Abstract
Modeling immunity in vitro has potential to be a powerful tool for investigating fundamental biological questions, informing therapeutics and vaccines, and providing new insight into disease progression. There are two major elements to immunity that are necessary to model: primary immune tissues, and healthy and diseased peripheral tissues with immune components. Here, we systematically review progress made along each of three strategies to modeling these elements: ex vivo cultures, which preserve native tissue structure, microfluidic devices, a versatile approach to providing physiologically relevant fluid flow and environmental control, and engineered tissues, which provide precise control of the chemistry of the 3D microenvironment and biophysical cues. While many models focus on diseases such as infection and cancer, more primary immune tissue models are necessary to progress the field. In the future, we anticipate that the expansion of patient-specific models may inform why immunity differs so greatly patient to patient and allow for the rapid comprehension and treatment of emerging diseases, such as SARS-CoV-2.
Keywords: explants, organ-on-chip, bioreactor, tumor, infection, lymphatics
Introduction
Interest in the immune system has grown exponentially in recent years, spreading from traditional immunologists to the realm of engineers and physical scientists. The success of cancer immunotherapy, named the Science Breakthrough of the Year in 2013,(1) heralded a new era of heightened attention to immunity throughout the scientific community. While much work has focused on “immunoengineering” in vivo responses, e.g. via engineered cells, vaccines, and immunotherapies,(2) efforts to generate faithful and robust models of immunity in vitro and ex vivo are less developed.
With human data mostly limited to the cells and molecules present in the bloodstream, experimental insights into tissue-level immunity largely come from in vivo, ex vivo, and in vitro models of immunity. At varying levels of reductionism, ex vivo and in vitro models offer the unique opportunity to dissect individual cell functions, cell-cell interactions, and tissue-level mechanisms of immunity. Such models range from “top down,” ex vivo cultures of intact human and animal tissues, to “bottom up,” engineered co-cultures in traditional well plates or advanced microfluidic systems. The models can synergize with a growing suite of computational models of immunity,(3, 4) to both inform the models and test their hypotheses in ways not possible in vivo. Applications of in vitro and ex vivo models of immunity range from drug screening and toxicity studies to mechanistic models of development and disease.
Despite a surge of recent progress, many aspects of the immune system remain unexplored and rarely or inadequately modelled in vitro. Below, we briefly outline the fundamentals that should be considered when creating a model of a particular facet of the immune system. Then, we present a summary of existing technologies and recent progress, while pointing out the many gaps that still remain.
1.1. Elements of immunity
1.1.1. Compartments
Development of immune cells begins in the bone marrow, where progenitors of T cells, B cells, natural killer (NK) cells, and myeloid cells such macrophages and dendritic cells are formed (Figure 1). Thus, the bone marrow is considered a primary immune tissue. From there, T cell progenitors migrate to the thymus, another primary immune tissue, where those of suitable antigen specificity and affinity are selected for survival. Naive T cells and naive B cells enter circulation in the blood stream, periodically exiting to pass through the lymph nodes and spleen.(5, 6) While there, lymphocytes sample cells called antigen presenting cells (APCs, e.g. \dendritic cells and macrophages) to determine which, if any, APCs are presenting their cognate (matching) antigen. Those antigens would have arrived in the lymph node from peripheral tissues via the lymphatic system, while the spleen collects only blood-borne antigens. These organs where the immune cells mature and perform their expected functions are referred to as secondary immune tissues.
Figure 1:
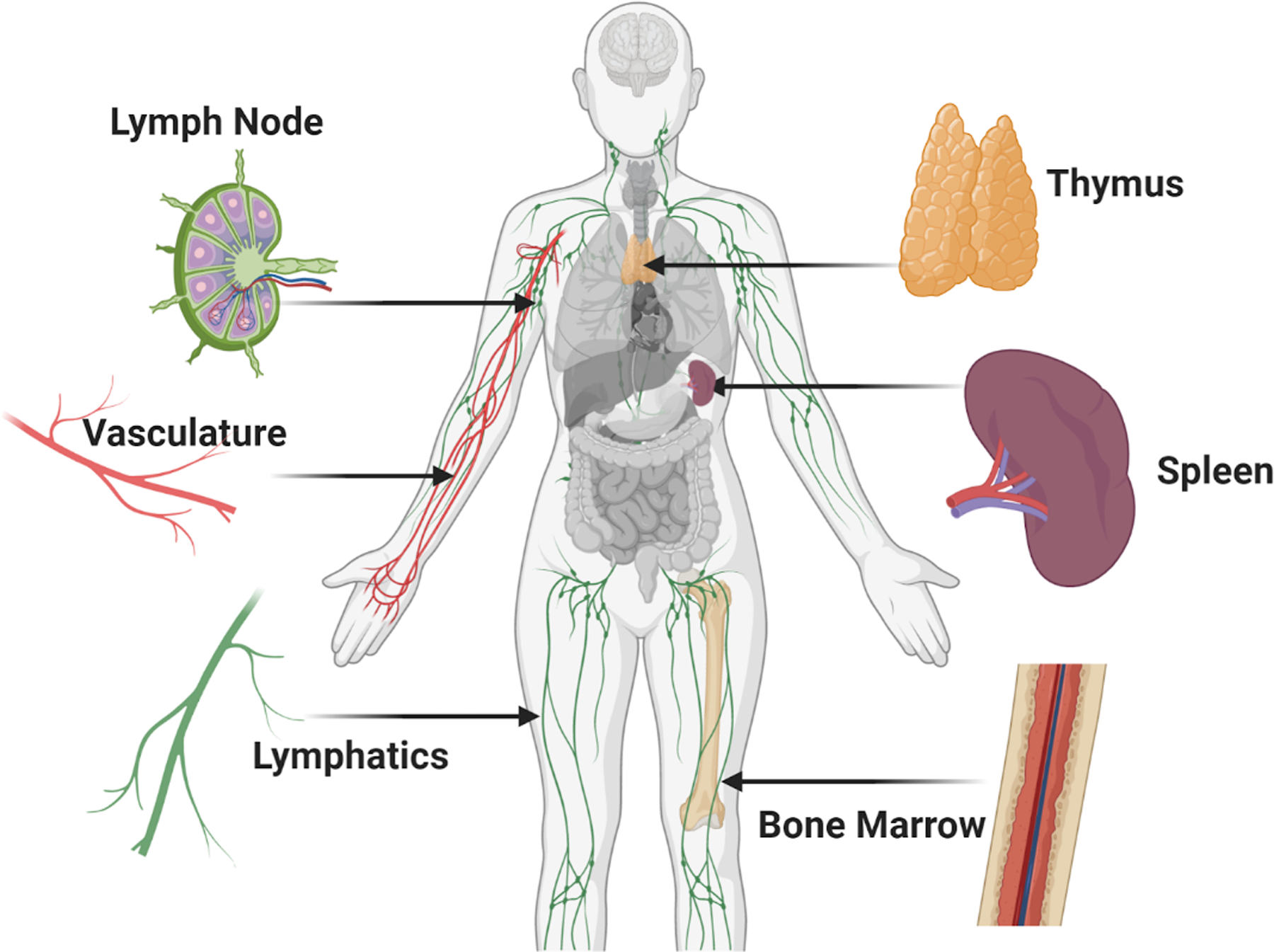
Overview of organs and vasculature central to immunity. Immune compartments are found throughout the body and are connected to each other and peripheral tissues through lymphatic and blood vasculature, forming a sophisticated and effective immune system. The primary and secondary immune organs discussed in this review are shown here, including the lymph node, thymus, spleen, and bone marrow. (Small figure)
If a naïve T cell finds its antigen on an activated APC, it becomes activated and begins a program of differentiation and/or proliferation ultimately leading to an effector immune response. For antibody-mediated immune responses (humoral immunity), specialized antigen-specific T cells called follicular helper T cells interact with B cells,(7, 8) that eventually mature and expand into memory B cells or antibody-producing plasma cells. Once activated, T cells and B cells travel via the bloodstream to other peripheral organs (skin, lungs, brain, gut, etc.) to respond to pathological conditions. There, they interact with tissue-resident immune cells such as macrophages as well as other white blood cells recruited during the initial innate immune response and inflammation (i.e. eosinophils, monocytes, NK cells, neutrophils). After the initial threat is cleared, memory cells reside in the infected tissue, the lymph node, or the bone marrow, where they serve as a pool for rapid reactivation upon future infection or antigen exposure.(9–12)
1.1.2. Timescales: Adaptive vs Innate Immunity
The immune response can be broken down into two phases, innate and adaptive.(13) The innate response initiates rapidly, within the first hours of exposure to an immunogenic stimulant. This response is not pathogen-specific and relies on the recognition of conserved motifs to initiate activation.(14, 15) Motifs include pathogen-associated molecular patterns, or PAMPs, such as bacterial peptidoglycans and lipopolysaccharide (LPS), as well as damage-associated molecular patterns, or DAMPs. Adaptive immunity, on the other hand, is highly specific to the antigens that initiated it. Antigen transport to the lymph node requires hours or days, and it may be weeks before T cells and B cells are sufficiently activated, differentiated, and proliferated to counter the invading threat.
1.2. Multi-faceted inputs
From a physical science and engineering perspective, the immune response is a fascinating integration of both biochemical and biophysical inputs (Figure 2). Cells of the immune system communicate largely via receptor-ligand binding, either via physical cell-cell contact or by exchange of secreted proteins, vesicles, and small molecules. Each type of immune cell secretes a dynamic combination of cytokines and chemokines, small proteins (most less than 20 kDa) that act in both autocrine and paracrine fashion to control cellular chemotaxis, differentiation, proliferation, and apoptosis. After secretion, cytokines and chemokines undergo diffusion, convection, active transport, and binding to the extracellular matrix (ECM);(16, 17) their local concentrations heavily influence tissue-level immune function.(18) Thus, composition of the ECM and the distribution or transport of secreted factors is a critical element of any in vitro model of immunity.
Figure 2:
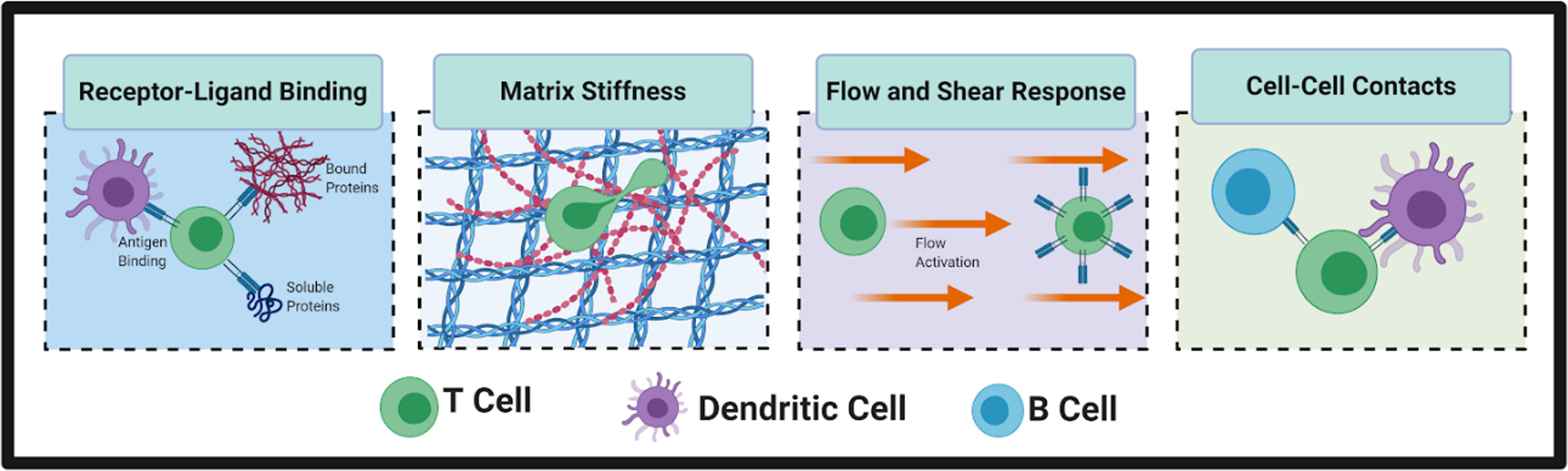
Cells of the immune system integrate biochemical and biophysical cues. For example, T cells and other cells experience receptor-ligand binding to antigens, bound proteins, and soluble proteins. Immune cells are also responsive to biophysical cues, including local tissue stiffness, the presence or absence of interstitial fluid flow, and physical contact with neighboring cells. For example, T cells physically engage with antigen-presenting dendritic cells to initiate an adaptive immune response and with B cells to initiate the cascade that leads to antibody production. (Small figure)
The role of the microenvironment does not stop at biochemical composition, but also includes biophysical aspects such as stiffness and fluid flow. T cells and other immune cells are responsive to the stiffness of their local microenvironment, a finding that is still underexplored in engineered models.(19) Immune cells are also responsive to flow-induced shear stress. T cells can respond to flow by proliferation,(20) or by migrating against the direction of flow.(21) Macrophages similarly migrate against the direction of flow and are polarized toward an M2 anti-inflammatory phenotype.(22) In contrast, neutrophils are shown to migrate in the direction of flow via the αvβ3 integrin,(23) demonstrating the varying responses of immune cells to flow.
1.3. Scope of review: Three approaches x two contexts
In this review, we will take a critical look at the state of ex vivo models of the immune system. We have identified two major contexts for which models are needed: models of primary immune tissues, and models of peripheral tissues (healthy or pathological) with an immune component. Within each context, we systematically review the development of three types of approaches: ex vivo cultures, microfluidic devices, and engineered models of tissue (Figure 3). The use of ex vivo cultures, including explants and slices, provide an important bridge between in vivo animal models and in vitro cell cultures. These tissues maintain the original structure and organization of the organ, while providing increased experimental accessibility. By removing the organs from the body, however, any native fluid flow is also removed. Microfluidic chips offer the possibility to add fluid flow and a controlled microenvironment to ex vivo and in vitro cultures, and provide a versatile experimental platform to study immune function.(24, 25) However, many chips are often designed for one specific function, meaning that each biological question requires a specific chip design. Tissue engineering offers the most flexible experimental platforms to study a variety of immune cell functions.(24, 26) By creating a model tissue from scratch, researchers may investigate specific cell-cell and cell-matrix interactions in a strictly controlled environment. These models offer the chance to manipulate many of the fundamental components that are inherent in ex vivo or microfluidic platforms, including the ECM and fluid dynamics. The introduction of novel techniques to model immunity offers the potential for new insights into its dynamic complexity and to inform new immunotherapeutic strategies.
