Abstract
Diabetes mellitus represents a group of diseases with persistent hyperglycemia. Exocrine disorders of the pancreas are increasingly recognized to cause or precede the onset of diabetes, which in this context is referred to as pancreatogenic or type 3c diabetes. The sequela of diabetes is observed across the spectrum of severity in acute pancreatitis and may be associated with other clinical complications. The pathophysiology of acute pancreatitis-related diabetes mellitus is poorly understood, and observations suggest it is likely multifactorial. In this Review, we discuss the epidemiology, pathophysiology, management considerations, and highlight knowledge gaps in diabetes mellitus following acute pancreatitis.
INTRODUCTION
Pancreatogenic diabetes mellitus (DM), also termed type 3c diabetes mellitus (T3cDM), refers to DM secondary to a condition or disease of the exocrine pancreas (Table)(1, 2). Even though the islet cells account for a very small proportion of the overall pancreas volume, many patients with a exocrine pancreatic disorder will develop endocrine dysfunction and dysregulated glucose metabolism. Although the different etiologies of pancreatogenic DM are collectively referred to under a single term, there are pathophysiological differences according to the underlying pancreatic disease; these characteristics have been previously described for chronic pancreatitis, pancreatic cancer, and cystic fibrosis(2–4). Considering the relatively higher frequency of acute pancreatitis compared to other diseases of the pancreas, it is likely the most common etiology of pancreatogenic DM(5).
Table.
Etiopathogenic subclassifications of diabetes mellitus secondary to exocrine pancreatic disease, collectively referred to as pancreatogenic diabetes mellitus, grouped according to their proposed mechanisms. Adapted from Hart et al(2).
| Congenital or acquired complete absence of islets: |
| Pancreatectomy (total) |
| Pancreatic agenesis |
|
Acquired partial absence or dysfunction of islets: |
| Acute pancreatitis |
| Chronic pancreatitis* |
| Cystic fibrosis |
| Hemochromatosis |
| Pancreatectomy (partial) |
|
Paraneoplastic: |
| Pancreatic ductal adenocarcinoma |
Includes tropical pancreatitis, which was previously referred to as fibrocalculous pancreatopathy.
Acute pancreatitis (AP) is an inflammatory disease with a global pooled incidence of approximately 34 cases per 100,000 in the general population per year, but which varies widely given the geographic location(6). Although the majority of patients with AP have mild disease, a subset have increased morbidity and prolonged hospitalization(7). Advances in early risk stratification, supportive care, and aggressive fluid resuscitation have decreased overall morbidity and mortality; but the mechanisms and associated risk factors that impact intermediate and long-term outcomes, such as AP-related DM, remain poorly characterized.
Herein, we review the epidemiology, pathophysiology, management considerations, and outline key knowledge gaps for future investigations regarding the relationships between AP and dysregulated glucose control. The mechanisms underlying development of DM after AP remain unknown, but may represent a combination of classic pancreatogenic (or type 3c) DM, type 2 DM, and type 1 DM. One set of diagnostic criteria for pancreatogenic DM have been proposed, requiring the presence of abnormal pancreatic imaging, exocrine pancreatic insufficiency, and absence of islet auto-antibodies(8). However, it is important to recognize these criteria have not been validated and modifications are likely necessary taking the underlying pancreatic disorder into consideration. To be consistent with the precedent established in cystic fibrosis, we propose and will use the term “acute pancreatitis-related DM (AP-DM)” to describe DM that develops during or following an episode of acute pancreatitis. We recognize the limitations of this nomenclature (i.e., it does not reflect the heterogeneity in the underlying pathophysiology), but believe it is helpful to distinguish from prevalent DM (i.e., DM preceding the development of acute pancreatitis). Prevalent DM is an established risk factor for AP, and is not discussed further to avoid confusion(9).
SEARCH STRATEGY
Potential references were identified for this narrative review using a sensitive search of PubMed for articles published between January 1980 and September 2020, using the following terms: (pancreatogenic OR pancreatogenous OR type 3c OR pancreatitis) AND (diabetes OR diabetes mellitus). Additional articles were identified with chaining, by examining the bibliographies of selected articles. Final references were selected with preference to original articles and those with high relevance to the defined scope of this Review (e.g., studies related to the relationship between diabetes medication usage and acute pancreatitis were not included).
EPIDEMIOLOGY OF ACUTE PANCREATITIS-RELATED DIABETES
Acute pancreatitis (AP) is an inflammatory disease of the exocrine pancreas that accounts for more than 300,000 hospitalizations, with health care costs exceeding $2 billion annually in the United States(10, 11). The global pooled incidence is 34 cases per 100,000 per year, with geographic variation. Specifically, the incidence is significantly higher in North America (e.g., 58.2 cases per 100,000 per year in the United States) than in Europe (e.g., 24.7 and 15.0 cases per 100,000 per year in Sweden and Denmark, respectively)(12–14). The crude mortality rate is approximately 1.2 per 100 000 person-years(6).
