Abstract
Introduction:
Understanding rural-urban variation in the diagnostic incidence and prevalence of Alzheimer’s disease and related dementias (ADRD) will inform policies to improve timely diagnosis and access to supportive services for older adults in rural communities.
Methods:
Using 2008 to 2015 national claims data for fee-for-service Medicare beneficiaries (roughly 170 million person-years), we computed unadjusted and adjusted diagnostic incidence and prevalence estimates for ADRD in metropolitan, micropolitan, and rural counties, and examined differences in survival rates.
Results:
Risk-adjusted ADRD diagnostic incidence was higher in rural versus metropolitan counties despite lower prevalence. Among beneficiaries diagnosed with ADRD in 2008, metropolitan county residents experienced longer survival compared to residents in rural and micropolitan counties.
Discussion:
These data suggest that older adults in rural communities may be underdiagnosed with ADRD, and/or diagnosed at later stages of dementia. Further work is needed to develop strategies to reduce this disparity.
Keywords: ADRD, diagnostic incidence, diagnostic prevalence, rural-urban disparity
1 ∣. BACKGROUND
Alzheimer’s disease and related dementias (ADRD) affect roughly 14% of adults over age 70 and more than 37% of adults aged 90 and older.1 Older adults are disproportionally represented among rural populations,2 suggesting that rural regions of the country may be disproportionally affected by ADRD in current and coming years as the population of older Americans grows. Additionally, individuals living in rural areas have higher rates of risk factors for ADRD, including chronic health conditions (eg, diabetes, obesity, depression), lifestyle factors such as alcohol and tobacco use, and lower educational attainment.2-4 Yet, little is known about differences in ADRD incidence and prevalence between rural and urban-dwelling Americans.
The expected increase in incidence and prevalence of ADRD is particularly concerning for rural areas where healthcare capacity is limited. There is growing evidence that disparities in disease burden as well as availability, accessibility, affordability, and acceptability of healthcare exist among older adults living in rural areas, thus contributing to the growing gap in life expectancy and survival witnessed between urban and rural communities.5-8 As the population ages, there is also expected to be a decrease in the availability of family and other informal caregivers who provide over 18 billion hours of unpaid care annually to people with ADRD.9 A decline in caregiver availability will increase demand for formal supportive services, yet availability and access to such services is limited in rural areas.2,10,11
This study aims to assess differences in the diagnostic incidence and prevalence of ADRD between metropolitan, micropolitan, and rural counties, and determine whether these differences are explained by underlying beneficiary demographics, prior healthcare utilization, and comorbid illness. Of note, we did not aim to measure actual ADRD incidence and prevalence as determined by specific diagnostic criteria as has been done through prospective cohort studies like the Aging, Demographics, and Memory Study (ADAMS).1 Rather, we used 2007 to 2015 Medicare claims to understand diagnostic patterns, broadly, in the entire fee-for-service (FFS) Medicare population. We also performed a survival analysis of FFS Medicare beneficiaries who were diagnosed with ADRD in 2008 to examine rural-urban differences in 10-year survival rates.
2 ∣. METHODS
2.1 ∣. Data sources
We used 2007 to 2015 FFS Medicare enrollment and Part A data. The Medicare Beneficiary Summary File (MBSF) contains enrollment data on all beneficiaries enrolled in or entitled to Medicare per year. It includes a Chronic Condition Warehouse (CCW) section with annual flags for 27 chronic conditions and 33 other chronic or potentially disabling conditions. All conditions also have the date of the first diagnosis. We linked these data with Medicare Part A inpatient, skilled nursing facility (SNF) and home health (HHA) claims.
We linked the Medicare data with the nursing home Minimum Data Set (MDS) and home health Outcome and Assessment Information Set (OASIS) to identify beneficiaries’ settings of care. The MDS is a federally-mandated assessment tool for all residents in Medicare- or Medicaid-certified nursing homes, completed at admission and at least quarterly thereafter. OASIS home healthcare assessments are completed at start of care, 60-day follow-ups, and discharge (and surrounding an inpatient or nursing facility stay).
These data all informed our Residential History File,12,13 a per-person chronological history of health service utilization and location of service based on claims and assessment data.
2.2 ∣. Study cohort
We examined all Medicare beneficiaries over the age of 65 who were enrolled in FFS Medicare between 2007 and 2015. For each year of observation, we excluded beneficiaries enrolled in Medicare Advantage in that year or the prior year because their diagnoses and healthcare utilization could not be identified. We calculated incidence and prevalence measures for 2008 to 2015, but used 2007 data as our prior year lookback window for 2008.
2.3 ∣. ADRD
We identified Medicare beneficiaries with ADRD, our main outcome variable, using the CCW flag for Alzheimer’s Disease, Related Disorders, or Senile Dementia. This flag captures 24 ICD-9 codes (for claims submitted prior to October 1, 2015) or 22 ICD-10 codes (for claims on or after October 1, 2015) for dementia present on one or more inpatient, SNF, HHA, hospital outpatient, or carrier claim over a 3-year lookback. Compared to clinically-diagnosed cases of dementia in the ADAMS study, the CCW ADRD algorithm had a sensitivity of 0.85 and specificity of 0.89.14 Validation studies in other populations have measured sensitivity between 0.51 and 0.85 and specificity between 0.77 and 0.92.15,16
For each year of observation, we used the annual CCW flags and the associated date of the first diagnosis to classify beneficiaries as not diagnosed with ADRD, newly diagnosed with ADRD in that year (ie, study year is the same as the year of first diagnosis), or started the year with an ADRD diagnosis (ie, study year is greater than the year of the first diagnosis). Because CCW flags are based on claims since 1999, the earliest possible date of ADRD diagnosis is January 1, 1999.
2.4 ∣. Metropolitan, micropolitan, and rural residence
We used the MBSF to determine each Medicare beneficiary’s county of residence. We then used the rural urban continuum codes (RUCC) to determine the rurality of a beneficiary’s county: metropolitan (RUCC 1 to 3), micropolitan (RUCC 4 to 7) and rural (RUCC 8 and 9).5,8,17,20 Detailed codes are presented in Appendix Table 1.
2.5 ∣. Covariates
Beneficiary demographic characteristics (sex, race, and Medicaid eligibility) came from the MBSF. We classified beneficiaries’ comorbidities using two measures1: a count of chronic and potentially disabling conditions identified in the CCW file for the previous year, and2 a hierarchical chronic condition (HCC) score derived from ICD-9 codes reported on all Part-A claims during the previous year. As an additional measure of clinical complexity, we also computed summary measures of each beneficiary’s healthcare utilization in the prior year,21,22 including the number of inpatient hospital admissions, days in a nursing home, and a count of home health assessments. The main reason for including prior healthcare utilization and comorbidities as risk adjusting variables is to ensure that differences in ADRD prevalence between rural and urban counties are not just a reflection of differences in overall health. Finally, to characterize beneficiaries’ residential neighborhood, we determined the share of Medicare beneficiaries eligible for Medicaid and the share of beneficiaries enrolled in Medicare Advantage for each beneficiary’s zip code.
2.6 ∣. Statistical analysis
Change in ADRD prevalence at a given point of time is determined by two factors: new incident cases and deaths in the population. In the first part of our analysis, we examined how the new inflow of ADRD cases differed from the existing population of ADRD cases in rural and urban counties. The diagnostic incidence is the percent of Medicare beneficiaries newly diagnosed with ADRD in a given year. The diagnostic prevalence is the percent of Medicare beneficiaries who started a given year with a diagnosis of ADRD. For both measures, the denominator consists of the entire FFS over age 65 Medicare population as of January 1 of a given year. We plotted these measures for the years 2008 to 2015 stratified by county rurality (metropolitan, micropolitan, vs rural). We calculated risk-adjusted incidence and prevalence using two steps. First, we estimated a multinomial logistic regression (mlogit command in Stata) in which the outcome was ADRD status (ie, not diagnosed with ADRD, newly diagnosed with ADRD, or started the year with a diagnosis of ADRD). The main explanatory variables were categories for rurality (metropolitan, micropolitan, or rural). We controlled for beneficiaries’ demographics, comorbidities, healthcare utilization, and zip code characteristics. Second, we calculated the adjusted likelihood of each category of outcome as the marginal effects of rurality categories (margins command in Stata). We performed these two steps for each year. We then plotted these predictive margins for a given outcome by year. An illustration of detailed steps of risk adjustment for year 2015 is presented in Appendix Table 4.
