Abstract
Background
In 2020 Lao PDR had low reported COVID-19 cases but it was unclear whether this masked silent transmission. A seroprevalence study was done August - September 2020 to determine SARS-CoV-2 exposure.
Methods
Participants were from the general community (n=2433) or healthcare workers (n=666) in five provinces and bat/wildlife contacts (n=74) were from Vientiane province. ELISAs detected anti- SARS-CoV-2 Nucleoprotein (N; n=3173 tested) and Spike (S; n=1417 tested) antibodies. Double-positive samples were checked by IgM/IgG rapid tests. Controls were confirmed COVID-19 cases (n=15) and pre-COVID-19 samples (n=265). Seroprevalence for the general community was weighted to account for complex survey sample design, age and sex.
Findings
In pre-COVID-19 samples, 5·3%, [95% CI=3·1-8·7%] were anti-N antibody single-positive and 1·1% [0·3-3·5%] were anti-S antibody single positive. None were double positive. Anti-N and anti-S antibodies were detected in 5·2% [4·2-6·5%] and 2·1% [1·1-3·9%] of the general community, 2·0% [1·1-3·3%] and 1·4% [0·5-3·7%] of healthcare workers and 20·3% [12·6-31·0%] and 6·8% [2·8-15·3%] of bat/wildlife contacts. 0·1% [0·02-0·3%] were double positive for anti-N and anti-S antibodies (rapid test negative).
Interpretation
We find no evidence for significant SARS-CoV-2 circulation in Lao PDR before September 2020. This likely results from early decisive measures taken by the government, social behavior, and low population density. High anti-N /low anti-S seroprevalence in bat/wildlife contacts may indicate exposure to cross-reactive animal coronaviruses with threat of emerging novel viruses.
Funding
Agence Française de Développement. Additional; Institut Pasteur du Laos, Institute Pasteur, Paris and Luxembourg Ministry of Foreign and European Affairs (“PaReCIDS II”).
Research in context.
Evidence before this study
We searched PubMed for research published up until 20th May 2021, using the terms “SARS-CoV-2” or “COVID-19” and “Laos” or “Lao People's Democratic Republic”. We found no previous SARS-CoV-2 seroprevalence studies from Lao PDR. In 2020, a time when many countries in the region and worldwide were struggling with a huge burden of COVID-19 cases, the Lao People's Democratic Republic (PDR) stood out as a country with low reported numbers of SARS-CoV-2 infections.
Added value of this study
In order to address whether or not there was an unseen circulation of SARS-CoV-2 within the Lao PDR in 2020, we carried out a seroprevalence study for anti-SARS-CoV-2 antibodies. Within our study population, only two out of 3173 were seropositive for both anti-N and anti-S SARS-CoV-2 antibodies. However, these participants were antibody rapid test negative. These extremely low numbers confirm that there was no widespread circulation of SARS-CoV-2 in Lao PDR. Nevertheless, a high prevalence of anti-N antibodies, particularly in individuals with close contact to bats (20·3%), coupled with low anti-S antibody seroprevalence, may indicate exposure to other alpha or beta coronaviruses within Lao PDR.
Implications of all the available evidence
Several factors may have contributed to the low number of COVID-19 cases in Lao PDR. The early, decisive action by the Government of Lao PDR is likely to have had a significant impact. As such, a three-month long lock-down was implemented on March 23, 2020, including shutting of schools and entertainment venues. International flights have since been greatly limited, and all international visitors must provide a SARS-CoV-2 negative test result prior to entry and quarantine for 14 days upon arrival. Societal and epidemiological factors may also be important, including low population density. Nevertheless, Lao PDR remains vulnerable, with a large number of susceptible individuals and a limited capacity for care and treatment. This was underlined by an outbreak of cases in mid-April 2021, believed to originate from illegal migrants crossing the border from Thailand. Evidence for significant exposure of bat/wildlife contacts to coronaviruses reminds us that the threat of emergence of novel viral pandemics is always present in the region. In the short term, the Government of Lao PDR needs to balance the strict control of international visitors with a need to open up the country again, for example by considering large vaccination campaigns, vaccine passports or by forming travel corridors with other countries where the situation is similarly controlled. In the longer term, as Lao PDR opens up to greater numbers of visitors, a strategy is needed to increase sustainable surveillance for COVID-19 and other infectious diseases and to increase biosafety and appropriate personal protective measures among at-risk populations including wildlife contacts.
Alt-text: Unlabelled box
1. Introduction
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the causative agent of COVID-19) emerged in Wuhan, China [1]. The virus spread worldwide and World Health Organization (WHO) characterized the COVID-19 outbreak as a pandemic on March 11th, 2020. In the middle of May 2021, there were more than 160, 000,000 confirmed cases recorded by the WHO, including more than 3,400,000 deaths [2].
Lao People's Democratic Republic (PDR) is a land-locked, developing country of more than seven million people bordering China, Myanmar, Thailand, Cambodia and Vietnam. The capacity of the healthcare system in Lao PDR is limited, with only 17666 healthcare staff working at health facilities in 2016, equivalent to 2·68/1000 inhabitants [3]. Furthermore, a large proportion of the country has limited access to healthcare facilities and the diagnostic and surveillance capacity is low for many diseases. As with other developing countries, a significant outbreak of COVID-19 could have devastating consequences on the fragile healthcare infrastructure. This concern, together with the close proximity to China and relatively porous borders, led to the establishment of a national task force committee on the 3rd of February 2020, led by the Deputy Prime-Minister and Minister for Finance and supported by a Secretariat headed by the Vice Minister for Health. In the middle of March 2020, the task force implemented restrictions at the country level such as school closure (until May 2020) and a lockdown was implemented the 20th of March until the 3rd of May. Domestic flights were cancelled until May 15, and a restriction on most international flights is ongoing [4].
From January 2020, testing for COVID-19, as recommended by WHO guidelines, was conducted on nasopharyngeal and oropharyngeal swabs using real-time reverse transcription polymerase chain reaction (RT-PCR). This testing was done on suspected cases – those with symptoms including fever and at least one of the following: cough, runny nose, sore throat, shortness of breath or difficulty to breath - and their contacts [5,6]. Travelers into and out of Lao PDR are also tested by the same technique and a 14-day quarantine is imposed on all arrivals.
As of the middle of 2021, almost 230,000 samples have been tested. These include all new arrivals into the country, in addition to symptomatic individuals who have close contact with people coming from endemic countries or with confirmed cases. Although the first case of COVID-19 in Lao PDR was confirmed early - on March 24, 2020 - there were less than 60 SARS-CoV-2 positive confirmed cases in total and no recorded COVID-19-related deaths by mid-April 2021, which increased rapidly to around 1800 cases and 2 deaths by mid-May [7]. Until mid-April, just over half of confirmed cases were imported (from Thailand, the United Kingdom, Papua New Guinea, Japan, Russia, India and Malaysia) but subsequently, low-level community transmission became the main route of infection. The low number of cases contrasts with those of several southeast Asian countries such as Myanmar, Indonesia, Thailand and the Philippines, and is among the lowest proportionally worldwide [8].
The low number of confirmed COVID-19 cases could largely be due to the strict control measures set in place by the Lao Government. Another reason could be the relatively low population density limiting the spread of the virus or the young age structure of the population; 51% are under 24 years old and less than 7% over 60 [9]. This young population may allow mild or asymptomatic infections (approximately 80% of COVID-19 cases) to go undetected [10], [11], [12]. This is particularly relevant in the context of countries, such as Lao PDR, where routine disease surveillance is challenging [13].