Figure 3:
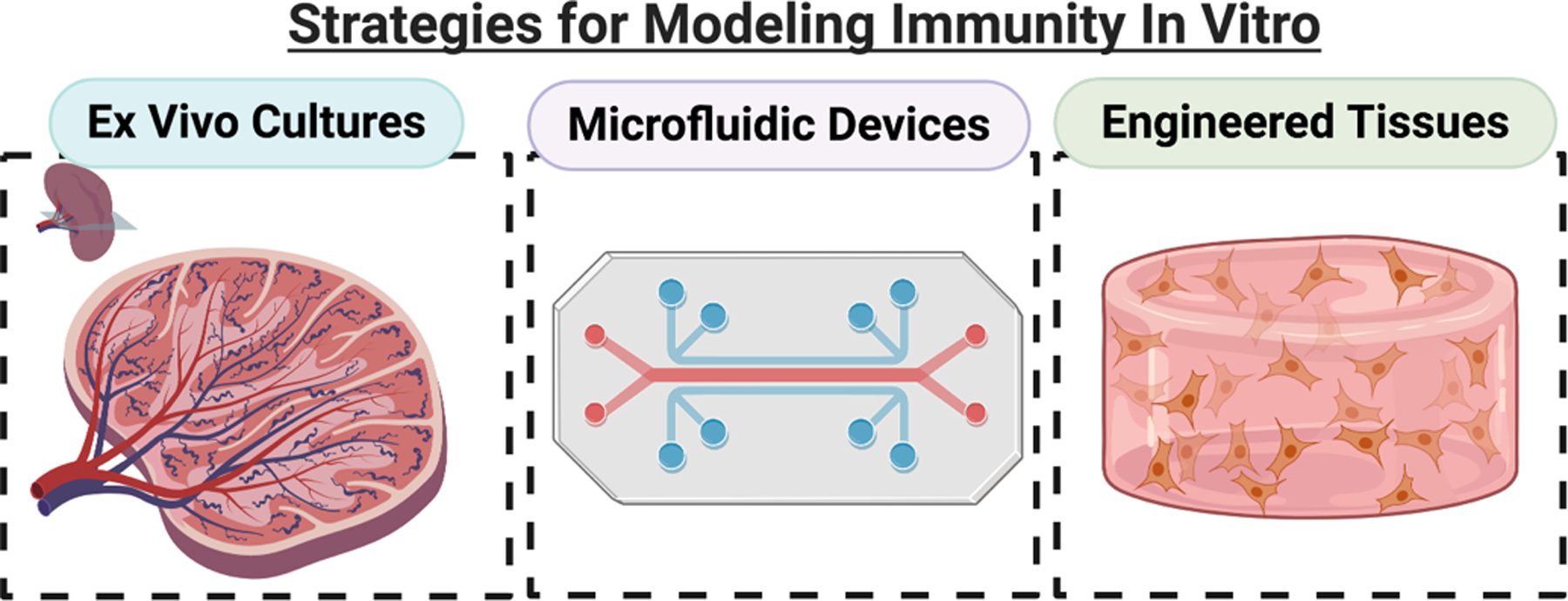
Overview of the three approaches to modeling immunity in vitro: ex vivo cultures, microfluidic devices, and engineered tissues. Ex vivo cultures, including slices and explants, preserve the tissue structure by removing live tissues from in vivo animals and culturing them. Microfluidic devices are versatile, miniaturized engineered platforms that incorporate fluid flow and provide dynamic control over the microenvironment. Engineered models can range from bioreactors to hydrogels, but typically incorporate a 3D extracellular matrix component and can include multiple cell types. Microfluidic devices can bridge the gap by incorporating slices or engineered tissues. (Small figure).
2. Approach I: Models of primary immune tissues
Modelling primary and secondary immune tissues gives insight into the development, maturation, and activation of immune cells and adaptive immune responses. Here we discuss models of immune tissues, including the bone marrow, thymus, lymph node, and spleen. We also include models of lymphatic vessels here.
2.1. Bone Marrow
The bone marrow stem cell niche is vital for maintaining multipotent hematopoietic stem and progenitor cells (HSPCs), which are the source for all blood cell types, including lymphocytes, dendritic cells and macrophages, NK cells, and granulocytes.(27) It is also the residence of long-lived plasma cells, which may live for decades in the bone marrow niche to preserve humoral memory.
2.1.1. Ex vivo cultures
Most experimental work with bone marrow begins with cell suspensions collected from bone marrow aspirates or biopsies; intact bone marrow samples are usually fixed for immunohistochemistry.(28) Thus, live slices and explants have not yet been used to study immunological functions of the bone marrow, although ex vivo cultures of bone, developed to study osteogenesis,(29) may potentially prove useful to study hematopoiesis as well.
2.1.2. Microfluidic chips
While there are many bone marrow-on-a-chip models, most are focused on developing a biomimetic scaffolding and culturing HSPCs rather than the differentiation into immune cells, one of the main functions of bone marrow. In Sieber et al., a multiorgan chip (MOC) developed previously(30) was used to culture human HSPCs in a ceramic scaffold.(31) The MOC consists of a single looped channel with recirculating flow and a compartment to culture the bone marrow niche model. To confirm proper HSPC function within the device for long-term culture (up to 28 days), bone marrow niche-related genes were expressed and compared to off-chip cells without the ceramic scaffold.(31) After a period of 4 weeks on the device, the cells were removed and tested, and were seen to maintain their ability to differentiate into different cell types (erythrocytes, granulocytes, and macrophages) comparable to freshly isolated HSPCs.(31) This bone marrow niche model could be cultured with additional organ models to generate a functioning MOC.
Torisawa et al. tested responses to radiation countermeasure drugs in a microfluidic device.(32) By integrating engineered bone marrow into a polydimethylsiloxane (PDMS) device, HSPCs can be cultured for at least 2 weeks, and normal bone marrow function was maintained with an increase in leukocytes and red blood cells (RBCs) over time.(32) In addition to modelling healthy bone marrow function, the bone marrow-on-a-chip was exposed to gamma radiation to be used as a model to test novel drugs. Initially, the gamma radiation significantly decreased the production of leukocytes, but the introduction of two potential therapeutics induced significant increases.(32) Along with generating a bone marrow model that integrated engineered tissue, Torisawa et al. successfully tested the effect of novel drugs on cells exposed to radiation.(33)
Chou et al. expanded the bone marrow-on-a-chip model to include a vascularized channel beneath the bone marrow niche 3D culture.(34) Bone marrow-derived stromal cells and CD34+ stem cells were co-cultured in a hydrogel channel, separated from a vascularized channel by a porous membrane. Similar to Torisawa et al.,(32) this bone marrow model was cultured long-term and exposed to radiation and chemotherapeutic drugs. The authors integrated cells from patients with Shwachman-Diamond syndrome, a genetic disorder that results in bone marrow failure, which resulted in neutrophil-maturation abnormalities.(34) In a 2019 study from Aleman et al., a bone marrow niche-on-a-chip integrated four major niche constructs (periarterial, perisinusoidal, mesenchymal, and osteoblastic) within a single bone marrow microenvironment.(35) Homing and retention of both healthy HSPCs and malignant lymphoma and leukemic cells were monitored in real-time on the NOC platform.(35) These approaches may provide a platform to test novel therapeutics on patient-specific cells, and eventually reduce, though not eliminate, the need for animal testing.
2.1.3. Engineered tissues
Modeling bone marrow through engineered tissues is primarily achieved via static 3D culture of hematopoietic or mesenchymal stem cells followed by proof of immune cell production. This strategy encapsulates the more spongy aspect of bone marrow. Mortera-Blanco et al created a bone marrow model by expanding cord blood mononuclear cells in type I collagen coated polyurethane scaffolds, creating a dynamic culture that contained erythroid precursors, HSPCs, maturing myeloid cells, T-lymphocytes, and megakaryocytes.(36) More recently, perfusion has been introduced via bioreactor, enabling the study of bone marrow models in dynamic conditions that recapitulate in vivo physiology. Nichols et al cultured hematopoietic stem cells in a 3D polyacrylamide scaffold with inverted colloidal crystal geometry in a rotating wall vessel and showed the production of B lymphocytes.(37) HSPCs have also been cultured in a hydroxyapatite bone-like scaffold under perfusion via bioreactor, and shown to produce natural killer cells.(38)
Ultimately, bone marrow models alone do not provide a complete picture of immunity and have thus far been mostly limited to modeling immune cell production. However, they provide a useful system for the study of disease such as leukemia and multiple myeloma. One such study examines multiple myeloma: Braham et al developed a bone marrow model consisting of mesenchymal stem cells (MSCs), osteogenic MSCs, and endothelial progenitor cells within Matrigel. Myeloma cells were added to the culture and engineered T cells were tested as a treatment, observing the killing response and how they migrated through the model.(39) Therefore, in vitro bone marrow models could provide a disease modeling platform for testing therapeutics.
2.2. Thymus
The thymus is a primary immune organ where naïve T cells are screened for antigen recognition. Removal of the thymus during development is lethal after 2–4 months,(40) and even in adulthood the removal of the thymus has been shown to correlate with a drop in circulating immune cells.(41) Thus indicating the crucial role of the thymus, not only in immune development, but throughout the lifetime of the organism.
2.2.1. Ex vivo cultures
Ex vivo thymus culture has provided invaluable insight into important characteristics of T cell development and tolerance. Mouse feotal thymus organ culture in particular set the stage for many early studies of the requirements for healthy T cell development.(42–46) Slices of mouse thymus tissue have allowed for the dynamic nature of T cell tolerance to be better understood.(47–52) By carefully tracking CD8 single positive thymocytes through the process of positive selection, Ross et al. identified distinct phases of selection using thymic slices.(47) Phases were distinguished by changes in thymocyte motility, surface marker and T cell receptor expression, and Ca2+ signaling, as well as their location within specific regions of the tissue.(47) This information has led to a comprehensive tool box to study T cell development in mouse models, recently slices of human thymus tissue have also been introduced to begin studying human T cells.(53) As new questions about T cell development come to light ex vivo slices and culture will continue to provide a platform to help answer them.
2.2.2. Engineered tissues
Attempts to engineer the thymus have been largely limited to organoids with focus on T cell generation. Poznansky et al seeded thymus fragments from mice onto Cell Foam until stromal cells had reached 80% confluency. Then, human bone marrow derived progenitor cells were added and shown to efficiently differentiate into T cells within 14 days.(54) The authors suggest a potential use of their system for ex vivo generation of T cells for the treatment of immunodeficiency. Seventeen years later, Seet et al aggregated stromal cells and HSPCs into organoids that were also capable of producing T cells, in this case, antigen-specific T cells.(55) These authors suggested their platform could be used for testing engineered T cells, demonstrating how the goals of modeling immunity have changed over time.
In contrast to organoid technology, Bredenkamp et al approached treating immunodeficiency via cell transfection and implantation for the in vivo generation of T cells. Fibroblasts were transfected with transcription factor forkhead box N1 (FOXN1) and reprogrammed into FOXN1-induced thymic epithelial cells (iTECs). The iTECs were able to produce CD4+ and CD8+ T cells from early T lineage progenitors in vitro. When combined with thymocytes and transplanted into mice, an organized and functional thymus was formed. The authors note that this iTEC system has the potential to serve as the basis for thymus transplantation therapies to treat immunocompromised patients.(56)
2.3. Lymph Node
In addition to the spleen, the lymph node is the primary location where adaptive immune responses initiate and mature. This organ is highly organized in distinct regions, and changes drastically during the course of an immune response.(57) Interactions within the lymph node help direct the strength of the immune response generated, including T cell polarization,(58, 59) antibody specificity, and adequate memory formation. Models of the lymph node may further our understanding of how immune responses are generated.
2.3.1. Ex vivo cultures
The lymph node is a highly organized organ, and appreciation of its dynamic cell-cell interactions, lymphocyte motility, and sophisticated mechanisms of antigen recognition first arose from live fluorescence imaging of explanted whole lymph nodes from mice and humans (Figure 4a).(60–62) Culture of these organs is facilitated by their small size (1–2 mm in mouse; 1–2 cm in human), particularly in mice, whose lymph nodes can be kept viable for several hours ex vivo by perfusing with oxygenated solution. When deeper portions of the organ must be visualized or manipulated, live slices 250 – 400 μm thick may be produced using a vibratome and similarly monitored by live imaging, though this technique is less common for lymph nodes than it is for thymus.(63, 64) One of the greatest strengths of tissue slice platforms is the ability to visualize individual cells over time (Figure 4b), while also allowing for monitoring of gross changes in surface marker expression, and measuring bulk cytokine secretion from the intact slice or explant to evaluate immune responses.(65–70) While ex vivo culture retains the spatial organization of the in vivo system, removing the tissue from the body does cut off blood and lymphatic flow, and prevents additional cells from homing to the tissue. Most ex vivo lymph node cultures are used for short-term studies of a few hours or overnight, in part because motile lymphocytes largely exit from open-face slices after 24 – 48 hr. However, human tonsil slices have been maintained for 4 – 7 days for informative models of viral infections including human immunodeficiency virus (HIV), measles, and West Nile, despite cell egress.(67) Recently, human tonsil slices were cleverly used to demonstrate that the lymph node stromal microenvironment inhibited T cell proliferation in response to stimulation, confirming the predictions of in vitro cell cultures.(71) Using a multi-compartment microfluidic device, Shim et al. modelled immunosuppression by co-cultured LN slices with tumor slices in recirculating fluid flow that allowed inter-organ communication via secreted factors.(72) LN slices cultured with the breast cancer tissue secreted more immunosuppressive cytokines than those cultured with healthy tissue, showing the initial effects of cancer on the peripheral immune organs and subsequently the immune system.(72) Thus, studies in ex vivo lymph node tissue provide visualization of immune system dynamics and a deeper understanding of early events in the immune response.