In the second part of our analysis, we compared Medicare beneficiaries in metropolitan, micropolitan, and rural counties who died between 2008 and 2015. We calculated the share of those who had an ADRD diagnosis (either new or existing) among all decedents, by the year of death. Finally, among the persons who were diagnosed with ADRD in 2008, we compared the probability of survival over ten years from the date of diagnosis for metropolitan, micropolitan, and rural counties. Here we focused on incident cases in 2008 because they had the longest follow-up in our dataset. In this survival analysis, the event is death. The cases were tracked at the end of 2017. Our survival analysis involves two steps. First, we estimated a Cox proportional hazards model (stcox command in Stata) including rurality indicators and the same set of covariates as described above in the Cox regression model. Second, we plotted the survival function (stcurve command in Stata) for metropolitan, micropolitan, and rural counties. Stata, version 15.1, was used for all statistical analysis.
3 ∣. RESULTS
We examined approximately 170 million person-years for Medicare FFS beneficiaries, 76% of whom (person-years) were in metropolitan counties, 14% in micropolitan counties, and 10% in rural counties. Beneficiaries residing in nonmetropolitan counties were slightly younger, less likely to be female and white, and had slightly fewer chronic conditions (Table 1), though the HCC score derived from inpatient claims was similar across all counties. Rural beneficiaries spent more time in nursing homes compared to beneficiaries residing in metropolitan and micropolitan counties. These differences were fairly consistent across non-ADRD, new-ADRD and existing ADRD cases (Appendix Table 2).
TABLE 1.
Characteristics of Medicare beneficiaries enrolled in fee-for-service Medicare between 2008 and 2015
| Metropolitan counties |
Micropolitan counties |
Rural counties |
|
|---|---|---|---|
| Person-years (N) | 129,624,353 | 23,649,523 | 17,282,079 |
| Age in calendar year, mean | 76.9 | 76.5 | 76.4 |
| Female (%) | 58.4% | 57.1% | 56.2% |
| Black (%) | 7.7% | 4.6% | 4.7% |
| Hispanic (%) | 5.2% | 2.4% | 1.7% |
| Other race (%) | 3.7% | 1.8% | 1.7% |
| Dual eligible (%) | 12.1% | 13.0% | 15.6% |
| No. of chronic conditions, mean | 4.22 | 4.01 | 3.86 |
| HCC score, mean | 0.66 | 0.64 | 0.64 |
| No. of hospitalized days, mean | 0.28 | 0.29 | 0.31 |
| No. of nursing home days, mean | 9.00 | 10.15 | 11.45 |
| No. of home health assessments, mean | 0.23 | 0.21 | 0.23 |
| Residential ZIP code Medicare Advantage penetration (%) | 23.03 | 15.46 | 13.32 |
| Residential ZIP code dual-eligible rate (%) | 16.17 | 18.15 | 19.79 |
Abbreviation: HCC, hierarchical chronic conditions.
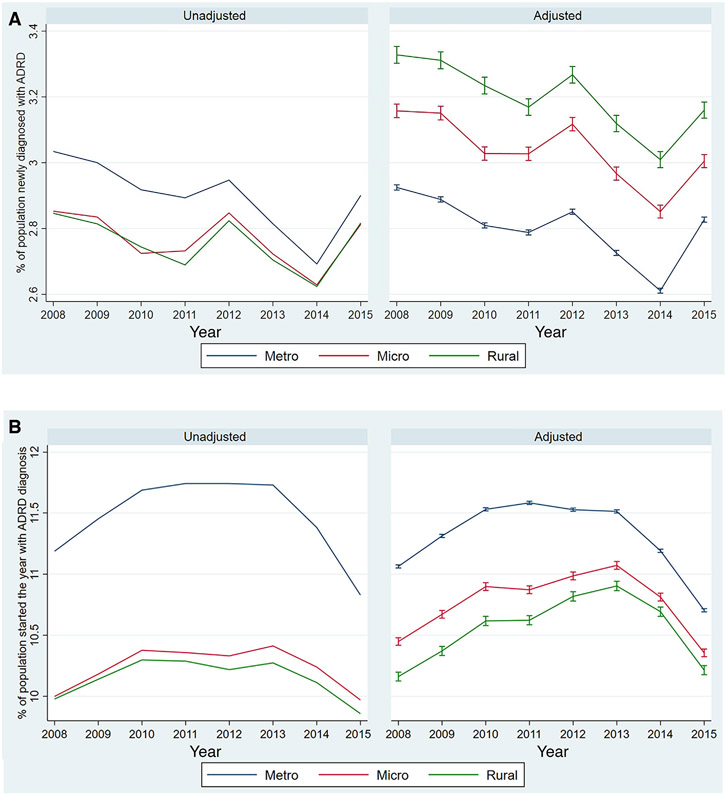
Unadjusted diagnostic incidence and prevalence were higher in metropolitan counties compared to micropolitan and rural counties throughout the study period (left panels of Figure 1). Between 2008 and 2015, ADRD diagnostic incidence declined, though there were small spikes in new diagnoses in 2012 and 2015. At the same time, diagnostic prevalence increased from 2008 to 2010, generally plateaued from 2010 to 2013, then decreased from 2013 to 2015. After adjustment for beneficiary and zip code characteristics, incidence was higher in rural and micropolitan counties compared to metropolitan counties (right panels of Figure 1). The difference in prevalence between rural and urban counties was also reduced substantially after risk adjustment (from 1 percentage point in the unadjusted rate to 0.5 percentage points following adjustment).
FIGURE 1.
Diagnostic incidence and prevalence of Alzheimer’s disease and related dementias (ADRD) in urban and rural counties in the US from 2008 to 2015. (A) ADRD diagnostic incidence. (B) ADRD diagnostic prevalence. Adjusted measures and the 95% confidence intervals are estimated separately for each year using two steps. First, we estimate a multinomial logit regression of ADRD diagnosis onto rurality of beneficiary’s residential county, beneficiary’s demographic, clinical and residential zip code characteristics (listed in Table 1). Second, we estimate the marginal effects of rurality. Abbreviations: Metro, metropolitan counties; Micro, micropolitan counties; Rural, rural counties
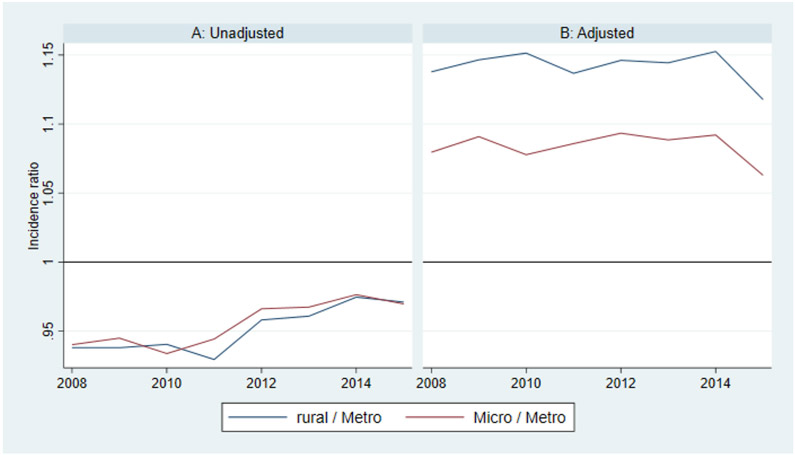
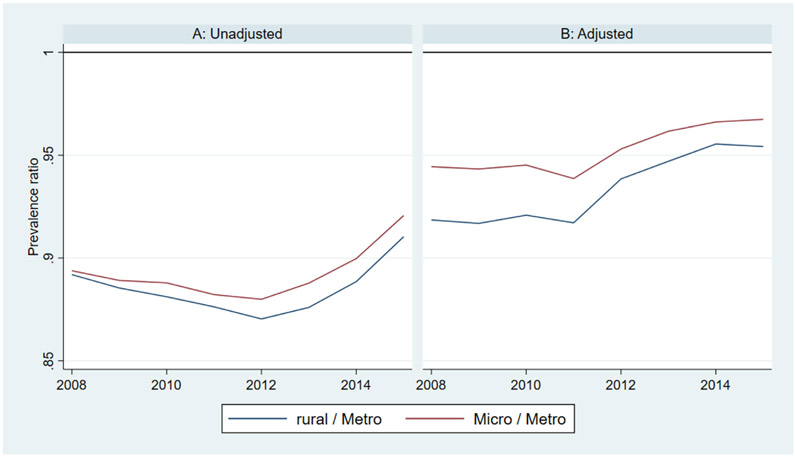
Differences in diagnostic prevalence and incidence between rural and urban counties can perhaps be better understood in terms of risk and prevalence ratios. For example, the adjusted diagnostic incidence is about 1.14 times higher in rural versus urban counties and 1.08 times higher in micropolitan versus urban counties. Conversely, the adjusted diagnostic prevalence is about 0.93 times lower in rural versus urban counties and 0.95 times lower in micropolitan versus urban counties. These ratios were fairly stable over time (Appendix Figures 1 and 2).
