Abstract
The inhibitory effect of estradiol (E2) on water intake has been recognized for 50 years. Despite a rich literature describing this phenomenon, we report here a previously unidentified dipsogenic effect of E2 during states of low fluid intake. Our initial goal was to test the hypothesis that the anti-dipsogenic effect of E2 on unstimulated water intake is independent of its anorexigenic effect in female rats. In support of this hypothesis, water intake was reduced during estrus, compared to diestrus, when food was present or absent. Water intake was reduced by E2 in ovariectomized rats when food was available, demonstrating a causative role of E2. Surprisingly, however, when food was removed, resulting in a significant reduction in baseline water intake, E2 enhanced drinking. Accordingly, we next tested the effect of E2 on water intake after an acute suppression of intake induced by exendin-4. The initial rebound drinking was greater in E2treated, compared to Oil-treated, rats. Finally, to reconcile conflicting reports regarding the effect of ovariectomy on water intake, we measured daily water and food intake, and body weight in ovariectomized and sham-operated rats. Predictably, ovariectomy significantly increased food intake and body weight, but only transiently increased water intake. Together these results provide further support for independent effects of E2 on the controls of water and food intake. More importantly, this report of bidirectional effects of E2 on water intake may lead to a paradigm shift, as it challenges the prevailing view that E2 effects on fluid intake are exclusively inhibitory.
Keywords: Estrogens, ovariectomy, food intake, drinking microstructure
INTRODUCTION
Over 50 years of research has demonstrated that the ovarian hormone estradiol (E2) exerts a suppressive effect on both fluid and food intake that is particularly well characterized in the female rat (Curtis, 2009; Eckel, 2004; Santollo and Daniels, 2015b, c). For example, water and saline intakes stimulated by treatments that mimic extracellular dehydration, such as treatment with angiotensin II (AngII) or isoproterenol, are reduced on the day of estrus (E), after circulating E2 levels peak on the afternoon of proestrus (Becker et al., 2005; Danielsen and Buggy, 1980; Findlay et al., 1979). In ovariectomized (OVX) rats, E2 treatment reduces water intake stimulated by AngII, isoproterenol, and water deprivation (Fregly, 1978; Fregly and Thrasher, 1978; Jonklaas and Buggy, 1984; Kisley et al., 1999; Krause et al., 2003; Thrasher and Fregly, 1977, 1978). E2 exerts its anti-dipsogenic and anti-natriorexigenic effects on stimulated intake through activation of multiple estrogen receptor (ER) subtypes by enhancing both postingestive and orosensory feedback signals (Santollo and Daniels, 2015a; Santollo et al., 2016; Thammacharoen et al., 2009). In addition to these fluid intake effects, daily food intake is lowest on the day of estrus (Blaustein and Wade, 1976; Drewett, 1973; Eckel et al., 2000; Schwartz and Wade, 1981). In OVX rats, E2-treatment reduces food intake by enhancing postingestive signals primarily through activation of estrogen receptor alpha (Eckel and Geary, 1999; Roesch, 2006; Santollo et al., 2007; Thammacharoen et al., 2009). In addition to this phasic anorexigenic effect, that occurs after the cyclic peak in endogenous E2 release or after exogenous E2 treatment, OVX results in a more sustained increase in daily food intake, related to the loss of persistent low levels of E2 (Blaustein and Wade, 1976; McElroy and Wade, 1987; Mook et al., 1972; Tarttelin and Gorski, 1971; Varma et al., 1999; Wade, 1975; Witte et al., 2010).
Daily unstimulated water intake also reaches its nadir during estrus and is reduced by E2 treatment in OVX rats (Antunes Rodrigues and Covian, 1963; Danielsen and Buggy, 1980; Eckel et al., 2000; Findlay et al., 1979; Santollo et al., 2013; Spiteri et al., 1980; Tarttelin and Gorski, 1971). Whether the anti-dipsogenic effect of E2 extends to daily unstimulated water intake, however, is complicated by the fact that rats are prandial drinkers; therefore, the reduction in water intake could be secondary to the reduction in food intake. Previous research, however, by Czaja and colleagues demonstrated independent effects of E2 on daily food and water intake in the female guinea pig (Czaja et al., 1983). Specifically, when food was rationed to 30% of ab libitum levels, E2-treatment reduced water intake without a concomitant change in food intake. A reduction in food intake was also observed when water was rationed to 30% of ab libitum levels. While this demonstrates that the reduction in daily water intake is not secondary to the anorexigenic effect of E2, and vice versa, important questions remain. Specifically, it is still unclear if E2 has independent effects on daily unstimulated water and food intake in female rats. This is important because the fluid intake effects of E2 are almost exclusively characterized in the female rat and there are species specific differences in the ingestive effects of E2 (Eckel, 2004; Roepke et al., 2010; Witte et al., 2010). Furthermore, if E2-mediated reductions in water intake are independent of feeding in rats, another open question is whether this effect involves changes in postingestive or orosensory feedback, which can be addressed by examining drinking microstructure patterns.
Here we tested the hypothesis that the anti-dipsogenic effect of E2 on daily unstimulated intake is independent of the anorexigenic effect of E2. We first tested this hypothesis in cycling female rats and then as a direct test of E2, in OVX rats with or without E2 replacement. In OVX rats, however, we revealed an unexpected dipsogenic effect of E2 during conditions that typically decrease water intake. Furthermore, while the orexigenic effect of OVX has been well reported, post-OVX changes in water intake are less well studied. Tarttelin and Gorski reported a dipsogenic effect of OVX that lasted for 12 days post-surgery, however, a later study by Findlay and colleagues reported that this dipsogenic effect was the result of increased body weight (Findlay et al., 1979; Tarttelin and Gorski, 1971). Accordingly, we also tested for changes in water intake as a result of OVX. An absence of or a temporal disassociation of any OVX induced changes in food and water intake would provide additional support for independent effects of E2 on daily water and food intake in the female rat.
MATERIALS AND METHODS
Animals
Adult female Long Evans rats (Envigo) were used throughout the experiments. Rats were between 75 and 120 days of age at the start of the experiments. Rats were singly housed in modified shoebox cages (two external lick blocks) with ad libitum access to food (Teklad 2018; Envigo), tap water, and 1.5% saline solution (Experiment 1b) unless otherwise noted. The temperature- and humidity-controlled colony rooms were maintained on a 12:12 h light-dark cycle (lights on at 0100 h Experiment 1, 2, & 3; lights on at 0700 h Experiment 4). All experimental protocols were approved by the Animal Care and Use Committee at the University of Kentucky, and the handling and care of the rats was in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Surgery
Rats in Experiments 2–4 were ovariectomized. Briefly, rats were anesthetized with isoflurane and given 5 mg/kg carprofen (sc; Henry Schein, Dublin, OH). Rats were then bilaterally OVX using an intraabdominal approach as previously described (Santollo et al., 2019). Twelve rats in Experiment 4 received a SHAM surgery, where the ovaries were externalized but then placed back into the body cavity. After surgery, rats received 5 ml of sterile 0.9% saline (sc) and 24 h later received a second injection of 5 mg/kg carprofen. Rats were given two weeks to recover from surgery before hormone replacement and testing (Experiment 2). Data collection for rats in Experiment 4 continued throughout surgical recovery.
Intake and Lick Measurements
Water and food (when applicable) intakes were measured at the start and end of each test period. In Experiments 2 and 3, the distribution of drinking throughout the test was assessed by use of a custom designed contact lickometer (University of Kentucky, College of Arts and Sciences Electronics Shop) that recorded individual licks. The lickometer connected to modified shoebox cages, which have two external lick blocks. Bottle spouts were behind an electrically isolated metal plate with a 3.175 mm-wide opening, through which the rat needed to lick to reach the spout, minimizing non-tongue contacts (i.e. paw) with the spout. The lickometer interfaced with a computer using an integrated USB digital I/O device (National Instruments, Austin, TX) to record time-stamped licks, after which the data were processed in Excel. Licks across the testing period, therefore, could be analyzed in any size bin, to capture any changes in intake. The time stamp recorded licks also allows for the analysis of drinking microstructure, which was processed in Excel with a burst defined as at least 2 licks with an inter-lick-interval (ILI) of no more than 1 s. Burst size was defined as the average number of licks within a burst. If no bursts occurred, the burst size could not be calculated, and those subjects were not included in the burst size analysis.
Experiment 1: Is the reduction in fluid intake on estrus secondary to a reduction in food intake?
Body weight and vaginal cytology samples were monitored daily to determine estrous cycle stage (as previously described,(Santollo et al., 2019), in 23 female rats (Experiment 1a n = 12; Experiment 1b n = 11). In Experiment 1a, 23 h water intake was measured once a cycle on either diestrus 2 (D2) or estrus (E) in the presence or absence of food. When food was available, intake of food was also measured. Testing occurred for four cycles to achieve a repeated measures design. The testing protocol was repeated in a new group of rats in Experiment 1b, but with rats having access to 1.5% saline, in addition to water, because previous reports demonstrate cycle related decreases in saline intake (Antunes Rodrigues and Covian, 1963; Danielsen and Buggy, 1980).
