Abstract
Over the past decade, it has become clear that lipid homeostasis is central to cellular metabolism. Lipids are particularly abundant in the central nervous system (CNS) where they modulate membrane fluidity, electric signal transduction, and synaptic stabilization. Abnormal lipid profiles reported in Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and traumatic brain injury (TBI), are further support for the importance of lipid metablism in the nervous system. Cardiolipin (CL), a mitochondria-exclusive phospholipid, has recently emerged as a focus of neurodegenerative disease research. Aberrant CL content, structure, and localization are linked to impaired neurogenesis and neuronal dysfunction, contributing to aging and the pathogenesis of several neurodegenerative diseases, such as AD and PD. Furthermore, the highly tissue-specific acyl chain composition of CL confers it significant potential as a biomarker to diagnose and monitor the progression in several neurological diseases. CL also represents a potential target for pharmacological strategies aimed at treating neurodegeneration. Given the equipoise that currently exists between CL metabolism, mitochondrial function, and neurological disease, we review the role of CL in nervous system physiology and monogenic and neurodegenerative disease pathophysiology, in addition to its potential application as a biomarker and pharmacological target.
Keywords: cardiolipin, lipids, mitochondria, mitochondrial disease, nervous system, neurodegeneration
Highlights
The central nervous system is rich in lipids that support membrane fluidity, electric signal transduction, and synaptic stabilization.
Cardiolipin (CL) is a mitochondria-exclusive phospholipid essential for mitochondrial morphology, bioenergetics, dynamics, and signaling pathways.
Alterations in brain CL are associated with impaired neuronal function and neurodegeneration.
The highly tissue-specific acyl chain composition of CL confers it significant potential as a biomarker to diagnose and monitor the progression in several neurological diseases.
CL represents a potential target for pharmacological strategies aimed at treating neurodegeneration.
Lipids and the Central Nervous System
The central nervous system (CNS) is rich in lipids, which account for approximately 50% of the total brain dry weight [1]. They are primarily localized to biological membranes, where they sustain CNS architecture and function. Sphingolipids, glycerophospholipids, and cholesterol are the predominant species and participate in a broad range of physiological functions, including cellular signaling (e.g., myelination to enable neuronal communication and nerve conduction, and lipid raft formation), energy balance, blood–brain barrier formation, and inflammatory responses [2]. It is therefore unsurprising that dysregulation of the CNS lipidome is associated with a broad spectrum of neurodegenerative disorders, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [2].
Neuronal cellular functions have an extremely high metabolic rate, with the brain consuming up to 20% of the total body energy [2]. To fulfil this extensive energy requirement, neurons rely on glucose metabolism and mitochondrial oxidative phosphorylation (OXPHOS). Indeed, mitochondria function as sophisticated energy sensors that rapidly modulate their morphology and activity according to cellular energy demands. In addition to energy metabolism, mitochondria also participate in numerous biochemical and signaling pathways that are crucial for brain homeostasis, including cell death signaling, generation of free radical species, and lipid synthesis [3]. As a consequence of their bacterial ancestry, mitochondria have distinctive features that include multiple copies of a circular genome (mitochondrial DNA, mtDNA), the ability to divide independently from the cell, and the mitochondria-exclusive membrane phospholipid cardiolipin (CL). Despite representing just 1–3% of the total phospholipids in the CNS [1], CL is currently a major focus of neurodegenerative research.
Cardiolipin Is a Mitochondria-Exclusive Phospholipid
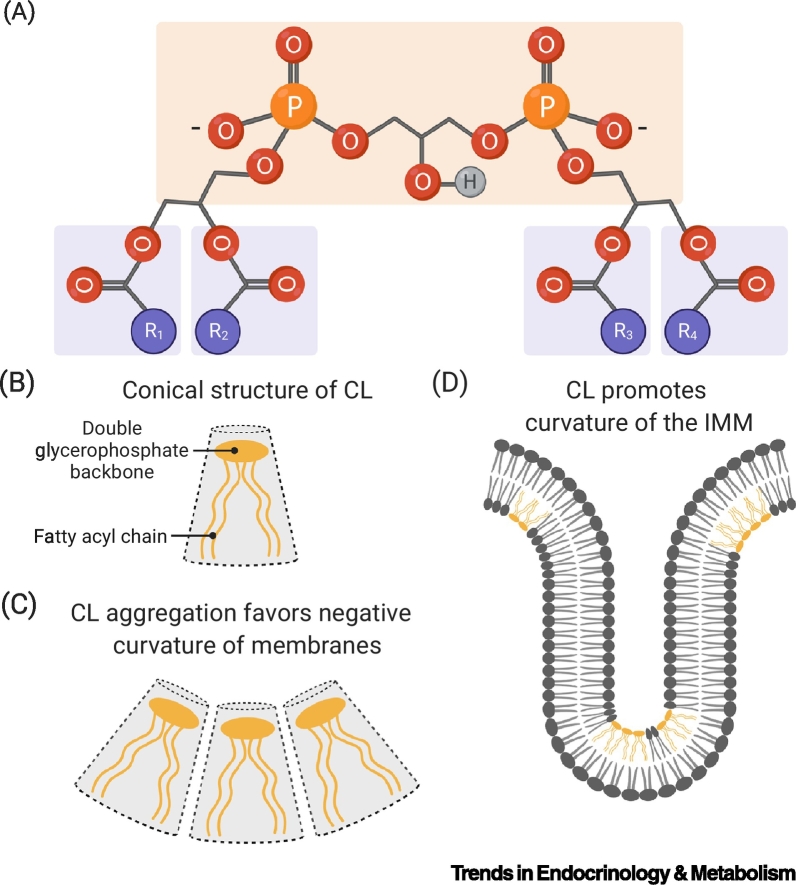
CL is a unique nonbilayer-forming glycerophospholipid present in the membranes of prokaryotes and the mitochondrial membranes of eukaryotes [4]. Its distinctive conical shape is defined by a double glycerophosphate backbone and four fatty acyl side chains, rather than the canonical two fatty acyl side chains commonly found in the phospholipid structure (Figure 1). Studies on human and murine tissues confirm that CL fatty acid (FA) composition is highly tissue specific [5,6]. Notably, the brain displays a unique and diverse acyl chain profile that is enriched by long-chained FA (i.e., 20:4 and 22:6), unlike other mammalian tissues (e.g., heart, skeletal muscle, and liver) that display a much more homogenous acyl chain pattern, defined by the preferential incorporation of linoleic acid (18:2) [5]. A systemic analysis of murine tissues has revealed that the diversity of CL species observed in multiple organs does not result from a stochastic process, but rather depends on the oleic (18:1) and linoleic (18:2) acid balance within individual cells. Consequently, the enrichment in longer-chained FA in the cerebrum and cerebellum might result from reduced import of FA 18:2 across the blood–brain barrier and subsequent incorporation of FA 18:1, and long-chained FA (i.e., 20:4 and 22:6) [5]. This study has opened new inroads in the tissue-specific composition of CL. However, further work is necessary to fully understand the regulatory mechanisms and biological significance of CL acyl chain diversity.
Figure 1.
Major Characteristics of Cardiolipin (CL).
(A) The chemical structure of CL is defined by its double glycerophosphate backbone and four fatty acyl chains (R1–R4). (B) The presence of a double glycerophosphate backbone and four fatty acyl side chains confers a conical shape to CL. (C) CL present in a lipid bilayer induces a negative curvature. (D) CL promotes the formation of highly curved regions within the inner mitochondrial membrane (IMM).
