Abstract
Previous studies on mouse embryo limbs have established that interzone mesenchymal progenitor cells emerging at each prescribed joint site give rise to joint tissues over fetal time. These incipient tissues undergo structural maturation and morphogenesis postnatally, but underlying mechanisms of regulation remain unknown. Hox11 genes dictate overall zeugopod musculoskeletal patterning and skeletal element identities during development. Here we asked where these master regulators are expressed in developing limb joints and whether they are maintained during postnatal zeugopod joint morphogenesis. We found that Hoxa11 was predominantly expressed and restricted to incipient wrist and ankle joints in E13.5 mouse embryos, and became apparent in medial and central regions of knees by E14.5, though remaining continuously dormant in elbow joints. Closer examination revealed that Hoxa11 initially characterized interzone and neighboring cells and was then restricted to nascent articular cartilage, intra joint ligaments and structures such as meniscal horns over prenatal time. Postnatally, articular cartilage progresses from a nondescript cell-rich, matrix-poor tissue to a highly structured, thick, zonal and mechanically competent tissue with chondrocyte columns over time, most evident at sites such as the tibial plateau. Indeed, Hox11 expression was intimately coupled to such morphogenetic processes and, in particular, to the topographical rearrangement of chondrocytes into columns within the intermediate and deep zones of tibial plateau that normally endures maximal mechanical loads. Revealingly, these expression patterns were maintained even at 6 months of age. In sum, our data indicate that Hox11 genes remain engaged well beyond embryonic synovial joint patterning and are specifically tied to postnatal articular cartilage morphogenesis into a zonal and resilient tissue. The data demonstrate that Hox11 genes characterize adult, terminally differentiated, articular chondrocytes and maintain region-specificity established in the embryo.
Keywords: Synovial joint formation, Limb development, Articular cartilage growth and morphogenesis, Gdf5, Hox genes, Wholemount imaging, RNAscope, Mouse
Graphical Abstract
Introduction
Synovial joints are essential for body movement, and each is uniquely shaped to maximize range and type of motion based on anatomical location (Mow & Sugalski, 2001). Much has been learned about the structure and function of adult joint tissues (articular cartilage, meniscus and intra joint ligaments) including the biomechanical and lubricating roles each tissue plays in sustaining motion and resilience (Longobardi et al., 2015). In comparison, relatively little is known about how the diverse joint tissues are established and organized during embryonic development and, in particular, how they acquire their morphological traits and 3D architecture to generate a single functioning unit in adults (Pacifici et al., 2018; Rux et al., 2019). In developing limb buds, the first overt sign of incipient joint formation is the emergence of tightly-associated and elongated mesenchymal progenitor cells collectively known as the interzone (Holder, 1977; Mitrovic, 1978). Original genetic lineage tracing studies conducted by our group and collaborators first established that the embryonic interzone cells specify and give rise to all of the diverse structures of synovial joints including the capsule, articular cartilage, meniscus and intra joint ligaments, thus serving as specialized joint-forming progenitors (Koyama et al., 2008; Rountree et al., 2004). Notably, all of the structures of adult joints are distinguishable at birth but are still relatively immature, and much of their morphogenesis and terminal organizational processes occur during postnatal life in coordination with growth of the skeleton (Decker et al., 2017; Decker, 2017; L. Li et al., 2017). In particular, postnatal morphogenesis of articular cartilage is complex since it needs to eventually establish a highly structured, anisotropic tissue critical for joint mechanical function and resistance to injury- and age-related diseases (Gannon et al., 2015; Hunziker et al., 2007; Julkunen et al., 2009).
In adults, the fully mature articular cartilage displays a stereotypic zonal organization of both cells and extracellular matrix (ECM) that includes: (i) a top superficial zone of flat cells juxtaposed with the synovial space and parallel to the surface, producing ECM components including lubricin/Prg4; (ii) an intermediate zone of round chondrocytes amidst randomly aligned ECM components; (iii) a thick deep zone of polarized chondrocytes oriented into columns and containing abundant ECM rich in collagen II and aggrecan, aligned perpendicular to the surface; and (iv) a bottom calcified zone with large hypertrophic-like chondrocytes that conjoins articular cartilage to the underlying subchondral bone (Hunziker et al., 2007). Surprisingly little is known of prenatal and postnatal mechanisms that lead to specification and phenotypic characterization of each of these zones (Decker, 2017; Rux et al., 2019). To address this issue, we recently described the basic tenets of postnatal morphogenesis of articular cartilage in mouse knee joints (Decker et al., 2017). We found that at birth, nascent articular cartilage was matrix-poor, disorganized, but highly proliferative. Thereafter, cell proliferation decreased rapidly while the tissue thickened by 2–3 weeks of age through matrix production/accumulation and increases in average cell size. A stereotypic zonal organization with chondrocyte columns became apparent and established by 6–8 weeks of age. Further, using genetic lineage tracing of single cells (rosa-confetti reporter), we found that each column was made up of non-daughter cells. This is notably distinct from growth plate organization in which each chondrocyte column has been shown to derive from a single progenitor residing near/at the reserve zone (Decker et al., 2017; Mizuhashi et al., 2018; Newton et al., 2019). Thus, our data suggested a distinct and active mechanism of chondrocyte column formation during articular cartilage morphogenesis in which the cells would translocate and become realigned into patterned stacks over postnatal time (Decker et al., 2017). It should be underlined here that a full understanding of postnatal articular cartilage morphogenesis would be critical to inform how joint developmental defects/diseases are established and, as importantly, how defective and damaged joint tissues could be repaired and regenerated in the general patient population.
