Abstract
Streptococcus agalactiae, also known as Group B Streptococcus (GBS), is increasingly recognized as a major cause of soft tissue and invasive diseases in the elderly and diabetic populations. Antibiotics like penicillin are used with great frequency to treat these infections, although antimicrobial resistance is increasing among GBS strains and underlines a need for alternative methods not reliant on traditional antibiotics. GBS granadaene pigment is related to the hemolysin/cytolysin of GBS, which is critical for the pathogenesis of GBS diseases. Here, we show that photobleaching granadaene dampens the hemolytic activity of GBS. Furthermore, photobleaching of this antioxidant was found to increase GBS susceptibility to killing by reactive oxygen species like hydrogen peroxide. Treatment with light was also shown to affect GBS membrane permeability and contribute to increased susceptibility to the cell membrane-targeting daptomycin. Overall, our study demonstrates a dual effect of photobleaching on the virulence and antimicrobial susceptibility of GBS and suggests a novel approach for the treatment of GBS infection.1
INTRODUCTION
Gram-positive streptococci are responsible for a wide variety of medical conditions, ranging from more mild infections like strep throat to more serious infections such as meningitis, myositis, and necrotizing fasciitis (2–4). Among the many species within the Streptococcus genus, Streptococcus agalactiae (commonly referred to as group B streptococcus or GBS) is a β-hemolyic, catalase negative facultative anaerobe which is best known for causing neonatal invasive diseases (5, 6). In recent years, there has been a greater appreciation for GBS as a leading cause of skin and soft tissue infections as well as invasive disease in the elderly and diabetic patients (7). While the risk of neonatal GBS infections has significantly decreased as a result of preventive intrapartum antibiotic prophylaxis (8, 9), the rate of GBS infection within non-pregnant adults has increased as evidence by a two-fold rise in incidence of invasive GBS disease in adults between 1990 and 2007 (10). Compared to S. pneumoniae invasive diseases (42 per 100,000), a leading cause of mortality in the elderly population, the incidence of GBS invasive disease among elderly is remarkable at 25 per 100,000, based on active, population-based surveillance data from the ABCs Emerging Infections Program Network on cases of and deaths associated with infection due to several Gram-positive pathogens (7).
As the rate of infection increases, treatment of GBS infection has become more reliant on the usage of antibiotics like penicillin V, ampicillin, and daptomycin (11, 12). However, even with optimal antibiotic treatment, management of skin infections can be difficult especially in patients suffering from diabetes (13). Compounding this therapeutic challenge, GBS has developed resistance to commonly used antibiotics like erythromycin and clindamycin, and has also shown the capacity to develop resistance to β-lactam antibiotics, threatening future use of current first-line drugs (14, 15). Because of these challenges, there is a growing need to develop novel GBS therapeutics that are less reliant on antibiotics.
One of the key reasons why GBS is highly infectious is its multitude of virulence factors that endow it with the capability to subvert suboptimal host defenses, allowing the bacterium to better adapt to its environment and cause severe infection (16). Some of these GBS virulence factors include the β hemolysin/cytolysin; the immune system evasion factor sialic acid capsular polysaccharide; as well as antimicrobial resistance factors like penicillin binding proteins and pili (17). Amongst all these different virulence factors, one of the most enigmatic is the ornithine rhamno-polyene pigment known as granadaene, which is localized within the plasma membrane of GBS and found within 97% of human clinical GBS isolates (18, 19). As the source of the characteristic red color of GBS, granadaene contains a linear chain of twelve conjugated C=C bonds, providing the pigment with an absorption spectrum nearly identical to most carotenoids, with absorption peaks present at 430, 460, 490, and 520 nm (19). This similarity in conjugated double bonds provides granadaene with antioxidant characteristics shared by traditional carotenoids, and provides it with the ability to quench and detoxify reactive oxygen species (ROS) like hydrogen peroxide (H2O2), singlet oxygen and superoxide (20). Previous studies have shown that pigment increases GBS resistance to ROS like H2O2 and superoxide by 5- to 10-fold compared to pigment deficient mutants, as well as significantly contributing to the survival within phagocytes by shielding the bacteria from oxidative damage (21). In addition to enhancing survival under oxidative stress, studies have indicated a close relationship between the pigment expression and hemolytic activity of GBS, both of which are controlled by the cyl operon (22, 23). Recent studies have indicated that the molecule responsible for pigmentation and the molecule responsible for hemolytic activity may be one and the same, as findings shown that increased pigment production correlates with increased hemolysin production and that isolated and stabilized granadaene pigment exhibits hemolytic activity, strongly indicate a close linkage between the two virulence determinants (23–25).
Based on the multiple conjugated bonds located within the structure of granadaene, the granadaene pigment is highly likely to exhibit similar chemical reactivity to traditional carotenoids, such as the increased photosensitivity and photodegradation of the carotenoid structure in response to near UV and blue light exposure (26). Previous experiments have demonstrated that the staphyloxanthin (STX) carotenoid pigment within methicillin-resistant S. aureus (MRSA) is capable of undergoing photolysis when exposed to 460 nm light. Such photolysis results in the formation of pores within the membrane of MRSA that not only increase the susceptibility of MRSA to reactive oxygen species, but also sensitizes MRSA to conventional antibiotics the bacteria have become resistant to (27, 28).
Considering the structural similarity between carotenoids and the granadaene pigment, we hypothesize that granadaene is capable of exhibiting similar photochemical reactions to blue light sources. In this paper, we present a novel granadaene photobleaching approach for reducing virulence and increasing the antimicrobial susceptibility of infectious S. agalactiae. By degrading the pigment within the membrane, we significantly reduced the hemolytic activity of GBS and increased GBS susceptibility to oxidative stress. In addition, we demonstrate that photobleaching of GBS leads to improved effectiveness of antibiotics such as daptomycin. Based on its capacity for reducing bacterial virulence and increasing antimicrobial susceptibility, photobleaching potentially represents an effective adjunct therapeutic for the treatment of GBS skin and soft tissue infections.
MATERIALS AND METHODS
Source of blue light:
Pulsed blue light was administered through a short-pulsed Opolette HE 355 LD laser (OPOTEK). The laser is tunable from 410 nm to 2200 nm at a repetition rate of 20 Hz and a pulse width of ~5 ns. At 430 nm, the system has a maximum pulse energy of 9 mJ. While the standard beam diameter of the system is ~2 mm, a collimator attached to an optical fiber was used to expand the diameter of the beam to 10 mm. With these parameters, the pulsed laser offers a power output of ~100 mW/cm2.
Bacterial strains:
S. agalactiae Lehmann and Neumann (ATCC 12386) were purchased from the American Type Culture Collection, and GBS grown in a Group B Streptococcus Carrot Broth was used for initial granadaene pigment extraction. For bacterial susceptibility and hemolytic assays, a highly pigment S. agalactiae strain (NCTC 10/84) and its non-pigmented isogenic ΔCylE mutant were used (22).
Pigment extraction:
Extraction of the granadaene pigment was performed based on a modified extraction protocol published by Rosa-Fraile et al (18). To summarize, GBS (ATCC 12386) was grown in 4 mL of New Granada Media/Strep B Carrot Broth (Z40, Hardy Diagnostics) at 37°C for 48 to 72 hours until the broth turned a rich orange-red color. Bacterial suspension was centrifuged and washed three times with 1 mL of distilled water and two times with 1 mL of DMSO. Following washing, the bacterial pellet was resuspended in 0.5 to 1 mL of DMSO with 0.1% Trifluoroacetic acid (TFA) overnight. The next day, the suspension was centrifuged and the 0.5 to 1 mL of supernatant containing the extracted pigment was collected with measured OD490 ranging from 0.2 to 0.08 respectively.
Photobleaching and spectroscopic measurements:
Photobleaching and subsequent absorbance measurements of extracted pigment and bacteria samples were performed by placing 90 μL of extracted pigment suspended in DMSO/0.1% TFA (OD490 = 0.08) or concentrated GBS bacteria suspended in PBS (OD600 = 1.5) in a 96 well plate. Extracted pigment solution (OD490 = 0.08) was initially treated with 60 J/cm2 of blue light ranging from 420 nm to 490 nm to determine the most effective photobleaching wavelength based on the reduction of the 490 nm absorbance peak. For 430 nm photobleaching, both extracted pigment solution (OD490 = 0.08) and concentrated GBS bacteria (OD600 = 1.5) were exposed to 430 nm light for different time intervals to provide a range of light dosages (0, 30, 60, 120 J/cm2). The absorbance spectrum of each sample was then measured through a plate reader spectrometer.
