Abstract
Background:
Carcinogenesis is governed by a series of genetic alterations and epigenetic changes that lead to aberrant patterns in neoplastic cells. Sirtuin-1(SIRT1), an NAD+-dependent protein deacetylase, is capable of deacetylating histones and non-histone substrates that regulate various physiological activities during tumorigenesis. Recent studies have identified the role of SIRT1 in different stages of cancer, including genome instability, tumor initiation, proliferation, metabolism, and therapeutic response. However, the action of SIRT1 has been reported to be both oncogenic and tumor suppressive during carcinogenesis. Consequently, the biological functions of SIRT1 in cancer remain controversial.
Scope of review:
We highlight the most recent findings on SIRT1 in different stages of tumorigenesis, and update the current status of SIRT1 small molecule modulators in clinical application of cancer treatment.
Major conclusion:
By targeting both tumor suppressors and oncogenic proteins, SIRT1 has a bifunctional role at different stages of tumorigenesis. The impact of SIRT1 on tumorigenesis is also distinct at different stages and is dependent on its dosages. SIRT1 suppresses tumor initiation through its functions in promoting DNA repair, increasing genome stability, and inhibiting inflammation at the pre-cancer stage. However, SIRT1 enhances tumor proliferation, survival, and drug resistance through its roles in anti-apoptosis, pro-tumor metabolism, and anti-inflammation (inhibition of anti-tumor immunity) at the stages of tumor progression, metastasis, and relapse. Consequently, both SIRT1 inhibitors and activators have been explored for cancer treatment.
General significance:
Better understanding the dose- and stage-dependent roles of SIRT1 in each cancer type can provide new avenues of exploration for therapy development.
Keywords: Sirtuins, SIRT1, Cancer, Cancer Stem Cell, Metabolism, Bifunctional functions, Therapeutic Response
INTRODUCTION
Cancer incidence remains a major health problem worldwide. In the United States, cancer is the second leading cause of death, responsible for approximately 608,570 deaths and 1,898,160 new cases projected in 2021 [1]. Cancer is considered a multifaceted disease and is associated with a range of genetic mutations, chromosomal translocations and epigenetic modifications responsible for tumor formation and metastasis [2]. Despite recent advancements in cancer therapy, neoplastic cells are capable of bypassing treatments through cell cycle alterations, apoptosis induction, efflux alterations, DNA methylation, and genetic mutations. These adaptations allow them to acquire resistance to treatment, directly affecting the outcome of patients. Therefore, novel target-based approaches are needed to improve therapy response.
Sirtuins (SIRTs), a family of highly conserved nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylases that are also known as the class III HDACs, actively participate in heterochromatin formation, gene silencing, metabolism, and aging [3], and are well poised to fundamentally influence carcinogenesis. SIRTs arose after their founding member Sir2, a corresponding protein product of sir2 in Saccharomyces cerevisiae, was identified as an NAD+-dependent HDAC essential for transcription silencing, DNA repair, and lifespan regulation [4]. Conserved throughout evolution, SIRTs are present in both eukaryotic and prokaryotic cells, with seven SIRTs (SIRT1- SIRT7) being identified in the mammalian genome [4] (Figure 1). These seven members are respectively located in distinct subcellular compartments, including the nucleus, mitochondria, and cytoplasm (Figure 1). SIRT1, SIRT6 and SIRT7 are commonly present in the nucleus; SIRT2 is present in the cytoplasm; and SIRT3, SIRT4 and SIRT5 are typically presented in the mitochondria. However, SIRTs are known to shuffle between subcellular compartments [5]. For example, SIRT1 is typically found in the nucleus but can also be present in the cytoplasm, and SIRT2 and SIRT3 can also be found in the nucleus [5, 6]. Despite structural similarities, each SIRT is composed of a core domain with distinct N- and C- terminal regions that reflect functional distinctions, and individual tissue expression patterns, along with diverse substrate specificities unique to each SIRT [4].
Figure 1.

Localizations and biochemical functions of sirtuins. A visual representation of the seven mammalian sirtuins. Each SIRT is composed of a core domain with distinct N- and C- terminal regions that reflect functional distinctions and individual tissue expression patterns, along with diverse substrate specificities unique to each SIRT [4]. SIRT1, SIRT6 and SIRT7 are commonly present in the nucleus; SIRT2 is present in the cytoplasm; while SIRT3, SIRT4 and SIRT5 are typically in the mitochondria (Created with BioRender.com).
As the most conserved mammalian member of the SIRTs family, SIRT1 shares the greatest homology with Sir2 [4, 7]. Using NAD+ as a co-substrate, SIRT1 deacetylates histones and non-histone proteins, such as key transcription factors and cofactors, essential for fundamental biological activities (Figure 2). Specifically, SIRT1 can regulate chromatin structures and gene transcription through deacetylation of histones, including H1 lysine 26 (H1K26ac), H3 lysine 9 (H3K9ac), H3 lysine 56 (H3K56ac), and H4 lysine 16 (H4K16ac) [8]. SIRT1 can also control the activity of non-histone proteins involved in DNA damage response, chromatin remodeling, gene expression, cell differentiation and proliferation, aging, endocrine signaling, stress response, metabolism, and carcinogenesis [3].
Figure 2.

Tumor Promoting and Tumor Suppressing Actions of SIRT1. Schematic representation of the role of SIRT1 in both tumor suppressing and tumor promoting activities. SIRT1 can deacetylase a broad range of transcription targets that can influence tumor development both positively and negatively. Tumor suppressing targets enhanced by SIRT1 are found in the upper left: Ku70, XPC, XPA, PARP1, WRN; tumor promoting targets inhibited by SIRT1 are on the bottom left: NF-κB, STAT3, β-catenin, HIF-1α, APE1; while tumor suppressing targets inhibited by SIRT1 are found in the upper right: p53, HIC1, Rb, FOXO, PTTEN, AR, E2F1, XRCC1; and tumor promoting targets enhanced by SIRT1 are in the bottom right: Ras, Myc, PGC-1α, CTTN, Akt. Together, they represent the different ways in which SIRT1 can either promote or inhibit tumor development (Created with BioRender.com).
TUMOR-PROMOTING AND TUMOR-SUPPRESSING ACTIONS OF SIRT1
Extensive studies have been undertaken in order to improve our understanding of the mechanisms governed by SIRT1 in human malignancies. However, SIRT1 displays both tumor suppressive and oncogenic features by targeting different protein substrates at different stages of tumor progression; therefore, the precise role of SIRT1 in cancer is extremely complex (Figure 2). On the one hand, SIRT1 deacetylase activity can directly affect DNA damage enzymes like WRN, Ku70, APE1, XPA, and XPC to improve DNA repair [9–13]. SIRT1 has also been shown to suppress tumor development through inhibition of nuclear factor kappa B (NF-κB), a transcription factor known to regulate a large range of genes involved in innate and adaptive immune response during carcinogenesis [14, 15]. On the other hand, SIRT1 is known to promote tumor development through deacetylation and suppression of many tumor suppressors like hypermethylated in cancer 1 (HIC1), tumor protein p53 (p53), p53 like transcription factor p73 (p73), Forkhead transcription factors (FOXO), Retinoblastoma (Rb), and E2F transcription factor 1 (E2F1) [14, 16–20]. Additionally, many important nuclear receptors and cofactors involved in regulation of tumorigenesis, such as androgen receptor (AR), estrogen receptor-alpha (ER-α), PPARγ, and PPARγ coactivator 1α (PGC-1α) [21–24], as well as a number of oncogenes including HIF-1α, β-catenin, cortacin (CTTN), c-Myc, and N-Myc [15, 25–28], can be modulated by SIRT1 negatively and/or positively depending upon cell types, tissue origins, and environmental stresses. These activities of SIRT1 affect a diverse array of anti- and pro-tumor cellular pathways (Figure 2), exerting complex impacts on tumor progression. The role of SIRT1 in tumors is even more complicated because of the observed dose-dependent impact of SIRT1 on different cellular pathways and tumor outcomes [29, 30]. In this review, we will discuss recent findings concerning SIRT1 on tumor suppressors and oncogenic genes in different stages of tumorigenesis.
THE BIFUNCTIONAL ROLE OF SIRT1 AT DIFFERENT STAGES OF TUMORIGENESIS
SIRT1 in DNA repair and genome stability
Tumor initiation and development are often triggered by DNA mutations and genome instability [31]. SIRT1 has a consistent role in improving DNA repair and increasing genome stability in different cancer types. Specifically, SIRT1 has been implicated in promoting both double-stranded breaks (DSBs) repair and single stranded DNA lesion repair.
