Abstract
Diabetes mellitus is a metabolic disorder projected to afflict 700 million people globally by 2045. Fundamental to the progression of diabetes is an insufficient supply of insulin to meet metabolic demand. The MIN6-K8 cell line is a mouse insulinoma model of pancreatic β-cells frequently used to study the mechanisms of insulin secretion. Here, we evaluated the effects of short-term exposure to dimethyl sulfoxide (DMSO), a polar aprotic solvent commonly used in drug screening, on physiological characteristics of MIN6-K8 cells. Short-term exposure of MIN6-K8 cells to DMSO enhanced glucose-induced and tolbutamide-stimulated insulin secretion without significant effects on basal secretion or potassium responsiveness. Calcium influx was enhanced during glucose and tolbutamide treatments, suggesting that DMSO’s mechanism of action is upstream of calcium-dependent insulin granule exocytosis. Based on these studies, investigators should use caution when conducting experiments with DMSO in the MIN6-K8 cell line and should report all DMSO concentrations when used as a solvent.
Keywords: Insulin secretion, diabetes, drug screening, dimethyl sulfoxide, glucose, drug discovery
Introduction
By 2045 approximately 700 million individuals worldwide are projected to have type 2 diabetes mellitus(T2D)(Saeedietal. 2019). Fundamentally, T2D progression involves a combination of insulin resistance and a failure of pancreatic β-cells to effectively compensate with increased insulin secretion. This imbalance of insulin supply versus demand manifests as dysregulated glucose homeostasis and hyperglycemia. To enhance the levels of endogenous insulin secretion from β-cells, multiple classes of pharmaceutical agents have been developed, including sulfonylureas, glinides, and glucagon-like peptide-1 analogues. Despite the widespread use of these and other agents in the treatment of T2D, many patients remain hyperglycemic and progress to more severe stages of the disease, while others are often plagued by a variety of medication-associated side effects (Seino et al. 2017; Spollett et al. 2016). Therefore, novel insulin secretagogues are needed with better performance and risk profiles.
The MIN6-K8 cell line is a highly physiological β-cell model system derived from a male mouse on the IT6 background, that has been used extensively to uncover novel regulators of insulin secretion (Iwasaki et al. 2010). There are two main phases of glucose-induced insulin secretion: the triggering pathway and the amplifying pathway. In the triggering pathway, glucose entry into the cell and its metabolism raises the ATP:ADP ratio, which induces closure of ATP-sensitive potassium (KATP) channels and depolarization of the plasma membrane. Voltage-dependent calcium channels sense the change in membrane potential and open, flooding the cytosol with calcium. This influx of calcium triggers insulin granule exocytosis. The amplifying pathway augments elements of the triggering pathway independent of changes to the ATP:ADP ratio via a complex web of signaling pathways (Henquin 2009; Kibbey et al. 2007; Ferdaoussi et al. 2015; Kalwat and Cobb 2017; Gheni et al. 2014).
Dimethyl sulfoxide(DMSO)is a polar, aprotic solvent with an interesting and complex history. It has been used topically for over 50 years by veterinarians to reduce swelling induced by acute trauma (Brayton 1986) and is currently approved by the Food and Drug Administration for clinical use in the treatment of interstitial cystitis (Rawls et al. 2017). DMSO has also played a pivotal role in research, acting as a multipurpose solvent for rapid screening of candidate small-molecule compounds with therapeutic potential in many model systems (Raphemot et al. 2013).
Administration of DMSO to cultured cells has a wide range of reported effects across different concentrations and model systems. Its physical impacts include enhancing membrane flexibility and producing transient or stable pores in biological membranes (Gurtovenko and Anwar 2007). These mechanical effects have been observed both experimentally and in silico, with transient membrane pore formation observed in molecular dynamics simulations using DMSO concentrations of approximately 0.1 mole fraction (Notman and Anwar 2013), translating to approximately 30% v/v in aqueous solutions. Experimentally, far lower concentrations of DMSO, as low as 0.1% v/v, can significantly enhance the permeability of biological membranes and affect membrane transport (Heetal.2012). In high-throughput screening publications using mouse insulinoma cell lines, the concentrations of DMSO used sometimes go unreported (Szabat et al. 2015; Burns et al. 2015). Here, we investigated the effects of DMSO at varying concentrations on insulin secretion and calcium dynamics in the MIN6-K8 cell line.