For Raman measurements, a Raman spectroscopy system (1221, LABRAM HR EVO, Horiba) with a 40x Olympus objective and an excitation wavelength of 532 nm was used to detect changes in pigment presence within GBS. Images were acquired under a 30 second acquisition time with a laser ND filter of 10%. Pigment-expressing GBS was concentrated in 50 μL of PBS and a 2 μL droplet was aliquoted and sandwiched between two cover slides (48393-230, VWR International). Changes in pigment presence were determined by measuring the change in Raman peak amplitudes associated with conjugated double bonds located at the 1126 cm−1 and 1510 cm−1 before and after light treatment. For Raman measurements collected with high background peaks, Raman Spectrum Baseline Removal Matlab Code was utilized to better isolate Raman peaks (29, 30).
Hemolytic activity assay:
Hemolytic activity of broth-cultured NCTC 10/84 GBS was measured. Colonies of pigment expressing GBS were removed from agar plates and incubated overnight at 37°C in Todd Hewitt broth. After incubation, the bacteria were washed and resuspended in 1x PBS to an OD600 of 0.4. A 15 μL aliquot of bacterial suspension was exposed to 120 J/cm2 of 430 nm light. Following the light exposure, the suspension was diluted with 35 μL of PBS and then further combined with 200 μL of 1% fresh human blood solution. Blood samples were acquired through the Boston Children’s Hospital Blood Donor Center. For positive controls, 200 μL of blood solution was mixed with 50 μL of 0.1% Triton X. For negative controls, 200 μL of blood solution was mixed with 50 μL of 1x PBS. Blood samples were cultured at 37°C for 2 hours, after which the samples were centrifuged and the supernatant were collected. The absorbance of the supernatant was measured at 420 nm. Percent hemolytic activity was determined by subtracting the GBS supernatant absorbance by the negative control absorbance, followed by dividing it all by the difference between the positive and negative control absorbance measurements.
In vitro potentiation assessment between photobleaching and reactive oxygen species in GBS:
S. agalactiae NCTC 10/84 strains (WT and non-pigment expressing ΔCylE mutant) were streaked and cultured on trypticase soy agar plates in a static 37°C incubator for 24 to 48 hours. To maximize colony size and pigment expression in NCTC 10/84, bacterial suspensions of GBS were diluted in PBS and droplets of GBS dilutions were placed on the plate to incubate. Once pigment-expressing colonies were formed, colonies were picked with a bacterial loop and then wash and resuspended in 1x PBS. A 10 μL aliquot of this bacterial suspension was placed onto a cover slide and exposed to 100 mW/cm2 430 nm pulsed light for various time intervals. Following exposure, the droplets were removed from the cover slide and mixed with 990 μL of PBS supplemented with different concentrations of hydrogen peroxide. These tubes were cultured for 1 hour. Once culturing was complete, serial 10-fold dilutions were performed using a 96 well plate. These dilutions were plated on tryptic soy agar plates in triplicate to quantify the number of surviving GBS colonies. Plates were incubated at 37°C overnight before counting CFU/mL.
Membrane permeability assay:
S. agalactiae NCTC 10/84 colonies cultured on agar plates were removed, washed, and resuspended in in 100 μL of 1x PBS. A 10 μL aliquot of suspension was placed on a cover slide and exposed to 30 J/cm2 of 430 nm light. Following exposure, the exposed droplet was combined with 980 μL of sterile water and 10 μL of 0.5 mM SYTOX Green (S7020, Thermo Fisher Scientific) solution before being added to a 96 well plate (200 μL solution per well). For untreated GBS, a 10 μL aliquot of bacterial suspension from the same stock was mixed in 980 μL of sterile water and 10 μL of 0.5 mM SYTOX Green. Using a plate reader, the fluorescence of each well was measured over the course of 1 hour at 5-minute intervals. The excitation wavelength was set at 488 nm while the emission wavelength was set at 525 nm. Increasing emission intensity was indicative of greater membrane permeability.
In vitro potentialization assessment between photobleaching and antibiotics:
For initial minimum inhibitory concentration determinations, agar plate-grown S. agalactiae NCTC 10/84 colonies were washed and resuspended in 1x PBS. A 5 μL aliquot of this bacterial suspension was placed onto a cover slide and exposed to 100 mW/cm2 430 nm pulsed light for specific time intervals. Following exposure, the droplets were removed from the cover slide and mixed with 5 mL of Todd Hewitt Broth. (T1438-100G, Sigma Aldrich). 100 μL of GBS containing broth was added to a 96 well plate, after which an additional 100 μL of GBS broth was added to the first row of the plate alongside an initial starting concentration of antibiotics daptomycin (103060-53-3, Acros Organics), penicillin V (1504489, Sigma Aldrich), ampicillin (A9518, Sigma Aldrich). 2-fold serial dilutions were performed and the plate was allowed to incubate overnight. Following overnight culture, the plate was removed and measured through both visual means and through a plate reader to determine the minimum inhibitory concentration of different antibiotics. For daptomycin studies, initial broth cultures were supplemented with 50 μg/mL of CaCl2.
To determine possible potentialization, S. agalactiae NCTC 10/84 colonies were removed from agar plates and then washed and resuspended in 1x PBS to an OD600 between 0.4 and 0.5. A 10 μL aliquot of this bacterial suspension was placed onto a cover slide and exposed to 430 nm pulsed light for various time intervals. Following exposure, the droplets were removed from the cover slide and mixed with 990 μL of PBS supplemented with differing concentrations of daptomycin, penicillin V, and ampicillin. For daptomycin studies, cultures were supplemented with 50 μg/mL of CaCl2. These culture tubes were incubated at 37°C and CFU was measured through serial dilutions at various time intervals.
Confocal imaging and tracking of daptomycin membrane integration:
To visualize the integration of daptomycin into the membrane of GBS using confocal imaging, daptomycin was conjugated to the fluorescent marker BODIPY STP-ester. To prepare this drug-dye combination, 10 mg of daptomycin was dissolved in 1 mL of 0.1 M NaHCO3 solution. 100 μL of 1 mg/mL BODIPY STP-ester (B10006, Thermo Fisher Scientific) was added to the daptomycin solution drop by drop and then stirred for 1 hour at room temperature. Following this, the solution underwent dialysis utilizing 0.1 M NaHCO3 solution overnight. Once dialysis was complete, the drug-dye complex was lyophilized.
In order to prepare samples for imaging, pigment expressing GBS colonies were removed from agar plates, centrifuged, washed, and resuspended in 100 μL of PBS. A 10 μL aliquot of GBS was placed on a cover slide and exposed to 60 to 90 J/cm2 of 430 nm light. Following exposure, the 10 μL aliquot was combined with 970 μL of Todd Hewitt Broth, 10 μL of 5 mg/mL CaCl2, and 10 μL of 3 mg/mL Daptomycin-BODIPY. The GBS culture was incubated for 30 minutes at 37°C, after which the culture was washed with PBS and fixed in 50 μL of 10% formalin. A 2.5 μL aliquot of GBS fixed in formalin was sandwiched between a Poly-L lysine coated glass slide and a cover slide.
For confocal imaging, a FV3000 Confocal Laser Scanning Microscope (Olympus) was used to image and detect the presence of daptomycin within the membrane of GBS. Confocal images were acquired under an excitation wavelength of 488 nm and an emission detection wavelength range of 510 nm to 560 nm. Images were taken under the following confocal settings: 100x Objective, 2.85x Zoom, a PMT Voltage of 600 V, and a laser ND Filter of 10%. Images were processed through ImageJ utilizing thresholding and particle analysis in order to obtain the average intensities of individual cells.
Statistical analysis:
CFU and hemolytic assays were performed in triplicate (n = 3). Results are presented with the mean associated standard deviation. All comparative data was analyzed utilizing a student t-test to obtain significance between data sets. All figures and analysis were performed using ImageJ or GraphPad PRISM version 7.
