Abstract
Background:
There is limited information about outcomes associated with stopping asthma biologics.
Objective:
To compare outcomes in people who stopped or continued asthma biologics.
Methods:
We identified a cohort of people with asthma who stopped or continued asthma biologics in the Optum Labs Database Warehouse, using a propensity matching method for case and control groups with the variables age, sex, race, region, insurance, income, specialist access, Charlson comorbidity, specific medical conditions, pre-index exacerbation count, pre-index rescue inhaler pharmacy fills, and pre-index inhaled corticosteroid +/− long-acting β-agonist pharmacy fills. The primary outcome used to assess failure of stopping was an increase of 50% or more in the asthma exacerbation rate in the six months after discontinuing the biologic compared to the six -month period before biologic initiation.
Results:
Among a cohort of 4,960 asthma biologic users, 1,249 were observed to stop use after 6–12 months of use. We identified a matched cohort of 1,247 stoppers and 1,247 people who continued biologic use for at least 18 months. In the first 6 months after stopping/sham stopping, 10.2% of stoppers and 9.5% of continuers had an increase of 50% or more in asthma exacerbations. We found a similar adjusted odds of failing among stoppers and continuers [OR: 1.085; (95% CI 0.833 to 1.413)].
Conclusion:
An increase in asthma exacerbations is infrequently observed in people who stopped asthma biologics and was observed at similar rates as in matched controls who continued asthma biologics.
Keywords: asthma, biologics, monoclonal antibodies, step down treatment
INTRODUCTION
Asthma is a common, costly, and sometimes fatal disease.1–3 Medications can help individuals control asthma,4 with the leading goal of medication management being to use the least amount and safest medication to control symptoms and reduce risks of exacerbations. One strategy to achieve this goal involves “stepping down” asthma medications when disease stability permits, which could include stopping the medication, reducing the frequency of use, or reducing the dose. Current asthma guidelines suggest that asthma controller medications can be stepped down after 3–12 months of disease stability.4 Studies of stepping down asthma medications have focused primarily on inhaled corticosteroids and long-acting β-agonists (ICS/LABA).5
Asthma biologics are a class of asthma controller medications that are produced from living organisms. The 5 currently FDA-approved biologic medications for asthma are omalizumab (approved 2003), mepolizumab (2015), reslizumab (2016), benralizumab (2017), and dupilumab (2017). Recent guidelines address many key questions surrounding when to start and how to select asthma biologic medications.6 However, there is limited information on outcomes associated with stepping down asthma biologic medications.7–12 We compared individuals who stopped to those who continued asthma biologics, using an observational health insurance claims data set derived from clinical practice in a broad variety of settings in the United States.
METHODS
We used data from the OptumLabs Data Warehouse (OLDW), a database of longitudinal administrative health data including medical and pharmacy claims, laboratory results, and enrollment records for commercial and Medicare Advantage (MA) enrollees from across the United States. We identified a cohort of people with asthma from February 2003 to June 2020 using a modified version of the Healthcare Effectiveness Data and Information Set (HEDIS) definition of persistent asthma.13 The cohort includes people who had a) at least one ED visit or inpatient encounter with a principal diagnosis of asthma, or b) at least 4 outpatient encounters on different days with an asthma diagnosis (any position) plus at least two asthma medication fills, or c) at least four asthma medication fills in a rolling 365 -day period. For criterion c), if all asthma medication fills were for leukotriene modifiers or antibody inhibitors, at least 1 asthma diagnosis for any type of visit (ED, inpatient, or outpatient) was also required.
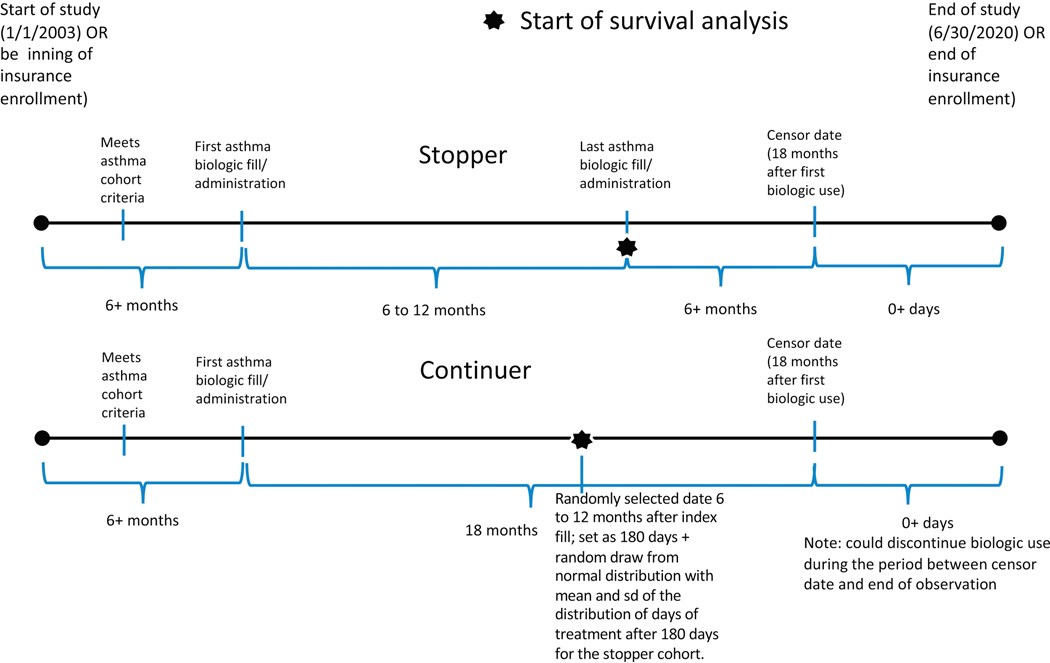
From the cohort of people with asthma, we identified those who had at least one administration or pharmacy fill of one of the five asthma biologic drugs. We created episodes of biologic use, starting with the first observed administration or pharmacy fill of a particular biologic drug, and continuing until the patient had a break of 120+ days in treatment or had filled or been administered a different asthma biologic drug. To ensure we were capturing the beginning of the biologic use episode, we required 6 months of medical and pharmacy coverage with no asthma biologic claims prior to the start of the episode. To limit bias from differential periods of follow-up, we censored all people at 18 months after their index date (first administration/fill of an asthma biologic). See Supplementary Materials for a diagram illustrating these requirements (Figure E1).