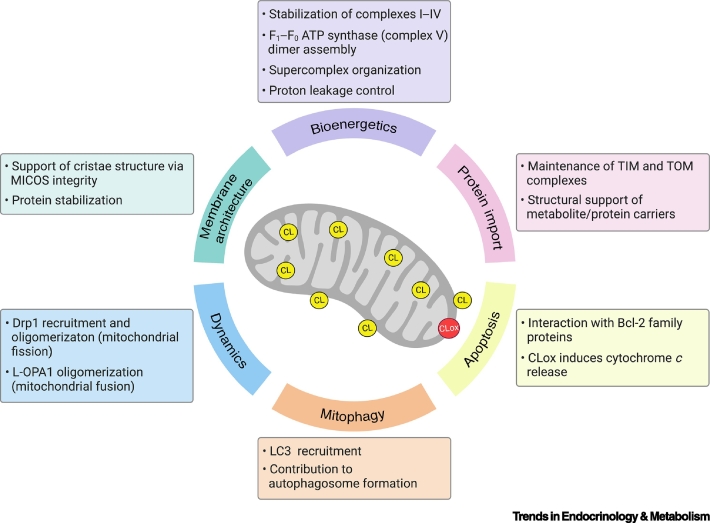
Mitochondria are characterized by a double membrane system, comprising the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM). This creates two aqueous compartments, the mitochondrial intermembrane space (IMS) and a matrix region, while infolding of the IMM forms cristae [7]. The structure of mitochondrial membranes is linked to the cellular metabolic state and characterized by a unique lipid composition that sustains the energetic cellular requirements. Importantly, mitochondrial membranes contain elevated levels of phospholipids, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), and CL, whereas sphingolipids and sterols are less abundant [4]. The abundance and the distribution of these phospholipids varies among mitochondrial compartments and is tightly connected to the specialized roles of different regions of the two membranes [4]. CL accounts for 10–15% of the mitochondrial phospholipid content [8] and is primarily localized in the IMM, where it is essential to maintain membrane integrity and cristae morphology [6]. In addition to its role in membrane architecture, CL participates in a wide range of mitochondrial processes, including the formation and maintenance of protein–protein and protein–membrane interactions [9,10], stabilization of the mitochondrial respiratory chain complexes (I–IV) [11., 12., 13., 14.], assembly of F1–F0 ATP synthase (complex V) dimers [15], and organization of supercomplexes [16]. Under stress, CL is externalized on the OMM and functions as a signaling molecule to promote mitophagy [17,18] and apoptotic signaling pathways [19,20]. The crucial role of CL in mitochondrial function and brain homeostasis is further highlighted by the increasing number of neurological disorders linked with CL abnormalities [21., 22., 23.] (Table 1). Moreover, the embryonic lethality of murine models deficient in enzymes involved in CL biosynthesis emphasizes the significance of CL in brain development [24,25]. A summary of the roles of CL in mitochondrial function is provided in Box 1 and illustrated in Figure 2.
Table 1.
Cardiolipin Abnormalities in Patients with Neurological Disordersa
| Condition | Model/biological sample | Cardiolipin abnormalities | Refs |
|---|---|---|---|
| BTHS | Skeletal muscle, heart and platelets from BTHS patients | • 80% (skeletal muscle and platelets) and 20% (heart tissue) reduction of total CL | [23] |
| FTD | Serum from 40 FTD patients | • ∼ 20% decrease of total CL | [22] |
| TBI | Brain tissue from the pericontusional area of 10 TBI patients | • Increased CLox (1 h after TBI) • Increased MLCL by hydrolysis (4 and 24 h after TBI) • Increased TAZ expression (4 and 24 h after TBI) |
[21] |
Abbreviations: BTHS, Barth syndrome; CL, cardiolipin; CLox, oxidized CL; FTD, frontotemporal dementia; MLCL, monolysocardiolipin; TBI, traumatic brain injury; TAZ, tafazzin.
Box 1. Roles of Cardiolipin (CL).
Membrane Architecture
The unique conical shape of CL contributes towards the structural organization of mitochondria [87]. Under defined pH and ionic strength conditions, CL arranges into local hexagonal phase structures that promote the formation of highly bent regions within the IMM (see Figure 1 in the main text) [88]. In healthy cells, CL is primarily present in the IMM in close proximity to the protein complexes of oxidative phosphorylation (OXPHOS). Emerging in vivo and in vitro studies demonstrate that impaired CL biosynthesis is associated with altered cristae morphology, affecting the shape of mitochondria and their ability to adapt to cellular energy demands through OXPHOS [24,25,89,90]. The master regulator of cristae junctions and mitochondrial morphology is the mitochondrial contact site and cristae organizing system (MICOS) [91]. Recently, several proteins of the MICOS complex have been reported to interact with CL and preserve cristae architecture synergistically [92,93], thus highlighting the crucial role of CL in membrane bending and mitochondrial respiration.
Protein Import
Mitochondrial-related proteins have dual genomic expression; 13 OXPHOS proteins are encoded by the mitochondrial genome, while the remainder of the mitochondrial proteome (~1500 in humans) is encoded by nuclear DNA. Nuclear mitochondrial proteins are synthesized within the cytosol before they are imported to different mitochondrial compartments via multiple mechanisms [3]. The majority of such precursors gain entry into mitochondria through the translocase of the outer membrane (TOM) and translocase of the inner mitochondrial membrane 23 (TIM23) complexes. CL in the IMM and OMM (<1% of total phospholipid content [94]) is crucial to the activity and assembly of these mitochondrial translocases. Deficient CL models display perturbed TOM and TIM23 assembly, with resultant impaired protein import [10,95]. Moreover, a large body of evidence shows that CL stabilizes and preserves the activity of ADP/ATP carrier [9,96,97]. The stabilization and activity of other mitochondrial carriers, including phosphate carrier, pyruvate carrier, tricarboxylate carrier, the carnitine/acylcarnitine translocases, and calcium uniporter also require CL [98., 99., 100.]. Finally, CL is necessary for Fe-S cluster biogenesis and iron homeostasis [101], most likely through its contribution to the maturation of Yfh1/frataxin intermediate form within the mitochondrion, as observed in yeast and mammalian models of CL deficiency [102].
Bioenergetics
CL stabilizes the structure of mitochondrial respiratory chain complexes (I–IV) and supports efficient OXPHOS activity [11., 12., 13., 14.]. Recent cryogenic electron microscopy studies in bovine mitochondria have also revealed that CL binds tightly to the membrane domain of complex V and stabilizes its interaction with the IMM, thus controlling proton leakage and improving ATP generation, in addition to participating in dimer assembly [15]. Moreover, theoretical simulations show that transient binding of CL to complex V lubricates the ATP synthase rotor [103]. Furthermore, CL supports the organization of OXPHOS complexes into supercomplexes that, in turn, remodel and stabilize CL [104,105]. Finally, a recent study has shown that the mitochondrial ribosome binds CL, thereby stabilizing the IMM association of the protein translation machinery and supporting the biogenesis of mitochondrial OXPHOS proteins [106].
Mitochondrial Dynamics
Mitochondria respond to the cellular metabolic demand by modulating their number, morphology, and distribution through fragmentation (fission) and fusion events [107]. During fission, CL favors the recruitment of the GTPase dynamin-related protein 1 (Drp1) to the OMM and stimulates Drp1 activity to promote membrane remodeling and mitochondrial division [108]. Conversely, fusion of the OMM is executed by MFN1 and 2 (mitofusins), while the short (S) and long (L) isoforms of optic atrophy 1 (OPA1) perform this function for the IMM. Recently, CL has been shown to be necessary for L-OPA1 dimerization and IMM fusion [109]. Finally, CL can influence the activity of other fission-related proteins. For example, CL binds to α-synuclein, which is linked to PD, to induce mitochondrial fission and membrane fragmentation [66,68].
Mitophagy
In addition to mitochondrial fission and fusion, overall mitochondrial fitness is maintained through the selective elimination of damaged mitochondria by mitophagy. Mitophagy is a multistep process regulated by a consistent number of protein complexes [110]. A study conducted in neuronal cells showed that in response to mitochondrial damage, CL translocates from the IMM to the OMM to recruit microtubule-associated protein 1A/1B-light chain 3 (LC3), in addition to other proteins implicated in mitophagy, to mediate the formation of the autophagosome and fusion with lysosomes and eliminate the dysfunctional mitochondria [17,18].
Alt-text: Box 1
Figure 2.
Roles of Cardiolipin (CL) in Mitochondria.
CL is primarily localized within the inner mitochondrial membrane (IMM), where it contributes to maintenance of mitochondrial membrane architecture, bioenergetics, and the stability of protein carriers. In the outer mitochondrial membrane (OMM), a small fraction of CL serves as a signaling molecule for selective elimination of damaged mitochondria via mitophagy. CL is also an essential mediator of apoptosis through two mechanisms involving cytochrome c release that is triggered by CL peroxidation and externalization, and binding to Bcl-2 family protein Bid to induce Bax and Bak oligomerization. Finally, CL in the OMM and IMM regulates mitochondrial fission and fusion dynamics. Figure partially created with BioRender.com. Abbreviations: Bak, Bcl-2 antagonist/killer1; Bax, Bcl-2 associated X protein; Bid, BH3 interacting domain death agonist; CDP-DAG, cytidine diphosphate-diacylglycerol; CLox, oxidized cardiolipin; Drp1, dynamin-related protein 1; L-OPA1, long isoforms of optic atrophy 1; LC3, microtubule-associated protein 1A/1B-light chain 3; MICOS, mitochondrial contact site and cristae organizing system; TIM, translocase of the inner membrane; TOM, translocase of the outer membrane.