Healthcare workers are a particular risk group who may come into contact with COVID-19 patients. Importantly, the seroprevalence of anti-SARS-CoV-2 antibodies within the healthcare settings can offer insight into the effectiveness of infection prevention and control measures [14], [15], [16], [17], [18]. Another potential risk group for infection are individuals in close contact with wildlife including bats, one of the natural reservoirs of coronaviruses, possibly including SARS-CoV-2 or its progenitor [19,20]. The large diversity of bat species in Lao PDR and the geographical closeness of the Hubei province in China where SARS-CoV-2 was first detected suggest that such viruses could naturally circulate in Lao PDR.
In this context, the seroprevalence of antibodies against SARS-CoV-2 is a crucial indicator of virus exposure and can guide prevention measures. Recent studies have demonstrated an heterogeneous anti-SARS-CoV-2 seroprevalence depending on the location [10,[21], [22], [23], [24]] and serological studies can indicate the circulation of SARS-CoV-2 in the absence of detection by routine surveillance [25].
Thus, the aim of this study was to determine the seroprevalence of anti-SARS-CoV-2 antibodies in the general population, healthcare workers, and individuals with close contact to bats/wildlife in Lao PDR, late 2020.
2. Methods
2.1. Participants
Three cross-sectional seroprevalence surveys were conducted in parallel from August to September 2020: a population-based survey and two sub-surveys focusing on two high-risk groups: healthcare workers and guano-collectors, wildlife traders and people in close contacts with bats.
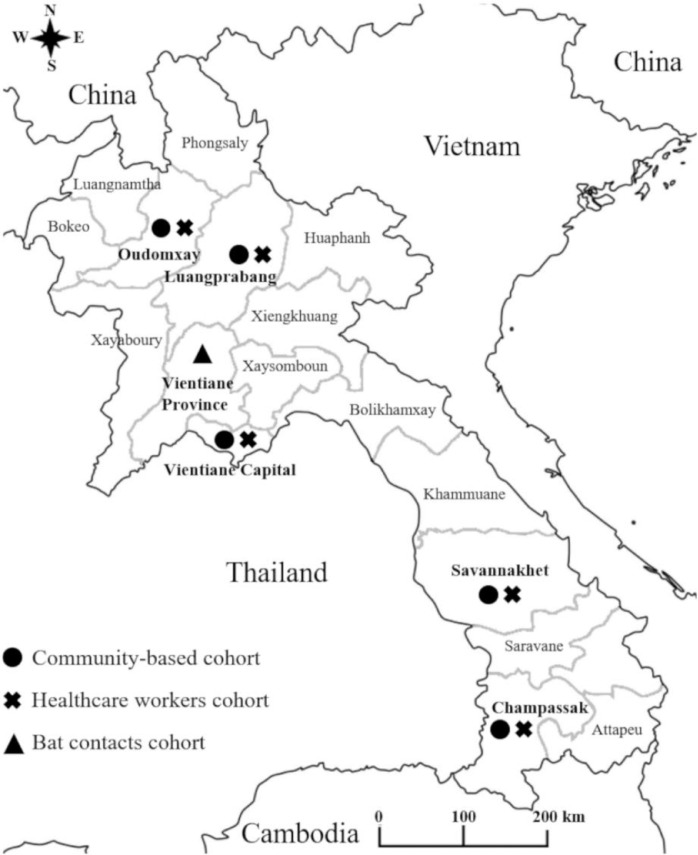
For the population-based survey and the healthcare workers survey, five provinces were selected: Vientiane Capital, two provinces in the North-Luang-Prabang, Oudomxay and two provinces in the South -Savanakhet and Champassak (Figure 1).
Figure 1.
Map of Lao PDR showing study locations.
2.2. Sample size calculation
The study was designed in March 2020, at a time when little was known about the spread of the SARS-CoV-2 in Lao PDR and neighboring countries.
In order to facilitate the recruitment of participants and to maximize the chances of obtaining a representative sample of the age structure of the population, it was decided to use a cluster sampling design in which all members of a selected household were invited to participate. The study was carried out in three “regions”: Vientiane Capital, the North and the South. The sample size was calculated for each region. We considered a prevalence of 1% of SARS-CoV-2-specific antibodies in the general population, with a precision of 1% at a 95% confidence level. Assuming an overall design effect of two to account for the variance inflation due to correlations among residents in the same household and anticipating a 75% response rate, 900 participants were targeted in each region. For the Northern and Southern regions, two provinces were selected in each with a sample size of 450 participants per province.
For the high-risk groups, we aimed to recruit around 900 healthcare workers in the three regions and 100 bat/wildlife contacts in Vientiane Province.
In order to establish the cut-off values and validate the anti-N and anti-S ELISAs, 265 pre-COVID-19 samples were tested. These samples were collected as part of a separate hospital-based serostudy in the South of Lao PDR in 2018 (ethics approval number NECHR018/2017). In addition, serum from 15 confirmed SARS-CoV-2 patients from Lao PDR, collected between four and nine weeks after diagnosis and ten longitudinal samples from one patient (diagnosed in France and living in Lao PDR) were used after consent was obtained.
2.3. Participant recruitment
Participants of the population-based survey were selected using a multistage cluster sampling design to select households in the five provinces. In each province, districts were randomly selected and considered as strata. In each district, villages were then randomly selected using probability-proportional-to-size (PPS) sampling method using official lists of villages based on the population data from the 2015 census [26]. A fixed number of households were then randomly selected in each village and in each household; all inhabitants aged five and above were invited to participate. If a designated village could not be reached due to obstacles such as inaccessibility during the rainy season, the nearest village accessible was selected as an alternative. In Vientiane Capital, four districts were randomly selected, then nine villages were selected using the PPS method and six households were randomly selected. In the four other provinces, three districts were selected per province; six villages and five households of each village were selected (see sample size calculation).
In Vientiane Capital, the survey focusing on healthcare workers was conducted in four central hospitals (three major public referral hospitals and one major military hospital) and in two district hospitals. In the four other provinces, only healthcare workers working in the provincial hospitals were included. All staff assigned to dedicated COVID-19 teams were invited to participate -including clinical and non-clinical staff (e.g administrative staff, drivers, cleaners etc). In central and provincial hospitals, nurses, doctors and lab-technicians working in emergency departments (ER), outpatient departments (OPD), intensive care units (ICU), infectious disease departments, inpatient departments (IPD) and paediatric departments were randomly selected maintaining the nurses to doctors ratio in each department. In the two district hospitals, staff working in the OPD and ER were randomly selected.
Four villages in Feung District, Vientiane province, were visited in September 2020. The villages were selected as they are locations where the profession of bat guano collecting - for selling as fertilizer – is common. Villagers who had contact with bats or other wildlife were informed about the study by the head of their village and invited to participate.
Surveyors visited households after informing local health authorities and obtaining the authorisation from head of each village. Healthcare workers were recruited in their working site. After obtaining both oral and written informed consent, participants answered a series of questions regarding their socio-demographic characteristics, history of symptoms compatible with COVID-19 (e.g. fever, chills, respiratory symptoms, digestive symptoms, anosmia or agueusia), known contact with a confirmed or suspected case of COVID-19 and knowledge and practices regarding COVID-19 prevention measures. Specific questions were asked to healthcare workers about their risk of exposure and utilization of personal protective equipment (PPE). People in close contacts with bats were asked specific questions about their exposure to bats. The answers were recorded directly on tablets using Kobotoolbox® and anonymized data were saved on a secure server at the Institut Pasteur du Laos.