We excluded biologic use episodes that lasted less than 6 months, which is generally considered an adequate trial period for people who can tolerate a biologic treatment. We then categorized patients into stoppers and continuers. Stoppers discontinued biologic use after 6 to 12 months of treatment and had 6+ months of follow-up after discontinuing. Continuers remained on biologic treatment for at least 18 months (see Table 1 for description of full cohort).
Table 1.
Cohort description
| Full Cohort | After Propensity Matching (k=0.2, w/o replacement) | Standardized Differences* | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Continuer | Stopper | p value | Continuer | Stopper | p value | Full Cohort | Matched Cohort |
| N | 3,104 | 1,410 | 1,394 | 1,394 | ||||
| Days on Treatment, Mean (SD) | 547 (0) | 263.35 (54.1) | <0.0001 | 547 (0) | 263.64 (54.22) | <0.0001 | 7.414 | 7.391 |
| Pre-Index Exacerbation Count, Mean (SD) | 0.65 (0.96) | 0.67 (0.96) | 0.5806 | 0.67 (0.97) | 0.66 (0.96) | 0.9843 | 0.018 | 0.001 |
| Pre-Index Severe Exacerbation Count Mean (SD) | 0.18 (0.53) | 0.19 (0.52) | 0.599 | 0.21 (0.58) | 0.19 (0.51) | 0.3325 | 0.017 | 0.037 |
| Age, Mean (SD) | 47.48 (17.03) | 52.38 (17.37) | <0.000 1 | 52.03 (17.64) | 52.32 (17.42) | 0.6571 | 0.285 | 0.017 |
| Age Group | ||||||||
| 0–17 | 242 (7.8) | 65 (4.6) | <0.000 1 | 84 (6) | 65 (4.7) | 0.2774 | 0.132 | 0.061 |
| 18–64 | 2,399 (77.3) | 945 (67) | 919 (65.9) | 934 (67) | 0.231 | 0.023 | ||
| 65+ | 463 (14.9) | 400 (28.4) | 391 (28) | 395 (28.3) | 0.331 | 0.006 | ||
| Sex | ||||||||
| Female | 1,902 (61.3) | 860 (61) | 0.8566 | 852 (61.1) | 850 (61) | 0.9381 | 0.006 | 0.003 |
| Male | 1,202 (38.7) | 550 (39) | 542 (38.9) | 544 (39) | 0.006 | 0.003 | ||
| Race | ||||||||
| Unknown/Missing | 127 (4.1) | 88 (6.2) | 0.0025 | 74 (5.3) | 79 (5.7) | 0.9556 | 0.097 | 0.016 |
| Asian | 104 (3.4) | 53 (3.8) | 54 (3.9) | 53 (3.8) | 0.022 | 0.004 | ||
| Black | 291 (9.4) | 159 (11.3) | 157 (11.3) | 159 (11.4) | 0.063 | 0.005 | ||
| Hispanic | 245 (7.9) | 115 (8.2) | 106 (7.6) | 115 (8.2) | 0.010 | 0.024 | ||
| White | 2,337 (75.3) | 995 (70.6) | 1,003 (72) | 988 (70.9) | 0.106 | 0.024 | ||
| Region | ||||||||
| Midwest | 796 (25.6) | 301 (21.3) | 0.0039 | 310 (22.2) | 300 (21.5) | 0.6591 | 0.101 | 0.017 |
| Northeast | 354 (11.4) | 174 (12.3) | 151 (10.8) | 171 (12.3) | 0.029 | 0.045 | ||
| South | 1,460 (47) | 728 (51.6) | 709 (50.9) | 719 (51.6) | 0.092 | 0.014 | ||
| West | 492 (15.9) | 204 (14.5) | 222 (15.9) | 202 (14.5) | 0.039 | 0.040 | ||
| Insurance | ||||||||
| Commercial | 2,550 (82.2) | 862 (61.1) | <0.000 1 | 878 (63) | 861 (61.8) | 0.5063 | 0.480 | 0.025 |
| MedicareAdvantage | 554 (17.8) | 548 (38.9) | 516 (37) | 533 (38.2) | 0.480 | 0.025 | ||
| Household Income | ||||||||
| <$40,000 | 420 (13.5) | 277 (19.6) | <0.000 1 | 268 (19.2) | 274 (19.7) | 0.7764 | 0.165 | 0.011 |
| $40,000-$74,999 | 632 (20.4) | 316 (22.4) | 337 (24.2) | 314 (22.5) | 0.050 | 0.039 | ||
| $75,000–124,999 | 766 (24.7) | 333 (23.6) | 314 (22.5) | 333 (23.9) | 0.025 | 0.032 | ||
| $125,000$199,999 | 534 (17.2) | 158 (11.2) | 170 (12.2) | 158 (11.3) | 0.172 | 0.027 | ||
| $200,000+ | 428 (13.8) | 135 (9.6) | 139 (10) | 135 (9.7) | 0.131 | 0.010 | ||
| Unknown/Missing | 324 (10.4) | 191 (13.5) | 166 (11.9) | 180 (12.9) | 0.096 | 0.030 | ||
| Allergist/Pulmonologist visit | 2,542 (81.9) | 1,198 (85) | 0.0112 | 1,172 (84.1) | 1,183 (84.9) | 0.5652 | 0.083 | 0.022 |
| Charlson Conditions** | ||||||||
| 0–1 | 2,416 (77.8) | 973 (69) | <0.000 1 | 986 (70.7) | 970 (69.6) | 0.8031 | 0.201 | 0.025 |
| 2–3 | 510 (16.4) | 302 (21.4) | 281 (20.2) | 292 (20.9) | 0.128 | 0.020 | ||
| 4+ | 178 (5.7) | 135 (9.6) | 127 (9.1) | 132 (9.5) |
0.145 | 0.012 | ||
| Baseline Comorbidities | ||||||||
| Chronic Urticaria | 302 (9.7) | 155 (11) | 0.1921 | 145 (10.4) | 152 (10.9) | 0.6674 | 0.041 | 0.016 |
| Atopic Dermatitis | 747 (24.1) | 277 (19.6) | 0.001 | 284 (20.4) | 277 (19.9) | 0.7409 | 0.107 | 0.013 |
| GERD | 1,368 (44.1) | 627 (44.5) | 0.8039 | 631 (45.3) | 626 (44.9) | 0.8491 | 0.008 | 0.007 |
| Rhinitis | 2,493 (80.3) | 1,063 (75.4) | 0.0002 | 1,039 (74.5) | 1,056 (75.8) | 0.4563 | 0.119 | 0.028 |
| Sinusitis | 1,938 (62.4) | 789 (56) | <0.000 1 | 789 (56.6) | 784 (56.2) | 0.8485 | 0.132 | 0.007 |
| COPD | 929 (29.9) | 536 (38) | <0.000 1 | 513 (36.8) | 526 (37.7) | 0.6106 | 0.171 | 0.019 |
| Depression | 828 (26.7) | 426 (30.2) | 0.0139 | 415 (29.8) | 418 (30) | 0.9012 | 0.078 | 0.005 |
| Number of Reliever Fills in 6 months pre, Mean (SD) | 2.09 (2.82) | 2.31 (3.07) | 0.0206 | 2.24 (3.01) | 2.28 (3.05) | 0.6709 | 0.073 | 0.016 |