In response to DNA DSB damage, SIRT1 has been shown to be recruited to the damaged sites to induce chromatin remodeling and promote repair [32]. In a chronic myelogenous leukemia (CML) cell line, the DSB-induced association of SIRT1 with DNA damage sites can promote homologous recombination (HR) through the Werner helicase (WRN) [9]. SIRT1 has also been shown to enhance HR in transformed human cells by deacetylation of NBS1, a component of a conserved nuclease complex required for DNA damage sensing [33]. In addition to HR, SIRT1 can promote non-homologous end joining (NHEJ) by deacetylating Ku70 in CML cells [10].
SIRT1 has also been shown to be important for single stranded DNA lesion repair in different cancer types. Apurinic/apyrimidinic endonuclease-1 (APE1), an essential enzyme in the base excision repair (BER) pathway, is a target of SIRT1 in human cancer cells [11]. Activation of SIRT1 deacetylates APE1, promoting binding of APE1 to the BER protein X-ray cross-complementing-1 (XRCC1). Conversely, knockdown of SIRT1 increases cellular basic DNA content, which is rescued by overexpression of APE1 [11]. SIRT1 also plays an important role in the regulation of nucleotide excision repair (NER) pathway. In lung cancer cells, SIRT1 interacts with xeroderma pigmentosum group A (XPA), a core NER factor essential for NER process, in response to UV irradiation [12]. This interaction leads to deacetylation of XPA, resulting in an optimal NER activity and enhanced resistance to UV irradiation [12]. In skin cancer cells, SIRT1 enhances the expression of another core NER factor, xeroderma pigmentosum group C (XPC), by reducing AKT-dependent nuclear localization of the E2F4-p130 suppressor complex on the promoter of XPC gene [13]. Inhibition of SIRT1 impairs global genome NER. Consistently, in human skin tumors, SIRT1 levels are reversely correlated with tumor progression in patients [13]. When taken together, these studies all suggest that activation of SIRT1 in the pre-cancer stage could help to increase genome stability and suppress tumor initiation.
Although DNA damage-induced SIRT1 recruitment on the damage sites improves DNA repair and genome stability, studies have shown that after DNA repair, SIRT1 can interact with gene silencing factors including EZH2, an H3K27me3 methyltransferase, and DNMT1 and DNMT3B, two DNA methyltransferases, near the repaired sites, leading to the onset of aberrant CpG island DNA methylation that is frequently associated with silencing of tumor suppressors in cancer [34–36]. Particularly, a special SIRT1-containing Polycomb Repressive Complex (PRC) complex, PRC4, has been found to be present only in undifferentiated pluripotent cells and cancer cells, and this complex is overexpressed in breast, colon, and prostate cancers [35]. Moreover, in colon cancer cells, the PRC4 complex can be targeted to transcriptionally active promoter CpG islands upon oxidative stress induced by H2O2 treatment. This action in turn reduces histone activation marks H4K16ac and H3K4me3, increases histone repression mark H3K27me3 and DNA methylation, and contributes to cancer-related abnormal gene silencing and shifts in DNA methylation [36]. Taken together, these observations indicate that SIRT1-mediated histone deacetylation and subsequent aberrant gene silencing upon chronic exposure to oxidative DNA damage could promote tumorigenesis.
SIRT1 in cancer cell proliferation and progression
In established cancer cells and different types of cancers, SIRT1 plays a major role in regulation of cancer cell proliferation through different mechanisms.
In breast cancer, SIRT1 interacts with hormone signaling pathways to regulate breast cancer cell proliferation. In estrogen receptor (ER) positive breast cancer cells, it has been reported that ERα is acetylated by p300 following E2 activation, and SIRT1 is able to deacetylate ERα and inhibits estrogen-dependent signaling [22]. ERα has also been reported to recruit FOXO family members, which have shown to also be targeted by SIRT1. ERα interacts with FOXN3, which in turn recruits SIRT1 to induce SIRT1-mediated deacetylation and repression of ERα signaling [37]. SIRT1-mediated inhibition of ERα signaling represses the proliferation of ER positive breast cells. However, in ER negative breast cancer cells, the expression of SIRT1 can be regulated by hormone signaling through G-protein coupled ER (GPER), which is known to mediate estrogens and antiestrogens in both malignant and normal cells in tumor microenvironment [38]. It has been shown that upon activation by E2, GPER induces SIRT1 expression via activation of the EGFR/ERK/c-fos/Ap-1 transduction pathway [39], which in turn promotes tumor cell survival after DNA damage [40]. Therefore, SIRT1 could function as both tumor suppressor and oncogene in breast cancer cells, depending on the status of ER.
SIRT1 has a bifunctional role in tumor progression by interacting with known tumor suppressors and oncogenes in a number of other cancer types. According to some reports, SIRT1 is an oncogene. High expression of SIRT1 has been reported in colon cancer (CRC) cell lines [41], melanoma cell lines [42], chronic leukemia [43], and lymphoblastic leukemia and lymphoblastic lymphomas [44, 45]. In CRC, the increased expression of SIRT1 can upregulate c-Myc, aiding in malignant prognosis [46]. In melanoma cells, SIRT1 increases resistance to stress induced by deadhesion by associating with DNMT3B to silence MXD dimerization protein 1 (Mxd1), which leads to increased activity of Myc and drives melanoma progression [47]. Additionally, the SIRT1- AMP-activated protein kinase (AMPK) axis has been shown to maintain survival and proliferation of primary effusion lymphoma [48] and SIRT1 overexpression enhances proliferation, upregulates pro-inflammatory cytokines, and inhibits apoptosis in mouse B lymphocytes BaF3 cells [49]. In line with these observations, deletion of SIRT1 specifically in intestinal epithelial cells results in less development of intestinal tumors in Apcmin mutant mice and AOM/DSS-induced colon cancer model [50, 51]. Inhibition of SIRT1 transcription by interferon regulatory family IRF9 activates p53 and reduces cell growth of acute myeloid leukemia [52]. Knockdown of SIRT1 by shRNA or inhibition of SIRT1 activity by small molecule inhibitors also increases p53 transcriptional activity and enhances mitochondria oxidative phosphorylation in these cells [53, 54].
A growing body of evidence suggests that SIRT1 can also function as a tumor suppressor as well as aiding tumor growth. For instance, overexpressed SIRT1 can also induce cell cycle arrest by indirectly activating E2F1, which further induces cyclins and pRb, and repress colon cancer cell proliferation and tumor formation [55]. Transgenic mice modestly overexpressing SIRT1 in the intestine reduces the development of polyps caused by Apcmin mice [56]. In this model, SIRT1 deacetylases β-catenin, leading to suppression of activities that drive cellular proliferation [56]. Consistently, SIRT1+/− mice have been shown to increase tumor development when crossed to a p53+/− background [57], further suggesting that SIRT1 suppresses CRC. These observations were supported by immunohistochemistry from normal human colonic mucosa, adenoma, adenocarcinoma, and metastatic tissue samples that indicated SIRT1 expression gradually decreases during normal adenocarcinoma metastasis [58]. The expression of SIRT1 in cores from 349 colorectal cancer patients further suggested that SIRT1 overexpression is a good prognostic factor for CRC [59].
Although the contexts relating the bifunctional roles of SIRT1 remain unclear, a recent study revealed that the dose-dependent regulation of tumor metabolism and apoptosis by SIRT1 mechanistically contributes to the observed dual roles of SIRT1 in CRC progression [29]. This study revealed that different SIRT1-regulated cellular pathways have distinct sensitivities to changes of SIRT1 dosages. For instance, deletion of one copy of Sirt1 gene induces c-Myc expression, enhancing glutamine metabolism and subsequent proliferation, autophagy, stress resistance, and cancer formation. In contrast, deletion of both copies of Sirt1 gene triggers cellular apoptotic pathways, increases cell death, diminishes autophagy, and reduces cancer formation. Consistently, intestine-specific Sirt1 heterozygous mice have enhanced intestinal tumor formation, whereas intestine-specific Sirt1 homozygous knockout mice have reduced development of colon cancer [29]. These dose-dependent impacts of SIRT1 on tumor progression have also been observed in a skin cancer mouse model, where heterozygous deletion of Sirt1 promotes while homozygous deletion of Sirt1 suppresses cancer development [30]. Therefore, these studies indicate that the distinct sensitivities of different pathways to SIRT1 dosages differentiate the outcomes in cancer cell proliferation and growth, contributing to observed dual functions of SIRT1 in tumorigenesis. Taken together, current studies demonstrate an intriguing dual role of SIRT1 in regulation of cancer cell metabolism, stress resistance, and proliferation. These findings further point to the importance of maintaining a suitable SIRT1 dosage for metabolic and tissue homeostasis and suggest that potent SIRT1 inhibitors and modest SIRT1 activators will be beneficial for treatment of human cancers.
SIRT1 in epithelial-to-mesenchymal-transition (EMT) and cancer metastasis
The dual role SIRT1 exhibited in different cancer types has come into play in epithelial to mesenchymal transition (EMT), an important process in metastatic progression [60]. Activation of EMT program allows cancer cells to propagate from the primary tumor to adjacent tissues, and subsequently metastasize by spreading through the lymphatic system and/or bloodstream. Mechanistically, EMT exhibits downregulation of epithelial markers such as E-cadherin and cytokeratin, with upregulation of mesenchymal markers such as N-cadherin, Vimentin and β-catenin along with transcription regulators such as Zeb1 and Snail [60].
