Abstract
Background:
The US older adult population (≥65 years) is increasing and may be at increased risk for severe anaphylaxis. Little is known about the healthcare utilization for acute allergic reactions (AAR), including anaphylaxis, among older adults.
Objectives:
To characterize trends in emergency department (ED) visits and hospitalizations for AAR and anaphylaxis among US older adults from 2006–2014, and to examine factors associated with severe anaphylaxis.
Methods:
We performed cross-sectional analyses of trends in ED visits and hospitalizations among older adults using data from the Nationwide Emergency Department Sample and the National (Nationwide) Inpatient Sample in 2006–2014. We used International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic codes to identify visits for AAR, including anaphylaxis. Multivariable logistic regression modeling was used to identify factors associated with severe anaphylaxis (cardiac arrest, intubation and death).
Results:
In 2006–2014, older adults experienced approximately 1,019,967 AAR-related ED visits; 173,844 AAR-related hospitalizations; 93,795 anaphylaxis-related ED visits; and 72,677 anaphylaxis-related hospitalizations. While AAR-related ED visit and hospitalization rates remained stable (P-trends=0.28 and 0.16, respectively), anaphylaxis-related ED visit and hospitalization rates significantly increased over time (37 visits per 100,000 population in 2006 to 51 in 2014, P-trend<0.001; 13 hospitalizations per 100,000 population in 2006 to 23 in 2014, P-trend<0.001), especially hospitalization rates for drug-related anaphylaxis (47 hospitalizations per 100,000 population in 2006 to 85 in 2014, P-trend<0.001). Risk factors for anaphylaxis-related death included older age and drug-related trigger.
Conclusions:
In a nationally representative sample of US older adults, the rate of anaphylaxis-related ED visits and hospitalizations increased over time. Drug-related triggers represented a substantial portion of the increased healthcare utilization and are a growing risk in this vulnerable population.
Keywords: anaphylaxis, allergy, allergic reaction, older adult, elderly, emergency department, hospitalization
Introduction
Nearly 46 million older adults (age ≥65 years) currently live in the United States (US). The older adult population is rapidly growing, projected to more than double in size by 2060.1 Anaphylaxis is potentially life-threatening, and fatal anaphylaxis has been associated with older age, especially among those with multiple co-morbidities.2–4 While an increase in anaphylaxis-related visits have been observed in younger populations, little is known about the trends in acute allergic reactions (AAR), including anaphylaxis, among US older adults.5 Few studies have examined risk factors for severe anaphylaxis in this vulnerable population.6, 7
The objective of this study was to characterize temporal trends in emergency department (ED) visits and hospitalizations for AAR (including anaphylaxis) among US older adults and to examine factors associated with severe anaphylaxis. It is important to better understand the trends in anaphylaxis, healthcare utilization, and severe outcomes in this rapidly growing US older adult population to inform future policy and clinical guidelines.
Methods
We performed a series of cross-sectional analyses and conducted a retrospective cohort study using discharge data from 2006 to 2014 from the Nationwide Emergency Department Sample (NEDS) and the National (Nationwide) Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality.8–10 NEDS is the largest all-payer ED database in the US and provides nationally-representative data from approximately 145 million ED visits each year.8 NIS is a large, publicly available all-payer inpatient US healthcare database, which contain data from approximately 7 million hospital stays per year and provides national estimates of inpatient utilization and outcomes.9 This study was approved by the Mass General Brigham Institutional Review Board and the data are protected by a data use agreement.
Definitions
We defined adults as patients ≥18 years of age at the time of the ED visit or hospitalization and older adults as patients who were ≥65 years of age at the time of the ED visit or hospitalization. We used the following definitions for older adult age subgroups: early-old (65–74 years), middle-old (75–84 years), late-old (≥85 years).11 We used the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes to determine cases.
Acute allergic reactions
We used the first-listed discharge diagnostic code to determine cases for acute allergic reactions using ICD-9-CM codes for drug allergy, venom allergy, and other allergy (see Table E1 in the Online Repository). Drug allergy was defined by ICD-9-CM codes 693.0, 995.2, 995.1, 995.4.12–14 Venom allergy was defined by first-listed ICD-9-CM code 989.5.15 Food allergy was defined by 693.1, 995.60–70.15, 16 Other allergy was defined by 995.0, 995.3, 708.0,12 999.4, 999.41, 999.42, 999.49.17
Anaphylaxis
We defined anaphylaxis-related visits using two methods (see Table E2 and Figure E1 in the Online Repository).6, 14 For the first method, we used the following ICD-9-CM diagnostic codes: 995.60–995.69, 995.0 listed in any of the reported discharge diagnoses. For the second method, we used a previously validated algorithm for identification of anaphylaxis cases based on ICD-9-CM diagnostic codes and external cause-of-injury codes (E-codes)18. We excluded cases with the following E-codes: E905.0, E905.1, E905.6. We used ICD-9-CM codes to categorize anaphylaxis cases according to trigger (drug, venom, food) based on previously described methods:14, 16 drug (693.0, 995.2, 995.4, E930-E930.9, E931-E931.9), venom (989.5, E905.3, E905.5), and food (995.60–69, 995.70). Anaphylaxis-related visits that were not included in the above categories were classified as other, including visits with multiple trigger types.
Covariates
Covariates included year of discharge, patient age, sex, race/ethnicity (only available in NIS), primary health insurance, quartile of median household income based on ZIP code of residence, season of discharge, hospital region, urban/rural and teaching status of hospital, and weekend presentation. Administration of epinephrine in the ED was determined by current procedural terminology (CPT) code J0170 (2006–2010) and J0171 (2011–2014). Presence of any chronic conditions was identified using the Chronic Condition Indicator (maintained by HCUP), which identifies chronic conditions likely present prior to admission.19 The Elixhauser comorbidity index was also used to capture the comorbidity burden and specific comorbidities were included (e.g. chronic pulmonary disease) in NIS.20, 21
Outcomes
The outcomes included hospitalization (NEDS only), cardiac arrest, intubation, and death, which were proxies used for severe anaphylaxis. Hospitalization was defined using NEDS disposition variable. Cardiac arrest was defined using ICD-9-CM code 427.5.22 Endotracheal intubation was defined using procedural code of invasive mechanical ventilation (96.7) and endotracheal intubation (96.04).23 Death was determined using disposition variables in NEDS or NIS dataset, which only includes death in the hospital (either ED or inpatient).
Statistical analysis
NEDS and NIS do not include patient-level identifiers; therefore, the unit of analysis was ED visit or hospitalization rather than patient. To account for the complex sampling design, yearly trend (for 2006 to 2011) or discharge (for 2012 to 2014) weights provided by HCUP were used to provide estimates for the entire US population.24, 25 Analyses were conducted using Stata IC 15.1 (StataCorp, College Station, TX). We present weighted frequencies, means (for continuous variables), percentages (for categorical variables), and 95% confidence intervals. Incidence per 100,000 population were calculated using age-specific population estimates from the US Census Bureau. We used ordinary least squares regression to test for linear trends over time. To determine factors associated with severe anaphylaxis, multivariable logistic regression models were constructed for demographic and clinical risk factors, accounting for the survey weighting in all models. Separate models were fit using complete case analysis for each data source and outcome.