RESULTS AND DISCUSSION
Photobleaching of granadaene pigment in GBS
A previous study by Rosa-Fraile et al. established that the granadaene pigment expressed by S. agalactiae is a ornithine rhamno-polyene that contains a linear chain of twelve conjugated C=C bonds (Figure 1a) (18). Based on the protocol established by that study, we extracted the granadaene pigment from GBS (ATCC 12386) incubated in Strep B Carrot broth. Using the extracted pigment solution, the absorbance profile of the pigment was measured and found to exhibit the absorbance peaks at 460 nm, 490 nm, and 520 nm (Figure 1b). In addition to these three explicit peaks, a small peak was observed at the 430 nm wavelength, which was also described in a previous report (19). The consistency of our observed absorption peaks with previously established literature indicates that our protocol did successfully extract a solution containing the bacterial pigment granadaene. As such, photobleaching was then performed on the extracted solution to determine the impact of light exposure on the pigment.
Figure 1.
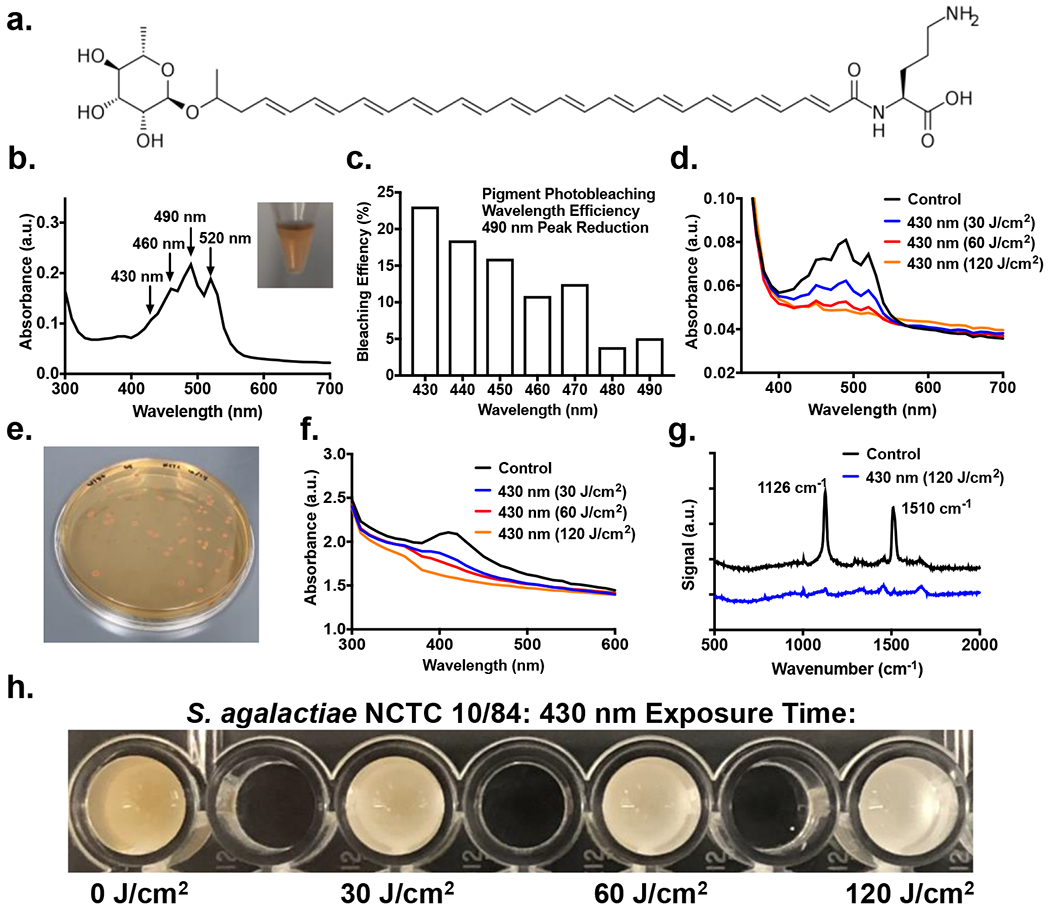
Extraction and photobleaching of granadaene pigment within S. agalactiae. (a) Chemical structure of granadaene. (b) Characteristic orange-red color of granadaene extracted from S. agalactiae (ATCC 12386) resuspended in DMSO with 0.1% Trifluoroacetic acid. Absorption spectrum of extracted granadaene exhibits characteristic peaks at 430 nm, 460 nm, 490 nm, and 520 nm. (c) Bleaching efficiency of different wavelengths of nanosecond pulsed light in the bleaching of granadaene pigment solution. Exposure to 60 J/cm2 of 430 nm light resulted in greatest decrease in the 490 nm absorption peak from an untreated control. (d) Absorption spectrum of extracted granadaene following exposure to 0 (black), 30 (blue), 60 (red), and 120 J/cm2 (yellow) of nanosecond pulsed 430 nm blue light. Absorption peaks initially present at 490 nm disappears upon light exposure. (e) Granadaene-expressing S. agalactiae (NCTC 10/84) exhibits a strong orange-red color when grown on agar plates. (f) Absorption spectrum of S. agalactiae (NCTC 10/84) colonies following exposure to 0 (black), 30 (blue), 60 (red), and 120 J/cm2 (yellow) of 430 nm pulsed blue light. Absorption peak for pigment within bacteria appears to shift to 410 nm, which begins to disappear upon light exposure. (g) Raman spectroscopy of S. agalactiae (NCTC 10/84) following exposure to 120 J/cm2 of pulsed blue light. Characteristic peaks associated with carotenoid structures at 1126 cm−1 and 1510 cm−1 disappear following light exposure. (h) Photobleaching of granadaene results in visible change in color from orange to white in GBS colonies. Bleaching increases with increasing light exposure. Black wells present within the image are empty.
To determine overall photobleaching efficiency, we used pulsed light (60 J/cm2) with differing wavelengths and measured the greatest decrease in pigment peak. 430 nm light exhibited the greatest decrease in 490 nm pigment peak, with a peak decrease of 23.01% (Figure 1c). Other wavelengths of light tested, such as 460 nm and 490 nm, were only found to decrease the pigment peak by only 10.86% and 5.1% respectively, indicating that the pigment appeared most susceptible to 430 nm light. Exposure to 30 J/cm2, 60 J/cm2, and 120 J/cm2 of 430 nm blue light resulted in a 23.2%, 35.1%, and 39.6% reduction of the 490 nm absorbance peak respectively (Figure 1d). By the time the pigment solution had been exposed to 60 J/cm2 of 430 nm light exposure, the entire peak profile of the pigment had completely disappeared.
Following photobleaching of the extracted pigment solution, the impact of photobleaching on the granadaene pigment still present within the membrane of the GBS bacteria was examined. Due to its naturally high pigment expression on agar plates without the usage of pigment enhancers like methotrexate, as well as its higher virulence and hemolytic activity, the S. agalactiae clinical isolate strain NCTC 10/84 was used for the bacterial photobleaching and subsequent studies (Figure 1e). When these bacterial colonies were washed and concentrated in PBS, the absorbance profile of GBS colonies did not exhibit the expected absorption peaks found in the extracted pigment solution. Instead, the bacterial solution was found to express only a single absorption peak at 410~420 nm. While these results do not show the same three peak characteristics observed in the extracted pigment, a previous study by Rosa-Fraile et al. demonstrated that at high pH, the absorbance peaks of the granadaene were consolidated into a single peak at 420 nm (18). Thus, the 410 to 420 nm absorbance peak detected in the bacterial solution corresponds to the presence of the pigment in the bacteria. Exposure of this bacterial solution to 30 J/cm2, 60 J/cm2, and 120 J/cm2 of 430 nm blue light was found to result in a 12.9%, 17.3%, and 24.1% reduction of the 410 nm absorbance peak respectively, thus exhibiting similar photobleaching capabilities to the extracted pigment (Figure 1f).