Materials and Methods
Cell culture:
MIN6-K8 cells (passages 24–30) were cultured at 37°C with 5% CO2 in Dulbecco’s Modified Eagle’s Medium (#5796, Sigma-Aldrich, St. Louis, MO) containing 25 mM glucose, 10% heat-inactivated fetal bovine serum (Origin: United States, #16000–069, Gibco, Thermo Fisher Scientific, Waltham, MA), 1 mM sodium pyruvate (#S8636–100ML, Sigma-Aldrich, St. Louis, MO), and 55 μM 2-mercaptoethanol (#O3446I-100, Sigma-Aldrich, St. Louis, MO). This cell line was generously provided by Professor Jun-ichi Miyazaki of Osaka University Medical School.
Insulin Secretion:
Insulin secretion experiments were performed as described previously (Carmean et al. 2018; Hashim et al. 2018; Gheni et al. 2014). MIN6-K8 cells were pre-incubated for 30 min in Krebs-Ringer bicarbonate HEPES solution (KRBH) with 2.8 mM glucose and then incubated for 30 min in KRBH containing 0 – 2.5% v/v DMSO (#D8418, purity: 99.9%, Sigma Aldrich, St. Louis, MO) in the presence of 2.8 – 16.7 mM glucose, 2.8 mM glucose + 60 mM K+, or 2.8 mM glucose + 100 μM tolbutamide (#T0891, Sigma Aldrich, St. Louis, MO, USA). Insulin was quantified using the Cisbio Ultra-sensitive HTRF assay (#62IN2PEH, Codolet, France).
Calcium Dynamics:
MIN6-K8 cells were pre-loaded with 1 mM FURA-2-AM Ca2+ binding reagent (#F1225, Invitrogen, Carlsbad, CA) in 0.5% DMSO for 30 min in KRBH containing 2.8 mM glucose. Cells were rinsed and stimulation solution was added to each well. Ca2+ influx was measured by calculating the ratio of the fluorescence emission values at 510 nm resulting from excitation at 340 and 380 nm. For measurements shown,the Ca2+ signal detected for unstimulated conditions at each time point was subtracted from the stimulated wells’ signals to obtain the values shown.
DNA Determination:
Double-stranded DNA (dsDNA) in the assay supernatant was quantified using the Quant-iT PicoGreen dsDNA Assay kit (#P7589, Invitrogen, Carlsbad, CA). Following the normal 30-minute stimulation of the insulin secretion assay (described above), supernatant was collected and then diluted 40-fold with assay buffer (10mM Tris-HCl, 1mM EDTA, pH=7.5). PicoGreen reagent was added to samples at room temperature. After 5 minutes, fluorescence was measured (excitation at 480nm, emission at 520nm).
Human and animal rights:
No human subjects or animals were utilized directly for the present study. Historical details of the MIN6-K8 cell line derivation have been reported (Iwasaki et al. 2010).
Statistics:
In charts where only one comparison was made (+/− DMSO comparisons for tolbutamide- and potassium-stimulated insulin secretion), a non-parametric Mann-Whitney U-test was performed. Where multiple tests were desired for either different concentrations of DMSO, different concentrations of glucose, or across a time course of stimulation, a 2-way ANOVA was performed to discover overall treatment effects followed by Sidak’s multiple comparison test for DMSO effects at each measurement interval. P<0.05 was considered statistically significant. Figures were prepared and statistical tests were conducted in GraphPad Prism version 9.0.
