Childhood asthma is a heterogenous condition with multiple endophenotypes, with most treatments focused on type 2 allergic/eosinophilic inflammation. Despite the use of treatments targeting type 2 inflammation, a subset of children continue to have asthma exacerbations. A cross-sectional analysis of adults enrolled into the National Institutes of Health/National Heart, Lung, and Blood Institute sponsored Severe Asthma Research Program-3 (SARP-3) cohort study by Peters et al. demonstrated that high plasma IL-6 is associated with metabolic dysfunction, reduced lung function, and greater asthma severity, independent of body mass index (BMI)(1). Prospective analysis of this cohort also found a strong association between plasma IL-6 and an increased rate of asthma exacerbations over a 3-year period(2). Very few studies exist evaluating peripheral blood IL-6 as a biomarker for asthma morbidity and severity in children/adolescents(3).
To determine if baseline plasma IL-6 is associated with increased asthma morbidity and severity in a pediatric cohort, data from the multicenter SARP-3 study of 155 children with asthma (median age 11.4 years, range 6.1-18.4) who completed the 3-year longitudinal study and had complete data for BMI assessment, baseline plasma IL-6 level, and asthma outcome measures were included in this analysis. The main objective of the SARP-3 study was to advance the understanding of severe asthma through integration of mechanistic studies with phenotype classification. SARP-3 included two baseline visits in which participants underwent detailed characterization studies and provided blood and induced sputum samples. Details of the SARP-3 protocol have previously been described(1). Plasma IL-6 was measured with a high sensitivity assay with a LLOD of 0.16pg/mL (Quantikine ELISA Kit, R&D Systems, Minneapolis, MN).
A pediatric reference interval of normal does not exist for plasma IL-6. The distribution of plasma IL-6 levels is displayed in Figure E1. The median value was 1.188 pg/mL (interquartile range, 0.803–1.806 pg/mL). Given the unknown threshold of IL-6 risk levels and the skewness of the distribution, children were stratified into quartiles according to baseline IL-6 levels: Low IL-6 (<0.805pg/mL); Med-Low IL-6 (0.805–1.19pg/mL); Med-High IL-6 (1.2–1.828pg/mL); and High IL-6 (≥1.828pg/mL). Linear, logistic, and negative binomial regression models assessed relationships between plasma IL-6 groups and BMI percentile, inflammatory markers, lung function and asthma outcomes. Longitudinal analysis evaluating the association between baseline IL-6 and asthma outcomes was performed using linear and generalized linear mixed models with a random intercept at the participant level. All analyses were adjusted for BMI and gender. IL-6 levels were significantly higher in females than males; the proportion of females was larger in the Med-High (54%) and High (42%) IL-6 groups compared to the Med-Low (26%) and Low (31%) IL-6 groups (χ2(3)=7.82, p=0.049). There were no between-group differences for other potential confounders including age, race, ethnicity, household income, and ICS use (all p>0.05). Analyses were performed using STATA 16.1.
Comparable proportions of participants were Black (41%) or White (41%). Forty-three percent of the participants were healthy weight, 21% overweight, and 35% obese. On average, baseline lung function showed an obstructive pattern, with a mean FEV1/FVC ratio of 76.7%. Ninety-five percent of participants reported using ICS over the prior 12 months. Baseline characteristics of this study population are detailed in Table E1.
Plasma IL-6 positively correlates with BMI in adults(1). Our findings were comparable in children, as increasing plasma IL-6 was associated with higher BMI percentiles (p<0.001)(Table 1). No other cross-sectional associations between IL-6 and asthma outcomes were appreciated. In contrast to what was previously reported in the adult SARP-3 cohort(1) and recent analysis of the Asthma Phenotypes in the Inner-City (APIC) pediatric cohort within the Inner-City Asthma Consortium study(3), there was no association between IL-6 and total white blood cells or blood/sputum neutrophils(Table 1). Additionally, IL-6 was not associated with type 2 inflammatory markers including blood/sputum eosinophils, serum IgE measures, or FENO, consistent with findings from adult and pediatric studies(1, 3)(Table 1). Higher levels of CRP were observed with increasing IL-6 levels (p<0.001)(Table 1). Elevated CRP is associated with metabolic syndrome and a predictor for diabetes(4) and cardiovascular disease. Thus, IL-6 may be a useful biomarker for the early signs of metabolic dysfunction in children with asthma, supporting a distinct asthma endotype.