Results
Acute allergic reactions
Emergency Department Visits
In 2006–2014, the total number of ED visits for AAR that occurred among older adults was 1,019,967 (95%CI: 984,410–1,055,525). The number of ED visits for AAR among older adults increased from 106,073 in 2006 to 127,493 visits in 2014 (P-trend<0.001). The rate of ED visits for AAR for older adults did not increase over time (285 visits per 100,000 population in 2006 and 276 visits per 100,000 population in 2014; P-trend=0.28). ED visit rates for AAR among early-old adults was slightly higher than the rate for middle-old adults and late-old adults (e.g., 2014: early-old, 294 visits per 100,000 population; middle-old, 272; and late-old, 205; see Figure E2 in the Online Repository). The rate of ED visits for AAR for early-old, middle-old, and late-old subgroups did not significantly increase over time (P-trends=0.28, 0.81, 0.09). The rate of ED visits for drug-related AAR among older adults was much higher than the rate of ED visits for venom-related and food-related AAR (e.g., 2014: drug, 95 visits per 100,000 population; venom, 57 visits per 100,000; and food 9 visits per 100,000). ED visit rates for drug-related AAR slightly decreased over time, while venom-related and food-related ED visit rates were stable over time (P-trends=0.02, 0.71, and 0.70, respectively, see Figure E3 in the Online Repository).
Hospitalizations
In 2006–2014, the total number of hospitalizations for AAR that occurred among older adults was 173,844 (95%CI: 169,243–178,444). The number of hospitalizations for AAR among older adults increased from 14,856 in 2006 to 19,550 in 2014 (P-trend=0.001). The rate of hospitalizations for AAR was stable from 2006 to 2014 (P-trend=0.16). The hospitalization rates for AAR among late-old adults were higher than that of middle-old and early-old adults (e.g., 2014: late-old, 105 visits per 100,000; middle-old, 73 per 100,000; early-old, 74 per 100,000; see Figure E4 in the Online Repository). Hospitalization rates for drug-related AAR among older adults were much higher compared to venom-related or food-related AAR (e.g., 2014: drug, 20 visits per 100,000; venom, 2 visits per 100,000; food, 1 visit per 100,000; see Figure E5 in the Online Repository). Hospitalization rates for food-related AAR marginally decreased over time while venom-related and drug-related AAR hospitalization rates were stable over time (P-trends=0.003, 0.10, and 0.55, respectively).
Anaphylaxis
Emergency Department Visits
From 2006–2014, the unweighted number of ED visits for anaphylaxis among US older adults was 20,989 visits, with survey weighting resulting in an overall estimate of 93,795 (95%CI: 89,797–97,794) ED visits for anaphylaxis occurring among US older adults during this time period (Table I). The majority of these ED visits for anaphylaxis were patients ages 65–74 years, female, with public insurance, and visiting urban hospitals (Table I). Approximately 2% of anaphylaxis-related ED visits among older adults had documentation of epinephrine use, which was less than that for younger adults ages 18–64 years (7%; see Table E3 in the Online Repository). The majority of anaphylaxis-related ED visits among older adults involved patients with any chronic condition (87%), which was more frequent than that for younger adults (54%). Drug-related triggers were more frequent among older adults compared to younger adults (35% vs 18%, respectively). Food-related triggers were less frequent among older adults compared to younger adults (6% vs 19%, respectively). Among ED visits for anaphylaxis among older adults, 59% of these visits resulted in hospitalization, 17% of these visits involved intubation/ventilation, and 3% of these visits resulted in inpatient or ED death (Table I).
Table I.
Characteristics of Emergency Department visits and hospitalizations for anaphylaxis among US older adults (age ≥65 years), 2006–2014 National Emergency Department Sample and National Inpatient Sample
| ED Visits | Hospitalizations | |||||||
|---|---|---|---|---|---|---|---|---|
| Unweighted Frequency | Weighted Frequency | Unweighted Frequency | Weighted Frequency | |||||
| Characteristics | Frequency | % | (95% CI) | Frequency | % | (95% CI) | ||
| Overall number | 20989 | 93795 | 100 | 14990 | 72677 | 100 | ||
| Age | ||||||||
| 65–74 years | 11504 | 51385 | 55 | 54–56 | 7509 | 36387 | 50 | 49–51 |