Experiment 2: Is the reduction in water intake in OVX rats after EB-treatment secondary to a reduction in food intake?
Experiment 2a:
11 OVX rats were treated with oil or 10 μg estradiol benzoate (EB, Sigma) at 0900 h once a day for two days. Immediately prior to dark onset on day two, rats were given pre-weighed water bottles and intake and licks were measured for 23 h in the presence or absence of food. When food was available, intake of food was also measured. The testing protocol occurred once a week for four weeks to achieve a repeated measured design. The hormone replacement regimen was chosen based on past research examining the fluid intake effects of E2 (Findlay et al., 1979; Graves et al., 2011; Jones et al., 2012; Krause et al., 2003; Spiteri et al., 1980).
Experiment 2b:
To replicate and extend the findings from Experiment 2a, 12 additional OVX rats were tested only in the absence of food. Again, rats were treated with oil or 10 μg EB at 0900 h once a day for two days. To test the effect of EB + progesterone (P), rats also received an injection of 500 mg P (Sigma) on day two, resulting in three treatment groups (Oil, EB, EB +P). Immediately prior to dark onset on day two, rats were given pre-weighed water bottles and intake and licks were measured for 23 h. The testing protocol occurred once a week for three weeks to achieve a repeated measures design.
Experiment 2c:
Next, we determined if EB reduced food intake in the absence of water. A subset of rats (n = 9) from Experiment 2a were treated with oil or 10 μg EB at 0900 h once a day for two days. Immediately prior to dark onset on day two, water was removed, rats were given pre-weighed food, and intake was measured 23 h later. The testing protocol occurred once a week for two weeks to achieve a repeated measures design.
Experiment 3: Does EB increase water intake after an acute suppression of intake caused by exendin-4 treatment?
Next, we explored changes in water intake as a function of EB treatment after an acute suppression of intake. To do so, we treated rats with the GLP-1 receptor agonist Exendin-4, which has previously been reported to temporarily suppress water intake in male rats by enhancing postingestive feedback signals (McKay and Daniels, 2013; McKay et al., 2011). Eleven OVX rats from Experiment 2 were treated with oil or 10 μg EB at 0900 h once a day for two days. Thirty min before dark onset on day two, rats were injected (sc) with 3 μg/kg exendin-4 (Ex-4, GLP-1 receptor agonist, Tocris). Immediately prior to dark onset, rats were given pre-weighed water bottles and food, and intake and licks were measured for 23 h. The testing protocol occurred once a week for two weeks to achieve a repeated measures design. This dose of Ex-4 was chosen because it has been previously reported to temporarily decrease dark phase water intake for ~4 h in male rats (McKay et al., 2011).
Experiment 4: What is the time course for the increase in water and food intake after OVX?
Daily body weight, water, and food intake were recorded in 24 female rats for one week. Rats then received either a sham or OVX surgery (n = 12/group). Daily body weight, water and food intake were recorded for another 5 weeks after surgery.
Data Analysis
The statistical software package Statistica was used to analyze all data. Data are presented as MEANS ± SEM throughout. Data normality was verified with Q-Q plots of the residuals. When data sets were not normally distributed, a log transformation was applied (Experiment 1a: food intake, Experiment 1b: saline and total fluid intake, Experiment 2b: licks, Experiment 3: Licks across time, Experiment 4: add data), except for the analysis of food intake in Experiment 1b and burst number in Experiment 2a, which were analyzed with a Wilcoxon Matched Pairs Test. Water intake, saline intake, total fluid intake, and total licks (Experiments 1 & 2a) were analyzed with a 2 factor repeated measures ANOVA (food access by cycle stage or hormone). Water intake and licks (Experiment 2b) were analyzed with a one-way repeated measures ANOVA (group). Percent change in water intake, food intake, burst size, burst number, and latency to consume 1 ml of water (Experiments 1, 2, & 3) were analyzed with paired t-tests. Licks across the test period or dark phase (Experiment 2a & 3) were analyzed by a 2 factor repeated measures ANOVA (hormone by time). Daily body weight, food intake, and water intake (Experiment 4) were analyzed with a 2 factor ANOVA (group by day). Newman-Keuls post hoc tests were used to follow up any significant main or interactive effects. Effect sizes were calculated using Cohen’s d for t-tests (μ1-μ2/SD) or eta squared (η2 = SSeffect/SStotal) for ANOVA.
RESULTS
Experiment 1: Is the reduction in fluid intake on estrus secondary to a reduction in food intake?
Water intake in the presence or absence of food was analyzed in D2 and E rats. Water intake was influenced by main effects of cycle stage (F1,11 = 12.91, p = 0.004, η2 = 0.12) and food access (F1,11 = 33.66, p = 0.0002, η2 = 0.50), but there was no interaction between cycle stage and food access (F1,11 = 3.90, p = n.s., η2 = 0.03; Figure 1A). D2 rats drank more water than E rats (p = 0.004) and rats with access to food drank more water than when food was not available (p = 0.0003). The magnitude of the decrease in water intake from D2 to E did not differ as a function of food availability (t11 = 1.91, p = 0.08, Cohen’s d = 0.77; Figure 1B). As expected, D2 rats consumed more food than E rats (t11 = 2.46, p = 0.0168, Cohen’s d = 0.32; Figure 1C). Next, water and 1.5% saline intake in the presence or absence of food was analyzed in D2 and E rats. Both water and saline intakes were influenced by a main effect of food access (F1,10 = 5.56 and 8.07, p = 0.0401 and 0.017, η2 = 0.20 and 0.20, respectively), but there was no effect of cycle stage nor an interaction between cycle stage and food access for either water or saline intake (Table 1). Rats with access to food drank more water (p = 0.04), but less saline (p = 0.018), than when food was not available. There were no main or interactive effects on total fluid (water + saline) intake. Again, as expected, D2 rats consumed more food than E rats (Z10 = 2.80, p = 0.005, Cohen’s d = 0.67).
Figure 1. Water and food intake during E was reduced compared to intake during D2.
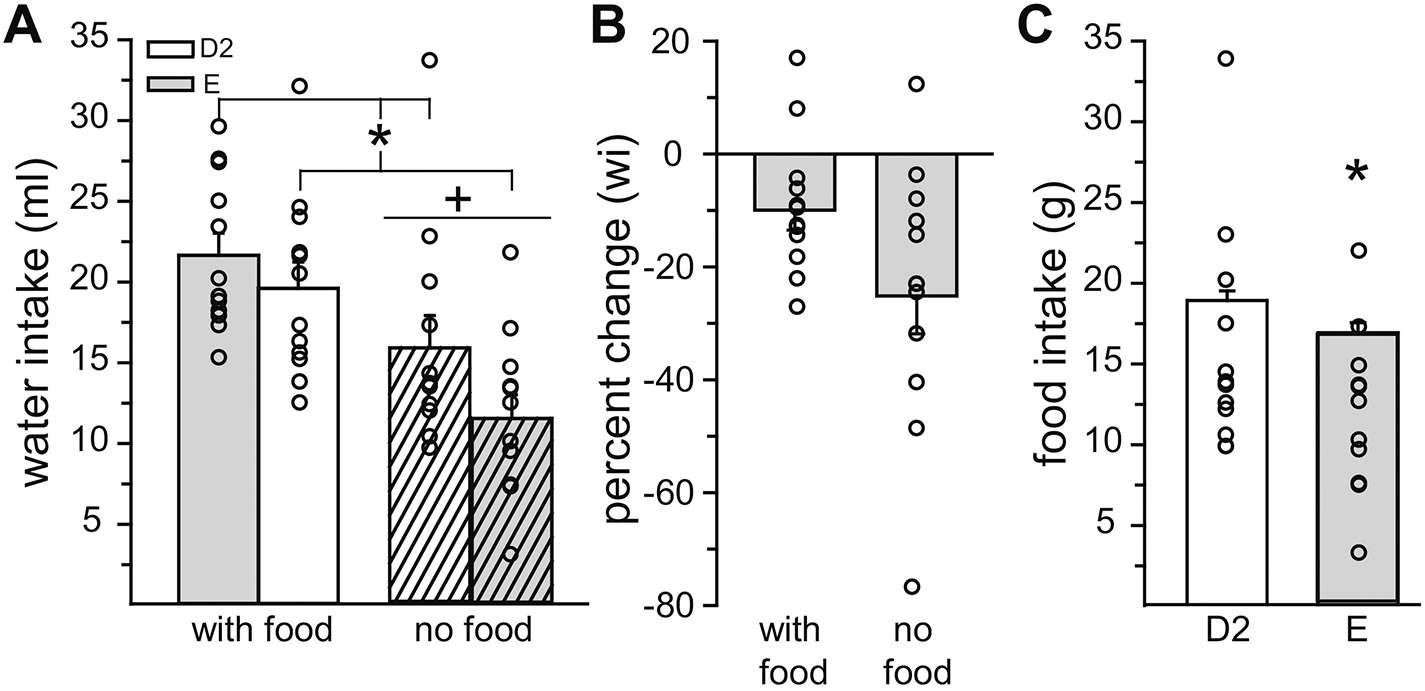
(A) Water intake was lower during E, compared to D2, regardless of food access. Regardless of cycle stage, water intake was greater when food was available. (B) The magnitude of the reduction in water intake (wi) between D2 and E was not affected by food availability. (C) Food intake was lower during E, compared to D2. *Less than D2, p < 0.05. +Less than when food was available, p < 0.05.