Approximately 80% of patients have a mild clinical course with a hospitalization lasting less than a week, while those with moderately-severe or severe AP experience pancreatic necrosis and/or organ failure and a protracted hospital course(7). Long-term sequelae include exocrine pancreatic insufficiency, complications from walled-off pancreatic necrosis, and recurrent episodes of AP in up to 20% of patients(14–16). Hyperglycemia was historically considered a merely transient phenomenon in AP during hospitalization. However, two recent meta-analyses have shed new light on the chronicity and high incidence rate of AP-related DM reporting that approximately 23% of AP patients will develop DM within three years of discharge(17, 18). Importantly, these estimates did not include patients with pre-existing DM (also referred to as prevalent DM), which may exist in approximately 25% of AP patients(19). A recent prospective study reported the development of DM in 3%, 7%, 9%, and 11% at 6, 12, 18, and 24 months following an AP episode, but larger studies are needed to validate these findings(20). In longitudinal studies with at least five years follow-up, the cumulative incidence rate of new onset AP-related DM increased up to 40% (18). Despite this, the precise time course for endocrine dysfunction following AP is incompletely described, with some studies demonstrating complete temporal resolution or marked improvements, while others indicate persistent endocrine dysfunction(21–23). In summary, the development of dysregulated glucose metabolism is common in patients with AP, but may have a reversible component.
Numerous factors have been associated with an increased risk of developing AP-related DM. One of the strongest predictors is the development of severe AP, particularly with necrosis. Indeed, the distribution of clinical severity is an important consideration when interpreting incident rates of AP-related DM. For example, one study reported endocrine dysfunction in 56% of subjects with severe AP in comparison to 23% with mild AP(24). However, the development of DM in patients with mild, non-necrotizing AP suggests there are other contributing factors. The presence of canonical risk factors for type 2 DM, such as family history of type 2 DM, excess adiposity, and physical inactivity, likely contribute, but were not routinely reported in many of the prior studies. Factors predisposing to type 1 DM (e.g., the presence of autoantibodies and/or susceptible genetic polymorphisms) were also not routinely measured. More often, pancreatitis-related risk factors for the development of AP-related DM were documented, and included exocrine pancreatic insufficiency and a non-biliary etiology(18).
While prior studies have provided key observations regarding AP-related DM, there is a need for further, definitive investigations that overcome limitations in the existing data. For example, most published studies have used a clinical diagnosis or diagnostic coding for DM case ascertainment via chart reviews or administrative databases, respectively. While important to generate preliminary data, this methodology will inherently underestimate the true incidence of AP-related DM due to undercoding of DM and/or not attributing it to AP. Conversely, overrepresentation of patients with severe AP in many studies may inflate the incidence rate, and limits the ability to identify predictive factors for patients with mild AP, which reflects the majority of patients in clinical practice. The heterogeneity in study designs is reflected in two similar meta-analyses estimating the incidence of AP-related DM in which there was high statistical heterogeneity that was not explained with subgroup or sensitivity analyses(17, 18). Therefore, definitive studies on the incidence rate and risk factors for AP-related DM require prospective, longitudinal follow-up with serial assessments of glycemic status and evaluation of the impact of potential patient and disease related factors; yet have not been undertaken, likely due to the substantial costs and other resources required.
PATHOPHYSIOLOGY OF ACUTE PANCREATITIS-RELATED DIABETES
The pathophysiology of AP-related DM is incompletely understood, but is likely multifactorial with varying potential contributions in an individual patient arising from: a) loss of islet cell mass, b) AP-induced autoimmunity, c) shared etiologic risk factors, d) local and systemic inflammatory response, and/or e) alterations in the insulin-incretin axis (Figure). It is particularly important to understand the relative contributions from these, as well as potential genetic predispositions, to identify opportunities for prevention of AP-related DM.
Figure.