TABLE 1.
Associations between Plasma IL-6, Asthma Outcome Measures, and Baseline Markers of Inflammation
| Low IL-6 | Med-Low IL-6 | Med-High IL-6 | High IL-6 | p-value** | |
|---|---|---|---|---|---|
| Cross-sectional* | (n=39 ) | (n= 39) | (n= 39) | (n= 38) | |
| Clinical Outcome Measures | |||||
| BMI Percentile, mean (SD) | 63.2 ± 27.4 | 79.3 ± 20.8a | 84.8 ± 19.3a | 78.1 ± 30.8a | <0.001 |
| Asthma exacerbation in the past yr, mean (SD)*** | 1.1 ± 1.2 | 2.1±2.8 | 2.5±3.3 | 1.8±1.9 | 0.13 |
| Health care utilization for asthma in the past yr, n (%) | 23 (59) | 24 (62) | 23 (59) | 24 (63) | 0.98 |
| ER visit for asthma in the past yr, n (%) | 16 (41) | 22 (56) | 23 (59) | 18 (47) | 0.72 |
| Hospitalization for asthma in the past yr, n (%) | 9 (23) | 12 (31) | 7 (18) | 11 (29) | 0.40 |
| Missed days of school in the past yr, mean (SD) | 3.8 ± 8.6 | 2.8 ± 6.6 | 4.8 ± 14.7 | 2.0 ± 4.1 | 0.64 |
| Severe asthma, n (%)§ | 20 (51) | 25 (64) | 21 (54) | 23 (61) | 0.54 |
| Spirometry Outcome Measures | |||||
| FEV1 (% predicted), mean (SD) | 93.8 ± 11.4 | 89.8 ± 13.6 | 90.2 ± 16.1 | 88.1 ± 15.0 | 0.22 |
| FVC (% predicted), mean (SD) | 105.5 ± 9.9 | 102.3 ± 15.1 | 104.0 ± 10.5 | 102.2 ± 15.9 | 0.21 |
| FEV1/FVC, mean (SD) | 77.8 ± 7.3 | 77.0 ± 9.2 | 75.7 ± 9.5 | 76.1 ± 9.3 | 0.85 |
| FEV1 (% predicted) max. absolute reversibility, mean (SD) | 10.8 ± 11.5 | 11.2 ± 9.5 | 15.6 ± 15.8 | 11.7 ± 12.3 | 0.50 |
| Non-Th2 Biomarkers | |||||
| Plasma CRP log10, mean (SD) | 10.9 ± 1.3 | 11.7 ± 1.3 | 12.4 ± 1.1b | 12.9 ± 1.5b | <0.001 |
| Total white blood cells (K/uL), mean (SD) | 6.3 ± 2.2 | 6.8 ± 2.8 | 7.1 ± 1.9 | 7.5 ± 2.0 | 0.71 |
| Blood neutrophils (cells/uL), mean (SD) | 2933 ± 2068 | 3332 ± 2467 | 3587 ± 1673 | 3945 ± 1713 | 0.71 |
| Sputum neutrophils (%), mean (SD) | 35.4 ± 17.7 | 49.4 ± 25.9 | 42.2 ± 24.2 | 58.6 ± 21.7 | 0.27 |
| Th2 Biomarkers | |||||
| Blood eosinophils (cells/uL), mean (SD) | 472 ± 332 | 362 ± 268 | 362 ± 290 | 383 ± 285 | 0.45 |
| Sputum eosinophils (%), mean (SD) | 17.2 ± 25.4 | 5.2 ± 7.8 | 5.7 ± 15.2 | 3.3 ± 5.0 | 0.54 |
| Serum IgE Measures | |||||
| IgE (kU/L), mean (SD) | 916 ± 1186 | 740 ± 1356 | 867 ± 1614 | 938 ± 1141 | 0.96 |
| ≥ 1 positive specific IgE, n (%) | 36 (92) | 32 (84) | 36 (95) | 34 (92) | 0.43 |
| FENO (ppb), mean (SD) | 40.2 ± 37.8 | 35.5 ± 25.1 | 35.7 ± 41.9 | 31.2 ± 44.5 | 0.84 |
=p<0.01 vs. Low IL-6;
= p<0.001 vs. Low IL-6
There was full data for all outcomes (n=155) with exception of 40 sputum samples, 146 IgE samples, 152 specific IgE, and 152 FENO samples
The p values were generated from linear, logistic, and negative binomial regression models, adjusted for BMI status (healthy weight, overweight, obese) and gender.