Table 1.
Water + Saline intake during D2 and E
| Water (ml) | Saline (ml) | Total Fluids (ml) | Food (g) | |
|---|---|---|---|---|
| With Food: | ||||
| D2 | 17.05 ± 1.60 | 16.15 ± 3.20 | 33.19 ± 3.04 | 19.25 ± 0.81 |
| E | 17.04 ± 2.79 | 18.34 ± 3.97 | 35.37 ± 4.01 | 17.31 ± 0.87* |
| No Food: | + | # | ||
| D2 | 12.60 ± 1.44 | 27.44 ± 5.73 | 40.04 ± 6.32 | - |
| E | 11.38 ± 1.46 | 23.68 ± 4.91 | 35.06 ± 5.67 | - |
Water intake with food > water intake without food, p < 0.05
Saline intake with food < saline intake without food, p < 0.05
D2 food intake > E food intake, p < 0.05
Experiment 2: Is the reduction in water intake after EB-treatment secondary to a reduction in food intake?
As a direct test of the anti-dipsogenic effect of E2, water intake in the presence or absence of food was analyzed in Oil and EB-treated rats. Water intake was influenced by a main effect of food access (F1,10 = 101.5, p = 0.000001, η2 =0.76) and an interactive effect of food access and hormone (F1,10 = 27.65, p = 0.0004, η2 = 0.08; Figure 2A). Regardless of hormone treatment, when food was available rats drank more water than when food was not available (p = 0.0001). When food was available, rats treated with EB consumed less water than Oil-treated rats (p = 0.049). Surprisingly, when food was not available, rats treated with EB consumed more water than Oil-treated rats (p = 0.0005). Analysis of total licks showed similar patterns of statistical results. Total licks were influenced by main effects of food access (F1,10 = 94.93, p = 0.000002, η2 =0.71) and hormone (F1,10 = 5.18, p = 0.045, η2 =0.02), and an interactive effect of food access and hormone (F1,10 = 32.89, p = 0.0001, η2 =0.13; Figure 2B). Again, when food was available, rats treated with EB made fewer licks for water than Oil-treated rats (p = 0.0368). When food was not available, rats treated with EB made more licks for water than Oil-treated rats (p = 0.0002). As expected, Oil-treated rats consumed more food than EB-treated rats (t10 = 3.29, p = 0.008, Cohen’s d = 1.17; Figure 2C). To replicate the unexpected finding that, under these circumstances, EB enhanced water intake, in Expt. 2b we repeated the no food access portion of this experiment in a new group of rats and also tested the effect of P on the dipsogenic effect of EB. Analysis of water intake revealed a main effect of group (F2,22 = 6.26, p = 0.007, η2 = 0.36; Figure 2D). EB and EB + P treatment significantly increased water intake, compared to Oil treatment (p = 0.013), but there was no difference in water intake between EB and EB + P treatment. Analysis of total licks also revealed a main effect of group (F2,22 = 4.42, p = 0.0244, η2 =0.29; data not shown). Again, EB and EB + P treatment significantly increased licks for water, compared to Oil treatment (p = 0.0336). In a separate experiment (Expt. 2c) to determine whether the anorexigenic effect of E2 is secondary to the anti-dipsogenic effect, EB treatment significantly reduced 23 h food intake when water was not present (t8 = 4.82, p = 0.0013, Cohen’s d = 1.32; Figure 2E).
Figure 2. The direction of the fluid intake effect of E2 was influenced by food access in OVX rats.
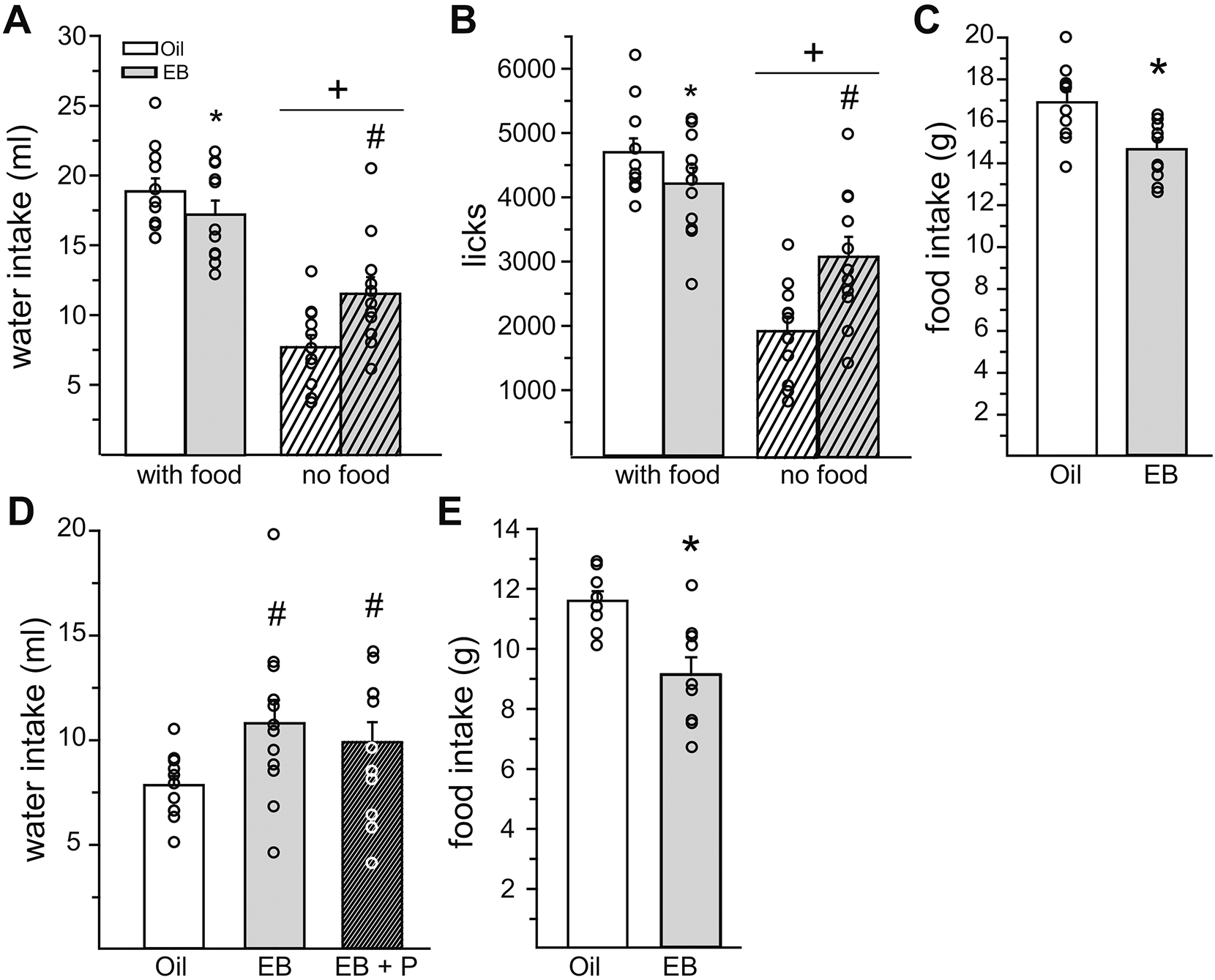
(A) When food was available, EB treatment reduced water intake. When food was not available, EB treatment increased water intake. Regardless of hormone treatment, water intake was greater when food was available. (B) When food was available, EB-treatment reduced licks for water. When food was not available, EB-treatment increased licks for water. Regardless of hormone treatment, licks for water were greater when food was available. (C) EB-treatment reduced food intake. (D) When food was not available, both EB and EB+P treatment increased water intake. (E) When water was not available, EB treatment reduced food intake. *Less than Oil, p < 0.05. #Greater than Oil, p < 0.05. +Less than when food was available, p < 0.05.
Next, to further explore the nature of the change in intake as a function of EB, we analyzed drinking microstructure and licks as a function of time (Experiment 2a). Because EB differentially influenced water intake depending on food availability, we performed separate analysis for when food was available and when it was not available. When food was present, there was no effect of EB treatment on burst number (t10 = 0.29, p = n.s., Cohen’s d = 0.11) or burst size (t10 = 0.94, p = n.s., Cohen’s d = 0.38) during the 23 h test period (Figure 3A/B). Analysis of licks in 6 h bins revealed a main effect of hormone (F1,10 = 7.49, p = 0.00398, η2 =0.004) and time (F3,30 = 83.66, p = 1.13E-14, η2 =0.83), but no interaction of hormone and time (F3,30 = 0.07, p = n.s., η2 =0.0005; Figure 3C). We predicted that EB would influence intake in discrete time bins, however, a priori post hoc analysis did not reveal a selective effect of EB treatment in any specific time bin. Analysis of drinking microstructure within the 6 h bins demonstrated that during the 4th bin, EB treatment reduced burst number (Z10 = 2.52, p = 0.012; Cohen’s d = 0.97; Figure 3D). Nine out of eleven rats in the EB group took no bursts during bin 4, which prevented a statistical analysis of burst size (Figure 3E).