Figure 4:
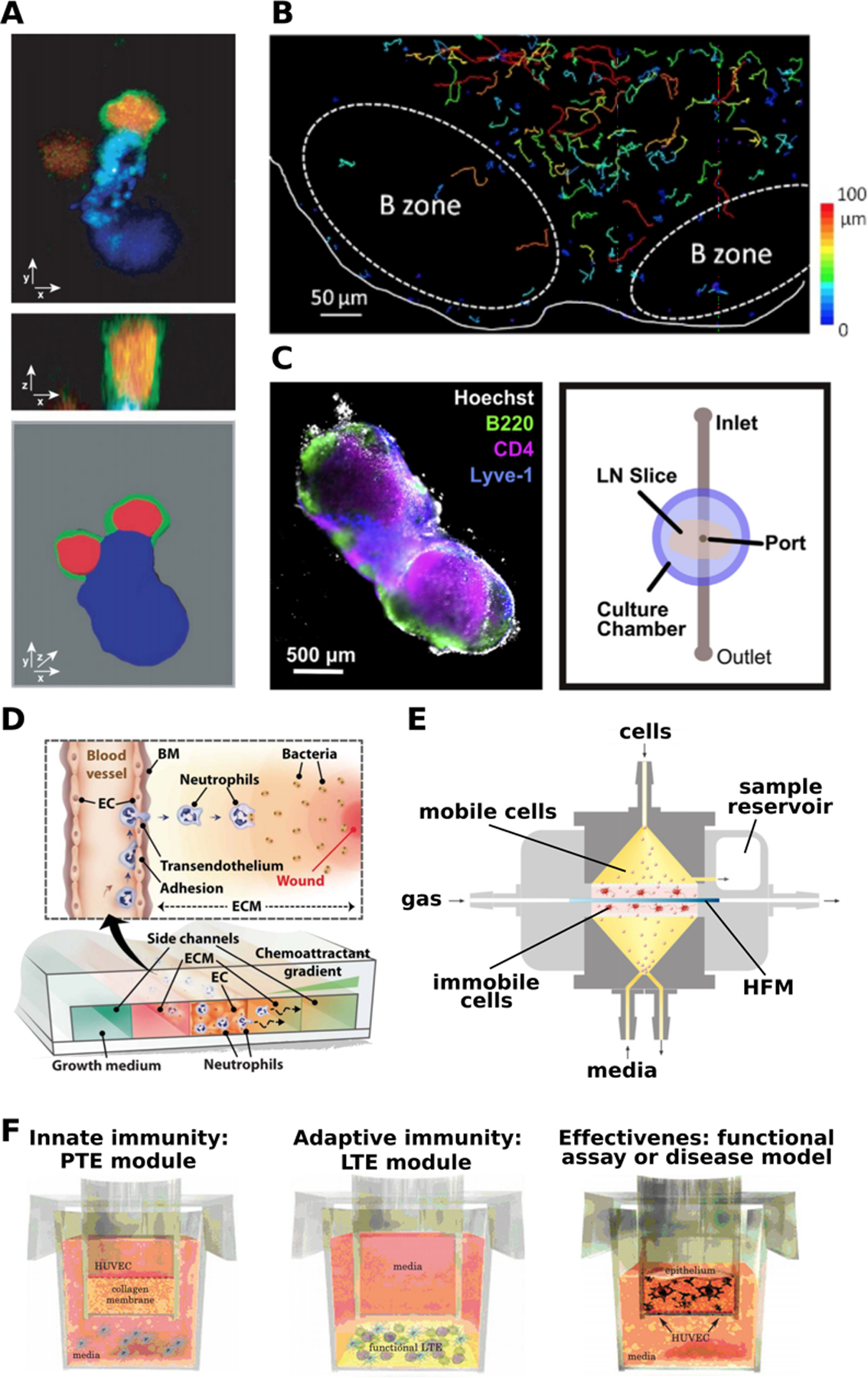
Experimental models of the lymph node. (a) Intact explanted lymph nodes provided a view of the molecular organization of the immune synapse between a dendritic cell (blue) and a T cell (red cytoplasmic label; green on membrane-bound CD43). Shown in confocal cross-sections (upper, middle) and in 3D view (lower image). Presented with permission from Stoll et al.(61) (b) Ex vivo lymph node slices have been used to track T cell motility. Colored lines indicate cell tracks during time-lapse microscopy. Presented with permission from Salmon et. al.(63) (c) Live lymph node slices were combined with microfluidics to deliver local stimulation to the tissue. (left) Tissue was immunostained for B220 (B cells, green), CD4 (T cells, pink), and Lyve-1 (lymphatics, blue), with Hoechst nuclear counterstain (gray). (right) Top-view schematic of the microfluidic device used for local fluid delivery and analysis of diffusion. Presented with permission from Ross and Pompano.(73) (d) A schematic of a microfluidic device modeling neutrophil migration in the presence of a chemoattractant gradient, presented with permission from Han et al.(78) (e) The human artificial lymph node bioreactor (HuALN). Gas perfuses the system horizontally in a hollow fiber membrane (HFM) surrounded by a hydrogel matrix that contains antigen presenting cells (APCs), forming the central culture space (pink). The peripheral culture space (yellow) contains media flowing vertically and a cycling B cell suspension. Presented with permission from Giese et al.(82) (f) The MIMIC system to model immunity in vitro, with three of four main components shown. The innate immunity module, or Peripheral Tissue Equivalent (PTE) module, consists of a HUVEC-coated hydrogel and PBMCs. The adaptive immunity module, or the Lymphoid Tissue Equivalent (LTE) module, consists of a sequential application of dendritic cells, follicular dendritic cells, T cells and B cells. The functional assay modules are customized for each experimental design, to assess the responses of the PTE and LTE against a stimulus. Presented with permission from Higbee et al.(89)
2.3.2. Microfluidic chips
Microfluidic chips have been used to model the lymph node both in combination with slice cultures and by supporting cellular-scale studies. Combining lymph node slices with chips provides a way to access the full range of cell types and tissue organization, along with fluid flow control. So far, work has focused on local delivery and diffusion within the tissue slice, not whole organ immune function. In a study from 2017, Ross and Pompano developed a user-friendly microfluidic platform, dubbed Microfluidic Integrated Optical Imaging, that was used to study diffusion of cytokines through the lymph node slice to gain a better understanding of cell-cell communication and drug efficacy (Figure 4c).(73) The simple PDMS-based device was used to deliver picograms of fluorescently-labelled cytokines (tumor necrosis factor-a (TNF-a), interferon-γ (IFN- γ), and interleukin-2 (IL-2)) to specific regions of the tissue slice through a small port beneath the slice. The diffusion of each individual cytokine was quantified via widefield fluorescence microscopy, and was the first method to directly measure cytokine transport in live tissue slices.(73) A second generation of the device provided user-defined locations for the delivery and stimulation by incorporating movable elements.(74)
Other microfluidic models have focused at the level of lymphocyte function rather than whole organ function. In Moura Rosa et al., a microfluidic device was used to study perfusing T cells as they interacted with adherent dendritic cells as well as the effects of varying shear stress within the device.(75) This device aimed to model the T cell region within the lymph node, where dendritic cells prime and activate T cells during an immune response. Another main function of T cells, and other lymphocytes like neutrophils, is migration in the presence of a chemoattractant gradient. The immune synapse was further studied by Faley et al., where T cell-APC interactions were observed in a microfluidic cell trap device.(76) Various bucket-like structures within the device passively trapped perfusing cells, where cell-cell interactions can be observed for up to 24hrs. To mimic the immune synapse, primary T cells and LPS-matured dendritic cells (DCs) were introduced into the device and shown to increase calcium signaling in T cells post-interaction.(76) A 2006 study from Lin and Butcher used a simple Y channel in a microfluidic device to study T cell chemotaxis and migration in the presence of single and competing gradients of two cytokines, CCL19 and CXCL12.(77) While T cell chemotaxis is a main function of the adaptive immune response, transendothelial migration (TEM) of leukocytes is crucial for both innate and adaptive immunity. Similar to the Lin and Butcher device, TEM of neutrophils is modeled on a microfluidic device by culturing the leukocytes in the presence of a chemoattractant gradient.(78, 79)
In Han et al., neutrophils were housed in a central channel surrounded by hydrogel ECM, with perfusing media and a chemoattractant gradient running parallel (Figure 4d). As neutrophils responded to the gradient and migrate towards the regions with higher concentrations, they can be tracked within the device.(78) Similar to the previously described device, a 2015 study from Wu et al. cultured neutrophils in a hydrogel support in the presence of chemoattractant gradients, but this device integrated endothelial cells into the hydrogel as well as competing chemoattractant gradients to develop a more biomimetic model of TEM.(79) In a 2013 study, Mitra et al. integrated DC chemotaxis with T cell activation by co-culturing these cell types in adjacent compartments on a PDMS-based microfluidic device.(80) The DC migration was observed in the presence of a chemokine gradient, and T cell activation was quantified by measuring cellular calcium levels. This device was used to study various stages of the immune response, from DC maturation and migration to T cell activation.(80) While these devices modelled key components of the lymph node immune response, there is still no integrated microphysiological (organ-on-chip) model of a lymph node that replicates immune function of the organ, though several groups are working towards that goal.
2.3.3. Engineered tissues
Interestingly, engineered tissues for the study of immunity have largely focused on replicating specific functions, as compared to other engineered systems that focus on tissue structure.(26) As such, lymph node organoids are designed after organic development from precursor cell types and aim to mimic specific functions, such as antibody production. These organoids provide a platform to control the cell and matrix elements of the microenvironment around cultured immune cells and to better replicate in vivo conditions, although they usually do not incorporate fluid flow. A 2017 study from Purwada et al produced ex vivo immune organoids, consisting of B cells from mouse spleen, that replicated the germinal center reaction of secondary lymphoid organs (SLOs).(81) In vivo, B cells are activated through a variety of signals, including the CD40L ligand presented by follicular T helper cells, B cell activating factor (BAFF) presenting by follicular dendritic cells (FDCs), and the cytokine IL-4. To replicate these signals without incorporating T cells or true FDCs, fibroblasts were transduced with CD40L and BAFF, and the culture was supplemented with IL-4. The ECM was designed to mimic in vivo conditions by using crosslinked, RGD-presenting gelatin. In this immune organoid platform, the naive B cells were successfully differentiated into the germinal center phenotype and were capable of antibody class switching. Further, they were more proliferative and viable compared to 2D systems, providing a successful platform to study B-cell development in SLOs.(81) This study demonstrates the advantages of organoid technology, allowing precise control of ECM and cell-cell contacts to replicate in vivo immune organs.
Adding another layer of complexity to immune organoids, bioreactors introduce the element of flow. The HuALN (human artificial lymph node) bioreactor, developed by Giese et al is used extensively as an in vitro model of the lymph node.(82) The HuALN incorporates several cell types (T cells, B cells, dendritic cells), a hydrogel matrix, and micro-organoid structures. It has enabled reproduction of functions such as antigen specific antibody production and pro and anti-inflammatory cytokine secretion (Figure 4e).(82) The HuALN bioreactor has also been applied to the study of immunogenicity of protein aggregates. Patients can produce anti-drug antibodies when a drug has high immunogenicity. This can be impacted by patient specific factors such as allergies and the route of administration, but also by drug formulation and protein aggregates within the drug.(83) Two monoclonal antibodies - Adalimumab (also known as HUMIRA - an immunosuppressive drug used to treat arthritis, psoriasis, and Crohn’s disease) and Bevacizumab (also known as Avastin - used to treat colorectal, lung, glioblastoma, kidney, cervical, and ovarian cancer) were turned into protein aggregates by exposure to light, mechanical, and heat stress. A proinflammatory immune response was triggered by Bevacizumab after heat exposure. Though this result has not been proven to have clinical relevance yet, the authors point out that with further optimization, the HuALN can be a useful predictive tool for evaluating immunogenicity in humans.(83) Another bioreactor system - designed for bone marrow cultures - was adapted to culture immune cells isolated from tonsils. Immune cells including monocytes, dendritic cells, B cells, and T cells were viable over several weeks without the addition of specific growth factors, produced antigen specific antibodies, and self-organized into cell-specific aggregates.(84) These results demonstrate the importance of fluid flow to immune function.
Despite being a quintessential tissue engineering approach, cells in hydrogels have been limited in their use as models of the lymph node. Interestingly, 3D hydrogels have been mainly applied to modeling fibroblastic reticular cells in the T zone of the lymph node. In vivo, T zone fibroblastic reticular cells (FRCs) deposit and remodel ECM to guide lymphocyte migration and secrete CCL19 and CCL21 to direct immune cell interaction. Tomei et al encapsulated FRCs in type I collagen and matrigel on macroporous polyurethane scaffolds and applied interstitial flow (1–23 μL/min).(85) This 3D in vitro system recapitulated the morphology of FRCs in vivo, and confirmed the importance of flow for lymph node function, showing that flow enhanced FRC organization, and in its absence, the FRCs did not produce CCL21.(85) Since that study, more work using 3D hydrogels to model the T zone and stromal cells of the lymph node have been published,(86, 87) validating the benefits of a 3D matrix format that is less dense than organoid cultures. In a patient-specific model developed by Votanopoulos et al, matched lymph node and melanoma cells were aggregated together into immune-enhanced patient tumor organoids (iPTOs) for testing cancer drug efficacy. The iPTOs were able to replicate 85% (6/7) of patient immune responses. The iPTOs were then used to activate naive T cells before transfer to melanoma-only tumor organoids, where they were able to promote tumor cell death.(88)
Apart from bioreactors and 3D hydrogels, the MIMIC system provides a sophisticated way to recapitulate patient-specific immune response.(89) The MIMIC system has four components: a leukocyte module, a peripheral tissue equivalent (PTE) module to simulate innate immune responses, a lymphoid tissue equivalent (LTE) module to simulate adaptive immune responses, and a functional assay module (Figure 4f). In the first module, leukocytes are collected from donors and cryopreserved. In the PTE module, human umbilical vein endothelial cells (HUVECs) are cultured to confluence above a layer of collagen, after which donor peripheral blood mononuclear cells (PBMCs) are applied and differentiated into dendritic cells, antigen presenting cells, and macrophage-like cells. In the LTE module, dendritic cells, follicular dendritic cells, T cells and B cells are applied sequentially. Finally, the functional assay module is customized for each study to confirm how effective the immune responses from the PTE and LTE modules are against the antigen.(89) MIMIC incorporates multiple elements of immunity and is highly patient-specific, serving as a useful in vitro clinical trial system. So far, engineered lymph node models have focused on recapitulating immune function and have proven successful. However, future models with a focus on structure would enable the investigation of lingering biological questions about lymph node structure and function.
2.4. Spleen
The spleen is the single largest secondary immune organ in the body and is often the preferred source of primary immune cells from animal models for in vitro culture. The organ itself acts as a filter for blood pathogens and maintains roles in hematopoiesis and red blood cell clearance.(6) With a unique cellular organization that is somewhat distinct from that of the lymph node, the spleen also functions as the primary site for immune activation against blood-borne pathogens. Therefore, models of the spleen and its immune functions may inform novel therapies for blood-borne infections.
2.4.1. Ex vivo cultures
Protocols to slice mouse spleen were described during the 1970s,(90) and recently renewed interest in this organ has resulted in updated technologies to study spleen biology.(91) Human spleen sections have also been described, and interestingly, significant differences in cytokine secretion were observed between bulk splenocyte culture and slices of tissue, similar to the lymph node above.(65, 66) The difference between slices and suspension cultures may be attributable to the preservation of the spatial organization of the tissue, or to preservation of rare or matrix-adhesive cell types. Organotypic cultures of spleen sections supplemented with different amino acids have been shown to have distinct proliferation and differentiation profiles,(92) highlighting the need for further studies of the spleen, especially within intact tissue samples, to fully place the organ in the appropriate immune context.