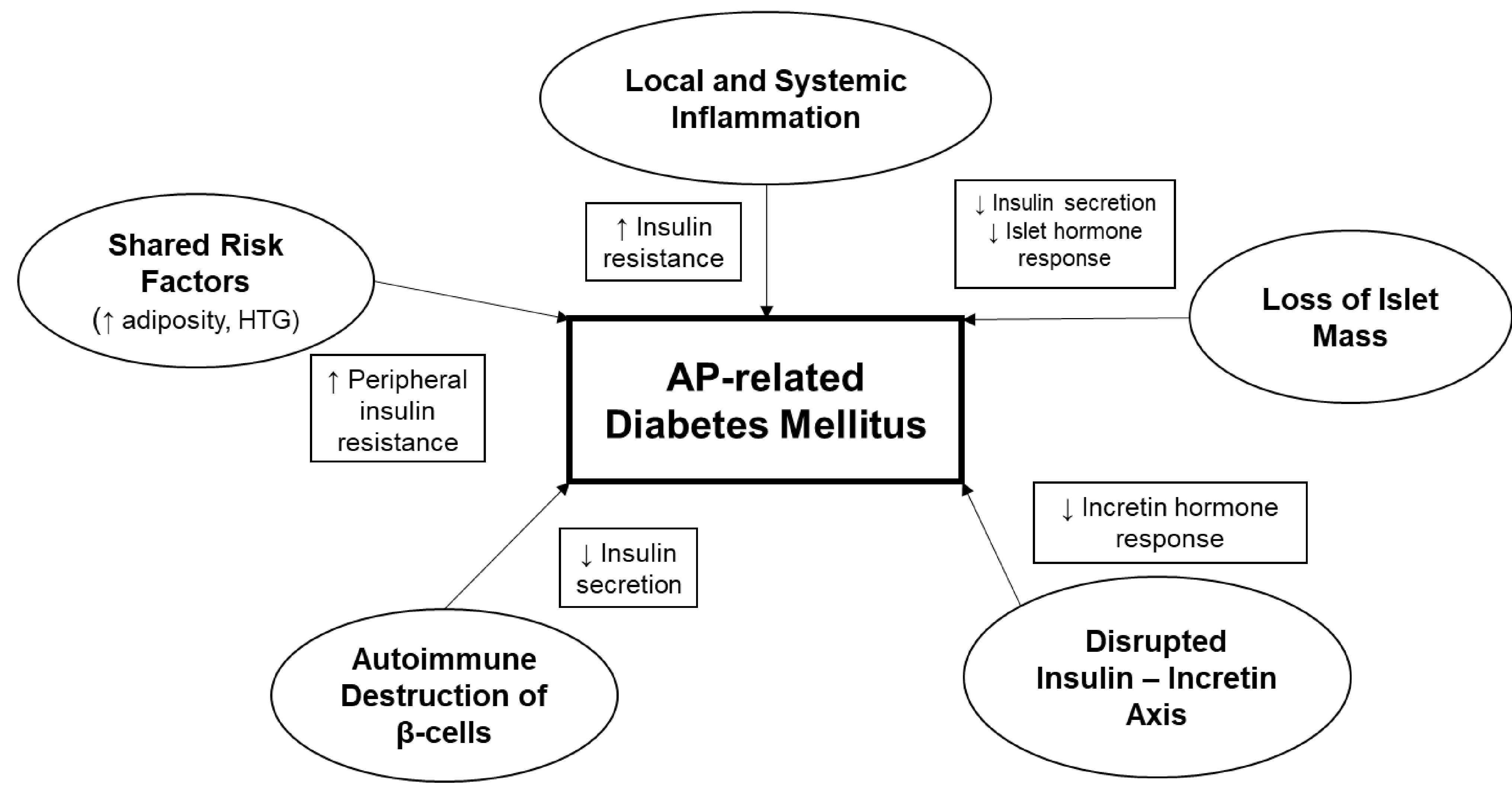
Proposed contributing factors to the pathophysiology of diabetes following acute pancreatitis (acute pancreatitis-related diabetes mellitus), including potential mechanisms of dysglycemia. HTG, hypertriglyceridemia.
Loss of islet mass
In patients experiencing extensive pancreatic necrosis, there is a loss of overall islet mass that results in relative insulin deficiency, which is analogous to pancreatogenic DM following pancreatic surgery. These patients experience the onset of DM early in the course of the disease, which persists over time. Data supporting this mechanism have shown that patients with necrotizing pancreatitis have a higher incidence of AP-related DM compared to subjects without necrosis (37% vs. 11%, P < 0.001) (18). As a result, pancreatic necrosis is expected to result in a reduction or loss of the production and secretion of insulin as well as other islet hormones (e.g., pancreatic polypeptide, amylin, somatostatin, and glucagon). Since >10% of patients with non-necrotizing AP develop DM during short term follow-up, the pathophysiology of AP-related DM is likely dependent on factors other than immediate loss of islets for many patients(18).
Autoimmunity
The clinical observation that insulin requirements in AP-related DM are similar to type 1 DM (T1D), has raised the possibility of disease overlap(5). In T1D, patients develop one or more autoantibodies following a trigger of immune activation. The majority of patients have antibodies to glutamic acid decarboxylase (GAD65) (70–80%), insulin (IAA), insulinoma-associated autoantigen 2 (IA2A), zinc transporter (ZnT8A), and/or tetraspanin 7. These autoimmune markers represent a hallmark of T1D and are produced in response to autoreactive T cells recognizing β-cell self-antigens and neo-antigens. In the absence of AP, it may take months to years for patients to develop overt DM after autoantibody detection, indicating a latent phase of disease(25). The possibility that AP could lead to similar immune activation has not been extensively studied, but there are sparse reports of β-cell autoantibody positivity in patients with AP-related DM(26). Nevertheless, we speculate a robust local (and systemic) inflammatory response in AP could result in post-translational modification of endogenous β-cell proteins, such as insulin and islet nucleic acids. Indeed, such modified neoepitopes have recently been recognized as a contributor to β-cell death in T1D and their production is enhanced under conditions of endoplasmic reticulum stress and inflammation(27). These proteins could act as autoantigens, inducing an autoimmune response with resultant reduction in insulin secretion and ultimately overt diabetes. To date, no studies have systematically evaluated the frequency of autoimmunity during and following an AP episode.
