A.M., et al. Volume-controlled ventilation does not prevent injurious inflation during spontaneous effort. Am J Respir Crit Care Med. 2017;196(5):590–601. doi: 10.1164/rccm.201610-1972OC. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida T., Uchiyama A., Matsuura N., Mashimo T., Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med. 2012;40(5):1578–1585. doi: 10.1097/CCM.0b013e3182451c40. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida T., Torsani V., Gomes S., et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188(12):1420–1427. doi: 10.1164/rccm.201303-0539OC. [DOI] [PubMed] [Google Scholar]
- 66.Cereda M., Foti G., Marcora B., et al. Pressure support ventilation in patients with acute lung injury. Crit Care Med. 2000;28(5):1269–1275. doi: 10.1097/00003246-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Putensen C., Zech S., Wrigge H., et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164(1):43–49. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 68.Ferguson N.D., Villar J., Slutsky A.S. Understanding high-frequency oscillation: lessons from the animal kingdom. Intensive Care Med. 2007;33(8):1316–1318. doi: 10.1007/s00134-007-0706-z. [DOI] [PubMed] [Google Scholar]
- 69.Wise M.P., Saayman A.G., Gillies M.A. High-frequency oscillatory ventilation and acute respiratory distress syndrome: at the crossroads? Thorax. 2013;68(5):406–408. doi: 10.1136/thoraxjnl-2013-203466. [DOI] [PubMed] [Google Scholar]
- 70.Gattinoni L., Caironi P., Pelosi P., Goodman L.R. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164(9):1701–1711. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 71.Amato M.B., Meade M.O., Slutsky A.S., et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 72.Gattinoni L., Caironi P., Cressoni M., et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354(17):1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 73.Lee C.C., Lee M.G., Hsu T.C., et al. A population-based cohort study on the drug-specific effect of statins on sepsis outcome. Chest. 2018;153(4):805–815. doi: 10.1016/j.chest.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 74.Gattinoni L., Chiumello D., Caironi P., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 76.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ziehr D.R., Alladina J., Petri C.R., et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schenck E.J., Hoffman K., Goyal P., et al. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc. 2020;17(9):1158–1161. doi: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan E., Beitler J.R., Brochard L., et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8(8):816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cook D.I., Gebski V.J., Keech A.C. Subgroup analysis in clinical trials. Med J Aust. 2004;180(6):289–291. doi: 10.5694/j.1326-5377.2004.tb05928.x. [DOI] [PubMed] [Google Scholar]
- 83.Cui L., Hung H.M., Wang S.J., Tsong Y. Issues related to subgroup analysis in clinical trials. J Biopharm Stat. 2002;12(3):347–358. doi: 10.1081/bip-120014565. [DOI] [PubMed] [Google Scholar]
- 84.Robertson S.E., Leith A., Schmid C.H., Dahabreh I.J. Assessing heterogeneity of treatment effects in observational studies. Am J Epidemiol. 2021;190(6):1088–1100. doi: 10.1093/aje/kwaa235. [DOI] [PubMed] [Google Scholar]
- 85.Sinha P., Calfee C.S., Cherian S., et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med. 2020;8(12):1209–1218. doi: 10.1016/S2213-2600(20)30366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]