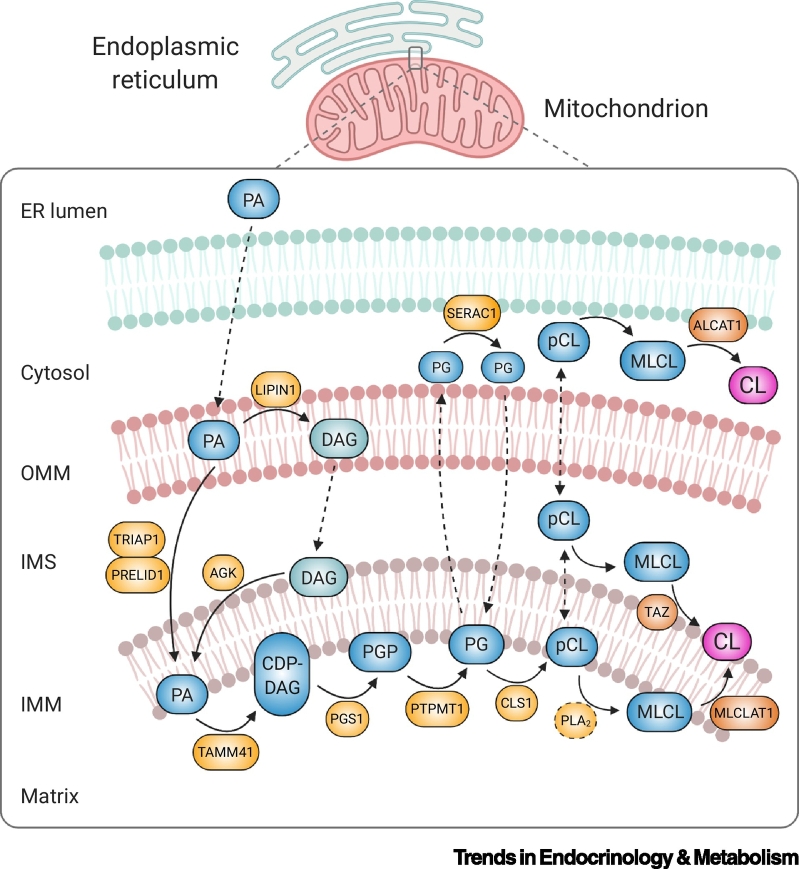
The endoplasmic reticulum (ER) represents the principal site of lipid biosynthesis and provides significant levels of triacylglycerol, cholesterol, phospholipids, and precursors for CL biosynthesis in the mitochondrion [26]. Thus, the communication between mitochondria and the ER via mitochondria–ER membrane contact sites is crucial to mitochondrial lipid biosynthesis and exchange [26]. In mammalian cells, de novo synthesis of CL (Figure 3) begins at the matrix leaflet of the IMM with phosphatidic acid (PA) [6]. PA is synthesized via several distinct enzymes that reside in the ER or on the OMM [125] and possibly in the IMM by acylglycerol kinase [126]. PA made in or transported to the OMM from the ER is moved across the IMS by the heterodimeric complex TP53-regulated inhibitor of apoptosis 1-protein of relevant evolutionary and lymphoid interest domain (TRIAP1-PRELID1) [27], before combining with cytidine triphosphate (CTP), in a reaction catalyzed by TAM41 translocator assembly and maintenance homologue (TAMM41), to generate cytidine diphosphate-diacylglycerol (CDP-DAG). CDP-DAG is fused with glycerol-phosphate by phosphatidylglycerol phosphate synthase (PGS1) to produce phosphatidylglycerol phosphate (PGP). PGP is dephosphorylated by the protein-tyrosine phosphatase mitochondrial 1 (PTPMT1) to phosphatidylglycerol (PG). Finally, PG reacts with a second molecule of CDP-DAG to form premature CL in a reaction catalyzed by CL synthase (CLS1). Mature CL is generated via the remodeling of its acyl chains, a process that is likely initiated by a phospholipase A2 (PLA2) that catalyzes the formation of the intermediate monolysocardiolipin (MLCL). MLCL is reacylated by tafazzin (TAZ) and, through a series of deacylation/reacylation reactions, the characteristic four acyl chains of mature CL are formed [6]. Two additional enzymes, monolysocardiolipin acyltransferase 1 (MLCLAT1) [28] and acyl-CoA:lysocardiolipin acyltransferase 1 (ALCAT1) [29], are also capable of attaching FAs to MLCL. Defects in genes involved in the CL biosynthetic and remodeling pathway are now a well-recognized cause of human disease. However, the components necessary for CL metabolism have not been fully resolved (indicated by broken lines in Figure 3). Thus, the true contribution of CL-related genes to human pathophysiology is unknown. A summary of the recognized CL-related monogenic disorders is provided in Box 2.
Figure 3.
Schematic Representation of Cardiolipin (CL) Biosynthesis and Remodeling.
CL is synthesized in the inner mitochondrial membrane (IMM). Biosynthesis occurs within the matrix leaflet of the IMM, while the final remodeling step can be catalyzed by three different enzymes, including tafazzin (TAZ) in the intermembrane space (IMS)-facing leaflet, monolysocardiolipin acyltransferase 1 (MLCLAT1) on the matrix-leaflet of the IMM, and acyl-CoA:lysocardiolipin acyltransferase 1 (ALCAT1) on the endoplasmic reticulum (ER). Phosphatidic acid (PA) is transported from the ER to the IMM, where it is converted to CL via a series of enzymatic reactions. PG that is generated on the matrix-leaflet of the IMM is trafficked out of the mitochondrion (broken line) and remodeled by SERAC1 on the ER side. Remodeled PG may translocate back to the matrix leaflet of the IMM (broken line) to serve as substrate for CL synthesis. PA can also be dephosphorylated into diacylglycerol (DAG) by the phosphatase LIPIN1 in the outer mitochondrial membrane (OMM). DAG is then able to traffic across the OMM (broken line) and be phosphorylated by acylglycerol kinase (AGK), forming PA in the IMS-side of the IMM. Figure created with BioRender.com. Abbreviations: CLS1, CL synthase; MLCL, monolysocardiolipin; pCL, premature CL; PG, phosphatidylglycerol; PGP, phosphatidylglycerol phosphate; PGS1, phosphatidylglycerol phosphate synthase; PLA2, phospholipase A2; PRELID1, protein of relevant evolutionary and lymphoid interest domain; PTPMT1, protein-tyrosine phosphatase mitochondrial 1; SERAC1, serine active site containing 1; TAMM41, TAM41 translocator assembly and maintenance homolog; TRIAP1, TP53-regulated inhibitor of apoptosis 1.
Box 2. Barth Syndrome (BTHS) and Other Cardiolipin (CL)-Related Monogenic Disorders.
BTHS is an ultra-rare (200–300 cases worldwide) [111], X-linked recessive disorder caused by mutations in the tafazzin (TAZ) gene. TAZ encodes a transacylase that catalyzes CL remodeling within the IMM [112] (see Figure 3 in the main text). The principal clinical features of BTHS include cardiomyopathy (usually dilated, but hypertrophic cardiomyopathy has also been observed), skeletal myopathy, growth delay, and neutropenia [113]. Biochemically, an increase in MLCL:CL ratio (caused by the accumulation of MLCL and usually a decrease in CL) is universally observed in BTHS, thereby representing a reliable diagnostic biomarker for the disease [23,82,114]. Additional biochemical abnormalities observed in patients with BTHS include increased levels of plasma 3-methylglutaconic acid (3-MGC) and increased urinary 3-MGC, 3-methylglutaric acid, and 2-ethylhydracrylic acid [115].