Results and Discussion
The effects of short-term (30 min) DMSO exposure on β-cell physiology were evaluated in the MIN6-K8 mouse insulinoma cell line. Exposure to 1.25% or 2.5% DMSO for 30 min did not affect basal insulin secretion (Fig 1a) but significantly increased glucose-induced insulin secretion at 11.2 mM (Fig 1b) with no effects on total insulin content (data not shown). The highest concentration of DMSO tested, 2.5%, enhanced glucose-induced insulin secretion at 11.2 mM and 16.7 mM glucose by 39% and 62%, respectively (Fig 1e).
Fig. 1.
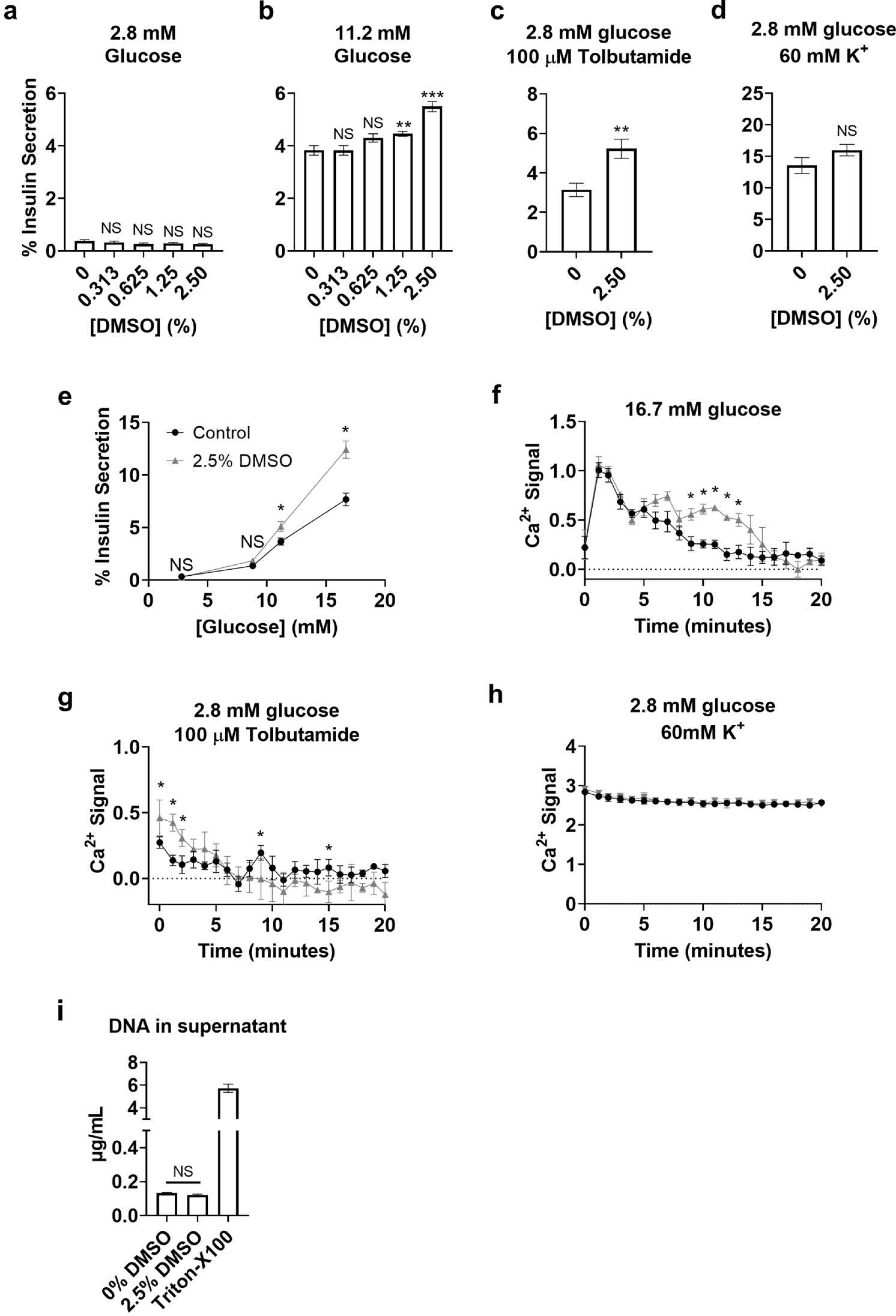
DMSO effects on MIN6-K8 cells. Insulin secretion due to DMSO co-treatment with 2.8mM glucose (a),11.2mM glucose (b), 2.8mM glucose + 100μM tolbutamide (c), 2.8mM glucose + 60mM K+ (d). Insulin secretion due to 2.5% DMSO co-treatment with various concentrations of glucose (e). Ca2+ influx +/− 2.5% DMSO co-stimulated with 16.7mM glucose (f), 2.8mM glucose + 100μM tolbutamide (g), or 2.8mM glucose + 60mM K+ (h). DNA in supernatant following 30 minutes of stimulation with 16.7mM glucose +/− 2.5% DMSO (i). Gray triangles: 2.5% DMSO, black circles: Controls (e-h). Statistics: 2-way ANOVA with Sidak’s Multiple Comparison test (a, b, e-h); Mann-Whitney U-test (c, d, i). *p<0.05, **p<0.01, ***p<0.001 .