2.4.2. Microfluidic chips
Efforts to model the spleen on a microfluidic device have focused primarily on the main function of the spleen, filtering red blood cells (RBCs), rather than on the immune functions of the splenocytes. To model spleen function, micro-constraints are integrated into microfluidic devices in the form of small posts that healthy RBCs can deform to fit through, while stiff, unhealthy RBCs are trapped.(93, 94) In a 2014 study from Rigat-Brugarolas et al., the spleen-on-a-chip model was used to filter out malaria-infected RBCs from healthy RBCs; future integration of immune functions into such a model may enhance the ability to study the outcomes and mechanisms of blood-borne infections.(94)
2.5. Lymphatic Vessels
An emphasis on lymphatic-immune interactions is an essential step toward recapitulating immunity in vitro. Lymphatic vessels run through tissue along-side blood vessels and act as paths for lymphatic fluid and immune cells to travel between organs. These vessels were traditionally viewed as maintaining a passive role in the immune response, or were even excluded as a formal part of the immune system. Therefore, models of the lymphatics so far have focused primarily on fluid flow and biomechanics, as reviewed previously by Dixon et al.(95) However, new studies are emerging highlighting the important roles that lymphatic endothelial cells play in presenting antigen to the immune system, and regulating cell entry to the lymphatic network.(96, 97) Due to the lack of basement membrane, initial lymphatic vessels are highly responsive to interstitial fluid flow – a benefit that allows for easy immune cell trafficking.(98) Lymphatics have now been implicated in anti-tumor immune responses, autoimmune diseases, and the regulation of inflammation.(98) Indeed, lymphatics have been added to co-cultures of breast cancer cells and mammary fibroblasts to recreate initial lymphatic-tumor cell interactions during metastatic spread, showing that LECs reduce therapeutic efficacy.(99) No ex vivo model of the full immune system would be complete without considering the role of lymphatic vessels, but so far this aspect of immune modelling is highly incomplete.
2.5.1. Ex vivo cultures
Experimental models to investigate lymphatic vessels ex vivo were recently reviewed.(100) While many excellent studies focused on the physical functionality (contractility, fluid flow etc.) of the vessels under different pressurized conditions,(101) further study into how immune responses affect the lymphatic system is required to help provide a full picture of the systemic nature of immunity. Lymphatic vessels have not been examined in the context of the whole tissue ex vivo, however with most studies focusing on single explanted lymphatic vessels. The lymphatic system is intimately involved in immune responses and lymphatic endothelial cells (LECs) the fundamental unit of vessels are an immunologically-active cell.(96) Thus, further study into how immune responses interact with, and depend upon, the lymphatic system is required to help provide a full picture of the systemic nature of immunity.
2.5.2. Microfluidic chips
With its control over fluid flow and cellular-scale microenvironments, microfluidic technology is naturally suited to model vascular networks. Models of the lymphatic system often use hydrogels as scaffolding, and can be used to study lymphatic drainage, lymphangiogenesis, and barrier function. Primarily, due to these approaches, initial lymphatics have been modeled, with little existing to model the collecting lymphatics, which feature smooth muscle cell mediated pumping and an intricate valve system. One established and robust strategy to micropattern lymphatic vessels is to insert a rod or a wire into liquid hydrogel and remove it when solidified, leaving behind a physiologically relevant circular hydrogel channel that is ideal for endothelial cell culture. Thompson et al formed lymphatic vessels in this manner in collagen scaffolds with and without lymphatic endothelial cells, leaving one end of the channel closed to model drainage under lymphatic-like pressure.(102) A 2019 study from Gong et al further studied lymphatic drainage and barrier function in a microfluidic device fabricated in a similar style, called μLYMPH.(103) A lumen rod was used to intersect the hydrogel region, and once the tubular channel had been formed, the LECs were seeded, forming a lymphatic vessel model that mimicked physiological structure and function. This study began to address the immunological function of the lymphatics, by quantifying the secretion of various growth factors and inflammatory mediators (follistatin, granulocyte colony-stimulating factor, hepatocyte growth factor, etc.), as well as the vessel response in the presence of exogenous stimuli (vascular endothelial growth factor (VEGF)-C, VEGF-D, and IL-6).(103)
Kim et al. cultured LECs in two fluidic chambers that contained perfusing media, separated by a central fibrin gel matrix.(104) On either side of the LEC fluidic chambers, stromal fibroblasts were cultured within a separate fibrin gel matrix. This microfluidic device closely mimicked the interstitial flow, biochemical cues, and cell-cell interactions found in the lymphatic vessel microenvironment in vivo, and was used to model sprouting lymphangiogenesis. In the presence of pro- and anti-lymphangiogenic stimuli, increased and decreased LEC sprouting, respectively, was observed in the fibroblast gel channels.(104) LEC-lined channels or monolayers also can serve as an interface in which to examine the role of the lymphatic system in more complex tissues and microenvironments, including its interaction with blood vessels(105) and the tumor microenvironment.(106, 107)
2.5.3. Engineered tissues
Engineering of lymphatics in vitro is structure-focused and has been performed in both decellularized scaffolds and hydrogels. While fluid flow in the lymphatic system is highly complex and governed by biological and mechanical phenomena,(95) engineered lymphatic systems are more often simple models that focus on whether cells or vessels assemble into the correct morphology. For example, LECs have been cultured in poly glycolic acid scaffolds,(108) VEGF-Fibrin-Collagen matrices,(109) and in collagen alone,(110) to quantify the extent of networking and morphology of LECs. These systems did not form lymphatic vessels with open lumens or luminal flow, limiting their physiological relevance. However, when exposed to interstitial flow through the culture matrix, the LECs did show drastic morphological differences compared to blood endothelial cells (BECs), forming large vacuoles and long extensions, thus providing insight into the fundamental behavior of LECs.(110) In a more macroscopic study, human adipose derived stem cells were differentiated into LECs and cultured on decellularized arterial scaffolds, and maintained vessel-like morphology for ten days.(111) The lymphatic models discussed above demonstrate the feasibility of culturing LECs into lymphatic vessels in vitro. By adding fluid flow and immune cells, such as T or B cells, these models may be used to address and understand lymphatic-immune interactions.
3. Approach II: Models of peripheral tissues with immune elements
All tissues interact with immune cells, not just the primary and secondary organs of the immune system discussed above. Each tissue contains populations of tissue-resident antigen-presenting cells for immunosurveillance, e.g. the so-called conventional dendritic cells and macrophages in peripheral organs and the microglia in the brain.(112) In addition, each organ experiences a constant flux of circulating naïve T and B cells that patrol tissues in search of their cognate antigen. Effector T and B cells are recruited to the sites of inflammation or infection, along with innate cells such as NK cells, neutrophils, and macrophages via the blood stream.
So far, efforts to incorporate components of the immune system into models of tissues focused most frequently on the incorporation of macrophages or cytokines into pre-existing tissue models, both of normal and pathological conditions. More recently, efforts have been made to include elements of adaptive immunity as well, but this area is in its infancy. The addition of an immune component to models of healthy tissue has the potential to reveal not only the importance of the immune system in maintaining the tissue of interest, but also the dynamic cross-talk between immunity and other body systems. Here we discuss in more detail models of the gut, brain, lungs, and vasculature with an integrated immune element. The role of macrophages has also been examined in models of osteogenesis and muscle repair, but were outside the scope of this review.(113–117)
3.1. Gut
The gut not only digests the food that you eat, but plays an important role in maintaining immunity. The large mucosal surface area, passage of food-borne antigens, and the presence of commensal bacteria populations mean the intestinal immune system interacts with more potential antigens than any other part of the body. The immune system of the gut is large enough to merit its own name, the gut-associated lymphoid tissue (GALT), and includes specialized lymph-node-like structures known as Peyer’s patches.(118) The GALT provides balance and triggers protective immunity in the presence of commensal and pathogenic microbiota, and is a critical component to modelling immune homeostasis in the gut.
3.1.1. Ex vivo cultures
There is a close relationship between the gut and the immune system, however the use of explanted gut tissue to investigate this relationship is rare, despite previous popularity as a platform to study toxicology and drug development.(119, 120) In one recent study, mouse gut explants were used to study Peyer’s patches and the gut-brain-immune axis.(121) The organotypic tissue cultures maintained the structural integrity of the intestine, Peyer’s patches, and neurons ex vivo, which may allow for future studies into complex gastrointestinal function. Samples of human gut tissue can be collected during colonoscopies, providing a valuable platform for translational study. For example, Schwerdtfeger et al used human gut cultures to investigate the effects of antibiotics and immunoprotection on exposure to Salmonella enterica bacteria.(122, 123) Thus, ex vivo gut culture has the potential to facilitate deep investigations of the complex interactions between the gut and the immune system.
3.1.2. Microfluidic chips
While many gut-on-a-chip microfluidic models have been developed, they originally focused on replicating the intricate structural and barrier functions of the gut. Building on that foundation, there is a more recent push to include immunity in these models.(124) Maurer et al developed a microphysiological model of the intestine that integrated resident innate immune cells, including mucosal macrophages and DCs.(125) Endothelial and epithelial cell layers were separated by a porous membrane within the device, and formed villus- and crypt-like structures normally found within the human intestine.(125) Mucosal macrophages and DCs were integrated into the endothelial and epithelial cell layers, respectively. This model was used to quantify changes in barrier functionality and immunotolerance in response to lipopolysaccharide (LPS), e.g. to model endotoxemia,(125) thus opening the door to future studies with more complex immune-related challenges. A 2013 study from Ramadan et al. integrated immune cells into a miniaturized artificial gastrointestinal tract, titled NutriChip, to study the immunomodulatory function of dairy in the diet.(126) The NutriChip device co-cultured an epithelial monolayer with macrophages, separated by a permeable membrane, with an on-chip sandwich immunoassay located downstream to detect pro-inflammatory cytokine secretion. In the future, the authors plan to use the device to test the inflammatory response in the presence of dairy to evaluate the influence of food on human health.(126)
While there are many gut-on-a-chip models, so far there are only a few that integrate immunity with a specifically disease-focused gut model, e.g. to study the inflammatory events in Inflammatory Bowel Disease (IBD). Kim et al. studied the different contributing factors of inflammation and villus damage using a gut-on-a-chip model that incorporated elements from the gut microbiome, immune cells, and epithelial deformation to model a diseased state.(127) Human epithelial cells were cultured in a microfluidic device with luminal-like fluid flow that encourages villi formation. When immune cells and LPS endotoxin were introduced into the system, the epithelial barrier produced pro-inflammatory cytokines that induced villus damage and compromised the barrier integrity, similar to in vivo studies.(127)
3.1.3. Engineered Tissues
Similar to chip-based models, many tissue engineered bowel models focus on recapitulating the inflammation associated with IBD. Roh et al fabricated a 3D model of the large intestine using spongy silk materials.(128) The inner layer of the scaffold was seeded with non-transformed human colon organoid cells and the outer layer of the scaffold was seeded with monocyte-derived macrophages. The engineered bowel formed microvilli and mucus layers. The presence of macrophages enhanced the engineered bowel’s response to inflammatory stimuli, including E.Coli, LPS, and interferon gamma, specifically upregulating the secretion of inflammatory cytokines associated with IBD: CXCL10, interleukin-1β (IL-1β), interleukin-6 (IL-6), monocyte chemoattractant protein-2 (MCP-2), and macrophage inflammatory protein-1β (MIP-1β).(128) Another area of interest in bowel tissue engineering is modeling the inflamed intestinal mucosa for drug screening and safety testing. The Lehr group utilized a 3D co-culture system of macrophages, dendritic cells, and Caco-2 epithelial cells to test inflammatory responses to bacteria, drugs, and nanomaterials.(129–131) 3D in vitro models of the inflamed bowel have been shown to be an effective and easily implemented tool for the study of inflammation and drug screening in the bowel.
3.2. Central Nervous System
The brain is an immunoprivileged organ, with naïve peripheral immune cells being largely excluded from the brain tissue. Adaptive immune cells found within the brain are associated with disease. The brain parenchyma contains a tissue-resident macrophage counterpart, microglia, which populate the brain tissue during development and provide immune function as an innate immune cell for immune protection throughout the life of the organism. Immune-brain interactions are increasingly examined experimentally, due to recent findings of more complex neuroimmune connections in vivo. Immune cells moving into the brain must cross through one of the barriers, be it through the blood vasculature and the blood-brain barrier (BBB, Sidebar 1), the choroid plexus, or via the newly discovered meningeal and subdural lymphatics.
Sidebar 1: Blood-Brain Barrier
Normal brain function is maintained by preserving the ionic and cellular composition of the neural environment and is maintained by a unique anatomical and physical barrier called the blood-brain barrier (BBB).(132–134) This barrier separates the central nervous system from peripheral blood circulation by tightly regulating the passive and active transport of cells, molecules, and ions.(133, 134) Along with the physical barrier, there is an immunological barrier that regulates the recruitment and transport of leukocytes and other innate immune elements.(134) The BBB is comprised of a highly specialized monolayer of endothelial cells (ECs) and is a part of larger structure called the Neurovascular Unit.(134) The ECs are linked by tight junction proteins and form a capillary, surrounded by the basal lamina, a collagen-based basement membrane.(132–134) Surrounding the basement membrane are pericytes, astrocytes, microglia, and neurons, all forming the highly regulated barrier that is essential for maintaining cerebrospinal fluid homeostasis.(132, 134) In the presence of locally-secreted inflammatory cytokines like IL-6 and TNF- a, the tight junctions between the ECs are disrupted, leading to increased leukocyte adhesion and migration.(135) There is increasing evidence that inflammation in the brain is involved in the pathogenesis of neurological diseases like Alzheimer’s and Multiple Sclerosis.(135)
3.2.1. Ex vivo cultures
Brain slices are used extensively in the neuroscience field to study brain function and biology. With the increased interest in neuro-immunity, microglia in particular are a recent intensive focus of investigation in both rat and mouse brain slices.(136–141) Neural inflammation is a concern for many brain diseases, and the immune response to inflammatory stimuli has been investigated in brain slices.(142, 143) T cell interactions with neurons are also a topic of interest in brain slice studies.(144, 145) For example, Nitsch et al. monitored interactions between activated proteolipid protein-specific T cells and neurons in a model of multiple sclerosis, and reported that these interactions resulted in neuron death.(144) The ability to clear viruses from the brain have also been described for both cytomegalovirus (CMV)(146) and zika.(147) Lyme disease clearance has also been studied in brain slices.(148) By studying how tissues clear both pathogens and respond to self-antigens more targeted therapies can be designed, off-target damage can be limited, and patient-specific effects may be determined. These studies highlight the importance of investigating all components of the immune system when preparing models of brain immunity, to provide insight into normal homeostatic and disease-specific states.