SIRT1 has been shown to play a major role in promoting tumor metastasis. In triple-negative breast cancer (TNBC), SIRT1 interacts with EMT proteins, allowing for predictions of tumor invasiveness. Consistently, siRNA-mediated SIRT1 knockdown suppresses tumor invasion while amending protein expression patterns related to EMT [61]. Several non-EMT related proteins have also shown to be regulated by SIRT1 in breast cancer. For instance, SIRT1 transcriptionally regulates the expression of the frizzle family (FZD7), a factor that promotes cellular proliferation [62], in response to Wnt signaling during breast cancer metastasis [63]. Inhibition of SIRT1 reduces β-catenin and c-jun levels, which in turn decreases the expression of FZD7 and suppresses breast cancer metastasis [63]. In support of these observations, the deacetylation activity of SIRT1 is positively correlated with MMP-2 protein levels in BC patient samples and is directly associated with tumor stage and patient survival [63].
The expression of SIRT1 in cancers is under control of various micro-RNAs (miRNA) that impact its ability to regulate metastasis [64–66]. For example, the miR-200 family has been shown to be essential regulators of EMT in breast cancer, where overexpression of SIRT1 is followed by a decrease in miR-200 promotes distant breast cancer metastasis [67]. Conversely, the reduced activity of SIRT1 caused by the influence of these miRNAs leads to increase acetylation of p53 [66]. SIRT1 is also a direct target of miR-22, whose downregulation results in SIRT1 overexpression in breast cancer [68]. Consistently, activation of miR-22 in MCF-7 BC cells decreases proliferation, invasion, and migration, whereas overexpression of SIRT1 reverses the anti-metastatic phenotype observed upon miR-22 activation [68].
SIRT1 has also been shown to promote EMT and metastasis in several other cancer types, including liver cancer [69] and prostate cancer [70], through induction of the activity of EMT regulator Snail and Twist, or inhibition of the expression of E-cadherin. SIRT1 also has the ability to accelerate autophagic degradation of E-cadherin by deacetylation of Beclin 1 in melanoma [71].
Again, the role of SIRT1 in EMT appears to be bifunctional and context dependent. It has been shown that SIRT1 is able to suppress EMT in HMLER breast cancer cells by deacetylating Smad4 and repressing the TGF-beta signaling [72]. This relationship between SIRT1 and tumor metastasis provides a link between SIRT1 overexpression and the poor prognosis observed in cancer patients with increase SIRT1 expression. Nonetheless, further studies are needed in order to understand SIRT1 regulation of EMT during tumorigenesis in different types of cancer.
SIRT1 in cancer drug resistance
Cancer drug resistance is governed by both innate and acquired responsive mechanisms that involve intrinsic and extrinsic factors. Innate resistance can mediate drug intake mutations on drug transporters while promoting drug efflux via upregulation of ATP-binding cassette (ABC) transporters such as multidrug resistance protein 1 (MDR1) together with partial tumor penetrance [73]. Furthermore, activation of Cytochrome P450 enzymes, glutathione S-transferase along with mechanisms that directly affect cell cycle checkpoints and DNA repair machineries also contribute to innate resistance [74]. Acquired resistance develops as a result of treatment and is mediated via genetic mutations associated with innate resistance [75]. The ability to acquire genetic mutations that defer drug efficacy remains elusive.
Recent studies have shown that SIRT1 contributes to multiple aspects of cancer drug resistance. For example, SIRT1 has been shown to enhance mitochondrial metabolism through PGC-1α, which is associated with resistance to therapy in lung cancer and CRC [76, 77]. High levels of nuclear SIRT1 in ovarian cancer patient have also been associated with resistance to chemotherapy [78]. Moreover, SIRT1 has been shown to promote mutation acquisition in cancer by serving as an intermediate of oncogenic function genes that alter DNA damage repair [79]. Furthermore, a recent study has suggested that SIRT1 can interact with XRCC1 and disrupt acetylation-dependent interaction of XRCC1 with β-TrCP E3 ligase, which extends the half-life of XRCC1 to promote chemoresistance in lung cancer [80]. Additionally, the cooperation between SIRT1 and hedgehog (Hh) signaling pathway is an important mechanism of drug resistance in myeloma. In bortezomib-resistant myeloma cells, SIRT1 deacetylates and stabilizes GLI2, a key transcription factor of the Hh signaling pathway, and consequentially activates the Hh signaling [81]. Consistently, SIRT1-specific inhibitors partially impede Hh signaling and confer bortezomib sensitivity. Clinically, SIRT1 expression is elevated in relapsed patients and patients with high SIRT1 expression have poor outcomes [81].
Although inhibition of SIRT1 could provide innovative approached to overcome dug resistance, it may not necessarily be effective in different tumor stages. Further studies are necessary to better understand the mechanistic function of SIRT1 in tumor initiation, proliferation, and metastasis. While SIRT1 has shown bifunctional roles depending on tissue context and cancer type, SIRT1 can also display different expression patterns at different tumor stages.
SIRT1 in cancer stem cell maintenance and proliferation
Cancer stem cells (CSCs) are a small population of a tumor that possess the ability to self-renew and differentiate into all the cell types in a cancer [82]. This ability allows them to generate a new tumor, leading to tumor relapse and metastasis. CSCs are generally resistant to conventional chemotherapy and radiotherapy that are designed to primarily target the differentiated or differentiating cancer cells. Development of therapy against CSCs, therefore, would greatly improve the survival and quality of life for certain cancer patients, particularly patients with metastatic tumors.
SIRT1 has been shown to be critical for stemness and survival of CSCs and is thereby oncogenic for a number of solid tumors (Figure 3). For instance, SIRT1 is highly expressed in glioma stem cells (GSCs), where it is required for their maintenance, as well as oncogenic transformation, through suppressing p53-dependent tumor surveillance [83, 84]. Consistently, knocking down SIRT1 enhances the sensitivity of GCSs to radiation [83]. SIRT1 is also highly expressed in breast cancer stem cells (BCSCs), where it is a direct target of miR-34a [85]. It has been shown that reduction of miR-34a leads to high expression of SIRT1, an important factor for the expression of CSC markers and maintenance of BSCS pool [85]. In liver CSCs, high levels of SIRT1 inhibit DNMT3A, reducing the DNA methylation level in the SOX2 promoter and promoting SOX2 expression [86]. This action of SIRT1 has been shown to be important for the maintenance of their self-renewal and tumorigenic potential [86]. It has been recently reported that SIRT1 is also important for liver CSC stemness by increasing mitochondrial respiratory capacity, as SIRT1 is able to deacetylate and promote mitochondrial translocation of mitochondrial ribosomal protein S5 (MRPS5) [87]. In colorectal CD133+ CSC-like cells, SIRT1 induces the expression of several stemness-associated genes, thereby promoting tumorigenesis of colorectal cancer cells in vitro and in vivo [88]. In addition, SIRT1 promotes the hypoxia-induced CSC-like properties in human ovarian cancer cell lines [89].
Figure 3.
