Abstract
Hematopoietic stem cells are the most illustrious inhabitants of the bone marrow. Direct visualization of endogenous hematopoietic stem cells in this niche is essential to study their functions. Until recently this was not possible in live animals. Recent studies, using state-of-the-art technologies, including sophisticated in vivo inducible genetic approaches in combination with two-photon laser scanning microscopy, allow the follow-up of endogenous hematopoietic stem cells’ behavior in their habitat. Strikingly, the new findings reveal that quiescent hematopoietic stem cells are more mobile than previously thought, and link their retained steady state within the niche to a mobile behavior. The arising knowledge from this research will be critical for the therapy of several hematological diseases. Here, we review recent progress in our understanding of hematopoietic stem cell biology in their niches.
Keywords: hematopoietic stem cells, niche, microenvironment, two-photon laser scanning microscopy
INTRODUCTION
HEMATOPOIETIC STEM CELLS
The bone marrow is, presently, well-established as the primary postnatal site of new blood cells formation, generating approximately 10 billion leukocytes, 200 billion red cells, and 400 billion platelets daily during our whole life [1]. Nevertheless, this bone marrow’s capacity was only first experimentally discovered at the second half of the 19th century by a German pathologist Ernst Neumann [2]. He also proposed the controversial, at that time, concept that one cell type may originate all other blood cells in the bone marrow [2]. This pioneer theory introduced the field of hematopoietic stem cell biology. Early works from the 50s demonstrated that transplantation of bone marrow cells could protect the organism from some of the damages caused by irradiation, avoiding hematopoietic failure [3–6], suggesting the existence of a cell with reconstitutive ability in the middle of bone marrow cells. In the 60s, James Till, Ernest McCulloch, and their colleagues brought the initial experimental proof of the existence of hematopoietic stem cells. They demonstrated that there were cells in the bone marrow with capacity to generate all blood cell types and make more of themselves [7–12]. Since then, in the clinic, intravenous transplantation of bone marrow cells has proven to be effective to treat patients with several blood-related diseases, such as leukemia [13–15]. In leukemic patients, bone marrow transplantation has revolutionized therapeutic options, and now is widely used in the clinic, allowing bone marrow cells from healthy donors to repopulate the bones of patients with leukemia after aggressive chemotherapy [16–20].
Nowadays, hematopoietic stem cells can be isolated from the bone marrow highly enriched by using multiple specific molecular markers [21]. Scientists are constantly searching for new markers to isolate subsets of purified hematopoietic stem cells. It is well accepted that stem cells capable of hematopoietic reconstitution are positive for Sca-1, a membrane glycoprotein [22] and c-Kit, a tyrosine kinase receptor (CD117), concomitantly being negative for lineage markers (Lin-), including Gr-1, Ter119, Mac-1, B220, CD4 and CD8 [23–25]. Additionally, these characteristics are combined with strategies stablished by different groups to isolate purified hematopoietic stem cells [24, 26], such as their status of expression of Thy1.1, Flk2, CD34, Endoglin (CD105) [27], Tie-2 [28], endothelial protein C receptor (EPCR) [29], CD244, CD48, and/or CD150 [24]. The exclusion of fluorescent dyes is an additional method that has proven advantageous to select for cells enriched with hematopoietic stem cells activity [22, 23, 30, 31].
One obstacle in the hematopoietic stem cells’ isolation is that the number of available compatible bone marrow donors still limits the usage of hematopoietic stem cells for transplantation. Although hematopoietic stem cells are maintained throughout all our life in their niche in vivo, we still are unable to multiply and expand effectively hematopoietic stem cells in vitro under suitable conditions. Therefore, a deeper understanding of hematopoietic stem cells biology will be essential for the better efficiency of bone marrow transplantation in the future. In this review, we discuss the recent progress in our understanding of hematopoietic stem cell biology in their niches, focusing on hematopoietic stem cells’ heterogeneity and interactions with other cells in the context of recent findings. Furthermore, we shed light on the gaps in the field and highlight important open questions.
HEMATOPOIETIC STEM CELLS WITHIN THE BONE MARROW NICHE
Hematopoietic stem cells reside predominantly within the bone marrow [32]. The hematopoietic stem cells’ bone marrow niche regulates the behavior of those cells [33]. Hematopoietic stem cell fate is decided by the pro-quiescence, pro-renewal, or pro-differentiation intrinsic and extrinsic regulators inside the niche [34]. Multiple genetically engineered mouse models have been extensively used to explore the complexity of the hematopoietic stem cell niche within the bone marrow. These investigations established diverse components as niche-supporting cells for hematopoietic stem cells, providing many molecules, such as cytokines, to control hematopoietic stem cell function [35]. Experimental proof has revealed that intervention in the key niche regulators may lead to various hematologic pathologic processes [32, 36]. Thus, understanding hematopoietic stem cells’ behavior in their niche, as well as their interactions with other niche constituents, is of crucial significance.
Direct visualization of hematopoietic stem cells in their niche is necessary to study their activity in vivo. This was possible with the advancement of deep confocal microscopic imaging that helped determine hematopoietic stem cell niche architecture. Several studies analyzed the localization of hematopoietic stem cells relative to distinct niche components [37–39]. In most studies, the hematopoietic stem cells behavior was analyzed in bone marrow biopsies, in which hematopoietic stem cells can be precisely identified using a combination of molecular markers by immunohistochemistry [37–39]. Nevertheless, remains the open question whether hematopoietic stem cell behavior is the same within the bones of live animals. Other works analyzed the behavior of pre-labeled hematopoietic stem cells in recipient live mice [40–42]. Nevertheless, it is not clear whether the non-physiological behavior of these introduced hematopoietic stem cells is the same as of endogenous stem cells. Additionally, for the efficiency of transplantation, recipient animals receive treatments that affect the bone marrow microenvironment, bringing the possibility of changes in hematopoietic stem cell behavior due to niche disruption.