To evaluate potential KATP channel involvement, cells were co-stimulated with 2.8 mM glucose plus tolbutamide, which closes KATP channels in the presence of absence of 2.5% DMSO. DMSO significantly enhanced tolbutamide-induced insulin secretion by 66% (Fig 1c). In contrast, administration of DMSO did not significantly affect potassium-induced insulin secretion (Fig 1d).
Ca2+ influx is a necessary and sufficient stimulus-secretion coupling mechanism in β-cells that induces insulin granule exocytosis. To test whether DMSO affected Ca2+ dynamics, the FURA-2A Ca2+-sensitive dye was pre-loaded into MIN6-K8 cells and then the time-resolved influx of Ca2+ was measured after administration of DMSO with co-stimuli. DMSO enhanced glucose-induced Ca2+ influx significantly 9 min after the onset of glucose stimulation (Fig 1f). Interestingly, treatment with DMSO in the presence of tolbutamide showed an immediate and significant enhancement of Ca2+ influx that waned after a few min and ultimately decreased significantly lower than controls (Fig 1h). There were no significant differences in Ca2+ dynamics during incubation in the presence of 2.8 mM glucose (data not shown) or 60 mM K+ stimulation in the presence of 2.8 mM glucose (Fig 1h).
To test the possibility that DMSO exposure during glucose stimulation might enhance the amount of insulin released into the supernatant by lysing cells, dsDNA in the supernatant was measured (Fig 1i). The detergent Triton-X100 (0.1% v/v) was added to a separate set of wells as a positive control for cell lysis. We observed that cells exposed to 2.5% DMSO and co-stimulated with 16.7mM glucose (the same condition that increased insulin release by 62%), did not change the amount of dsDNA detected in the supernatant, providing strong direct evidence that DMSO did not lyse cells during treatment.
To the best of our knowledge, this is the first report of the dose-dependent effects of DMSO on insulin secretion and Ca2+ flux in the MIN6-K8 cell line. Though the specific mechanisms by which DMSO augments insulin secretion remain to be resolved, there are some indications from the present study. Its lack of effects on insulin secretion and Ca2+ flux at 2.8 mM glucose levels, as well as its lack of effectsondsDNAinthe supernatant under 16.7mM glucose conditions, provide strong, overlapping evidence that DMSO at 2.5% v/v does not directly induce cell lysis, activate voltage-gated Ca2+ channels, or form large pores in MIN6-K8 cells (though this has been demonstrated for other cell types previously) (Morley and Whitfield 1993). Whether the observed effects during stimulation arise from alterations in ion channel sensitivity, membrane fluidity, or changes in the redox state of the cell areamong several potentialmechanisms. Alternatively, DMSO’s mechanism of action may be entirely novel, raising the possibility that the study of DMSO per se may yield novel targets for the treatment of T2D.