After completion of the questionnaire, 5mL of whole blood was collected into dry collection tubes and allowed to clot. Serum was separated by centrifugation and stored at 4°C for a maximum of seven days until storage at -80°C.
2.4. Antibody detection
SARS-CoV-2-specific anti-nucleocapsid (N) and anti-spike (S) antibodies were detected by ELISA as previously described [27]. 96-well plates (ThermoFisher MaxiSorp 439454) were coated with 1μg/mL of N or S recombinant proteins (Institut Pasteur, Paris) in PBS (Sigma Aldrich, pH 7·4) and incubated overnight at 4°C. Plates were washed three times with 300μL of PBS/Tween (0·1%). Sera diluted 1:200 in PBS/Tween/milk (3%) were incubated in duplicate in their respective wells for 75 minutes at 37°C. After washing with PBS/Tween, the plates were incubated with HRP-labeled anti-human Ig (SouthernBiotech 2010-05) at 1:4000 for 75 minutes at 37°C. After washing, 100μL of chromogenic substrate TMB (Sera Care 5120-0047) was added and incubated for 10 minutes in the dark at room temperature. Finally, 100μL of 1M H3PO4 was added and the absorbance determined at 450nm with the reference at 620nm.
IgM/IgG rapid tests (BioMedomics, Morrisville, USA) were done according to manufacturer's instructions. These tests are reported by the manufacturer to have sensitivity and specificity of 100% and approximately 99%, respectively.
2.5. Calculation of cut-off and testing algorithm
Due to the low expected seroprevalence in Lao PDR, cut-offs of 95% and 99% were calculated against anti-N and S antibodies, respectively using our data from pre-COVID-19 samples. These cut-offs were also confirmed to be appropriate for defining seropositive results in the longitudinal serology from the French patient and, furthermore, are in accordance with previous studies [27,28]. We considered any samples that were positive in the ELISA for both anti-N and anti-S to be SARS-CoV-2 previous infected if they were also rapid test positive.
All samples were tested for anti-N antibodies. Due to reagent limitations, anti-S antibodies were only tested in the following samples; all anti-N antibody positive samples; all bat/wildlife contacts; randomly selected 1061 samples from the general population and 282 samples from the healthcare worker group.
2.6. Statistical analysis
Baseline characteristics of participants were described separately for each group (general population, healthcare workers and people in close contacts with bats/wildlife). The seroprevalence of SARS-CoV-2-specific antibodies was first estimated as crude frequencies of positive tests, by age and sex, as a proportion of the total sample size for the general population. Prevalence in groups and categories were compared using Fisher exact, Pearson chi square or Kruskall-Wallis non-parametric test when appropriate. For the community-based survey, anti-N and anti-S antibody seroprevalence were then weighted to account for the complex survey sample design and for age and sex, using the 2015 population and household census in Lao PDR. Seroprevalence data are expressed as percentage and 95% confidence intervals (CI). All statistical analyses were done with STATA software (version 14) or GraphPad Prism 8 (GraphPad Software, LLC). Given the different selection methods of the three sub-populations, statistical comparison of seroprevalence between them was not indicated.
2.7. Ethics approval
The study protocol was approved by all participating institutes and by the Lao National Ethics Committee for Health Research (NECHR) (Ref #052/2020). Oral and written informed consent was obtained from people aged 15 years and older. Parental consent was taken for children aged between 5 and 11 years, and assent from children aged between 12 and 14 years in addition to parental consent, before the survey [29]. Given the uncertainties concerning the duration of the detection of SARS-CoV-2 antibodies and the correlates of protection, it was decided that individual results of the antibody testing would not be given to participants to avoid misinterpretation. The full study proposal can be found at https://www.pasteur.la/static/COVID-19serostudyinLaoPDREnglishProposal.docx.
Role of the funding source The main funding was received from Agence Française de Développement (AFD), with additional support from Institut Pasteur du Laos, Institute Pasteur, Paris and Luxembourg Ministry of Foreign and European Affairs (“PaReCIDS II” grant).
The funders had no role in the study design, implementation or interpretation.
3. Results
3.1. Characteristics of participants
A total of 3173 participants were recruited between the 12th of August and the 25th of September 2020. This consisted of participants from the general population (n=2433; mean age 42·6 years (range 5-55) and healthcare workers (n=666; mean age 36·8 years (range 20-65)) from two Northern, one Central and two Southern provinces (Table 1 and Figure 1). Seventy-four serum samples were collected from bat/wildlife contacts; 12 bat guano collectors, five wildlife animal vendors and 57 bat contacts, catchers, or bat guano collectors' families in Vientiane Province (mean age 43·5 years (range 17-86)).
Table 1.
Characteristics of study participants
| Pre-COVID-19 n (%) | Community-based n (%) | Healthcare workers n (%) | Bat/wildlife contacts n (%) | Total n (%) | |
|---|---|---|---|---|---|
| Age | |||||
| ≤ 18 years | 119 (44·9) | 233 (9·6) | - | 5 (6·8) | 357 (10·4) |
| 19-40 years | 80 (30·2) | 849 (34·9) | 448 (67·3) | 30 (40·5) | 1407 (40·9) |
| 41-60 years | 49 (18·5) | 1000 (40·1) | 217 (32·6) | 28 (37·8) | 1294 (37·6) |
| > 60 years | 17 (6·4) | 351 (14·4) | 1 (0·1) | 11 (14·9) | 380 (11·1) |
| Sex | |||||
| Female | 129 (48·7) | 1414 (58·1) | 518 (77·8) | 39 (52·7) | 2100 (61·1) |
| Male | 136 (51·3) | 1019 (41·9) | 148 (22·2) | 35 (47·3) | 1338 (38·9) |
| Province | |||||
| Vientiane Capital | - | 746 (30·7) | 410 (61·6) | - | 1156 (33·6) |
| Luangprabang | - | 465 (19·1) | 47 (9·2) | - | 512 (14·9) |
| Oudomxay | - | 396 (16·3) | 31 (4·6) | - | 427 (12·4) |
| Savanakhet | - | 412 (16·9) | 106 (15·9) | - | 518 (15·1) |
| Champassak | - | 414 (17·0) | 72 (10·8) | - | 486 (14·1) |
| Vientiane Province | - | - | - | 74 (100) | 74 (2·2) |
| Saravan Province | 265 | - | - | - | 265 (7·7) |
| Travel outside of Lao PDR since December 2019?* | - | 101 (4·1) | 42 (6·3) | 4 (5·4) | 147 (4·3) |
| Thailand | - | 87 (3·6) | 29 (4·4) | 3 (4·1) | 119 (3·5) |
| China/Vietnam/Korea | - | 11 (0·5) | 12 (1·8) | 1 (1·4) | 24 (0·7) |
| Total | 265 | 2433 | 666 | 74 |
Missing country of travel for 4 participants
1593 individuals were unavailable for the study due to reasons such as “working” or “at school” and were therefore replaced by other randomized participants from the same location. Only two healthcare workers and six people from the community-based cohort refused to participate in the study due to fear of needles and unwillingness to answer questions.