3.2.2. Microfluidic chips
Immune cell trafficking into the central nervous system via the BBB has been a primary focus for microfluidic models of immunity in the brain.(135, 149) In a 2011 study, Cucullo et al. altered their existing dynamic in vitro blood-brain barrier model to introduce immune cell trafficking.(135) The device utilized artificial capillaries to culture a layer of endothelial cells with abluminal astrocytes to form a biomimetic BBB model. Microholes were formed in the artificial capillary, allowing monocyte extravasation across the vascular endothelial layer, shown by a significant increase of cytokine levels as well as a loss of barrier integrity.(135) Another BBB model from Mossu et al. cultured brain-like endothelial cells on a nanoporous silicon nitride membrane to form an in vitro cerebrovascular barrier (CVB) model, termed μSiM-CVB.(149) While the device only incorporated brain-like endothelial cells and not additional cell types found in the neurovascular unit, pericyte-conditioned media was used to make a more physiologically relevant model. When co-cultured with T cells, μSiM-CVB was able to capture the multi-step migration process using live imaging.(149)
3.2.3. Engineered tissues
While the microfluidic models focused on barrier function at the BBB, engineered tissue models using hydrogels for 3D culture are well suited to model the brain parenchyma. Modeling immunity in tissue engineered brain models has focused largely on microglial function and inflammatory response. Many studies have observed microglia in 3D hydrogels and their response to inflammation induced by LPS.(150–153) These studies confirmed that microglial function in 3D more closely resembles in vivo responses compared to 2D cultures. Though these models included only one cell type, they provide a simple platform to study microglial activation in response to inflammatory stimuli and validate the necessity of 3D cultures for in vitro studies of brain immunity. A more multi-cellular model, presented by Abreu et al, utilized induced pluripotent stem cell-derived microBrainSpheres (μBS). Neural progenitor cells are aggregated into spheroids with and without immortalized microglia and tested for inflammatory response and cell viability. The μBS were shown to contain astrocyte, oligodendrocyte, and neuronal populations. To study inflammatory response, the μBS with and without microglia were treated with LPS for 24 hours.(154) The inflammatory response to LPS differed greatly based on which cell types were included in the model. In the absence of microglia, the μBS had a very minimal response. Microglia-only spheroids upregulated CCL2 and TNF-⍺ at 12 hours. In combination microglia-μBS cultures, IL-1, IL-6, and IL-10 were upregulated at 12 hours and downregulated at 24 hours, and the microglia-only response was not observed.(154) The authors point to crosstalk between microglia and the other cell populations as the cause for these altered responses. The LPS treatment also caused an increase in dividing microglia, and a decrease in viability in both microglia and the μBS cells.(154) A recent study performed a triculture of neurons, microglia, and astrocytes in a 3D microfluidic platform.(155) This model successfully recapitulated important aspects of Alzheimer’s Disease (AD), including beta-amyloid aggregation, phosphorylated tau accumulation, and neuroinflammatory activity and can be used in the future to study the pathogenesis of AD and to develop therapeutics. Bioprinted glioblastoma models (BGMs) that incorporated microglia or macrophages were shown to recapitulate patient survival profiles, including tumor invasion and drug resistance.(156) When compared to 2D monolayer cultures, glioblastoma cells in BGMs with macrophages were more resistant to drugs.(157) More complex tissue engineered models of the brain incorporating microglia allow more physiologically relevant inflammatory responses, while enabling the examination of cell-cell crosstalk to answer more specific mechanistic questions.
3.3. Lungs
Similar to the gut, the lungs are a mucosal layer that is exposed to foreign particles on a daily basis. Many airborne pathogens enter the body through the lungs, including viruses and solid particulates. These organs pose a particular challenge to regulating immunity, as collateral damage to the surrounding tissue as a result of an excessive immune response can severely affect their critical role as a gas exchange surface. Immunity within the lung is a closely regulated balance between the innate and adaptive responses, and understanding this balance in healthy tissue is important towards designing effective vaccines against airborne pathogens.(158)
3.3.1. Ex vivo cultures
Lung immunity is an area of intense study, as a result, precision cut lung slices have been well-characterized for immune-related functions.(159–161) In one application, lymphatic vessels were visualized within healthy tissue to help further understand immune cell trafficking. Kretschmer et. al. investigated the role of CD90/Thy-1 as a marker for both healthy and inflamed lymphatic vessels in lung slices (Figure 5 a). By using this marker researchers were able to highlight a possible route for immune cells to leave the lung, by tracking T cell infiltration in response to dust mite antigen.(160) Human lung sections have even been characterized to act as a screening platform for vaccines and pharmaceuticals.(162, 163) Additional studies have been completed in the context of infection as ex vivo tissue culture is often the only means to study the human immune response in a fully intact tissue setting. This is particularly timely with the current investigation of immune responses related to SARS-CoV-2 (COVID-19). Respiratory infections have been extensively modelled in lung slices, including responses to the flu(164) and flu vaccines.(165, 166)
Figure 5:
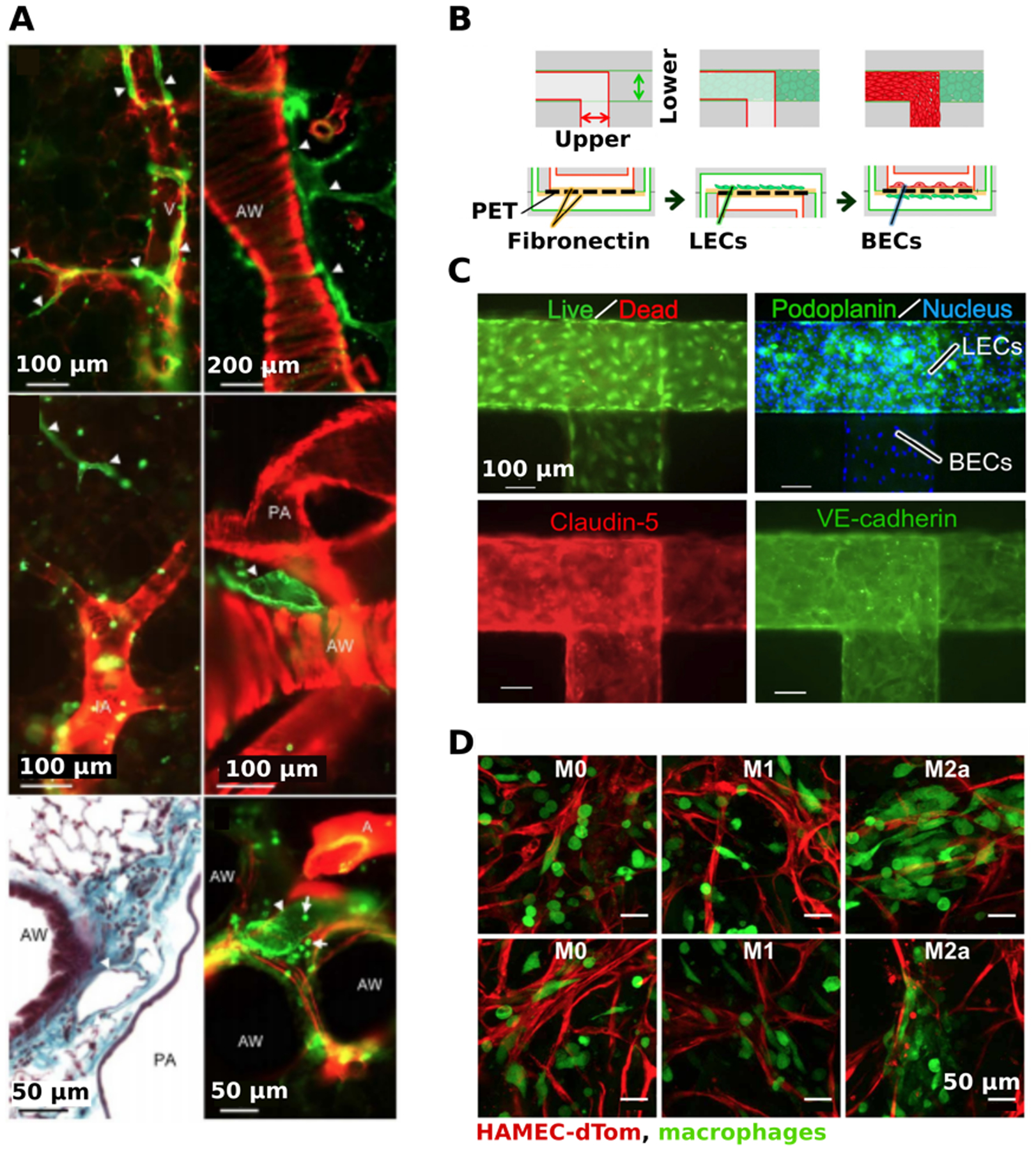
Experimental models of immune-related vasculature. (a) Visualization of lymphatic vessels in slices of lung tissue. Double labelling of precision-cut lung slices with anti-CD90/Thy-1 (green) and anti-α-smooth muscle actin (red). Lymphatic vessels (white arrowheads) were found near veins (V), muscular airways (AW), and pulmonary arties (PA) and were not associated with intra-acinar arteries (IA). Bottom left panel was paraffin-embedded and stained with Masson Goldner stain. Presented with permission from Kretschmer et al.(160) (b) A microcirculation-on-a-chip model was developed with lymphatic endothelial cells (LECs) in the lower channel and blood endothelial cells (BECs) in the upper channel, separated by a PET membrane. The membrane was initially coated with fibronectin before co-culturing the LECs and BECs, respectively. (c) Visualization of LECs and BECs within the microfluidic device. High cell viability was shown using Calcien AM (green) and ethidium homodimer (red). Both LECs and BECs nuclei were labelled with Hoescht33342 (blue), LECs were labeled using anti-podoplanin (green), and BECs were labeled with claudin-5 (red). Both channels were stained for an endothelial-specific adhesion molecule, VE-cadherin (green), to check for barrier stability. Presented with permission from Sato et al.(167) (d) The effects of macrophages of different polarizations on angiogenesis. Engineered blood vessels (red) were formed on Gelfoam by culturing human microvascular endothelial cells expressing tD-Tomato (HAMEC-dTOMs) and mesenchymal stem cells. After 3 days, suspensions of M0, M1, or M2a macrophages (green) were added to the vessels and imaged 1 day later. Presented with permission from Graney et al.(168)
3.3.2. Microfluidic chips
Immunity is crucial for maintaining health and proper function within the lungs, as this tissue is exposed to foreign particles daily, and immune cells are naturally found throughout this tissue. While there are many microfluidic devices that model healthy and diseased lung tissue,(169, 170) they don’t include an immune element, which would be crucial for making a lung model that accurately mimics in vivo function.
3.3.3. Engineered tissues
Engineered models of the lung aim to recapitulate the respiratory tract to study the effects of particles, such as potentially toxic nanoparticles released by industrial processes,(171, 172) or gold nanoparticles that have potential for drug delivery.(173) Harrington et al developed an immunocompetent model of the respiratory tract that incorporated epithelial cells, fibroblasts, and dendritic cells onto electrospun polyethylene terephthalate scaffolds. Stimulation with LPS and Papain, an allergen that disrupts tight junctions in the respiratory tract, caused dendritic cell migration and mimicked the in vivo response.(174) More recently, lung organoids have been applied to the global COVID-19 pandemic caused by the novel SARS-CoV-2 virus.(175, 176) These models provide useful tools to investigate drug delivery and the effects of allergens or harmful particles in the lung.
3.4. Vasculature
The body is connected by an intricate vascular system composed of blood vessels of varying sizes, from the aorta down to capillaries. The immune system and vasculature interface in many contexts. During an immune response, immune cells travel to an inflamed site through the vasculature. Many in vitro models focus on understanding and recapitulating the migration, adhesion, and extravasation of immune cells in blood vessels. Immune cells have also been implicated in regulating angiogenesis, the formation of new blood vessels.(177)
3.4.1. Ex vivo cultures
Regarding ex vivo systems, studies of immune-related vascular angiogenesis and lymphangiogenesis have been confined to immunostaining slices of fixed tissue.
3.4.2. Microfluidic chips
Many vascular microfluidic models integrate immunity by studying blood vessel function and interaction alongside lymphatic vessels. The interaction of both blood and lymphatic vessels may be key to gain a further understanding of inflammation and cancer metastases.(105) To study the cooperative effects of vascular angiogenesis (VA) and lymphangiogenesis (LA), Osaki et al. modeled blood and lymphatic vessels side by side on a microfluidic device.(105) The in vitro vessels were fabricated using sacrificial molding to form two circular channels in PDMS. To induce VA and LA in the microfluidic device, phorbol 12-myristate 13-acetate and VEGF (C or A) were introduced. Angiogenic sprouting for both the blood and lymphatic vessels were observed. In addition to inducing angiogenesis, anti-angiogenic factors such as VEGF-R3, a VEGF antagonist, were introduced, resulting in reduced angiogenic sprouting.(105) A 2015 study from Sato et al. developed a microcirculation-on-a-chip that co-cultures BECs and LECs in upper and lower channels, separated by a porous membrane (Figure 5b).(167) Both claudin-5 and VE-cadherin were used to detect cell-cell junctions of both the lymphatic and blood vessel channels, confirming barrier integrity (Figure 5c). Histamine is a compound generally released by cells as a part of the inflammatory response, and was introduced to the microcirculation model. This reduced tightness of the cell-cell junctions of both the BECs and the LECs, which led to an increase in vascular permeability.(167)
Vasculature-on-a-chip has also been used to study migration and adhesion of lymphocytes and other immune cells to endothelial cells. In a 2011 study, Srigunapalan et al. developed a microfluidic device that modelled the vascular microenvironment, incorporating hemodynamic shear stress via perfusion, circulating cytokines, extracellular matrix proteins, and monocyte interactions.(178) A monolayer of aortic endothelial cells was cultured across a membrane within the device, and monocytes were introduced into perfusing media to quantify cell adhesion and transmigration.(178) Monocyte adhesion and transmigration can be increased with the introduction of specific inflammatory cytokines (TNF-a) and chemoattractant proteins (monocyte chemoattractant protein-1). To gain a better understanding of leukocyte adhesion to endothelial cells, Kim et al. cultured an endothelial monolayer in a microfluidic channel to model in vitro vasculature to study cell-cell binding with activated and inactivated T cells.(179) Immunosuppressive drugs were also perfused through the device, decreasing the frequency of T cell-endothelial cell interactions.(179)
Utilizing a similar device design, Zhang et al. cultured endothelial monolayers within a multi-channel microfluidic device. Coupling this device with a multiplexed perfusion platform they could run up to 4 devices at once.(180) The branched channels within each device better mimicked the geometric configuration of natural blood vessels. Immune cell adhesion was tested with this platform to validate proper function by introducing monocytes with and without TNF-ɑ.(180) Wu et al, coupled another function of vascular angiogenesis, endothelial migration, with neutrophil migration in a hydrogel scaffold on a microfluidic chip.(181) A controllable chemical gradient ran parallel to the cultured cells induced cell migration through the gel support, showing that this model can be used to gain a better understanding of dynamic cellular interactions.(181)
3.4.3. Engineered tissues
It is well established that macrophages are beneficial to angiogenesis. A 2018 review by Moore and West summarized how macrophages have been utilized in tissue engineering of vasculature.(182) Recent work by Graney et al showed the importance of macrophage phenotype (see Sidebar 2) in regards to EC behavior.(168) When added to 3D tissue engineered blood vessels, M1 macrophages were elongated and found in proximity with the leading front of sprouts (Figure 5d). Further, at day 1, M1 macrophages supported vascularization, but caused vessel regression by day 3. M2 macrophages had a paracrine effect of upregulated pericyte differentiation genes, and were found wrapped around blood vessels in the 3D tissue engineered model (Figure 5d). Overall, this study showed that macrophage impact on EC behavior greatly relies on phenotype, and raises questions of temporal regulation of angiogenesis via macrophage polarization. Angiogenesis can be triggered by hypoxia, and hypoxic conditions lead to inflammation and immune cell invasion. Therefore, the immune system plays an important role in blood vessel formation. Modeling the interaction between blood vessels and immune cells in vitro provides a platform that could be used to study hypoxic tissues, tissue expansion, or tissue regeneration.