| ICS/LABA MPR in 6 months pre index, Mean (SD) | 0.37 (0.37) | 0.37 (0.37) | 0.9701 | 0.36 (0.37) | 0.37 (0.37) | 0.9027 | 0.001 | 0.005 |
| Response to biologic (% reducing exacerbations by 50% after 6 months biologic use) | 861 (27.7) |
474 (33.6) | <0.000 1 | 387 (27.8) | 468 (33.6) | 0.0009 | 0.128 | 0.126 |
| Response to biologic (% reducing severe exacerbations by 50% after 6 months biologic use) | 361 (11.6) | 165 (11.7) | 0.9443 | 181 (13) | 164 (11.8) | 0.3282 | 0.002 | 0.037 |
Standardized differences are commonly provided to quantify the success of propensity score matching in reducing differences between two cohorts. The standardized difference is the mean difference divided by its standard deviation.
Analyses included binary indicator variables for each Charlson condition. Proportions with each condition are reported in Supplemental Table E1.
We estimated consistency of ICS/LABA controller medication use by calculating a medication possession ratio (MPR) over the six months prior to index asthma biologic use. The MPR was calculated as the sum of days supplied for use during the period divided by the number of days in the period; previously filled medications were carried over from prior fills, and medications available for use after the period ended were excluded. We assessed whether patients had accessed specialist care for asthma by looking for one or more visits to a pulmonologist or allergist in the 6 months before index biologic use.
Treatment response in the initial 6 months of biologic use was defined as a reduction of at least 50% in the number of exacerbations, compared to the six months prior to index biologic use. People with no exacerbations during the pre-period could not achieve treatment response, by this definition. Exacerbations were defined as a hospital or emergency department visit with asthma in first diagnostic position (or second if another respiratory diagnosis was in the first position), or a systemic corticosteroid fill associated with an outpatient visit. In secondary analyses, we looked only at severe exacerbations, defined as hospital or emergency department only (i.e., excluding those who met exacerbation criteria by outpatient systemic corticosteroid criterion).
Because the decision to stop or continue biologic use was not random, we used propensity score matching to create a matched cohort of continuers and stoppers balanced on observed characteristics other than treatment time. We estimated propensity scores for stopping treatment, with the following variables included in the propensity score equation: pre-index exacerbation count, age, sex, race/ethnicity, geographic region, insurance type (commercial vs. Medicare Advantage [MA]), household income, allergist or pulmonologist visit, flags for each Charlson comorbidity, chronic idiopathic urticaria, atopic dermatitis, GERD, rhinitis, sinusitis, COPD, the number of pre-index reliever fills, and pre-index ICS/LABA MPR. Charlson conditions were assessed using claims from the 6 months prior to the index date, requiring a single instance of one of the diagnoses defining the conditions.14–16 We selected a matched cohort of continuers and stoppers with adequate propensity score overlap for analysis of outcomes after biologic discontinuation. One-to-one nearest neighbor matching without replacement was used, with a caliper width of 0.2. We assigned continuers a sham “stopping date” to allow comparison with stoppers. The sham stopping date for continuers was calculated by assigning each continuer the same treatment time as the matched stopper.
To protect the anonymity of people whose insurance claims are included in the OLDW, researchers are not allowed to disclose any patient counts between 1 and 10. In the final step of cohort creation, a small number (<11) of people who stopped asthma biologic use were not matched to continuers. To allow presentation of as much information as possible throughout the rest of the manuscript, we have removed those people from all patient counts in the cohort flow and tables.
Outcomes
The primary outcome used to assess failure of discontinuation was an increase of 50% or more in the asthma exacerbation rate in the six months after discontinuing the biologic (or after the sham discontinuation date) compared to the six -month period before biologic initiation. The secondary outcomes were time to exacerbation for stoppers vs. continuers, with continuers assigned a sham end date as the index date for the analysis, as described below; and severe exacerbations, defined as exacerbations involving an ED visit or hospitalization.