3.2. Anti-N and anti-S antibody serology
3.2.1. Calculation of cut-offs and assay validation
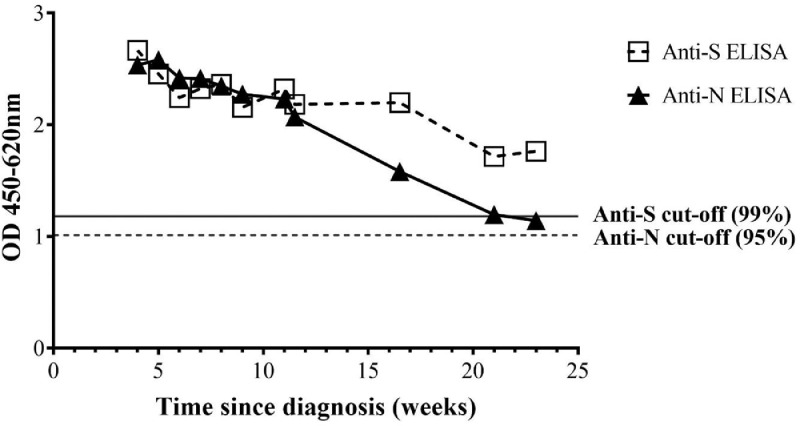
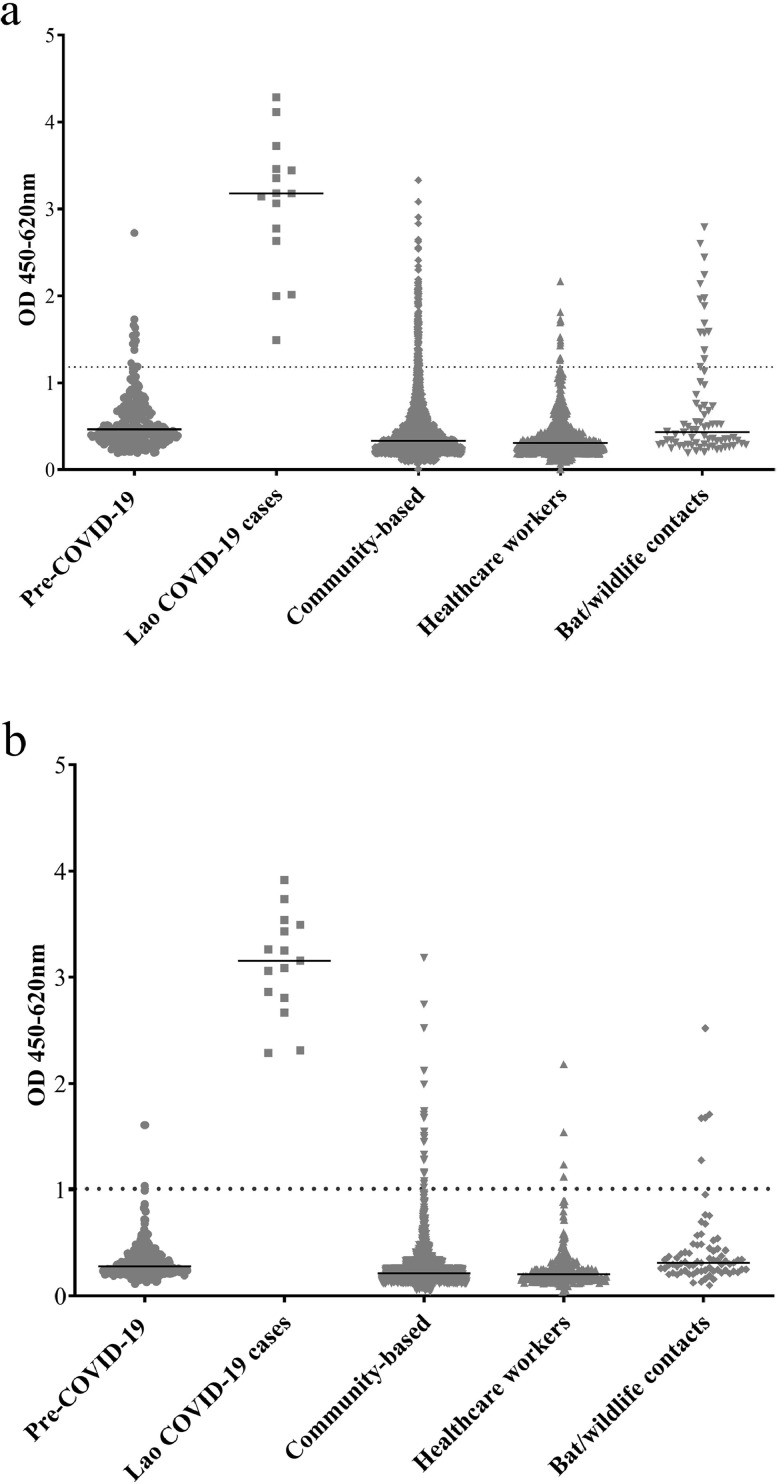
Cut-offs of 95% and 99% were set for defining anti-N and S antibody seropositivity, using pre-COVID-19 samples taken from individuals in the South of Lao PDR in 2018. Thus, 14/265 (5·3% [3·1-8·7%]) pre-COVID-19 samples were positive for anti-N antibodies. Three pre-COVID-19 samples were positive for anti-S antibodies (1·1% [0·3-3·5%]). No pre-COVID-19 samples were double-positive for anti-N and anti-S antibodies and 3 double-negative samples (1·1%) were anti-SARS-CoV-2 IgM positive (1·1%) and one IgG positive (0·4%) by rapid test. Longitudinal samples from the French confirmed case with mild symptoms showed double-positive N and S antibody seropositivity and were rapid test positive until at least 21 weeks post diagnosis despite a decline in ELISA OD (Figure 2). Samples from confirmed COVID-19 cases identified by the national COVID-19 surveillance in Lao PDR (n=15) were all double-positive for anti N- and S-antibodies and rapid test positive (Figure 3 and Supplementary Figure 1).
Figure 2.
Longitudinal anti-N and anti-S antibody serology. Data represent mean of duplicate results from a COVID-19 positive individual (diagnosed in France, end of August 2020). 11 samples were obtained from the end of September 2020 until early February 2021.
Figure 3.
a. Anti-N antibodies b. Anti-S antibodies. Data show average of duplicate OD values for each sample. The dotted line represents the OD of 95% cut-off for anti-N antibodies and 99% cut-off for anti-S antibodies, established from the pre-COVID-19 samples. The solid lines represent the median OD.
3.3. Participant serology data
In the participant samples from 2020, anti-N antibodies were detected in only 131/2433 (5·2% [4·2-6·5%]) of the general population, 13/666 (2·0% [1·3-3·3%]) of the healthcare workers but 15/74 (20·3% [12·6-31·0%]) of the bat/wildlife contacts (Figure 3 and Supplementary Figure 1).
Only 3/159 (1·9% [0·6-5·7%]) of the anti-N antibody positive samples were positive by rapid test (one IgM single positive and two IgM/IgG double positive). These samples were all anti-S antibody negative and therefore they were not considered as true “COVID-19 cases”.