The present stud ymay have limited applicability to islet biology. Investigators have previously reported that mouse islets are resistant to insulin-secretory effects of acute exposure to DMSO within the range of concentrations used in the present manuscript (Sandler and Andersson 1982). To our knowledge it remains unreported whether DMSO affects human islets and/or primary dispersed pancreatic β-cells in culture within this concentration range. Based on the present results, we believe that this topic warrants investigation.
The data presented herein provide important evidence that DMSO enhances regulated insulin secretion in MIN6-K8 cells at concentrations that may be employed during high-throughput screening or other physiological assays. Based on the present study, we suggest using ≤0.625% v/v DMSO in MIN6-K8 cells to limit solvent-based complications. Importantly, this concentration is below those reported in some small-molecule screening research using mouse insulinoma cell lines (Briscoe et al. 2006). Collectively, the assembled data should alert investigators to the important physiological effects of DMSO in β-cell models to enhance experimental quality regarding high-throughput screening and other physiological investigations. The authors recommend that all investigators utilizing DMSO as a solvent in mouse insulinoma cell lines report the concentrations used to allay any concerns about potential DMSO-induced physiological disruptions.
Acknowledgements
Many thanks to Rui Tang and Eben Alsberg for their technical assistance during the described study. The authors would also like to thank Graeme Bell and Susumu Seino for their constructive feedback and guidance.
Funding:
This work was supported by the National Institutes of Health (R01-ES-028879; P30-ES-027792; and P30-DK-020595).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Declarations
Conflict of interest/Competing interests: RMS has received honoraria from CVS/Health and the American Medical Forum. The authors have no conflicts of interest orcompeting interests to disclose related to this work.
Ethics approval: Not Applicable
Consent to participate: Not Applicable
Consent for publication: The corresponding author gives their consent for the article to be published in Histochemistry and Cell Biology.
Availability of data: The datasets generated during the current study are available from the corresponding author on reasonable request.
Code availability: Not Applicable
References
- Brayton CF (1986) Dimethyl sulfoxide (DMSO): a review. Cornell Vet 76 (1):61–90 [PubMed] [Google Scholar]
- Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S (2006) Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol 148 (5):619–628. doi: 10.1038/sj.bjp.0706770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SM, Vetere A, Walpita D, Dančík V, Khodier C, Perez J, Clemons PA, Wagner BK, Altshuler D (2015) High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metab 21 (1):126–137. doi: 10.1016/j.cmet.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Carmean CM, Yokoi N, Takahashi H, Oduori OS, Kang C, Kanagawa A, Kirkley AG, Han G, Landeche M, Hidaka S, Katoh M, Sargis RM, Seino S (2018) Arsenic modifies serotonin metabolism through glucuronidation in pancreatic β-cells. Am J Physiol Endocrinol Metab. doi: 10.1152/ajpendo.00302.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, Ilkayeva O, Smith N, Miller N, Hajmrle C, Spigelman AF, Wright RC, Plummer G, Suzuki K, Mackay JP, van de Bunt M, Gloyn AL, Ryan TE, Norquay LD, Brosnan MJ, Trimmer JK, Rolph TP, Kibbey RG, Manning Fox JE, Colmers WF, Shirihai OS, Neufer PD, Yeh ET, Newgard CB, MacDonald PE (2015) Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. J Clin Invest 125 (10):3847–3860. doi: 10.1172/JCI82498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheni G, Ogura M, Iwasaki M, Yokoi N, Minami K, Nakayama Y, Harada K, Hastoy B, Wu X, Takahashi H, Kimura K, Matsubara T, Hoshikawa R, Hatano N, Sugawara K, Shibasaki T, Inagaki N, Bamba T, Mizoguchi A, Fukusaki E, Rorsman P, Seino S (2014) Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep 9 (2):661–673. doi: 10.1016/j.celrep.2014.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtovenko AA, Anwar J (2007) Modulating the structure and properties of cell membranes: the molecular mechanism of action of dimethyl sulfoxide. J Phys Chem B 111 (35):10453–10460. doi: 10.1021/jp073113e [DOI] [PubMed] [Google Scholar]
