Abstract
A concurrent human papilloma virus (HPV) infection potentiates the efficacy of ionizing radiation for treatment of head and neck cancer by promoting apoptosis. Studies in cell culture indicated an opposite effect for photodynamic therapy (PDT) when this leads to mitochondrial and ER photodamage. The explanation for this difference in PDT efficacy remains to be established. While apoptosis was impaired in HPV(−) cells, such cells can be killed via photodamage directed at the ER: this leads to a non-apoptotic death pathway termed paraptosis. No differences in photosensitizer uptake or reactive oxygen species (ROS) production were observed in HPV(+) vs. HPV(−) tumors. We now provide evidence that death pathways initiated by ER/mitochondrial photodamage leading to either paraptosis or apoptosis are impaired in an HPV(+) head and neck cell line. These results illustrate the complex determinants of PDT efficacy, a topic that has yet to be fully explored.
Graphical Abstract
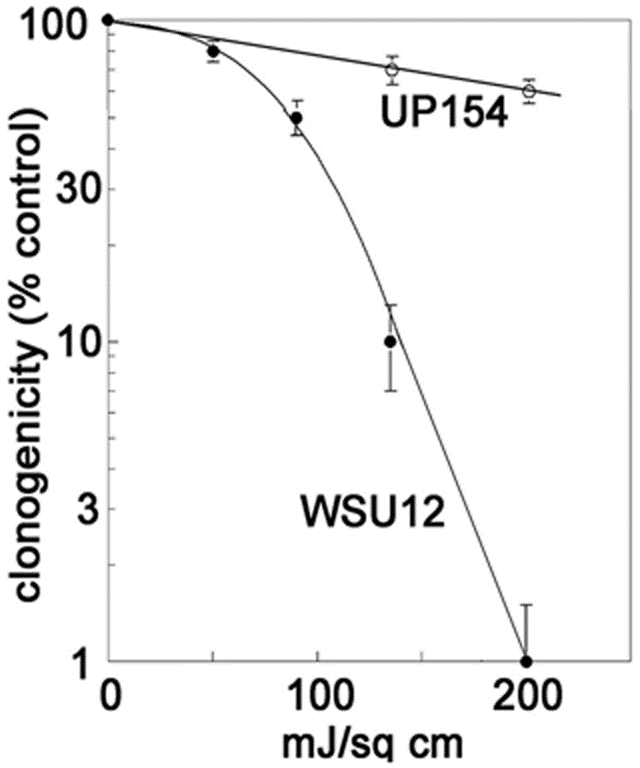
This image demonstrates the ability of photodynamic therapy (PDT) directed at ER/mitochondria to eradicate a head & neck cancer cell line (WSU12) from a patient with a human papilloma virus infection. These cells were relatively unresponsive to ionizing radiation, typical of HPV (+) tumors. In contrast, cells from an HPV (−) patient (UP154) were responsive to ionizing radiation but not to PDT.
INTRODUCTION
Photodynamic therapy (PDT) involves the use of photosensitizing agents that target specific sub-cellular loci. Irradiation at wavelengths corresponding to an absorbance band of a photosensitizer can trigger pathways to cell-death [1]. A study involving tumor cells derived from patients with head and neck cancer explored response patterns to ionizing radiation (IR) vs. photodynamic therapy (PDT) using benzoporphyrin derivative (BPD, Visudyne) as the photosensitizing agent. Tumor cells derived from patients infected with the human papilloma virus (HPV) showed an enhanced response to IR but an impaired response to PDT when compared with cells from uninfected patients [2]. The poor response to PDT in HPV(+) tumor cells could not be attributed to impaired BPD accumulation or reduced reactive oxygen species (ROS) formation associated with PDT [2]. While apoptosis was impaired in HPV(−) cells, such cells can be killed via photodamage directed at the ER: this leads to a non-apoptotic death pathway termed paraptosis. We now provide further information on determinants of PDT efficacy as a function of sub-cellular PDT targets and the HPV status of the patient from whom tumor was derived.