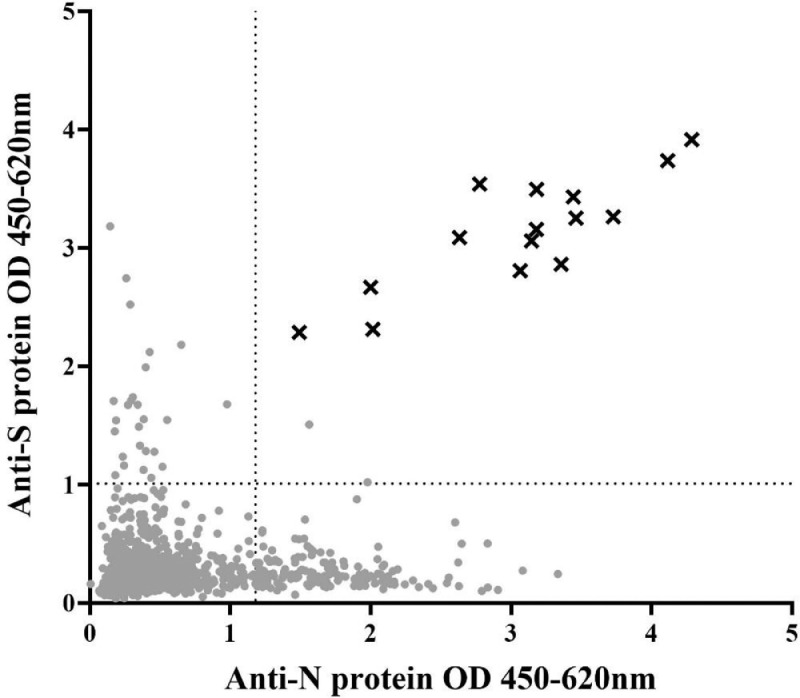
Due to limited reagents, anti-S antibody ELISAs were done on a smaller selection of participants (n=1417), including all anti-N antibody positive participants and all bat/wildlife contacts plus a randomly selected sample from the general population and healthcare workers. Anti-S antibodies were detected in only 19/1061 (2·1% [1·1-3·9%]) of the general population, 4/282 (1·4% [0·3-3·7%]) of the healthcare workers and 5/74 (6·8% [2·8-15·3%]) of the bat/wildlife contacts. Only two out of all 2020 participant samples (2/3173; 0·1%) were double-positive for both anti-N and anti-S antibodies (Figure 4). These two samples were from the general population and were negative by anti-SARS-CoV-2 IgM/IgG rapid test and therefore also considered unlikely to be true “COVID-19 cases”.
Figure 4.
Relationship between anti-N and anti-S antibody amounts. Points show mean OD of individual samples from the general population, healthcare workers, and bat/wildlife contacts. Crosses represent mean OD of 15 confirmed SARS-CoV-2 cases, identified by the Lao national surveillance. Dotted lines represent 95% cut-off value for anti-N antibodies and 99% cut-off for anti-S antibodies.
Only one of the anti-S antibody positive samples from the 2020 participants was rapid test positive (IgG). This participant was a bat/wildlife contact (anti-N antibody negative) aged 74 years. He had worked previously as a guano collector in caves. Two members of his family tested negative for anti-N and anti-S antibodies although his daughter had travelled to Thailand five months previously.
Within the general population, anti-N antibody positive status increased with age from 4·2% before 18 years to 9·3% above 60 years (p<0·0001, using score test for trend of odds). No other parameters were associated with anti-N or S antibody status in any cohort (Tables 2 and 3 and Supplementary Table 1).
Table 2.
N-protein seroprevalence and associated variables
| Cohort |
Community-based |
Healthcare workers |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample tested, n | S-ELISA positive,n | Crude seroprevalence %,95%CI | Weighted1seroprevalence %, 95%CI | OR | Sample tested, n | S-ELISA positive,n | Crude seroprevalence %,95%CI | OR | |
| Total | 1061 | 19 | 1·8 (1.1-2.8) | 2·1 (1.1-3.9) | 282 | 4 | 1·4 (0.5-3.7) | ||
| Age | |||||||||
| ≤ 18 years | 95 | 0 | 0 | - | - | ||||
| 19-40 years | 382 | 6 | 1·6 (0·7-3·4) | 1·9 (0·7-4·7) | - | 181 | 3 | 1·7 (0·5-5·0) | NA |
| 41-60 years | 435 | 8 | 1·8 (0·9-3·6) | 1·5 (0·7-3·4) | 1·5 (0·5-4·3)x | 101 | 1 | 1·0 (0·1-6·8) | |
| > 60 years | 149 | 5 | 3·4 (1·4-7·8) | 4·5 (1·8-10·8) | 2·7 (0·8-9·1)x | 0 | 0 | ||
| Sex | |||||||||
| Female | 601 | 10 | 1·7 (0·9-3·0) | 1·5 (0·7-3·3) | - | 209 | 4 | 1·9 (0·7-5·0) | NA |
| Male | 460 | 9 | 2·0 (1·0-3·7) | 2·6 (1·2-5·9) | 1·2 (0·5-2·9) | 73 | 0 | ||
| Travel history | |||||||||
| Yes | 32 | 0 | - | - | NA | 17 | 1 | 5·9 (0·8-33·6) | - |
| No | 1029 | 19 | 1·8 (1·1-2·8) | 2·2 (1·2-4·0) | 265 | 3 | 1·1 (0·4-2·5) | 0·2 (0·02-1·9) | |
| Province | |||||||||
| Vientiane Capital | 318 | 3 | 1·0 (0·3-2·9) | 1·3 (0·3-5·6) | - | 176 | 2 | 1·1 (0·3-4·5) | NA |
| Luangprabang | 196 | 4 | 2·0 (0·7-5·2) | 2·4 (0·6-8·7) | 2·1 (0·5-9·7) | 23 | 1 | 4·3 (0·6-26·2) | |
| Oudomxay | 170 | 4 | 2·3 (0·9-6·0) | 1·9 (0·6-6·0) | 2·5 (0·5-11·2) | 12 | 0 | ||
| Savanakhet | 181 | 4 | 2·2 (0·8-5·6) | 2·7 (0·8-8·8) | 2·3 (0·5-10·5) | 31 | 0 | ||
| Champassak | 180 | 4 | 2·2 (0·8-5·6) | 3·0 (1·0-8·8) | 2·3 (0·5-10·5) | 40 | 1 | 2·5 (0·3-16·2) | |
| Vientiane Province | - | - | |||||||
Weighted by age, sex and complex sample design
OR was calculated after creating new age-groups (≤ 40; 41-60; >60)
Table 3.