| 75–84 years | 6784 | 30282 | 32 | 32–33 | 5105 | 24636 | 34 | 33–35 |
| >85 years | 2701 | 12095 | 13 | 12–13 | 2376 | 11530 | 16 | 15–17 |
| Sex | ||||||||
| Male | 8200 | 36801 | 39 | 39–40 | 5988 | 29025 | 40 | 39–41 |
| Female | 12786 | 56973 | 61 | 60–61 | 9001 | 43570 | 60 | 59–61 |
| Missing | <10 | <10 | ||||||
| Race/Ethnicity | ||||||||
| White | N/A | 9661 | 46916 | 73 | 72–74 | |||
| Black | N/A | 1949 | 9387 | 15 | 14–15 | |||
| Hispanic | N/A | 861 | 4185 | 7 | 6–7 | |||
| Asian | N/A | 356 | 1724 | 3 | 2–3 | |||
| Other | N/A | 391 | 1890 | 3 | 3–3 | |||
| Missing | 1772 | |||||||
| Primary Health Insurance | ||||||||
| Public | 18515 | 24608 | 88 | 87–89 | 13398 | 64834 | 89 | 89–90 |
| Private | 2083 | 25118 | 10 | 9–11 | 1332 | 6566 | 9 | 9–10 |
| Self-pay | 173 | 22070 | 0.9 | 0.7–1 | 86 | 423 | 0.6 | 0.4–0.8 |
| Other | 199 | 20029 | 1.0 | 0.8–1 | 156 | 769 | 1 | 0.9–1.3 |
| Missing | 19 | 18 | ||||||
| Median household income quartile | ||||||||
| 1 (lowest) | 5577 | 24608 | 27 | 26–28 | 4105 | 19821 | 28 | 27–29 |
| 2 | 5575 | 25118 | 27 | 26–28 | 3739 | 18183 | 26 | 25–26 |
| 3 | 4916 | 22070 | 24 | 23–25 | 3499 | 16923 | 24 | 23–25 |
| 4 (highest) | 4480 | 20029 | 22 | 20–23 | 3342 | 16266 | 23 | 22–24 |
| Missing | 441 | 305 | ||||||
| Season of Discharge | ||||||||
| First quarter (Jan - Mar) | 4836 | 21632 | 23 | 22–24 | 3601 | 17448 | 24 | 23–25 |
| Second quarter (Apr - Jun) | 5161 | 23100 | 25 | 24–25 | 3818 | 18528 | 26 | 25–26 |
| Third quarter (Jul - Sep) | 5903 | 26398 | 28 | 28–29 | 3761 | 18251 | 25 | 24–26 |
| Fourth quarter (Oct - Dec) | 5086 | 22654 | 24 | 24–25 | 3804 | 18421 | 25 | 25–26 |
| Missing | <10 | <10 | ||||||
| Hospital Region | ||||||||
| Northeast | 3364 | 1565 | 17 | 15–18 | 2720 | 13375 | 18 | 17–20 |
| Midwest | 4492 | 2229 | 24 | 22–26 | 3790 | 18382 | 25 | 24–27 |
| South | 9707 | 4035 | 43 | 41–45 | 5706 | 27471 | 38 | 36–39 |
| West | 3426 | 1551 | 17 | 15–18 | 2774 | 13448 | 19 | 17–20 |
| Hospital Designation | ||||||||
| Rural | 3577 | 16985 | 18 | 17–19 | 1820 | 8775 | 12 | 11–13 |
| Urban | 17412 | 76810 | 82 | 81–83 | 13091 | 63536 | 88 | 87–89 |
| Missing | 79 | |||||||
| Hospital Status | ||||||||
| Metropolitan, Non-teaching | 9333 | 39070 | 42 | 40–44 | 5869 | 28284 | 39 | 38–41 |
| Metropolitan Teaching | 8079 | 37740 | 40 | 38–43 | 7222 | 35252 | 49 | 47–50 |
| Non-Metropolitan | 3577 | 16985 | 18 | 17–19 | 1820 | 8775 | 12 | 11–13 |
| Missing | 79 | |||||||
| Hospital Bedsize | ||||||||
| Small | N/A | 2144 | 10019 | 14 | 13–15 | |||
| Medium | N/A | 3804 | 18605 | 26 | 25–27 | |||
| Large | N/A | 8963 | 43686 | 60 | 59–62 | |||
| Missing | 79 | |||||||
| Medication Use | ||||||||
| Diphenhydramine | 1306 | 5802 | 6 | 6–7 | N/A | |||
| Methylprednisolone | 1352 | 5998 | 6 | 6–7 | N/A | |||
| Epinephrine Use | 418 | 1933 | 2 | 1.8–2.3 | N/A | |||
| Length of Stay (Inpatient), mean days | N/A | 14988 | 6.4 | 6.3–6.6 | ||||
| Missing | <10 | |||||||
| Number of ED procedures | ||||||||
| 0 | 19757 | 88143 | 94 | 93–95 | N/A | |||
| 1 | 493 | 2203 | 2 | 2–3 | N/A | |||
| 2 or more | 739 | 3415 | 4 | 3–4 | N/A | |||
| Number of chronic conditions | ||||||||
| 0 | 2795 | 12544 | 13 | 13–14 | 134 | 729 | 1.0 | 0.8–1.2 |
| 1–2 | 4410 | 19701 | 21 | 20–22 | 1364 | 6628 | 9 | 9–10 |
| 3 or more | 13784 | 61550 | 66 | 65–67 | 13492 | 65321 | 90 | 89–90 |
| Any chronic condition | 18194 | 81230 | 87 | 86–87 | ||||
| Comorbidities | ||||||||
| Alcohol | N/A | 407 | 1977 | 3 | 2–3 | |||
| Congestive Heart Failure | N/A | 2353 | 11347 | 16 | 15–16 | |||
| Chronic Pulmonary Disease | N/A | 4423 | 21416 | 29 | 29–30 | |||
| Diabetes | N/A | 3950 | 19085 | 26 | 26–27 | |||
| Hypertension | N/A | 10655 | 51565 | 71 | 70–72 | |||
| Liver Disease | N/A | 282 | 1372 | 1.9 | 1.7–2.1 | |||
| Obesity | N/A | 1700 | 8263 | 11 | 11–12 | |||
| Oncologic | N/A | 1271 | 6145 | 8 | 8–9 | |||
| Renal Failure | N/A | 2754 | 13333 | 18 | 18–19 | |||
| Rheumatoid arthritis/ collagen vascular diseases | N/A | 517 | 2518 | 3 | 3–4 | |||
| Elixhauser Comorbidity Index, mean | N/A | 14990 | 8.1 | 7.9–8.3 | ||||
| Severe Anaphylaxis | 12494 | 55965 | 60 | 59–61 | 4409 | 21370 | 29 | 29–30 |
| Hospitalization | 12446 | 55761 | 59 | 58–61 | N/A | |||
| Cardiac arrest | 380 | 1724 | 1.8 | 1.6–2.1 | 443 | 2150 | 3 | 2.7–3.3 |