Sidebar 2: Macrophage polarization
Macrophages subtypes offer differential effects in tissues, and though recent evidence presents these phenotypes as existing on a spectrum, the defined polarizations are useful for categorizing behaviors. The classically activated M1 phenotype is considered pro-inflammatory, while the alternatively activated M2 phenotype is considered anti-inflammatory.(168) However, M2 macrophages themselves have recently been identified to have varying subtypes. M2a macrophages are implicated in stabilizing blood vessels,(183) M2c macrophages secrete matrix metalloproteinases,(184) and M2f macrophages secrete anti-inflammatory signals in response to phagocytosis of apoptotic cells.(185)
4. Conclusions and future directions
Overall, the development of in vitro and ex vivo models of immunity is expanding, especially in recent years. We have described here several types of systems in which to study and model immune interactions, including ex vivo cultures, chips, and engineered tissues. Many of these systems overlap, with the inclusion of engineered tissues or slices with microfluidics, resulting in multi-modal systems that recapitulate more complex immune interactions. Interestingly, we found that the majority of systems that incorporate immunity are focused on a narrow set of disease states (e.g. tumors and infection), on incorporation of only a single aspect of immunity (e.g. macrophages or T cells). While such models are important, the in vitro systems are difficult to validate without a definitive knowledge of their fundamental immune-tissue or immune organ interactions and functions. In addition, the use of a small number of macrophage cell lines to replicate the vast diversity of antigen-presenting cells is a practical simplification that is useful in the short term, but will hinder the biological relevance of the models for more complex scenarios. Thus, we propose that development of primary immune organs and healthy tissues that adequately recapitulate native immune functions, including use of primary or stem-cell derived cell types, are vital to the future creation and use of immune-disease systems. In addition, most models so far focus on immunity within a single organ; additional models that replicate the systemic and multi-organ nature of immune responses will be valuable advances.
In vitro and ex vivo models of immunity may be best grounded in the use of patient-specific tissues, both for comparison to validate the model’s functions, and as a source of a diversity of cells. In patients, we know there are myriad baseline immune states, immune responses, and immune profiles that we are only just beginning to understand. The use of in vitro systems to model patient specific organs and tissues will expand our knowledge of how and why immunity varies from patient-to-patient. With the recent pandemic crisis, where we see that patient-to-patient responses are widely varied with resultant tragic health consequences,(186) the development of these types of high-throughput, immune-incorporating, rapidly usable in vitro systems is now more important than ever.
5. Acknowledgements
This publication was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) under Award Number U01EB029127 through the National Institutes of Health (NIH), with co-funding from the National Center for Advancing Translational Sciences (NCATS). Additional support was provided by the National Institute of Allergy and Infectious Diseases under Award Number R01AI131723 to RRP, and by the National Cancer Institute under Award Number R37CA222563 to JMM. M. C. Belanger was supported in part by the Immunology Training Grant at the University of Virginia (NIH, 5T32AI007496-23). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Figures 1 - 3 were created BioRender.com.
6 References
- 1.Couzin-Frankel J 2013. Cancer Immunotherapy. Science. 342(6165):1432–33 [DOI] [PubMed] [Google Scholar]
- 2.Hotaling NA, Tang L, Irvine DJ, Babensee JE. 2015. Biomaterial Strategies for Immunomodulation. Annu. Rev. Biomed. Eng 17(1):317–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Germain RN, Meier-Schellersheim M, Nita-Lazar A, Fraser IDC. 2011. Systems Biology in Immunology: A Computational Modeling Perspective. Annu. Rev. Immunol 29(1):527–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makaryan SZ, Cess CG, Finley SD. 2020. Modeling immune cell behavior across scales in cancer. WIREs Syst. Biol. Med 12(4):e1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter MC, Teijeira A, Halin C. 2016. T Cell Trafficking through Lymphatic Vessels. Front. Immunol 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis SM, Williams A, Eisenbarth SC. 2019. Structure and function of the immune system in the spleen. Sci. Immunol 4(33):eaau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinuesa CG, Linterman MA, Yu D, MacLennan ICM. 2016. Follicular Helper T Cells. Annu. Rev. Immunol 34(1):335–68 [DOI] [PubMed] [Google Scholar]
- 8.Crotty S 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity. 41(4):529–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol 22:745–63 [DOI] [PubMed] [Google Scholar]
- 10.Kurosaki T, Kometani K, Ise W. 2015. Memory B cells. Nat. Rev. Immunol 15(3):149–59 [DOI] [PubMed] [Google Scholar]
- 11.Steinbach K, Vincenti I, Merkler D. 2018. Resident-Memory T Cells in Tissue-Restricted Immune Responses: For Better or Worse? Front. Immunol 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing Z, Afkhami S, Bavananthasivam J, Fritz DK, D’Agostino MR, et al. 2020. Innate immune memory of tissue-resident macrophages and trained innate immunity: Revamping vaccine concept and strategies. J. Leukoc. Biol [DOI] [PubMed] [Google Scholar]
- 13.Sompayrac L 2016. How the immune system works. Chichester, West Sussex ; Ames, Iowa: John Wiley & Sons, Ltd. Fifth edition ed. [Google Scholar]
- 14.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. 2002. Innate Immunity. Mol. Biol. Cell 4th Ed. [Google Scholar]
- 15.Mogensen TH. 2009. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev 22(2):240–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proudfoot AEI. 2006. The biological relevance of chemokine–proteoglycan interactions. Biochem. Soc. Trans 34(3):422–26 [DOI] [PubMed] [Google Scholar]
- 17.Jin T, Xu X, Hereld D. 2008. Chemotaxis, chemokine receptors and human disease. Cytokine. 44(1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pompano RR, Chiang AH, Kastrup CJ, Ismagilov RF. 2017. Conceptual and Experimental Tools to Understand Spatial Effects and Transport Phenomena in Nonlinear Biochemical Networks Illustrated with Patchy Switching. Annu. Rev. Biochem 86(1):333–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Zerda A, Kratochvil MJ, Suhar NA, Heilshorn SC. 2018. Review: Bioengineering strategies to probe T cell mechanobiology. APL Bioeng. 2(2):021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chittur KK, Mcintire LV, Rich RR. 1988. Shear Stress Effects on Human T Cell Function. Biotechnol. Prog 4(2):89–96 [Google Scholar]
- 21.Valignat M-P, Theodoly O, Gucciardi A, Hogg N, Lellouch AC. 2013. T Lymphocytes Orient against the Direction of Fluid Flow during LFA-1-Mediated Migration. Biophys. J 104(2):322–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Serrano JC, Xing H, Lee TA, Azizgolshani H, et al. 2018. Interstitial flow promotes macrophage polarization toward an M2 phenotype. Mol. Biol. Cell 29(16):1927–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rainger GE, Buckley CD, Simmons DL, Nash GB. 1999. Neutrophils sense flow-generated stress and direct their migration through α V β 3 -integrin. Am. J. Physiol.-Heart Circ. Physiol 276(3):H858–64 [DOI] [PubMed] [Google Scholar]
- 24.Giese C, Marx U. 2014. Human immunity in vitro — Solving immunogenicity and more. Adv. Drug Deliv. Rev 69–70:103–22 [DOI] [PubMed] [Google Scholar]
- 25.Ramadan Q, Ting FCW. 2016. In vitro micro-physiological immune-competent model of the human skin. Lab. Chip 16(10):1899–1908 [DOI] [PubMed] [Google Scholar]
- 26.Gosselin EA, Eppler HB, Bromberg JS, Jewell CM. 2018. Designing natural and synthetic immune tissues. Nat. Mater 17(6):484–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro-Filho AC, Levy D, Ruiz JLM, Mantovani M da C, Bydlowski SP. 2019. Traditional and Advanced Cell Cultures in Hematopoietic Stem Cell Studies. Cells. 8(12): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mokhtari Z, Mech F, Zehentmeier S, Hauser AE, Figge MT. 2015. Quantitative image analysis of cell colocalization in murine bone marrow. Cytometry A. 87(6):503–12 [DOI] [PubMed] [Google Scholar]
- 29.Bellido T, Delgado-Calle J. 2020. Ex Vivo Organ Cultures as Models to Study Bone Biology. JBMR Plus. 4(3): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner I, Materne E-M, Brincker S, Süßbier U, Frädrich C, et al. 2013. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab. Chip 13(18):3538. [DOI] [PubMed] [Google Scholar]
- 31.Sieber S, Wirth L, Cavak N, Koenigsmark M, Marx U, et al. 2018. Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J. Tissue Eng. Regen. Med 12(2):479–89 [DOI] [PubMed] [Google Scholar]
- 32.Torisawa Y, Mammoto T, Jiang E, Jiang A, Mammoto A, et al. 2016. Modeling Hematopoiesis and Responses to Radiation Countermeasures in a Bone Marrow-on-a-Chip. Tissue Eng. Part C Methods 22(5):509–15 [DOI] [PubMed] [Google Scholar]
- 33.Torisawa Y, Spina CS, Mammoto T, Mammoto A, Weaver JC, et al. 2014. Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro. Nat. Methods 11(6):663–69 [DOI] [PubMed] [Google Scholar]
- 34.Chou DB, Frismantas V, Milton Y, David R, Pop-Damkov P, et al. 2020. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng 4(4):394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aleman J, George SK, Herberg S, Devarasetty M, Porada CD, et al. 2019. Deconstructed Microfluidic Bone Marrow On-A-Chip to Study Normal and Malignant Hemopoietic Cell–Niche Interactions. Small. 15(43):1902971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mortera-Blanco T, Mantalaris A, Bismarck A, Aqel N, Panoskaltsis N. 2011. Long-term cytokine-free expansion of cord blood mononuclear cells in three-dimensional scaffolds. Biomaterials. 32(35):9263–70 [DOI] [PubMed] [Google Scholar]
- 37.Nichols JE, Cortiella J, Lee J, Niles JA, Cuddihy M, et al. 2009. In vitro analog of human bone marrow from 3D scaffolds with biomimetic inverted colloidal crystal geometry. Biomaterials. 30(6):1071–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourgine PE, Klein T, Paczulla AM, Shimizu T, Kunz L, et al. 2018. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc. Natl. Acad. Sci 115(25):E5688–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braham MVJ, Minnema MC, Aarts T, Sebestyen Z, Straetemans T, et al. 2018. Cellular immunotherapy on primary multiple myeloma expanded in a 3D bone marrow niche model. OncoImmunology. 7(6):e1434465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller JFAP Haddow A. 1962. Effect of neonatal thymectomy on the immunological responsiveness of the mouse. Proc. R. Soc. Lond. B Biol. Sci 156(964):415–28 [Google Scholar]
- 41.Miller JF a. P. 1965. Effect of Thymectomy in Adult Mice on Immunological Responsiveness. Nature. 208(5017):1337–38 [DOI] [PubMed] [Google Scholar]
- 42.Mandel T, Russell PJ. 1971. Differentation of foetal mouse thymus. Ultrastructure of organ cultures and of subcapsular grafts. Immunology. 21(4):659–74 [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi Y, Horiuchi T, Hamamura K, Sugimoto T, Yagita H, Okumura K. 1991. Role of CD4 molecule in intrathymic T-cell development. Immunology. 74(2):183–90 [PMC free article] [PubMed] [Google Scholar]
- 44.Oh S-H, Kim K. 1999. Expression of interleukin-1 receptors in the later period of foetal thymic organ culture and during suspension culture of thymocytes from aged mice. Immunol. Cell Biol 77(6):491–98 [DOI] [PubMed] [Google Scholar]
- 45.Robinson JH, Owen JJT. 2008. Pillars Article: Generation of T-cell Function in Organ Culture of Foetal Mouse Thymus. I. Mitogen Responsiveness. J. Immunol 181(11):7437–44 [PubMed] [Google Scholar]
- 46.Sahni H, Ross S, Barbarulo A, Solanki A, Lau C-I, et al. 2015. A genome wide transcriptional model of the complex response to pre-TCR signalling during thymocyte differentiation. Oncotarget. 6(30):28646–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross JO, Melichar HJ, Au-Yeung BB, Herzmark P, Weiss A, Robey EA. 2014. Distinct phases in the positive selection of CD8+ T cells distinguished by intrathymic migration and T-cell receptor signaling patterns. Proc. Natl. Acad. Sci 111(25):E2550–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurd N, Robey EA. 2016. T-cell selection in the thymus: a spatial and temporal perspective. Immunol. Rev 271(1):114–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross JO, Melichar HJ, Halkias J, Robey EA. 2016. Studying T Cell Development in Thymic Slices. In T-Cell Development: Methods and Protocols, ed Bosselut R, Vacchio MS, pp. 131–40. New York, NY: Springer; [DOI] [PubMed] [Google Scholar]
- 50.Sood A, Dong M, Melichar HJ. 2016. Preparation and Applications of Organotypic Thymic Slice Cultures. J. Vis. Exp. JoVE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lancaster JN, Ehrlich LIR. 2017. Analysis of Thymocyte Migration, Cellular Interactions, and Activation by Multiphoton Fluorescence Microscopy of Live Thymic Slices. In T-Cell Trafficking: Methods and Protocols, ed Rainger GE, Mcgettrick HM, pp. 9–25. New York, NY: Springer; [DOI] [PubMed] [Google Scholar]
- 52.Lancaster JN, Thyagarajan HM, Srinivasan J, Li Y, Hu Z, Ehrlich LIR. 2019. Live-cell imaging reveals the relative contributions of antigen-presenting cell subsets to thymic central tolerance. Nat. Commun 10(1):2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hale LP, Neff J, Cheatham L, Cardona D, Markert ML, Kurtzberg J. 2020. Histopathologic assessment of cultured human thymus. PLOS ONE. 15(3):e0230668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poznansky MC, Evans RH, Foxall RB, Olszak IT, Piascik AH, et al. 2000. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat. Biotechnol 18(7):729–34 [DOI] [PubMed] [Google Scholar]