Asthma exacerbation was defined as a burst of systemic corticosteroid lasting ≥ 3 days for treatment of worsening asthma control.
The classification of severe asthma was determined using criteria from the American Thoracic Society/European Thoracic Society guidelines.
Abbreviations: BMI = body mass index; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; FEF25-75 = forced expiratory flow between the 25th and 75th percentile of forced vital capacity; SD = standard deviation; CRP = C-reactive protein; IgE = Immunoglobulin E; FENO = fraction of exhaled nitric oxide; ppb = parts per billion
The SARP-3 study design included a 3-year longitudinal follow-up period, allowing us to detect significant differences between plasma IL-6 groups, prospectively. Longitudinal analysis demonstrated relationships between plasma IL-6 and measures of asthma severity including propensity for asthma exacerbations requiring systemic corticosteroids and lung function impairment during follow-up. Since the low IL-6 group had markedly fewer prospective exacerbations, we combined the three medium/high IL-6 groups and found that IL-6 levels greater than or equal to 0.805pg/mL were associated with twice as many exacerbations compared to levels less than 0.805pg/mL (IRR=2.09, 95%CI [1.02, 4.28],p=0.04)(Table 2). Likewise, IL-6 levels greater than or equal to 0.805pg/mL were associated with lower FEV1 and FVC values, β=−5.8 [−11.2,-0.5] FEV1 and β=−6.4 [−11.6,−1.16] FVC compared to levels less than 0.805pg/mL (p=0.03 and p=0.01, respectively)(Table 2). Jackson et al. demonstrated that children with higher IL-6 had increased metabolic dysfunction and risk of asthma exacerbations during a 1-year longitudinal period; however, they found no association between IL-6 and lung function(3), potentially reflecting the longer follow-up period in this study. Our lung function findings are consistent with the adult SARP-3 cohort results, and provides new evidence that children with higher plasma IL-6 levels have worse lung function, risk of exacerbation, and increased severity, adjusting for BMI.
TABLE 2.
Associations between Plasma IL-6 and Asthma Outcome Measures During 3-Year Longitudinal Follow-Up (three 1-year measurements after baseline, n=8 subjects missing follow-up data)
| Low IL-6 | Med-High IL-6§ | Effect size (95% CI) | p-value* | |
|---|---|---|---|---|
| Longitudinal | (n=37) | (n=110) | ||
| Clinical Outcome Measures | ||||
| Asthma exacerbation rate per yr, mean (SD)** | 0.40 (0.87) | 0.97 (1.90) | IRR = 2.09 (1.02, 4.28) | 0.04 |
| One-year follow-up | 0.33 (0.79) | 1.19 (2.40) | ||
| Two-year follow-up | 0.53 (1.11) | 0.91 (1.64) | ||
| Three-year follow-up | 0.33 (0.66) | 0.75 (1.33) | ||
| Proportion of participants with ER visit for asthma per yr, % (n) | 12 (12) | 27 (79) | OR = 3.41 (0.88, 13.1) | 0.08 |
| One-year follow-up | 17 (6) | 33 (36) | ||
| Two-year follow-up | 12 (4) | 26 (26) | ||
| Three-year follow-up | 7 (2) | 21 (17) | ||
| Spirometry Outcome Measures | ||||
| FEV1 (% predicted), mean (SD) | 93.1 (9.6) | 88.3 (16.2) | β = −5.8 (−11.2, −0.5) | 0.03 |
| One-year follow-up | 93.2 (10.2) | 88.6 (15.5) | ||
| Two-year follow-up | 95.1 (9.6) | 87.4 (16.8) | ||
| Three-year follow-up | 91.0 (8.9) | 88.9 (16.5) | ||
| FVC (% predicted), mean (SD) | 104.8 (11.3) | 101.4 (15.2) | β = −6.4 (−11.6, −1.16) | 0.01 |
| One-year follow-up | 105.0 (11.2) | 101.3 (14.3) | ||
| Two-year follow-up | 105.2 (11.4) | 101.1 (15.3) | ||
| Three-year follow-up | 104.0 (11.5) | 101.9 (16.2) | ||
IL-6 levels greater than or equal to 0.805pg/mL
The p values were generated from mixed effects linear, logistic, and negative binomial regression models, adjusted for year, BMI status (healthy weight, overweight, obese), gender, and participant random intercept.