Figure 3. The anti-dipsogenic effect of E2 decreased water intake by reducing burst number.
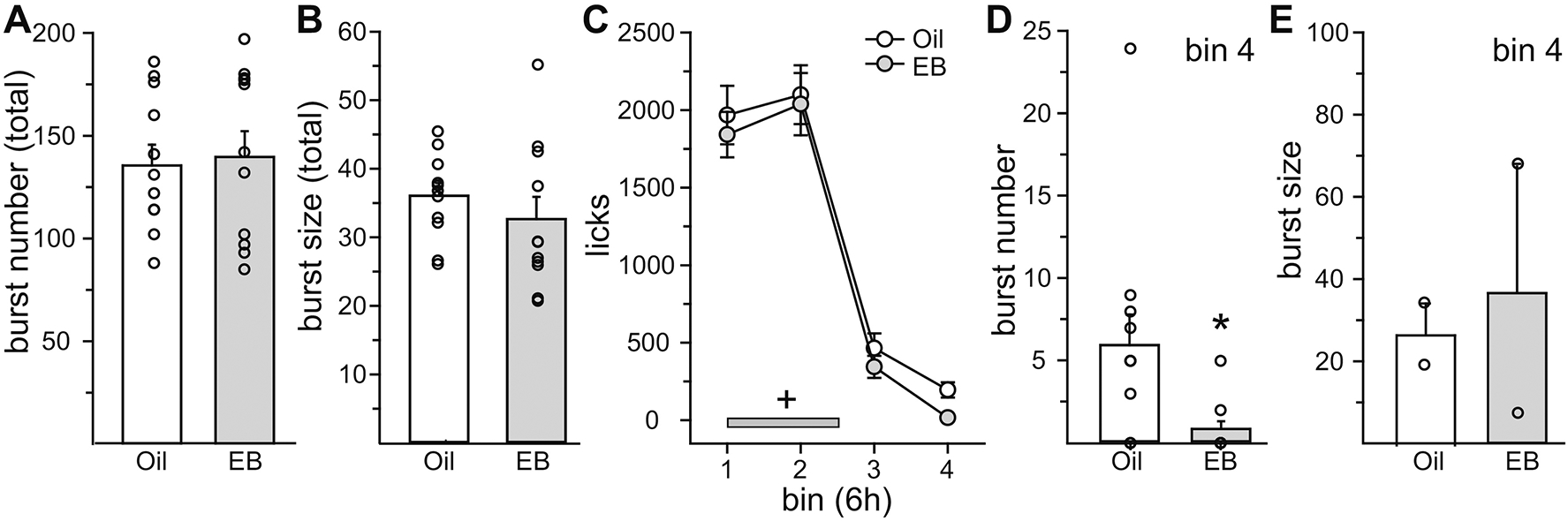
Lick analysis was conducted in Oil- and EB-treated rats in the presence of food. (A/B) There was no effect of EB-treatment on burst number or burst size across the entire test period. (C) EB-treatment did not reduce licks for water in any specific 6 h bin. The reduction in licks was due to a main effect of EB-treatment. Licks for water during the dark phase (grey bar) were significantly greater than licks for water during the light phase. (D/E) During the last 6 h bin (bin 4), EB treatment reduced burst number, with no effect on burst size. +Greater than light phase, p < 0.05. *Less than Oil, p < 0.05.
When food was not present, EB treatment significantly increased burst number (t10 = 2.8, p = 0.0185, Cohen’s d = 0.85), but had no effect on burst size (t10 = 1.84, p = n.s., Cohen’s d = 0.58) during the 23 h test period (Figure 4A/B). Analysis of licks in 6 h bins revealed a main effect of hormone (F1,10 = 25.78, p = 0.00048, η2 =0.06) and time (F3,30 = 20.60, p = 1.93E-07, η2 = 0.51), but no interaction of hormone and time (F3,30 = 2.56, p = n.s., η2 = 0.03; Figure 4C). Again, we predicted that EB would influence intake in discrete time bins, and a priori post hoc analysis revealed that EB treatment increased intake in the first two 6 h bins (p = 0.02). Analysis of drinking microstructure within the 6 h bins demonstrated that during the 2nd bin, EB treatment increased burst number (t10 = 2.34, p = 0.0413; Cohen’s d = 0.71; Figure 4D) but had no effect on burst size (t10 = 0.26, p = n.s., Cohen’s d = 0.09; Figure 3E).
Figure 4. The dipsogenic effect of E2 increased water intake by increasing burst number.
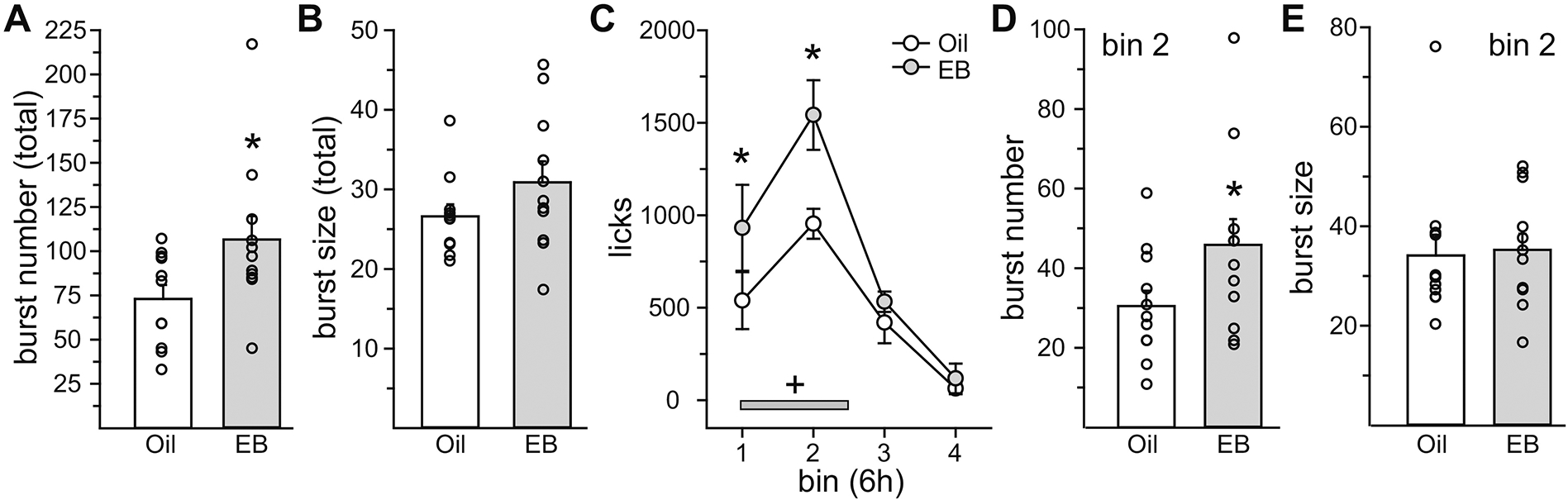
Lick analysis was conducted in Oil- and EB-treated rats in the absence of food. (A/B) EB-treatment increased burst number, but had no effect on burst size, across the entire test period. (C) EB-treatment increased licks for water during the first two 6h bins. Licks for water during the dark phase (grey bar) were significantly greater than licks for water during the light phase. (D/E) During the second 6 h bin (bin 2), EB treatment increased burst number, with no effect on burst size. *Greater than Oil, p < 0.05. +Greater than light phase, p < 0.05.
Experiment 3: Does EB increase water intake after an acute suppression of intake?
To explore changes in water intake as a function of EB treatment after an acute suppression of intake, rats were treated prior to dark onset with the GLP-1 receptor agonist Exendin-4, which has previously been reported to suppress water intake for approximately 4 h in male rats (McKay et al., 2011). Food intake was greater in Oil-treated, compared to EB-treated, rats (t10 = 5.62, p = 0.00022, Cohen’s d = 1.17; data not shown). Neither total fluid intake or total licks were influenced by EB treatment (t10 = 0.12 or 0.40, p = n.s., Cohen’s d = 0.04 or 0.09, respectively; data not shown). Next, we analyzed dark phase licks in 1 h bins to assess EB effects on rebound drinking after the suppressive effects of Exendin-4 had abated. Licks were influenced by a main effect of time (F11,110 = 28.461, p = 1.00E-10, η2 = 0.56) and an interaction between time and hormone (F11,110 = 4.205, p = 3.40E-05, η2 = 0.07; Figure 5A). Licks were suppressed during the first 4 h of the dark phase in both EB-treated (p = 0.005) and Oil-treated rats (p = 0.00001). During the first h of rebound drinking (5h post dark onset), EB-treated rats made more licks than Oil-treated rats (p = 0.012). This was also true during the third h of rebound drinking (p = 0.0001; 7h post dark onset). During the second h of rebound drinking (6h post dark onset), Oil-treated rats made more licks than EB-treated rats (p = 0.0004). There were no other timepoints where licks differed between Oil and EB treated rats. Analysis of the latency to lick for 1 ml of water (cumulative licks = 1ml/drop size) revealed that while 90% of EB-treated rats consumed 1 ml of water by the 5th h of the dark phase, only 36% of Oil-treated rats consumed 1 ml of water by this time. All rats drank to the 1 ml criterion by the 8th h of the dark phase (Figure 5B). The latency to drink 1 ml of water was shorter after EB, compared to Oil, treatment (t10 = 2.61, p < 0.0261, Cohen’s d = 0.70).