Shared etiologic risk factors
Obesity and hypertriglyceridemia are risk factors for the development of both T2DM and AP. Their presence prior to the onset of AP results in an increased risk for developing subsequent DM following AP, and may potentially accelerate the onset of overt DM(28, 29). Both factors are independently associated with an increased risk for more advanced clinical severity of AP, which may explain part of the risk for AP-related DM(30, 31). Previous studies have shown the prevalence of obesity in subjects with AP-related DM (42%) approximates that observed in T2DM (48%) providing epidemiological support for their relationship(5). Similar comparative data regarding hypertriglyceridemia are not available, and its relative contribution to the development of AP-related DM is expected to be smaller than other factors discussed. Nevertheless, as a reversible risk factor it is important to consider, particularly for those with severe, persistent hypertriglyceridemia who may experience subsequent episodes of AP. Similar to observations in T2DM, it is also possible these factors may contribute to the insulin resistance observed in AP-related DM(32).
Local and systemic inflammatory response
Acute pancreatitis generates a robust local and systemic inflammatory response, even in clinically mild episodes. This inflammatory response is usually transient; however, it may persist in a subgroup of patients with ongoing pancreatic stimulation, clinically severe disease, and/or an underlying genetic predisposition. Since a pro-inflammatory milieu is observed in patients with T2DM, this is an important pathway to investigate(33). In a preliminary study, increased circulating interleukin-6 (IL-6) levels, one of the aforementioned pro-inflammatory cytokines increased in obesity, was associated with the presence of hyperglycemia and insulin resistance following AP(34, 35). Unfortunately, due to the inaccessibility of the pancreas and limitations with collecting pancreas tissue or fluid from patients with AP, future investigations into the local inflammatory response will likely be limited to animal models.
Disruption of the insulin-incretin axis
Exocrine pancreatic insufficiency occurs in up to 30% of patients within three years after an episode of AP(36, 37). In patients with exocrine pancreatic insufficiency, the incretin-insulin axis is disrupted, leading to impaired incretin hormone secretion. This contributes to hyperglycemia due to altered secretion of the incretin hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). Previous studies in subjects with chronic pancreatitis-related DM have shown that pancreatic enzyme replacement therapy (PERT) partially reverses this deficit(2). Therefore, understanding the contribution of exocrine pancreatic dysfunction to AP-related DM may have treatment implications.
Genetic risk factors
Emerging data supports a potential association between known DM genetic risks and the development of pancreatogenic DM. In a study of 2,685 subjects enrolled in the North American Pancreatitis Study 2, the presence of single nucleotide polymorphisms (SNPs) associated with T2DM were investigated in chronic pancreatitis (CP) patients of which 27% had DM(38). The genetic risk score composed of validated T2DM SNPs was similar in the 321 CP-related DM patients as in 423 subjects with T2DM. This finding suggests that among those CP patients who developed DM relatively early in their disease course, the same risk factors prevailed as in patients with T2DM without a history of CP. It is unknown whether similar genetic risks exist in patients with acute pancreatitis who subsequently develop DM. It would be appropriate to include SNPs that have been associated with T1D as well in such a study.
Future investigations
A systematic epidemiological, clinical, and biochemical characterization of the relationship between the endocrine and exocrine pancreas is needed to advance our understanding of AP-related DM and provide potential diagnostic biomarkers and preventive and therapeutic strategies. Specifically, dynamic assessments of insulin secretion, insulin sensitivity, and pancreatic and incretin hormone responsiveness are needed to guide investigations into these proposed pathogenic mechanisms. Dynamic studies of glucose homeostasis will also be helpful to prioritize interventions for treatment of AP-related DM. For example, if there are similarities with T2DM (e.g., increased peripheral insulin resistance), then lifestyle interventions along with an insulin sensitizer (e.g., metformin or a thiazolidinedione) may be beneficial. On the other hand, if there is predominantly a deficit in insulin secretion (similar to T1D), then early insulin introduction would be favored. There is an opportunity to refine the current animal models of acute pancreatitis, which are typically designed with short term endpoints(39). Extending the duration of these models may provide additive value to investigate the pathophysiology of AP-related DM and other delayed complications.