Consistent with the multiple roles of CL in mitochondria, impaired OXPHOS, increased oxidative stress, and abnormal cristae morphology have been reported in patient-derived tissues and in vitro and in vivo models of BTHS [116]. Recently, cognitive and neurological manifestations accompanied by hippocampal degeneration have also been observed in a mouse model of BTHS, thus supporting the importance of CL in maintaining normal function of mitochondria in the CNS [117]. The complexity of BTHS, combined with the inconsistent correlation that exists between the underlying genotype and associated clinical phenotype (a variable phenotypic spectrum can exist within the same family), have contributed to the lack of approved therapies that delay the onset and/or slow progression of the disease. However, several therapeutic strategies have been investigated to alleviate cardiac dysfunction and exercise intolerance, including: (i) CL stabilization and reduction of mitochondrial dysfunction using elamipretide (Bendavia, MTP-131, SS-31), a mitochondrially targeted tetrapeptide [85]; (ii) restoration of physiological levels of mature CL via adeno-associated virus-mediated replacement of TAZ gene [118,119]; and (iii) stimulation of mitochondrial metabolism by increasing mitochondrial biogenesis through the repurposing of bezafibrate, an activator of peroxisome proliferator-activated receptors, which is widely used in the treatment of hyperlipidemia [120].
There are other, less well characterized, monogenic disorders linked with abnormalities in the biosynthesis and maintenance of CL and other mitochondrial phospholipids. For example, Senger syndrome is caused by mutations in an acylglycerol kinase (AGK) that catalyzes the synthesis of PA, an essential precursor of CL biosynthesis (see Figure 3 in the main text). However, AGK null cells do not exhibit CL deficiency, suggesting compensatory mechanisms exist to partially counter impaired PA production in the IMM [121]. Dilated cardiomyopathy with ataxia (DCMA, 3-methylglutaconic aciduria, type V) is an autosomal recessive disorder caused by loss of DNAJC19, an IMM protein thought to facilitate CL remodeling by TAZ, which is associated with changes in CL acyl chain composition, albeit not total CL levels [122,123]. Finally, MEGDEL syndrome (3-methylglutaconic aciduria, deafness, encephalopathy, and Leigh-like syndrome) is caused by variants in the serine active site containing 1 gene (SERAC1) [124], the protein product of which is implicated in the remodeling of PG, an important precursor of CL (see Figure 3 in the main text).
Alt-text: Box 2
Cardiolipin Oxidation and Apoptosis
Free Radical Oxidation
The brain’s high metabolic activity, regenerative limitations, and abundancy of polyunsaturated fatty acids (PUFA), which are particularly sensitive to oxidation, make it susceptible to oxidative damage. There is also extensive evidence that PUFA oxidation contributes towards the progressive neuronal loss associated with neurodegenerative diseases, such as in AD and PD [30]. The brain has therefore acquired a number of protective mechanisms against reactive oxygen species (ROS) to maintain homeostasis [31]. However, when the CNS incurs damage, the balance between ROS production and scavenging is lost, initiating a cascade of detrimental molecular events that ultimately leads to cell death and tissue damage [30]. CL is particularly susceptible to oxidative damage due to its high composition of unsaturated acyl chains, proximity to the mitochondrial electron transport chain, which is recognized as the primary source of ROS within mitochondria, and physical association with cytochrome c, which, in the presence of ROS, becomes a CL peroxidase [32]. Uncontrolled CL oxidation generates conformational changes that affect the physical properties of the IMM and OXPHOS activity [33]. For instance, there is evidence that oxidation of CL affects mitochondrial bioenergetics and the activity of complexes I, III, and IV [34]. Moreover, oxidized CL (CLox) favors the release of cytochrome c and other apoptotic factors into the cytosol, leading to cell death (see Apoptotic Signaling) [35]. Finally, the oxidized form of CL can be hydrolyzed by mitochondrial calcium-independent phospholipase A2 (iPLA2), thus triggering the production of FA second messengers, as observed in mitochondria isolated from transgenic mouse liver and cardiac tissue, which can regulate the inflammatory response [36,37].
Apoptotic Signaling
Apoptosis is a complex mechanism of programmed cell death that is initiated by a variety of internal or external cellular events with the intention of eliminating damaged cells [38]. Mitochondria act as a critical hub for apoptotic signaling molecules because they contain cytochrome c and apoptosis-inducing factor (AIF), in addition to other proapoptotic factors. Recently, CL has emerged as an important player in the execution of the mitochondrial apoptotic signaling cascade via two different mechanisms. First, in the early stages of apoptosis and in the presence of ROS, CL in complex with cytochrome c is peroxidized in the IMM. CLox, which has a low binding affinity for cytochrome c, translocates from the IMM to the OMM where it initiates the apoptotic cascade most probably via the interaction with Bcl-2 family proteins, OMM permeabilization, and release of apoptotic factors into the cytosol [19]. Second, upon apoptotic stimuli, CL translocates to the cytoplasmic side of the OMM where, potentially at contact sites between the IMM and OMM, it forms a platform for caspase 8 recruitment and activation, resulting in the cleavage of the proapoptotic protein BH3 interacting domain death agonist (Bid) to its truncated form (t-Bid). This triggers oligomerization of the proteins Bcl-2 associated X protein (Bax) and Bcl-2 antagonist/killer 1 (Bak), thus inducing OMM permeabilization and cytochrome c release [20].
Cardiolipin, Aging, and Neurodegeneration
Alterations in lipid metabolism have been associated with a broad range of detrimental responses in the CNS leading to neurodegeneration. Consistent with this mechanistic link, dysregulation of CL metabolism in the brain is rapidly emerging as a critical factor in the pathogenesis of several neurodegenerative states. Consequently, fully understanding the role of defective CL metabolism in nervous system homeostasis and brain function is likely to yield significant insights into the pathophysiology of neurodegenerative processes. The major CL abnormalities associated with neurodegenerative disorders are highlighted in Table 1, Table 2.
Table 2.
Cardiolipin Abnormalities in Animal Models of Aging and Neurological Disordersa
| Condition | Model/biological sample | Cardiolipin abnormalities | Refs |
|---|---|---|---|
| AD | Brain from 3xTg-AD mice (3 months old) | • Lower CL content in synaptic mitochondria • No change in CL saturation |
[59] |
| Aging | Mouse brain cortex (3 and 17 months) | • 21% decrease of total CL in synaptic-mitochondria in 17-month- old mice | [41] |
| Brain from 24-month-old rats | • 31% decrease of total CL | [42] | |
| Brain from 20–24-month-old rats | • 25% decrease of total CL | [40] | |
| ALS | Motor cortex and spinal cord from asymptomatic (SOD1-G93A 70 days) and symptomatic (SOD1-G93A 120 days) ALS rats | • Reduced CL levels in the spinal cord of symptomatic rats | [52] |
| BTHS | Brain tissue from TAZ-KD mice | • Increased MLCL (19-fold) and decreased CL | [117] |
| PD | Brain tissue from Parkin-KO mice (2 and 24 months old) | • No change in total CL levels • CL remodeling defects with increase of short saturated CL acyl- chains in 24-month-old mice |
[71] |
| Brain and plasma from rats exposed to rotenone | • Increase of CLox in the substantia nigra • Increase of PUFA-containing CL in the plasma |
[83] | |
| TBI | Brain tissue and plasma from rats after controlled cortical impact (CCI) | • Decreased cortical CL (4 and 24 h after TBI) • Decreased CL in noncontusional areas (hippocampus and thalamus) • Increased in plasma levels of brain-specific CL (24 h after TBI) |
[73,74,77] |
Abbreviations: AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; BTHS, Barth syndrome; CL, cardiolipin; CLox, oxidized CL; KD, knockdown; KO, knockout; MLCL, monolysocardiolipin; PD, Parkinson’s disease; PUFA, polyunsaturated fatty acids; SOD1, superoxide dismutase 1; TBI, traumatic brain injury; TAZ, tafazzin.
Aging
Aging is an irreversible physiological process associated with the decline of organism cellular functions. CNS lipid metabolism has been strongly linked with aging and during senescence the brain undergoes a slow but progressive decline in lipid content [39]. Several studies have shown that murine brains display an age-dependent decrease in CL content associated with aberrant mitochondrial bioenergetics and loss of motor neurons [40., 41., 42.]. There is also evidence of increased ROS production and CL peroxidation in the brains of aged rats [40,42., 43., 44.]. However, although impaired mitochondrial function and increased ROS production represent molecular hallmarks of aging, their direct contribution remains unclear, as do the potential benefits of using antioxidants to extend lifespan [45]. Further research is therefore required to determine whether CL peroxidation has causal implications in cellular damage and aging, or whether it is part of a mitohormetic mechanism, a mitochondrial adaptive defense response triggered by exposure to mild stress, that improves mitochondrial function and longevity [46].