To better validate the presence of pigment within the cultured GBS colonies and photobleaching induced by light exposure, Raman spectroscopy was utilized to chemically detect the presence of pigment with GBS colonies (Figure 1g). Previous Raman studies have indicated that carotenoids exhibit intense spectral peaks within the 900 to 1600 cm−1 regions (31). In carotenoids, a strong peak at 1520 cm−1 (v1) peak is associated with the C=C stretching vibration, while a strong 1157 cm−1 (v2) peak is usually associated with C-C stretching. For our data, we observed that pigmented GBS exhibit significant Raman resonance peaks at the 1126 cm−1 and 1510 cm−1 wavenumbers. These results are consistent with the carotenoid Raman literature, as the 1510 cm−1 peak observed can be attributed to the C=C stretching vibration. While the 1126 cm−1 peak observed might appear inconsistent with the expected C-C stretching peak at 1160 cm−1, this can be explained by the lack of CH3 groups present on the granadaene pigment. Prior studies have indicated that the presence of CH3 groups on the carbon backbone interferes with C-C stretching, artificially increasing the resonance wavenumber. In polyacetylenic molecules that lack such CH3 groups, such as granadaene, the C-C peak can be expected to be generally 20 cm−1 lower than for carotenoids with the same chain length (31). Given this information, the observed resonance peak observed in GBS at 1126 cm−1 could indeed be attributed to C-C stretching, further confirming detection of the polyene structure of granadaene within the bacterial sample. With the strong presence of these polyene peaks within the bacterial sample established, it was determined that exposure to 120 J/cm2 was able to nearly eradicate the 1520 cm−1 and 1126 cm−1 peaks present within the sample, with a decrease in peak height of 90.2% and 88.4%, respectively. In addition to reducing the absorbance and Raman peaks, the impact of 430 nm light could also be observed visually, as increasing exposure dosages of 430 nm light resulted in an increased loss of the distinct orange-red color associated with GBS, eventually reaching a blank white color after 120 J/cm2 of light exposure (Figure 1h). These results collectively reveal a previously unknown aspect of granadaene pigment, namely that the granadaene pigment is vulnerable to photobleaching through blue light exposure, with 430 nm light being the most effective method for photobleaching. Aside from the observed changes in absorbance peaks observed in both the extracted pigment and the GBS bacteria, the reduction in Raman resonance peaks in the light treated GBS further confirms that 430 nm light photobleaches the granadaene pigment specifically by breaking up the linear chain of twelve conjugated C=C bonds present within granadaene.
Granadaene photobleaching reduces GBS hemolytic activity
Having established that exposure to 430 nm light alters the granadaene pigment present within GBS, we next investigated the potential for photobleaching to inhibit the hemolytic capabilities of GBS. As previously discussed, recent studies have indicated that the GBS β-hemolysin/cytolysin and the granadaene pigment may be the same molecule due to their close correlation with one another. (24). With this in mind, the impact of photobleaching on hemolytic activity was quantified for the extremely hemolytic GBS NCTC 10/84 strain. GBS was cultured on tryptic soy agar plates and pigment production was allowed to accumulate over 48 hours. These colonies were then removed and allowed to grow overnight in Todd Hewitt Broth. Using a 1% blood solution, the hemolytic activity of non-exposed and 430 nm (120 J/cm2) exposed bacteria was calculated based on positive and negative controls utilizing 0.1% Triton X detergent and 1x PBS (Figure 2a). Based on these results, the hemolytic activity of non-exposed and 430 nm (120 J/cm2) exposed bacteria was calculated. Untreated GBS was found to express a hemolytic activity of 88.8%, while photobleached GBS expressed 48.3% hemolytic activity (Figure 2b). The application of photobleaching was found to immediately reduce hemolytic activity by 40.5% in GBS.
Figure 2.
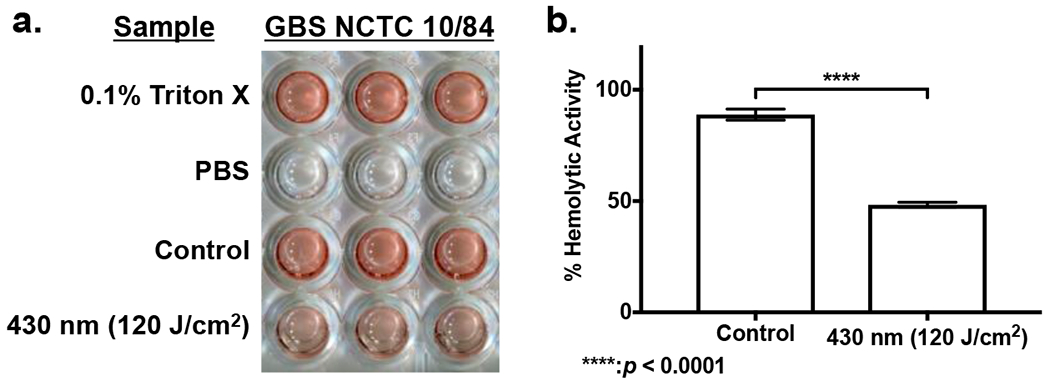
Granadaene photobleaching reduces Hemolytic activity in S. agalactiae. (a) Hemolytic assay of overnight cultured GBS treated with 120 J/cm2 of 430 nm light alongside untreated GBS sample, a positive 0.1% Triton X control, and a negative PBS control. (b) Percent hemolytic activity of non-exposed and blue light exposed GBS. ****: p < 0.0001.
These results reveal that pigment photobleaching is capable of significantly reducing the hemolytic activity of GBS, providing a potentially non-invasive method of reducing the virulence of GBS without the use of antimicrobial agents. The loss of hemolytic activity from photobleaching may be due to disruption of the double conjugated backbone of the granadaene pigment by the 430 nm light, as previous studies have found that the granadaene polyene chain length is critical to hemolytic activity, and that shortening or cleaving this polyene chain results in decreased hemolytic activity.(32) Given that our previous Raman spectroscopy data indicated that photobleaching results in a significant decrease in both C=C and C-C stretching, it appears that photobleaching is capable of specifically targeting the hemolytic activity of granadaene by disrupting its double conjugated bonds. These results not only indicate that photobleaching is capable of significantly reducing the hemolytic activity of GBS, but it also further confirms that granadaene does indeed contribute to the overall hemolytic activity of GBS.
Granadaene photobleaching sensitizes GBS to H2O2
Previous work by our group have demonstrated that the granadaene pigment production in GBS confers resistance to reactive oxygen species (ROS) (21). With this in mind, it was found that application of 60 J/cm2 of 430 nm light and 12 mM of H2O2 resulted in the eradication of pigment expressing wild type (WT) GBS (Figure 3a). Light exposure alone was found to have no significant effect on the survival of WT GBS, while the addition of 12 mM of H2O2 resulted in only a 1 log, or 92.5%, reduction in CFU/mL. In contrast, when non-pigmented ΔCylE GBS mutant cultures were tested, there was no significant difference found between the ΔCylE GBS treated with only 12 mM of H2O2 and the ΔCylE GBS treated with both light and H2O2. (Figure 3b) When the effectiveness of H2O2 in killing either the pigmented or non-pigmented GBS was compared, it was observed that, for H2O2 only samples, WT GBS CFU were reduced by about 1 log, while ΔCylE GBS CFU was reduced by around 1.5 log, indicating that ΔCylE GBS was generally more susceptible to ROS consistent with previous studies.
Figure 3.
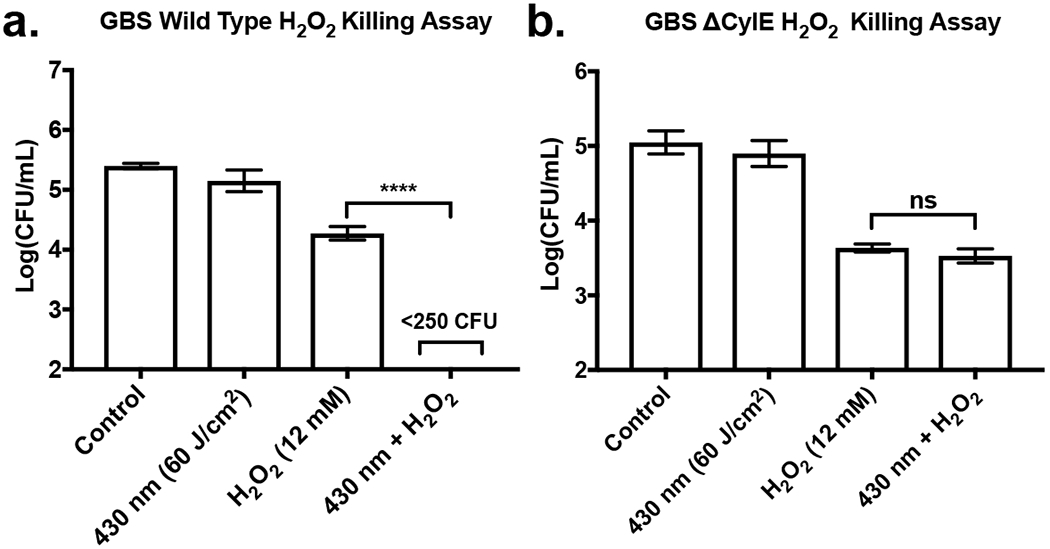
H2O2 killing assays of wild type and mutant GBS, exhibited through differences in mean bacterial CFU population and standard deviation following incubation within H2O2 environment. (a) Pigment expressing GBS exposed to 60 J/cm2 of 430 nm nanosecond pulsed light and incubated with 12 mM of H2O2 for 1 hour. Combination of 430 nm light exposure and H2O2 resulted in eradication of GBS. (b) Pigment deficient ΔCylE GBS exposed to 60 J/cm2 of pulsed light and incubated with 12 mM of H2O2 for 1 hour. Combination of 430 nm light exposure and H2O2 resulted in no significant improvement in H2O2 antimicrobial activity. ****: p < 0.0001.