Figure 5. E2 increased water intake after an acute suppression by Exendin-4.
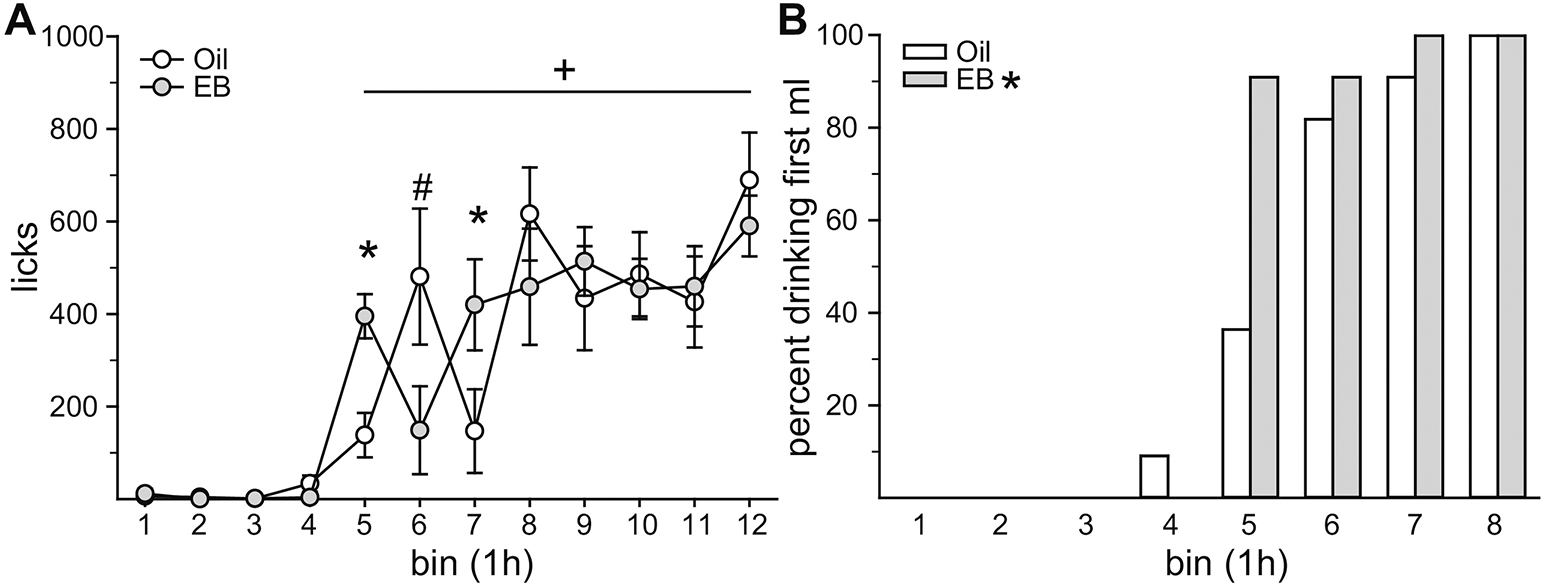
(A) Ex-4 temporarily suppressed water intake. Drinking resumed during the 5th h of the dark phase. During this time, intake in EB-treated rats was greater than intake in Oil-treated rats. Intake during the 7th h of the dark phase was also greater in EB-treated rats. Intake during the 6th h of the dark phase was greater in Oil-treated, compared to EB-treated, rats. (B) The latency to consumed 1 ml of water was significantly shorter in EB-treated rats. *Greater than Oil, p < 0.05. #Greater than EB, p < 0.05. +Greater than bins 1–4, p < 0.05.
Experiment 4: What is the time course for the increase in water and food intake after OVX?
To reconcile the discrepancies in the literature regarding a dipsogenic effect of OVX, we measured daily body weight, food intake, and water intake in OVX and SHAM rats. Prior to surgery, there were no group differences in daily body weight (F1,22 = 0.0001, p = n.s., η2 = 0.0002), food intake (F1,22 = 1.94, p = n.s., η2 = 0.009), or water intake (F1,21 = 0.07, p = n.s., η2 = 0.002; Figure 6). Analysis of body weight throughout the baseline week, day of surgery, and 5 weeks post-surgery revealed a main effect of group (F1,22 = 10.00, p = 0.0045, η2 = 0.16) and day (F42, 924 = 175.3, p = 0.00E+00, η2 = 0.37), and an interaction between group and day (F42, 924 = 37.50, p = 0.00E+00, η2 = 0.08; Figure 6A). As expected, OVX rats weighed more than sham rats (p = 0.0046). This separation in body weights became significant 22 days after surgery and OVX rats weighted significantly more for the duration of the study (p = 0.040– 0.003). Analysis of daily food intake throughout the baseline week, day of surgery, and 5 weeks post-surgery revealed a main effect of group (F1,22 = 6.25, p = 0.02, η2 = 0.026) and day (F42, 924 = 27.06, p = 0.00E+00, η2 = 0.47), and an interaction between group and day, (F42, 924 = 1.47, p = 0.029, η2 = 0.06; Figure 6B). As expected OVX rats ate more than sham rats (p = 0.020). Despite a significant group by day interaction, post hoc analysis did not reveal OVX vs sham differences on any specific days. To aid in the visualization of the difference in food intake between the OVX and sham groups, Figure 6C plots the daily difference in mean food intake. The noteworthy increase in food intake on day 29 is likely the result of fresh food being added to the cages. Analysis of daily water intake throughout the baseline week, day of surgery, and 5 weeks post-surgery revealed a main effect of day (F42, 882 = 2.87, p = 8.71E-09, η2 = 0.10), but no main effect of group (F1,21 = 0.01, p = n.s., η2 = 5.05E-05; Figure 6D) nor an interaction between group and day (F42, 882 = 1.40, p = 0.05, η2 = 0.05). Figure 6E plots the daily difference in mean water intake. In an attempt to reconcile our null finding and the dipsogenic effect of OVX reported by Tarttelin (Tarttelin and Gorski, 1971), we limited our data analysis to the 12 days after surgery during which time Tarttelin reported an effect of OVX. Analysis of daily intake for the 12 days post-surgery revealed an effect of group (F1,21 = 4.53, p = 0.045, η2 = 0.071). Findlay and colleagues reported no difference in water intake during the third week post-surgery, therefore we also performed an analysis of daily water intake during this time (Findlay et al., 1979). Analysis of water intake during the third week after surgery demonstrated no effect of group on intake (F1,21 = 0.004, p = n.s., η2 = 2.95E-05). One SHAM rat was dropped from the analysis due to observable leakage from the water bottle, resulting in daily intake greater than 2 standard deviations above the mean.
Figure 6. Ovariectomy increased body weight and food intake.