MANAGEMENT OF DIABETES FOLLOWING ACUTE PANCREATITIS
Although there is a paucity of data regarding the clinical management of AP-related DM, there is some rationale to guide decisions with the understanding that refinement will be required as additional epidemiological and interventional studies become available. Aspects of management include prevention, screening, and treatment.
Considering the strong association with severity of AP on the subsequent development of DM, one method to potentially avoid this sequela is to prevent the progression from mild to severe AP. In addition to early, aggressive IV hydration, there are a number of potential therapies under investigation that are beyond the current scope of discussion; yet those with local or systemic anti-inflammatory effects may be particularly useful to reverse the immunopathogenicity leading to hyperglycemia (either transient or chronically). As shared risk factors that are modifiable, management of obesity and hypertriglyceridemia using lifestyle and/or pharmacological interventions should be recommended for all patients with either of these factors present.
Screening for AP-related DM is not explicitly suggested in societal guidelines related to AP. This likely stems from a primary focus on the initial management of AP. Indeed, particularly for gastroenterologists and surgeons, the primary emphasis following hospitalization is on interventions to lower a patient’s risk for recurrent AP (e.g., cholecystectomy for gallstone pancreatitis and cessation of alcohol) and management of symptomatic necrosis. Nevertheless, there is a need for physician awareness of the intermediate and long-term complications of acute pancreatitis. In view of the emerging data regarding the clinically relevant risk of AP-related DM, we believe it is reasonable to consider screening for DM in all patients with acute pancreatitis. While this is particularly true for patients with acute necrotizing pancreatitis or recurrent episodes of AP (who are at increased risk for development of chronic pancreatitis), there is also increased risk for patients with mild AP. In regards to the frequency of screening, a proposed approach involves more intensive glycemic monitoring for the first year after initial hospital discharge (e.g., hemoglobin A1c at 6 months), followed by annual screening thereafter. The rationale for more frequent screening during the first 12 months is based on the observation that approximately 20% of patients will develop pre-diabetes or DM within 6 months, which requires additional clinical evaluation(20). Subsequent studies regarding the economic costs and benefits of screening will be useful to inform this practice at a population level. Refinement of an approach to screening will occur as additional data are generated to more accurately predict the incidence of AP-related DM at the time of hospital discharge. This manner of risk stratification will also be informative to guide more aggressive attempts at education, lifestyle modifications, and potential therapeutic interventions.
In the absence of clinical trials, there is no evidence-based guidance regarding treatment of AP-related DM. Therefore, current treatment is typically adapted using a similar paradigm to the treatment of T2DM with consideration of the potential risk profile of medications. In a large, population-based study a higher proportion of patients with AP-related DM were on insulin therapy within 5 years compared to those with T2DM (20.9% vs. 4.1%), and experienced worse glycemic control (defined as HbA1c ≥7%)(5). Whether the poor glycemic control led to the initiation of insulin or persisted despite insulin therapy cannot be ascertained given the study design. As previously mentioned, there is a need for understanding the mechanisms of hyperglycemia in AP-related DM to guide investigations into treatment options. Although increasingly utilized in the treatment of T2DM, there is concern regarding the use of incretin-based therapies (i.e., GLP-1 receptor agonists and DPP-4 inhibitors) in patients with a history of acute pancreatitis. This caution relates to early reports that these medication classes may precipitate AP. Results from meta-analyses have yielded mixed results regarding the association with DPP-4 inhibitors and acute pancreatitis, but there does not appear to be an association with GLP-1 receptor agonists(40–42). Since many study participants did not have a preceding history of AP, it is uncertain whether or not we can extrapolate these results to patients with a known history of AP. Additional post-marketing surveillance studies are needed to further understand the safety of these medications.
Another consideration in the management of AP-related DM is the potential need to monitor for concurrent exocrine pancreatic dysfunction. As previously mentioned, up to one-third of patients with AP will develop exocrine pancreatic insufficiency(36, 37). A recent population-based study demonstrated the use of pancreatic enzymes (as a surrogate measure of exocrine pancreatic dysfunction/insufficiency) was associated with concurrent AP-related DM; however, the interactions between the endocrine and exocrine pancreatic dysfunction following AP remain unexplained(43). Although screening for exocrine pancreatic insufficiency is not currently recommended for patients with AP, this would be reasonable considering its relatively common occurrence. While unproven, there is a possibility treating exocrine pancreatic insufficiency could improve glycemic control if there is a contribution from disruption of the insulin-incretin axis, as previously observed in chronic pancreatitis-related diabetes. A more recent concern relates to a potentially increased risk for pancreatic cancer among patients with acute pancreatitis compared to the general population(44). Two different population-level studies suggest this risk is further elevated in those patients who develop AP-related DM compared to AP without DM(45, 46). There is risk for clinical misdiagnosis of occult pancreatic cancer as acute pancreatitis, which is part of the rationale for the recommendation to perform short term imaging follow-up for adults with unexplained AP(47). These studies have reduced this type of bias by utilizing eligibility criteria that require a predefined washout period between the diagnoses, which increases the validity of the findings. Although longitudinal imaging surveillance cannot be recommended at this time, maintaining an increased index of clinical suspicion for this complication remains prudent.