Frontotemporal Dementia, Amyotrophic Lateral Sclerosis, and Overlap Syndromes
Frontotemporal dementia (FTD) is the second most common cause of early-onset dementia [47]. It is a clinically heterogenous disorder characterized by focal atrophy of frontal and temporal regions, accompanied by cognitive, behavioral, and motor impairments [48]. Several disease-causing genes associated with brain homeostasis, including chromosome 9 open reading frame 72 (C9ORF72), microtubule-associated protein tau (MAPT), progranulin (GRN), and fused-in-sarcoma (FUS), have been linked to the development of FTD and aberrant mitochondrial function [48]. Interestingly, a recent analysis of serum lipids derived from patients with FTD confirmed impaired mitochondrial bioenergetics in the setting of a significant decrease of CL content. These findings suggest a potential role for CL as a biomarker for FTD and other neurodegenerative diseases [22].
Amyotrophic lateral sclerosis (ALS) is a life-limiting neurodegenerative disorder caused by the loss of upper and lower motor neurons and consequent degeneration of the motor cortex and spinal cord [49]. The majority of ALS is sporadic. Of the 5–10% of ALS that has a genetic component, pathogenic variants are most commonly detected in superoxide dismutase 1 (SOD1), C9ORF72, FUS, and TAR DNA-binding protein 43 (TARDBP) [50]. There is increasing evidence that alterations of lipid metabolism are linked to ALS pathogenesis [51]. However, the implications of lipidome dysregulation in ALS progression remains unresolved. Using a transgenic rat model of ALS (SOD1-G93A), a recent lipidomic analysis revealed a significant decrease of CL content in the spinal cord of symptomatic ALS rats, in addition to other lipidomic changes [52]. Alterations in CL content may also mirror the loss of mitochondrial integrity observed in several ALS models [49]. Interestingly, spinal cord and, to a lesser extent, brain mitochondria of SOD1-G93A transgenic mice display increased CL peroxidation, impaired OXPHOS activity, and enhanced cytochrome c release from the IMM, consistent with the low binding affinity of CLox for cytochrome c [53]. These emerging lines of evidence suggest that aberrant CL metabolism plays a broader role in ALS pathogenesis that requires further investigation.
Clinical and molecular overlap between FTD and ALS exists with mutations in C9orf72, TARDBP, FUS, TANK-binding kinase 1 (TBK1), valosin containing protein (VCP), coiled-coil-helix-coiled-coil-helix domain containing 10 (CHCHD10), and sequestosome-1 (SQSTM1) [54], suggesting that the two disorders share common pathophysiological mechanisms. Interestingly, aberrant ER–mitochondria tethering has recently been observed with TARDBP mutations [55]. Given the central role of ER–mitochondria contact sites in lipid translocation and biosynthesis, disruption to this axis potentially has detrimental effects on lipid flux and CL biosynthesis. However, further work is required to fully understand the link between altered ER–mitochondrial signaling, CL content/metabolism, and FTD/ALS pathophysiology.
Alzheimer’s Disease
AD is the most prevalent cause of dementia in aging people [56]. Clinically, the disease is characterized by a progressive loss of memory, decline of learning abilities, disorientation, and mood swings [56]. Over the past three decades, substantial evidence has been generated to support the notion that the synaptic loss and neuronal impairment observed in AD is causally related to the co-presence of extracellular amyloid-ß plaques and toxic neurofibrillary tangles of tau protein in the brain [56]. Moreover, the abnormal accumulation of misfolded tau protein and amyloid-ß aggregates observed in familial AD (<5% of cases) have been associated with mutations in the amyloid precursor protein (APP) and presenilin 1 and 2 (PSEN1 and 2) [57]. By contrast, sporadic AD, which occurs with much higher prevalence, is hypothesized to result from complex interactions between genetic, environmental, and lifestyle factors [56]. Nevertheless, the complexity of AD suggests that additional players are involved in the pathogenesis of the disease. Changes in the brain and plasma lipidome have been extensively observed in people with AD [58]. However, knowledge concerning the role of CL is limited. A reduction in total CL, associated with mitochondrial synaptic dysfunction and oxidative stress, has been observed in a mouse model of AD, suggesting a contribution of CL to the disease pathogenesis [59]. Additionally, in vivo and in vitro models of AD have shown that mitochondrial membranes are particularly vulnerable to tau aggregates, leading to neuronal toxicity [60], and that tau protein preferentially binds to CL-rich regions of the OMM, inducing mitochondrial swelling, cytochrome c release, and decreased membrane potential [61]. Finally, a critical driver of AD is the aberrant neuroinflammatory response, induced by chronic microglial activation and release of proinflammatory cytokines, and a potential protective role of CL in the AD inflammatory response has been proposed [62]. Indeed, CL extracellularly released by damaged cells may regulate microglia function by upregulating the phagocytosis of amyloid-ß deposits, thus enhancing neuronal survival [62]. Collectively, these observations suggest a causal association between CL and AD pathophysiology that requires further study.
Parkinson’s Disease
PD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra and the presence of intraneuronal aggregates of misfolded α-synuclein. The majority of PD is idiopathic, with familial PD accounting for 5–10% of cases [63]. Several molecular factors contribute to the pathogenesis of PD, including toxic α-synuclein deposition, oxidative stress, and impaired mitochondrial function (i.e., biogenesis, bioenergetics, and mitophagy) [63]. Numerous in vitro and in vivo studies confirm that the neurotoxicity of the intracellular aggregates is associated with the ability of misfolded α-synuclein to interact with CL, thus impacting mitochondrial membrane integrity [64,65]. Moreover, the co-presence of CL and α-synuclein oligomers favors the formation of ion-permeable pores, inducing mitochondrial membrane permeabilization and cytochrome c release [66], in addition to disrupting bioenergetics [64,67]. However, CL exposure in the OMM can also promote α-synuclein refolding, thereby reducing the toxicity of the intracellular aggregates, preventing neuronal loss [68]. Indeed, α-synuclein can form a triple complex with CL and cytochrome c, which prevents cytochrome c release and delays neuronal damage [69]. Reduced α-synuclein oligomerization may also be facilitated by the inhibition of ALCAT1, an enzyme involved in pathologic CL remodeling and formation of highly oxidizable CL species [70] (Figure 3). Abnormalities in CL content have also been associated with impaired mitophagy [18,71] and defects of complex I activity [72] in multiple PD models. Together, these data confirm an important role for CL in PD. However, further work is necessary to determine the precise balance between the detrimental and protective implications of CL in PD pathogenesis.
Traumatic Brain Injury
Traumatic brain injury (TBI) is the leading cause of death in young adults. From a pathophysiological perspective, the neurological damage that occurs is divided into primary and secondary injuries. Primary brain injuries derive from the immediate mechanical impact, whereas secondary injuries occur within hours or weeks from the initial insult as a consequence of a wide range of signaling responses, including oxidative stress, lipid peroxidation, apoptosis, and inflammation [35]. In a controlled cortical impact (CCI) rat model of TBI, loss of CL content has been observed post-TBI in the contusional and pericontusional regions [73], while the unique long-chain PUFA of CL present in the brain are readily oxidized (CLox) [74., 75., 76.], hydrolyzed, and released into the systemic circulation, where they activate a large number of well-characterized inflammatory signaling molecules [21]. Consequently, these oxidized FAs have been proposed as mediators and/or regulators of the inflammatory response to the injury [74]. Moreover, given that in murine models brain CL is detectable in the blood within hours of the insult, it potentially represents a biomarker to assess the severity and progression of the injury [77]. The procoagulant effects of CL present in circulating brain-derived microparticles have also been linked with TBI-associated coagulopathy in mice, a feature of TBI that is associated with a poorer outcome in humans [78]. Finally, preliminary evidence suggests that CL is externalized from the IMM to the OMM following TBI, thereby eliminating damaged brain mitochondria through mitophagy. This early protective mechanism is activated during the first few hours after the injury before rapidly being replaced by uncontrolled apoptotic signaling pathways. Accordingly, the development of small molecules that modulate the mitophagy response might prevent the extensive neuronal loss that occurs, thereby representing a potential therapeutic strategy in TBI [79].