- Hashim M, Yokoi N, Takahashi H, Gheni G, Okechi OS, Hayami T, Murao N, Hidaka S, Minami K, Mizoguchi A, Seino S (2018) Inhibition of SNAT5 Induces Incretin-Responsive State From Incretin-Unresponsive State in Pancreatic β-Cells: Study of β-Cell Spheroid ClustersasaModel. Diabetes 67 (9):1795–1806. doi: 10.2337/db17-1486 [DOI] [PubMed] [Google Scholar]
- He F, Liu W, Zheng S, Zhou L, Ye B, Qi Z (2012) Ion transport through dimethyl sulfoxide (DMSO) induced transient water pores in cell membranes. Mol Membr Biol 29 (3–4):107–113. doi: 10.3109/09687688.2012.687460 [DOI] [PubMed] [Google Scholar]
- Henquin JC (2009) Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 52 (5):739–751. doi: 10.1007/s00125-009-1314-y [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Minami K, Shibasaki T, Miki T, Miyazaki J, Seino S (2010) Establishment of new clonal pancreatic β-cell lines (MIN6-K) useful for study of incretin/cyclic adenosine monophosphate signaling. J Diabetes Investig 1 (4):137–142. doi: 10.1111/j.2040-1124.2010.00026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwat MA, Cobb MH (2017) Mechanisms of the amplifying pathway of insulin secretion in the β cell. Pharmacol Ther 179:17–30. doi: 10.1016/j.pharmthera.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI (2007) Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab 5 (4):253–264. doi: 10.1016/j.cmet.2007.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley P, Whitfield JF (1993) The differentiation inducer, dimethyl sulfoxide, transiently increases the intracellular calcium ion concentration in various cell types. J Cell Physiol 156 (2):219–225. doi: 10.1002/jcp.1041560202 [DOI] [PubMed] [Google Scholar]
- Notman R, Anwar J (2013) Breaching the skin barrier--insights from molecular simulation of model membranes. Adv Drug Deliv Rev 65 (2):237–250. doi: 10.1016/j.addr.2012.02.011 [DOI] [PubMed] [Google Scholar]
- Raphemot R, Weaver CD, Denton JS (2013) High-throughput screening for small-molecule modulators of inward rectifier potassium channels. J Vis Exp (71). doi: 10.3791/4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls WF, Cox L, Rovner ES (2017) Dimethyl sulfoxide (DMSO) as intravesical therapy for interstitial cystitis/bladder pain syndrome: A review. Neurourol Urodyn 36 (7):1677–1684. doi: 10.1002/nau.23204 [DOI] [PubMed] [Google Scholar]
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- Sandler S, Andersson A (1982) Short- and long-term effects of dimethyl sulfoxide on mouse pancreatic islet B-cell function in vitro. Cryobiology 19 (3):299–305. doi: 10.1016/0011-2240(82)90158-4 [DOI] [PubMed] [Google Scholar]
- Seino S, Sugawara K, Yokoi N, Takahashi H (2017) beta-Cell signalling and insulin secretagogues: A path for improved diabetes therapy. Diabetes Obes Metab 19 Suppl 1:22–29. doi: 10.1111/dom.12995 [DOI] [PubMed] [Google Scholar]
- Spollett G, Edelman SV, Mehner P, Walter C, Penfornis A (2016) Improvement of Insulin Injection Technique: Examination of Current Issues and Recommendations. Diabetes Educ 42 (4):379–394. doi: 10.1177/0145721716648017 [DOI] [PubMed] [Google Scholar]
- Szabat M, Modi H, Ramracheya R, Girbinger V, Chan F, Lee JT, Piske M, Kamal S, Carol Yang YH, Welling A, Rorsman P, Johnson JD (2015) High-content screening identifies a role for Na(+) channels in insulin production. R Soc Open Sci 2 (12):150306. doi: 10.1098/rsos.150306 [DOI] [PMC free article] [PubMed] [Google Scholar]