Asthma exacerbation was defined as a burst of systemic corticosteroid lasting ≥ 3 days for treatment of worsening asthma control.
Abbreviations: FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; IRR=incidence rate ratio; OR = odds ratio; β = beta; CI = confidence interval; SD = standard deviation
The mechanism through which peripheral blood IL-6 plays a role in asthma pathogenesis is largely unknown, although multiple processes have been suggested(5). Recent data suggest IL-6 is directly linked to airway inflammation (6–8). IL-6 blockade may be a potential target for asthma treatment; tocilizumab, a humanized monoclonal antibody against the IL-6 receptor, was an effective treatment in two pediatric patients with severe persistent asthma(9).
Strengths of our study include a well-phenotyped cohort of children with severe asthma, analysis of plasma IL-6 with identical assay used in the adult SARP-3 cohort allowing for direct comparisons with adult data, and a 3-year prospective follow-up period. Limitations include a modest sample size, lack of control cohort without asthma, and a longer recall from baseline potentially explaining the lack of cross-sectional findings. We did not find a statistically significant association between plasma IL-6 and total WBC and blood neutrophil levels as was demonstrated in the APIC pediatric cohort(3). One plausible reason may be that the cohorts are phenotypically different at baseline. In contrast to the APIC cohort, the SARP-3 pediatric cohort was enriched with children having severe asthma and higher baseline levels of total serum IgE, FeNO, and blood eosinophil, suggesting a more skewed Th2 population. Nonetheless, higher IL-6 quartiles trended towards elevated total WBC and blood neutrophil levels, and perhaps a larger sample size would have provided greater statistical power needed to demonstrate significant associations.
In conclusion, higher plasma IL-6 levels in children are significantly associated with elevated BMI, early signs of metabolic dysfunction evidenced by high CRP, and greater asthma severity with risk for both asthma exacerbation and lower lung function based on longitudinal analyses. This is consistent with previous work in adults with asthma and the first to show lower lung function in children with high IL-6 levels. Further mechanistic and therapeutic IL-6 studies are needed to determine the clinical relevance of these findings.