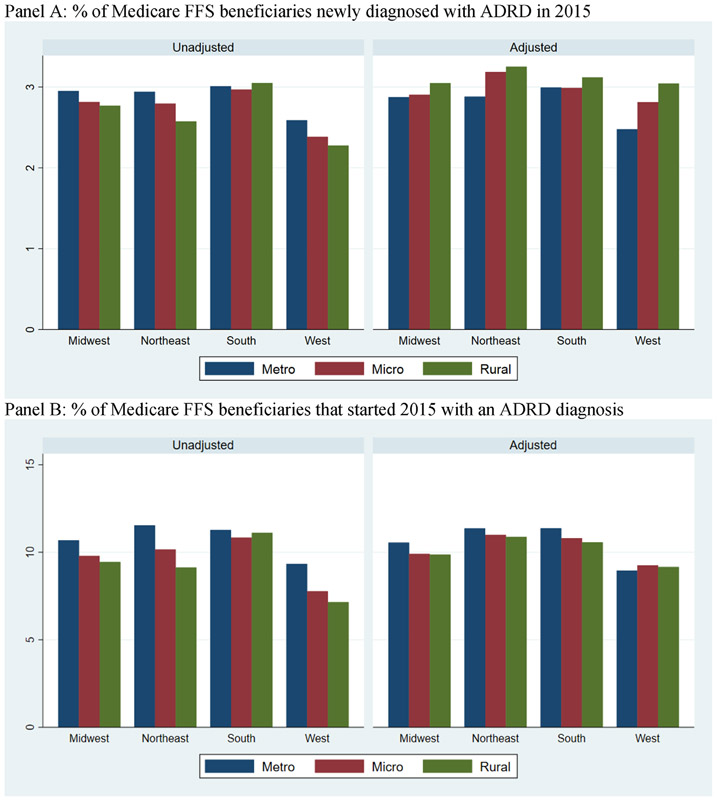
Appendix Figure 3 compares ADRD diagnostic incidence and prevalence between rural and urban counties in different regions of the US in 2015. In general, patterns are fairly similar across regions. We observed a lower unadjusted incidence and prevalence in rural counties, a higher adjusted incidence in rural counties, and a decline in the rural-urban difference in prevalence after adjustment.
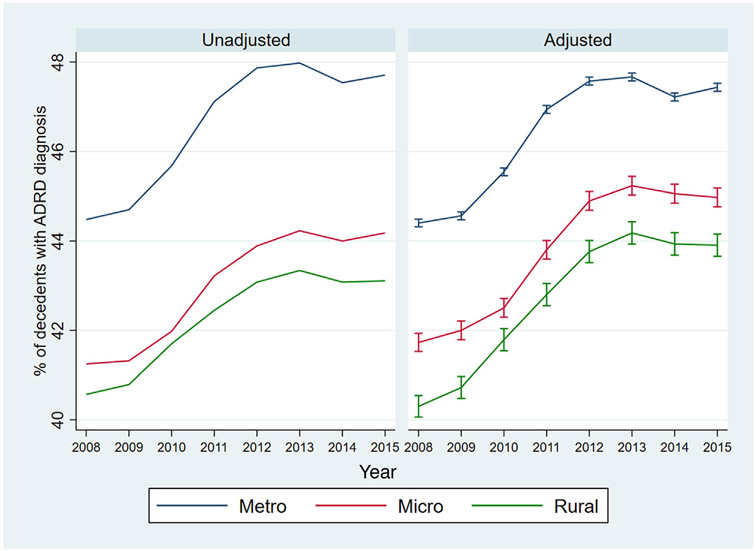
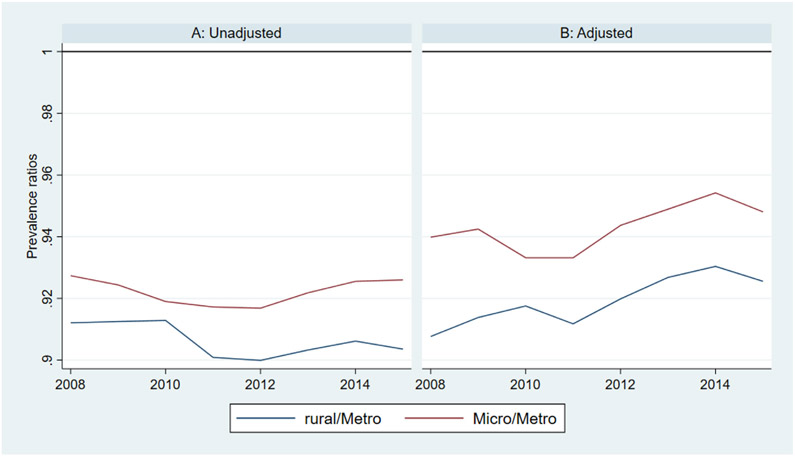
Between 2008 and 2015, there was an increase in the share of Medicare beneficiaries dying with ADRD across metropolitan, micropolitan, and rural counties (Figure 2). In unadjusted analyses, a larger percentage of decedents in metropolitan and micropolitan counties had ADRD compared to decedents in rural counties (metropolitan counties were about four percentage points higher than rural counties in all years). In adjusted analyses, the overall trends remained consistent but the difference in prevalence between metropolitan and rural counties decreased. Similar to diagnostic prevalence ratios in the entire population, adjusted prevalence among decedents was about 0.92 times lower in rural counties and 0.95 times lower in micropolitan counties relative to urban counties (Appendix Figure 4). Table 2 displays the characteristics of decedents in metropolitan, micropolitan, and rural counties. Average age at death was 0.7 years lower in micropolitan/rural counties compared to metropolitan counties. Decedents in rural counties spent significantly more days in hospitals and nursing homes during the last year of life compared to decedents in metropolitan and micropolitan counties.
FIGURE 2.
Proportion of deceased Medicare beneficiaries with Alzheimer’s disease and related dementias (ADRD), 2008 to 2015. Adjusted measures and the 95% confidence intervals are estimated separately for each year using two steps. First, we estimate a logit regression of ADRD diagnosis onto rurality of beneficiary’s residential county, beneficiary’s demographic, clinical and residential zip code characteristics (listed in Table 2). Second, we estimate the marginal effects of rurality. Abbreviations: Metro, metropolitan counties; Micro, micropolitan counties; Rural, rural counties
TABLE 2.