MATERIALS AND METHODS
Chemicals and supplies.
BPD (benzoporphyrin derivative, Verteporfin) was purchased from VWR (Cat No 1711461), Radnor PA. NPe6 was provided by Prof. Kevin M. Smith, Department of Chemistry, Louisiana State University. Fluorescent probes were purchased from Thermo Fisher Scientific, Waltham MA. Other reagents were obtained from MilliporeSigma, St. Louis MO.
Cell cultures.
Maintenance of OVCAR-5 cells, an ovarian malignancy of human origin, has been described [3] as have sources and propagation of head & neck cancer cell lines [2]. The WSU12 cell line, negative for HPV, was established at Wayne State University. UP154, an HPV(+) cell line, was obtained from the University of Pittsburgh. The latter two cell lines were cultured in RPMI1640 and MEM, respectively, using media supplemented with 10% fetal bovine serum.
Photodynamic therapy.
Cultures were incubated at 37°C in growth medium containing 0.5 μM BPD or 20 μM NPe6 for 1 h. The medium was replaced and cultures irradiated at 690 nm (BPD) or 660 nm (NPe6) using specified light doses. The irradiation source was a QH lamp with the wavelengths limited by a 10 cm path through distilled water followed by an interference filter (bandwidth = 10 nm). This source provides a 2 mW/cm2 light dose at this bandwidth as determined by a Scientech H410 Power & Energy meter.
Ionizing radiation.
Cells were irradiated with 6 Gy using a gantry mounted Best Theratronics Gammabeam 500 at a dose rate of 1 Gy/min. This was carried out at room temperature under atmospheric oxygen conditions. The dose was confirmed with a Farmer ionization chamber.
Microscopy.
Images were acquired with a Nikon E-600 microscope using a Rolera EM-CCD camera and MetaMorph software as described in Ref. 4. Phase-contrast microscopy was used to characterize the cytoplasmic vacuole formation associated with paraptosis [5]. The florescent probe Mitotracker orange (MTO) was used to assess effects of photodamage on ΔΨm [6].
Clonogenic survival.
Effects of photodynamic therapy or ionizing radiation were determined as described in Ref. 4. Colonies were fixed in 70% ethanol, stained with 1% crystal violet (2 h, ambient temperature) and counted using an Oxford Optronix Ltd. GelCount system. All such studies were performed in triplicate.
RESULTS
Efficacy of PDT vs. IR
Clonogenic studies revealed that WSU12 was more responsive to the photodynamic effects initiated by BPD (Table 1). WSU12 cells were more readily killed by BPD-mediated photodamage than U154 cells. When NPe6 was used to target lysosomes for photodamage, the HPV(+) UP154 cell line was, however, more responsive than WSU12. BPD is known to target ER and mitochondria while NPe6 is specific for lysosomes [6]. Prior studies had indicated an impaired pathway to apoptotic death in the HPV (−) cell line after ionizing radiation [2]: a 6 Gy radiation dose was shown to kill ~80% of a WSU12 population but > 98% of UP154 cultures. We had also shown [2] that uptake of BPD and production of singlet oxygen were essentially identical in the two cell lines.
Table 1.
Clonogenicity
| Conditions | Dose | WSU12 | UP154 | OVCAR-5 |
|---|---|---|---|---|
| PDT (BPD) | 90 mJ/cm2 | 60 ± 4 | 78 ± 4 | 32 ± 3 |
| 135 mJ/cm2 | 9 ± 2 | 68 ± 4 | 8 ± 2 | |
| 200 mJ/cm2 | 5 ± 1 | 55 ± 4 | 2 ± 1 | |
| PDT (NPe6) | 270 mJ/cm2 | 52 ± 5 | 28 ± 2 | 15 ± 2 |
| IR | 6 Gy | 28 ± 3 | 5 ± 2 | --- |
Cells were treated with IR or photosensitized with BPD or NPe6 and irradiated as described in the text. Clonogenicity was determined by colony counting (mean ± SD for three experiments).