The eradication of WT GBS following photobleaching indicates that photobleaching is capable of significantly reducing antioxidant activity present within GBS, and by doing so, significantly reduce the ability for GBS to resist oxidative stress, a phenomena that is not observed to the same extent in ΔCylE GBS. While the granadaene pigment contributes significantly to antioxidant activity in GBS, it is not the only antioxidant factor in GBS, as the GBS genome has been found to encode peroxidases like thiol peroxidase and alkylhydroperoxide reductase, with GBS NADH peroxidase have been shown to exhibit anti-oxidant activity. (33, 34) For the nonpigmented ΔCylE GBS, while the bacterium exhibited greater sensitivity to H2O2 than the pigmented bacterium, the survival of the non-pigmented GBS can potentially be attributed to possible upregulation of other antioxidant mechanisms in response to the lack of pigment presence, an aspect that would not occur in the clinically relevant pigment expressing wild type GBS. Overall, these results demonstrate that photobleaching of GBS granadaene can significantly reduces the ability of GBS to survive oxidative stress, allowing for the improved effectiveness and activity of ROS like H2O2.
Granadaene photobleaching sensitizes GBS to daptomycin
Since granadaene pigment is localized within the membrane of S. agalactaie, we sought next to quantify the impact of photobleaching on bacterial membrane permeability using SYTOX Green (Figure 4a). Removing or breaking up the pigment should theoretically result in an increase in membrane permeability. The results demonstrate that exposure to 430 nm light results in a near instant increase in the SYTOX Green fluorescence, with a 35.9% increase in measured fluorescence following 30 J/cm2 dosages from the control at time point t = 0 minutes. By 60 minutes, the fluorescence of GBS exposed to 30 J/cm2 of light increased by an average of 44.2% respectively. As a fluorescent dye with a high affinity for nucleic acids, changes in SYTOX Green fluorescence is an ideal measurement to quantify alteration in membrane permeability of bacteria (35). Since SYTOX is normally membrane impermeable, the dye only attaches and fluoresces to nucleic acids found in membrane compromised bacteria. Thus, the significant increases in measured fluorescence for 430 nm is indicative of the membrane disrupting effects of granadaene photobleaching.
Figure 4.
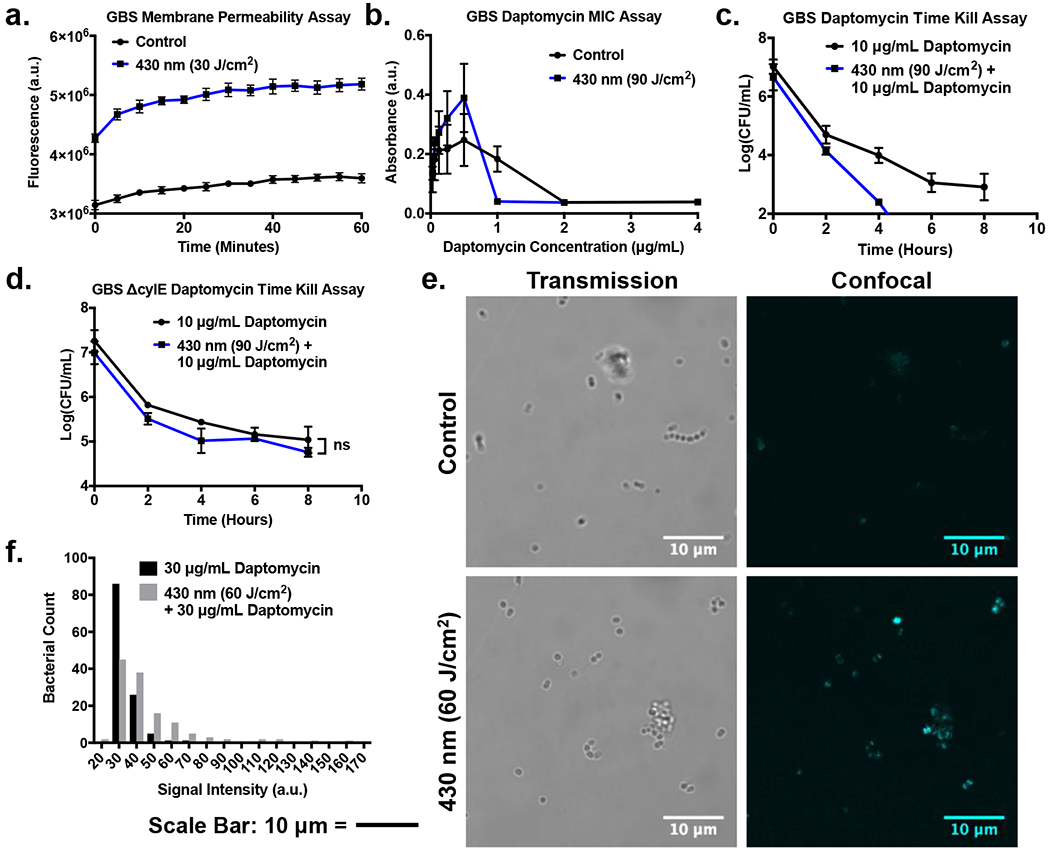
Granadaene photobleaching facilitates cellular uptake and antimicrobial activity of daptomycin. (a) Membrane permeability assay of GBS exposed to 430 nm nanosecond pulsed light utilizing SYTOX Green. Instantaneous increase in SYTOX Green fluorescence following light exposure indicates immediate membrane permeabilization. (b) Minimum Inhibitory Concentration (MIC) of daptomycin on GBS photobleached with blue light decreases 2-fold from 2 μg/mL to 1 μg/mL. (c) CFU count of time killing assay of GBS exposed to 90 J/cm2 of pulsed blue light. GBS exhibits faster response to daptomycin following initial light treatment. (d) CFU count of time killing assay of pigment deficient ΔCylE GBS exposed to 90 J/cm2 of pulsed blue light and treated with 10 μg/mL for 8 hours. Light exposed ΔCylE GBS lacks the same daptomycin enhancement observed in the pigmented strain. (e) Confocal imaging of light exposed GBS treated with 30 μg/mL of daptoymcin-BODIPY for 30 minutes. The light treated GBS exhibits greater BODIPY fluorescence compared to the control, indicating higher daptomycin intake. (f) Histogram of signal intensities of GBS bacteria treated with 30 μg/mL of daptoymcin-BODIPY for 30 minutes. Light treated GBS exhibits a greater number of bacteria with higher signal intensities compared to the control.
Since photobleaching increased membrane permeability of GBS, we next studied the impact of increased membrane permeability on the effectiveness of various antibiotics such as daptomycin, penicillin V, and ampicillin. Following exposure to 90 J/cm2 of 430 nm light GBS showed a twofold decrease in daptomycin minimal inhibitory concentration (MIC), from 2 μg/mL to 1 μg/mL, (Figure 4b). A time kill assay of GBS treated with 10 μg/mL of daptomycin and 90 J/cm2 of 430 nm light demonstrated an additional 2 log reduction in CFU with light therapy and daptomycin compared to daptomycin alone after 4 hours in a TSB solution (Figure 4c). To query the role of the granadaene pigment in the improved daptomycin performance, non-pigment expressing ΔCylE GBS was evaluated. In contrast to wild type GBS, a time kill assay of untreated and light treated ΔCylE GBS found no significant enhancement of daptomycin over the course of 8 hours (Figure 4d). To examine more into the potential impact of light exposure on the effectiveness of daptomycin on pigment expressing GBS, light exposed and non-light exposed GBS was treated with BODIPY dye conjugated daptomycin and imaged using a confocal microscope (Figure 4e). Based on the images taken, the thresholding and particle tracking capabilities of ImageJ were used to obtain the average intensities of individual cells within the exposed and non-exposed GBS groups, all of which were charted onto a histogram (Figure 4f). Based on the histogram, the average cell intensity of the daptomycin only treated GBS was 33.12 ± 6.6, while the average intensity of the daptomycin plus light treated GBS was 46.11 ± 22.71.