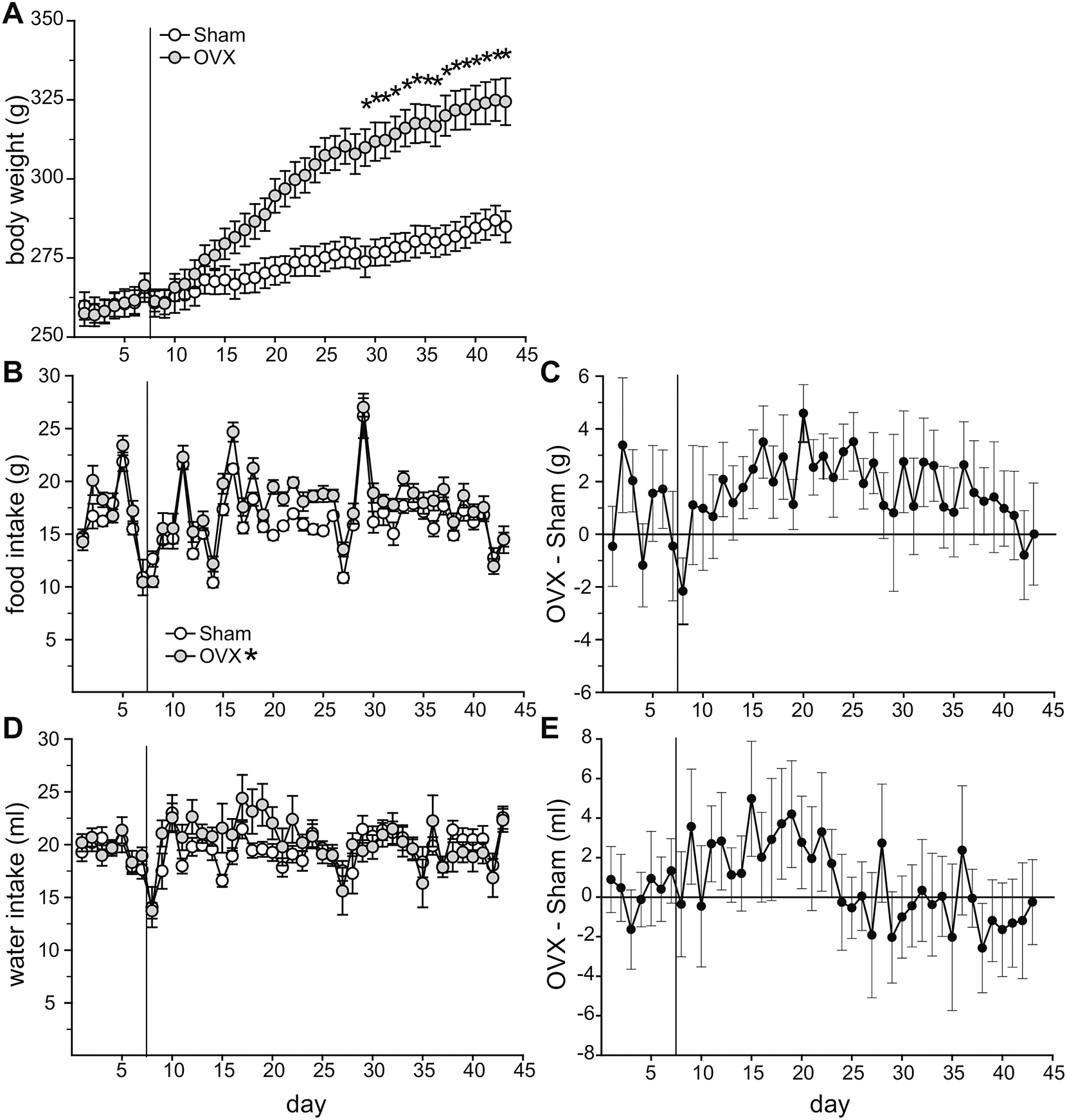
(A) At 22 days post-surgery, OVX rats weighed significantly more than SHAM rats, a different that remained for the duration on the study. (B/C) Daily food intake was significantly greater in OVX compared to SHAM rats. (D/E) Daily water intake was not significantly different between OVX and SHAM rats throughout the entire experiment. During the 12-day period post-surgery, water intake in OVX rats was greater than in SHAM rats. The vertical line in each graph denotes the surgery. *Greater than SHAM, p < 0.05.
DISCUSSION
The original goal of this series of experiments was to test the hypothesis that the inhibitory effect of E2 on unstimulated water intake is independent of the anorexigenic effect of E2. Furthermore, we wanted to understand whether changes in postingestive or orosensory signals are involved in the reduction in water intake when the effects of prandial drinking were removed. As expected, we found support for the hypothesis that the E2 mediated reduction in water intake is not secondary to the anorexigenic effect of E2 and vice versa, that the anorexigenic effect of E2 is observed in the absence of water (Czaja et al., 1983). We also found, however, that under certain testing conditions E2 can increase water intake. This was unexpected, as the inhibitory effects of E2 on fluid intake have been reported since the 1970s, yet no reports, to our knowledge, have demonstrated a dipsogenic effect of E2 (Fregly, 1978; Jonklaas and Buggy, 1984; Thrasher and Fregly, 1977, 1978). This finding is, however, timely as reports within the last two years have shown that E2 also has bidirectional effects on both feeding and thermogenesis in females despite long held views that E2 inhibits feeding and enhances thermogenesis (Lapid et al., 2019; Yu et al., 2020). This finding provides additional evidence for a paradigm shift regarding our understanding of estrogenic action, by which bidirectional effects of E2, depending on the internal or motivational state of the animal, contribute to the defense of homeostasis in the face of various environmental challenges. In cycling rats, water intake was reduced during E regardless of food access. Furthermore, there was no difference in the magnitude of the decrease in water intake when food was absent or present. This provides further support for the original findings from Czaja and colleagues demonstrating independent effects of E2 on food and water intake in the female guinea pig (Czaja et al., 1983). When rats had access to both water and saline, however, the fluid intake effects of E2 disappeared in our study. It is unclear why the inhibitory effect on water intake was not observed when saline was available, however, others have failed to report cycle related effects on unstimulated fluid intake. For example, Eckel and colleagues found reductions in water intake on E when rats had access to a running wheel, but no cycle related changes in the absence of running wheels (Eckel et al., 2000). Findlay and colleagues also did not report any cycle related changes in unstimulated saline intake, however, this was likely due to the saline concentration (Findlay et al., 1979). While intake of 2.7% saline does not change across the cycle, others have observed changes with lower, more palatable concentrations, specifically 2 and 1.8% saline (Antunes Rodrigues and Covian, 1963; Danielsen and Buggy, 1980). It is unclear why cycle related decreases in intake are not always observed, as our concentration (1.5%) was also in the lower, more palatable range. Strain differences can at least be ruled out, as E associated decreases in saline intake have been previously reported in both Long Evans and Wistar rats (Antunes Rodrigues and Covian, 1963; Danielsen and Buggy, 1980). The present data in combination with the reports from Eckel and Findlay highlight how multiple variables, perhaps across different labs, can influence whether cycle related changes in unstimulated water and saline intake are observed.
Next, we used OVX rats to determine a causative role of E2 in the estrus associated reductions in unstimulated water intake and to determine whether postingestive or orosensory signals contribute to the change. As expected, E2 reduced water intake in the presence of food, a change that was associated with a reduction in burst number late in the test period. This confirms our previous report demonstrating changes in burst number on unstimulated water intake in the presence of food (Santollo et al., 2013). When food was absent and baseline levels of intake were reduced, however, E2 surprisingly increased water intake. This change in water intake was also associated with a change in burst number. Changes in burst number reflect changes in postingestive feedback (i.e., satiety hormones, gastric distension, gastric emptying; (Davis, 1989; Davis et al., 1999). Together this suggests that when food is available, E2 decreases water intake through enhanced postingestive feedback signals but when food is not available, E2 increases water intake through decreased postingestive feedback signals. We also demonstrated that in the absence of water, EB treatment reduces food intake, which rules out the possibility that the anorexigenic effect is secondary to the anti-dipsogenic effect of E2. This finding also confirms the previous work by Czaja et al. (Czaja et al., 1983). It should be noted that our dose of EB was picked based on previous research examining the fluid intake effects of E2 (Findlay et al., 1979; Jones and Curtis, 2009; Krause et al., 2003), however it may produce serum levels of E2 that are slightly higher than in cycling females (Asarian and Geary, 2002). Nevertheless, the dose is selected to be high enough to uncover effects that may be subtle in the cycling female but low enough to not lead to an aversion-like suppression of intake (de Beun et al., 1991).
Why was a dispogenic effect of hormone observed in OVX but not cycling rats? Because OVX removes all ovarian-produced hormones and not just E2, we tested if the addition of P would alter water intake in the absence of food. Water intake after treatment with E2 + P, however, did not augment the dipsogenic effect of E2. Instead, we propose that the bidirectional effects of E2 on water intake is influenced by baseline levels of intake, which could reflect the motivational state of the rat. Oil-treated OVX rats consumed significantly less water, compared to D2 rats when food was not available (7.82 ± 0.87 vs 15.79 ± 2.01; t(21) = 3.53, p < 0.001). This suggests that when baseline water intake is low, which could potentially put an animal at a future risk for dehydration, E2 enhances intake. Additional studies will be necessary to test this hypothesis, especially since the experiments in this study were not designed to compare intakes between cycling and OVX rats. In addition to understanding the bidirectional effect of E2, future studies will also be necessary to understand why in the absence of food, baseline water intake is significantly lower in OVX compared to cycling rats. One possibility could be that E2 exerts a tonic stimulatory effect on daily water intake which is lost after OVX. In the presence of food, water intake in D2 rats was slightly greater than Oil-treated OVX rats (21.65 ± 1.37 vs 19.01 ± 0.93), but this difference was not statistically significant (t(21) = 1.56, p = 0.18). But again, the experiments in this study were not designed to compare intakes between cycling and OVX rats, therefore future research is needed to address these questions.
Because E2 enhanced water intake during a condition when baseline intake was low, we next tested whether E2 would enhance drinking after an acute suppression of intake which would decrease the drive to drink but not be severe enough to induce dehydration. To achieve an acute suppression of intake, rats were treated just prior to dark onset with exendin-4, which has previously been reported to inhibit dark phase water intake for 4 h in male rats and, similar to E2, reduces water intake through changes in postingestive feedback (McKay and Daniels, 2013; McKay et al., 2011; Santollo et al., 2013). Indeed, a suppression of intake during h 1–4 of the dark phase was observed in female rats in our study. When rats were treated with EB, intake during the first h of rebound drinking (h 5 of the dark phase) was greater than Oil-treated rats. In addition, the latency to consume 1 ml of water was shorter in EB-treated rats. This provides further support for the hypothesis that when the drive to consume water is reduced, E2 enhances intake. It should be noted that GLP-1 has significant effects on insulin and glucose metabolism (Gribble and Reimann, 2021) in addition to its anorexigenic effects (Daniels and MietlickiBaase, 2019). Since both insulin and glucose metabolism can affect water intake, these factors could potentially confound the interpretation of our results and future studies are necessary to disentangle these factors.