KNOWLEDGE GAPS
At a fundamental level the pathophysiology of AP-related DM represents the key limitation to our knowledge of this disease. For example, the current classification approach involves broadly categorizing patients as having AP-related DM based on the chronologic onset of DM in relation to the AP episode. We anticipate that further studies defining the pathophysiology will enable more tailored approaches to prevention and potentially treatment. Furthermore, these studies, when combined with prospective studies, will enable more definitive epidemiological estimates of the incidence rates and associated factors to improve the accurate of risk stratification. Comparisons and contrasts with other, more common, DM subtypes (i.e., T1D and T2DM) will likely provide the ability to accelerate knowledge gains by leveraging existing animal models and results from clinical trials amongst larger patient populations. In the end, developing tailored approaches to screening, prevention, and treatment will require the collaborative efforts of interdisciplinary teams.
FUTURE STUDIES
There are a number of ongoing international efforts to more precisely define the epidemiology and pathophysiology of this disease. For example, the National Institute of Diabetes and Digestive and Kidney diseases (NIDDK) of the United States’ National Institutes of Health has recently formed a collaborative network of 10 clinical centers and one data coordinating center, referred to as Type 1 Diabetes after Acute Pancreatitis Consortium (T1DAPC), to collaboratively address a number of these knowledge gaps. The overriding objective of this research program is to undertake an observational cohort study of patients with acute pancreatitis to investigate the incidence, etiology, and pathophysiology of diabetes following acute pancreatitis with a particular emphasis on the auto-immune processes that result in Type 1 DM. The prospective study will include serial monitoring for diabetes and collection of biological specimens. This rich dataset will provide the opportunity to more precisely estimate the incidence, time course, and relationship to pancreatitis severity, as well as the roles of pancreatitis etiology, genetic and genomic risk factors, environmental and biological factors and potentially discover and/or validate diagnostic or predictive biomarkers. Furthermore, through the acquisition of a cohort of well characterized patients monitored over time for diabetes onset and T1D-associated autoantibodies, and collected biospecimens (e.g., blood, saliva, urine, pancreatic fluid, and stool), the proposed clinical research consortium will provide the resources and collaborative opportunities necessary to identify the interrelationship between the exocrine and the endocrine pancreas in the development of AP-related DM. The consortium will also provide a multidisciplinary environment to conduct both cross sectional and longitudinal studies to further understand the pathophysiology. Additionally, as previously mentioned, there is need to develop animal models that accurately replicate AP-related DM to permit more detailed characterization of its pathogenic mechanisms and provide a platform to accelerate the investigation of potential therapeutic interventions. These, and other, investigations are needed to address a number of remaining clinical questions, including how to diagnose AP-related DM (in contrast to type 2 DM), define its natural history, and optimize the approaches to monitoring and treatment.
CONCLUSIONS
AP-related DM is increasingly recognized as a sequelae of AP. While it is more common in patients with necrotizing or severe AP, patients with mild AP are also at increased risk for developing this complication. The pathophysiology is poorly understood, and may represent a combination of type 1 DM, type 2 DM, and pancreatogenic DM. It is expected that efforts of the newly formed T1DAPC in the United States and ongoing efforts worldwide will shed light on the incidence, risk factors, and mechanisms of AP-related DM, which will allow development of evidence-based guidelines for screening, prevention, and treatment of AP-related DM.