Management and Treatment Strategies
Dysregulated phospholipid levels in the brain and serum have been associated with acute and chronic pathological conditions. The development of liquid chromatography-tandem mass spectrometry (LC-MS/MS) lipidomics approaches have already generated important data concerning altered CL species in human and animal models of neurodegenerative diseases [59,80,81]. Detection of reduced CL content and increased MLCL expressed as a ratio of MLCL:CL is currently used in clinical practice as a diagnostic biomarker for Barth syndrome (BTHS) [82]. Furthermore, increasing information in humans and in animal models indicates that CL species detected in the systemic circulation have the potential to act as biomarkers that measure disease progression in other neurological disorders, for example, FTD, PD, and TBI [22,74,83]. A summary of the CL species detected in the serum of human and animal models is summarized in Table 1, Table 2, respectively.
Rapid advances in current understanding of the novel roles of CL in brain homeostasis and neurodegeneration represent an exciting opportunity to develop new treatment strategies aimed at targeting various neurodegenerative processes. Small molecules that preserve mitochondrial CL content and integrity have the potential to prevent and/or attenuate the mitochondrial damage associated with neurodegeneration. For example, elamipretide (Bendavia, MTP-131, SS-31) is a mitochondrial-targeted synthetic tetrapeptide that concentrates at the IMM and binds to 12 CL-binding proteins [84]. While the precise molecular mechanism is unclear, elamipretide is proposed to stabilize CL, thus improving OXPHOS efficiency. A Phase III trial of elamipretide in primary mitochondrial myopathy did not meet its primary end-points. However, statistically significant improvements in exercise tolerance, measured using a 6-minute walk test, and BTHS symptom scores at 36 weeks were recorded during the open label extension of a Phase II/III study in BTHS [85]. There is also promising preclinical data for elamipretide as a disease-modifying molecule in TBI [86].
Concluding Remarks
Over recent years, clinical and experimental studies from human and animal models have provided compelling evidence that links aberrant CL metabolism with neurological dysfunction. Alterations in CL profiles measured in several neurodegenerative disorders further emphasizes the important role of CL in brain homeostasis and neuronal physiology. Given the equipoise that currently exists between CL, mitochondria, and neurological disease, there is critical need to fully understand the factors that regulate CL acyl chain composition and whether abnormalities in CL are a direct cause, or downstream effect, of the underlying pathological process. Moreover, despite significant advances in lipidomic techniques and in vivo approaches, the pathways involved with normal CL metabolism remain poorly understood (see Outstanding Questions). Consequently, CL may still contribute to numerous, as yet uncharacterized, aspects of cellular homeostasis and human health. In future, the application of innovative, multi-research domain approaches will be crucial when evaluating these roles and their contribution to nervous system homeostasis and brain function.
Outstanding Questions.
What is the functional role of cardiolipin (CL) long-chained fatty acids in the brain?
What are the missing steps in the CL biosynthetic pathway?
What are the molecular mechanisms underpinning aberrant CL composition in human disease states?
Could CL represent a biomarker and pharmacological target in CL-related monogenic and neurodegenerative disorders?
Alt-text: Outstanding Questions
Acknowledgments
Acknowledgments
The University College London Hospitals/University College London Queen Square Institute of Neurology sequencing facility receives a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. The clinical and diagnostic 'Rare Mitochondrial Disorders' Service in London is funded by the UK National Health Service (NHS) Highly Specialised Commissioners. M.F. and R.D.S.P. are supported by a Medical Research Council (UK) Clinician Scientist Fellowship (MR/S002065/1). M.G.H. and R.D.S.P. are funded by a Medical Research Council (UK) strategic award to establish an International Centre for Genomic Medicine in Neuromuscular Diseases (ICGNMD) (MR/S005021/1).
Declaration of Interests
No interests are declared.
References
- 1.Sastry P.S. Lipids of nervous tissue: composition and metabolism. Prog. Lipid Res. 1985;24:69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Fantini J., Yahi N. Elsevier; 2015. Brain Lipids in Synaptic Function and Neurological Disease: Clues to Innovative Therapeutic Strategies for Brain Disorders. [Google Scholar]
- 3.Pfanner N. Mitochondrial proteins: from biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019;20:267–284. doi: 10.1038/s41580-018-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Meer G. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oemer G. Phospholipid acyl chain diversity controls the tissue-specific assembly of mitochondrial cardiolipins. Cell Rep. 2020;30:4281–4291. doi: 10.1016/j.celrep.2020.02.115. [DOI] [PubMed] [Google Scholar]
- 6.Schlame M., Greenberg M.L. Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:3–7. doi: 10.1016/j.bbalip.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannella C.A. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta, Mol. Cell Res. 2006;1763:542–548. doi: 10.1016/j.bbamcr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Colbeau A. Enzymac characterization and lipid composition of rat liver subcellular membranes. Biochim. Biophys. Acta Biomembr. 1971;249:462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- 9.Claypool S.M. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 2008;182:937–950. doi: 10.1083/jcb.200801152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebert N. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr. Biol. 2009;19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwall C.T. The stability and activity of respiratory complex II is cardiolipin-dependent. Biochim. Biophys. Acta Bioenerg. 2012;1817:1588–1596. doi: 10.1016/j.bbabio.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Fiedorczuk K. Atomic structure of the entire mammalian mitochondrial complex I. Nature. 2016;538:406–410. doi: 10.1038/nature19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinzawa-Itoh K. Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 2007;26:1713–1725. doi: 10.1038/sj.emboj.7601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez B., Robinson N.C. Phospholipase digestion of bound cardiolipin reversibly inactivates bovine cytochrome bc1. Biochemistry. 1999;38:9031–9038. doi: 10.1021/bi990603r. [DOI] [PubMed] [Google Scholar]
- 15.Spikes T.E. Structure of the dimeric ATP synthase from bovine mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2020;117:23519–23526. doi: 10.1073/pnas.2013998117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M. Gluing the respiratory chain together: cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 17.Chu C.T. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu C.T. LC3 binds externalized cardiolipin on injured mitochondria to signal mitophagy in neurons: implications for Parkinson disease. Autophagy. 2014;10:376–378. doi: 10.4161/auto.27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagan V.E. Cytochrome C acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 20.Lutter M. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 21.Chao H. Disentangling oxidation/hydrolysis reactions of brain mitochondrial cardiolipins in pathogenesis of traumatic injury. JCI Insight. 2018;3 doi: 10.1172/jci.insight.97677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan K. Uncovering pathophysiological changes in frontotemporal dementia using serum lipids. Sci. Rep. 2020;10:3640. doi: 10.1038/s41598-020-60457-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlame M. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann. Neurol. 2002;51:634–637. doi: 10.1002/ana.10176. [DOI] [PubMed] [Google Scholar]
- 24.Kasahara T. Cardiolipin is essential for early embryonic viability and mitochondrial integrity of neurons in mammals. FASEB J. 2020;34:1465–1480. doi: 10.1096/fj.201901598R. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J. Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis. Cell Metab. 2011;13:690–700. doi: 10.1016/j.cmet.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scharwey M. Mitochondrial lipid transport at a glance. J. Cell Sci. 2013;126:5317–5323. doi: 10.1242/jcs.134130. [DOI] [PubMed] [Google Scholar]
- 27.Potting C. TRIAP1/PRELI complexes prevent apoptosis by mediating intramitochondrial transport of phosphatidic acid. Cell Metab. 2013;18:287–295. doi: 10.1016/j.cmet.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Taylor W.A., Hatch G.M. Purification and characterization of monolysocardiolipin acyltransferase from pig liver mitochondria. J. Biol. Chem. 2003;278:12716–12721. doi: 10.1074/jbc.M210329200. [DOI] [PubMed] [Google Scholar]
- 29.Cao J. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J. Biol. Chem. 2004;279:31727–31734. doi: 10.1074/jbc.M402930200. [DOI] [PubMed] [Google Scholar]