Effects of BPD-catalyzed photodamage on ΔΨm
A prior study involving the OVCAR-5 cell line revealed that a 90 mJ/sq cm light dose substantially suppressed the mitochondrial membrane potential (ΔΨm) as indicated by fluorescence microscopy, using MTO as the fluorescent probe [6]. This light dose had a similar effect on WSU12 cells. A significantly higher (200 mJ/sq cm) light dose was, however, needed to suppress ΔΨm in the UP154 cell line (Fig. 1). Loss of ΔΨm indicates photodamage to the mitochondrial membrane that can lead to loss of cytochrome c into the cytoplasm and initiation of apoptosis [5]. These results are consistent with impaired photokilling by BPD in UP154 cells as shown in Table 2.
Figure 1.
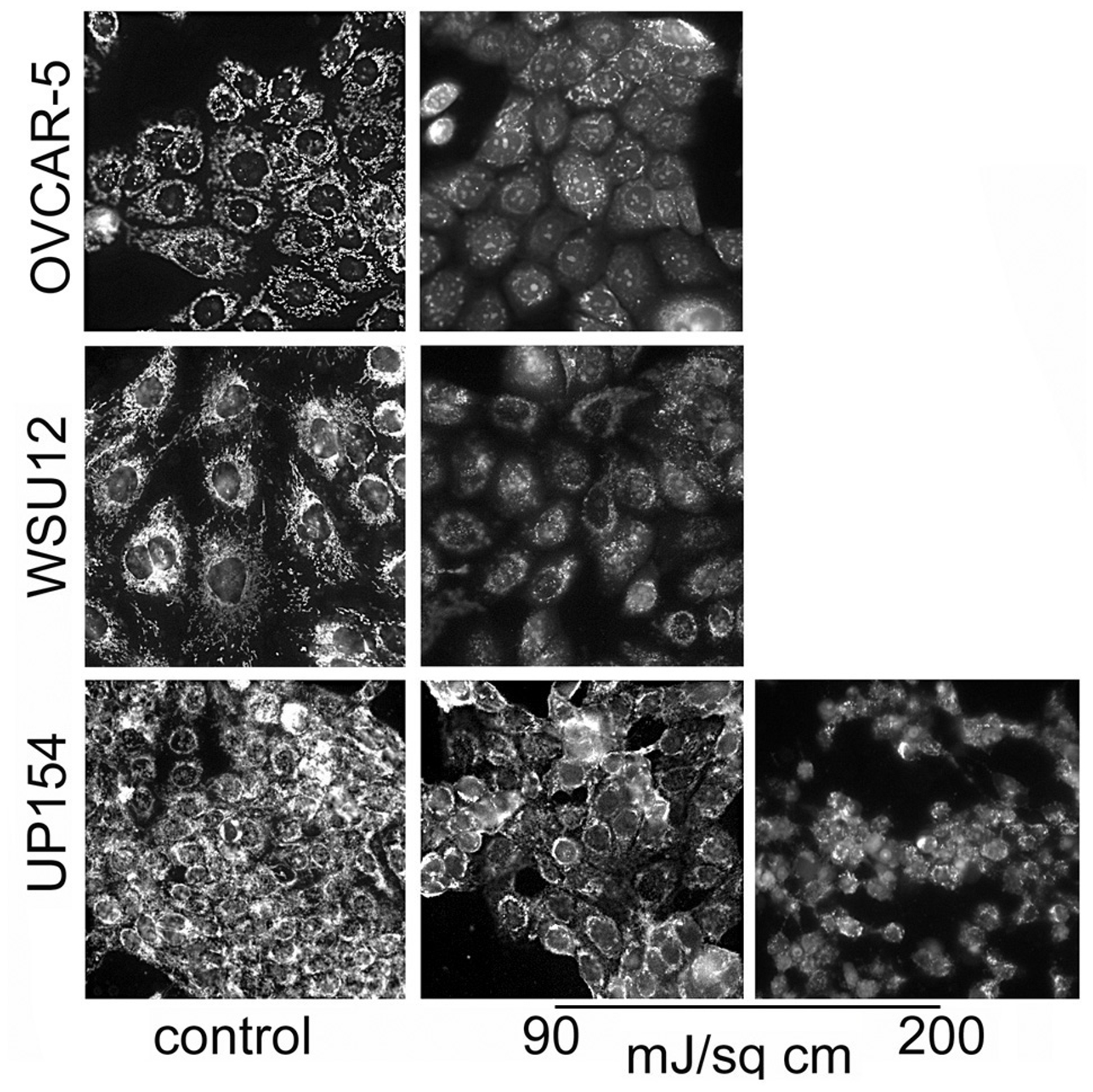
Loss of the mitochondrial membrane potential (ΔΨm) in OVCAR-5, WSU12 and UP154 cells as detected by Mitotracker Orange (MTO). Cells were photosensitized with BPD; light doses are indicated. Images were acquired directly after irradiation.