Now, in a recent article in Cell Stem Cell, Upadhaya and colleagues demonstrated elegantly how endogenous adult hematopoietic stem cells behave in the bone marrow in live animals [43]. Using state-of-the-art technologies, including sophisticated in vivo inducible genetic approaches, such as lineage-tracing Cre/loxP mediated technologies, in combination with two-photon laser scanning microscopy, the authors selectively followed the behavior of single adult hematopoietic stem cells for several hours. The authors analyzed the bone marrow of a mouse model in which specifically endogenous hematopoietic stem cells produce red fluorescence, Pdzk1ip1-CreER/TdTomato mice. Behaviors of hematopoietic stem cells and macrophages, which were detected by their autofluorescence, were compared. These experiments revealed that hematopoietic stem cells present a constantly changing not-rounded shape extending cytoplasmatic projections, in contrast to the perfectly round cells as previously thought. Surprisingly, hematopoietic stem cells moved 7.5 times more than resident macrophages in steady state conditions [43]. Importantly, the authors confirmed that Pdzk1ip1-expressing cells were bona fide hematopoietic stem cells by confirming that the investigated cells were also Fgd5+ in Pdzk1ip1-CreER/TdTomato/Fgd5-ZsGreen mice. Upadhaya and colleagues also reported, as previously known, that hematopoietic stem cells are located in the perivascular space, and physically interact with stem cell factor (SCF)-expressing pericytes in the bone marrow. Strikingly, mobilization of the hematopoietic stem cells from the bone marrow niche by drugs that block C-X-C chemokine receptor type 4 (CXCR4) receptor and integrin signaling inhibited hematopoietic stem cell mobility as well as its form fluctuations within the niche [43]. This study reveals that hematopoietic stem cells are more mobile than previously thought, and links their retained steady state within the niche to a mobile behavior. Here, we discuss the findings from this work and evaluate recent advances in our understanding of the hematopoietic stem cell microenvironment.
PERSPECTIVES / FUTURE DIRECTIONS
HEMATOPOIETIC STEM CELLS HETEROGENEITY
Hematopoietic stem cells are not homogeneous. There have been shown subpopulations based on their life span [44], specific surface markers [45], differentiation capacities [46], and level of self-renewal [47]. Although great advances were made regarding our knowledge of the bone marrow niche components, how extrinsic regulators act on hematopoietic stem cell subsets remains completely unknown. Interestingly, Upadhaya and colleagues analyzed only about one-fifth of hematopoietic stem cells, as this is approximately the amount labeled in Pdzk1ip1-CreER/TdTomato mice [43]. It remains unclear whether in these transgenic mice a subpopulation of rapidly moving hematopoietic stem cells is selected or whether all hematopoietic stem cells display approximately the same rate of movement. Future studies should study the behavior of not-expressing Pdzk1ip1 hematopoietic stem cells.
Hematopoietic stem cells modify their differentiation capacity during aging, losing gradually their self-renewal ability, becoming increasingly myeloid-biased [48, 49]. The changes perceived in old hematopoietic stem cells were speculated to be exclusively due to hematopoietic stem cell-intrinsic alterations [50, 51]. Nonetheless, recent results show the critical function of several extrinsic molecules inducing hematopoietic stem cell aging as well [52]. It will be interesting to explore how hematopoietic stem cells’ behavior changes in live animals with aging, and whether myeloid-biased hematopoietic stem cells behave differently from the others.
OTHER HEMATOPOIETIC STEM CELL NICHES
During embryonic development, hematopoiesis occurs at specific anatomical sites that change with the developmental age [53–56]. This happens because of the migration of hematopoietic stem cells throughout the embryo [57]. The hematopoietic activity starts in the extraembryonic yolk sac at embryonic day 7.5; then, at day 9, it advances to the dorsal aorta-gonad-mesonephros (AGM region), the para-aortic splanchnopleura, and chorioallantoic placenta [58]; at day 10, it arrives to vitelline and umbilical arteries, spleen, skeletal muscle surrounding the developing long bones, and the fetal liver, where hematopoietic stem cells expand exponentially [54, 55, 59–70]. Lastly, at day 15, hematopoietic stem cells from the fetal liver move through the circulation to the bone marrow cavity, which turns into the dominant niche for hematopoietic stem cells throughout the whole adult life [53, 62]. Hematopoietic stem cells in adults can also appear outside the medullary spaces. This phenomenon is termed extramedullary hematopoiesis. was reported in adults in the periosteum, spleen, liver, heart, kidney, adrenal glands, fatty tissue, intra-spinal tissue, para-vertebral regions, pre-sacral region, nasopharyngeal region, paranasal sinuses, and in multiple types of cancers [71–81]. Although it normally indicates a pathologic state of the organ, recent works show the extramedullary hematopoiesis may occur under physiologic conditions as well. Elegant studies have shown the presence of hematopoietic stem cells in the pulmonary microenvironment under physiologic circumstances [36, 82]. Future studies using modern technologies such as two-photon laser scanning microscopy adapted to the specific organs will reveal how hematopoietic stem cells behave in these extramedullary niches.