Acknowledgments
Grant Support:
Research reported in this publication was supported by the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award numbers U01DK127388 (PH, DB, DC, KD, GP), U01DK108327 (PH, DB, DC), U01DK126300 (MB), U01DK127367 (MB), U01DK108306 (DY), and U01DK127377 (DY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AP
acute pancreatitis
- AP-related DM
diabetes following acute pancreatitis
- CP
chronic pancreatitis
- DM
diabetes mellitus
- HTG
hypertriglyceridemia
- T1D
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
- T3cDM
Type 3c diabetes mellitus
Footnotes
Conflicts of interest/disclosures: No conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes care. 2020;43(Suppl 1):S14–S31. [DOI] [PubMed] [Google Scholar]
- 2.Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1(3):226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moran A, Becker D, Casella SJ, Gottlieb PA, Kirkman MS, Marshall BC, et al. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes care. 2010;33(12):2677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granados A, Chan CL, Ode KL, Moheet A, Moran A, Holl R. Cystic fibrosis related diabetes: Pathophysiology, screening and diagnosis. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2019;18 Suppl 2:S3–S9. [DOI] [PubMed] [Google Scholar]
- 5.Woodmansey C, McGovern AP, McCullough KA, Whyte MB, Munro NM, Correa AC, et al. Incidence, demographics, and clinical characteristics of diabetes of the exocrine pancreas (Type 3c): A retrospective cohort study. Diabetes care. 2017;40(11):1486–93. [DOI] [PubMed] [Google Scholar]
- 6.Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. The lancet Gastroenterology & hepatology. 2016;1(1):45–55. [DOI] [PubMed] [Google Scholar]
- 7.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nature reviews Gastroenterology & hepatology. 2019;16(8):479–96. [DOI] [PubMed] [Google Scholar]
- 8.Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World journal of gastroenterology. 2013;19(42):7276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, He Z, Tang X, Liu J. Type 2 diabetes mellitus and the risk of acute pancreatitis : a meta-analysis. European journal of gastroenterology & hepatology. 2013;25(2):225–31. [DOI] [PubMed] [Google Scholar]
- 10.Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156(1):254–72 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagenholz PJ, Fernandez-del Castillo C, Harris NS, Pelletier AJ, Camargo CA Jr. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35(4):302–7. [DOI] [PubMed] [Google Scholar]
- 12.Nojgaard C. Prognosis of acute and chronic pancreatitis - a 30-year follow-up of a Danish cohort. Danish medical bulletin. 2010;57(12):B4228. [PubMed] [Google Scholar]
- 13.Lindkvist B, Appelros S, Manjer J, Borgstrom A. Trends in incidence of acute pancreatitis in a Swedish population: is there really an increase? Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2004;2(9):831–7. [DOI] [PubMed] [Google Scholar]
- 14.Yadav D, O'Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. The American journal of gastroenterology. 2012;107(7):1096–103. [DOI] [PubMed] [Google Scholar]
- 15.Yadav D, Lee E, Papachristou GI, O'Connell M. A population-based evaluation of readmissions after first hospitalization for acute pancreatitis. Pancreas. 2014;43(4):630–7. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed Ali U, Issa Y, Hagenaars JC, Bakker OJ, van Goor H, Nieuwenhuijs VB, et al. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14(5):738–46. [DOI] [PubMed] [Google Scholar]
- 17.Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63(5):818–31. [DOI] [PubMed] [Google Scholar]
- 18.Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, Li L. Incidence of New Onset Diabetes Mellitus Secondary to Acute Pancreatitis: A Systematic Review and Meta-Analysis. Frontiers in physiology. 2019;10:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brindise E, Elkhatib I, Kuruvilla A, Silva R. Temporal Trends in Incidence and Outcomes of Acute Pancreatitis in Hospitalized Patients in the United States From 2002 to 2013. Pancreas. 2019;48(2):169–75. [DOI] [PubMed] [Google Scholar]
- 20.Bharmal SH, Cho J, Alarcon Ramos GC, Ko J, Stuart CE, Modesto AE, et al. Trajectories of glycaemia following acute pancreatitis: a prospective longitudinal cohort study with 24 months follow-up. Journal of gastroenterology. 2020;55(8):775–88. [DOI] [PubMed] [Google Scholar]
- 21.Ibars EP, Sanchez de Rojas EA, Quereda LA, Ramis RF, Sanjuan VM, Peris RT. Pancreatic function after acute biliary pancreatitis: does it change? World journal of surgery. 2002;26(4):479–86. [DOI] [PubMed] [Google Scholar]
- 22.Angelini G, Cavallini G, Pederzoli P, Bovo P, Bassi C, Di Francesco V, et al. Long-term outcome of acute pancreatitis: a prospective study with 118 patients. Digestion. 1993;54(3):143–7. [DOI] [PubMed] [Google Scholar]
- 23.Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of Diabetes Mellitus after First-Attack Acute Pancreatitis: A National Population-Based Study. Am J Gastroenterol. 2015;110(12):1698–706. [DOI] [PubMed] [Google Scholar]