| Intubation/V entilation | 3592 | 15974 | 17 | 16–18 | 4130 | 20026 | 28 | 27–28 |
| Death | 525 | 2420 | 3 | 2–3 | 689 | 3306 | 5 | 4–5 |
| Type of Trigger | ||||||||
| Drug | 7428 | 33013 | 35 | 34–36 | 5,537 | 26,821 | 37 | 36–38 |
| Venom | 1267 | 5711 | 6 | 5.7–6.5 | 258 | 1,272 | 1.8 | 1.5–2.0 |
| Food | 1304 | 5920 | 6 | 6.0–6.7 | 595 | 2,956 | 4.1 | 4.1–4.4 |
| Other/multiple | 10990 | 49152 | 52 | 52–53 | 8,600 | 41,628 | 57 | 56–58 |
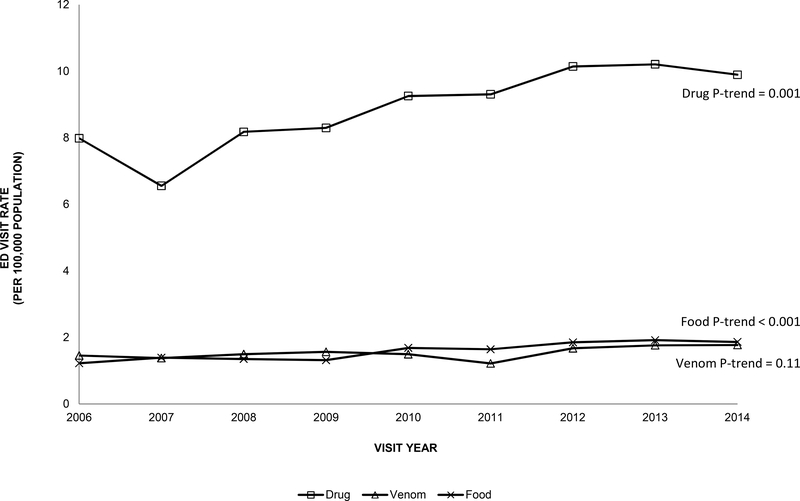
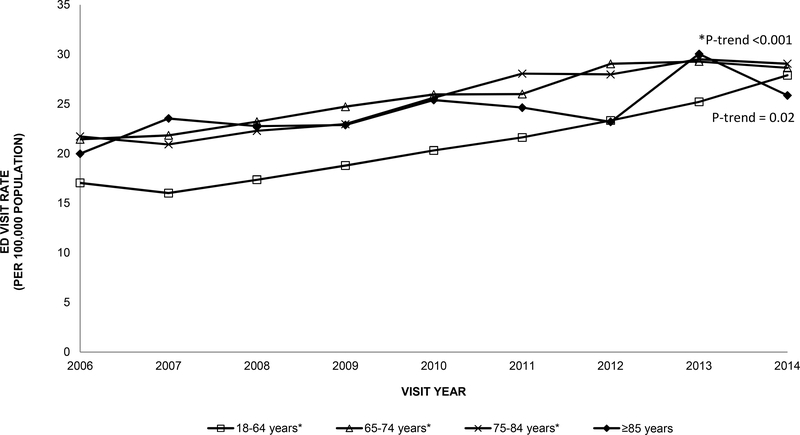
The number of ED visits for anaphylaxis among older adults increased more than 50% from 7941 visits in 2006 to 13,127 visits in 2014 (P-trend<0.001). The rate of ED visits for anaphylaxis also increased over time among older adults from 37 visits per 100,000 population in 2006 to 51 visits per 100,000 in 2014 (P-trend<0.001). A similar trend was observed among younger adults 18–64 years old (P-trend<0.001, Figure I). There was a slight increase in ED visits over time in early-old and middle-old adults (P-trends <0.001). Among older adults, the ED visit rate for drug-related anaphylaxis were higher than for venom-related or food-related causes across all years (e.g., 2014: drug, 10 visits per 100,000; venom, 2 visits per 100,000; food, 2 visits per 100,000). Drug-related ED visit rates and food-related ED visit rates for anaphylaxis increased over time among older adults (P-trends=0.001 and <0.001, respectively, Figure II). Drug-related ED visit rates for anaphylaxis among older adults were nearly double the rates for all adults ≥18 years old across the study period (e.g., 2014: 10 visits per 100,000 population; 6 visits per 100,000, respectively), and food-related ED visit rates for anaphylaxis among older adults were about half the rates for all adults ≥18 years old (e.g., 2014: 2 visits per 100,000 population; 5 visits per 100,000 population, respectively).
Figure I.
Rate of emergency department visits per 100,000 population for anaphylaxis among younger adults (18–64 years) and older adults (≥65 years) in the US, 2006–2014 National Emergency Department Sample
Figure II.
Rate of emergency department visits per 100,000 population for anaphylaxis by trigger among older adults (≥65 years) in the US, 2006–2014 National Emergency Department Sample
Hospitalizations
From 2006–2014, the unweighted number of hospitalizations for anaphylaxis among US older adults was 14,990 visits, with survey weighting resulting in an overall estimate of 72,677 (95%CI: 70,450–74,904) hospitalizations for anaphylaxis among older adults during this time period (Table I). The majority of these hospitalizations for anaphylaxis were patients ages 65–74 years, female, white race, with public insurance, and visiting large hospitals in urban areas (Table I). The mean length of stay for these hospitalizations was 6.4 days. Around 90% of these hospitalizations were from patients with 3 or more chronic conditions. Of the total hospitalizations for anaphylaxis occurring between 2006–2014 among older adults, 28% of these visits involved intubation/ventilation, 3% involved cardiac arrest, and 5% of these visits resulted in inpatient death.
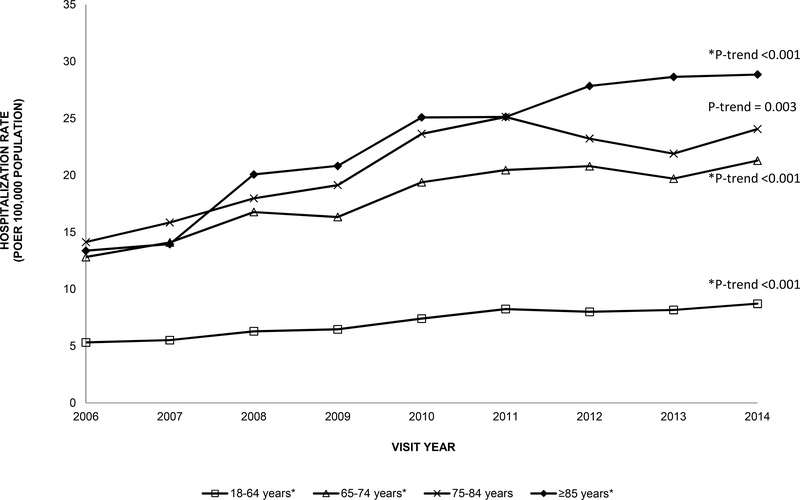
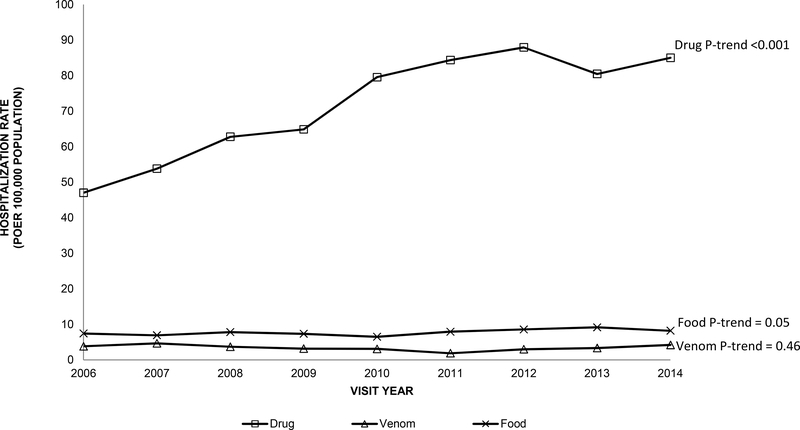
Among older adults, the number of hospitalizations for anaphylaxis more than doubled from 4,973 anaphylaxis-related hospitalizations in 2006 to 10,680 hospitalizations in 2014 (P-trend<0.001). The anaphylaxis-related hospitalization rate for older adults was more than double the rate for younger adults across all years and increased from 13 visits per 100,000 population in 2006 to 23 visits per 100,000 population in 2014 (P-trend<0.001, Figure III). There was an increase in the hospitalization rate for anaphylaxis over time in early-old and late-old adults (P-trends<0.001, Figure III). The hospitalization rates for drug-related anaphylaxis among older adults were much higher compared to hospitalization rates for venom-related or food-related anaphylaxis (e.g., 2014: drug, 85 visits per 100,000 population; food, 8 visits; venom, 4 visits, respectively, Figure IV). Among older adults, drug-related anaphylaxis hospitalizations significantly increased over time (P-trend<0.001) as compared to hospitalizations for venom-related or food-related (P-trends=0.46 and 0.05, respectively).
Figure III.