Analysis
The primary outcome was assessed in the propensity-matched cohort, comparing the proportion of stoppers and continuers who had at least 50% more exacerbations in the 6 months after stopping/sham stopping compared to the six months before biologic treatment. The odds ratio was calculated, along with a 95% confidence interval, and McNemar’s chi-squared test was completed.A non-parametric analysis of the secondary outcome of time from stopping/sham stopping date to exacerbation was conducted, with Kaplan-Meier survival curves plotted for stoppers vs. continuers.Hypothesis tests were considered statistically significant if the p-value was less than 0.05; distributions of cohort characteristics were compared using t-tests for continuous variables, a two-sample test of proportions, or chi-squared tests for categorical variables with 3 or more levels. Analysis was performed using SAS v. 9.4 and R v. 3.6.3.
RESULTS
Cohort description
We identified a cohort of 4,958 biologic users with asthma, of whom 1,247 (25.1%) were observed to stop biologic use before being censored by the end of the study period or discontinuation of insurance enrollment (Table 1). Additional detail about the individual elements of the Charlson index are available in the supplement (Table E1). Because it was on the market the longest, omalizumab was the most frequently used drug (67.5%, N=3,347), followed by dupilumab (19.8%; N=980), mepolizumab (10.2%; N=503), benralizumab (1.7%; N=82), and reslizumab (0.9%; N=46). Stoppers were more likely to have MA insurance than continuers (MA patients were 22.0% of the stopper cohort and 18.8% of the continuer cohort). They were also more likely to have seen an allergist or pulmonologist in the pre-period (6 months before index biologic use), more likely to have COPD, and had slightly more reliever fills in the period 6 months prior to index biologic use. Stoppers and continuers were similar in their ICS/LABA use in the pre-period, as estimated by the MPR (0.33 [sd 0.36] vs. 0.36 [sd 0.37]; p=0.0580), but neither group had high adherence using that measure (Table 2). When treatment response was defined using all exacerbations, stoppers had somewhat higher rates of treatment response in the first six months of biologic use (31.6% compared to 25.6%, p<0.001). However, when looking only at severe exacerbations, stoppers and continuers had similar rates of treatment response (10.5% compared to 10.3%, p=0.8533).
Table 2:
Asthma severity indicators before, during, and after biologic treatment episode; includes N=3,711 continuers and N=1,247 stoppers
| Measure | Continuers: before biologic initiation (6 months) | Continuers: during biologic treatment* | Stoppers: before biologic initiation (6 months) | Stoppers: during biologic treatment* | Stoppers: after stopping (6 months) | |
|---|---|---|---|---|---|---|
| Exacerbations | Mean (SD) | 0.65 (0.96) | 0.36 (0.6) | 0.67 (0.97) | 0.5 (0.82) | 0.33 (0.69) |
| Median (Q1, Q3) | 0 (0, 1) | 0 (0, 0.33) | 0 (0, 1) | 0 (0, 0.77) | 0 (0, 0) | |
| ICS/LABA MPR | Mean (SD) | 0.37 (0.37) | 0.37 (0.36) | 0.37 (0.37) | 0.36 (0.37) | 0.32 (0.35) |
| Median (Q1, Q3) | 0.3 (0, 0.66) | 0.27 (0, 0.67) | 0.28 (0, 0.67) | 0.24 (0, 0.67) | 0.16 (0, 0.59) | |
| Reliever Fills | Mean (SD) | 2.09 (2.82) | 1.45 (2.15) | 2.31 (3.07) | 1.67 (2.64) | 1.37 (2.46) |
| Median (Q1, Q3) | 1 (0, 3) | 0.67 (0, 2.01) | 1 (0, 3) | 0.64 (0, 2.21) | 0 (0, 2) |
Presented as rate per 6 months across the treatment time
Descriptive analysis of response to treatment and discontinuation
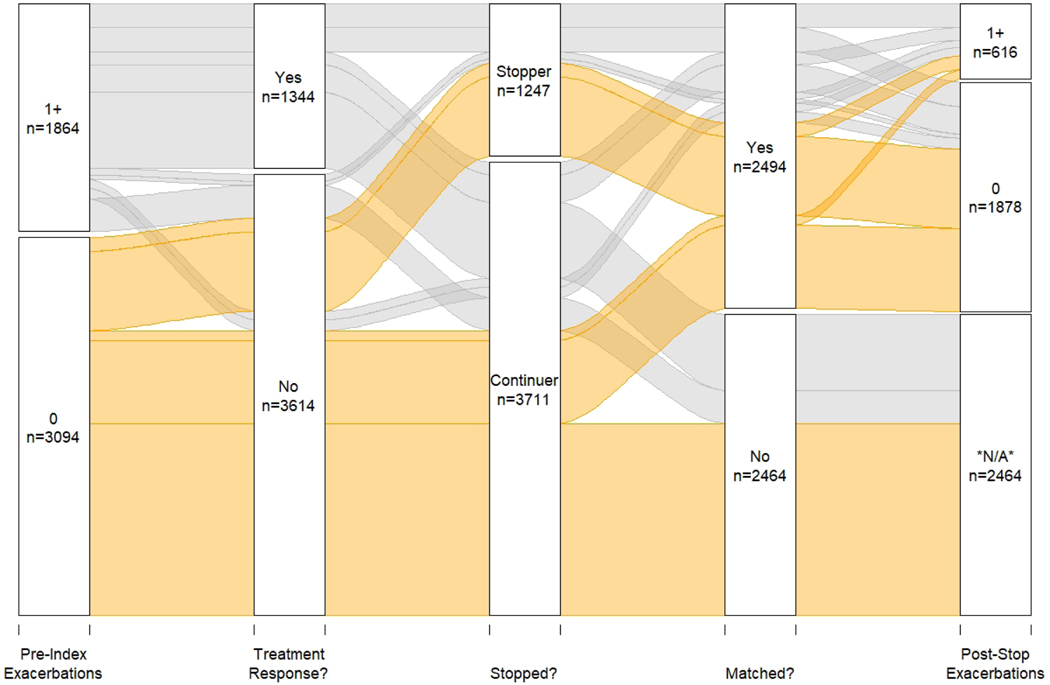
An alluvial flow diagram depicts the full cohort’s asthma biologic experience (Figure 1). Of 1,864 people who had one or more exacerbations in the 6 months before index biologic use, 1,344 (72.1%) achieved treatment response during their biologic treatment period of 6 or more months (a reduction of 50% or more in the 6-month rate of exacerbations). Of these, 950 (70.7%) continued biologic use until censored at 547 days (i.e., 18 months) after treatment initiation; 394 (29.3%) of treatment responders stopped biologic treatment after 6–12 months of treatment, averaging 257.1 days of treatment (sd 53.1).