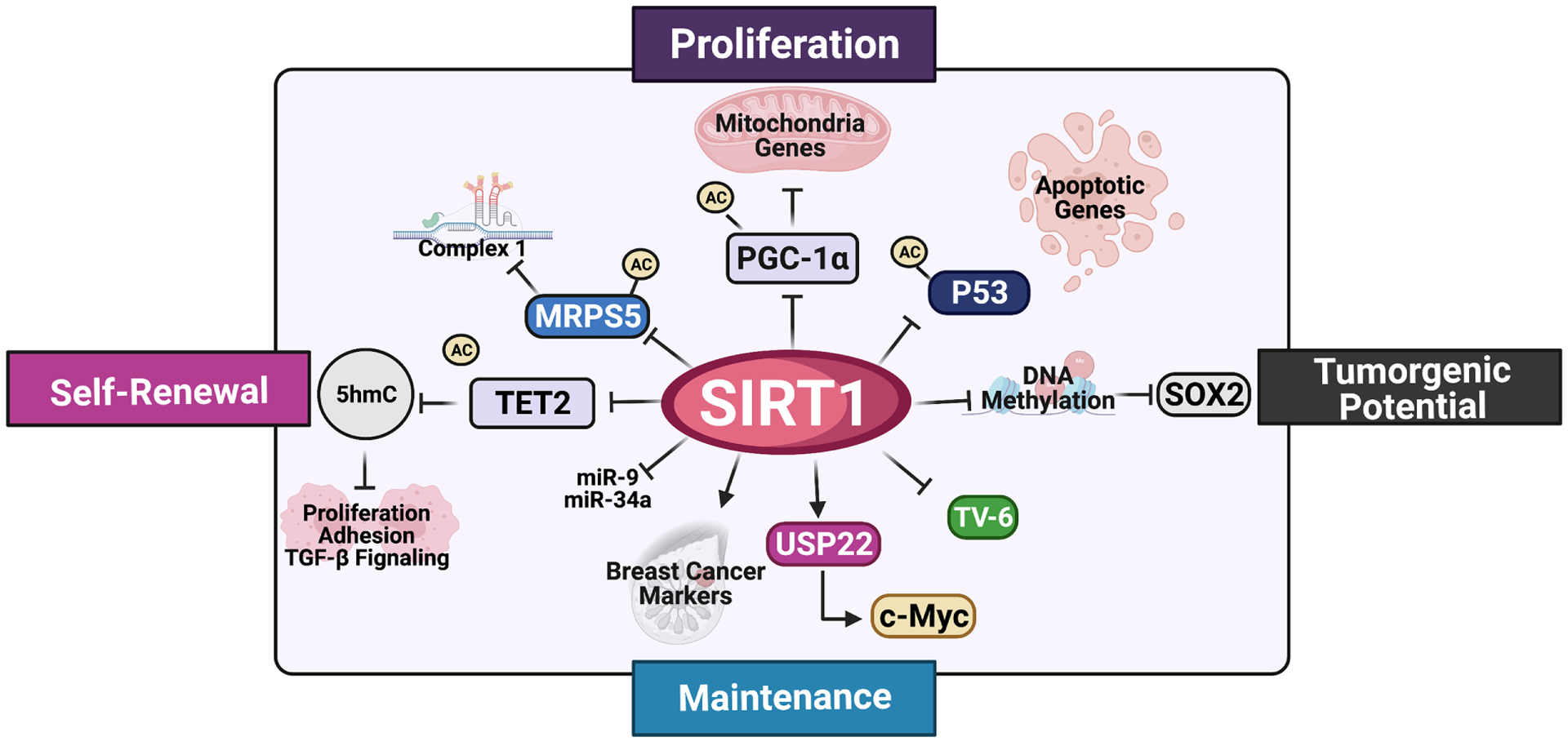
SIRT1 in Cancer Stem-cell maintenance and Proliferation. Schematic representation of how SIRT 1 exerts tumorigenic response in different cancer stem cells. (Created with BioRender.com).
SIRT1 is also important for survival and proliferation of leukemic stem cells (LSCs) [90–92]. It has been reported that SIRT1 protein level is increased in LSCs in both chronic myeloid leukemia (CML) and acute myeloid leukemia (AML), by either transcriptional induction in CML or by c-Myc-USP22 mediated stabilization of SIRT1 protein in AML [93]. The increase in SIRT1 protein in LSCs inhibits p53 signaling, aiding their survival and proliferation [91, 92]. Consistently, reducing SIRT1 activity either by SIRT1 knockdown or by Tenovin-6 (TV6), a small molecule inhibitor of SIRT1, decreases LSC proliferation, enhances apoptosis, and impairs their colony-forming ability [91, 92]. Consequently, the combined use of TV6 with kinase inhibitors [92, 94] enhances inhibition of LSCs and improves survival of animals receiving CML LSCs transplantation or xenografted with imatinib-resistant blast crisis CML patient sample [91, 92].
Although SIRT1 is important for stemness and survival of most CSCs, it also functions to disrupt maintenance of Myelodysplastic Syndrome (MDS) stem and progenitor cells (HSPC) [95]. In MDS HSPCs, two miRNAs, miR-9 and miR-34a, help to keep SIRT1 protein levels low. Activation of SIRT1 by a SIRT1 agonist SRT1720 or overexpression of SIRT1 leads to deacetylation and activation of TET2, which repress the expression a number of genes involved in proliferation, adhesion, and TGF-β signaling by increasing 5-hydroxymethylcytosine (5hmC) on their enhancers, which then in turn inhibits MDS HSPC proliferation and functions [95]. In addition to MDS HSPC, SIRT1 also functions to inhibit chemoresistance and eliminate CSC properties of gastric cancer cells through FOXO3 and AMPK [96]. These results indicate that SIRT1 exerts a bifunctional role in different cancer stem cells (Figure 3), depending on its deacetylation targets.
SIRT1 in inflammation and anti-tumor immunity in tumor microenvironment
Tumor microenvironment, a complex tumor-surrounding niche consisting of immune cells, stromal cells, vascular networks, and many other cellular and noncellular components, has been increasingly shown to be important in regulation of tumor initiation, progression, and therapeutic responses [97, 98]. Particularly, the tumor microenvironment is enriched with both innate and adaptative immune cells, including immunosuppressive tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T (Treg) cells, as well as anti-tumor cytotoxic CD8 T cells, CD4 Th1, and natural killer cells [98]. These immune components, together with their molecular products, such as cytokines, chemokines, and selectins, have a dual role in carcinogenesis. On the one hand, chronic inflammatory conditions have been associated with carcinogenesis in many types of cancers, and anti- inflammatory therapies have been shown to reduce the risk for various cancers [99]. On the other hand, activation of cytotoxic CD8 T cells, CD4 Th1, and natural killer cells has been associated with an enhanced anti-tumor activity and is key in determining the therapeutic response of the tumor to immunotherapy [97, 98].
SIRT1 has a well-known activity in repression inflammation and immune cell activation by targeting different transcription factors in immune cells. For example, SIRT1 is known to directly deacetylate the p65 subunit of NF-κB to inhibit its transactivation [15]. Specific deletion of SIRT1 in myeloid cells increases NF-κB-mediated activation of pro-inflammatory genes and results in enhanced chronic inflammation upon high fat feeding in mice [100]. SIRT1 also directly interacts with and represses c-Fos and c-Jun, the major components of AP-1, in peritoneal macrophages, thus reducing the expression an AP-1 target gene of COX-2 and subsequent production of prostaglandin E (2) [101]. SIRT1 also blunts the Th17 differentiation of CD4+ T cells by deacetylation of STAT3 and reduction of the expression of Rorc [102]. Additionally, SIRT1 has been shown to reduce the proinflammatory activation of MDSCs [103] and limits the Th9 differentiation of CD4+ T cells [104] through inhibition of mTOR-HIF-1α-dependent glycolysis. However, due to the dual role of inflammation and immunity in carcinogenesis, SIRT1-mediated immunosuppression again has a bifunctional impact on tumor development and progression in the tumor microenvironment. First, the suppression of chronic inflammation by SIRT1 in myeloid cells [100] may reduce the risk of tumor-promoting chronic inflammatory diseases such as colitis, chronic pancreatitis, chronic bronchitis, and hepatitis [99]. Consistently, overexpression of SIRT1 in peritoneal macrophages improves their phagocytosis and tumoricidal functions [101], and activation of SIRT1 by small molecule activators reduces the expression of cytokines important for VEGF-A production and angiogenesis in CD4+ Th17 cells, which limits tumor growth in mice [102]. Therefore, activation of SIRT1 in tumor microenvironment is able to suppress inflammation and tumorigenesis. Second, SIRT1-mediated suppression of the proinflammatory activation of MDSCs [103] and the Th9 differentiation of CD4+ T cells [104] have been shown to enhance the immunosuppressive tumor microenvironment, reduce the anti-tumor immunity, and increase the tumor growth.
TARGETING SIRT1 IN CANCER
The bifunctional roles of SIRT1 in tumor progression leads to a suggestion that both SIRT1 activators and inhibitors could be used for cancer treatment [55, 105]. The observed dose-dependent impact of SIRT1 on different cellular pathways and tumor outcomes [29, 30] further suggest that potent SIRT1 inhibitors and modest SIRT1 activators would be suitable for anti-cancer treatment.
Extensive investigation of a number of SIRT1 inhibitors in anti-cancer research indicates that inhibiting SIRT1 has direct anti-cancer effects (reviewed in [106]). One of the most well-studied SIRT1 inhibitors is nicotinamide, the earliest sirtuin inhibitor that can inhibit not only SIRT1, but also SIRT2, SIRT3, SIRT5, and SIRT6 with IC50 from 50 to 184 μM. Nicotinamide has been shown to block proliferation and promote apoptosis in chronic lymphocytic leukemia cells and prostate cancer cells [53, 107], and sensitizes lung cancer cells to cisplatin-induced apoptosis [80]. In a phase 1 proof-of-principle clinical trial, the combination of nicotinamide with a pan-class I/II HDAC inhibitor vorinostat results in a response in 24% of patients with relapsed or refractory lymphoma; among those patients, 57% experienced disease stabilization [108]. In a randomized phase III trial in patients with clinical stage T2–4 laryngeal carcinoma under accelerated radiotherapy, the addition of carbogen and nicotinamide has been shown to have improved recurrence-free survival of patients with anemia [109]. Currently, a number of clinical trials exploring the impact of nicotinamide on lung cancer, skin cancer, and chronic lymphocytic leukemia are ongoing (www.clinicaltrials.gov).