Rate of hospitalizations per 100,000 population for anaphylaxis among younger adults (18–64 years) and older adults (≥65 years) in the US, 2006–2014 National Inpatient Sample
Figure IV.
Rate of hospitalizations per 100,000 population for anaphylaxis among older adults (≥65 years) in the US by trigger, 2006–2014 National Inpatient Sample
Severe anaphylaxis
Demographic factors associated with hospitalization, a proxy used for severe anaphylaxis, for anaphylaxis-related ED visits included older age, male sex, public insurance, lowest quartile of median household income based on ZIP code of residence, or visit at a metropolitan hospital, compared to respective counterparts (Table II). Clinical factors associated with hospitalization for anaphylaxis-related ED visits included a drug-related or other trigger, no documentation of receiving epinephrine in the ED, or having a chronic condition compared to the respective counterparts.
Table II.
Clinical risk factors for severe anaphylaxis (hospitalization, cardiac arrest/intubation, or death) among US older adults, 2006–2014 National Emergency Department Sample
| Ho spitalization | cardiac arrest/intubation | Death | ||||
|---|---|---|---|---|---|---|
| Risk Factors | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Year | 0.95 | 0.93–0.97 | 1.03 | 1.01–1.05 | 0.99 | 0.95–1.03 |
| Age | ||||||
| 65–74 years | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| 75–84 years | 1.43 | 1.33–1.54 | 1.08 | 0.99–1.19 | 1.55 | 1.27–1.91 |
| >85 years | 2.19 | 1.96–2.45 | 0.98 | 0.86–1.11 | 2.96 | 2.34–3.75 |
| Sex | ||||||
| Male | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Female | 0.83 | 0.78–0.89 | 080 | 0.74–0.88 | 0.92 | 0.77–1.11 |
| Primary Health Insurance | ||||||
| Private | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Public | 1.31 | 1.16–1.48 | 1.23 | 1.04–1.45 | 1.12 | 0.74–1.70 |
| Self-pay | 1.18 | 0.79–1.78 | 1.10 | 0.64–1.88 | 0.77 | 0.17–3.54 |
| Other | 0.94 | 0.63–1.40 | 1.11 | 0.67–1.83 | 0.78 | 0.22–2.70 |
| Median household income quartile | ||||||
| 1 (lowest) | 1.23 | 1.09–1.39 | 1.55 | 1.35–1.78 | 1.35 | 1.00–1.81 |
| 2 | 1.09 | 0.97–1.22 | 1.27 | 1.11–1.46 | 1.28 | 0.97–1.70 |
| 3 | 0.98 | 0.87–1.10 | 1.11 | 0.96–1.27 | 1.04 | 0.77–1.40 |
| 4 (highest) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Season of Discharge | ||||||
| First quarter (Jan - Mar) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Second quarter (Apr - Jun) | 0.95 | 0.86–1.05 | 0.92 | 0.81–1.03 | 0.88 | 0.68–1.13 |
| Third quarter (Jul - Sep) | 0.95 * | 0.86–1.04 | 0.84 | 0.75–0.95 | 0.94 | 0.73–1.22 |
| Fourth quarter (Oct - Dec) | 1.01 | 0.91–1.11 | 0.97 | 0.86–1.08 | 0.90 | 0.70–1.16 |
| Hospital Region | ||||||
| Northeast | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Midwest | 0.67 | 0.58–0.79 | 0.75 | 0.64–0.89 | 0.59 | 0.43–0.80 |
| South | 0.54 | 0.47–0.63 | 0.85 | 0.73–0.99 | 0.64 | 0.48–0.86 |
| West | 0.76 | 0.64–0.89 | 0.97 | 0.83–1.15 | 0.75 | 0.54–1.05 |
| Hospital Status | ||||||
| Non-Metropolitan | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Metropolitan, Non-teaching | 1.77 | 1.57–1.99 | 1.91 | 1.61–2.26 | 1.34 | 0.83–2.16 |
| Metropolitan Teaching | 1.98 | 1.75–2.24 | 2.50 | 2.11–2.97 | 1.64 | 1.03–2.61 |
| Weekend presentation | ||||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Yes | 0.92 | 0.85–0.99 | 0.96 | 0.88–1.05 | 0.85 | 0.68–1.06 |
| Type of Trigger | ||||||
| Food | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Drug | 2.75 | 2.35–3.21 | 5.21 | 4.11–6.60 | 5.64 | 2.60–12.25 |
| Venom | 0.47 | 0.38–0.59 | 0.29 | 0.19–0.46 | 0.23 | 0.03–1.86 |
| Other | 1.48 | 1.28–1.71 | 1.04 | 0.82–1.31 | 3.66 | 1.68–7.97 |
| Epinephrine use in ED | ||||||
| Yes | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| No | 9.89 | 6.48–15.09 | 9.62 | 3.76–24.62 | 2.24 | 0.54–9.27 |
| Any chronic condition | ||||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Yes | 15.61 | 13.37–18.21 | 21.57 | 13.72–33.91 | 14.68 | 5.29–40.72 |
Note: Number of chronic conditions had to be collapsed due to unstable estimates for the 2 or more group Survey weights were used to construct this model. Unweighted n = 20,989; weighted n = 93,795.
Using NEDS data, demographic factors associated with cardiac arrest/intubation included male sex, public insurance, lower quartiles of median household income based on ZIP code of residence, or visit at a metropolitan hospital, compared to respective counterparts. Clinical factors associated with cardiac arrest/intubation included drug-related trigger, no documentation of receiving epinephrine in the ED, or having a chronic condition compared to the respective counterparts.
Using NEDS data, demographic factors associated with death after anaphylaxis-related ED visits included older age or visit at a metropolitan teaching hospital, and clinical factors associated with death after anaphylaxis-related ED visits included drug-related or other trigger or having a chronic condition compared to respective counterparts.
Using NIS data, demographic factors associated with cardiac arrest/intubation included male sex, Black race, lowest quartiles of median household income based on ZIP code of residence, or visit at a metropolitan hospital, compared to respective counterparts (Table III). Clinical factors associated with cardiac arrest/intubation include drug-related or other trigger, or having a co-morbidities of alcohol abuse, diabetes, hypertension, or obesity, compared to respective counterparts. Demographic factors associated with death after anaphylaxis-related hospitalization in the NIS cohort included older age or other race, and clinical factors associated with death after anaphylaxis-related hospitalization included drug-related or other trigger, compared to respective counterparts. A sensitivity analysis with patients only identified by method I for anaphylaxis-related visits was performed using both NEDS and NIS data, which showed similar results (See Table E4 and Table E5 in the Online Repository).
Table III.