Table 2.
Clonogenicity
| Conditions | Light dose (mJ/cm2) | WSU12 | UP154 | OVCAR-5 |
|---|---|---|---|---|
| PDT (BPD) | 90 | 60 ± 4 | 78 ± 4 | 32 ± 3 |
| 135 | 9 ± 2 | 68 ± 4 | 8 ± 2 | |
| 200 | 5 ± 1 | 55 ± 4 | 2 ± 1 | |
| PDT (NPe6) | 270 | 52 ± 5 | 28 ± 2 | 15 ± 2 |
Cells were photosensitized with BPD or NPe6 and irradiated as described in the text. Clonogenicity was determined via colony counting (mean ± SD for three experiments).
Apoptosis and paraptosis.
Morphologic studies carried out 4 hr after irradiation, using BPD as the photosensitizing agent, revealed that a 90 mJ/sq cm light dose led to the appearance of a substantial number of WSU12 cells with paraptotic morphology (Fig. 2). This light and drug dose had a similar effect on OVCAR-5 cells [3]. A higher light dose was, however, needed to initiate paraptosis in UP154 cells. An examination of chromatin condensation and fragmentation 24 h after irradiation revealed a similar result. A 90 mJ/sq cm light dose led to a greater number of apoptotic cells in WSU12 than in UP154. At a higher (200 mJ/cm2) light dose, apoptosis was substantially enhanced in UP154 cells while the effect in WSU12 had reached a plateau (Fig. 3). When NPe6 was used as the photosensitizer, more apoptosis was observed in UP154 cells than in WSU12, an effect correlated with photokilling data (Table 1).
Figure 2.
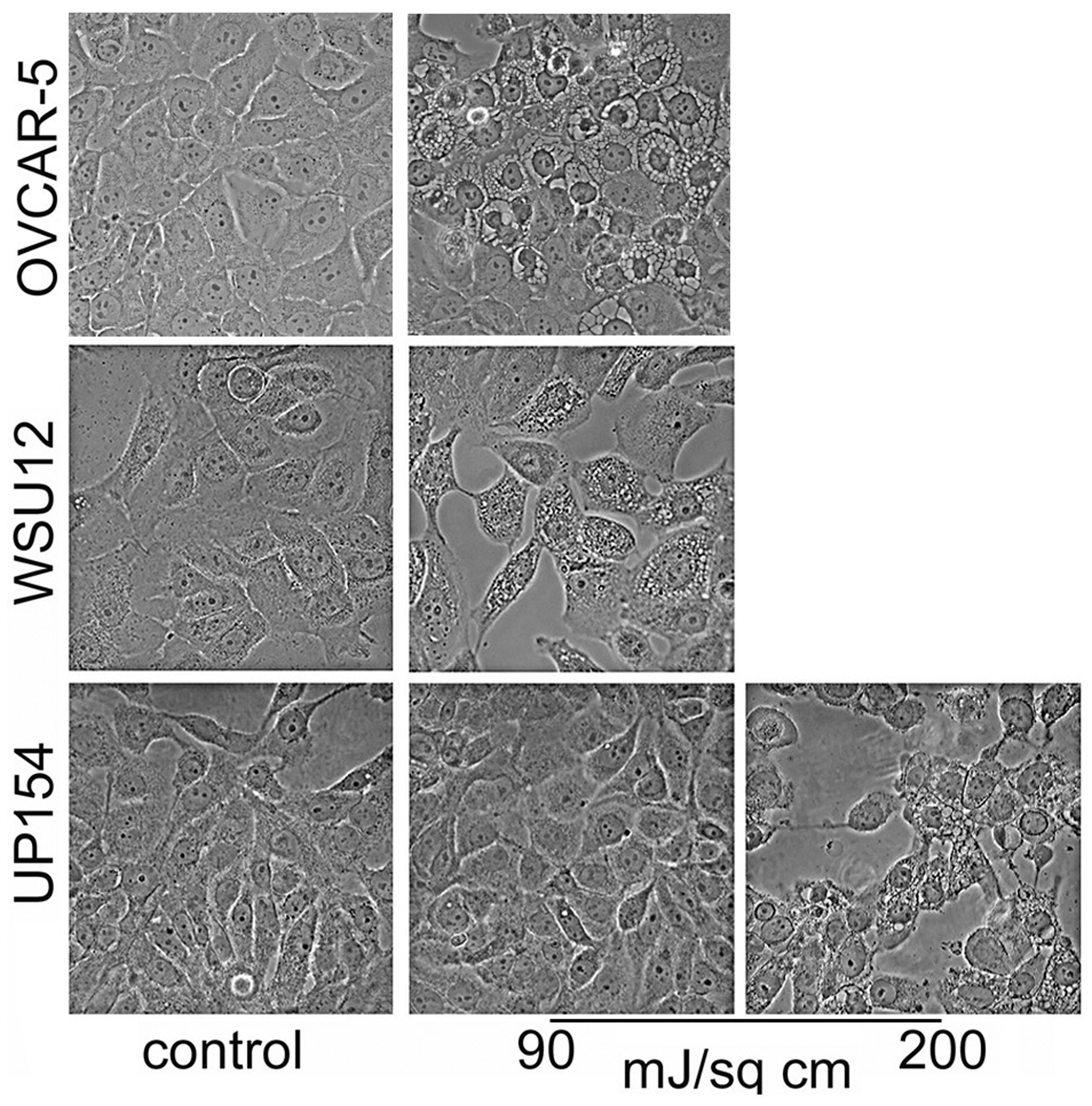
Effects of BPD-induced photodamage on initiation of paraptosis in OVCAR-5, UP154 and WSU12 cells. Images were acquired 4 hr after irradiation at specified light doses.
Figure 3.
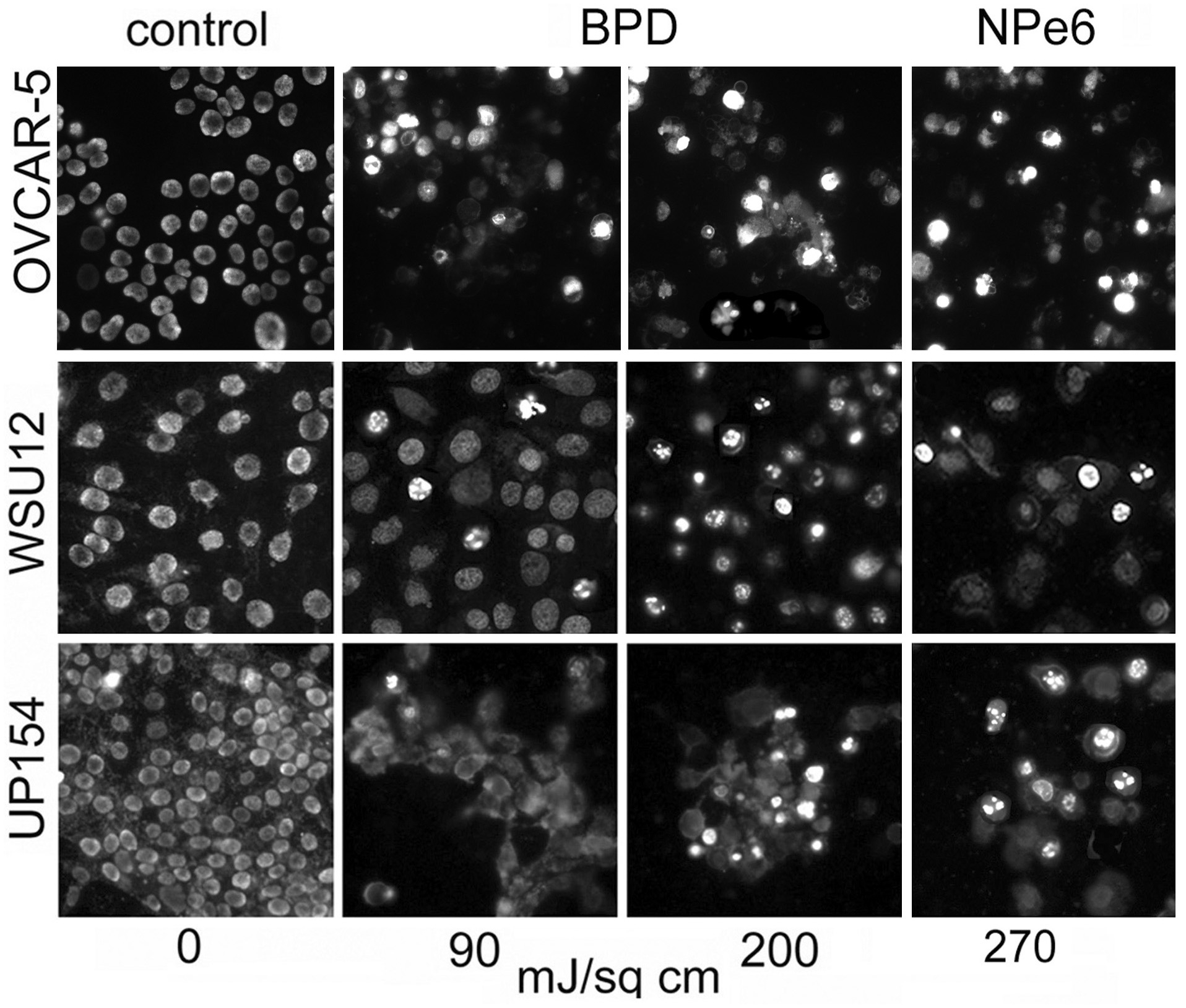
Initiation of apoptosis 24 h after BPD or NPe6-induced photodamage using specified light doses. Chromatin condensation and fragmentation were detected by labeling with Hö33342.
DISCUSSION
Infection with HPV tends to enhance the clinical response of head and neck cancer to ionizing radiation [7–10]. An earlier study involving cell cultures revealed that a line (UP154) derived from a patient with an HPV infection was less responsive to PDT (with BPD as photosensitizer) than another cell line (WSU12) that came from an HPV(−) patient [2]. Table 1 depicts survival as a function of HPV status after exposure to PDT. We interpret these data, combined with more recent studies, to suggest that an HPV infection promotes the apoptotic response to IR but impairs the effects of ER/mitochondrial photodamage to initiate both apoptosis and paraptosis. Effects of lysosomal photodamage mimic IR: apoptotic death is more readily evoked in HPV(+) cells.