Figure 1: Cohort experience with biologic treatment.
This alluvial flow diagram shows the full cohort’s experience. Treatment response is defined as having achieved a 50% or more decrease in exacerbations during 6-month treatment period. People are then sorted based on stopping, matching, and post-stopping asthma exacerbations.
Of the 520 patients with 1 or more asthma exacerbations in the pre-period who did not respond to treatment (i.e., did not achieve at least a 50% reduction in exacerbation rate during treatment), 91 (17.5%) stopped biologic treatment (mean 300.2 days of treatment [sd 45.4]), while 429 (82.5%) continued biologic treatment until censored at 547 days of treatment (i.e., 18 months).
Primary outcome analysis
Among all stoppers, 127 (10.2%) failed discontinuation of the asthma biologic in the 6 months after stopping, defined as an increase of 50% or more in exacerbations. In the propensity matched cohort, the odds of failing discontinuation were slightly higher among stoppers compared to continuers assessed after a sham end treatment stop date, but the difference was not statistically significant (OR: 1.087 CI: 0.825, 1.435). This OR reflects observed discontinuation failure rates of 10.2% of stoppers and 9.5% of continuers. In a secondary analysis using only severe exacerbations, stoppers and continuers had similar odds of discontinuation failure (OR: 1.216 CI: 0.770, 1.932).
We also conducted a sensitivity analysis with the propensity matched cohort that defined stepdown failure as any increase in asthma exacerbations compared to the pre-period. We found, similarly, that odds of failing discontinuation were slightly higher among stoppers compared to continuers assessed after a sham end treatment stop date, but the difference was not statistically significant (OR: 1.109 CI: 0.850, 1.448). This change in definition did not affect the analysis looking only at severe exacerbations, identifying the same number of treatment failures, and thus the same odds of discontinuation failure as the analysis defining treatment failure as a 50% increase in exacerbation rate (OR: 1.216 CI: 0.787, 1.879).
Finally, because asthma exacerbations have a seasonal component, we conducted a sensitivity analysis calculating 12-month exacerbation rates in the pre-period and in the period after stopping or sham stopping. After propensity score calculation, we were able to achieve matches for 625 stoppers. In the propensity matched cohort, the odds of failing discontinuation were slightly higher among stoppers compared to continuers assessed after a sham end treatment stop date, but the difference was not statistically significant (OR: 1.125 CI: 0.792, 1.601). Looking only at severe exacerbations, stoppers again had slightly higher but insignificant odds of discontinuation failure (OR: 1.500 CI: 0.890, 2.566) when compared to continuers.