- 55.Seet CS, He C, Bethune MT, Li S, Chick B, et al. 2017. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat. Methods 14(5):521–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bredenkamp N, Ulyanchenko S, O’Neill KE, Manley NR, Vaidya HJ, Blackburn CC. 2014. An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nat. Cell Biol 16(9):902–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grant SM, Lou M, Yao L, Germain RN, Radtke AJ. 2020. The lymph node at a glance – how spatial organization optimizes the immune response. J. Cell Sci 133(5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swain SL. 1995. T-Cell Subsets: Who does the polarizing? Curr. Biol 5(8):849–51 [DOI] [PubMed] [Google Scholar]
- 59.Cameron SB, Stolte EH, Chow AW, Savelkoul HFJ. 2003. T helper cell polarisation as a measure of the maturation of the immune response. Mediators Inflamm. 12(5):285–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller MJ, Wei SH, Parker I, Cahalan MD. 2002. Two-Photon Imaging of Lymphocyte Motility and Antigen Response in Intact Lymph Node. Science. 296(5574):1869–73 [DOI] [PubMed] [Google Scholar]
- 61.Stoll S, Delon J, Brotz TM, Germain RN. 2002. Dynamic Imaging of T Cell-Dendritic Cell Interactions in Lymph Nodes. Science. 296(5574):1873–76 [DOI] [PubMed] [Google Scholar]
- 62.Huang JH, Cárdenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, et al. 2007. Requirements for T lymphocyte migration in explanted lymph nodes. J. Immunol. Baltim. Md 1950 178(12):7747–55 [DOI] [PubMed] [Google Scholar]
- 63.Salmon H, Rivas-Caicedo A, Asperti-Boursin F, Lebugle C, Bourdoncle P, Donnadieu E. 2011. Ex vivo Imaging of T Cells in Murine Lymph Node Slices with Widefield and Confocal Microscopes. J. Vis. Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katakai T 2018. Live Imaging of Interstitial T Cell Migration Using Lymph Node Slices. In Intravital Imaging of Dynamic Bone and Immune Systems : Methods and Protocols, ed Ishii M, pp. 29–42. New York, NY: Springer; [DOI] [PubMed] [Google Scholar]
- 65.Hoffmann P, Skibinski G, James K. 1995. Organ culture of human lymphoid tissue. I. Characteristics of the system. J. Immunol. Methods 179(1):37–49 [DOI] [PubMed] [Google Scholar]
- 66.Skibinski G, Hoffmann P, Radbruch A, James K. 1997. Organ culture of human lymphoid tissue. II. Marked differences in cytokine production and proliferation between slice and suspension cultures of human spleen. J. Immunol. Methods 205(2):115–25 [DOI] [PubMed] [Google Scholar]
- 67.Grivel J-C, Margolis L. 2009. Use of human tissue explants to study human infectious agents. Nat. Protoc 4(2):256–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giger B, Bonanomi A, Odermatt B, Ladell K, Speck RF, et al. 2004. Human tonsillar tissue block cultures differ from autologous tonsillar cell suspension cultures in lymphocyte subset activation and cytokine gene expression. J. Immunol. Methods 289(1–2):179–90 [DOI] [PubMed] [Google Scholar]
- 69.Belanger MC, Kinman AWL, Catterton MA, Ball AG, Groff BD, et al. 2019. Acute lymph node slices are a functional model system to study immunity ex vivo. bioRxiv, p. 865543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Groff BD, Kinman AWL, Woodroof JF, Pompano RR. 2019. Immunofluorescence staining of live lymph node tissue slices. J. Immunol. Methods 464:119–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knoblich K, Cruz Migoni S, Siew SM, Jinks E, Kaul B, et al. 2018. The human lymph node microenvironment unilaterally regulates T-cell activation and differentiation. PLOS Biol. 16(9):e2005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shim S, Belanger MC, Harris AR, Munson JM, Pompano RR. 2019. Two-way communication between ex vivo tissues on a microfluidic chip: application to tumor–lymph node interaction. Lab. Chip 19(6):1013–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ross AE, Pompano RR. 2018. Diffusion of cytokines in live lymph node tissue using microfluidic integrated optical imaging. Anal. Chim. Acta 1000:205–13 [DOI] [PubMed] [Google Scholar]
- 74.Catterton MA, Dunn AF, Pompano RR. 2018. User-defined local stimulation of live tissue through a movable microfluidic port. Lab. Chip 18(14):2003–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moura Rosa P, Gopalakrishnan N, Ibrahim H, Haug M, Halaas Ø. 2016. The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device. Lab. Chip 16(19):3728–40 [DOI] [PubMed] [Google Scholar]
- 76.Faley S, Seale K, Hughey J, Schaffer DK, VanCompernolle S, et al. 2008. Microfluidic platform for real-time signaling analysis of multiple single T cells in parallel. Lab. Chip 8(10):1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin F, Butcher EC. 2006. T cell chemotaxis in a simple microfluidic device. Lab Chip. 6(11):1462–69 [DOI] [PubMed] [Google Scholar]
- 78.Han S, Yan J-J, Shin Y, Jeon JJ, Won J, et al. 2012. A versatile assay for monitoring in vivo-like transendothelial migration of neutrophils. Lab. Chip 12(20):3861. [DOI] [PubMed] [Google Scholar]
- 79.Wu X, Newbold MA, Haynes CL. 2015. Recapitulation of in vivo-like neutrophil transendothelial migration using a microfluidic platform. The Analyst. 140(15):5055–64 [DOI] [PubMed] [Google Scholar]
- 80.Mitra B, Jindal R, Lee S, Dong DX, Li L, et al. 2013. Microdevice integrating innate and adaptive immune responses associated with antigen presentation by dendritic cells. RSC Adv. 3(36):16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Purwada A, Singh A. 2017. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat. Protoc 12(1):168–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giese C, Lubitz A, Demmler CD, Reuschel J, Bergner K, Marx U. 2010. Immunological substance testing on human lymphatic micro-organoids in vitro. J. Biotechnol 148(1):38–45 [DOI] [PubMed] [Google Scholar]
- 83.Kraus T, Lubitz A, Schließer U, Giese C, Reuschel J, et al. 2019. Evaluation of a 3D Human Artificial Lymph Node as Test Model for the Assessment of Immunogenicity of Protein Aggregates. J. Pharm. Sci 108(7):2358–66 [DOI] [PubMed] [Google Scholar]
- 84.Kuzin I, Sun H, Moshkani S, Feng C, Mantalaris A, et al. 2011. Long-term immunologically competent human peripheral lymphoid tissue cultures in a 3D bioreactor. Biotechnol. Bioeng 108(6):1430–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA. 2009. Fluid Flow Regulates Stromal Cell Organization and CCL21 Expression in a Tissue-Engineered Lymph Node Microenvironment. J. Immunol 183(7):4273–83 [DOI] [PubMed] [Google Scholar]
- 86.Kim J, Wu B, Niedzielski SM, Hill MT, Coleman RM, et al. 2015. Characterizing natural hydrogel for reconstruction of three-dimensional lymphoid stromal network to model T-cell interactions: 3D LYMPHOID STROMAL NETWORK. J. Biomed. Mater. Res. A 103(8):2701–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murakami T, Kim J, Li Y, Green GE, Shikanov A, Ono A. 2018. Secondary lymphoid organ fibroblastic reticular cells mediate trans-infection of HIV-1 via CD44-hyaluronan interactions. Nat. Commun 9(1):2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Votanopoulos KI, Forsythe S, Sivakumar H, Mazzocchi A, Aleman J, et al. 2020. Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study. Ann. Surg. Oncol 27(6):1956–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Higbee RG, Byers AM, Dhir V, Drake D, Fahlenkamp HG, et al. 2009. An Immunologic Model for Rapid Vaccine Assessment — A Clinical Trial in a Test Tube. Altern. Lab. Anim 37(1_suppl):19–27 [DOI] [PubMed] [Google Scholar]
- 90.Willerson JT, Wakeland JR, Stone MJ, Wildenthal K. 1975. Maintenance of mouse spleen in organ culture and assessment of certain functional capabilities. Cytologia (Tokyo). 40(2):433–40 [DOI] [PubMed] [Google Scholar]
- 91.Finetti F, Capitani N, Manganaro N, Tatangelo V, Libonati F, et al. 2020. Optimization of Organotypic Cultures of Mouse Spleen for Staining and Functional Assays. Front. Immunol 11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kontcevaya EA, Linkova NS, Chalisova NI, Dudkov AV, Sinyachkin DA. 2012. Effect of Amino Acids on Expression of Signal Molecules in Organotypic Culture of the Spleen. Bull. Exp. Biol. Med 153(4):573–76 [DOI] [PubMed] [Google Scholar]
- 93.Picot J, Ndour PA, Lefevre SD, El Nemer W, Tawfik H, et al. 2015. A biomimetic microfluidic chip to study the circulation and mechanical retention of red blood cells in the spleen: Red blood cells deformability in a spleen-like-microfluidic-chip. Am. J. Hematol 90(4):339–45 [DOI] [PubMed] [Google Scholar]
- 94.Rigat-Brugarolas LG, Elizalde-Torrent A, Bernabeu M, De Niz M, Martin-Jaular L, et al. 2014. A functional microengineered model of the human splenon-on-a-chip. Lab Chip. 14(10):1715–24 [DOI] [PubMed] [Google Scholar]
- 95.Nipper ME, Dixon JB. 2011. Engineering the Lymphatic System. Cardiovasc. Eng. Technol 2(4):296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Card CM, Yu SS, Swartz MA. 2014. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Invest 124(3):943–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Randolph GJ, Ivanov S, Zinselmeyer BH, Scallan JP. 2017. The Lymphatic System: Integral Roles in Immunity. Annu. Rev. Immunol 35:31–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shields JD. 2011. Lymphatics: At the Interface of Immunity, Tolerance, and Tumor Metastasis: Lymphatics in Cancer and Inflammation. Microcirculation. 18(7):517–31 [DOI] [PubMed] [Google Scholar]
- 99.Harris AR, Perez MJ, Munson JM. 2018. Docetaxel facilitates lymphatic-tumor crosstalk to promote lymphangiogenesis and cancer progression. BMC Cancer. 18(1):718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zawieja SD, Castorena-Gonzalez JA, Dixon B, Davis MJ. 2017. Experimental Models Used to Assess Lymphatic Contractile Function. Lymphat. Res. Biol 15(4):331–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maejima D, Nagai T, Bridenbaugh EA, Cromer WE, Gashev AA. 2014. The Position- and Lymphatic Lumen-Controlled Tissue Chambers to Study Live Lymphatic Vessels and Surrounding Tissues ex Vivo. Lymphat. Res. Biol 12(3):150–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thompson RL, Margolis EA, Ryan TJ, Coisman BJ, Price GM, et al. 2018. Design principles for lymphatic drainage of fluid and solutes from collagen scaffolds: Design principles for lymphatic drainage. J. Biomed. Mater. Res. A 106(1):106–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gong MM, Lugo-Cintron KM, White BR, Kerr SC, Harari PM, Beebe DJ. 2019. Human organotypic lymphatic vessel model elucidates microenvironment-dependent signaling and barrier function. Biomaterials. 214:119225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim S, Chung M, Jeon NL. 2016. Three-dimensional biomimetic model to reconstitute sprouting lymphangiogenesis in vitro. Biomaterials. 78:115–28 [DOI] [PubMed] [Google Scholar]
- 105.Osaki T, Serrano JC, Kamm RD. 2018. Cooperative Effects of Vascular Angiogenesis and Lymphangiogenesis. Regen. Eng. Transl. Med 4(3):120–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ozcelikkale A, Shin K, Noe-Kim V, Elzey BD, Dong Z, et al. 2017. Differential response to doxorubicin in breast cancer subtypes simulated by a microfluidic tumor model. J. Controlled Release 266:129–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harris AR, Yuan JX, Munson JM. 2018. Assessing multiparametric drug response in tissue engineered tumor microenvironment models. Methods. 134–135:20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.ting Dai T, hua Jiang Z, li Li S, dong Zhou G, Kretlow JD, et al. 2010. Reconstruction of lymph vessel by lymphatic endothelial cells combined with polyglycolic acid scaffolds: A pilot study. J. Biotechnol 150(1):182–89 [DOI] [PubMed] [Google Scholar]
- 109.Helm C-LE, Zisch A, Swartz MA. 2007. Engineered blood and lymphatic capillaries in 3-D VEGF-fibrin-collagen matrices with interstitial flow. Biotechnol. Bioeng 96(1):167–76 [DOI] [PubMed] [Google Scholar]
- 110.Ng CP, Helm C-LE, Swartz MA. 2004. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc. Res 68(3):258–64 [DOI] [PubMed] [Google Scholar]
- 111.Yang Y, Yang J, Chen X, Qin B, Li F, et al. 2018. Construction of tissue-engineered lymphatic vessel using human adipose derived stem cells differentiated lymphatic endothelial like cells and decellularized arterial scaffold: A preliminary study. Biotechnol. Appl. Biochem 65(3):428–34 [DOI] [PubMed] [Google Scholar]
- 112.Castell-Rodríguez A, Piñón-Zárate G, Herrera-Enríquez M, Jarquín-Yáñez K, Medina-Solares I. 2017. Dendritic Cells: Location, Function, and Clinical Implications. Biol. Myelomonocytic Cells [Google Scholar]
- 113.Romero-López M, Li Z, Rhee C, Maruyama M, Pajarinen J, et al. 2020. Macrophage Effects on Mesenchymal Stem Cell Osteogenesis in a Three-Dimensional In Vitro Bone Model. Tissue Eng. Part A ten.tea.2020.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang H, Husch JFA, Zhang Y, Jansen JA, Yang F, Beucken JJJP. 2019. Coculture with monocytes/macrophages modulates osteogenic differentiation of adipose-derived mesenchymal stromal cells on poly(lactic-co-glycolic) acid/polycaprolactone scaffolds. J. Tissue Eng. Regen. Med 13(5):785–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.He X-T, Wu R-X, Xu X-Y, Wang J, Yin Y, Chen F-M. 2018. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 71:132–47 [DOI] [PubMed] [Google Scholar]
- 116.Juhas M, Abutaleb N, Wang JT, Ye J, Shaikh Z, et al. 2018. Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration. Nat. Biomed. Eng 2(12):942–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sesia SB, Duhr R, Medeiros da Cunha C, Todorov A, Schaeren S, et al. 2015. Anti-Inflammatory/Tissue Repair Macrophages Enhance the Cartilage-Forming Capacity of Human Bone Marrow-Derived Mesenchymal Stromal Cells: MACROPHAGES MODULATE MSC CHONDROGENESIS. J. Cell. Physiol 230(6):1258–69 [DOI] [PubMed] [Google Scholar]
- 118.Zheng D, Liwinski T, Elinav E. 2020. Interaction between microbiota and immunity in health and disease. Cell Res. 30(6):492–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Randall KJ, Turton J, Foster JR. 2011. Explant culture of gastrointestinal tissue: a review of methods and applications. Cell Biol. Toxicol 27(4):267–84 [DOI] [PubMed] [Google Scholar]