Characteristics of Medicare beneficiaries who died between 2008 and 2015
| Metropolitan counties |
Micropolitan counties |
Rural counties |
|
|---|---|---|---|
| N | 7,609,611 | 1,403,745 | 1,036,497 |
| Age at death, mean | 82.7 | 82.0 | 81.9 |
| Female (%) | 56.13% | 54.58% | 53.67% |
| Black (%) | 8.47% | 5.29% | 5.45% |
| Hispanic (%) | 4.46% | 2.01% | 1.47% |
| Other race (%) | 2.56% | 1.57% | 1.65% |
| Dual eligible (%) | 20.94% | 24.44% | 28.14% |
| No. of Chronic Conditions, mean | 6.34 | 6.06 | 5.93 |
| HCC score, mean | 1.40 | 1.31 | 1.29 |
| No. of Hospitalization Days, mean | 0.93 | 0.91 | 0.98 |
| No. of Nursing Home Days, mean | 47.03 | 52.08 | 57.17 |
| No. of Home Assessments, mean | 0.75 | 0.70 | 0.72 |
| Residential zip code Medicare Advantage penetration (%) | 23.65 | 15.84 | 13.72 |
| Residential zip code dual-eligible rate (%) | 16.87 | 18.78 | 20.42 |
Abbreviation: HCC, hierarchical chronic conditions.
Among decedents with ADRD, age at death was 0.5 years younger and age at diagnosis of ADRD was 0.4 years younger in micropolitan/rural counties compared to metropolitan counties (Appendix Table 3). As a result, the mean time from diagnosis to death was about 40 days shorter in rural/micropolitan counties compared to metropolitan counties (Appendix Table 3).
In the survival analysis, out of the 655,440 subjects, 556,136 experienced the event of death. To evaluate the proportional hazard assumption, we assessed Kaplan-Meier observed versus expected survival curves, which appeared to be quite close for each type of county implying that the rurality variable satisfies the proportional hazard assumption. We evaluated the fit of the model by using the Cox-Snell residuals. We graphed the Nelson-Aalen cumulative hazard function and the Cox-Snell residuals so that we can compare the hazard function to the diagonal line. The hazard function follows the 45 degree line, implying that it approximately has an exponential distribution with a hazard rate of one and that the model fits the data well.
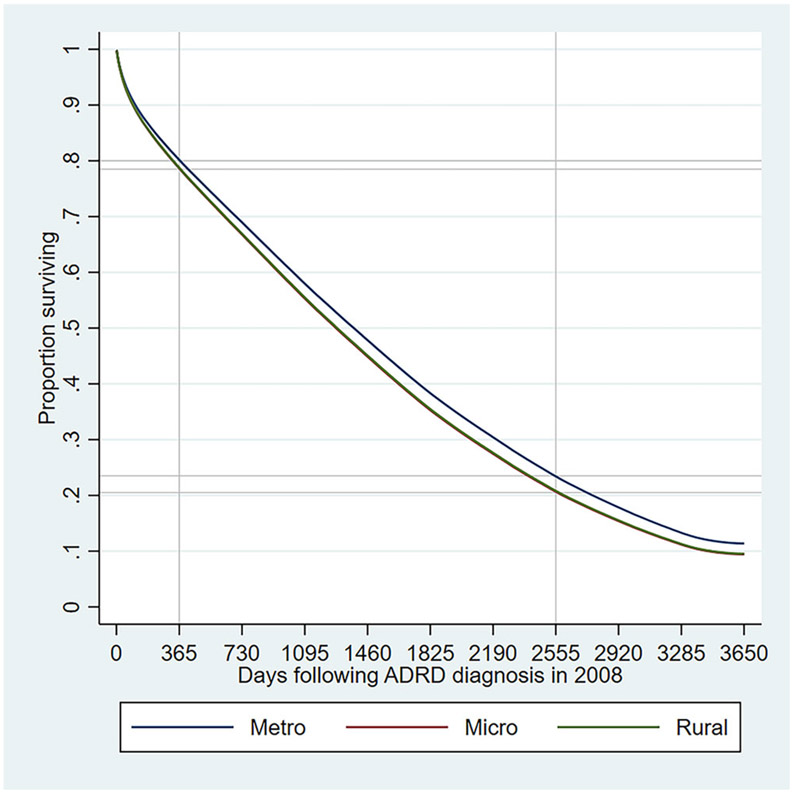
Figure 3 plots the adjusted likelihood of survival in days following ADRD diagnosis in 2008 in the different types of counties. The adjusted share of beneficiaries who survived 365 days following diagnosis was 80% in metropolitan counties and 79% in rural and micropolitan counties. This implies that 365-day survival rates were about 1.013 times or 1% higher in metropolitan counties compared to nonmetropolitan counties. The adjusted share of beneficiaries who survived 2555 days (ie, 7 years) following diagnosis was 24% in metropolitan counties and 21% in rural and micropolitan counties. The 2555-day survival rates were about 14% (ie, 1.14 times) higher in metropolitan counties compared to nonmetropolitan counties. Thus, the gap in likelihood of survival between rural/micropolitan and metropolitan counties increased over the follow-up years. We observed similar patterns in terms unadjusted survival curves as well.
FIGURE 3.
Adjusted survival functions among beneficiaries newly diagnosed with Alzheimer’s disease and related dementias (ADRD) in 2008. Survival functions are plotted based on Cox regression onto rurality of beneficiary’s residential county, beneficiary’s demographic, clinical and residential zip code characteristics (listed in Table 1). Detailed results of the Cox regression are presented in Appendix Table 5. Second, we estimate the marginal effects of rurality. Lines for rural (green) and micropolitan (red) counties are almost identical and overlapping in this figure. Abbreviations: Metro, metropolitan counties; Micro, micropolitan counties; Rural, rural counties
4 ∣. DISCUSSION
To our knowledge, this is the first national prospective cohort study to assess differences in ADRD diagnostic prevalence and incidence between metropolitan, micropolitan, and rural counties in the United States. We found that, while unadjusted estimates suggest lower incidence and prevalence of ADRD in rural counties, after adjustment, ADRD incidence was higher in rural counties compared to metropolitan counties, despite lower prevalence in these rural counties. We also found that beneficiaries with ADRD in metropolitan counties typically had a longer survival following diagnosis compared to beneficiaries with ADRD in rural and micropolitan counties.
ADRD diagnostic prevalence in the overall FFS Medicare population increased from 2008 to 2010, plateaued from 2010 to 2013, then declined from 2013 to 2015. Prevalence in 2015 was lower than in 2008, though this decline was more prominent in metropolitan counties versus micropolitan and rural counties. This overall trend is similar to previous findings from the Health and Retirement Study23 which demonstrated a decline in dementia prevalence between two time points, 2000 and 2012. At the same time, we found a general decline in diagnostic incidence of ADRD between 2008 and 2015, despite some slight disruptions in that trend in 2012 and 2015. Our findings of overall declining ADRD diagnostic incidence are consistent with previous findings of declining dementia incidence among participants of the Framingham Heart Study.24 Both the Framingham and Health and Retirement Studies cited an increase in educational attainment as partial explanation for the trends they observed.
We found a significant increase in the proportion of deceased Medicare beneficiaries with ADRD between 2008 and 2015, comporting with existing evidence from the Centers for Disease Control and Prevention (CDC) showing an increase in mortality rates due to Alzheimer’s disease over time.25 Importantly, our findings reflect the proportion of Medicare beneficiaries who died with ADRD as captured on a lookback of claims data, rather than as reported on death certificates as an underlying cause of death, which is what the CDC uses. Our estimates are significantly higher than the CDC estimates, which is likely due to dementia being underreported on death certificates.26 Additionally, the CDC estimates are only for Alzheimer’s disease, whereas the CCW algorithm for Medicare claims captures multiple types of dementia.