Because daptomycin has an unique membrane-dependent mechanism of action, other antibiotics commonly used to treat GBS infections, such as ampicillin and penicillin V, were investigated to examine the potential effect of granadaene photobleaching on their effectiveness (36). For ampicillin, a time kill assay on both pigment-expressing GBS (Figure 5a) and the isogenic pigment deficient ΔCylE GBS (Figure 5b) showed that light exposure had no statistically significant impact on the effectiveness of ampicillin. For penicillin V treatment, 60 J/cm2 of 430 nm exposure resulted in a minor, less then 1 log change in the CFU count of both GBS (Figure 5c) and pigment deficient ΔCylE GBS after 6 hours of incubation (Figure 5d)
Figure 5.
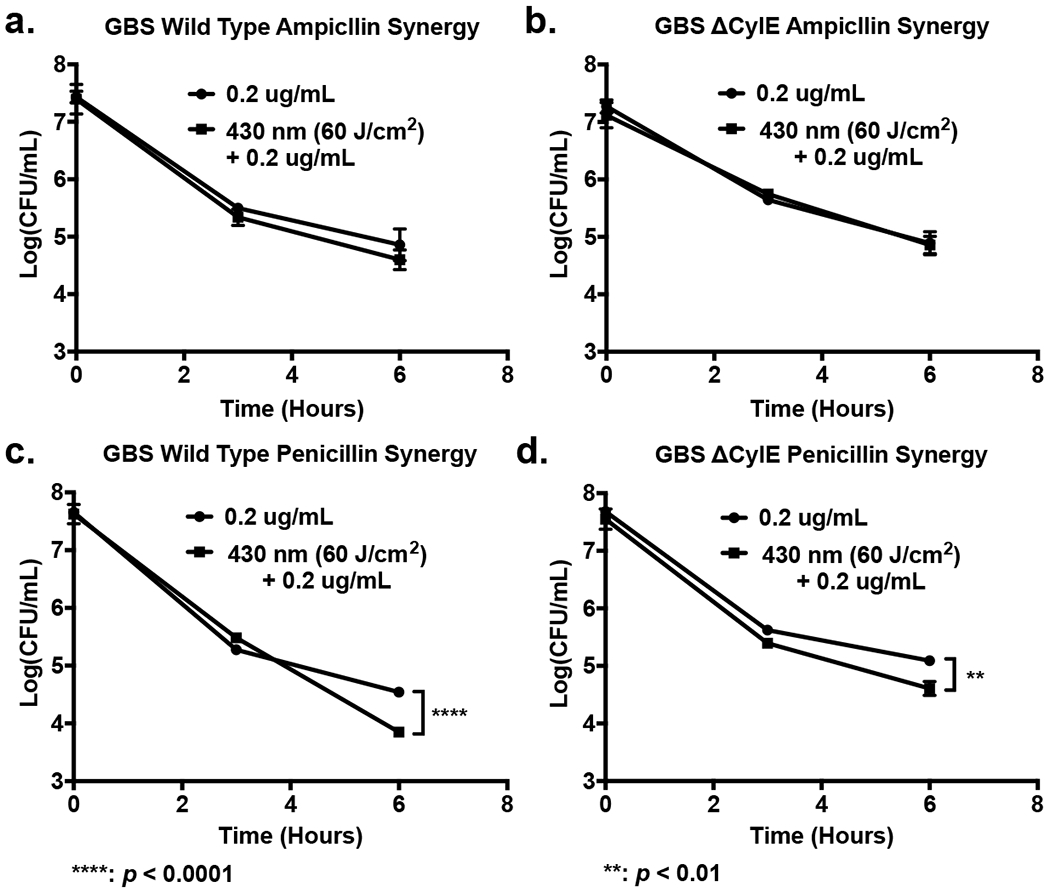
Granadaene photobleaching exerts little impact on beta-lactam antibiotic activity on GBS. (a) CFU Time kill assay of GBS exposed to 430 nm pulsed light and treated with 0.2 μg/mL of ampicillin. No significant changes in ampicillin performance observed. (b) CFU Time kill assay of ΔCylE GBS exposed to 430 nm pulsed light and treated with 0.2 μg/mL of ampicillin. No significant changes in ampicillin performance observed. (c) CFU Time kill assay of GBS exposed to 430 nm pulsed light and treated with 0.2 μg/mL of penicillin V. Only minor enhancement of penicillin V activity observed after 6 hours of incubation. (d) CFU Time kill assay of ΔCylE GBS exposed to 430 nm pulsed light and treated with 0.2 μg/mL of penicillin V. Only minor enhancement of penicillin V activity observed after 6 hours of incubation, although not as large as the enhancement observed in the pigment expressing GBS. ****: p < 0.0001; **: p < 0.01.
When examining these results, it is apparent that granadaene photobleaching can increase the effectiveness of daptomycin in clearance of GBS, but has no significant impact of the effectiveness of penicillin or ampicillin. Starting with daptomycin, it appears that the improvement in daptomycin activity for the light treated pigment expressing GBS, and the absence of improved daptomycin activity observed in the photobleached pigment deficient ΔCylE GBS suggests that the removal of the pigment is responsible for the improvement in daptomycin performance. As a lipopeptide antibiotic, daptomycin is known to induce damage on Gram-positive bacteria through its ability to disrupt the cell membrane of bacteria (12, 37). By binding to and aggregating within the cell membrane, daptomycin is able to alter the membrane curvature and generate ion leaks within the membrane, rapidly altering the membrane potential and inhibiting protein synthesis, causing cell death (38). Given the membrane disrupting capabilities of granadaene photobleaching, it was confirmed through confocal imaging that the membrane insertion and aggregation capabilities of daptomycin are enhanced given following photobleaching of GBS. For the BODIPY-daptomycin treated and light treated GBS, both the average cell intensity and standard deviation were found to be higher then that of the non-light treated GBS. While the higher average intensity of the daptomycin plus light treated GBS cells indicates higher average daptomycin intake, the larger standard deviation observed can be attributed to the contribution of individual high intensity (120+) GBS cells as well, which were only observed within the light treated GBS samples. In addition, a greater number of light treated GBS exhibited higher average intensities than the non-treated GBS, with 47.66% of photobleached GBS exhibiting average signal intensities above 40, compared with the 8.4% observed for control GBS. Given these trends, it is clear that photobleached GBS are capable of improved daptomycin aggregation based on increased fluorescence, allowing for improved efficiency and performance of daptomycin in 430 nm light exposed GBS. Thus, these results indicate that granadaene photobleaching is capable of improving the antimicrobial activity of daptomycin against pigment expressing GBS by disrupting the membrane integrity of the GBS bacterium and allowing for improved daptomycin incorporation within the membrane.
Moving on the ampicillin and penicillin data, it appears that photobleaching had little impact on the antimicrobial activity of β-lactam antibiotics. Given that β -lactam antibiotics induce antibacterial activity by inhibiting the synthesis of the peptidoglycan layer in bacteria by binding to penicillin binding proteins responsible for catalyzing peptidoglycan crosslinks, the lack of improved activity observed with both the ampicillin and the penicillin may be due to how the penicillin binding proteins are often found on the plasma membrane of bacteria, meaning that there is less need for the β -lactam antibiotics to necessarily penetrate the membrane in order to induce its antimicrobial mechanism of action. (39, 40). This means that membrane permeability is less of an issue for β -lactam antibiotics, especially since as a gram-positive bacterium; GBS lacks a protective outer membrane like gram-negative organisms. However, given the increasing development of reduced penicillin susceptibility in GBS, it is possible that photobleaching may be able to eventually provide an avenue to reduce potential penicillin resistance in GBS if it were to become widespread within the population (41, 42). However, it is clear with the current results that the pigment photobleaching process is more effective with antimicrobials like daptomycin that are dependent on membrane insertion and disruption.