Effects of PDT using BPD vs. NPe6 (Table 1) reflect differences in the target(s) for photodamage. BPD is known to partition to ER and mitochondria [6], a pattern that can lead to apoptosis and paraptosis, whichever is available. With apoptosis impaired in WSU12 cells, paraptosis becomes the pathway to cell death after ER photodamage [5,11]. Photodamage to lysosomes leads to only apoptosis [3,4]. Compared with ER/mitochondrial targeting by BPD (Table 1) the lethality of NPe6-directed PDT was greater in HPV(+) cells, since apoptosis is an available death mechanism. Because of an impaired apoptotic pathway, WSU12 cells were less responsive to the effects of lysosomal photodamage )Table 1(. While the UP154 cell line did show a greater phototoxic response than WSU12, photokilling after lysosomal photodamage was limited even at a relatively high light dose. Prior studies [2] had shown the PDT dose-response curve for WSU12 had a prominent shoulder, consistent with the cytoprotective effect of autophagy. No such shoulder was observed for UP154. There is no evidence of impaired BPD accumulation by UP154 cells. Moreover, the rate of formation of singlet molecular oxygen during irradiation was similar in both lines [2]. A summary of information relating to responses of these cell lines to IR vs. PDT is shown in Table 3.
Table 3.
Summary
| Cell line | HPV status | IR (6Gy) lethality | apoptosis after IR | Loss of ΔΨm after PDT (BPD) | PDT efficacy | BPD | ||
|---|---|---|---|---|---|---|---|---|
| BPD* | NPe6† | uptake | 1O2 formation | |||||
| WSU12 | − | 80% | − | +++ | +++ | + | + | + |
| UP154 | + | 95% | +++ | 0 | ± | ++ | + | + |
Comparison of WSU12 vs. UP154 cells
Cell death occurs with the morphology of paraptosis
Apoptotic death observed
Determinants of PDT efficacy have yet to be fully explored. Casas et al summarized a collection of potential factors [12]. These include [a] impaired photosensitizer accumulation, [b] altered sub-cellular localization, [c] enhanced capacity for DNA repair, [d] variation in induction of early-response genes and signal transduction pathways, [e] quenching of reactive oxygen species, [f] altered expression of heat shock proteins, [g] resistance mediated by NO formation or survivin. With regard to cell lines examined here, prior results [2] rule out [a], [b] and [e]. Effects of an HPV infection on the other pathways remain to be explored.
Tasso et al [13] reported a correlation between rates of photobleaching vs. photodamage to artificial membranes. These authors reported finding no correlation between PDT efficacy and ROS formation and therefore proposed that the extent of photobleaching might be a more pertinent determinant of efficacy. Since no examples of actual photokilling were examined, it is not clear that these results reflect the role of PDT as a means to tumor eradication. Several studies have indicated that hypoxia can limit photokilling [14,15], indicating that ROS formation is required. Affinity of photosensitizing agents for sub-cellular membranes is, however, a determinant of efficacy since ROS formed during irradiation will have a very limited lifetime.
There are metabolic effects associated with an HPV infection that could affect the ability of photodamage to eradicate a cell population [14]. These include decreased glucose metabolism and more dependence on mitochondrial respiration. There was also a report that infection with Epstein/Barr and HPV can lower both glutathione peroxidase and superoxide dismutase levels which would be expected to promote photokilling [15]. No data was acquired from cells lacking both infections, so there the ‘base-line’ values were missing. The potential role of such metabolic differences in the lethality of both ionizing radiation and PDT remains to be explored. Lockshin [16] has postulated that a lethally damaged cell will take any available pathway to death. Examples shown in this report relating to apoptosis and paraptosis provide further evidence for this proposal.
In reviews of PDT efficacy in clinical studies, there have been no reports of decreased responsiveness when there is a concurrent HPV infection [17–19]. The ability of PDT to affect multiple factors related to tumor survival may account for this observation. In addition to direct cell killing, PDT can also shut down the tumor vasculature and stimulate antitumor immunologic responses [1].
ACKNOWLEDGEMENTS
This research was supported by NIH grants CA 123362 and DE023181 (H-R.C.K), CA 23378 (DK), and by funds from the Office of the Vice President for Research. Excellent technical assistance was provided by Summera Kanwal.