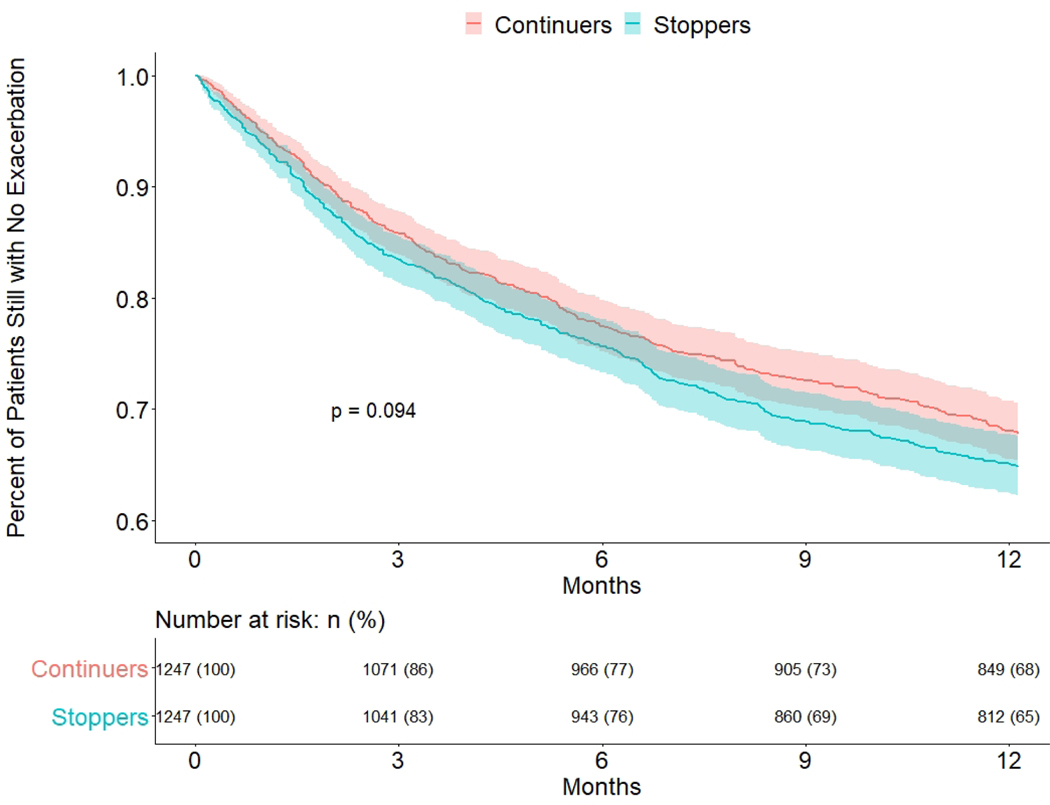
Time to first exacerbation after stopping treatment
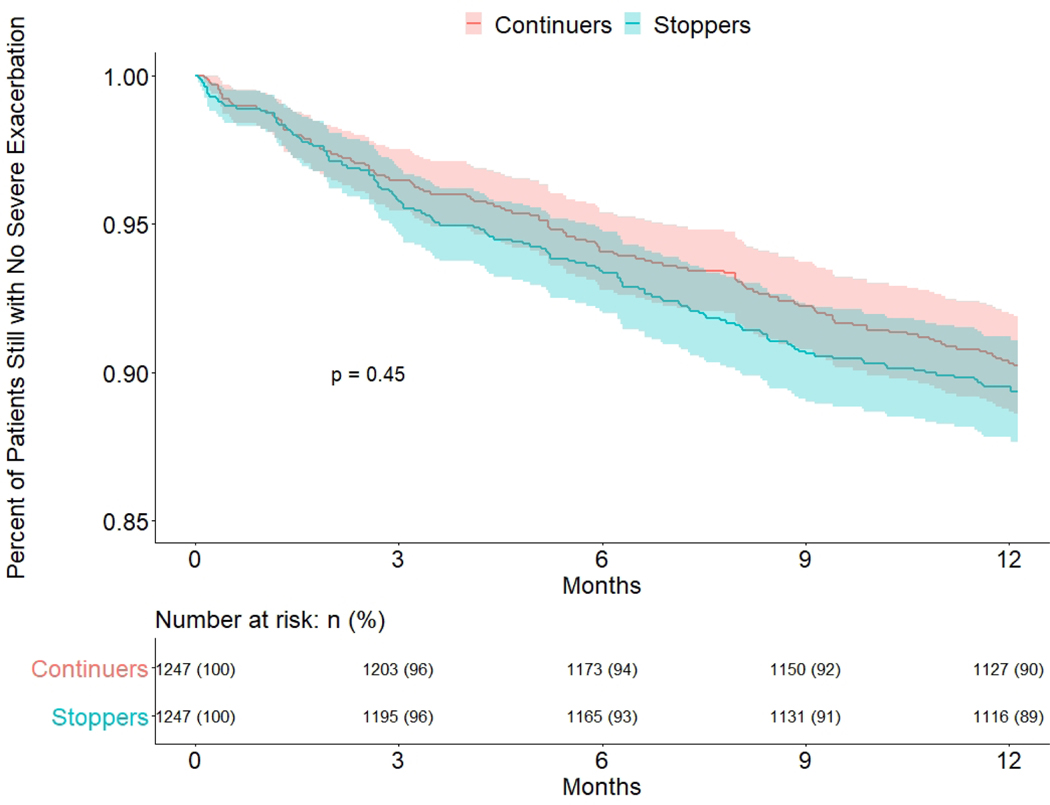
To compare risk of early asthma exacerbation after stopping biologic treatment, we plotted Kaplan-Meier survival curves for stoppers versus continuers (Figure 2). The first day at risk in this analysis was the stopping date for stoppers or the sham stopping date for continuers. We found no difference in time to first exacerbation between the groups in the main analysis (p=0.09) or in a secondary analysis using only severe exacerbations (p=0.45) (Figure 3).
Figure 2: Time to exacerbation after stopping date/sham stopping date, Stoppers vs Continuers: propensity score matched cohort.
Figure 3: Time to Severe exacerbation after stopping date/sham stopping date, Stoppers vs Continuers: propensity score matched cohort.
DISCUSSION
In this observational cohort study of people with asthma who used a biologic treatment for at least six months, we did not find statistically significant increases in asthma exacerbations or decreases in time to first asthma exacerbation after stopping biologic therapy as compared to a propensity-score-matched control group of people who continued asthma biologics. These data suggest that deciding to stop asthma biologic treatment could be a feasible option for many people with asthma.
Because this study used pre-existing administrative data, outcomes were limited to those that can be assessed using claims data. Asthma exacerbations are an important outcome frequently used in clinical trials, but they are not the only measure of treatment success. Uncontrolled asthma can reduce quality of life in the absence of asthma exacerbations, with sufferers missing school or work, having trouble sleeping, or having frequent symptoms like coughing, wheezing, and exercise intolerance. We observed many patients who discontinued biologic use after a good treatment response and many others who continued biologic use with no observed treatment response, when treatment response is measured by exacerbation reductions. These patients may have made treatment continuation decisions based on the effect of biologic therapy on other aspects of quality of life, including reduction of symptoms other than exacerbations, as well as factors that would lead to stopping, including cost, side effects, and access to care.