Variations in prevalence and incidence measures over time can be driven by multiple factors, not all of which are fully understood. Because we used Medicare data, we also have to consider how changes in administrative policies over time may have affected the coding of ADRD in claims. For example, the Centers for Medicare and Medicaid Services (CMS) expanded the allowable number of diagnosis codes hospitals could report from 9 to 25 in 2011, which has increased the reported number of comorbidities in hospital claims.27 This could potentially explain why we saw a spike in ADRD diagnostic incidence in 2012. Similarly, the spike in incident ADRD diagnoses in 2015 could potentially be due to the transition from ICD-9 to ICD-10, though this would have only affected claims after October 1, 2015, when ICD-10 took effect. Another policy change that may have increased clinical diagnoses of ADRD was the introduction of the annual Medicare wellness visit in 2011, which has enabled primary care providers to be reimbursed for preventative care, including a screening for cognitive impairment. This may also partially explain the spike in diagnostic incidence we found in 2012.
Our findings are similar to those by Abner and colleagues who, also using Medicare data, found that rural counties in Kentucky and West Virginia had lower adjusted diagnostic ADRD prevalence than urban counties in the same states.28 However, our findings differ from those of Weden and colleagues29 who found that the unadjusted dementia prevalence was higher in rural communities than urban communities (5.1% vs 4.4%) in 2010. They also found higher prevalence of cognitive impairment with no dementia (CIND) in rural versus urban communities (16.5% vs 14.9%). Forthatsame year, we found that the unadjusted dementia prevalence was lower in rural counties than urban counties (10.3% vs 11.7%). These differences are likely to be driven by differences in measurement and classification of dementia between the two studies. Weden et al. used data from the Health and Retirement Study, which relies on telephonic cognitive assessments with self or proxy reporting and the ADAMS dementia classification methodology.30 We identified dementia cases using the CCW indicator in Medicare claims, which is derived from an algorithm that has been validated against the ADAMS classification for dementia, but not for CIND. Medicare claims do not have separate indicators for CIND or mild cognitive impairment, so some of the discrepancy in unadjusted prevalence between our study results may be related to misclassification. Despite these differences in unadjusted prevalence, the risk adjustment models in both studies move the estimated rural-urban difference in the same direction.
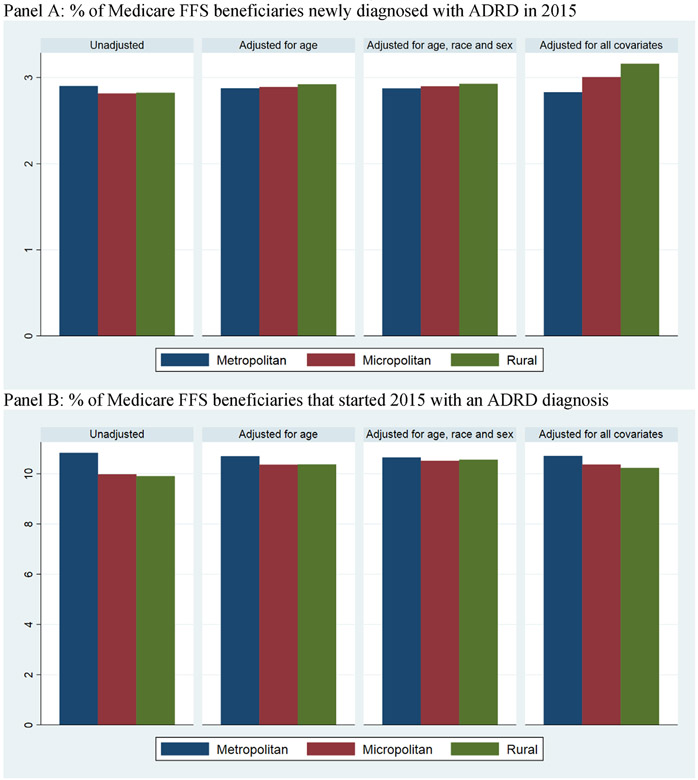
Rural-urban differences between our adjusted and unadjusted estimates are driven by several factors. First, age at ADRD diagnosis is about six months younger in rural and micropolitan counties compared to metropolitan counties (see Appendix Table 2 for newly diagnosed beneficiaries and Appendix Table 3 for decedents). As a result, adjusting for differences in age distribution between metropolitan, micropolitan, and rural counties alone reduces the gap in ADRD diagnoses (Appendix Figure 5). Second, it is possible that underdiagnosed ADRD is more prevalent in micropolitan and rural counties compared to metropolitan counties. Additionally, both Abner et al.28 and Weden et al.29 found fewer chronic conditions diagnosed in rural counties compared to metropolitan counties. In our data, we observed the number of chronic conditions to be about five percent lower despite higher healthcare utilization in rural beneficiaries compared to the urban beneficiaries (Tables 1 and 2). Abner et al.28 interpreted this lower comorbidity as underdiagnosis of chronic conditions in nonmetropolitan counties. As in the prior studies, we included the number of chronic conditions as a risk-adjusting variable because it is an important predictor of ADRD diagnosis. Additionally, because both ADRD and chronic conditions are likely to be underreported in rural counties, the bias in rural-urban difference in ADRD due to underreporting is likely to be smaller in the adjusted model when the number of chronic conditions is included as a risk adjustor.
The most perplexing aspect of our findings is that we observed lower diagnostic prevalence of ADRD in rural counties despite higher diagnostic incidence. This may be partially explained by the shorter time to death following ADRD diagnosis in rural counties. It may be that individuals in rural counties tend to be diagnosed at later stages of dementia because they have fewer encounters with healthcare providers, and thus fewer opportunities for a diagnosis to be made. Still, average age at ADRD diagnosis is about six months lower in rural counties compared to urban counties. Additionally, lower life expectancy in rural counties reduces the likelihood of ever having ADRD.6,8 That is, beneficiaries in rural counties may die of other causes before they can develop and/or be diagnosed with ADRD.
Our study has several limitations. First, we acknowledge that rurality is a continuous concept and we stratified rurality into three categories to simplify our presentation. We did not examine more detailed rurality categories because our findings for micropolitan and rural counties were similar. Second, the use of claims data to identify ADRD cases is a far less precise approach than has been undertaken in existing prospective cohort studies like ADAMS, the Framingham Heart Study, or the Health and Retirement Study in which participants receive comprehensive screenings and must meet specific diagnostic criteria to be considered a case. We recognize that there are presumably false-positive and false-negative ADRD cases in our sample; however, we chose this approach in order to be able to identify diagnostic patterns across a broad sample of individuals outside of research settings. Third, we excluded Medicare Advantage enrollees because of the lack of data. If ADRD diagnosis is associated with Medicare Advantage enrollment, lower Medicare Advantage penetration in rural counties may bias our estimates. We tried to minimize this bias by including Medicare Advantage penetration of beneficiary’s residential zip code in our models. Finally, we cannot measure many risk factors that may be associated with ADRD and vary by rurality such as life expectancy, education, and healthcare access.
In conclusion, we found higher adjusted diagnostic incidence, but not unadjusted diagnostic incidence in rural counties compared to metropolitan counties, suggesting underdiagnosis of ADRD in rural areas. We also found lower diagnostic prevalence and shorter time to death following ADRD diagnosis in rural counties compared to metropolitan counties, suggesting that rural beneficiaries may be diagnosed at later stages of disease. These rural-urban disparities raise unique challenges for policymakers given the declining availability of formal support services2,10,11 and primary care providers7,31 in rural areas. Further research is needed to better understand the factors driving underdiagnosis of ADRD among rural residents.
RESEARCH IN CONTEXT.
Systematic review: Rural-urban differences in the prevalence of Alzheimer’s disease and related dementias (ADRD) have been examined in only two previous studies, cited in this article. One found higher prevalence in rural versus urban communities in 2000 but no difference in 2010, based on self and proxy reporting, while another using Medicare data found lower adjusted diagnostic prevalence in rural compared with urban counties.