THE QUIESCENT STATE
The definition of quiescence emerged from the perception that each cell in a population proliferates at its own rate [83]. Thus, cells that are in a non-proliferative state are termed quiescent, even under certain stimuli they can enter the cell cycle and start proliferating. Unicellular organisms, which survive in adverse habitats, enter the quiescent state to not be extinct [84]. Similarly, stem cells exist in a quiescent state throughout our life to keep for as long as possible a reserve pool. Despite quiescence being considered as a dormant static state, quiescence seems to portray a state in which the stem cell is ready to be activated. Upadhaya and colleagues demonstrate that quiescent hematopoietic stem cells are not so “dormant”, being rather “awake” based on the movement that they present within the niche [43]. The reason for this augmented mobility of hematopoietic stem cells should be examined in future studies. It is interesting to explore the molecular mechanisms involved in this movement. It remains uncertain whether this migration is caused by active molecules that promote hematopoietic stem cell mobility or by the lack of specific anchoring factors. Are hematopoietic stem cells searching for a higher gradient of specific limited factors within the niche? Are other quiescent stem cells also behaving like hematopoietic stem cells in live mice? Also, as circadian rhythms influence hematopoietic stem cells [85], it will be attractive to examine whether hematopoietic stem cell behavior varies during light cycles.
INTERACTIONS WITHIN THE BONE MARROW NICHE
The bone marrow microenvironment defines the hematopoietic stem cell fate [34]. Experimental data has revealed that small changes in niche regulatory mechanisms affect directly hematopoietic stem cells [45]. Understanding exactly how hematopoietic stem cells are controlled by their niche is of fundamental importance. Upadhaya and colleagues showed the proximity of hematopoietic stem cells to the perivascular zones [43], as it has been previously reported [45]. Nevertheless, the perivascular niche itself is complex. Perivascular cells have been distinguished as essential components of the hematopoietic stem cell microenvironment [86, 87], and in vivo genetic elimination of those cells from the bone marrow directly affects hematopoietic stem cells [86]. There are two main subpopulations of bone marrow perivascular cells in regards to their vascular positions: sinusoidal and arteriolar pericytes [37]. Most of the quiescent hematopoietic stem cells reside closer to arterioles [37]. Upadhaya and colleagues did not determine whether their analyzes were done in the sinusoidal or arteriolar niches [43]. Future studies should explore whether hematopoietic stem cells behave differently in these two central niches within live mice.
Upadhaya and colleagues showed that the blockade of C-X-C motif chemokine 12 (CXCL12) signaling abrogates hematopoietic stem cell movement in the niche [43]. It is not clear, however, whether this is caused by a direct or indirect effect of the drug. Is the drug acting directly on hematopoietic stem cells or on a niche component? Interestingly, sinusoidal and arteriolar niches contribute with different cytokines for the maintenance of hematopoietic stem cells. CXCL12-derived from the arteriolar niche is essential for hematopoietic stem cells, but not the one derived from the sinusoidal niche. Thus, it would be important to analyze hematopoietic stem cell behavior in response to CXCL12 deletion only from arteriolar pericytes. In contrast, SCF from the sinusoidal niche, but not from the arteriolar, seems to be essential for hematopoietic stem cell functioning. Thus, future experiments should address how distinct niche regulatory molecules affect hematopoietic stem cells’ behavior in live animals.
Modern technologies provide the possibility of eliminating single cells from the tissue microenvironment and analyzing the behavior of the remaining cells [88–90]. Thus, it is possible to explore the effect of eliminating single components of the niche by using targeted two-photon irradiation and analyzing the effect on hematopoietic stem cells’ behavior by two-photon laser scanning microscopy. Alternatively, it will be interesting to evaluate what is the effect of the death of one hematopoietic stem cell on other neighboring hematopoietic stem cells. Thus, longitudinal imaging studies may advance significantly our knowledge on hematopoietic stem cell biology in the future.
Our better understanding of hematopoietic stem cells’ behavior in their normal bone marrow microenvironment leads to questions on how these cells behave in the bone marrow in different pathologies. Changes in the normal bone marrow niche may activate the appearance of pre-leukemic microenvironments [91]. How leukemic stem cells may affect this hematopoietic stem cell behavior, as well as how the leukemia stem cells themselves behave in live animals within their niches remains to be discovered.
CONCLUSION
In conclusion, the study by Upadhaya and colleagues reveals how the most illustrious residents of the bone marrow behave within their niche in live animals [43]. However, our understanding of the hematopoietic stem cells’ behavior in their niches still remains limited, and the complexity of interactions with all niche components should be elucidated in future studies. Despite the powerful experimental transgenic models that provide proof of concept for the hematopoietic stem cell biology within the bone marrow, we are still lacking direct demonstration of hematopoietic stem cell behavior within the human bone marrow cavity. The main question for the future is whether we can translate mice research into humans. Improving the availability of human bone marrow biopsies will be essential to reach this aim. The creation of bone marrow organoids from human induced pluripotent stem cells (iPSCs) may in the future support the data provided by elegant mouse studies.
Figure 1. Schematic illustrating hematopoietic stem cell movement within the bone marrow niche.