Extended Data
Fig E1.
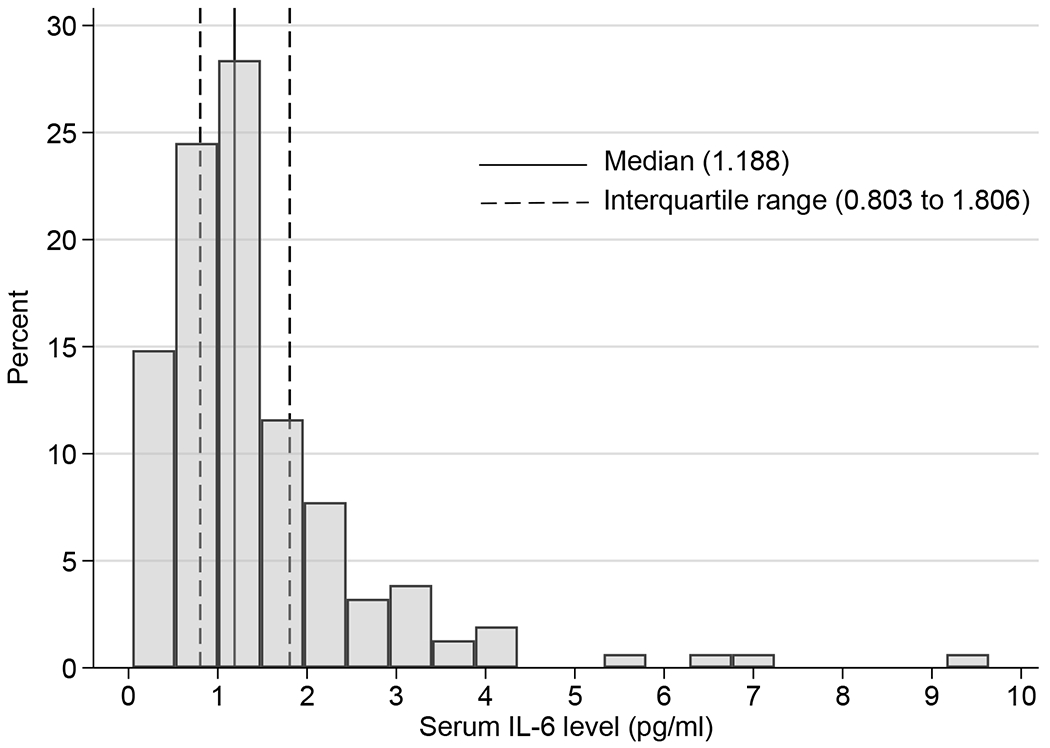
Distribution of plasma IL-6 level (N=155) with 25th and 75th percentiles (dashed lines); 0.16 pg/mL is the lower limit of detection.
TABLE E1.
Baseline Characteristics of Study Population (n = 155)
| Characteristic | No. (%) |
|---|---|
| Demographics | |
| Age (yrs), median (range) | 11.4 (6.1-18.4) |
| Male gender | 96 (62) |
| Race | |
| American Indian/Alaska Native | 1 (1) |
| Asian | 0 (0) |
| Black/African American | 63 (41) |
| Mixed race | 26 (17) |
| Native Hawaiian/Other Pacific Islander | 1 (1) |
| White | 64 (41) |
| Hispanic ethnicity | 22 (14) |
| Annual household income <$25,000 | 51 (37) |
| Clinical characteristics | |
| BMI category | |
| Underweight (<5th percentile) | 2 (1) |
| Normal weight (5th - <85th percentile) | 66 (43) |
| Overweight (85th - <95th percentile) | 33 (21) |
| Obese (≥95th percentile) | 54 (35) |
| ACT score, mean (SD)1 | 18.2 (4.5) |
| ≥ 1 asthma exacerbation in past year | 101 (66) |
| ICS use over prior 12 months | 147 (95) |
| Severe Asthma* | 89 (57) |
| Blood markers of atopy/inflammation | |
| ≥ 1 positive specific IgE3 | 138 (91) |
| Serum IgE conc (kU/L), mean (SD)4 | 868 (1328) |
| Blood eosinophil count (cells/uL), mean (SD)5 | 395 (296) |
| FENO (ppb), mean (SD)2 | 35.7 (37.8) |
| Pulmonary function testing6 | |
| FEV1 % predicted, pre-bronchodilator, mean (SD) | 90.5 (14.1) |
| FVC % predicted, pre-bronchodilator, mean (SD) | 103.5 (13.0) |
| FEV1/FVC, pre-bronchodilator, mean (SD) | 76.7 (8.8) |
| FEV1 % predicted max. absolute reversibility, mean (SD) | 12.3 (12.5) |
n = 155 ACT scores
n = 152 FENO samples
n = 152 specific IgE samples
n = 146 IgE samples
n = 155 CBC samples
n = 155 PFTs
The classification of severe asthma was determined using criteria from the American Thoracic Society/European Thoracic Society guidelines.
Abbreviations: BMI = body mass index; ACT = Asthma Control Test; ICS = inhaled corticosteroid; IgE = immunoglobulin E; FENO = fraction of exhaled nitric oxide; ppb = parts per billion; SD = standard deviation; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second
Clinical Implications:
Higher plasma IL-6 levels in children are significantly associated with elevated body mass index, early signs of metabolic dysfunction evidenced by high CRP, and greater asthma severity with risk for both asthma exacerbation and lower lung function.