Interpretation: Following the latter study, we expected lower diagnostic ADRD incidence and prevalence in rural areas in Medicare data due to underdiagnosis, since the relative risk of dementia is similar, if not higher, in rural versus urban communities.
Future directions: These results will inform efforts to improve ADRD screening to ensure timely diagnosis for older rural adults, so they may access supportive services and plan for long-term care needs, and will also provide data and background for larger studies to develop strategies to reduce rural-urban disparities.
Highlights.
ADRD diagnostic incidence was higher in rural vs. metropolitan counties.
ADRD diagnostic prevalence was lower in rural vs. metropolitan counties.
The share of decedents with ADRD diagnosis was lower in rural vs. metropolitan counties.
Survival following ADRD diagnosis was lower in rural vs. metropolitan counties
ACKNOWLEDGMENTS
This project was funded by grants from the National Institute on Aging, No. R03 AG054687-01 and No. P01AG027296.
APPENDIX
FIGURE A1.
Alzheimer’s disease and related dimentias (ADRD) incidence ratios among Medicare fee-for-service (FFS) beneficiaries
FIGURE A2.
Alzheimer’s disease and related dimentias (ADRD) prevalence ratios among Medicare fee-for-service (FFS) beneficiaries
FIGURE A3.
Diagnostic incidence and prevalence of Alzheimer’s disease and related dimentias (ADRD) among Medicare fee-for-service (FFS) beneficiaries in urban and rural counties in 2015 across different regions of the US. (A) Percent of Medicare FFS beneficiaries newly diagnosed with ADRD in 2015. (B) Percent of Medicare FFS beneficiaries that started 2015 with an ADRD diagnosis
FIGURE A4.
Alzheimer’s disease and related dimentias (ADRD) prevalence ratios among deceased Medicare fee-for-service (FFS) beneficiaries
FIGURE A5.
Diagnostic incidence and prevalence of Alzheimer’s disease and related dimentias (ADRD) among Medicare fee-for-service (FFS) beneficiaries in 2015 with different levels of risk adjustment. (A) Percent of Medicare FFS beneficiaries newly diagnosed with ADRD in 2015. (B) Percent of Medicare FFS beneficiaries that started 2015 with an ADRD diagnosis
TABLE A1.
Rurality categories of counties
| RUCC 2013 | Description | Category |
|---|---|---|
| 1 | Metro - Counties in metro areas of 1 million population or more | Metro |
| 2 | Metro - Counties in metro areas of 250,000 to 1 million population | Metro |
| 3 | Metro - Counties in metro areas of fewer than 250,000 population | Metro |
| 4 | Nonmetro - Urban population of 20,000 or more, adjacent to a metro area | Micro |
| 5 | Nonmetro - Urban population of 20,000 or more, not adjacent to a metro area | Micro |
| 6 | Nonmetro - Urban population of 2500 to 19,999, adjacent to a metro area | Micro |
| 7 | Nonmetro - Urban population of 2500 to 19,999, not adjacent to a metro area | Micro |
| 8 | Nonmetro - Completely rural or less than 2500 urban population, adjacent to a metro area | Rural |
| 9 | Nonmetro - Completely rural or less than 2500 urban population, not adjacent to a metro area | Rural |
Abbreviation: RRUC, rural urban continuum code.
TABLE A2.
Characteristics of Medicare beneficiaries enrolled in fee-for-service Medicare between 2008 and 2015 by ADRD status
| ADRD Status |
||||||
|---|---|---|---|---|---|---|
| No ADRD diagnosis |
Newly diagnosed with ADRD in the observation year |
Started the observation year with ADRD diagnosis |
||||
| Metro | Micro/Rural | Metro | Micro/Rural | Metro | Micro/Rural | |
| Person-years (N) | 111,000,409 | 35,626,357 | 3,758,537 | 1,131,654 | 14,865,407 | 4,173,591 |
| Age in calendar year, mean | 75.9 | 75.6 | 81.9 | 81.4 | 83.3 | 82.9 |
| Female (%) | 57.1% | 55.4% | 62.1% | 61.2% | 67.2% | 67.0% |
| Black (%) | 7.3% | 4.4% | 8.5% | 5.7% | 9.9% | 6.7% |
| Hispanic (%) | 5.1% | 2.1% | 5.0% | 2.0% | 5.8% | 2.2% |
| Other race (%) | 3.8% | 1.8% | 3.0% | 1.6% | 3.1% | 1.5% |
| Dual eligible (%) | 9.9% | 11.6% | 17.6% | 22.7% | 27.3% | 32.6% |
| No. of Chronic Conditions, mean | 3.77 | 3.56 | 7.47 | 7.10 | 6.77 | 6.40 |
| HCC score, mean | 0.59 | 0.59 | 0.91 | 0.89 | 1.08 | 1.03 |
| No. of Hospitalization Days, mean | 0.22 | 0.25 | 0.48 | 0.50 | 0.69 | 0.69 |
| No. of Nursing Home Days, mean | 2.09 | 2.95 | 11.02 | 15.43 | 60.13 | 75.50 |
| No. of Home Assessments, mean | 0.15 | 0.15 | 0.50 | 0.51 | 0.74 | 0.69 |
| Residential zip code Medicare Advantage penetration (%) | 23.0% | 14.6% | 23.2% | 14.4% | 23.5% | 14.6% |
| Residential zip code dual eligible rate (%) | 16.0% | 18.7% | 16.9% | 19.6% | 17.4% | 19.9% |
Note: These mean values are based on beneficiary year level data. Number of chronic conditions is calculated from the chronic condition warehouse (CCW) data segment of the beneficiary summary file. Community based HCC score was calculated using part-A claims data using algorithm provided by the Centers for Medicare and Medicaid Services. Care use rates are calculated using residential history algorithm applying claims and assessment data from the previous year.
Abbreviations: ADRD, Alzheimer’s disease and related dementias; HCC, hierarchical chronic conditions.
TABLE A3.
Characteristics of Medicare beneficiaries who died between 2008 and 2015 with and without ADRD diagnosis
| Non-ADRD |
ADRD |
|||
|---|---|---|---|---|
| Metro | Micro/Rural | Metro | Micro/Rural | |
| Person-years (N) | 4,061,189 | 1,398,525 | 3,548,422 | 1,041,717 |
| Age at death, mean | 80.1 | 79.6 | 85.7 | 85.2 |
| Female (%) | 50.3% | 48.2% | 62.8% | 62.2% |
| Black (%) | 7.9% | 4.8% | 9.1% | 6.2% |
| Hispanic (%) | 4.5% | 1.8% | 4.4% | 1.8% |
| Other race (%) | 2.8% | 1.8% | 2.3% | 1.4% |
| Dual eligible (%) | 15.6% | 19.9% | 27.1% | 34.2% |
| No. of Chronic Conditions, mean | 5.59 | 5.38 | 7.19 | 6.84 |
| HCC score, mean | 1.36 | 1.28 | 1.44 | 1.33 |
| No. of Hospitalization Days, mean | 0.88 | 0.91 | 0.99 | 0.97 |
| No. of Nursing Home Days, mean | 16.83 | 20.78 | 81.59 | 99.15 |
| No. of Home Assessments, mean | 0.64 | 0.63 | 0.89 | 0.82 |
| Residential zip code Medicare Advantage penetration (%) | 23.7% | 15.1% | 23.6% | 14.8% |
| Residential zip code dual eligible rate (%) | 16.8% | 19.2% | 17.0% | 19.9% |
| ADRD-specific variables | ||||
| Age at diagnosis | 82.9 | 82.5 | ||
| No. of days survived following ADRD diagnosis | 1383.4 | 1339.3 | ||
Note: These mean values are based on beneficiary year level data. Number of chronic conditions is calculated from the chronic condition warehouse (CCW) data segment of the beneficiary summary file. Community based HCC score was calculated using part-A claims data using algorithm provided by the Centers for Medicare and Medicaid Services. Care use rates are calculated using residential history algorithm applying claims and assessment data from the previous year.