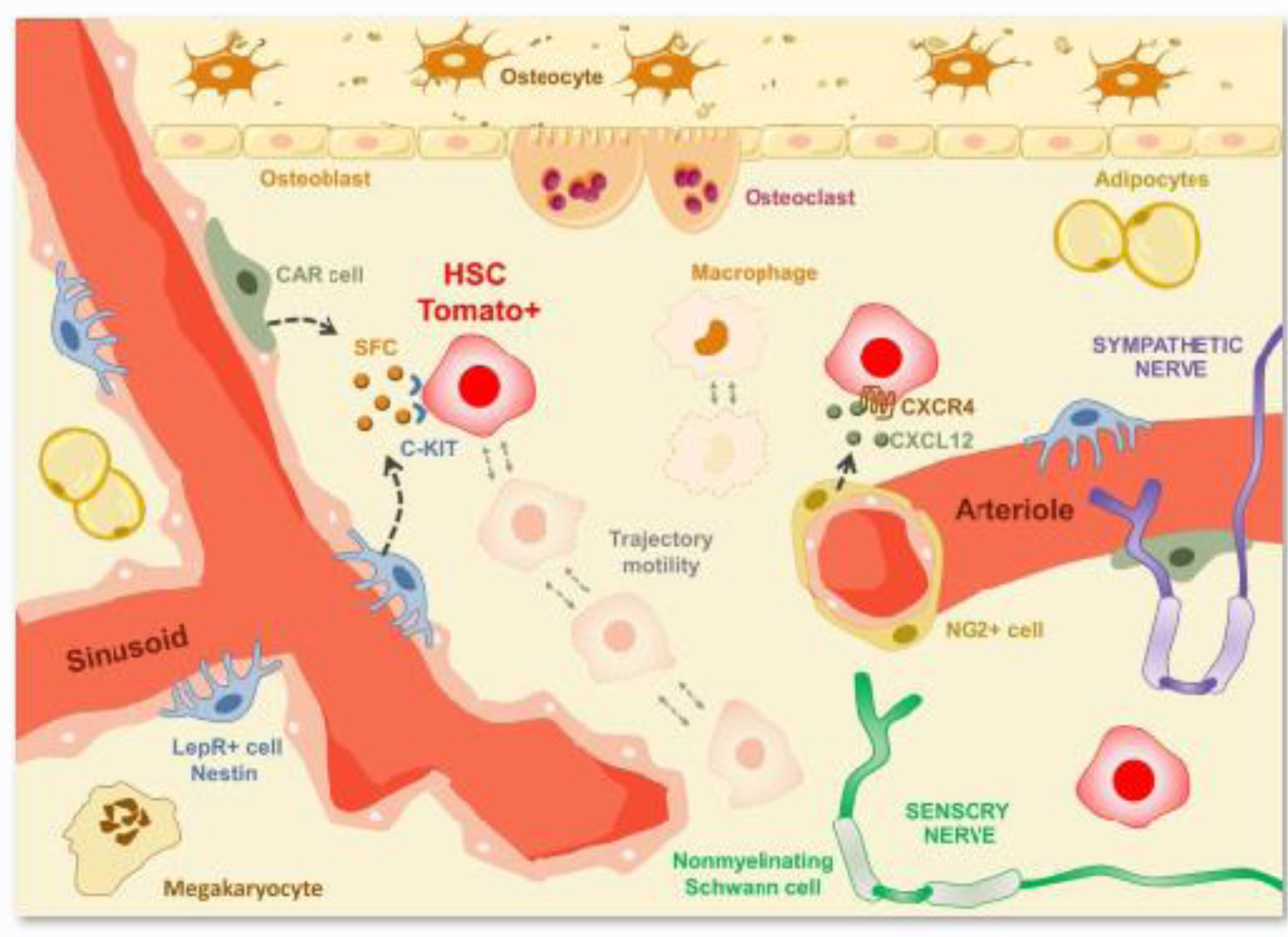
Hematopoietic stem cells (in red) present dynamic morphology (non-spherical) and complex motile behavior when compared to sessile resident macrophages (in brown) within the bone marrow cavity. Upadhaya and colleagues demonstrated that hematopoietic stem cells’ displacement velocity is 7.5 times faster than macrophages [43].
Figure 2. Hematopoietic stem cell retained steady state within the bone marrow niche is linked to a mobile behavior.