- 30.Shichiri M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014;54:151–160. doi: 10.3164/jcbn.14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baxter P.S., Hardingham G.E. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic. Biol. Med. 2016;100:147–152. doi: 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belikova N.A. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vähäheikkilä M. How cardiolipin peroxidation alters the properties of the inner mitochondrial membrane? Chem. Phys. Lipids. 2018;214:15–23. doi: 10.1016/j.chemphyslip.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Paradies G. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:35–41. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- 35.Lamade A.M. Mitochondrial damage & lipid signaling in traumatic brain injury. Exp. Neurol. 2020;329 doi: 10.1016/j.expneurol.2020.113307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu G.Y. The phospholipase iPLA2 is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling. J. Biol. Chem. 2017;292:10672–10684. doi: 10.1074/jbc.M117.783068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pizzuto M., Pelegrin P. Cardiolipin in immune signaling and cell death. Trends Cell Biol. 2020;30:892–903. doi: 10.1016/j.tcb.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Galluzzi L. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on cell death. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jové M. Lipids and lipoxidation in human brain aging. Mitochondrial ATP-synthase as a key lipoxidation target. Redox Biol. 2019;23 doi: 10.1016/j.redox.2018.101082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sen T. Depolarization and cardiolipin depletion in aged rat brain mitochondria: relationship with oxidative stress and electron transport chain activity. Neurochem. Int. 2007;50:719–725. doi: 10.1016/j.neuint.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Lores-Arnaiz S. Changes in motor function and brain cortex mitochondrial active oxygen species production in aged mice. Exp. Gerontol. 2019;118:88–98. doi: 10.1016/j.exger.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Petrosillo G. Mitochondrial dysfunction in rat brain with aging. Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem. Int. 2008;53:126–131. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Sen T. Lipid peroxidation associated cardiolipin loss and membrane depolarization in rat brain mitochondria. Neurochem. Int. 2006;49:20–27. doi: 10.1016/j.neuint.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Petrosillo G. Decline in cytochrome c oxidase activity in rat-brain mitochondria with aging. Role of peroxidized cardiolipin and beneficial effect of melatonin. J. Bioenerg. Biomembr. 2013;45:431–440. doi: 10.1007/s10863-013-9505-0. [DOI] [PubMed] [Google Scholar]
- 45.Phillips A.F. Single-molecule analysis of mtDNA replication uncovers the basis of the common deletion. Mol. Cell. 2017;65:527–538. doi: 10.1016/j.molcel.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Yun J., Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coyle-Gilchrist I.T.S. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panza F. Development of disease-modifying drugs for frontotemporal dementia spectrum disorders. Nat. Rev. Neurol. 2020;16:213–228. doi: 10.1038/s41582-020-0330-x. [DOI] [PubMed] [Google Scholar]
- 49.Hardiman O. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 2017;3:17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 50.Häkkinen S. Neuroimaging in genetic frontotemporal dementia and amyotrophic lateral sclerosis. Neurobiol. Dis. 2020;145 doi: 10.1016/j.nbd.2020.105063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tracey T.J. Neuronal lipid metabolism: multiple pathways driving functional outcomes in health and disease. Front. Mol. Neurosci. 2018;11:10. doi: 10.3389/fnmol.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaves-Filho A.B. Alterations in lipid metabolism of spinal cord linked to amyotrophic lateral sclerosis. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-48059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirkinezos I.G. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J. Neurosci. 2005;25:164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abramzon Y.A. The overlapping genetics of amyotrophic lateral sclerosis and frontotemporal dementia. Front. Neurosci. 2020;14:42. doi: 10.3389/fnins.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoica R. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun. 2014;5:3996. doi: 10.1038/ncomms4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheltens P. Alzheimer’s disease. Lancet. 2016;15:455–532. [Google Scholar]
- 57.Tanzi R.E. The genetics of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naudí A. Lipidomics of human brain aging and Alzheimer’s disease pathology. Int. Rev. Neurobiol. 2015;122:133–189. doi: 10.1016/bs.irn.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Monteiro-Cardoso V.F. Cardiolipin profile changes are associated to the early synaptic mitochondrial dysfunction in Alzheimer’s disease. J. Alzheimers Dis. 2014;43:1375–1392. doi: 10.3233/JAD-141002. [DOI] [PubMed] [Google Scholar]
- 60.Cheng Y., Bai F. The association of tau with mitochondrial dysfunction in Alzheimer’s disease. Front. Neurosci. 2018;12:163. doi: 10.3389/fnins.2018.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camilleri A. Tau-induced mitochondrial membrane perturbation is dependent upon cardiolipin. Biochim. Biophys. Acta Biomembr. 2019;1862 doi: 10.1016/j.bbamem.2019.183064. [DOI] [PubMed] [Google Scholar]
- 62.Pointer C.B. Extracellular cardiolipin regulates select immune functions of microglia and microglia-like cells. Brain Res. Bull. 2019;146:153–163. doi: 10.1016/j.brainresbull.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Poewe W. Parkinson disease. Nat. Rev. Dis. Prim. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 64.Ghio S. Interaction of α-synuclein with biomembranes in Parkinson’s disease - role of cardiolipin. Prog. Lipid Res. 2016;61:73–82. doi: 10.1016/j.plipres.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 65.Ugalde C.L. Misfolded α-synuclein causes hyperactive respiration without functional deficit in live neuroblastoma cells. DMM Dis. Model. Mech. 2020;13 doi: 10.1242/dmm.040899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghio S. Cardiolipin promotes pore-forming activity of alpha-synuclein oligomers in mitochondrial membranes. ACS Chem. Neurosci. 2019;10:3815–3829. doi: 10.1021/acschemneuro.9b00320. [DOI] [PubMed] [Google Scholar]
- 67.Ellis C.E. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Mol. Cell. Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryan T. Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat. Commun. 2018;9:817. doi: 10.1038/s41467-018-03241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bayir H. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome c. Protection against apoptosis versus delayed oxidative stress in Parkinson disease. J. Biol. Chem. 2009;284:15951–15969. doi: 10.1074/jbc.M900418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song C. Cardiolipin remodeling by ALCAT1 links mitochondrial dysfunction to Parkinson’s diseases. Aging Cell. 2019;18 doi: 10.1111/acel.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaudioso A. Lipidomic alterations in the mitochondria of aged Parkin null mice relevant to autophagy. Front. Neurosci. 2019;13:329. doi: 10.3389/fnins.2019.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vos M. Cardiolipin promotes electron transport between ubiquinone and complex I to rescue PINK1 deficiency. J. Cell Biol. 2017;216:695–708. doi: 10.1083/jcb.201511044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sparvero L.J. Imaging mass spectrometry reveals loss of polyunsaturated cardiolipins in the cortical contusion, hippocampus, and thalamus after traumatic brain injury. J. Neurochem. 2016;139:659–675. doi: 10.1111/jnc.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anthonymuthu T.S. Global assessment of oxidized free fatty acids in brain reveals an enzymatic predominance to oxidative signaling after trauma. Biochim. Biophys. Acta Mol. Basis Dis. 2017;863:2601–2613. doi: 10.1016/j.bbadis.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andriessen T.M.J.C. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 2010;14:2381–2392. doi: 10.1111/j.1582-4934.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bayir H. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Ann. Neurol. 2007;62:154–169. doi: 10.1002/ana.21168. [DOI] [PubMed] [Google Scholar]
- 77.Anthonymuthu T.S. Detection of brain specific cardiolipins in plasma after experimental pediatric head injury. Exp. Neurol. 2019;316:63–73. doi: 10.1016/j.expneurol.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Z. Cardiolipin-mediated procoagulant activity of mitochondria contributes to traumatic brain injury-associated coagulopathy in mice. Blood. 2016;127:2763–2772. doi: 10.1182/blood-2015-12-688838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chao H. Cardiolipin-dependent mitophagy guides outcome after traumatic brain injury. J. Neurosci. 2019;39:1930–1943. doi: 10.1523/JNEUROSCI.3415-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oemer G. Molecular structural diversity of mitochondrial cardiolipins. Proc. Natl. Acad. Sci. U. S. A. 2018;115:4158–4163. doi: 10.1073/pnas.1719407115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phan A.T. Propeller-type parallel-stranded G-quadruplexes in the human c-myc promoter. J. Am. Chem. Soc. 2004;126:8710–8716. doi: 10.1021/ja048805k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kulik W. Bloodspot assay using HPLC-tandem mass spectrometry for detection of Barth syndrome. Clin. Chem. 2008;54:371–378. doi: 10.1373/clinchem.2007.095711. [DOI] [PubMed] [Google Scholar]