- 24.Garip G, Sarandol E, Kaya E. Effects of disease severity and necrosis on pancreatic dysfunction after acute pancreatitis. World journal of gastroenterology. 2013;19(44):8065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahara T, Takamura T, Otoda T, Ishikura K, Matsushita E. Transient anti-GAD antibody positivity and acute pancreatitis with pancreas tail swelling in a patient with susceptible haplotype for type 1 diabetes mellitus. Internal medicine. 2009;48(21):1897–9. [DOI] [PubMed] [Google Scholar]
- 27.Purcell AW, Sechi S, DiLorenzo TP. The Evolving Landscape of Autoantigen Discovery and Characterization in Type 1 Diabetes. Diabetes. 2019;68(5):879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szentesi A, Parniczky A, Vincze A, Bajor J, Godi S, Sarlos P, et al. Multiple Hitsin Acute Pancreatitis: Components of Metabolic Syndrome Synergize Each Other's Deteriorating Effects. Frontiers in physiology. 2019;10:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu D, Xu Y, Zeng Y, Wang X. Endocrine pancreatic function changes after acute pancreatitis. Pancreas. 2011;40(7):1006–11. [DOI] [PubMed] [Google Scholar]
- 30.Smeets X, Knoester I, Grooteman KV, Singh VK, Banks PA, Papachristou GI, et al. The association between obesity and outcomes in acute pancreatitis: an individual patient data meta-analysis. European journal of gastroenterology & hepatology. 2019;31(3):316–22. [DOI] [PubMed] [Google Scholar]
- 31.Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology : official journal of the International Association of Pancreatology. 2020;20(5):795–800. [DOI] [PubMed] [Google Scholar]
- 32.Pendharkar SA, Singh RG, Bharmal SH, Drury M, Petrov MS. Pancreatic Hormone Responses to Mixed Meal Test in New-onset Prediabetes/Diabetes After Non-necrotizing Acute Pancreatitis. Journal of clinical gastroenterology. 2020;54(2):e11–e20. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-alpha and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine. 2016;86:100–9. [DOI] [PubMed] [Google Scholar]
- 34.Gillies N, Pendharkar SA, Asrani VM, Mathew J, Windsor JA, Petrov MS. Interleukin-6 is associated with chronic hyperglycemia and insulin resistance in patients after acute pancreatitis. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2016;16(5):748–55. [DOI] [PubMed] [Google Scholar]
- 35.Xu E, Pereira MMA, Karakasilioti I, Theurich S, Al-Maarri M, Rappl G, et al. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat Commun. 2017;8:14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollemans RA, Hallensleben NDL, Mager DJ, Kelder JC, Besselink MG, Bruno MJ, et al. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology : official journal of the International Association of Pancreatology. 2018;18(3):253–62. [DOI] [PubMed] [Google Scholar]
- 37.Huang W, de la Iglesia-Garcia D, Baston-Rey I, Calvino-Suarez C, Larino-Noia J, Iglesias-Garcia J, et al. Exocrine Pancreatic Insufficiency Following Acute Pancreatitis: Systematic Review and Meta-Analysis. Digestive diseases and sciences. 2019;64(7):1985–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodarzi MO, Nagpal T, Greer P, Cui J, Chen YI, Guo X, et al. Genetic Risk Score in Diabetes Associated With Chronic Pancreatitis Versus Type 2 Diabetes Mellitus. Clinical and translational gastroenterology. 2019;10(7):e00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorelick FS, Lerch MM. Do Animal Models of Acute Pancreatitis Reproduce Human Disease? Cellular and molecular gastroenterology and hepatology. 2017;4(2):251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forsmark CE. Incretins, Diabetes, Pancreatitis and Pancreatic Cancer: What the GI specialist needs to know. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2016;16(1):10–3. [DOI] [PubMed] [Google Scholar]
- 41.Giorda CB, Sacerdote C, Nada E, Marafetti L, Baldi I, Gnavi R. Incretin-based therapies and acute pancreatitis risk: a systematic review and meta-analysis of observational studies. Endocrine. 2015;48(2):461–71. [DOI] [PubMed] [Google Scholar]
- 42.Singh AK, Gangopadhyay KK, Singh R. Risk of acute pancreatitis with incretin-based therapy: a systematic review and updated meta-analysis of cardiovascular outcomes trials. Expert review of clinical pharmacology. 2020;13(4):461–8. [DOI] [PubMed] [Google Scholar]
- 43.Cho J, Scragg R, Pandol SJ, Petrov MS. Exocrine Pancreatic Dysfunction Increases the Risk of New-Onset Diabetes Mellitus: Results of a Nationwide Cohort Study. Clinical and translational science. 2020. [DOI] [PMC free article] [PubMed]
- 44.Kirkegard J, Cronin-Fenton D, Heide-Jorgensen U, Mortensen FV. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology. 2018;154(6):1729–36. [DOI] [PubMed] [Google Scholar]
- 45.Cho J, Scragg R, Petrov MS. Postpancreatitis Diabetes Confers Higher Risk for Pancreatic Cancer Than Type 2 Diabetes: Results From a Nationwide Cancer Registry. Diabetes care. 2020;43(9):2106–12. [DOI] [PubMed] [Google Scholar]
- 46.Kirkegard J, Mortensen FV, Heide-Jorgensen U, Cronin-Fenton D. Predictors of underlying pancreatic cancer in patients with acute pancreatitis: a Danish nationwide cohort study. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2020;22(4):553–62. [DOI] [PubMed] [Google Scholar]
- 47.Tenner S, Baillie J, DeWitt J, Vege SS, American College of G. American College of Gastroenterology guideline: management of acute pancreatitis. The American journal of gastroenterology. 2013;108(9):1400–15; 16. [DOI] [PubMed] [Google Scholar]