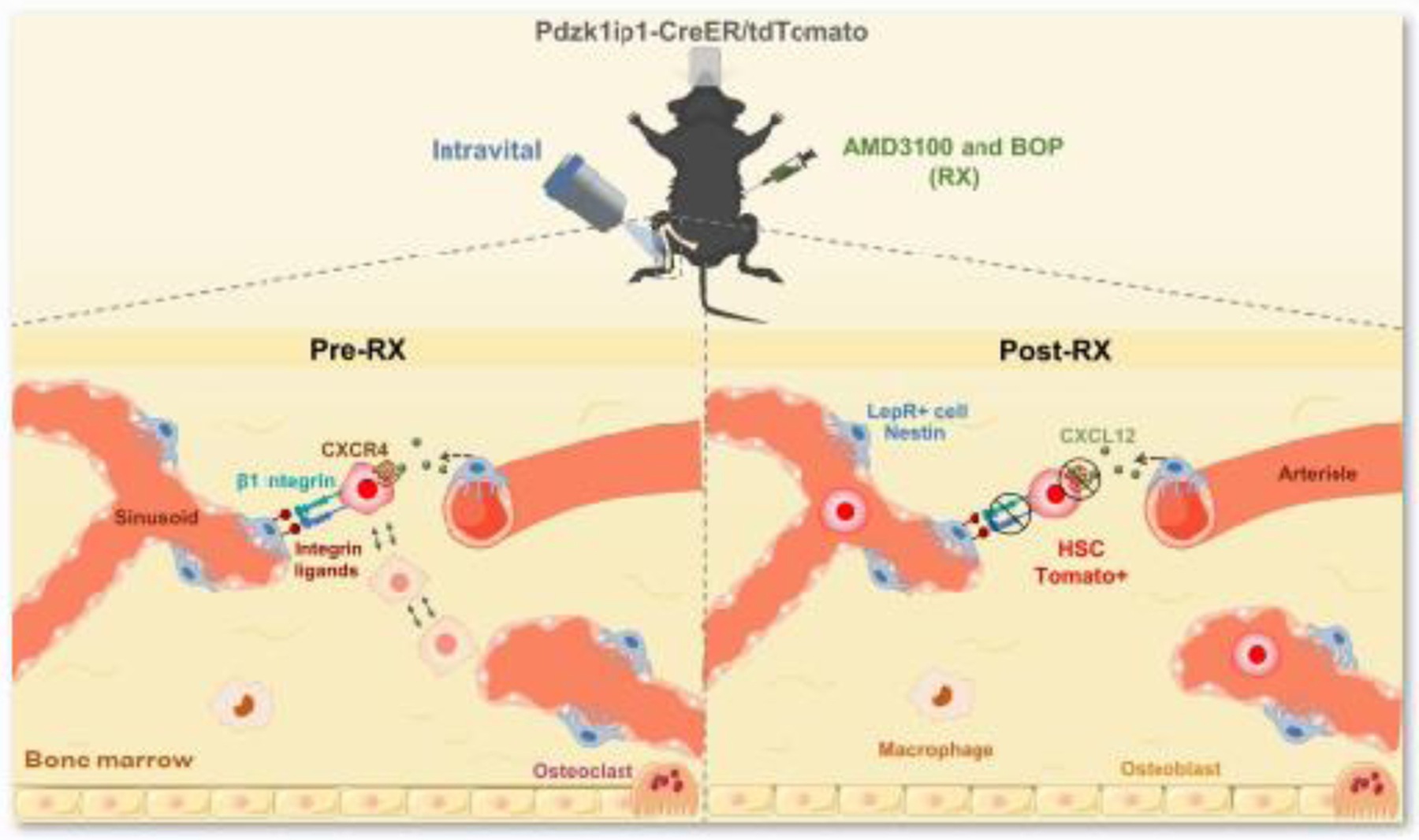
Mobilization of the hematopoietic stem cells from the bone marrow niche by drugs, that block CXCR4 (plerixafor, AMD3100) and integrin signaling [N-(Benzenesulfonyl)-L-prolyl-L-O-(1-pyrrolidinylcarbonyl) tyrosine, (BOP)] (AMD3100 + BOP, RX), inhibits hematopoietic stem cell mobility as well as its form fluctuations within the niche [43].
Highlights.
Hematopoietic stem cell retained steady state within the bone marrow niche is linked to a mobile behavior.
The heterogeneity of hematopoietic stem cells bone marrow niche
Hematopoietic stem cells’ displacement velocity in the bone marrow faster than macrophages
ACKNOWLEDGMENTS
Alexander Birbrair is supported by a grant from Instituto Serrapilheira/Serra-1708–15285, a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016), a grant from CNPQ (Universal, Process No. 405977/2018–2), a grant from National Institute of Science and Technology in Theranostics and Nanobiotechnology (CNPq/CAPES/FAPEMIG, Process No. 465669/2014–0), a grant from FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED-00570–16)], a grant from FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED-00313–16)], and a productivity fellowship from the National Council for Scientific and Technological Development (CNPq); Akiva Mintz is supported by the National Institute of Health (1R01CA179072–01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13–121-01-CDD). CCP and ACC are supported by doctoral fellowships from CAPES. PACC is supported by a postdoctoral fellowship (PNPD) from CAPES. GSPS is supported by a doctoral fellowship from CNPq. WNS and BGSR are supported by master fellowships from CAPES.
SHORT BIOGRAPHIES
Walison N. Silva is a Master student at Department of Pathology at the Federal University of Minas Gerais, Brazil, pursuing research in melanoma microenvironment and stem cells.
Alinne C. Costa, M.Sc., is a Ph.D. student in the Department of Pathology at the Federal University of Minas Gerais. Her research focuses on skeletal muscle regeneration, muscle development, stem cells, and cancer biology.
Caroline C. Picoli, M.Sc., is a Ph.D. student at the Department of Pathology at the Federal University of Minas Gerais. Her research focuses on the microenvironment of adipose perivascular cells.
Beatriz G. S. Rocha, is a Master student at Department of Pathology at the Federal University of Minas Gerais, pursuing research in perivascular cells influence on breast cancer progression.
Gabryella S. P. Santos, M.Sc. is a Ph.D. student in the Department of Pathology at the Federal University of Minas Gerais. Her research focuses on research in the prostate tumor microenvironment.
Pedro A. C. Costa, M.Sc., Ph.D., is a postdoctoral researcher in the Department of Pathology at the Federal University of Minas Gerais. He obtained his Ph.D. in Health Sciences from the René Rachou Research Center (CPqRR-Fiocruz Minas). His research focuses on the immunoregulation of the tumor microenvironment.
Parviz Azimnasab-sorkhabi M.Sc., is pursuing a Ph.D. in the Pathology Department at the Federal University of Mina Gerais. His research focuses on the role of cancer stem cells in the tumor microenvironment.