This cohort had low estimated ICS/LABA treatment consistency before starting biologic treatment, with an average MPR below 40%. Furthermore, more than half the cohort did not experience an exacerbation in the 6 months before index biologic use. This may indicate that patient selection for treatment is suboptimal.17 Alternatively, physicians and patients may be making rational treatment decisions based on factors other than exacerbations and ICS/LABA treatment use. Unlike ICS/LABA controllers which must be used every day, most biologics are dosed once or twice a month. MPRs and treatment persistence have been found to be higher in medications dosed weekly or monthly vs those dosed daily.18–20 Patients who find it difficult to maintain consistent use of a daily controller medication may want to try a less frequently administered controller. The findings from this observational study should be considered in the context of previous studies of stopping asthma biologics. Two randomized controlled trials assessed outcomes for stopping versus continuing asthma biologics. The XPORT trial randomly assigned 88 long-term omalizumab users to switch to placebo and assessed outcomes every 4 weeks for 1 year, assessing outcomes similar to ours: the proportion having one or more asthma exacerbation and time to first asthma exacerbation.7 In that study, stoppers were more likely to have one or more exacerbation in the year following randomization (53% vs 33% in the continuer cohort: OR 2.2). In the time-to-event analysis for XPORT, the separation between continuers and stoppers occurred primarily in the first 6 months, making the 6-month measurement period in our primary analysis a realistic time frame for assessment of differences. Stoppers in the XPORT trial experienced a greater hazard of exacerbation (hazard ratio for stoppers vs. continuers: 2.04). Our observational data found no statistically significant differences in either of these outcomes in a much larger sample (1861 per arm vs. 88 per arm in XPORT). Possible reasons for this discrepancy include 1) selection of most appropriate candidates for discontinuation (i.e., patients and clinicians of the patients who were at lowest risk were the ones who chose to stop using the biologic), 2) differences in post-biologic treatment (i.e., patients in XPORT received a placebo and no other changes to their treatment regimen, while patients in our study may have switched to an alternative medication), 3) biased ascertainment in our study (i.e., patients who discontinued biologics may have been less likely to be observed to have an exacerbation). However, our cohort experienced similar exacerbation rates to the XPORT continuer cohort, which may mean that biased ascertainment is not a major concern.
The second RCT of asthma biologic discontinuation (currently unpublished, but with results publicly posted) included people with at least 3 years of treatment with mepolizumab who were randomized to continue (n=141) or stop (n=151) for 1 year, with assessments every 3 months.21 At 6 months, the percentage of people who had an asthma exacerbation was lower in people who continued mepolizumab (32% in mepolizumab versus 49% in stoppers), results that are very similar to results seen for omalizumab in XPORT.6 When the asthma exacerbation definition was restricted to events involving ED or hospitalization only (excluding the criteria of systemic steroid given as outpatient), the percentage of people experiencing an asthma exacerbation was similar between stopping and continuing, and very infrequent (5% in mepolizumab and 6% in placebo). These trials, when taken together, show a consistent pattern of a small but statistically significant increase in asthma exacerbations in people randomly assigned to stop omalizumab or mepolizumab.
There are several non-controlled studies of stopping asthma biologics.8–12,22–25 These studies help inform exacerbation rates in people who are not participating in clinical trials. For example, Vennera et al report on a cohort of 49 people who stopped omalizumab, with 24% having an asthma exacerbation over 1 year of followup.22 Deschildre et al report a case-controlled nested study of 60 children who stopped and continued omalizumab (30 in each group), and found no asthma exacerbations in either group during a 12 month observation period.23 These studies, together with findings in this study, may suggest that patients and their doctors choosing to stop biologics are making appropriate decisions.
Limitations
Our study has some important limitations. Most importantly, this is an observational study and despite our efforts, there may be residual confounding. The choice to stop biologics was not randomly assigned and may have been driven by factors other than treatment effectiveness, including cost and access to biologic treatment. Second, we were limited to outcomes that can be ascertained with administrative data, and were unable to measure some important outcome domains, including quality of life. In addition, claims data do not allow us to know whether and when people used controller medications or whether they had controller medications received as samples, purchased medicines without insurance, or carried over from months or years before. Third, we performed the primary analysis in all people who stopped asthma biologics, not just people who had an initial treatment response. Future research to understand and further define response to asthma biologics may allow for better prediction of outcomes after biologic discontinuation. Fourth, the results apply best to people in the US who have commercial or Medicare Advantage insurance (and the other characteristics listed in Table 1). Fifth, the study design does not allow us to know the “reason” that people stopped or continued the asthma biologic. We suspect that insurance and costs of medication are important factors in deciding to stop and continue.13 Finally, we have fewer observations and thus less information on the newer asthma biologics (i.e., those other than omalizumab).
These limitations should be weighed against considerable strengths, including the inclusion of a large, generalizable cohort; randomized controlled trials of asthma biologics have had highly selected populations with fewer comorbidities and follow patients treated in academic medical centers with highly protocolized treatment regimens.26–29 Observational data may provide a more accurate depiction of treatment outcomes in the population that actually uses biologics. The propensity matched analysis allowed for a meaningful comparison of outcomes after stopping biologics. Although we were limited in the outcomes we could include, we were able to capture the primary outcome used in clinical trials for asthma biologics--asthma exacerbation--in our dataset, and also performed a time-to-event analysis as an alternative method to assess this outcome.
We identified a low overall risk in this study for stopping asthma biologics, a risk that is similar to continuing the asthma biologic. These data suggest that many people may be able to successfully stop asthma biologics. More studies in this area will help to better define this risk. It will be important to perform studies that may help predict which people fall into a high -risk category for stopping, and apply prediction rules to help providers and their patients with decisions about ongoing asthma biologic treatments. Also, it will be important to study outcomes over a longer -term period (years) and to incorporate additional outcomes that are important to people such as quality of life and control of day-to-day symptoms. For all of these reasons, we believe the data from this study can be useful to inform clinical guidelines by raising the possibility that stopping asthma biologics is possible. Nonetheless, the risk associated with randomly assigned stopping, based on data from 2 trials, is still likely higher than continuing, even if that risk overall is low and mostly related to risks of outpatient oral corticosteroid use rather than exacerbations leading to ED and hospital visits.
Conclusions
In this study using real world evidence to assess outcomes after stopping asthma biologic treatment, we did not find an increased risk of exacerbation associated with stopping biologics. The results from this study, along with results from previous studies, can be used to help patients and clinicians decide whether to stop or continue asthma biologic treatment.
Supplementary Material
HIGHLIGHTS:
What is already known about this topic?
Clinical trial data suggest that the risk of asthma exacerbation may increase after stopping asthma biologics.
What does this article add to our knowledge?