S-protein seroprevalence and associated variables
| Cohort |
Community-based |
Healthcare workers |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample size, n | N-ELISA positive, n | Crude seroprevalence%,95%CI | Weighted1seroprevalence %, 95%CI | OR | Sample size, n | N-ELISA positive, n | Crude seroprevalence%,95%CI | OR | |
| Total | 2433 | 131 | 5·4 (4·5-6·3) | 5·2 (4·2-6·5) | 666 | 13 | 1·9 (1·1-3·3) | ||
| Age | |||||||||
| ≤ 18 years | 233 | 9 | 3·9 (2·0-7·3) | 4·2 (2·0-8·4) | - | - | |||
| 19-40 years | 849 | 31 | 3·6 (2·6-5·1) | 3·6 (2·4-5·4) | 0·9 (0·4-2·0)* | 448 | 10 | 2·2 (1·2-4·1) | NA |
| 41-60 years | 1 | 60 | 6·0 (4·7-7·6) | 6·6 (5·0-8·6) | 1·6 (0·8-3·2)* | 217 | 3 | 1·4 (0·4-4·2) | |
| > 60 years | 351 | 31 | 8·8 (6·2-12·3) | 9·3 (6·4-13·4) | 2·4 (1·1-5·2)* | 1 | 0 | - | |
| Sex | |||||||||
| Female | 1414 | 69 | 4·9 (3·9-6·1) | 4·6 (3·4-6·2) | - | 518 | 8 | 1·5 (0·8-3·1) | 2·2(0·7-6·9) |
| Male | 1019 | 62 | 6·1 (4·8-7·3) | 5·8 (4·2-7·9) | 1·2 (0·9-1·8) | 148 | 5 | 3·4 (1·4-7·9) | |
| Travel history | |||||||||
| No | 2332 | 128 | 5·4 (4·6-6·4) | 5·5 (4·4-6·9) | - | 624 | 13 | 2·1 (1·2-3·6) | NA |
| Yes | 101 | 3 | 3·0 (0·9-8·8) | 1·2 (0·3-4·2) | 0·5 (0·2-1·7) | 42 | 0 | - | |
| Province | |||||||||
| Vientiane Capital | 746 | 36 | 4·8 (3·5-6·6) | 4·9 (3·3-7·2) | - | 410 | 10 | 2·4 (1·3-4·5) | NA |
| Luangprabang | 465 | 25 | 5·4 (3·6-7·8) | 6·2 (3·7-10·2) | 1·1 (0·6-1·9) | 47 | 0 | - | |
| Oudomxay | 396 | 21 | 5·3 (3·5-8·0) | 5·5 (3·2-9·2) | 1·1 (0·6-1·9) | 31 | 0 | - | |
| Savanakhet | 412 | 27 | 6·6 (4·5-9·3) | 5·7 (3·4-9·4) | 1·1 (0·6-1·9) | 106 | 2 | 2·8 (0·7-10·5) | |
| Champassak | 414 | 22 | 5·3 (3·5-7·9) | 4·8 (2·9-8·0) | 1·4 (0·8-2·3) | 72 | 1 | 0·9 (0·1-6·4) | |
| Vientiane Province | - | - | - | - | |||||
weighted by age, sex and complex survey design
p>0.0001 score test for trend of odds
4. Discussion
In this study, performed in Lao PDR from August until September 2020, the seroprevalence of anti-SARS-CoV-2 antibodies in the general population and healthcare workers was extremely low. These data likely reflect the low incidence of COVID-19 within the country and are in agreement with the few confirmed cases in 2020 and low reported rates of hospitalisation of patients with respiratory illnesses in Lao PDR [7]. Low seroprevalence in the general population in Lao PDR, 2020 is similar to studies from other countries in southeast Asia where COVID-19 cases were low [30] and in contrast to high incidence countries [31]. Healthcare workers are particularly at risk of infection and our data showing low seroprevalence are again in agreement with studies from countries with low reported COVID-19 cases [32], [33], [34], and in contrast to higher seroprevalence in countries with higher incidence of confirmed COVID-19 cases, including in East Asia [35].
Why has Lao PDR remained largely free of COVID-19, in contrast to many other countries? The answer is likely a combination of several factors. Firstly, the Government of Lao PDR implemented a robust control of visitors from outside the country, starting in March 2020. This included greatly restricting the number of commercial flights, and the requirement of all visitors to have proof of a negative SARS-CoV-2 RT-PCR test within the previous 72 hours, a negative test upon arrival, and to undertake a 14-day quarantine upon arrival. Even before such measures, Lao PDR had a relatively small number of international arrivals and, additionally, the land border point-of-entries are mostly connected to low-density areas of neighbouring countries. In addition, the rapid and decisive response of the Government of Lao PDR and other countries in the Lower Mekong region perhaps reflects a greater preparedness for pandemic outbreaks, born out of experience with SARS-CoV-1 in 2002-2003 and several avian influenza outbreaks in recent years [36,37].
An association between low population density and reduced COVID-19 incidence has been shown in some studies but not others [38], [39], [40]. Thus, even though the population is not evenly distributed throughout the country, the low population density in Lao PDR -approximately 32 people per km2 [41] - may have played some role in limiting the spread of COVID-19 to date. In addition, as younger people are more likely to be asymptomatic, the young age structure of the population may have reduced the number of symptomatic cases and disease-associated mortality [12]. The lack of a mass-transit system could be another factor.
We cannot discount that some cultural or societal factors played a role. Physical contact may be lower in Lao PDR, for example, shaking hands is not common and hand-holding in public is rare, while wearing of face-masks is often practiced. Lastly, it has been speculated that climate may have some role in the spread of SAR-CoV-2, with spread being limited in warmer and more humid environments [10]. However, this is unlikely to have made a significant impact in Lao PDR, given the high rates of infection in countries with similar climates.
N and S proteins from SARS-CoV-2 have been shown to be highly immunogenic, with anti-S antibodies being more specific [28,[42], [43], [44]]. In our study, pre-COVID-19 controls from 2018 had anti-N and anti-S antibody seroprevalence of 5·3% and 1·1%, respectively, with none seropositive for both although three (1·1%) were positive by rapid test. Seroprevalence studies in France observed similar pre-COVID-19 levels of anti-SARS-CoV-2 [42,44], likely representing cross-reaction with non-SARS-CoV-2 coronaviruses. In the 2020 study participants, we found that there were only 159/3173 (5·0%) seropositive for anti-N antibodies and only 28/1417 (2·0%) were seropositive for anti-S antibodies. Only two participants (0·1%) were double-seropositive for anti-S and -N antibodies. These participants were negative by IgM/IgG rapid test and likely represent false positives as the prevalence was similar to the pre-COVID-19 controls. Some of the “single-positives” may reflect true cross-reactivity with other unknown or seasonal alpha- and beta- coronaviruses such as HKU1, NL63, OC43 and 229E [23,44]. Notably, it is thought that most pre-existing cross-reactive antibodies do not confer protection against SARS-CoV-2 [43]. Although a prior infection with SARS-CoV-1, the causative agent of SARS, may confer cross-reactivity to SARS-CoV-2 due to neutralizing activity of antibodies against the highly homologous Receptor Binding Domain [43,44], such instances are probably insignificant in Lao PDR [45]. It is nevertheless possible that exposure to other coronaviruses occurs in Lao PDR given the frequent trade in bush meat and low biosafety awareness [46]. Indeed, it has been shown that bats and rodents from Lao PDR and bordering countries can be infected with a diverse array of alpha- and beta- coronaviruses including SARS-CoV-2–like viruses [47], [48], [49], [50], [51]. The participants in our study who had frequent contact with bats or other wildlife had high anti-N antibody seroprevalence. Also, one bat/wildlife contact whose daughter had been to Thailand five months previously was anti-S antibody positive, anti-N antibody negative and rapid test (IgG) positive. This could be due to exposure to coronaviruses from wildlife although we have no control group from the same location, and anti-S antibodies are thought to be less cross-reactive than anti-N antibodies. Neutralisation assays were not performed on these samples and it remains possible that there was a localized COVID-19 outbreak in this population. Therefore, in addition to coronavirus detection and typing among the bats and other wildlife populations, these particular populations deserve further studies.
Anti-SARS-CoV-2 antibodies in mild cases are thought to be lost rapidly – up to 20% of such cases in neighbouring Thailand were shown to be seronegative within two weeks after onset of symptoms [52]. It is therefore possible that some of the participants in the current study were infected with SARS-CoV-2 and did not seroconvert or had lost antibodies by the time of the study. However, similar to other studies [28,44], longitudinal samples from one patient who was infected in late August 2020 and presented only mild symptoms were seropositive for anti-N antibody until at least week 21 after diagnosis and longer for anti-S antibodies. Furthermore, the participant recruitment was done in August and September, 2020- only 4-5 months after the first COVID-19 case was identified in Lao PDR. Therefore, we believe that the majority of COVID-19 infected individuals would still have detectable anti-SARS-CoV-2 antibodies.