Clinical risk factors for severe anaphylaxis (cardiac arrest/intubation or death) among US older adults, 2006–2014 National Inpatient Sample
| Cardiac arrest/Intubation | Death | |||
|---|---|---|---|---|
| Risk Factors | OR | 95% CI | OR | 95% CI |
| Year | 1.02 | 1.00, 1.04 | 0.97 | 0.93, 1.01 |
| Age | ||||
| 65–74 years | 1.00 (referent) | 1.00 (referent) | ||
| 75–84 years | 1.04 | 0.94, 1.15 | 1.39 | 1.13, 1.71 |
| >85 years | 0.77 | 0.68, 0.89 | 2.21 | 1.75, 2.78 |
| Sex | ||||
| Male | 1.00 (referent) | 1.00 (referent) | ||
| Female | 0.89 | 0.81, 0.97 | 1.00 | 0.84, 1.20 |
| Race/Ethnicity | ||||
| White | 1.00 (referent) | 1.00 (referent) | ||
| Black | 1792 | 1.71, 2.16 | 1.12 | 0.86, 1.46 |
| Hispanic | U7 | 0.98, 1.39 | 1.09 | 0.77, 1.54 |
| Asian | 1.20 | 0.92, 1.58 | 1.05 | 0.62, 1.76 |
| Other | 1.28 | 1.00, 1.64 | 1.59 | 1.05, 2.41 |
| Primary Health Insurance | ||||
| Private | 1.00 (referent) | 1.00 (referent) | ||
| Public | 0.95 | 0.81, 1.10 | 1.02 | 0.74, 1.42 |
| Self-pay | 0.80 | 0.48, 1.31 | 1.33 | 0.41, 4.27 |
| Other | 0.74 | 0.47, 1.16 | 1.32 | 0.59, 2.96 |
| Median household income quartile | ||||
| 1 (lowest) | 1.27 | 1.11, 1.45 | 0.93 | 0.72, 1.20 |
| 2 | 1.12 | 0.98, 1.28 | 0.95 | 0.74, 1.21 |
| 3 | 1.11 | 0.97, 1.26 | 0.98 | 0.77, 1.26 |
| 4 (highest) | 1.00 (referent) | 1.00 (referent) | ||
| Season of Discharge | ||||
| First quarter (Jan - Mar) | 1.00 (referent) | 1.00 (referent) | ||
| Second quarter (Apr - Jun) | 1.08 | 0.96, 1.22 | 0.96 | 0.75, 1.22 |
| Third quarter (Jul - Sep) | 0.95 | 0.84, 1.07 | 0.90 | 0.71, 1.14 |
| Fourth quarter (Oct - Dec) | 1.05 | 0.93, 1.18 | 1.02 | 0.80, 1.30 |
| Hospital Region | ||||
| Northeast | 1.00 (referent) | 1.00 (referent) | ||
| Midwest | 0.84 | 0.73, 0.97 | 0.61 | 0.46, 0.82 |
| South | 0.92 | 0.81, 1.04 | 0.89 | 0.70, 1.14 |
| West | 1.06 | 0.92, 1.21 | 0.92 | 0.70, 1.21 |
| Hospital Status | ||||
| Non-Metropolitan | 1.00 (referent) | 1.00 (referent) | ||
| Metropolitan, Non-teaching | 1.50 | 1.27, 1.78 | 1.32 | 0.89, 1.96 |
| Metropolitan Teaching | 1.80 | 1.52, 2.13 | 1.44 | 0.98, 2.13 |
| Weekend presentation | ||||
| No | 1.00 (referent) | 1.00 (referent) | ||
| Yes | 1.02 | 0.93, 1.13 | 1.07 | 0.87, 1.30 |
| Type of Trigger | ||||
| Food | 1.00 (referent) | 1.00 (referent) | ||
| Drug | 2.75 | 2.19, 3.46 | 2.78 | 1.33, 5.78 |
| Venom | 050 | 0.31, 0.81 | 0.74 | 0.16, 3.55 |
| Other | 0.67 | 0.53, 0.84 | 2.11 | 1.02, 4.39 |
| Elixhauser Index Score | 1.04 | 1.04, 1.05 | 1.07 | 1.06, 1.08 |
| Any chronic condition | ||||
| No | 1.00 (referent) | 1.00 (referent) | ||
| Yes | 1.05 | 0.97, 1.14 | 1.18 | 0.97, 1.43 |
| Comorbidities (referent: no for each condition) | ||||
| Alcohol | 2.08 | 1.62, 2.67 | 1.29 | 0.79, 2.12 |
| Congestive Heart Failure | 1.05 | 0.92, 1.19 | 0.92 | 0.74, 1.15 |
| Chronic Pulmonary Disease | 1.06 | 0.96, 1.17 | 0.86 | 0.72, 1.04 |
| Diabetes | 1.16 | 1.05, 1.28 | 1.19 | 0.98, 1.44 |
| Hypertension | 1.45 | 1.30, 1.62 | 0.84 | 0.70, 1.01 |
| Liver Disease | 0.60 | 0.43, 0.82 | 1.08 | 0.65, 1.81 |
| Obesity | 1.69 | 1.49, 1.93 | 1.31 | 0.97, 1.77 |
| Oncologic | 0.88 | 0.74, 1.04 | 0.98 | 0.74, 1.30 |
| Renal Failure | 0.72 | 0.64, 0.80 | 0.80 | 0.64, 1.00 |
| Rheumatoid arthritis/collagen vascular diseases | 0.81 | 0.64, 1.03 | 0.91 | 0.56, 1.46 |
Survey weights were used to construct this model. Unweighted n =14,990; weighted n = 72,677.
Discussion
Little is known about national trends in health care utilization for AAR (including anaphylaxis) among older adults in the US. While the rate for ED visits and hospitalizations for AAR remained stable over the study period (2006–2014), we observed a significant increase in the rate of anaphylaxis-ED visits and hospitalizations among US older adults.
The observed increase may be secondary to an increase in the true prevalence of severe anaphylaxis in the older adult population or attributed to an increasing awareness of anaphylaxis and allergic disorders among clinicians and patients. Prior studies examining anaphylaxis-related US trends have examined the general adult population26, 27 or primarily focused on the pediatric population.16, 28, 29 Of the recent studies including anaphylaxis trends for older adults in the US, there have been conflicting data with a reported increase in anaphylaxis-related hospitalization rate in New York State6 and a stable rate across the US.26 This variability may be due to use of different case definitions of anaphylaxis; our study uses previously validated algorithms for anaphylaxis.12–14
While the older adult population had similar ED visit rates for anaphylaxis compared to younger adults, we found that the older adult population had higher anaphylaxis-related hospitalization rates compared to younger adults. Previous studies have noted that older adults may more likely be hospitalized30, 31 or experience severe anaphylaxis.7, 30, 32 Older adults may have comorbid conditions, atypical clinical presentations, receive delayed administration of epinephrine, or experience adverse effects from epinephrine use, leading to severe anaphylaxis.7, 33
Among older adults, we also found higher rates of ED visits and hospitalizations for drug-related AAR and anaphylaxis compared to venom-related or food-related events, which has been previously reported.6, 26, 34 Hospitalization rates for drug-related anaphylaxis for older adults also increased over the time period. Drug-related fatal anaphylaxis has been previously reported to have increased in the US from 1999 to 2010.35 Further studies will need to monitor this growing risk, especially in the older adult population.