This observational, controlled, and pragmatic analysis of US insurance claims data suggests that people who stop asthma biologics have a similar risk of increased asthma exacerbations as those who continue asthma biologics.
How does this study impact current management guidelines?
Current asthma guidelines are silent on if, when, or how to stop asthma biologics. Data from this study suggest that stopping asthma biologics may not be associated with an increased risk of asthma exacerbation for many people.
Acknowledgments
Funding: National Institutes of Health, National Heart, Lung, and Blood Institute (NIH R21 HL140287) and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Abbreviations:
- HEDIS
Healthcare Effectiveness Data and Information Set
- ICS
Inhaled corticosteroid
- LABA
long-acting β-agonist
- OLDW
OptumLabs Data Warehouse
- MA
Medicare Advantage
- MPR
medication possession ratio
Footnotes
Conflict of Interest Statement: No authors have any disclosures to make.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001–2010. Vital Health Stat 3. 2012;1–58. [PubMed] [Google Scholar]
- 2.Kamble S, Bharmal M. Incremental direct expenditure of treating asthma in the United States. J Asthma. 2009;46:73–80. [DOI] [PubMed] [Google Scholar]
- 3.Jang J, Gary Chan KC, Huang H, Sullivan SD. Trends in cost and outcomes among adult and pediatric patients with asthma: 2000–2009. Ann Allergy Asthma Immunol. 2013;111:516–22. [DOI] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2019. [Internet]. [cited 2020 Jun 19]. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf
- 5.Gionfriddo MR, Hagan JB, Rank MA. Why and how to step down chronic asthma drugs. BMJ. 2017;359:j4438. [DOI] [PubMed] [Google Scholar]
- 6.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J [Internet]. 2020;55. Available from: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 7.Ledford D, Busse W, Trzaskoma B, Omachi TA, Rosén K, Chipps BE, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162–9.e2. [DOI] [PubMed] [Google Scholar]
- 8.Baena-Cagnani CE, Teijeiro A, Canonica GW. Four-year follow-up in children with moderate/severe uncontrolled asthma after withdrawal of a 1-year omalizumab treatment. Curr Opin Allergy Clin Immunol. 2015;15:267–71. [DOI] [PubMed] [Google Scholar]
- 9.Kupryś-Lipińska I, Kuna P. Loss of asthma control after cessation of omalizumab treatment: real life data. Postepy Dermatol Alergol. 2014;31:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molimard M, Mala L, Bourdeix I, Le Gros V. Observational study in severe asthmatic patients after discontinuation of omalizumab for good asthma control. Respir Med. 2014;108:571–6. [DOI] [PubMed] [Google Scholar]
- 11.Nopp A, Johansson SGO, Ankerst J, Palmqvist M, Oman H. CD-sens and clinical changes during withdrawal of Xolair after 6 years of treatment. Allergy. 2007;62:1175–81. [DOI] [PubMed] [Google Scholar]
- 12.Nopp A, Johansson SGO, Adédoyin J, Ankerst J, Palmqvist M, Oman H. After 6 years with Xolair; a 3-year withdrawal follow-up. Allergy. 2010;65:56–60. [DOI] [PubMed] [Google Scholar]
- 13.Inselman JW, Jeffery MM, Maddux JT, Shah ND, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8:549–54.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 17.Jeffery MM, Shah ND, Karaca-Mandic P, Ross JS, Rank MA. Trends in Omalizumab Utilization for Asthma: Evidence of Suboptimal Patient Selection. J Allergy Clin Immunol Pract. 2018;6:1568–77.e4. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto H, Maehara M. Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos. 2015;10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruk ME, Schwalbe N. The relation between intermittent dosing and adherence: preliminary insights. Clin Ther. 2006;28:1989–95. [DOI] [PubMed] [Google Scholar]
- 20.Greene M, Yan T, Chang E, Hartry A, Touya M, Broder MS. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21:127–34. [DOI] [PubMed] [Google Scholar]
- 21.GlaxoSmithKline. Cessation Versus Continuation of Long-term Mepolizumab in Severe Eosinophilic Asthma Patients [Internet]. [cited 2020 Sep 22]. Available from: https://clinicaltrials.gov/ct2/show/NCT02555371
- 22.Vennera MDC, Sabadell C, Picado C, Spanish Omalizumab Registry. Duration of the efficacy of omalizumab after treatment discontinuation in “real life” severe asthma. Thorax. 2018;73:782–4. [DOI] [PubMed] [Google Scholar]
- 23.Deschildre A, Roussel J, Drumez E, Abou-Taam R, Rames C, Le Roux P, et al. Omalizumab discontinuation in children with severe allergic asthma: An observational real-life study. Allergy. 2019;74:999–1003. [DOI] [PubMed] [Google Scholar]
- 24.Haldar P, Brightling CE, Singapuri A, Hargadon B, Gupta S, Monteiro W, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol. 2014;133:921–3. [DOI] [PubMed] [Google Scholar]
- 25.Ortega H, Lemiere C, Llanos J-P, Forshag M, Price R, Albers F, et al. Outcomes following mepolizumab treatment discontinuation: real-world experience from an open-label trial. Allergy Asthma Clin Immunol. 2019;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briasoulis O, Breckenridge R, Nunn A. External validity of trials should be taken into account before asthma drug candidates reach market authorisation. Lancet Respir Med. 2016;4:601–3. [DOI] [PubMed] [Google Scholar]
- 27.Travers J, Marsh S, Williams M, Weatherall M, Caldwell B, Shirtcliffe P, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akenroye A, Keet C. Underrepresentation of blacks, smokers, and obese patients in studies of monoclonal antibodies for asthma. J Allergy Clin Immunol Pract. 2020;8:739–41.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albers FC, Müllerová H, Gunsoy NB, Shin J-Y, Nelsen LM, Bradford ES, et al. Biologic treatment eligibility for real-world patients with severe asthma: The IDEAL study. J Asthma. 2018;55:152–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.