Abbreviations: ADRD, Alzheimer’s disease and related dementias; HCC, hierarchical chronic conditions.
TABLE A4.
An illustration of how we calculated risk adjusted diagnostic incidence and prevalence
| For each year, risk-adjusted incidence and prevalence are calculated using two steps. | ||||||
| Step 1: Estimate a multinomial logit model of Alzheimer’s disease and related dementias (ADRD) diagnosis status (variable name adrd: 0 = no ADRD diagnosis, 1 = newly diagnosed with ADRD, 2 = started year with ADRD diagnosis) onto explanatory variables listed in Table 1 and rurality categories (variable name: F1406709; 1 = metropolitan, 2 = micropolitan, and 3 = rural). Below is the estimation for 2015. Here the control variables are the same as reported in Table 1 (Age in 2015, female indicator, indicators of different races: Black, Hispanic and other race, dual eligibility indicator, number of chronic conditions, hierarchical chronic conditions (HCC) score, number of hospitalized days, number of nursing home days, number of home health assessments, Medicare Advantage penetration in residential zip code and share of dual eligible in residential zip code). | ||||||
| . mlogit adrd age hksex black hispanic orace dual No_CCW_CC HCC_Community Hosp_count_PY Total_NH_Days_PY HHA_Assmnt_PY shma shdual i.F1406709 | ||||||
| Iteration 0: log likelihood = −9990884.3 | ||||||
| Iteration 1: log likelihood = −7959847.3 | ||||||
| Iteration 2: log likelihood = −7791967.1 | ||||||
| Iteration 3: log likelihood = −7363992.1 | ||||||
| Iteration 4: log likelihood = −7346301.1 | ||||||
| Iteration 5: log likelihood = −7341857.7 | ||||||
| Iteration 6: log likelihood = −7341854.9 | ||||||
| Iteration 7: log likelihood = −7341854.9 | ||||||
| Multinomial logistic regression | Number of obs = 21,446,383 | |||||
| LR chi2(30) = 5298058.90 | ||||||
| Prob > chi2 = 0.0000 | ||||||
| Log likelihood = −7341854.9 | Pseudo R2 = 0.2651 | |||||
| Adrd | Coef. | Std. Err. | z | P > ∣z∣ | [95% Conf. | Interval] |
|---|---|---|---|---|---|---|
| 0 | (base outcome) | |||||
| 1 | ||||||
| Age | .0875532 | .0001844 | 474.86 | 0.000 | .0871918 | .0879146 |
| Hksex | .1150767 | .0028127 | 40.91 | 0.000 | .1095638 | .1205896 |
| Black | .1244485 | .0053954 | 23.07 | 0.000 | .1138736 | .1350234 |
| Hispanic | −.0981258 | .007341 | −13.37 | 0.000 | −.1125139 | −.0837378 |
| Orace | −.1037752 | .0083075 | −12.49 | 0.000 | −.1200577 | −.0874928 |
| Dual | .3544636 | .0040938 | 86.58 | 0.000 | .3464398 | .3624873 |
| No_CCW_CC | .4018247 | .0004737 | 848.32 | 0.000 | .4008964 | .4027531 |
| HCC_Community | −.1036427 | .002543 | −40.76 | 0.000 | −.1086269 | −.0986586 |
| Hosp_count_PY | −.1042952 | .002525 | −41.31 | 0.000 | −.109244 | −.0993463 |
| Total_NH_Days_PY | .0042147 | .0000371 | 113.46 | 0.000 | .0041419 | .0042875 |
| HHA_Assmnt_PY | .0571485 | .0012526 | 45.62 | 0.000 | .0546935 | .0596035 |
| Shma | −.0984688 | .0110809 | −8.89 | 0.000 | −.1201869 | −.0767507 |
| Shdual | −.0167419 | .0147715 | −1.13 | 0.257 | −.0456935 | .0122097 |
| F1406709 | ||||||
| 2 | .054408 | .0041051 | 13.25 | 0.000 | .0463622 | .0624538 |
| 3 | .1059648 | .0047921 | 22.11 | 0.000 | .0965726 | .1153571 |
| _cons | −12.67833 | .0158433 | −800.23 | 0.000 | −12.70938 | −12.64728 |
| 2 | ||||||
| Age | .1136594 | .0001113 | 1021.18 | 0.000 | .1134412 | .1138775 |
| Hksex | .1799482 | .0017415 | 103.33 | 0.000 | .1765349 | .1833615 |
| Black | .1991068 | .0032032 | 62.16 | 0.000 | .1928287 | .2053849 |
| Hispanic | .0013809 | .0041882 | 0.33 | 0.742 | −.0068278 | .0095896 |
| Orace | −.1715693 | .004912 | −34.93 | 0.000 | −.1811965 | −.161942 |
| Dual | .8023836 | .0023737 | 338.03 | 0.000 | .7977313 | .8070359 |
| No_CCW_CC | .2736333 | .0002981 | 918.06 | 0.000 | .2730491 | .2742175 |
| HCC_Community | −.1242536 | .0015052 | −82.55 | 0.000 | −.1272037 | −.1213036 |
| Hosp_count_PY | .0684595 | .0014101 | 48.55 | 0.000 | .0656956 | .0712233 |
| Total_NH_Days_PY | .0104315 | .0000203 | 514.02 | 0.000 | .0103917 | .0104713 |
| HHA_Assmnt_PY | .1978542 | .0007169 | 276.00 | 0.000 | .1964492 | .1992592 |
| Shma | .054728 | .0067072 | 8.16 | 0.000 | .041582 | .0678739 |
| Shdual | −.1246478 | .0089432 | −13.94 | 0.000 | −.1421761 | −.1071194 |
| F1406709 | ||||||
| 2 | −.0434924 | .0025471 | −17.08 | 0.000 | −.0484846 | −.0385003 |
| 3 | −.0575522 | .0030114 | −19.11 | 0.000 | −.0634545 | −.0516499 |
| _cons | −13.06908 | .0096544 | −1353.68 | 0.000 | −13.088 | −13.05016 |
| Step 2: Generate predicted probabilities of each ADRD diagnosis status for different categories of rurality using predictive margins | ||||||
| . margins F1406709 | ||||||
| Predictive margins | Number of obs = 21,446,383 | |||||
| Model VCE: OIM | ||||||
| 1._predict: Pr(adrd = = 0), predict(pr outcome(0)) | ||||||
| 2._predict: Pr(adrd = = 1), predict(pr outcome(1)) | ||||||
| 3._predict: Pr(adrd = = 2), predict(pr outcome(2)) | ||||||
| Delta-method | ||||||
| Margin | Std. Err. | z | P > ∣z∣ | [95% Conf. Interval] | ||
| _predict#F1406709 ∣ | ||||||
| 1 1 | .8645893 | .0000712 | 1.2e+04 | 0.000 | .8644497 | .8647288 |
| 1 2 | .8662672 | .0001715 | 5051.37 | 0.000 | .8659311 | .8666033 |
| 1 3 | .8660872 | .0002069 | 4186.10 | 0.000 | .8656816 | .8664927 |
| 2 1 | .0282892 | .0000397 | 712.16 | 0.000 | .0282113 | .028367 |
| 2 2 | .0300535 | .0001005 | 298.93 | 0.000 | .0298565 | .0302506 |
| 2 3 | .031596 | .0001253 | 252.08 | 0.000 | .0313503 | .0318416 |
| 3 1 | .1071216 | .0000662 | 1619.33 | 0.000 | .1069919 | .1072512 |
| 3 2 | .1036793 | .0001588 | 653.04 | 0.000 | .1033681 | .1039905 |
| 3 3 | .1023169 | .0001901 | 538.35 | 0.000 | .1019444 | .1026894 |
Note: We performed these two steps for each year. We then plotted these predictive margins for a given outcome by year.
TABLE A5.