- 120.Russo I, Zeppa P, Iovino P, Del Giorno C, Zingone F, et al. 2016. The culture of gut explants: A model to study the mucosal response. J. Immunol. Methods 438:1–10 [DOI] [PubMed] [Google Scholar]
- 121.Schwerdtfeger LA, Ryan EP, Tobet SA. 2015. An organotypic slice model for ex vivo study of neural, immune, and microbial interactions of mouse intestine. Am. J. Physiol.-Gastrointest. Liver Physiol 310(4):G240–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pender SLF, Breese EJ, Günther U, Howie D, Wathen NC, et al. 1998. Suppression of T cell–mediated injury in human gut by interleukin 10: Role of matrix metalloproteinases. Gastroenterology. 115(3):573–83 [DOI] [PubMed] [Google Scholar]
- 123.Schwerdtfeger LA, Nealon NJ, Ryan EP, Tobet SA. 2019. Human colon function ex vivo: Dependence on oxygen and sensitivity to antibiotic. PLOS ONE. 14(5):e0217170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ambrosini YM, Shin W, Min S, Kim HJ. 2020. Microphysiological Engineering of Immune Responses in Intestinal Inflammation. Immune Netw. 20(2):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maurer M, Gresnigt MS, Last A, Wollny T, Berlinghof F, et al. 2019. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials. 220:119396. [DOI] [PubMed] [Google Scholar]
- 126.Ramadan Q, Jafarpoorchekab H, Huang C, Silacci P, Carrara S, et al. 2013. NutriChip: nutrition analysis meets microfluidics. Lab Chip. 13(2):196–203 [DOI] [PubMed] [Google Scholar]
- 127.Kim HJ, Li H, Collins JJ, Ingber DE. 2016. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. PNAS. 113(1):E7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roh TT, Chen Y, Paul HT, Guo C, Kaplan DL. 2019. 3D bioengineered tissue model of the large intestine to study inflammatory bowel disease. Biomaterials. 225:119517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leonard F 2012. Screening of budesonide nanoformulations for treatment of inflammatory bowel disease in an inflamed 3D cell-culture model. ALTEX. 29(3):275–85 [DOI] [PubMed] [Google Scholar]
- 130.Leonard F, Collnot E-M, Lehr C-M. 2010. A Three-Dimensional Coculture of Enterocytes, Monocytes and Dendritic Cells To Model Inflamed Intestinal Mucosa in Vitro. Mol. Pharm 7(6):2103–19 [DOI] [PubMed] [Google Scholar]
- 131.Susewind J, de Souza Carvalho-Wodarz C, Repnik U, Collnot E-M, Schneider-Daum N, et al. 2015. A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials. Nanotoxicology, pp. 1–10 [DOI] [PubMed] [Google Scholar]
- 132.Abbott NJ. 2013. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis 36(3):437–49 [DOI] [PubMed] [Google Scholar]
- 133.Serlin Y, Shelef I, Knyazer B, Friedman A. 2015. Anatomy and physiology of the blood–brain barrier. Semin. Cell Dev. Biol 38:2–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van der Helm MW, van der Meer AD, Eijkel JCT, van den Berg A, Segerink LI. 2016. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers. 4(1):e1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cucullo L, Marchi N, Hossain M, Janigro D. 2011. A Dynamic in vitro BBB Model for the Study of Immune Cell Trafficking into the Central Nervous System. J. Cereb. Blood Flow Metab 31(2):767–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME. 2002. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia. 37(3):229–40 [PubMed] [Google Scholar]
- 137.Kasahara Y, Koyama R, Ikegaya Y. 2016. Depth and time-dependent heterogeneity of microglia in mouse hippocampal slice cultures. Neurosci. Res 111:64–69 [DOI] [PubMed] [Google Scholar]
- 138.Basilico B, Pagani F, Grimaldi A, Cortese B, Angelantonio SD, et al. 2019. Microglia shape presynaptic properties at developing glutamatergic synapses. Glia. 67(1):53–67 [DOI] [PubMed] [Google Scholar]
- 139.Keaney J, Gasser J, Gillet G, Scholz D, Kadiu I. 2019. Inhibition of Bruton’s Tyrosine Kinase Modulates Microglial Phagocytosis: Therapeutic Implications for Alzheimer’s Disease. J. Neuroimmune Pharmacol 14(3):448–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ta T-T, Dikmen HO, Schilling S, Chausse B, Lewen A, et al. 2019. Priming of microglia with IFN-γ slows neuronal gamma oscillations in situ. Proc. Natl. Acad. Sci 116(10):4637–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Coleman LG, Zou J, Crews FT. 2020. Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling. J. Neuroinflammation 17(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huuskonen J, Suuronen T, Miettinen R, van Groen T, Salminen A. 2005. A refined in vitro model to study inflammatory responses in organotypic membrane culture of postnatal rat hippocampal slices. J. Neuroinflammation 2(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Foraker JE, Oh JY, Ylostalo JH, Lee RH, Watanabe J, Prockop DJ. 2011. Cross-talk between human mesenchymal stem/progenitor cells (MSCs) and rat hippocampal slices in LPS-stimulated cocultures: the MSCs are activated to secrete prostaglandin E2. J. Neurochem 119(5):1052–63 [DOI] [PubMed] [Google Scholar]
- 144.Nitsch R, Pohl EE, Smorodchenko A, Infante-Duarte C, Aktas O, Zipp F. 2004. Direct Impact of T Cells on Neurons Revealed by Two-Photon Microscopy in Living Brain Tissue. J. Neurosci 24(10):2458–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dombrowski Y, O’Hagan T, Dittmer M, Penalva R, Mayoral SR, et al. 2017. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci 20(5):674–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van den Pol AN, Reuter JD, Santarelli JG. 2002. Enhanced Cytomegalovirus Infection of Developing Brain Independent of the Adaptive Immune System. J. Virol 76(17):8842–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Onorati M, Li Z, Liu F, Sousa AMM, Nakagawa N, et al. 2016. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 16(10):2576–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ramesh G, Borda JT, Dufour J, Kaushal D, Ramamoorthy R, et al. 2008. Interaction of the Lyme Disease Spirochete Borrelia burgdorferi with Brain Parenchyma Elicits Inflammatory Mediators from Glial Cells as Well as Glial and Neuronal Apoptosis. Am. J. Pathol 173(5):1415–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mossu A, Rosito M, Khire T, Li Chung H, Nishihara H, et al. 2019. A silicon nanomembrane platform for the visualization of immune cell trafficking across the human blood–brain barrier under flow. J. Cereb. Blood Flow Metab 39(3):395–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cho HJ, Verbridge SS, Davalos RV, Lee YW. 2018. Development of an In Vitro 3D Brain Tissue Model Mimicking In Vivo-Like Pro-inflammatory and Pro-oxidative Responses. Ann. Biomed. Eng 46(6):877–87 [DOI] [PubMed] [Google Scholar]
- 151.Haw RT, Tong C, Yew A, Lee H, Phillips JB, Vidyadaran S. 2014. A three-dimensional collagen construct to model lipopolysaccharide-induced activation of BV2 microglia. J. Neuroinflammation 11(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pöttler M, Zierler S, Kerschbaum HH. 2006. An artificial three-dimensional matrix promotes ramification in the microglial cell-line, BV-2. Neurosci. Lett 410(2):137–40 [DOI] [PubMed] [Google Scholar]
- 153.Song Q, Jiang Z, Li N, Liu P, Liu L, et al. 2014. Anti-inflammatory effects of three-dimensional graphene foams cultured with microglial cells. Biomaterials. 35(25):6930–40 [DOI] [PubMed] [Google Scholar]
- 154.Abreu CM, Gama L, Krasemann S, Chesnut M, Odwin-Dacosta S, et al. 2018. Microglia Increase Inflammatory Responses in iPSC-Derived Human BrainSpheres. Front. Microbiol 9:2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park J, Wetzel I, Marriott I, Dréau D, D’Avanzo C, et al. 2018. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci 21(7):941–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang M, Xie Q, Gimple RC, Zhong Z, Tam T, et al. 2020. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hermida MA, Kumar JD, Schwarz D, Laverty KG, Di Bartolo A, et al. 2020. Three dimensional in vitro models of cancer: Bioprinting multilineage glioblastoma models. Adv. Biol. Regul 75:100658. [DOI] [PubMed] [Google Scholar]
- 158.The lungs at the frontlines of immunity. 2015. Nat. Immunol 16(1):17–17 [DOI] [PubMed] [Google Scholar]
- 159.Henjakovic M, Sewald K, Switalla S, Kaiser D, Müller M, et al. 2008. Ex vivo testing of immune responses in precision-cut lung slices. Toxicol. Appl. Pharmacol 231(1):68–76 [DOI] [PubMed] [Google Scholar]
- 160.Kretschmer S, Dethlefsen I, Hagner-Benes S, Marsh LM, Garn H, König P. 2013. Visualization of Intrapulmonary Lymph Vessels in Healthy and Inflamed Murine Lung Using CD90/Thy-1 as a Marker. PLOS ONE. 8(2):e55201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lyons-Cohen MR, Thomas SY, Cook DN, Nakano H. 2017. Precision-cut Mouse Lung Slices to Visualize Live Pulmonary Dendritic Cells. J. Vis. Exp. JoVE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Neuhaus V, Schaudien D, Golovina T, Temann U-A, Thompson C, et al. 2017. Assessment of long-term cultivated human precision-cut lung slices as an ex vivo system for evaluation of chronic cytotoxicity and functionality. J. Occup. Med. Toxicol 12(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Temann A, Golovina T, Neuhaus V, Thompson C, Chichester JA, et al. 2017. Evaluation of inflammatory and immune responses in long-term cultured human precision-cut lung slices. Hum. Vaccines Immunother 13(2):351–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Delgado-Ortega M, Melo S, Punyadarsaniya D, Ramé C, Olivier M, et al. 2014. Innate immune response to a H3N2 subtype swine influenza virus in newborn porcine trachea cells, alveolar macrophages, and precision-cut lung slices. Vet. Res 45(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Neuhaus V, Schwarz K, Klee A, Seehase S, Förster C, et al. 2013. Functional Testing of an Inhalable Nanoparticle Based Influenza Vaccine Using a Human Precision Cut Lung Slice Technique. PLOS ONE. 8(8):e71728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neuhaus V, Chichester JA, Ebensen T, Schwarz K, Hartman CE, et al. 2014. A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung. Vaccine. 32(26):3216–22 [DOI] [PubMed] [Google Scholar]
- 167.Sato M, Sasaki N, Ato M, Hirakawa S, Sato K, Sato K. 2015. Microcirculation-on-a-Chip: A Microfluidic Platform for Assaying Blood- and Lymphatic-Vessel Permeability. PLOS ONE. 10(9):e0137301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Graney PL, Ben-Shaul S, Landau S, Bajpai A, Singh B, et al. 2020. Macrophages of diverse phenotypes drive vascularization of engineered tissues. Sci. Adv 6(18):eaay6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. 2010. Reconstituting Organ-Level Lung Functions on a Chip. Science. 328(5986):1662–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stucki AO, Stucki JD, Hall SRR, Felder M, Mermoud Y, et al. 2015. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab. Chip 15(5):1302–10 [DOI] [PubMed] [Google Scholar]
- 171.Rothen-Rutishauser BM, Kiama SG, Gehr P. 2005. A Three-Dimensional Cellular Model of the Human Respiratory Tract to Study the Interaction with Particles. Am. J. Respir. Cell Mol. Biol 32(4):281–89 [DOI] [PubMed] [Google Scholar]
- 172.Klein SG, Serchi T, Hoffmann L, Blömeke B, Gutleb AC. 2013. An improved 3D tetraculture system mimicking the cellular organisation at the alveolar barrier to study the potential toxic effects of particles on the lung. Part. Fibre Toxicol 10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brandenberger C, Rothen-Rutishauser B, Mühlfeld C, Schmid O, Ferron GA, et al. 2010. Effects and uptake of gold nanoparticles deposited at the air–liquid interface of a human epithelial airway model. Toxicol. Appl. Pharmacol 242(1):56–65 [DOI] [PubMed] [Google Scholar]
- 174.Harrington H, Cato P, Salazar F, Wilkinson M, Knox A, et al. 2014. Immunocompetent 3D Model of Human Upper Airway for Disease Modeling and In Vitro Drug Evaluation. Mol. Pharm 11(7):2082–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Han Y, Yang L, Duan X, Duan F, Nilsson-Payant BE, et al. 2020. Identification of Candidate COVID-19 Therapeutics using hPSC-derived Lung Organoids. Microbiology [Google Scholar]
- 176.Suzuki T, Itoh Y, Sakai Y, Saito A, Okuzaki D, et al. 2020. Generation of human bronchial organoids for SARS-CoV-2 research. Cell Biology [Google Scholar]
- 177.Luque-González MA, Reis RL, Kundu SC, Caballero D. 2020. Human Microcirculation-on-Chip Models in Cancer Research: Key Integration of Lymphatic and Blood Vasculatures. Adv. Biosyst, p. 2000045. [DOI] [PubMed] [Google Scholar]
- 178.Srigunapalan S, Lam C, Wheeler AR, Simmons CA. 2011. A microfluidic membrane device to mimic critical components of the vascular microenvironment. Biomicrofluidics. 5(1):013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim SK, Moon WK, Park JY, Jung H. 2012. Inflammatory mimetic microfluidic chip by immobilization of cell adhesion molecules for T cell adhesion. The Analyst. 137(17):4062. [DOI] [PubMed] [Google Scholar]
- 180.Zhang B, Peticone C, Murthy SK, Radisic M. 2013. A standalone perfusion platform for drug testing and target validation in micro-vessel networks. Biomicrofluidics. 7(4):044125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wu X, Newbold MA, Gao Z, Haynes CL. 2017. A versatile microfluidic platform for the study of cellular interactions between endothelial cells and neutrophils. Biochim. Biophys. Acta BBA - Gen. Subj 1861(5):1122–30 [DOI] [PubMed] [Google Scholar]
- 182.Moore EM, West JL. 2019. Harnessing Macrophages for Vascularization in Tissue Engineering. Ann. Biomed. Eng 47(2):354–65 [DOI] [PubMed] [Google Scholar]
- 183.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, et al. 2014. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 35(15):4477–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lurier EB, Dalton D, Dampier W, Raman P, Nassiri S, et al. 2017. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology. 222(7):847–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest 101(4):890–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vardhana SA, Wolchok JD. 2020. The many faces of the anti-COVID immune response. J. Exp. Med 217(6): [DOI] [PMC free article] [PubMed] [Google Scholar]