Recent guidelines36 continue to recommend epinephrine as the first-line pharmacotherapy for anaphylaxis. We report a very low percentage of documented ED epinephrine use which could be secondary to decreased use of epinephrine in this population, pre-hospital or self-administered use of epinephrine, or lack of documentation due to the clinical acuity of ED cases. Previous studies have shown conflicting data regarding administration of epinephrine in the older adult population for anaphylaxis with reported increased use in a European registry7 and decreased use in a study examining US older adults presenting to ED for anaphylaxis.37 The older adult population have been noted to be less likely to fill a prescription for epinephrine autoinjectors after ED visit or hospitalization for anaphylaxis.30 Adverse cardiac complications after epinephrine use have also been noted in the older adult31, 37and >80 year old population33, so further work may need to elucidate these factors.
We found that the factors consistently associated with severe anaphylaxis outcomes (hospitalization, cardiac arrest/intubation, and death) after ED visit included drug-related triggers or having a chronic condition. We observed that older age and drug-related triggers were consistently associated with death, after controlling for multiple comorbidities. Both older age2, 35 and drug-related trigger2, 32, 38 have previously been noted to be a risk factor for fatal anaphylaxis. Patients who are older may be exposed to multiple drugs or have underlying comorbidities (e.g. cardiovascular disease) that predispose them to worse outcomes.
Interestingly, low median household income was associated with cardiac arrest/intubation. This could be due to poor access to care or delayed presentation to the ED. We also found that co-morbidities such as diabetes, hypertension, and obesity were risk factors for cardiac arrest/intubation, and these potential risk factors merit further investigation in severe anaphylaxis.
Limitations of this study include potential misclassification of AAR/anaphylaxis cases and triggers. However, we used previously validated algorithms14 and also conducted sensitivity analyses examining each ascertainment method for anaphylaxis and found similar trends. Coding errors and variation in documentation or reporting are a limitation when using administrative data; however, coding errors are likely random assuming no significant changes in coding over the years and typically not subject to systematic biases. Additionally, administration of epinephrine is not well documented and may have occurred during the pre-hospital setting, which may be subject to bias and misclassification. Our analyses were limited to factors reported in the administrative data, so other social factors (e.g. access to specialist), or clinical factors (e.g. timing of anaphylaxis), could not be measured. Additionally, NIS restructured the sampling strategy to be a sample of discharges in 2011 and for prior years it was a sample of hospitals; however, the new sampling strategy were thought to produce more precise estimates. Moreover, the study period ends in 2014, as diagnostic coding changes from ICD-9-CM to International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) in 2015 complicated interpretation of temporal trends in more recent years. Another limitation is a violation of the independence assumption in the regression models used given that the unit of analysis is at the visit-level rather than at the patient level, though the magnitude of this bias is likely small. Lastly, there may be potential residual confounding in the associations or unmeasured confounders.
The strengths of the study include the use of a large, nationally representative database to understand trends and utilization of AAR, including anaphylaxis, which is an uncommon event. Our study also focuses on older adults, a rapidly growing vulnerable population, which have been less studied in allergy and anaphylaxis research. Our results are generalizable to the US older adult population, and there have not been significant practice changes to anaphylaxis management guidelines affecting this population since 2014.36
Conclusion
Overall, in two nationally representative databases, we report an increase in anaphylaxis-related ED visits and hospitalizations, especially drug-related, among US older adults during 2006–2014. Older age and drug-related triggers were significant factors associated with fatal anaphylaxis. Given the rapidly growing older adult population in the US and other industrialized nations, these findings emphasize the importance of providing age-appropriate management and treatment of anaphylaxis for older adults. As more data accumulate on older adults with anaphylaxis, we anticipate the increasing need to establish age-appropriate clinical guidelines for anaphylaxis in this vulnerable population with unique exposures and comorbidities.
Supplementary Material
Highlights Box.
What is already known about this topic?
Little is known about national healthcare utilization patterns for acute allergic reactions, including anaphylaxis, in the US older adult population (age ≥65 years).
What does this article add to our knowledge?
Older adults had a higher anaphylaxis-related hospitalization rate than younger adults. Anaphylaxis-related ED visits and hospitalizations, especially drug-related, have increased over time. Risk factors for anaphylaxis-related death included older age, especially ≥85 years of age, and drug-related trigger.
How does this study impact current management guidelines?
Anaphylaxis, especially drug-related anaphylaxis, is a growing risk for the US older adult population. It may be important to establish age-appropriate clinical guidelines for anaphylaxis in this vulnerable population with unique exposures and comorbidities.
Acknowledgements
We acknowledge Janice A. Espinola, MPH for her statistical expertise and assistance with data management.
Funding Sources: A.C. Arroyo is supported by the National Institutes of Health award R25AI147369-02, and L.B. Robinson is supported by the National Institutes of Health award
T32HL116275. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Abbreviations:
- AAR
Acute allergic reaction
- ED
Emergency department
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- NEDS
Nationwide Emergency Department Sample
- NIS
National Inpatient Sample and Nationwide Inpatient Sample
- US
United States
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mather MJL, and Pollard KM. Aging in the United States. Population Bulletin; 2015. [Google Scholar]
- 2.Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal anaphylaxis: mortality rate and risk factors. The Journal of Allergy and Clinical Immunology: In Practice. 2017;5(5):1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberger PA, Rotskoff BD, Lifschultz B. Fatal anaphylaxis: postmortem findings and associated comorbid diseases. Annals of allergy, asthma & immunology. 2007;98(3):252–7. [DOI] [PubMed] [Google Scholar]
- 4.Mullins R, Wainstein B, Barnes E, Liew W, Campbell D. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clinical & Experimental Allergy. 2016;46(8): 1099–110. [DOI] [PubMed] [Google Scholar]
- 5.Shrestha P, Dhital R, Poudel D, Donato A, Karmacharya P, Craig T. Trends in hospitalizations related to anaphylaxis, angioedema, and urticaria in the United States. Ann Allergy Asthma Immunol. 2019;122(4):401–6 e2. [DOI] [PubMed] [Google Scholar]