Another class of SIRT1 inhibitors includes sirtinol, which has an IC50 of 4 0μM against human SIRT1 and has been shown to induce senescence-like growth arrest, promote apoptosis, and increase sensitivity to camptothecin and cisplatin in a number of human cancer cells (reviewed in [106]). EX-527, a cell permeable indole-containing SIRT1 inhibitor with an IC50 value in the range of 60 nM to 100 nM in vitro, has also be actively evaluated in anti-cancer studies. EX-527 is considered as a SIRT1-specific inhibitor, although it still has a weak potency to SIRT2 and SIRT3 [110]. Similar to sirtinol, EX-527 has a number of anti-tumor activities in cancer cells, including elevation of apoptosis, induction of cell cycle arrest, and sensitization of cancer cells to chemotherapy (reviewed in [111]). Another class of potent SIRT1 inhibitors includes tenovin-1 and its water-soluble analogue tenovin-6 that were discovered using a cell-based screen aimed at detecting small molecules that activate p53 [112, 113]. Tenovin-1 shows cytotoxic effects to the BL2 Burkitt’s lymphoma cells and ARN8 melanoma cells expressing wild type p53, and reduced tumor growth derived from those cells [112]. Tenovin-6 is more active than tenovin-1 to ARN8 melanoma cells [112]. In mice, tenovin-6 delays the progression of chronic myelogenous leukemia [114]. Again, tenovin-6 has a number of anti-tumor activities in a wide array of cancer cells like other SIRT1 inhibitors (reviewed in [111]). Despite these extensive in vitro and animal studies, however, none of above SIRT1 inhibitors except nicotinamide have been evaluated in clinical trials.
A number of SIRT1 activators, including natural polyphenol compound resveratrol and synthetic chemicals SRT1460, SRT1720, and SRT2183, have also been actively studied in aging and age-associated diseases, including cancer [111, 115]. Although the biochemical mechanisms underlying these activators remain unclear and controversial, they generally exert abilities in the areas of cell cycle arrest, cell death induction, cell proliferation inhibition, and EMT/metastasis suppression, in a wide arrange of cancer cells [111]. In a handful of clinical trials, resveratrol has been shown to inhibit Wnt pathway target gene expression in colonic mucosa and colon cancer [116] and increase apoptosis in malignant hepatic tissues [117]. Other synthetic SIRT1 activators have not been evaluated in clinical trials to date.
Given the observations that inhibiting SIRT1 has direct anti-cancer effects and that potent SIRT1 inhibitors are suitable for anti-cancer treatment, developing highly potent specific SIRT1 inhibitors is important for its therapeutic application.
CONCLUSION
As the most conserved mammalian sirtuin, SIRT1 targets a large number of protein factors including both tumor suppressors and oncogenic proteins. Although current literature supports a bifunctional role of SIRT1 in tumorigenesis, the impact of SIRT1 is distinct at different stages of this multi-step process. It is clear that SIRT1-mediated maintenance of DNA repair, genome stability, and anti-inflammation is tumor-preventing during the pre-cancer stage, while its roles in anti-apoptosis, glutamine metabolism and oxidative phosphorylation, anti-inflammation, and stress resistance are tumor promoting at the stages of tumor progression and metastasis. The latter actions of SIRT1 further enhance cancer stem cell maintenance and proliferation, leading to tumor drug resistance, relapse, and metastasis. Better understanding the dose- and stage-dependent roles of SIRT1 in each cancer type can provide new avenues of exploration for therapy development.
Table 1.
The impact of SIRT1 on tumorigenicity
| STAGES | FUNCTIONS | TARGETS | REFERENCES |
|---|---|---|---|
| DNA Repair & Genome Stability | Tumor suppression: Improve DNA repair and genome stability | WRN, Ju70, APE1, XPA, XPC, NBS1, Ku70, PARP1. | [9–13, 33] |
| Tumor promoting: Increase abnormal cancer-related gene silencing after DNA repair | PRC4 (EZH2, DNMT1, DNMT3B) | [34–36] | |
| Proliferation, Survival & Progression | Tumor suppression: Induce cell cycle arrest and suppress β-catenin-driven cell proliferation | Rb, E2F1, β-catenin | [55, 56] |
| Tumor promoting: Repress tumor suppressors, hormonal signaling, and activate other oncogenes | HIC1, p53, p73, FOXO, ERα, AR, PPARγ, PGC-1α; c-Myc, N-Myc, HIF-1α, β-catenin, cortacin, AMPK | [14–20, 22, 25–29, 46–49] | |
| EMT & Metastasis | Tumor suppression: Suppress the TGF-β signaling | Smad4 | [72] |
| Tumor promoting: Promote EMT and tumor invasiveness | β-catenin, c-Jun, FZD7, Wnt signaling, MMP2, miR-200, p53, Snail, Twist, Ecadherin, Beclin 1 | [61–63, 67–71] | |
| Drug Resistance | Tumor suppression: | No reports | |
| Tumor promoting: Enhance mitochondrial metabolism, promote mutations acquisition, activate Hh signaling, and increase resistance to therapy | PGC-1α, XRCC1, GLI2 | [76–81] | |
| CSC Maintenance & Proliferation | Tumor suppression: Repress TET2-mediated signaling in MDS HSPC. Inhibit chemoresistance and eliminate CSC properties of gastric cancer cells | TET2, FOXO3, AMPK | [95, 96] |
| Tumor promoting: Increase CSC self-renewal, maintenance, survival, and tumorigenic potential | p53, DNMT3A, MRPS5 | [83–94] | |
| Inflammation & Anti-Tumor Immunity | Tumor suppression: Inhibit tumor-promoting chronic inflammatory diseases, improve tumoricidal activity of macrophages, blunt Th17 differentiation of CD4+ T cells and angiogenesis in the tumor microenvironment | NF-κB, c-Fos, c-Jun, STAT3 | [14, 15, 101, 102] |
| Tumor promoting: Suppress the proinflammatory activation of MDSCs and the Th9 differentiation of CD4+ T cells by inhibiting mTOR-HIF-1α-dependent glycolysis | mTOR-HIF-1α | [103, 104] |
Highlights.
SIRT1 is an NAD+-dependent HDAC important for epigenetics, metabolism, and aging.
SIRT1 has a bifunctional role in tumorigenesis.
The impact of SIRT1 on tumorigenesis is distinct at different stages.
Both SIRT1 inhibitors and activators are under exploration for cancer treatment.