- 83.Tyurina Y.Y. LC/MS analysis of cardiolipins in substantia nigra and plasma of rotenone-treated rats: implication for mitochondrial dysfunction in Parkinson’s disease. Free Radic. Res. 2015;49:681–691. doi: 10.3109/10715762.2015.1005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chavez J.D. Mitochondrial protein interaction landscape of SS-31. Proc. Natl. Acad. Sci. U. S. A. 2020;117:15363–15373. doi: 10.1073/pnas.2002250117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thompson W.R. A phase 2/3 randomized clinical trial followed by an open-label extension to evaluate the effectiveness of elamipretide in Barth syndrome, a genetic disorder of mitochondrial cardiolipin metabolism. Genet. Med. 2020 doi: 10.1038/s41436-020-01006-8. Published online October 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu Y. SS-31 Provides neuroprotection by reversing mitochondrial dysfunction after traumatic brain injury. Oxidative Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/4783602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schlame M., Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim. Biophys. Acta Biomembr. 2009;1788:2080–2083. doi: 10.1016/j.bbamem.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ortiz A. Membrane fusion and the lamellar-to-inverted-hexagonal phase transition in cardiolipin vesicle systems induced by divalent cations. Biophys. J. 1999;77:2003–2014. doi: 10.1016/S0006-3495(99)77041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Acehan D. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J. Biol. Chem. 2011;286:899–908. doi: 10.1074/jbc.M110.171439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sustarsic E.G. Cardiolipin synthesis in brown and beige fat mitochondria is essential for systemic energy homeostasis. Cell Metab. 2018;28:159–174. doi: 10.1016/j.cmet.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van der Laan M. Mitochondrial contact site and cristae organizing system. Curr. Opin. Cell Biol. 2016;4:33–42. doi: 10.1016/j.ceb.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 92.Anand R. MIC26 and MIC27 cooperate to regulate cardiolipin levels and the landscape of OXPHOS complexes. Life Sci. Alliance. 2020;3 doi: 10.26508/lsa.202000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rampelt H. Assembly of the mitochondrial cristae organizer Mic10 is regulated by Mic26–Mic27 antagonism and cardiolipin. J. Mol. Biol. 2018;430:1883–1890. doi: 10.1016/j.jmb.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 94.Daum G., Vance J.E. Import of lipids into mitochondria. Prog. Lipid Res. 1997;36:103–130. doi: 10.1016/s0163-7827(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 95.Malhotra K. Cardiolipin mediates membrane and channel interactions of the mitochondrial TIM23 protein import complex receptor Tim50. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kunji E.R.S., Ruprecht J.J. The mitochondrial ADP/ATP carrier exists and functions as a monomer. Biochem. Soc. Trans. 2020;48:1419–1432. doi: 10.1042/BST20190933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mao X. Function-related asymmetry of the specific cardiolipin binding sites on the mitochondrial ADP/ATP carrier. Biochim. Biophys. Acta Biomembr. 2021;1863 doi: 10.1016/j.bbamem.2020.183466. [DOI] [PubMed] [Google Scholar]
- 98.Klingenberg M. Cardiolipin and mitochondrial carriers. Biochim. Biophys. Acta Biomembr. 2009;1788:2048–2058. doi: 10.1016/j.bbamem.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 99.Senoo N. Cardiolipin, conformation, and respiratory complex-dependent oligomerization of the major mitochondrial ADP/ATP carrier in yeast. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghosh S. An essential role for cardiolipin in the stability and function of the mitochondrial calcium uniporter. Proc. Natl. Acad. Sci. U. S. A. 2020;117:16383–16390. doi: 10.1073/pnas.2000640117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patil V.A. Loss of cardiolipin leads to perturbation of mitochondrial and cellular iron homeostasis. J. Biol. Chem. 2013;288:1696–1705. doi: 10.1074/jbc.M112.428938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y. Cardiolipin-deficient cells have decreased levels of the iron–sulfur biogenesis protein frataxin. J. Biol. Chem. 2020;295:11928–11937. doi: 10.1074/jbc.RA120.013960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duncan A.L. Cardiolipin binds selectively but transiently to conserved lysine residues in the rotor of metazoan ATP synthases. Proc. Natl. Acad. Sci. U. S. A. 2016;113:8687–8692. doi: 10.1073/pnas.1608396113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mileykovskaya E., Dowhan W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem. Phys. Lipids. 2014;179:42–48. doi: 10.1016/j.chemphyslip.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu Y. Assembly of the complexes of oxidative phosphorylation triggers the remodeling of cardiolipin. Proc. Natl. Acad. Sci. U. S. A. 2019;116:11235–11240. doi: 10.1073/pnas.1900890116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee R.G. Cardiolipin is required for membrane docking of mitochondrial ribosomes and protein synthesis. J. Cell Sci. 2020;133 doi: 10.1242/jcs.240374. [DOI] [PubMed] [Google Scholar]
- 107.Tilokani L. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62:341–360. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stepanyants N. Cardiolipin’s propensity for phase transition and its reorganization by dynamin-related protein 1 form a basis for mitochondrial membrane fission. Mol. Biol. Cell. 2015;26:3104–3116. doi: 10.1091/mbc.E15-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ban T. Molecular basis of selective mitochondrial fusion by heterotypic action between OPA1 and cardiolipin. Nat. Cell Biol. 2017;19:856–863. doi: 10.1038/ncb3560. [DOI] [PubMed] [Google Scholar]
- 110.Wade Harper J. Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 2018;19:93–108. doi: 10.1038/nrm.2017.129. [DOI] [PubMed] [Google Scholar]
- 111.Miller P.C. A Bayesian analysis to determine the prevalence of Barth syndrome in the pediatric population. J. Pediatr. 2020;217:139–144. doi: 10.1016/j.jpeds.2019.09.074. [DOI] [PubMed] [Google Scholar]
- 112.Bione S. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat. Genet. 1996;12:385–389. doi: 10.1038/ng0496-385. [DOI] [PubMed] [Google Scholar]
- 113.Barth P.G. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 1983;62:327–355. doi: 10.1016/0022-510x(83)90209-5. [DOI] [PubMed] [Google Scholar]
- 114.Bowron A. Barth syndrome without tetralinoleoyl cardiolipin deficiency: a possible ameliorated phenotype. J. Inherit. Metab. Dis. 2015;38:279–286. doi: 10.1007/s10545-014-9747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vernon H.J. Clinical laboratory studies in Barth syndrome. Mol. Genet. Metab. 2014;112:143–147. doi: 10.1016/j.ymgme.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 116.Bertero E. Cardiolipin remodeling in Barth syndrome and other hereditary cardiomyopathies. Biochim. Biophys. Acta Mol. basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165803. [DOI] [PubMed] [Google Scholar]
- 117.Cole L.K. Aberrant cardiolipin metabolism is associated with cognitive deficiency and hippocampal alteration in tafazzin knockdown mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3353–3367. doi: 10.1016/j.bbadis.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suzuki-Hatano S. AAV-mediated TAZ gene replacement restores mitochondrial and cardioskeletal function in Barth syndrome. Hum. Gene Ther. 2019;30:139–154. doi: 10.1089/hum.2018.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang S. AAV gene therapy prevents and reverses heart failure in a murine knockout model of Barth syndrome. Circ. Res. 2020;126:1024–1039. doi: 10.1161/CIRCRESAHA.119.315956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schafer C. The effects of PPAR stimulation on cardiac metabolic pathways in Barth syndrome mice. Front. Pharmacol. 2018;9:318. doi: 10.3389/fphar.2018.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mayr J.A. Lack of the mitochondrial protein acylglycerol kinase causes Sengers syndrome. Am. J. Hum. Genet. 2012;90:314–320. doi: 10.1016/j.ajhg.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davey K.M. Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition. J. Med. Genet. 2006;43:385–393. doi: 10.1136/jmg.2005.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Richter-Dennerlein R. DNAJC19, a mitochondrial cochaperone associated with cardiomyopathy, forms a complex with prohibitins to regulate cardiolipin remodeling. Cell Metab. 2014;20:158–171. doi: 10.1016/j.cmet.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 124.Wortmann S.B. Mutations in the phospholipid remodeling gene SERAC1 impair mitochondrial function and intracellular cholesterol trafficking and cause dystonia and deafness. Nat. Genet. 2012;44:797–802. doi: 10.1038/ng.2325. [DOI] [PubMed] [Google Scholar]
- 125.Acoba M.G. Phospholipid ebb and flow makes mitochondria go. J. Cell. Biol. 2020;219 doi: 10.1083/jcb.202003131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vukotic M. Acylglycerol kinase mutated in Sengers syndrome is a subunit of the TIM22 protein translocase in mitochondria. Mol. Cell. 2017;67:471–483.e7. doi: 10.1016/j.molcel.2017.06.013. [DOI] [PubMed] [Google Scholar]