It was first identified in the 1960s that fluid intake fluctuates across the estrous cycle, with studies in the late 70s and early 80s demonstrating a causative role of E2 on both stimulated and daily unstimulated water intake. Our present findings, that under certain conditions E2 can enhance water intake, represents a paradigm shift for our understanding of how E2 controls fluid intake. We propose that under conditions of copious intake, such as when animals are dehydrated or receive signals related to dehydration (as occurs after treatment with AngII or isoproterenol), E2 inhibits intake, which may aid in preventing overhydration. In turn, under conditions of low intake, such as intake in the absence of food or in rebound drinking after acute suppression of drinking with an anti-dipsogenic drug, E2 enhances intake, which may aid in preventing dehydration. These bidirectional effects of E2 on fluid intake, therefore, likely work in concert to defend fluid homeostasis at a given physiological set point. In this regard, there is some evidence in the literature to support the hypothesis that E2 has bidirectional effects on other factors that control fluid homeostasis. For example, while the depressor effect of E2 is well documented (Clark et al., 2004; Hinojosa-Laborde et al., 2004; Jonklaas and Buggy, 1984; Mirabito et al., 2014; Xue et al., 2007), E2 treatment in OVX rats attenuates the decrease in blood pressure after isoproterenol treatment (Krause et al., 2007). Furthermore, in humans there is a lower osmotic threshold for vasopressin release during the luteal phase, when ovarian hormones are elevated, which is independent of P effects (Calzone et al., 2001; Spruce et al., 1985; Vokes et al., 1988). This suggests that E2 enhances the conservation of body fluids during dehydration challenges.
Identification of a paradoxical effect of E2 on fluid intake is timely, given recent discoveries of bidirectional effects of E2 on the regulation of energy homeostasis. The anorexigenic effect of E2 was identified in the 1950s and a large research effort has focused on understanding the underlying mechanisms during the subsequent years (Blaustein and Wade, 1976; Eckel, 2004; Sullivan and Smith, 1957; Wade, 1975). These studies focused on the effect of E2 on ad libitum intake. Recent work by Yu and colleagues, however, eloquently characterized how E2 enhances intake, via membrane associated ERα signaling, during acute refeeding after food deprivation (Yu et al., 2020). In addition, another paradoxical effect of E2 on the control of thermogenesis was recently identified. While multiple groups have reported that E2 enhances thermogenesis in females housed at ambient temperature (Correa et al., 2015; Martinez de Morentin et al., 2014; Xu et al., 2011), in females exposed to chronic cold stress, E2 was recently reported to inhibit thermogenic browning of white adipose tissue (Lapid et al., 2019). Together these studies suggest that estrogenic control of ingestive behaviors and thermogenesis is bidirectional, which act to defend fluid and energy homeostasis, dependent on the environmental circumstances.
Post-OVX increases in body weight and daily food intake are well documented (Blaustein and Wade, 1976; McElroy and Wade, 1987; Mook et al., 1972; Tarttelin and Gorski, 1971; Varma et al., 1999; Wade, 1975; Witte et al., 2010). It is, therefore, not surprising that we also observed an increase in body weight and daily food intake in OVX rats. The effect of OVX on daily water intake, however, has only been reported three times with conflicting findings. Tarttelin and Gorski reported a dipsogenic effect of OVX that lasted for 12 days post-surgery (Tarttelin and Gorski, 1971). Zucker reported increased daily water intake after OVX, but attributed the change to increased body weight and food intake (Zucker, 1969). In addition, Findlay and colleagues measured daily water intake in the third week post-surgery and reported an increase in intake over pre-surgery levels in young adult rats, but no change when they repeated the protocol in a group of older rats (Findlay et al., 1979). This led them to conclude that the dipsogenic effect of OVX was the result of an increase in age and body weight. Unfortunately, the exact ages used in this study are unclear, but were somewhere between 40 and 120 days at the start of the experiment. Nevertheless, we did not find an effect of OVX when data for the entire test period was analyzed. We, similar to the Findlay report, also found no effect of OVX on water intake when we analyzed data from the third week post-surgery. Of note, rats used here were approximately 120 days at the start of the experiment, suggesting a similar age as those used in the “older” group tested in the Findlay study. We, similar to the Tarttelin report, also found an increase in daily water intake when we analyzed data for the first 12 days post-surgery. Together this suggests that after OVX, an increase in daily water intake can be observed, but this effect is transient. Of note, we recently reported in OVX rats that water intake does not correlate with body weight (Santollo and Edwards, 2021). This finding provides additional support for the dipsogenic effect of OVX being independent from other OVX-related changes in energy balance. This data set also highlights that an increase in food intake can be observed in the absence of an increase in water intake, providing additional evidence for a dissociation between food and water intake.
Here, we show for the first time that E2 can enhance water intake in rats under certain conditions and we provide further evidence to support the hypothesis that the anorexigenic and anti-dipsogenic effects of E2 are independent. Despite the surprising and exciting finding that E2 can enhance water intake, many open questions remain including further characterization of this behavioral phenomenon and understanding the underlying mechanism. Answering these questions will likely provide more insights in the estrogenic control of homeostasis, in general, and the estrogenic control of fluid homeostasis, in particular. Furthermore, this research will aid in our understanding of how the need for fluids changes across the female reproductive lifespan, particularly during pregnancy when water intake is increased in the face of increased ovarian hormone release (Atherton et al., 1982). While we demonstrated that the inhibition of unstimulated water intake is independent of changes in food intake, we were not able to identify whether the reduction in intake is mediated by changes in postingestive or orosensory feedback when removing any effects of prandial drinking. Future studies comparing the drinking microstructure of unstimulated water intake in D2 vs E rats in the absence of food are necessary to address this question. In conclusion, the main finding from this work showing that E2 can enhance water intake, depending on the conditions, adds to the growing evidence for bidirectional effects of E2 on physiology and behavior in the service of defending homeostasis.
Highlights.
Reductions in water intake on estrus are independent of reductions in food intake.
In ovariectomized rats, estradiol increased water intake when food was absent.
Estradiol reduced the latency to drink after a temporary suppression of intake.
The dipsogenic effect was mediated by changes in burst number.
ACKNOWLEDGMENTS
We thank Dr. Jeremy Van Cleve and Aviv Brokman for guidance on data visualization and analysis. This work was supported by NSF grant 2019346, NIH grant DA035150, and University of Kentucky Start-Up Funds.