The main limitation of this study was that only five of the 18 Lao provinces were purposively selected. However, these provinces were selected due to a perceived high risk of SARS-CoV-2 introduction (international flight arrivals into Luang Prabang and Vientiane Capital, large numbers of Chinese migrant workers in Oudomxay, and migrant workers returning to Lao PDR from Thailand in Champassack and Savannakhet). Nevertheless, it is possible that we missed areas where SARS-CoV-2 virus entered e.g. from neighbouring countries in the North. Therefore, our data should not be extrapolated nationwide. Other limitations included the non-representative nature of the age structure of the sampled population, although we controlled for this by weighting the results according to the Lao census data. Lastly, clarification regarding the cross-reactive nature of the few anti-N and anti-S antibodies detected in participants and pre-COVID-19 samples could be determined by neutralisation assays, which were not available to us at the time of the study. Despite these caveats, we believe that it is highly unlikely that the results would change in any meaningful way given the sample size and the three different tests used.
In summary, our data indicate that Lao PDR remained largely free of COVID-19, at least until late 2020. The Government of Lao PDR should be commended on the swift and decisive action taken to reduce the risk of SARS-CoV-2 introduction into the country. Nevertheless, Lao PDR remains at risk of virus introduction, either from neighboring countries or further afield. The borders cannot remain closed indefinitely and therefore other measures need to be strengthened such as active surveillance, vaccination of at-risk groups and vaccination status checks at points of entry. This was underlined by the importation of COVID-19 from Thailand following illegal entrants, resulting in an increase in cases in May 2021. With the rapid development and opening up of the country, such as the imminent launch of the high-speed rail system connecting China, Lao PDR and Thailand, this is as pertinent as ever. Lastly, the high seroprevalence of anti-N antibodies in bat/wildlife contacts in Lao PDR is a stark reminder that the threat of emergence of future pandemics is real and that steps need to be taken to mitigate and prepare for them.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgments
Acknowledgments
We would like to thank the study participants and the students of the Lao Tropical Public Health Institute and Thidachanh Soukhaserm, Namfon Mongkhonsinh, for their help with participant recruitment. Latdavone Khenkha, Bounta Vongphachanh and Nouna Innoula provided technical assistance in the laboratory. The authors thank Marion Gransagne (Global Health Department, Institut Pasteur), Stéphane Petres and staff of the Production and Purification of Recombinant Proteins Technological Platform, Institute Pasteur, Paris, for their invaluable help in N and S antigen design and production. Rapid tests were a donation from the European Union.
Author contributions
SV coordinated the participant recruitment and laboratory work, wrote the manuscript and verified the underlying data, VP formulated the study design, data analysis plan, wrote the manuscript and verified the underlying data, EC helped with study design and manuscript writing, VK helped coordinate participant recruitment and laboratory analysis, SS, SK, MM and PX helped with study design and manuscript formulation, ST, ME, NE and TR made the recombinant proteins, helped in data analysis and writing the manuscript, KV helped with participant recruitment and study design, JH, VL, SS and DP helped with the study design, data interpretation and writing the manuscript; PTB and APB designed and coordinated the study, interpreted the data and wrote the manuscript.
Data sharing
Anonymous participant data will be available upon request to the corresponding author. Requests will be reviewed and approved by the researcher, and staff on the basis of scientific merit and absence of competing interests. Once the request is approved, data can be transferred after the signing of a data access agreement and a confidentiality agreement.
Footnotes
Editor note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100197.
Appendix. Supplementary materials
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Coronavirus disease (COVID-19) pandemic [Internet]. 2021.
- 3.World Health Organization. Regional Office for the Western Pacific. Overview of Lao health system development 2009-2017. 2018;
- 4.World Health Organization (WHO). World Health Organization. The COVID-19 situation reports for Lao PDR [Internet]. [Internet].2021 Available from: www.who.int/laos.
- 5.World Health Organization. World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance (WHO/2019-nCoV/laboratory/2020.2) [Internet]. 2020 Available from: https://apps.who.int/iris/handle/10665/332300?locale-attribute=fr&.
- 6.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA - J Am Med Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 7.Lao Ministry of Health COVID-19 website [Internet]. 2021 Available from: https://www.covid19.gov.la/index.php.
- 8.Lim JT, Dickens BSL, Choo ELW, Chew LZX, Koo JRH, Tam C. Revealing regional disparities in the transmission potential of SARS-CoV-2 from interventions in Southeast Asia: SARS-CoV-2 regional disparities. Proc R Soc B Biol Sci. 2020;287(1933) doi: 10.1098/rspb.2020.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lao Statistics Bureau website [Internet]. 2021 Available from: https://www.lsb.gov.la/.
- 10.Rostami A, Sepidarkish M, Leeflang MMG, Riahi SM, Nourollahpour Shiadeh M, Esfandyari S. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect [Internet] 2020 doi: 10.1016/j.cmi.2020.10.020. Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China. Jama. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Davies NG, Klepac P, Liu Y, Prem K, Jit M, Pearson CAB. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 13.Nouanthong P, Hübschen JM, Billamay S, Mongkhoune S, Vilivong K, Khounvisith V. Varicella zoster and fever rash surveillance in Lao People's Democratic Republic. BMC Infect Dis. 2019;19(1):1–6. doi: 10.1186/s12879-019-3990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jespersen S, Mikkelsen S, Greve T, Kaspersen KA, Tolstrup M, Boldsen JK. Severe Acute Respiratory Syndrome Coronavirus 2 Seroprevalence Survey Among 17 971 Healthcare and Administrative Personnel at Hospitals, Prehospital Services, and Specialist Practitioners in the Central Denmark Region. Clin Infect Dis. 2020:1–8. doi: 10.1093/cid/ciaa1471. (Xx Xxxx) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant J, Wilmore S, McCann N, Donnelly O, Lai R, Kinsella M. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol. 2020;(June) doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebeiro P, Levinson K, Jolly L, Kassens E, Dizikes G, Steece R. Prevalence of IgG antibodies against the severe acute respiratory syndrome 2 coronavirus-2 among healthcare workers in Tennessee during May and June, 2020. medRxiv. 2020;(November) [Google Scholar]
- 17.Masuet-Aumatell C, Ramon-Torrell JM, Casanova-Rituerto A, Banqué-Navarro M, Dávalos-Gamboa M del R, Montaño-Rodríguez SL. Seroprevalence of varicella-zoster virus infection in children from Cochabamba: Tropical or temperate pattern? Trop Med Int Heal. 2013;18(3):296–302. doi: 10.1111/tmi.12040. [DOI] [PubMed] [Google Scholar]
- 18.Korth J, Wilde B, Dol S, Anastasiou OE, Krawczyk A, Jahn M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;(May) doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge XY, Li JL, Lou Yang X, Chmura AA, Zhu G, Epstein JH. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature [Internet] 2013;503(7477):535–538. doi: 10.1038/nature12711. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckerle I, Meyer B. SARS-CoV-2 seroprevalence in COVID-19 hotspots. Lancet [Internet] 2020;396(10250):514–515. doi: 10.1016/S0140-6736(20)31482-3. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X, Sun J, Nie S, Li H, Kong Y, Liang M. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med [Internet] 2020;26(8):1193–1195. doi: 10.1038/s41591-020-0949-6. Available from: [DOI] [PubMed] [Google Scholar]