Acknowledgements
We thank Drs. Stephen Shears and Robin Stanley, and members of the Li laboratory for critical reading of the manuscript. We also thank Christine Caufield-Noll, NIH Library, for manuscript editing assistance. The work related to this article was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences to X.L. (Z01 ES102205). We apologize to those colleagues whose work has not been cited due to the space limit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- [1].Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer Statistics, 2021, CA Cancer J Clin 71(1) (2021) 7–33. [DOI] [PubMed] [Google Scholar]
- [2].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144(5) (2011) 646–74. [DOI] [PubMed] [Google Scholar]
- [3].Haigis MC, Sinclair DA, Mammalian sirtuins: biological insights and disease relevance, Annu Rev Pathol 5 (2010) 253–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Frye RA, Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins, Biochem Biophys Res Commun 273(2) (2000) 793–8. [DOI] [PubMed] [Google Scholar]
- [5].Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y, Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1, J Biol Chem 282(9) (2007) 6823–32. [DOI] [PubMed] [Google Scholar]
- [6].Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I, Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins, Mol Biol Cell 16(10) (2005) 4623–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Imai S, Armstrong CM, Kaeberlein M, Guarente L, Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase, Nature 403(6771) (2000) 795–800. [DOI] [PubMed] [Google Scholar]
- [8].Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D, SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation, Nature 450(7168) (2007) 440–4. [DOI] [PubMed] [Google Scholar]
- [9].Uhl M, Csernok A, Aydin S, Kreienberg R, Wiesmuller L, Gatz SA, Role of SIRT1 in homologous recombination, DNA Repair (Amst) 9(4) (2010) 383–93. [DOI] [PubMed] [Google Scholar]
- [10].Jeong J, Juhn K, Lee H, Kim SH, Min BH, Lee KM, Cho MH, Park GH, Lee KH, SIRT1 promotes DNA repair activity and deacetylation of Ku70, Exp Mol Med 39(1) (2007) 8–13. [DOI] [PubMed] [Google Scholar]
- [11].Yamamori T, DeRicco J, Naqvi A, Hoffman TA, Mattagajasingh I, Kasuno K, Jung SB, Kim CS, Irani K, SIRT1 deacetylates APE1 and regulates cellular base excision repair, Nucleic Acids Res 38(3) (2010) 832–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fan W, Luo J, SIRT1 regulates UV-induced DNA repair through deacetylating XPA, Mol Cell 39(2) (2010) 247–58. [DOI] [PubMed] [Google Scholar]
- [13].Ming M, Shea CR, Guo X, Li X, Soltani K, Han W, He YY, Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C, Proc Natl Acad Sci U S A 107(52) (2010) 22623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen LF, Mu Y, Greene WC, Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB, EMBO J 21(23) (2002) 6539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW, Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase, EMBO J 23(12) (2004) 2369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA, hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase, Cell 107(2) (2001) 149–59. [DOI] [PubMed] [Google Scholar]
- [17].Dai JM, Wang ZY, Sun DC, Lin RX, Wang SQ, SIRT1 interacts with p73 and suppresses p73-dependent transcriptional activity, J Cell Physiol 210(1) (2007) 161–6. [DOI] [PubMed] [Google Scholar]
- [18].Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L, Mammalian SIRT1 represses forkhead transcription factors, Cell 116(4) (2004) 551–63. [DOI] [PubMed] [Google Scholar]
- [19].Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, Nemoto S, Finkel T, Gu W, Cress WD, Chen J, Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage, Nat Cell Biol 8(9) (2006) 1025–31. [DOI] [PubMed] [Google Scholar]
- [20].Wong S, Weber JD, Deacetylation of the retinoblastoma tumour suppressor protein by SIRT1, Biochem J 407(3) (2007) 451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fu M, Liu M, Sauve AA, Jiao X, Zhang X, Wu X, Powell MJ, Yang T, Gu W, Avantaggiati ML, Pattabiraman N, Pestell TG, Wang F, Quong AA, Wang C, Pestell RG, Hormonal control of androgen receptor function through SIRT1, Mol Cell Biol 26(21) (2006) 8122–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL, Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor, Mol Endocrinol 20(7) (2006) 1479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L, Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma, Nature 429(6993) (2004) 771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P, Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1, Nature 434(7029) (2005) 113–8. [DOI] [PubMed] [Google Scholar]
- [25].Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW, Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha, Mol Cell 38(6) (2010) 864–78. [DOI] [PubMed] [Google Scholar]
- [26].Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, Nicosia SV, Zhang X, Deacetylation of cortactin by SIRT1 promotes cell migration, Oncogene 28(3) (2009) 445–60. [DOI] [PubMed] [Google Scholar]
- [27].Marshall GM, Liu PY, Gherardi S, Scarlett CJ, Bedalov A, Xu N, Iraci N, Valli E, Ling D, Thomas W, van Bekkum M, Sekyere E, Jankowski K, Trahair T, Mackenzie KL, Haber M, Norris MD, Biankin AV, Perini G, Liu T, SIRT1 promotes N-Myc oncogenesis through a positive feedback loop involving the effects of MKP3 and ERK on N-Myc protein stability, PLoS Genet 7(6) (2011) e1002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Menssen A, Hydbring P, Kapelle K, Vervoorts J, Diebold J, Luscher B, Larsson LG, Hermeking H, The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop, Proc Natl Acad Sci U S A 109(4) (2012) E187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ren NSX, Ji M, Tokar EJ, Busch EL, Xu X, Lewis D, Li X, Jin A, Zhang Y, Wu WKK, Huang W, Li L, Fargo DC, Keku TO, Sandler RS, Li X, Haploinsufficiency of SIRT1 Enhances Glutamine Metabolism and Promotes Cancer Development, Curr Biol 27(4) (2017) 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ming M, Soltani K, Shea CR, Li X, He YY, Dual role of SIRT1 in UVB-induced skin tumorigenesis, Oncogene 34(3) (2015) 357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Negrini S, Gorgoulis VG, Halazonetis TD, Genomic instability--an evolving hallmark of cancer, Nat Rev Mol Cell Biol 11(3) (2010) 220–8. [DOI] [PubMed] [Google Scholar]
- [32].Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA, SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging, Cell 135(5) (2008) 907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yuan Z, Zhang X, Sengupta N, Lane WS, Seto E, SIRT1 regulates the function of the Nijmegen breakage syndrome protein, Mol Cell 27(1) (2007) 149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].O’Hagan HM, Mohammad HP, Baylin SB, Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island, PLoS Genet 4(8) (2008) e1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kuzmichev A, Margueron R, Vaquero A, Preissner TS, Scher M, Kirmizis A, Ouyang X, Brockdorff N, Abate-Shen C, Farnham P, Reinberg D, Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation, Proc Natl Acad Sci U S A 102(6) (2005) 1859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Hagan HM, Wang W, Sen S, Destefano Shields C, Lee SS, Zhang YW, Clements EG, Cai Y, Van Neste L, Easwaran H, Casero RA, Sears CL, Baylin SB, Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands, Cancer Cell 20(5) (2011) 606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xu Z, Yang Y, Li B, Li Y, Xia K, Yang Y, Li X, Wang M, Li S, Wu H, Checkpoint suppressor 1 suppresses transcriptional activity of ERalpha and breast cancer cell proliferation via deacetylase SIRT1, Cell Death Dis 9(5) (2018) 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lappano R, Maggiolini M, G protein-coupled receptors: novel targets for drug discovery in cancer, Nat Rev Drug Discov 10(1) (2011) 47–60. [DOI] [PubMed] [Google Scholar]
- [39].Santolla MF, Avino S, Pellegrino M, De Francesco EM, De Marco P, Lappano R, Vivacqua A, Cirillo F, Rigiracciolo DC, Scarpelli A, Abonante S, Maggiolini M, SIRT1 is involved in oncogenic signaling mediated by GPER in breast cancer, Cell Death Dis 6 (2015) e1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Santolla MF, Lappano R, De Marco P, Pupo M, Vivacqua A, Sisci D, Abonante S, Iacopetta D, Cappello AR, Dolce V, Maggiolini M, G protein-coupled estrogen receptor mediates the up-regulation of fatty acid synthase induced by 17beta-estradiol in cancer cells and cancer-associated fibroblasts, J Biol Chem 287(52) (2012) 43234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stunkel W, Peh BK, Tan YC, Nayagam VM, Wang X, Salto-Tellez M, Ni B, Entzeroth M, Wood J, Function of the SIRT1 protein deacetylase in cancer, Biotechnol J 2(11) (2007) 1360–8. [DOI] [PubMed] [Google Scholar]