REFERENCES
- 1.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC and Golab J (2011) Photodynamic therapy of cancer: an update. CA Cancer J. Clin 61, 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessel D, Cho WJ, Rakowski J, Kim H,E, and Kim H-RC (2020) Effects of HPV status on responsiveness to ionizing radiation vs. photodynamic therapy in head and neck cancer cell lines. Photochem. Photobiol 96, 62–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessel D (2019) Pathways to paraptosis after ER photodamage in OVCAR-5 cells. Photochem. Photobiol 95,1239–1242. [DOI] [PubMed] [Google Scholar]
- 4.Andrzejak M, Price M, and Kessel D (2011) Apoptotic and autophagic responses to photodynamic therapy in 1c1c7 murine hepatoma cells. Autophagy 7, 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessel D (2017) Apoptosis, paraptosis and autophagy: death and survival pathways associated with photodynamic therapy. Photochem. Photobiol 95,119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessel D (2017) Subcellular targeting as a determinant of the efficacy of photodynamic therapy. Photochem. Photobiol 93, 609–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A and Gillison ML (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst 100, 261–269. [DOI] [PubMed] [Google Scholar]
- 8.Hong AM, Dobbins TA, Lee CS, Jones D, Harnett GB, Armstrong BK, Clark JR, Milross CG, Kim J, O’Brien CJ and Rose BR (2010) Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br. J. Cancer 103, 1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S, Ali-Fehmi R, Franceschi S, Struijk L, van Doorn LJ, Quint W, Albashiti B, Ibrahim M and Kato I (2012) Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. Int. J. Cancer 131, 1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung YS, Najy AJ, Huang W, Sethi S, Snyder M, Sakr W, Dyson G, Hüttemann M, Lee I, Ali-Fehmi R, Franceschi S, Struijk L, Kim HE, Kato I and Kim H-C (2017) HPV-associated differential regulation of tumor metabolism in oropharyngeal head and neck cancer. Oncotarget 8, 51530–51541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperandio S, Poksay K, de Belle I, Lafuente MJ, Liu B, Nasir J and Bredesen DE (2004) Paraptosis: mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 11, 1066–1075. [DOI] [PubMed] [Google Scholar]
- 12.Casas A, Di Venosa G, Hasan T and Batlle A (2011). Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem 18, 2486–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tasso TT, Schlothauer JC, Junqueira HC, Matias TA, Araki K, Liandra-Salvador É, Antonio FCT, Homem-de-Mello P and Baptista MS (2019) Photobleaching efficiency parallels the enhancement of membrane damage for porphyrazine photosensitizers. J. Am. Chem. Soc 141,15547–15556. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Kwon N, Guo T, Liu Z and Yoon J (2018) Innovative strategies for hypoxic-tumor photodynamic therapy. Angew, Chem. Int. Ed. Engl 57, 11522–11531. [DOI] [PubMed] [Google Scholar]
- 15.Strycharz-Dudziak M, Fołtyn S, Dworzański J, Kiełczykowska M, Malm M, Drop B and Polz-Dacewicz M (2020) Glutathione peroxidase (GPx) and superoxide dismutase (SOD) in oropharyngeal cancer associated with EBV and HPV coinfection. Viruses 12, 1008. doi: 10.3390/v12091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockshin RA (2016) Programmed cell death 50 (and beyond). Cell Death Differ. 23,10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Civantos FJ, Karakullukcu B, Biel M, Silver CE, Rinaldo A, Saba NF, Takes RP, Vander Poorten V And Ferlito A (2018) A. A Review of Photodynamic Therapy for Neoplasms of the Head and Neck. Adv Ther. 35, 324–340. [DOI] [PubMed] [Google Scholar]
- 18.Jerjes W, Upile T, Akram S And Hopper C (2010) The surgical palliation of advanced head and neck cancer using photodynamic therapy. Clin. Oncol. (R. Coll. Radiol.) 22, 785–791. [DOI] [PubMed] [Google Scholar]
- 19.Rigual NR, Thankappan K, Cooper M, Sullivan MA, Dougherty T, Popat SR, Loree TR, Biel MA and Henderson B (2009) Photodynamic therapy for head and neck dysplasia and cancer. Arch. Otolaryngol. Head. Neck. Surg 135, 784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]