- 23.Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun [Internet] 2020;11(1):1–16. doi: 10.1038/s41467-020-18450-4. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koopmans M, Haagmans B. Assessing the extent of SARS-CoV-2 circulation through serological studies. Nat Med [Internet] 2020;26(8):1171–1172. doi: 10.1038/s41591-020-1018-x. Available from: [DOI] [PubMed] [Google Scholar]
- 25.Basavaraju S V, Patton ME, Grimm K, Rasheed MAU, Lester S, Mills L. Serologic testing of U.S. blood donations to identify SARS-CoV-2-reactive antibodies: December 2019-January 2020 Sridhar. Clin Infect Dis. 2020;(November) doi: 10.1093/cid/ciaa1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lao Statistics Bureau . Lao statistics Bureau; 2016. The 4th population and housing census 2015 [Internet]http://lao.unfpa.org/sites/default/files/pub-pdf/PHC-ENG-FNAL-WEB_0.pdf%0Ahttp://www.lsb.gov.la/lsb/pdf/PHC-ENG-FNAL-WEB.pdf Available from: [Google Scholar]
- 27.Grzelak L, Temmam S, Planchais C, Demeret C, Tondeur L, Huon C. A comparison of four serological assays for detecting anti–SARS-CoV-2 antibodies in human serum samples from different populations. Sci Transl Med. 2020;12(559) doi: 10.1126/scitranslmed.abc3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenwick C, Croxatto A, Coste AT, Pojer F, André C, Pellaton C. Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population- Based Seroprevalence Studies. J Virol. 2021;95(3):1–12. doi: 10.1128/JVI.01828-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lao People's Revolutionary Youth Union, Fund UNP . UNPFA, Lao PDR.; 2014. Adolescent and Youth Situation Analysis, Lao People's Democratic Republic. [Internet]https://lao.unfpa.org/en/publications/adolescent-and-youth-situation-analysis Available from: [Google Scholar]
- 30.Chen X, Chen Z, Azman AS, Deng X, Sun R, Zhao Z. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob Heal [Internet] 2021;9(5):e598–e609. doi: 10.1016/S2214-109X(21)00026-7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Megasari NLA, Utsumi T, Yamani LN, Juniastuti Gunawan E, Furukawa K. Seroepidemiological study of SARS-CoV-2 infection in East Java, Indonesia. PLoS One [Internet] 2021;16(5) doi: 10.1371/journal.pone.0251234. http://www.ncbi.nlm.nih.gov/pubmed/33956869 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nopsopon T, Pongpirul K, Chotirosniramit K, Jakaew W, Kaewwijit C, Kanchana S. Seroprevalence of hospital staff in a province with zero COVID-19 cases. PLoS One [Internet]. 2021;16(4) doi: 10.1371/journal.pone.0238088. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda H, Seyama K, Ito K, Ai T, Nojiri S, Hori S, et al. SARS-CoV-2 seroprevalence in healthcare workers at a frontline hospital in Tokyo. Sci Rep [Internet]. 2021;11(1):1–7. Available from: https://doi.org/ 10.1038/s41598-021-87688-9 [DOI] [PMC free article] [PubMed]
- 34.Tanaka A, Yamamoto S, Miyo K, Mizoue T, Maeda K, Sugiura W. Seroprevalence of antibodies against SARS-CoV-2 in a large national hospital and affiliated facility in Tokyo. Japan. J Infect. 2021;82(4):e1–e3. doi: 10.1016/j.jinf.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hossain A, Nasrullah SM, Tasnim Z, Hasan MK, Hasan MM. Seroprevalence of SARS-CoV-2 IgG antibodies among health care workers prior to vaccine administration in Europe, the USA and East Asia: A systematic review and meta-analysis. EClinicalMedicine. 2021:33. doi: 10.1016/j.eclinm.2021.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phommasack B, Moen A, Vongphrachanh P, Tsuyuoka R, Cox N, Khamphaphongphanh B. Capacity building in response to pandemic influenza threats: Lao PDR case study. Am J Trop Med Hyg. 2012;87(6):965–971. doi: 10.4269/ajtmh.2012.12-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corwin A, Plipat T, Phetsouvanh R, Mayxay M, Xangsayarath P, Quynh Mai LT. The Impact of Preparedness in Defying COVID-19 Pandemic Expectations in the Lower Mekong Region: A Case Study. Am J Trop Med Hyg [Internet] 2021:1–7. doi: 10.4269/ajtmh.20-1499. http://www.ncbi.nlm.nih.gov/pubmed/33534744 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamidi S, Sabouri S, Ewing R. Does Density Aggravate the COVID-19 Pandemic?: Early Findings and Lessons for Planners. J Am Plan Assoc [Internet] 2020;86(4):495–509. doi: 10.1080/01944363.2020.1777891. Available from: https://doi.org/ [DOI] [Google Scholar]
- 39.Bhadra A, Mukherjee A, Sarkar K. Impact of population density on Covid-19 infected and mortality rate in India. Model Earth Syst Environ [Internet] 2020;(0123456789) doi: 10.1007/s40808-020-00984-7. Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pequeno P, Mendel B, Rosa C, Bosholn M, Souza JL, Baccaro F. Air transportation, population density and temperature predict the spread of COVID-19 in Brazil. PeerJ. 2020;2020(6):1–15. doi: 10.7717/peerj.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worldometers.info [Internet]. 2021 [cited 2021 Feb 1]. Available from: https://www.worldometers.info/world-population/laos-population/
- 42.Grzelak L, Temmam S, Planchais C, Demeret C, Huon C, Guivel F. SARS-CoV-2 serological analysis of COVID-19 hospitalized patients, pauci-symptomatic individuals and blood donors. medRxiv. 2020 [Google Scholar]
- 43.Miyara M, Sterlin D, Anna F, Marot S, Mathian A, Atif M. Pre-COVID-19 humoral immunity to common coronaviruses does not confer cross- protection against SARS-CoV-2. medRxiv. 2020;(August) [Google Scholar]
- 44.Anna F, Goyard S, Lalanne AI, Nevo F, Gransagne M, Souque P. High seroprevalence but short-lived immune response to SARS-CoV-2 infection in Paris. Eur J Immunol. 2021;51(1):180–190. doi: 10.1002/eji.202049058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization (WHO). Cumulative Number of Reported Probable Cases of SARS [Internet]. Available from: https://www.who.int/csr/sars/country/2003_07_11/en/
- 46.Philavong C, Pruvot M, Reinharz D, Mayxay M, Khammavong K, Milavong P. Perception of health risks in Lao market vendors. Zoonoses Public Health. 2020;67(7):796–804. doi: 10.1111/zph.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIver DJ, Silithammavong S, Theppangna W, Gillis A, Douangngeun B, Khammavong K. Coronavirus surveillance of wildlife in the Lao People's Democratic Republic detects viral RNA in rodents. Arch Virol [Internet] 2020;165(8):1869–1875. doi: 10.1007/s00705-020-04683-7. Available from: https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacroix A, Duong V, Hul V, San S, Davun H, Omaliss K. Genetic diversity of coronaviruses in bats in Lao PDR and Cambodia. Infect Genet Evol. 2017;48:10–18. doi: 10.1016/j.meegid.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hul V, Delaune D, Karlsson EA, Hassanin A, Ou Tey P, Baidaliuk A. A novel SARS-CoV-2 related coronavirus in bats from Cambodia. bioRxiv [Internet] 2021 doi: 10.1038/s41467-021-26809-4. https://www.biorxiv.org/content/10.1101/2021.01.26.428212v1 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anthony SJ, Johnson CK, Greig DJ, Kramer S, Che X, Wells H. Global patterns in coronavirus diversity. Virus Evol. 2017;3(1):1–15. doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wacharapluesadee S, Tan CW, Maneeorn P, Duengkae P, Zhu F, Joyjinda Y. Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nat Commun [Internet] 2021;12(1) doi: 10.1038/s41467-021-21240-1. http://www.ncbi.nlm.nih.gov/pubmed/33563978 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kowitdamrong E, Puthanakit T, Jantarabenjakul W, Prompetchara E, Suchartlikitwong P, Putcharoen O. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS One [Internet] 2020;15(10 October):1–11. doi: 10.1371/journal.pone.0240502. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.