In order to confirm that the observed absorbance peaks and behaviors were indeed attributable to the pigment, the impact of 430 nm light exposure was also explored on pigment-deficient ΔCylE GBS. When the absorbance spectrum of ΔCylE GBS was examined, no significant peaks were observed in the control sample and light exposure had no effect on the absorbance spectrum (Figure S1a). However, when analyzed using the Raman spectroscopy, significant peaks were found to be present at the 1126 cm−1 and 1512 cm−1 (Figure S1b). These peaks were found to decrease by 66% when exposed to 60 J/cm2 of 430 nm light. In addition, membrane permeability studies found that, under 30 J/cm2 of 430 nm light, ΔCylE GBS exhibited a 19.8% increase in measured fluorescence immediately following bleaching at time point t = 0 minutes (Figure S1c). By 60 minutes, the fluorescence of GBS exposed to 30 J/cm2 of light increased by an average of 62.8% respectively. From the results, it appears that while the pigment coloration is no apparent, double conjugated bond structures are still present within the membrane of even the pigment deficient ΔCylE GBS. This observed behavior might be due to how the CylE gene is only responsible for the attachment of the L-ornithine to the end of the double conjugated fatty acid chain in granadaene synthesis (23). While the removal of the CylE gene does prevent the hemolytic activity and pigment coloration in GBS, the cyl operon may still be capable of forming fatty acid chains that are integrated into the membrane, explaining the increase in membrane permeability and decrease in Raman signal observed following 430 nm exposure, although not to the same extent as the pigment expressing wild type GBS. The lower amount of photosensitive conjugated fatty acid chains present in the membrane for the ΔCylE GBS may explain why light treatment had no significant impact on the ΔCylE GBS when treated with daptomycin. Research on this phenomenon can be explored in the future utilizing GBS strains with the cyl operon completely removed.
In summary, the current work presents a non-antibiotic reliant method of reducing the hemolytic and antioxidant activity of GBS, thereby reducing the potential virulence and infectivity of the bacteria. In addition, 430 nm exposure was also shown to disrupt membrane permeability of GBS and improve the activity of antimicrobials like H2O2 and daptomycin.
This discovery that the granadaene pigment is susceptible to photobleaching, and that this process can render GBS less virulent and more susceptible to antimicrobials is of particular interest within the clinical settings due to how prevalent pigment expressing GBS is within clinical infections, with pigment producing GBS being found within 97% of all GBS strains isolated from clinical specimens (19, 43). This aspect, combined with the fact that most GBS infections in adults manifest as skin and soft tissue infections, means that 430 nm light can easily access the area of the skin containing the GBS infection and therefore potentially enhance current treatment of S. agalactiae skin and soft tissue infection in adult patients, which would reduce the risk of life threatening invasive infections such as bacteremia and bone/joint infections (11, 44, 45). The improved daptomycin efficiency observed could be particularly useful for treatment of immunocompromised patients, such as elderly or diabetic individuals, with GBS infection (7, 46). The light applications protocols used within this study, in both fluence and dosages, all fall below the American National Standards Institute (ANSI) safety limitations for lasers applied to the skin (47). By operating below ANSI standards, the conditions tested within this paper have the potential of being applied directly to clinical settings, allowing for potentially easier implementation of this technology within healthcare settings.
While granadaene photobleaching has the dual effect of reducing virulence and increasing antimicrobial susceptibility, other potential applications of photo treatment exist. While photobleaching had no significant impact on β -lactam antibiotic activity, it is conceivable that photobleaching would have a more pronounced effect on GBS strains that are moderately resistant to antibiotics. Therefore, future photobleaching studies will focus on the potential to improve penicillin treatment in S. agalactiae strains that are resistant to penicillin. Additional studies will address if pigment photobleaching could resensitize resistant S. agalactiae strains to conventional antibiotics such as erythromycin and clindamycin (48). Overall, S. agalactiae granadaene photobleaching presents a potential method of reducing virulence and increasing antimicrobial susceptibility, creating a non-invasive way to improve upon current treatment of hard to treat GBS infections.
Supplementary Material
ACKNOWLEDGEMENTS:
We thank the Blood Donor Center at the Boston Children’s Hospital for providing blood samples and the Boston University Micro/Nano Imaging Facilities for usage of their equipment. This project was supported by the Boston University Start-Up Fund and NIH R01 AI 141439.
Footnotes
This manuscript was previously submitted as a bioRxiv Pre-Print (1)
REFERENCES:
- 1.Jusuf S, Dong P-T, Hui J, Ulloa ER, Liu GY and Cheng J-X (2020) Granadaene Photobleaching Reduces the Virulence and Increases Antimicrobial Susceptibility of Streptococcus agalactiae. bioRxiv 2020.03.31.019372. 10.1101/2020.03.31.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisno AL, Peter GS and Kaplan EL (2002) Diagnosis of Strep Throat in Adults: Are Clinical Criteria Really Good Enough? Clin Infect Dis 35, 126–129. 10.1086/342056. [DOI] [PubMed] [Google Scholar]
- 3.Arends JP and Zanen HC (1988) Meningitis Caused by Streptococcus suis in Humans. Rev Infect Dis 10, 131–137. 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- 4.Stevens DL (1992) Invasive Group A Streptococcus Infections. Clin Infect Dis 14, 2–13. 10.1093/clinids/14.1.2. [DOI] [PubMed] [Google Scholar]
- 5.Török E and Day N (2005) Staphylococcal and streptococcal infections. Medicine 33, 97–100. 10.1383/medc.33.5.97.64964. [DOI] [Google Scholar]
- 6.Edwards MS, Nizet V and Baker CJ (2011) CHAPTER 12 - Group B Streptococcal Infections. In Infectious Diseases of the Fetus and Newborn (Seventh Edition) (Edited by Remington JS, Klein JO, Wilson CB, Nizet V, and Maldonado YA), pp. 419–469. W.B. Saunders, Philadelphia. 10.1016/B978-1-4160-6400-8.00012-2. [DOI] [Google Scholar]
- 7.High KP, Edwards MS and Baker CJ (2005) Group B Streptococcal Infections in Elderly Adults. Clin Infect Dis 41, 839–847. 10.1086/432804. [DOI] [PubMed] [Google Scholar]
- 8.Barcaite E, Bartusevicius A, Tameliene R, Kliucinskas M, Maleckiene L and Nadisauskiene R (2008) Prevalence of maternal group B streptococcal colonisation in European countries. Acta Obstet Gynecol Scand 87, 260–271. 10.1080/00016340801908759. [DOI] [PubMed] [Google Scholar]
- 9.Benitz WE, Gould JB and Druzin ML (1999) Risk Factors for Early-onset Group B Streptococcal Sepsis: Estimation of Odds Ratios by Critical Literature Review. Pediatrics 103, e77–e77. 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- 10.Skoff TH, Farley MM, Petit S, Craig AS, Schaffner W, Gershman K, Harrison LH, Lynfield R, Mohle-Boetani J, Zansky S, Albanese BA, Stefonek K, Zell ER, Jackson D, Thompson T and Schrag SJ (2009) Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990-2007. Clin. Infect. Dis. 49, 85–92. 10.1086/599369. [DOI] [PubMed] [Google Scholar]
- 11.Tyrrell GJ, Senzilet LD, Spika JS, Kertesz DA, Alagaratnam M, Lovgren M and Talbot JA (2000) Invasive Disease Due to Group B Streptococcal Infection in Adults: Results From a Canadian, Population-Based, Active Laboratory Surveillance Study—1996. J Infect Dis 182, 168–173. 10.1086/315699. [DOI] [PubMed] [Google Scholar]
- 12.Steenbergen JN, Alder J, Thorne GM and Tally FP (2005) Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J Antimicrob Chemother 55, 283–288. 10.1093/jac/dkh546. [DOI] [PubMed] [Google Scholar]
- 13.Lipsky BA and Berendt AR (2000) Principles and practice of antibiotic therapy of diabetic foot infections. Diabetes/Metabolism Research and Reviews 16, S42–S46. . [DOI] [PubMed] [Google Scholar]