Maryam Soltani-asl, M.Sc., is a Ph.D. student at the Department of Pathology at the Federal University of Minas Gerais. Her research focuses on the melanoma metastasis microenvironment.
Rodrigo A. da Silva, M.Sc., Ph.D., is a postdoctoral researcher in the Department of Pathology at the Federal University of Minas Gerais. He obtained his PhD in Biochemistry from Campinas State University. His research focuses on biochemistry, molecular biology, and mechanisms of gene regulation mediated by epigenetic mechanisms.
Jaime Henrique Amorim M.Sc., Ph.D., is a professor in the Department of Biochemistry and Immunology at the Federal University of West Bahia. He obtained his PhD in Biotechnology at the University of São Paulo. His main research interests focus on vaccine development for cancer.
Rodrigo R. Resende, M.Sc., Ph.D., is a professor in the Department of Biochemistry and Immunology at the Federal University of Minas Gerais. He obtained his PhD in Biochemistry from the University of São Paulo. His research focuses on Neuroscience, Nanobiotechnology, Stem Cells, Calcium Signaling, and Tissue Engineering.
Akiva Mintz, M.D., Ph.D., is a Professor of Radiology at Columbia University Medical Center and Attending Radiologist at New York Presbyterian Hospital. He obtained his medical and graduate degrees at the Pennsylvania State University College of Medicine. Dr. Mintz’s cross-translational research efforts exploit nuclear-based molecular imaging and therapy techniques to personalize anti-cancer therapies.
Alexander Birbrair, Ph.D., is a Professor in the Department of Pathology at the Federal University of Minas Gerais. He obtained his Ph.D. in Neuroscience from Wake Forest School of Medicine. His laboratory is interested in understanding how the cellular components of different tissues function and control disease progression. His research is funded by the Serrapilheira Institute, CNPq, CAPES, and FAPEMIG. In 2018, Alexander was elected affiliate member of the Brazilian Academy of Sciences (ABC), and, in 2019, he was elected member of the Global Young Academy (GYA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Yoder MC, Blood cell progenitors: insights into the properties of stem cells. Anat Rec A Discov Mol Cell Evol Biol, 2004. 276(1): p. 66–74. [DOI] [PubMed] [Google Scholar]
- 2.Cooper B, The origins of bone marrow as the seedbed of our blood: from antiquity to the time of Osler. Proc (Bayl Univ Med Cent), 2011. 24(2): p. 115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobson LO, et al. , The role of the spleen in radiation injury and recovery. J Lab Clin Med, 1950. 35(5): p. 746–70. [PubMed] [Google Scholar]
- 4.Lorenz E, et al. , Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J Natl Cancer Inst, 1951. 12(1): p. 197–201. [PubMed] [Google Scholar]
- 5.Jacobson LO, et al. , Recovery from radiation injury. Science, 1951. 113(2940): p. 510–11. [DOI] [PubMed] [Google Scholar]
- 6.Main JM and Prehn RT, Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J Natl Cancer Inst, 1955. 15(4): p. 1023–9. [PubMed] [Google Scholar]
- 7.Till JE and Mc CE, A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res, 1961. 14: p. 213–22. [PubMed] [Google Scholar]
- 8.Wu AM, et al. , Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med, 1968. 127(3): p. 455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker AJ, Mc CE, and Till JE, Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature, 1963. 197: p. 452–4. [DOI] [PubMed] [Google Scholar]
- 10.Siminovitch L, McCulloch EA, and Till JE, The Distribution of Colony-Forming Cells among Spleen Colonies. J Cell Comp Physiol, 1963. 62: p. 327–36. [DOI] [PubMed] [Google Scholar]
- 11.Wu AM, et al. , A cytological study of the capacity for differentiation of normal hemopoietic colony-forming cells. J Cell Physiol, 1967. 69(2): p. 177–84. [DOI] [PubMed] [Google Scholar]
- 12.Wolf NS and Trentin JJ, Differential proliferation of erythroid and granuloid spleen colonies following sublethal irradiation of the bone marrow donor. J Cell Physiol, 1970. 75(2): p. 225–9. [DOI] [PubMed] [Google Scholar]
- 13.Thomas ED, et al. , Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med, 1957. 257(11): p. 491–6. [DOI] [PubMed] [Google Scholar]
- 14.Mathe G, et al. , [Transfusions and grafts of homologous bone marrow in humans after accidental high dosage irradiation]. Rev Fr Etud Clin Biol, 1959. 4(3): p. 226–38. [PubMed] [Google Scholar]
- 15.Mathe G, et al. , Haematopoietic Chimera in Man after Allogenic (Homologous) Bone-Marrow Transplantation. (Control of the Secondary Syndrome. Specific Tolerance Due to the Chimerism). Br Med J, 1963. 2(5373): p. 1633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wayne AS, Baird K, and Egeler RM, Hematopoietic stem cell transplantation for leukemia. Pediatr Clin North Am, 2010. 57(1): p. 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto S, et al. , Hematopoietic stem cell transplantation for pediatric acute promyelocytic leukemia in Japan. Pediatr Blood Cancer, 2020. 67(5): p. e28181. [DOI] [PubMed] [Google Scholar]
- 18.Khaddour K, Hana CK, and Mewawalla P, Hematopoietic Stem Cell Transplantation, in StatPearls. 2020: Treasure Island (FL). [PubMed] [Google Scholar]
- 19.Sanchez-Aguilera A and Mendez-Ferrer S, The hematopoietic stem-cell niche in health and leukemia. Cell Mol Life Sci, 2017. 74(4): p. 579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh AK and Cancelas JA, Gap Junctions in the Bone Marrow Lympho-Hematopoietic Stem Cell Niche, Leukemia Progression, and Chemoresistance. Int J Mol Sci, 2020. 21(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurenti E, et al. , Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell, 2008. 3(6): p. 611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada S, et al. , In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood, 1992. 80(12): p. 3044–50. [PubMed] [Google Scholar]