Detailed results of Cox regression for survival analysis rurality categories (variable name: F1406709; 1 = metropolitan, 2 = micropolitan, and 3 = rural). Here the control variables are the same as reported in Table 1 (Age in 2008, female indicator, indicators of different races: Black, Hispanic and other race, dual eligibility indicator, number of chronic conditions, hierarchical chronic conditions (HCC) score, number of hospitalized days, number of nursing home days, number of home health assessments, Medicare Advantage penetration in residential zip code and share of dual eligible in residential zip code)
| .stcox i.F1406709 age hksex black hispanic orace dual No_CCW_CC_HCC_Community Hosp_count_PY Total_NH_Days_PY HHA_Assmnt_PY shma shdual | ||||||
| failure _d: surv | ||||||
| analysis time_t: surv_days | ||||||
| Iteration 0: log likelihood= −7079801.6 | ||||||
| Iteration 1: log likelihood= −6999203.4 | ||||||
| Iteration 2: log likelihood= −6995488.5 | ||||||
| Iteration 3: log likelihood= −6995383 | ||||||
| Iteration 4: log likelihood= −6995382.8 | ||||||
| Refining estimates: | ||||||
| Iteration 0: log likelihood = −6995382.8 | ||||||
| Cox regression - Breslow method for ties | ||||||
| No. of subjects = 655,440 | Number of obs = 655,440 | |||||
| No. of failures = 556,136 | ||||||
| Time at risk =1031695650 | ||||||
| LR chi2(15)= 168837.53 | ||||||
| Log likelihood = −6995382.8 | Prob > chi2 = 0.0000 | |||||
| _t | Haz. Ratio | Sth. Err. | Z | P > ∣z∣ | [95% Conf. Interval] | |
|---|---|---|---|---|---|---|
| F1406709 | ||||||
| 2 | 1.004739 | .0056723 | 0.84 | 0.402 | .9936823 | 1.015918 |
| 3 | .9252037 | .004421 | −16.27 | 0.000 | .9165793 | .9339094 |
| Age | 1.060842 | .0002143 | 292.35 | 0.000 | 1.060422 | 1.061262 |
| Hksex | .7304632 | .0020683 | −110.92 | 0.000 | .7264207 | .7345282 |
| black | .9155114 | .0048876 | −16.53 | 0.000 | .9059818 | .9251413 |
| hispanic | .7045382 | .0054185 | −45.54 | 0.000 | .6939978 | .7152386 |
| orace | .6962504 | .006804 | −37.05 | 0.000 | .6830418 | .7097145 |
| dual | 1.030797 | .0040088 | 7.80 | 0.000 | 1.02297 | 1.038684 |
| No_CCW_CC | 1.063543 | .0005535 | 118.38 | 0.000 | 1.062459 | 1.064629 |
| HCC_Community | 1.307859 | .0035458 | 99.00 | 0.000 | 1.300928 | 1.314828 |
| Hosp_count_PY | .9816694 | .0017256 | -10.52 | 0.000 | .9782931 | .9850574 |
| Total_NH_Days_PY | 1.001207 | .0000206 | 58.51 | 0.000 | 1.001167 | 1.001248 |
| HHA_Assmnt_PY | 1.039136 | .0011753 | 33.94 | 0.000 | 1.036835 | 1.041442 |
| shma | 1.012407 | .0120466 | 1.04 | 0.300 | .9890693 | 1.036295 |
| shdual | .875022 | .0125622 | −9.30 | 0.000 | .8507438 | .899993 |
Footnotes
CONFLICT OF INTEREST
The authors declare there are no conflicts of interest or financial disclosures.
REFERENCES
- 1.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1-2):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meit M, Knudson A, Gilbert T, et al. The 2014 Update of the Rural-Urban Chartbook. Rural Health Reform Policy Research Center; 2014. [Google Scholar]
- 3.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. [DOI] [PubMed] [Google Scholar]
- 4.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin B Assessing Rural Health Disparities. Delmarva Foundation for Medical Care; 2014. [Google Scholar]
- 6.Singh GK, Siahpush M. Widening rural-urban disparities in all-cause mortality and mortality from major causes of death in the USA, 1969-2009. J Urban Health. 2014;91(2):272–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu S, Berkowitz SA, Phillips RL, Bitton A, Landon BE, Phillips RS. Association of Primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am J Prev Med. 2014;46(2):e19–29. [DOI] [PubMed] [Google Scholar]
- 9.Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387. [Google Scholar]
- 10.Vanderboom CP, Madigan EA. Relationships of rurality, home health care use, and outcomes. West J Nurs Res. 2008;30(3):365–384. [DOI] [PubMed] [Google Scholar]
- 11.Innes A, Morgan D, Kosteniuk J. Dementia care in rural and remote settings: a systematic review of informal/family caregiving. Maturitas. 2011;68(1):34–46. [DOI] [PubMed] [Google Scholar]
- 12.Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: studying nursing home residents’ long-term care histories. Health Serv Res. 2011;46(1 Pt 1):120–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman M, Norton EC, Grabowski DC. Do hospital-owned skilled nursing facilities provide better post-acute care quality?. J Health Econ. 2016;50:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor DH Jr, Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E, Gatz M, Tseng C, et al. Evaluation of Medicare claims data as a tool to identify dementia. J Alzheimers Dis. 2019;67(2):769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu CW, Ornstein KA, Cosentino S, Gu Y, Andrews H, Stern Y. Misidentification of dementia in medicare claims and related costs. J Am Geriatr Soc. 2019;67(2):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinn JS, Mor V. Nursing home special care units: distribution by type, state, and facility characteristics. Gerontologist. 1994;34(3):371–377. [DOI] [PubMed] [Google Scholar]
- 18.Banaszak-Holl J, Zinn JS, Mor V. The impact of market and organizational characteristics on nursing care facility service innovation: a resource dependency perspective. Health Serv Res. 1996;31(1):97–117. [PMC free article] [PubMed] [Google Scholar]
- 19.Hall MJ, Owings M. Rural and Urban Hospitals’ Role in Providing Inpatient Care, 2010. National Center for Health Statistics; 2014. NCHS Data Brief No. 147. [PubMed] [Google Scholar]
- 20.Medicare Payment Advisory Commission. Report to the Congress: Medicare and the Health Care Delivery System. MedPAC. 2012. [Google Scholar]
- 21.Meyers DJ, Mor V, Rahman M. Medicare Advantage enrollees more likely to enter lower-quality nursing homes compared to fee-for-service enrollees. Health Aff (Millwood). 2018;37(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Rahman M, Gozalo P, Keohane LM, Gold MR, Trivedi AN. Regional variations: the use of hospitals, home health, and skilled nursing in traditional Medicare and Medicare Advantage. Health Aff (Millwood). 2018;37(8):1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor CA, Greenlund SF, McGuire LC, Lu H, Croft JB. Deaths from Alzheimer’s disease - United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2017;66(20):521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero JP, Benito-León J, Louis ED, Bermejo-Pareja F. Under reporting of dementia deaths on death certificates: a systematic review of population-based cohort studies. J Alzheimers Dis. 2014;41(1):213–221. [DOI] [PubMed] [Google Scholar]
- 27.Ody C, Msall L, Dafny LS, Grabowski DC, Cutler DM. Decreases in readmissions credited to Medicare’s program to reduce hospital readmissions have been overstated. Health Aff (Millwood). 2019;38(1):36–43. [DOI] [PubMed] [Google Scholar]
- 28.Abner EL, Jicha GA, Christian WJ, Schreurs BG . Rural-Urban Differences in Alzheimer’s Disease and Related Disorders Diagnostic Prevalence in Kentucky and West Virginia. J Rural Health. 2016;32(3):314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weden MM, Shih RA, Kabeto MU, Langa KM. Secular trends in dementia and cognitive impairment of U.S. rural and urban older adults. Am J Prev Med. 2018;54(2):164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i162–i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenblatt RA, Hart LG. Physicians and rural America. West J Med. 2000;173(5):348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]