Acknowledgments
Funding:
This study was conducted with the support of grants that were awarded by the NHLBI to the Severe Asthma Research Program Principal Investigators, Clinical Centers, and Data Coordinating Center as follows: Brigham and Women’s Hospital (U10 HL109172); University of California San Francisco (U10 HL109146); University of Wisconsin (U10 HL109168); Cleveland Clinic (U10 HL109250); Emory University (U10 HL109164); Washington University (U10 HL109257). Additionally, this study was conducted with support of grants that were awarded by the NIH: K24 AI 106822 (WP), K23 AI 123517 (PP), K23 HL 138303 (MP), K23 AI 125785 (JCC).
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Conflict of Interest:
Dr. Permaul has nothing to disclose. Dr. Peters reports grants from NIH/NHLBI and grants from Boeringer-Ingelheim, during the conduct of the study; grants from AstraZeneca, Boehringer-Ingelheim, Genentech, GSK, Sanofi-Genzyme-Regeneron, and Teva, outside the submitted work. Mr. Petty has nothing to disclose. Dr. Cardet reports personal fees from AstraZeneca and from Genentech outside the submitted work. Dr. Ly reports grants from Vertex, grants from Gilead, outside the submitted work. Dr. Ramratnam reports grants from AstraZeneca, during the conduct of the study. Dr. Ross reports grants from NHLBI, grants from AstraZeneca, during the conduct of the study; grants and non-financial support from TEVA, non-financial support from GSK, non-financial support from Merck, grants from Flamel, grants from Jazz, grants from AstraZeneca, grants from Boehringer Ingelheim, and grants from Novartis, outside the submitted work. Dr. Fitzpatrick has nothing to disclose. Dr. Israel reports grants from AstraZeneca, non-financial support from GSK, during the conduct of the study; personal fees from AB Science, grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants and personal fees from Avillion, personal fees from Biometry, personal fees from Equillium, personal fees from Merck, grants and personal fees from Novartis, personal fees from 4D Pharma, personal fees from Pneuma Respiratory, personal fees from PPS Health, personal fees from Regeneron, personal fees from Sanofi Genzyme, personal fees from Sienna Biopharmaceutical, other from Vorso Corp, grants, personal fees and non-financial support from Genentech, personal fees and non-financial support from GSK, personal fees and non-financial support from TEVA, grants from Gossamer Bio, grants and non-financial support from Circassia, non-financial support from Boehringer Ingelheim, outside the submitted work. Dr. Bacharier reports grants from NIH/NIADI, during the conduct of the study; personal fees from GSK, personal fees from Genentech/Novartis, personal fees and non-financial support from Merck, personal fees from DBV Technologies, personal fees and non-financial support from Teva, personal fees and non-financial support from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from WebMD/Medscape, personal fees from Sanofi/Regeneron, personal fees from Vectura, personal fees from Circassia, outside the submitted work. Dr. Phipatanakul reports other trial support from AstraZeneca, during the conduct of the study; grants and personal fees from Genentech/Novartis, grants and personal fees from Sanofi/Regeneron, other trial support and medications from Merck, other trial support and reagents from Alk-Abello, personal consulting fees from GSK, other trial support and medications from Kaleo, grants from NIH, other trial support and medications from CSL-Behring, outside the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters MC, Mauger D, Ross KR, Phillips B, Gaston B, Cardet Jc, et al. Evidence for Exacerbation-Prone Asthma and Predictive Biomarkers of Exacerbation Frequency. Am J Respir Crit Care Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson DJ, Bacharier LB, Calatroni A, Gill MA, Hu J, Liu AH, et al. Serum IL-6: A biomarker in childhood asthma? J Allergy Clin Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. [DOI] [PubMed] [Google Scholar]
- 5.Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8(9):1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jevnikar Z, Östling J, Ax E, Calvén J, Thörn K, Israelsson E, et al. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol. 2019;143(2):577–90. [DOI] [PubMed] [Google Scholar]
- 7.Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med. 2016;22(9):1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harb H, Stephen-Victor E, Crestani E, Benamar M, Massoud A, Cui Y, et al. A regulatory T cell Notch4-GDF15 axis licenses tissue inflammation in asthma. Nat Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esty B, Harb H, Bartnikas LM, Charbonnier LM, Massoud AH, Leon-Astudillo C, et al. Treatment of severe persistent asthma with IL-6 receptor blockade. J Allergy Clin Immunol Pract. 2019;7(5):1639–42 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]