- 23.Challen GA, et al. , Mouse hematopoietic stem cell identification and analysis. Cytometry A, 2009. 75(1): p. 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiel MJ, Radice GL, and Morrison SJ, Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell, 2007. 1(2): p. 204–17. [DOI] [PubMed] [Google Scholar]
- 25.Dykstra B, et al. , High-resolution video monitoring of hematopoietic stem cells cultured in single-cell arrays identifies new features of self-renewal. Proc Natl Acad Sci U S A, 2006. 103(21): p. 8185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiel MJ, et al. , Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature, 2007. 449(7159): p. 238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C-Z, et al. , Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proceedings of the National Academy of Sciences, 2002. 99(24): p. 15468–15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arai F, et al. , Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell, 2004. 118(2): p. 149–161. [DOI] [PubMed] [Google Scholar]
- 29.Balazs AB, et al. , Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood, 2006. 107(6): p. 2317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodell MA, et al. , Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med, 1997. 3(12): p. 1337–45. [DOI] [PubMed] [Google Scholar]
- 31.Pearce DJ, et al. , Multiparameter analysis of murine bone marrow side population cells. Blood, 2004. 103(7): p. 2541–6. [DOI] [PubMed] [Google Scholar]
- 32.Birbrair A and Frenette PS, Niche heterogeneity in the bone marrow. Annals of the New York Academy of Sciences, 2016. 1370(1): p. 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schofield R, The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells, 1978. 4(1–2): p. 7–25. [PubMed] [Google Scholar]
- 34.Rashidi NM, et al. , In vivo time-lapse imaging shows diverse niche engagement by quiescent and naturally activated hematopoietic stem cells. Blood, 2014. 124(1): p. 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asada N, et al. , Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol, 2017. 19(3): p. 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borges IDT, et al. , Lung as a Niche for Hematopoietic Progenitors. Stem Cell Reviews and Reports, 2017. [DOI] [PMC free article] [PubMed]
- 37.Kunisaki Y, et al. , Arteriolar niches maintain haematopoietic stem cell quiescence. Nature, 2013. 502(7473): p. 637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asada N, et al. , Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol, 2017. [DOI] [PMC free article] [PubMed]
- 39.Acar M, et al. , Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature, 2015. 526(7571): p. 126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo Celso C, et al. , Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature, 2009. 457(7225): p. 92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewandowski D, et al. , In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood, 2010. 115(3): p. 443–52. [DOI] [PubMed] [Google Scholar]
- 42.Takizawa H, et al. , Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. J Exp Med, 2011. 208(2): p. 273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Upadhaya S, et al. , Intravital Imaging Reveals Motility of Adult Hematopoietic Stem Cells in the Bone Marrow Niche. Cell Stem Cell, 2020. [DOI] [PMC free article] [PubMed]
- 44.Yang L, et al. , Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood, 2005. 105(7): p. 2717–23. [DOI] [PubMed] [Google Scholar]
- 45.Birbrair A and Frenette PS, Niche heterogeneity in the bone marrow. Ann N Y Acad Sci, 2016. 1370(1): p. 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller-Sieburg CE, et al. , Stem cell heterogeneity: implications for aging and regenerative medicine. Blood, 2012. 119(17): p. 3900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ema H, et al. , Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev Cell, 2005. 8(6): p. 907–14. [DOI] [PubMed] [Google Scholar]
- 48.Mendelson A and Frenette PS, Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med, 2014. 20(8): p. 833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pang WW, et al. , Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A, 2011. 108(50): p. 20012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geiger H, de Haan G, and Florian MC, The ageing haematopoietic stem cell compartment. Nat Rev Immunol, 2013. 13(5): p. 376–89. [DOI] [PubMed] [Google Scholar]
- 51.Birbrair A, et al. , Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol, 2013. 305(11): p. C1098–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura-Ishizu A and Suda T, Aging of the hematopoietic stem cells niche. Int J Hematol, 2014. 100(4): p. 317–25. [DOI] [PubMed] [Google Scholar]
- 53.Khan JA, et al. , Fetal liver hematopoietic stem cell niches associate with portal vessels. Science, 2016. 351(6269): p. 176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Drees MA, et al. , Making Blood: The Haematopoietic Niche throughout Ontogeny. Stem Cells Int, 2015. 2015: p. 571893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palis J, et al. , Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development, 1999. 126(22): p. 5073–84. [DOI] [PubMed] [Google Scholar]
- 56.Tavian M and Peault B, Embryonic development of the human hematopoietic system. Int J Dev Biol, 2005. 49(2–3): p. 243–50. [DOI] [PubMed] [Google Scholar]