- [42].Singh CK, Panackal JE, Siddiqui S, Ahmad N, Nihal M, Combined Inhibition of Specific Sirtuins as a Potential Strategy to Inhibit Melanoma Growth, Front Oncol 10 (2020) 591972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bhalla S, Gordon LI, Functional characterization of NAD dependent de-acetylases SIRT1 and SIRT2 in B-Cell Chronic Lymphocytic Leukemia (CLL), Cancer Biol Ther 17(3) (2016) 300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jin Y, Cao Q, Chen C, Du X, Jin B, Pan J, Tenovin-6-mediated inhibition of SIRT1/2 induces apoptosis in acute lymphoblastic leukemia (ALL) cells and eliminates ALL stem/progenitor cells, BMC Cancer 15 (2015) 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li L, Ye S, Yang M, Yu W, Fan Z, Zhang H, Hu J, Liang A, Zhang W, SIRT1 downregulation enhances chemosensitivity and survival of adult T-cell leukemia-lymphoma cells by reducing DNA double-strand repair, Oncol Rep 34(6) (2015) 2935–42. [DOI] [PubMed] [Google Scholar]
- [46].Kriegl L, Vieth M, Kirchner T, Menssen A, Up-regulation of c-MYC and SIRT1 expression correlates with malignant transformation in the serrated route to colorectal cancer, Oncotarget 3(10) (2012) 1182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meliso FM, Micali D, Silva CT, Sabedot TS, Coetzee SG, Koch A, Fahlbusch FB, Noushmehr H, Schneider-Stock R, Jasiulionis MG, SIRT1 regulates Mxd1 during malignant melanoma progression, Oncotarget 8(70) (2017) 114540–114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].He M, Tan B, Vasan K, Yuan H, Cheng F, Ramos da Silva S, Lu C, Gao SJ, SIRT1 and AMPK pathways are essential for the proliferation and survival of primary effusion lymphoma cells, J Pathol 242(3) (2017) 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang Q, Yan C, Xin M, Han L, Zhang Y, Sun M, Sirtuin 1 (Sirt1) Overexpression in BaF3 Cells Contributes to Cell Proliferation Promotion, Apoptosis Resistance and Pro-Inflammatory Cytokine Production, Med Sci Monit 23 (2017) 1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Leko V, Park GJ, Lao U, Simon JA, Bedalov A, Enterocyte-specific inactivation of SIRT1 reduces tumor load in the APC(+/min) mouse model, PLoS One 8(6) (2013) e66283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lo Sasso G, Ryu D, Mouchiroud L, Fernando SC, Anderson CL, Katsyuba E, Piersigilli A, Hottiger MO, Schoonjans K, Auwerx J, Loss of Sirt1 function improves intestinal anti-bacterial defense and protects from colitis-induced colorectal cancer, PLoS One 9(7) (2014) e102495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tian WL, Guo R, Wang F, Jiang ZX, Tang P, Huang YM, Sun L, The IRF9-SIRT1-P53 axis is involved in the growth of human acute myeloid leukemia, Exp Cell Res 365(2) (2018) 185–193. [DOI] [PubMed] [Google Scholar]
- [53].Audrito V, Vaisitti T, Rossi D, Gottardi D, D’Arena G, Laurenti L, Gaidano G, Malavasi F, Deaglio S, Nicotinamide blocks proliferation and induces apoptosis of chronic lymphocytic leukemia cells through activation of the p53/miR-34a/SIRT1 tumor suppressor network, Cancer Res 71(13) (2011) 4473–83. [DOI] [PubMed] [Google Scholar]
- [54].Abraham A, Qiu S, Chacko BK, Li H, Paterson A, He J, Agarwal P, Shah M, Welner R, Darley-Usmar VM, Bhatia R, SIRT1 regulates metabolism and leukemogenic potential in CML stem cells, J Clin Invest 129(7) (2019) 2685–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kabra N, Li Z, Chen L, Li B, Zhang X, Wang C, Yeatman T, Coppola D, Chen J, SirT1 is an inhibitor of proliferation and tumor formation in colon cancer, J Biol Chem 284(27) (2009) 18210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, Hahn WC, Guarente LP, Sinclair DA, The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth, PLoS One 3(4) (2008) e2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B, Jia R, Zheng ZM, Appella E, Wang XW, Ried T, Deng CX, Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice, Cancer Cell 14(4) (2008) 312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jang SH, Min KW, Paik SS, Jang KS, Loss of SIRT1 histone deacetylase expression associates with tumour progression in colorectal adenocarcinoma, J Clin Pathol 65(8) (2012) 735–9. [DOI] [PubMed] [Google Scholar]
- [59].Jung W, Hong KD, Jung WY, Lee E, Shin BK, Kim HK, Kim A, Kim BH, SIRT1 Expression Is Associated with Good Prognosis in Colorectal Cancer, Korean J Pathol 47(4) (2013) 332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ye X, Weinberg RA, Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression, Trends Cell Biol 25(11) (2015) 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chung SY, Jung YY, Park IA, Kim H, Chung YR, Kim JY, Park SY, Im SA, Lee KH, Moon HG, Noh DY, Han W, Lee C, Kim TY, Ryu HS, Oncogenic role of SIRT1 associated with tumor invasion, lymph node metastasis, and poor disease-free survival in triple negative breast cancer, Clin Exp Metastasis 33(2) (2016) 179–85. [DOI] [PubMed] [Google Scholar]
- [62].Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, Somlo G, Yen Y, FZD7 has a critical role in cell proliferation in triple negative breast cancer, Oncogene 30(43) (2011) 4437–46. [DOI] [PubMed] [Google Scholar]
- [63].Simmons GE Jr., Pandey S, Nedeljkovic-Kurepa A, Saxena M, Wang A, Pruitt K, Frizzled 7 expression is positively regulated by SIRT1 and beta-catenin in breast cancer cells, PLoS One 9(6) (2014) e98861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kiga K, Fukuda-Yuzawa Y, Tanabe M, Tsuji S, Sasakawa C, Fukao T, Comprehensive silencing of target-sharing microRNAs is a mechanism for SIRT1 overexpression in cancer, RNA Biol 11(11) (2014) 1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Karbasforooshan H, Roohbakhsh A, Karimi G, SIRT1 and microRNAs: The role in breast, lung and prostate cancers, Exp Cell Res 367(1) (2018) 1–6. [DOI] [PubMed] [Google Scholar]
- [66].Yarahmadi S, Abdolvahabi Z, Hesari Z, Tavakoli-Yaraki M, Yousefi Z, Seiri P, Hosseinkhani S, Nourbakhsh M, Inhibition of sirtuin 1 deacetylase by miR-211–5p provides a mechanism for the induction of cell death in breast cancer cells, Gene 711 (2019) 143939. [DOI] [PubMed] [Google Scholar]
- [67].Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q, miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells, J Biol Chem 286(29) (2011) 25992–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Corzo C, Barrientos Santillan N, Westin SN, Ramirez PT, Updates on Conservative Management of Endometrial Cancer, J Minim Invasive Gynecol 25(2) (2018) 308–313. [DOI] [PubMed] [Google Scholar]
- [69].Hao C, Zhu PX, Yang X, Han ZP, Jiang JH, Zong C, Zhang XG, Liu WT, Zhao QD, Fan TT, Zhang L, Wei LX, Overexpression of SIRT1 promotes metastasis through epithelial-mesenchymal transition in hepatocellular carcinoma, BMC Cancer 14 (2014) 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Byles V, Zhu L, Lovaas JD, Chmilewski LK, Wang J, Faller DV, Dai Y, SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis, Oncogene 31(43) (2012) 4619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sun T, Jiao L, Wang Y, Yu Y, Ming L, SIRT1 induces epithelial-mesenchymal transition by promoting autophagic degradation of E-cadherin in melanoma cells, Cell Death Dis 9(2) (2018) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Simic P, Williams EO, Bell EL, Gong JJ, Bonkowski M, Guarente L, SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis, Cell Rep 3(4) (2013) 1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Minchinton AI, Tannock IF, Drug penetration in solid tumours, Nat Rev Cancer 6(8) (2006) 583–92. [DOI] [PubMed] [Google Scholar]
- [74].Townsend DM, Tew KD, The role of glutathione-S-transferase in anti-cancer drug resistance, Oncogene 22(47) (2003) 7369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Apperley JF, Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia, Lancet Oncol 8(11) (2007) 1018–29. [DOI] [PubMed] [Google Scholar]
- [76].Sun J, Li G, Liu Y, Ma M, Song K, Li H, Zhu D, Tang X, Kong J, Yuan X, Targeting histone deacetylase SIRT1 selectively eradicates EGFR TKI-resistant cancer stem cells via regulation of mitochondrial oxidative phosphorylation in lung adenocarcinoma, Neoplasia 22(1) (2020) 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Vellinga TT, Borovski T, de Boer VC, Fatrai S, van Schelven S, Trumpi K, Verheem A, Snoeren N, Emmink BL, Koster J, Rinkes IH, Kranenburg O, SIRT1/PGC1alpha-Dependent Increase in Oxidative Phosphorylation Supports Chemotherapy Resistance of Colon Cancer, Clin Cancer Res 21(12) (2015) 2870–9. [DOI] [PubMed] [Google Scholar]
- [78].Shuang T, Wang M, Zhou Y, Shi C, Over-expression of Sirt1 contributes to chemoresistance and indicates poor prognosis in serous epithelial ovarian cancer (EOC), Med Oncol 32(12) (2015) 260. [DOI] [PubMed] [Google Scholar]
- [79].Wang Z, Yuan H, Roth M, Stark JM, Bhatia R, Chen WY, SIRT1 deacetylase promotes acquisition of genetic mutations for drug resistance in CML cells, Oncogene 32(5) (2013) 589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yousafzai NA, Zhou Q, Xu W, Shi Q, Xu J, Feng L, Chen H, Shin VY, Jin H, Wang X, SIRT1 deacetylated and stabilized XRCC1 to promote chemoresistance in lung cancer, Cell Death Dis 10(5) (2019) 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Xie Y, Liu J, Jiang H, Wang J, Li X, Wang J, Zhu S, Guo J, Li T, Zhong Y, Zhang Q, Liu Z, Proteasome inhibitor induced SIRT1 deacetylates GLI2 to enhance hedgehog signaling activity and drug resistance in multiple myeloma, Oncogene 39(4) (2020) 922–934. [DOI] [PubMed] [Google Scholar]