- 6.Patel C, Haque M, Waqar O, Kline M, Jongco A. New York State cases of anaphylaxis in elderly patients from 2000 to 2010. Ann Allergy Asthma Immunol. 2020;125(4):410–7 e2. [DOI] [PubMed] [Google Scholar]
- 7.Aurich S, Dölle-Bierke S, Francuzik W, Bilo MB, Christoff G, Fernández-Rivas MFR, et al. Anaphylaxis in Elderly Patients-Data from the European Anaphylaxis Registry. Frontiers in Immunology. 2019;10:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HCUP Nationwide Emergency Department Sample (NEDS). Healthcare Cost and Utilization Project (HCUP). 2007, 2009. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/nedsoverview.jsp. [Google Scholar]
- 9.HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012-2015. Agency for Healthcare Research and Quality R, MD. www.hcup-us.ahrq.gov/nisoverviewjsp
- 10.(NIS). HNIS, Healthcare Cost and Utilization Project (HCUP). 2006-2011. Agency for Healthcare Research and Quality R, MD. www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed]
- 11.Roberts AW, Ogunwole Stella U., Laura Blakeslee, and Rabe Megan A., “The Population 65 Years and Older in the United States: 2016,” American Community Survey Reports, ACS-38, U.S. Census Bureau, Washington, DC, 2018. [Google Scholar]
- 12.Saff RR, Li Y, Santhanakrishnan N, Camargo CA Jr, Blumenthal KG, Zhou L, et al. Identification of inpatient allergic drug reactions using ICD-9-CM codes. The Journal of Allergy and Clinical Immunology: In Practice. 2019;7(1):259–64. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saff RR, Camargo CA Jr, Clark S, Rudders SA, Long AA, Banerji A. Utility of ICD-9-CM codes for identification of allergic drug reactions. The Journal of Allergy and Clinical Immunology: In Practice. 2016;4(1):114–9. e1. [DOI] [PubMed] [Google Scholar]
- 14.Harduar-Morano L, Simon MR, Watkins S, Blackmore C. A population-based epidemiologic study of emergency department visits for anaphylaxis in Florida. Journal of allergy and clinical immunology. 2011;128(3):594–600. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark S, Gaeta TJ, Kamarthi GS, Camargo CA. ICD-9-CM coding of emergency department visits for food and insect sting allergy. Annals of epidemiology. 2006;16(9):696–700. [DOI] [PubMed] [Google Scholar]
- 16.Rudders SA, Arias SA, Camargo CA. Trends in hospitalizations for food-induced anaphylaxis in US children, 2000–2009. Journal of Allergy and Clinical Immunology. 2014;134(4):960–2. e3. [DOI] [PubMed] [Google Scholar]
- 17.Walsh KE, Cutrona SL, Foy S, Baker MA, Forrow S, Shoaibi A, et al. Validation of anaphylaxis in the Food and Drug Administration’s Mini-Sentinel. Pharmacoepidemiology and drug safety. 2013;22(11):1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harduar-Morano L, Simon MR, Watkins S, Blackmore C. Algorithm for the diagnosis of anaphylaxis and its validation using population-based data on emergency department visits for anaphylaxis in Florida. J Allergy Clin Immunol. 2010;126(1):98–104 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HCUP Chronic Condition Indicator (CCI). Healthcare Cost and Utilization Project (HCUP). 2009. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed August 3, 2020. [PubMed]
- 20.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data. Medical care. 2017;55(7):698–705. [DOI] [PubMed] [Google Scholar]
- 21.Thompson NR, Fan Y, Dalton JE, Jehi L, Rosenbaum BP, Vadera S, et al. A new Elixhauser-based comorbidity summary measure to predict in-hospital mortality. Med Care. 2015;53(4):374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossestreuer AV, Gaieski DF, Donnino MW, Nelson JI, Mutter EL, Carr BG, et al. Cardiac arrest risk standardization using administrative data compared to registry data. PloS one. 2017;12(8):e0182864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerlin MP, Weissman GE, Wonneberger KA, Kent S, Madden V, Liu VX, et al. Validation of administrative definitions of invasive mechanical ventilation across 30 intensive care units. American journal of respiratory and critical care medicine. 2016;194(12):1548–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houchens RLRD, Elixhauser A. Using the HCUP National Inpatient Sample to Estimate Trends. 2015. HCUP Methods Series Report # 2006–05 ONLINE. January 4, 2016. U.S. Agency for Healthcare Research and Quality. Available:, http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp. [Google Scholar]
- 25.Trend Weights for 1993–2011 HCUP NIS Data. Healthcare Cost and Utilization Project (HCUP). 2015. Agency for Healthcare Research and Quality R, MD. www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Accessed August 3, 2020. [PubMed]
- 26.Shrestha P, Dhital R, Poudel D, Donato A, Karmacharya P, Craig T. Trends in hospitalizations related to anaphylaxis, angioedema and urticaria in the United States. Annals of Allergy, Asthma & Immunology. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Motosue MS, Bellolio MF, Van Houten HK, Shah ND, Campbell RL. Increasing emergency department visits for anaphylaxis, 2005–2014. The Journal of Allergy and Clinical Immunology: In Practice. 2017;5(1):171–5. e3. [DOI] [PubMed] [Google Scholar]
- 28.Rudders SA, Banerji A, Vassallo MF, Clark S, Camargo CA. Trends in pediatric emergency department visits for food-induced anaphylaxis. Journal of Allergy and Clinical Immunology. 2010;126(2):385–8. [DOI] [PubMed] [Google Scholar]
- 29.Motosue MS, Bellolio MF, Van Houten HK, Shah ND, Campbell RL. National trends in emergency department visits and hospitalizations for food-induced anaphylaxis in US children. Pediatric Allergy and Immunology. 2018;29(5):538–44. [DOI] [PubMed] [Google Scholar]
- 30.Clark S, Wei W, Rudders SA, Camargo CA Jr. Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. Journal of Allergy and Clinical Immunology. 2014;134(5):1125–30. [DOI] [PubMed] [Google Scholar]
- 31.Campbell RL, Hagan JB, Li JT, Vukov SC, Kanthala AR, Smith VD, et al. Anaphylaxis in emergency department patients 50 or 65 years or older. Annals of Allergy, Asthma & Immunology. 2011;106(5):401–6. [DOI] [PubMed] [Google Scholar]
- 32.Motosue MS, Bellolio MF, Van Houten HK, Shah ND, Campbell RL. Risk factors for severe anaphylaxis in the United States. Ann Allergy Asthma Immunol. 2017;119(4):356–61 e2. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien ME, Koehl JL, Raja AS, Erickson TB, Hayes BD. Age-related cardiovascular outcomes in older adults receiving epinephrine for anaphylaxis in the emergency department. J Allergy Clin Immunol Pract. 2019;7(8):2888–90. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Hess EP, Lohse C, Gilani W, Chamberlain AM, Campbell RL. Trends, characteristics, and incidence of anaphylaxis in 2001–2010: a population-based study. Journal of Allergy and Clinical Immunology. 2017;139(1):182–8. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jerschow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999–2010: temporal patterns and demographic associations. Journal of allergy and clinical immunology. 2014;134(6):1318–28. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145(4):1082–123. [DOI] [PubMed] [Google Scholar]
- 37.Kawano Y, Kawasaki T, Kawazoe N, Abe I, Uezono K, Ueno M, et al. Circadian variations of urinary dopamine, norepinephrine, epinephrine and sodium in normotensive and hypertensive subjects. Nephron. 1990;55(3):277–82. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-de-Olano D, Lombardo C, Gonzalez-Mancebo E. The difficult management of anaphylaxis in the elderly. Curr Opin Allergy Clin Immunol. 2016;16(4):352–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.