- 57.Bowman TV and Zon LI, Lessons from the Niche for Generation and Expansion of Hematopoietic Stem Cells. Drug Discov Today Ther Strateg, 2009. 6(4): p. 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhodes KE, et al. , The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell, 2008. 2(3): p. 252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swain A, et al. , Intrinsic and extrinsic regulation of mammalian hematopoiesis in the fetal liver. Histol Histopathol, 2014. 29(9): p. 1077–82. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka Y, et al. , Embryonic Hematopoietic Progenitor Cells Reside in Muscle before Bone Marrow Hematopoiesis. PLoS One, 2015. 10(9): p. e0138621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medvinsky AL, et al. , An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature, 1993. 364(6432): p. 64–7. [DOI] [PubMed] [Google Scholar]
- 62.Medvinsky A, Rybtsov S, and Taoudi S, Embryonic origin of the adult hematopoietic system: advances and questions. Development, 2011. 138(6): p. 1017–31. [DOI] [PubMed] [Google Scholar]
- 63.Baron MH, Early patterning of the mouse embryo: implications for hematopoietic commitment and differentiation. Exp Hematol, 2005. 33(9): p. 1015–20. [DOI] [PubMed] [Google Scholar]
- 64.Baron MH, Isern J, and Fraser ST, The embryonic origins of erythropoiesis in mammals. Blood, 2012. 119(21): p. 4828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barminko J, Reinholt B, and Baron MH, Development and differentiation of the erythroid lineage in mammals. Dev Comp Immunol, 2016. 58: p. 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumaravelu P, et al. , Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonadmesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development, 2002. 129(21): p. 4891–9. [DOI] [PubMed] [Google Scholar]
- 67.Muller AM, et al. , Development of hematopoietic stem cell activity in the mouse embryo. Immunity, 1994. 1(4): p. 291–301. [DOI] [PubMed] [Google Scholar]
- 68.Medvinsky A and Dzierzak E, Definitive hematopoiesis is autonomously initiated by the AGM region. Cell, 1996. 86(6): p. 897–906. [DOI] [PubMed] [Google Scholar]
- 69.Sugiyama D and Tsuji K, Definitive hematopoiesis from endothelial cells in the mouse embryo; a simple guide. Trends Cardiovasc Med, 2006. 16(2): p. 45–9. [DOI] [PubMed] [Google Scholar]
- 70.Lux CT, et al. , All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood, 2008. 111(7): p. 3435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sohawon D, et al. , Extra-medullary haematopoiesis: a pictorial review of its typical and atypical locations. J Med Imaging Radiat Oncol, 2012. 56(5): p. 538–44. [DOI] [PubMed] [Google Scholar]
- 72.Johns JL and Christopher MM, Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol, 2012. 49(3): p. 508–23. [DOI] [PubMed] [Google Scholar]
- 73.Tsamandas AC, et al. , Extramedullary hematopoiesis in the allograft liver. Mod Pathol, 1995. 8(6): p. 671–4. [PubMed] [Google Scholar]
- 74.Vassiliou V, et al. , Presacral Extramedullary Hematopoiesis in a Patient with Rectal Adenocarcinoma: Report of a Case and Literature Review. J Gastrointest Cancer, 2012. 43 Suppl 1: p. S131–5. [DOI] [PubMed] [Google Scholar]
- 75.Macki M, et al. , Presacral extramedullary hematopoiesis: an alternative hypothesis. J Clin Neurosci, 2013. 20(12): p. 1664–8. [DOI] [PubMed] [Google Scholar]
- 76.Inra CN, et al. , A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature, 2015. 527(7579): p. 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bozzini CE, et al. , Studies on medullary and extramedullary erythropoiesis in the adult mouse. Am J Physiol, 1970. 219(3): p. 724–8. [DOI] [PubMed] [Google Scholar]
- 78.Bowen JM, et al. , Extramedullary hematopoiesis in a sentinel lymph node as an early sign of chronic myelomonocytic leukemia. Case Rep Pathol, 2015. 2015: p. 594970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schnuelle P, et al. , Idiopathic myelofibrosis with extramedullary hematopoiesis in the kidneys. Clin Nephrol, 1999. 52(4): p. 256–62. [PubMed] [Google Scholar]
- 80.Woodward N, et al. , Renal myelofibrosis: an unusual cause of renal impairment. Nephrol Dial Transplant, 2000. 15(2): p. 257–8. [DOI] [PubMed] [Google Scholar]
- 81.Lewis DJ, et al. , Perirenal liposarcoma containing extramedullary hematopoiesis associated with renal cell carcinoma. Urology, 1994. 43(1): p. 106–9. [DOI] [PubMed] [Google Scholar]
- 82.Lefrancais E, et al. , The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature, 2017. 544(7648): p. 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheung TH and Rando TA, Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol, 2013. 14(6): p. 329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gray JV, et al. , “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol Mol Biol Rev, 2004. 68(2): p. 187–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mendez-Ferrer S, et al. , Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol, 2009. 16(4): p. 235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mendez-Ferrer S, et al. , Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature, 2010. 466(7308): p. 829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pinho S, et al. , PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med, 2013. 210(7): p. 1351–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berthiaume AA, et al. , Dynamic Remodeling of Pericytes In Vivo Maintains Capillary Coverage in the Adult Mouse Brain. Cell Rep, 2018. 22(1): p. 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santos GSP, et al. , Pericyte Plasticity in the Brain. Neurosci Bull, 2019. 35(3): p. 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prazeres P, et al. , Ablation of sensory nerves favours melanoma progression. J Cell Mol Med, 2020. [DOI] [PMC free article] [PubMed]
- 91.Konopleva MY and Jordan CT, Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol, 2011. 29(5): p. 591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