- 14.Farley MM and Strasbaugh LJ (2001) Group B Streptococcal Disease in Nonpregnant Adults. Clin Infect Dis 33, 556–561. 10.1086/322696. [DOI] [PubMed] [Google Scholar]
- 15.Dahesh S, Hensler ME, Van Sorge NM, Gertz RE, Schrag S, Nizet V and Beall BW (2008) Point Mutation in the Group B Streptococcal pbp2x Gene Conferring Decreased Susceptibility to β-Lactam Antibiotics. Antimicrob Agents Chemother 52, 2915–2918. 10.1128/AAC.00461-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maisey HC, Doran KS and Nizet V (2008) Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev Mol Med 10, e27. 10.1017/S1462399408000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajagopal L (2009) Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiology 4, 201–221. 10.2217/17460913.4.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa-Fraile M, Rodríguez-Granger J, Haidour-Benamin A, Cuerva JM and Sampedro A (2006) Granadaene: Proposed Structure of the Group B Streptococcus Polyenic Pigment. Appl Environ Microbiol 72, 6367–6370. 10.1128/AEM.00756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merritt K and Jacobs NJ (1978) Characterization and incidence of pigment production by human clinical group B streptococci. Journal of Clinical Microbiology 8, 105–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahl W and Sies H (2003) Antioxidant activity of carotenoids. Molecular Aspects of Medicine 24, 345–351. 10.1016/S0098-2997(03)00030-X. [DOI] [PubMed] [Google Scholar]
- 21.Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, Tissi L and Nizet V (2004) Sword and shield: Linked group B streptococcal β-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. PNAS 101, 14491–14496. 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE and Nizet V (2001) Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol Microbiol 39, 236–247. 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 23.Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Waldorf KMA and Rajagopal L (2013) A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. Journal of Experimental Medicine 210, 1265–1281. 10.1084/jem.20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tapsall JW (1987) Relationship between pigment production and haemolysin formation by Lancefield group B streptococci. J. Med. Microbiol. 24, 83–87. 10.1099/00222615-24-1-83. [DOI] [PubMed] [Google Scholar]
- 25.Rosa-Fraile M, Dramsi S and Spellerberg B (2014) Group B streptococcal haemolysin and pigment, a tale of twins. FEMS Microbiol Rev 38, 932–946. 10.1111/1574-6976.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.N VKB, Kampe B, Rösch P. and Popp J (2015) Characterization of carotenoids in soil bacteria and investigation of their photodegradation by UVA radiation via resonance Raman spectroscopy. Analyst (Cambridge, U. K.) 140, 4584–4593. 10.1039/C5AN00438A. [DOI] [PubMed] [Google Scholar]
- 27.Dong P-T, Mohammad H, Hui J, Leanse LG, Li J, Liang L, Dai T, Seleem MN and Cheng J-X (2019) Photolysis of Staphyloxanthin in Methicillin-Resistant Staphylococcus aureus Potentiates Killing by Reactive Oxygen Species. Adv. Sci. 6, 1900030. 10.1002/advs.201900030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui J, Dong P-T, Liang L, Mandal T, Li J, Ulloa ER, Zhan Y, Jusuf S, Zong C, Seleem MN, Liu GY, Cui Q and Cheng J-X (2020) Photo-Disassembly of Membrane Microdomains Revives Conventional Antibiotics against MRSA. Advanced Science 7, 1903117. 10.1002/advs.201903117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Rumaithi A (2019) Raman Spectrum Baseline Removal - File Exchange - MATLAB Central. Mathworks File Exchange. Available at: https://www.mathworks.com/matlabcentral/fileexchange/69649. Accessed on 5 September 2019.
- 30.Schulze HG, Foist RB, Okuda K, Ivanov A and Turner RFB (2012) A Small-Window Moving Average-Based Fully Automated Baseline Estimation Method for Raman Spectra. Appl. Spectrosc., AS 66, 757–764. [DOI] [PubMed] [Google Scholar]
- 31.Merlin JC (1985) Resonance Raman spectroscopy of carotenoids and carotenoid-containing systems. Pure and Applied Chemistry 57, 785–792. 10.1351/pac198557050785. [DOI] [Google Scholar]
- 32.Armistead B, Herrero-Foncubierta P, Coleman M, Quach P, Whidbey C, Justicia J, Tapia R, Casares R, Millán A, Haidour A, Granger JR, Vornhagen J, Santana-Ufret V, Merillat S, Adams Waldorf K, Cuerva JM and Rajagopal L (2020) Lipid analogs reveal features critical for hemolysis and diminish granadaene mediated Group B Streptococcus infection. Nature Communications 11, 1502. 10.1038/s41467-020-15282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaser P, Rusniok C, Buchrieser C, Chevalier F, Frangeul L, Msadek T, Zouine M, Couvé E, Lalioui L, Poyart C, Trieu-Cuot P and Kunst F (2002) Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Molecular Microbiology 45, 1499–1513. 10.1046/j.1365-2958.2002.03126.x. [DOI] [PubMed] [Google Scholar]
- 34.Korir ML, Flaherty RA, Rogers LM, Gaddy JA, Aronoff DM and Manning SD (2018) Investigation of the Role That NADH Peroxidase Plays in Oxidative Stress Survival in Group B Streptococcus. Front Microbiol 9, 2786. 10.3389/fmicb.2018.02786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lebaron P, Catala P and Parthuisot N (1998) Effectiveness of SYTOX Green Stain for Bacterial Viability Assessment. Appl Environ Microbiol 64, 2697–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim KS (1985) Antimicrobial Susceptibility of GBS. Neonatal Group B Streptococcal Infections 35, 83–89. 10.1159/000410363. [DOI] [PubMed] [Google Scholar]
- 37.Baltz RH (2009) Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Current Opinion in Chemical Biology 13, 144–151. 10.1016/j.cbpa.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 38.Pogliano J, Pogliano N and Silverman JA (2012) Daptomycin-Mediated Reorganization of Membrane Architecture Causes Mislocalization of Essential Cell Division Proteins. J Bacteriol 194, 4494–4504. 10.1128/JB.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donowitz GR and Mandell GL (1988) Beta-Lactam Antibiotics. New England Journal of Medicine 318, 419–426. 10.1056/NEJM198802183180706. [DOI] [PubMed] [Google Scholar]
- 40.Georgopapadakou NH and Liu FY (1980) Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother 18, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimura K, Suzuki S, Wachino J, Kurokawa H, Yamane K, Shibata N, Nagano N, Kato H, Shibayama K and Arakawa Y (2008) First Molecular Characterization of Group B Streptococci with Reduced Penicillin Susceptibility. Antimicrobial Agents and Chemotherapy 52, 2890–2897. 10.1128/AAC.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gizachew M, Tiruneh M, Moges F and Tessema B (2019) Streptococcus agalactiae maternal colonization, antibiotic resistance and serotype profiles in Africa: a meta-analysis. Ann Clin Microbiol Antimicrob 18, 14. 10.1186/s12941-019-0313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noble MA, Bent JM and West AB (1983) Detection and identification of group B streptococci by use of pigment production. J Clin Pathol 36, 350–352. 10.1136/jcp.36.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tazi A, Morand PC, Réglier-Poupet H, Dmytruk N, Billoët A, Antona D, Trieu-Cuot P and Poyart C (2011) Invasive group B streptococcal infections in adults, France (2007–2010). Clinical Microbiology and Infection 17, 1587–1589. 10.1111/j.1469-0691.2011.03628.x. [DOI] [PubMed] [Google Scholar]
- 45.Lefebvre N, Forestier E, Mohseni-Zadeh M, Remy V, Lesens O, Kuhnert C, Poindron V, Riegel P, Piémont Y, Christmann D and Hansmann Y (2007) [Invasive Streptococcus agalactiae infections in non-pregnant adults]. Med Mal Infect 37, 796–801. 10.1016/j.medmal.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Yanai H, Hamasaki H, Tsuda N, Adachi H, Yoshikawa R, Moriyama S, Masui Y and Mishima S (2012) Group B streptococcus infection and diabetes: A review. JMA 4, 1–5. 10.5897/JMA11.088. [DOI] [Google Scholar]
- 47.ANSI (2014) American National Standard for Safe Use of Lasers ANSI Z136. 1–2014. [Google Scholar]
- 48.Betriu C, Culebras E, Gómez M, Rodríguez-Avial I, Sánchez BA, Ágreda MC and Picazo JJ (2003) Erythromycin and Clindamycin Resistance and Telithromycin Susceptibility in Streptococcus agalactiae. Antimicrobial Agents and Chemotherapy 47, 1112–1114. 10.1128/AAC.47.3.1112-1114.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