- [82].Clevers H, The cancer stem cell: premises, promises and challenges, Nat Med 17(3) (2011) 313–9. [DOI] [PubMed] [Google Scholar]
- [83].Chang CJ, Hsu CC, Yung MC, Chen KY, Tzao C, Wu WF, Chou HY, Lee YY, Lu KH, Chiou SH, Ma HI, Enhanced radiosensitivity and radiation-induced apoptosis in glioma CD133-positive cells by knockdown of SirT1 expression, Biochem Biophys Res Commun 380(2) (2009) 236–42. [DOI] [PubMed] [Google Scholar]
- [84].Lee JS, Park JR, Kwon OS, Lee TH, Nakano I, Miyoshi H, Chun KH, Park MJ, Lee HJ, Kim SU, Cha HJ, SIRT1 is required for oncogenic transformation of neural stem cells and for the survival of “cancer cells with neural stemness” in a p53-dependent manner, Neuro Oncol 17(1) (2015) 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ma W, Xiao GG, Mao J, Lu Y, Song B, Wang L, Fan S, Fan P, Hou Z, Li J, Yu X, Wang B, Wang H, Wang H, Xu F, Li Y, Liu Q, Li L, Dysregulation of the miR-34a-SIRT1 axis inhibits breast cancer stemness, Oncotarget 6(12) (2015) 10432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cheng F, Su L, Yao C, Liu L, Shen J, Liu C, Chen X, Luo Y, Jiang L, Shan J, Chen J, Zhu W, Shao J, Qian C, SIRT1 promotes epithelial-mesenchymal transition and metastasis in colorectal cancer by regulating Fra-1 expression, Cancer Lett 375(2) (2016) 274–283. [DOI] [PubMed] [Google Scholar]
- [87].Wei Z, Jia J, Heng G, Xu H, Shan J, Wang G, Liu C, Xia J, Zhou H, Wu M, Yang Z, Wang M, Xiong Z, Huang H, Liu L, Qian C, Sirtuin-1/Mitochondrial Ribosomal Protein S5 Axis Enhances the Metabolic Flexibility of Liver Cancer Stem Cells, Hepatology 70(4) (2019) 1197–1213. [DOI] [PubMed] [Google Scholar]
- [88].Chen X, Sun K, Jiao S, Cai N, Zhao X, Zou H, Xie Y, Wang Z, Zhong M, Wei L, High levels of SIRT1 expression enhance tumorigenesis and associate with a poor prognosis of colorectal carcinoma patients, Sci Rep 4 (2014) 7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Qin J, Liu Y, Lu Y, Liu M, Li M, Li J, Wu L, Hypoxia-inducible factor 1 alpha promotes cancer stem cells-like properties in human ovarian cancer cells by upregulating SIRT1 expression, Sci Rep 7(1) (2017) 10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, Huberman K, Cheng J, Viale A, Socci ND, Heguy A, Cherry A, Vance G, Higgins RR, Ketterling RP, Gallagher RE, Litzow M, van den Brink MR, Lazarus HM, Rowe JM, Luger S, Ferrando A, Paietta E, Tallman MS, Melnick A, Abdel-Wahab O, Levine RL, Prognostic relevance of integrated genetic profiling in acute myeloid leukemia, N Engl J Med 366(12) (2012) 1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Li L, Wang L, Li L, Wang Z, Ho Y, McDonald T, Holyoake TL, Chen W, Bhatia R, Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib, Cancer Cell 21(2) (2012) 266–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Li L, Osdal T, Ho Y, Chun S, McDonald T, Agarwal P, Lin A, Chu S, Qi J, Li L, Hsieh YT, Dos Santos C, Yuan H, Ha TQ, Popa M, Hovland R, Bruserud O, Gjertsen BT, Kuo YH, Chen W, Lain S, McCormack E, Bhatia R, SIRT1 activation by a c-MYC oncogenic network promotes the maintenance and drug resistance of human FLT3-ITD acute myeloid leukemia stem cells, Cell Stem Cell 15(4) (2014) 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao B, Dong H, Wei J, Song J, Zhang DD, Fang D, USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development, Mol Cell 46(4) (2012) 484–94. [DOI] [PubMed] [Google Scholar]
- [94].Kindler T, Lipka DB, Fischer T, FLT3 as a therapeutic target in AML: still challenging after all these years, Blood 116(24) (2010) 5089–102. [DOI] [PubMed] [Google Scholar]
- [95].Sun J, He X, Zhu Y, Ding Z, Dong H, Feng Y, Du J, Wang H, Wu X, Zhang L, Yu X, Lin A, McDonald T, Zhao D, Wu H, Hua WK, Zhang B, Feng L, Tohyama K, Bhatia R, Oberdoerffer P, Chung YJ, Aplan PD, Boultwood J, Pellagatti A, Khaled S, Kortylewski M, Pichiorri F, Kuo YH, Carlesso N, Marcucci G, Jin H, Li L, SIRT1 Activation Disrupts Maintenance of Myelodysplastic Syndrome Stem and Progenitor Cells by Restoring TET2 Function, Cell Stem Cell 23(3) (2018) 355–369 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].An Y, Wang B, Wang X, Dong G, Jia J, Yang Q, SIRT1 inhibits chemoresistance and cancer stemness of gastric cancer by initiating an AMPK/FOXO3 positive feedback loop, Cell Death Dis 11(2) (2020) 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF, Understanding the tumor immune microenvironment (TIME) for effective therapy, Nat Med 24(5) (2018) 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Duan Q, Zhang H, Zheng J, Zhang L, Turning Cold into Hot: Firing up the Tumor Microenvironment, Trends Cancer 6(7) (2020) 605–618. [DOI] [PubMed] [Google Scholar]
- [99].Coussens LM, Werb Z, Inflammation and cancer, Nature 420(6917) (2002) 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X, Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress, Mol Cell Biol 30(19) (2010) 4712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhang R, Chen HZ, Liu JJ, Jia YY, Zhang ZQ, Yang RF, Zhang Y, Xu J, Wei YS, Liu DP, Liang CC, SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages, J Biol Chem 285(10) (2010) 7097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Limagne E, Thibaudin M, Euvrard R, Berger H, Chalons P, Vegan F, Humblin E, Boidot R, Rebe C, Derangere V, Ladoire S, Apetoh L, Delmas D, Ghiringhelli F, Sirtuin-1 Activation Controls Tumor Growth by Impeding Th17 Differentiation via STAT3 Deacetylation, Cell Rep 19(4) (2017) 746–759. [DOI] [PubMed] [Google Scholar]
- [103].Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang X, Liu H, Lu Y, Liao J, Chen X, Chu Y, SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1alpha-dependent glycolysis, Cancer Res 74(3) (2014) 727–37. [DOI] [PubMed] [Google Scholar]
- [104].Wang Y, Bi Y, Chen X, Li C, Li Y, Zhang Z, Wang J, Lu Y, Yu Q, Su H, Yang H, Liu G, Histone Deacetylase SIRT1 Negatively Regulates the Differentiation of Interleukin-9-Producing CD4(+) T Cells, Immunity 44(6) (2016) 1337–49. [DOI] [PubMed] [Google Scholar]
- [105].Carafa V, Rotili D, Forgione M, Cuomo F, Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M, Mai A, Altucci L, Sirtuin functions and modulation: from chemistry to the clinic, Clin Epigenetics 8 (2016) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hu J, Jing H, Lin H, Sirtuin inhibitors as anticancer agents, Future Med Chem 6(8) (2014) 945–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Jung-Hynes B, Nihal M, Zhong W, Ahmad N, Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition?, J Biol Chem 284(6) (2009) 3823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Amengual JE, Clark-Garvey S, Kalac M, Scotto L, Marchi E, Neylon E, Johannet P, Wei Y, Zain J, O’Connor OA, Sirtuin and pan-class I/II deacetylase (DAC) inhibition is synergistic in preclinical models and clinical studies of lymphoma, Blood 122(12) (2013) 2104–13. [DOI] [PubMed] [Google Scholar]
- [109].Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, Chin A, Takes RP, de Bree R, Hoogsteen IJ, Bussink J, Span PN, Kaanders JH, Improved recurrence-free survival with ARCON for anemic patients with laryngeal cancer, Clin Cancer Res 20(5) (2014) 1345–54. [DOI] [PubMed] [Google Scholar]
- [110].Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS, Huber LJ, Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage, Mol Cell Biol 26(1) (2006) 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lee YT, Tan YJ, Mok PY, Subramaniam AV, Oon CE, The bifunctional roles of sirtuins and their therapeutic potential in cancer, Book-Sirtuin Biology in Cancer and Metabolic Disease (2021) 153–177. [Google Scholar]
- [112].Lain S, Hollick JJ, Campbell J, Staples OD, Higgins M, Aoubala M, McCarthy A, Appleyard V, Murray KE, Baker L, Thompson A, Mathers J, Holland SJ, Stark MJ, Pass G, Woods J, Lane DP, Westwood NJ, Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator, Cancer Cell 13(5) (2008) 454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].McCarthy AR, Pirrie L, Hollick JJ, Ronseaux S, Campbell J, Higgins M, Staples OD, Tran F, Slawin AM, Lain S, Westwood NJ, Synthesis and biological characterisation of sirtuin inhibitors based on the tenovins, Bioorg Med Chem 20(5) (2012) 1779–93. [DOI] [PubMed] [Google Scholar]
- [114].Yuan H, Wang Z, Li L, Zhang H, Modi H, Horne D, Bhatia R, Chen W, Activation of stress response gene SIRT1 by BCR-ABL promotes leukemogenesis, Blood 119(8) (2012) 1904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hubbard BP, Sinclair DA, Small molecule SIRT1 activators for the treatment of aging and age-related diseases, Trends Pharmacol Sci 35(3) (2014) 146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Nguyen AV, Martinez M, Stamos MJ, Moyer MP, Planutis K, Hope C, Holcombe RF, Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer, Cancer Manag Res 1 (2009) 25–37. [PMC free article] [PubMed] [Google Scholar]
- [117].Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ, Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics, Cancer Prev Res (Phila) 4(9) (2011) 1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]