Support: NSF grant 2019346, NIH grant DA035150, and University of Kentucky Start-Up Funds
Footnotes
Disclosure Summary: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antunes Rodrigues J, Covian MR, 1963. Hypothalamic Control of Sodium Chloride and Water Intake. Acta physiologica latino americana 13, 94–100. [PubMed] [Google Scholar]
- Asarian L, Geary N, 2002. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Hormones and behavior 42, 461–471. [DOI] [PubMed] [Google Scholar]
- Atherton JC, Dark JM, Garland HO, Morgan MR, Pidgeon J, Soni S, 1982. Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. The Journal of physiology 330, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E, 2005. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146, 1650–1673. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN, 1976. Ovarian influences on the meal patterns of female rats. Physiology & behavior 17, 201–208. [DOI] [PubMed] [Google Scholar]
- Calzone WL, Silva C, Keefe DL, Stachenfeld NS, 2001. Progesterone does not alter osmotic regulation of AVP. American journal of physiology. Regulatory, integrative and comparative physiology 281, R2011–2020. [DOI] [PubMed] [Google Scholar]
- Clark JT, Chakraborty-Chatterjee M, Hamblin M, Wyss JM, Fentie IH, 2004. Estrogen depletion differentially affects blood pressure depending on age in Long-Evans rats. Endocrine 25, 173–186. [DOI] [PubMed] [Google Scholar]
- Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL, Ingraham HA, 2015. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep 10, 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis KS, 2009. Estrogen and the central control of body fluid balance. Physiology & behavior 97, 180–192. [DOI] [PubMed] [Google Scholar]
- Czaja JA, Butera PC, McCaffrey TA, 1983. Independent effects of estradiol on water and food intake. Behavioral neuroscience 97, 210–220. [DOI] [PubMed] [Google Scholar]
- Daniels D, Mietlicki-Baase EG, 2019. Glucagon-Like Peptide 1 in the Brain: Where Is It Coming From, Where Is It Going? Diabetes 68, 15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen J, Buggy J, 1980. Depression of ad lib and angiotensin-induced sodium intake at oestrus. Brain research bulletin 5, 501–504. [DOI] [PubMed] [Google Scholar]
- Davis JD, 1989. The microstructure of ingestive behavior. Annals of the New York Academy of Sciences 575, 106–119; discussion 120–101. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP, Singh B, 1999. A microstructural analysis of the control of water and isotonic saline ingestion by postingestional stimulation. Physiology & behavior 66, 543–548. [DOI] [PubMed] [Google Scholar]
- de Beun R, Jansen E, Smeets MA, Niesing J, Slangen JL, van de Poll NE, 1991. Estradiol-induced conditioned taste aversion and place aversion in rats: sex- and dose-dependent effects. Physiology & behavior 50, 995–1000. [DOI] [PubMed] [Google Scholar]
- Drewett RF, 1973. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Animal behaviour 21, 772–780. [DOI] [PubMed] [Google Scholar]
- Eckel LA, 2004. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiology & behavior 82, 35–41. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Geary N, 1999. Endogenous cholecystokinin’s satiating action increases during estrus in female rats. Peptides 20, 451–456. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Houpt TA, Geary N, 2000. Spontaneous meal patterns in female rats with and without access to running wheels. Physiology & behavior 70, 397–405. [DOI] [PubMed] [Google Scholar]
- Findlay AL, Fitzsimons JT, Kucharczyk J, 1979. Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. The Journal of endocrinology 82, 215–225. [DOI] [PubMed] [Google Scholar]
- Fregly MJ, 1978. Attenuation of thirst in estrogen-treated rats. Federation proceedings 37, 2694–2698. [PubMed] [Google Scholar]
- Fregly MJ, Thrasher TN, 1978. Attenuation of angiotensin-induced water intake in estrogen-treated rats. Pharmacology, biochemistry, and behavior 9, 509–514. [DOI] [PubMed] [Google Scholar]
- Graves NS, Hayes H, Fan L, Curtis KS, 2011. Time course of behavioral, physiological, and morphological changes after estradiol treatment of ovariectomized rats. Physiology & behavior 103, 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Reimann F, 2021. Metabolic Messengers: glucagon-like peptide 1. Nat Metab 3, 142–148. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K, 2004. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44, 405–409. [DOI] [PubMed] [Google Scholar]
- Jones AB, Bass EE, Fan L, Curtis KS, 2012. Estradiol selectively reduces central neural activation induced by hypertonic NaCl infusion in ovariectomized rats. Physiology & behavior 107, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AB, Curtis KS, 2009. Differential effects of estradiol on drinking by ovariectomized rats in response to hypertonic NaCl or isoproterenol: Implications for hyper- vs. hypo-osmotic stimuli for water intake. Physiology & behavior 98, 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonklaas J, Buggy J, 1984. Angiotensin-estrogen interaction in female brain reduces drinking and pressor responses. The American journal of physiology 247, R167–172. [DOI] [PubMed] [Google Scholar]
- Kisley LR, Sakai RR, Ma LY, Fluharty SJ, 1999. Ovarian steroid regulation of angiotensin II-induced water intake in the rat. The American journal of physiology 276, R90–96. [DOI] [PubMed] [Google Scholar]
- Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ, 2003. Estrogen influences stimulated water intake by ovariectomized female rats. Physiology & behavior 79, 267–274. [DOI] [PubMed] [Google Scholar]
- Krause EG, Curtis KS, Markle JP, Contreras RJ, 2007. Oestrogen affects the cardiovascular and central responses to isoproterenol of female rats. The Journal of physiology 582, 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapid K, Lim A, Berglund ED, Lu Y, 2019. Estrogen receptor inhibition enhances coldinduced adipocyte beiging and glucose tolerance. Diabetes Metab Syndr Obes 12, 1419–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, Ruiz-Pino F, Liu J, Morgan DA, Pinilla L, Gallego R, Saha AK, Kalsbeek A, Fliers E, Bisschop PH, Dieguez C, Nogueiras R, Rahmouni K, TenaSempere M, Lopez M, 2014. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell metabolism 20, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy JF, Wade GN, 1987. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiology & behavior 39, 361–365. [DOI] [PubMed] [Google Scholar]
- McKay NJ, Daniels D, 2013. Glucagon-like peptide-1 receptor agonist administration suppresses both water and saline intake in rats. Journal of neuroendocrinology 25, 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay NJ, Kanoski SE, Hayes MR, Daniels D, 2011. Glucagon-like peptide-1 receptor agonists suppress water intake independent of effects on food intake. American journal of physiology. Regulatory, integrative and comparative physiology 301, R1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabito KM, Hilliard LM, Head GA, Widdop RE, Denton KM, 2014. Pressor responsiveness to angiotensin II in female mice is enhanced with age: role of the angiotensin type 2 receptor. Biology of sex differences 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook DG, Kenney NJ, Roberts S, Nussbaum AI, Rodier WI 3rd, 1972. Ovarianadrenal interactions in regulation of body weight by female rats. Journal of comparative and physiological psychology 81, 198–211. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Ronnekleiv OK, Kelly MJ, 2010. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology 151, 4926–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch DM, 2006. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiology & behavior 87, 39–44. [DOI] [PubMed] [Google Scholar]
- Santollo J, Daniels D, 2015a. Activation of G protein-coupled estrogen receptor 1 (GPER-1) decreases fluid intake in female rats. Hormones and behavior 73, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Daniels D, 2015b. Control of fluid intake by estrogens in the female rat: role of the hypothalamus. Frontiers in systems neuroscience 9, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Daniels D, 2015c. Multiple estrogen receptor subtypes influence ingestive behavior in female rodents. Physiology & behavior 152, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Edwards AA, 2021. How predictive is body weight on fluid intake in rats? It depends on sex. Physiology & behavior 229, 113262. [DOI] [PubMed] [Google Scholar]
- Santollo J, Marshall A, Curtis KS, Speth RC, Clark SD, Daniels D, 2016. Divergent effects of ERalpha and ERbeta on fluid intake by female rats are not dependent on concomitant changes in AT1R expression or body weight. American journal of physiology. Regulatory, integrative and comparative physiology 311, R14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Marshall A, Daniels D, 2013. Activation of membrane-associated estrogen receptors decreases food and water intake in ovariectomized rats. Endocrinology 154, 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Myers KE, Rainer IL, Edwards AA, 2019. Gonadal hormones in female rats protect against dehydration-induced memory impairments in the novel object recognition paradigm. Hormones and behavior 114, 104547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Wiley MD, Eckel LA, 2007. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. American journal of physiology. Regulatory, integrative and comparative physiology 293, R2194–2201. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Wade GN, 1981. Effects of estradiol and progesterone on food intake, body weight, and carcass adiposity in weanling rats. The American journal of physiology 240, E499–503. [DOI] [PubMed] [Google Scholar]
- Spiteri NJ, Drewett RF, Padel U, 1980. Behavioral effects of ethynyl estrogens in the female rat. Physiology & behavior 25, 409–415. [DOI] [PubMed] [Google Scholar]
- Spruce BA, Baylis PH, Burd J, Watson MJ, 1985. Variation in osmoregulation of arginine vasopressin during the human menstrual cycle. Clin Endocrinol (Oxf) 22, 37–42. [DOI] [PubMed] [Google Scholar]
- Sullivan LW, Smith TC, 1957. Influence of estrogens on body growth and food intake. Proc Soc Exp Biol Med 96, 60–64. [DOI] [PubMed] [Google Scholar]
- Tarttelin MF, Gorski RA, 1971. Variations in food and water intake in the normal and acyclic female rat. Physiology & behavior 7, 847–852. [DOI] [PubMed] [Google Scholar]
- Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L, 2009. Divergent effects of estradiol and the estrogen receptor-alpha agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain research 1268, 88–96. [DOI] [PubMed] [Google Scholar]
- Thrasher TN, Fregly MJ, 1977. Responsiveness to various dipsogenic stimuli in rats treated chronically with norethynodrel, ethinyl estradiol and both combined. The Journal of pharmacology and experimental therapeutics 201, 84–91. [PubMed] [Google Scholar]
- Thrasher TN, Fregly MJ, 1978. Effect of chronic treatment with an estrogen-progestogen combination on beta adrenergic-induced thirst. Pharmacology, biochemistry, and behavior 8, 177–183. [DOI] [PubMed] [Google Scholar]
- Varma M, Chai JK, Meguid MM, Laviano A, Gleason JR, Yang ZJ, Blaha V, 1999. Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiology & behavior 68, 99–107. [DOI] [PubMed] [Google Scholar]
- Vokes TJ, Weiss NM, Schreiber J, Gaskill MB, Robertson GL, 1988. Osmoregulation of thirst and vasopressin during normal menstrual cycle. The American journal of physiology 254, R641–647. [DOI] [PubMed] [Google Scholar]
- Wade GN, 1975. Some effects of ovarian hormones on food intake and body weight in female rats. Journal of comparative and physiological psychology 88, 183–193. [DOI] [PubMed] [Google Scholar]
- Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM, 2010. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. General and comparative endocrinology 166, 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ, 2011. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell metabolism 14, 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Pamidimukkala J, Lubahn DB, Hay M, 2007. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. American journal of physiology. Heart and circulatory physiology 292, H1770–1776. [DOI] [PubMed] [Google Scholar]
- Yu K, He Y, Hyseni I, Pei Z, Yang Y, Xu P, Cai X, Liu H, Qu N, Liu H, He Y, Yu M, Liang C, Yang T, Wang J, Gourdy P, Arnal JF, Lenfant F, Xu Y, Wang C, 2020. 17beta-estradiol promotes acute refeeding in hungry mice via membrane-initiated ERalpha signaling. Mol Metab 42, 101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker I, 1969. Hormonal determinants of sex differences in saccharin preference, food intake, and body weight. Physiology & behavior 4, 595–602. [Google Scholar]