The Hox genes are a subset of ancient and evolutionarily conserved homeobox- containing transcription factors required for patterning during embryonic development (Duboule, 2007; Mallo et al., 2010). They are critical for specifying positional characteristics of the body plan in a range of organisms including (but not limited to) mammals, insects, fish and birds which has been accomplished evolutionarily by expansion of the Hox gene cluster from a single common ancestor. Loss of Hox gene function results in drastic patterning defects during embryonic development that disrupt the identity and formation of specific skeletal segments (Chisaka & Capecchi, 1991; Lewis, 1978; Wellik & Capecchi, 2003) and can also undermine segment-specific joint development (Albrecht et al., 2002; Koyama et al., 2010a, 2010b; Reno et al., 2016; Villavicencio-Lorini et al., 2010). Mammals have 39 Hox genes subdivided into 13 paralogous groups (Hox1–13) and arranged in 4 clusters (Hoxa-d) that are expressed and function along the anterior-posterior body axis collinear with their genomic arrangement (Wellik, 2007). Hox1 genes are expressed earlier in development and pattern the more anterior regions of the body, whereas Hox13 genes are expressed the latest and function in the most caudal regions. In addition, posterior Hox paralogous genes (Hox9–13) function to pattern the developing limbs along the proximal to distal axis (Davis et al., 1995; Fromental-Ramain et al., 1996a, 1996b; Pineault & Wellik, 2014; Wellik & Capecchi, 2003). Hox9 and Hox10 genes pattern the stylopod (humerus/femur) (Froment-Ramain et al., 1996a; Raines et al., 2015; Wellik & Capecchi, 2003), Hox11 genes pattern the zeugopod (radius/ulna and tibia/fibula) (Davis et al., 1995; Swinehart et al., 2013; Wellik & Capecchi, 2003), and Hox13 genes pattern the autopod (carpals/metacarpals and tarsals/metatarsals) (Fromental-Ramain et al., 1996b). Importantly, members of each paralogous group are functionally redundant and can compensate for each other if one is ablated or mutated, underscoring their critical function in development.
The Hox11 genes have received focused attention leading to new important findings, insights and concepts on overall roles for Hox genes in musculoskeletal development and maintenance. For example, global loss of Hoxa11 and Hoxd11 was found to cause severe shortening and malformation of forelimb zeugopod skeletal elements and their joints as well as absence or fusion of neighboring muscle groups, tendons and ligaments (Swinehart et al., 2013; Wellik & Capecchi, 2003). Triple Hoxa11, Hoxc11 and Hoxd11 mouse embryo mutants displayed shortening and malformation of both hindlimb and forelimb zeugopod skeletal elements and their joints, including wrist, ankle, knee and elbow (Koyama et al., 2010a, 2010b). In these triple mutants, the wrists and ankles displayed missing and fused bones, the knees were disrupted by inclusion of the fibula in the joint, and the elbow displayed a patella-like element in place of the olecranon attached to the ulna by an ectopic ligament (Koyama et al., 2010a). The spectrum, penetrance and multi-faceted consequences of Hox11 paralogous gene ablation on the zeugopod developing joints themselves raise the possibility that the genes could not only have overall joint patterning roles embryonically but could exert additional, local and distinct roles in postnatal joint tissue development, maturation and structuring. This idea is supported by recent work by one of us and colleagues providing the first evidence that Hox11 paralogous genes are not only continuously expressed through postnatal skeletal development and into adulthood but also participate in skeletal regeneration following injury (Rux et al., 2016, 2017). To extend those studies, we investigated here whether Hox11 genes are expressed during postnatal development and growth of limb joints and whether the patterns are dynamically modulated during joint tissue morphogenesis. To this end, we utilized a Hoxa11eGFP live reporter mouse line in combination with RNAscope and morphogenetic/phenotypic approaches to define the expression patterns of all Hox11 paralogous genes. The Hoxa11eGFP murine model was first created and used to monitor Hox11 paralogous group gene expression in prenatal and postnatal zeugopod structures, revealing also that the genes normally characterize mesenchymal progenitors within perichondrium, muscles, tendons and ligaments but not the differentiated cells of these tissues (Nelson et al., 2008; Pineault et al., 2019; Rux et al., 2016; Swinehart et al., 2013). The data we report below demonstrate that Hox11 genes display highly dynamic expression patterns in developing zeugopod joints and are specifically modulated in conjunction with morphogenesis of juvenile mouse articular cartilage into morphologically distinct zones, prominently persisting in adults through at least 6 months of age.
Material and Methods
Animals.
All studies involving mice were reviewed by our institutional IACUC at The Children’s Hospital of Philadelphia. All animals were handled, treated and cared for according to the approved protocols. Hoxa11eGFP (Stock no. 011036) and Rosa-TdTomato mice (Stock no. 007914) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Gdf5Cre mice (kindly provided by Dr. D. Kingsley) were described previously (Rountree et al., 2004) and line B was used in the present study. Wildtype littermate animals were used for controls.
Tissue Processing.
Tissues from fluorescent reporter animals were processed for frozen sectioning. Embryos were harvested and fixed overnight in 4% paraformaldehyde (PFA), washed in PBS, equilibrated in 30% sucrose overnight and embedded in OCT. Postnatal and adult limbs were harvested and joints were injected with 4% PFA before fixing for 2 days. Tissues were decalcified in 20% EDTA for up to 2 weeks (depending on age) washed in PBS overnight, equilibrated in 30% sucrose for 16–36 hours (depending on age) and embedded in OCT. 10 μm sections were collected from all samples; adult samples were collected on Kawamoto’s cryofilm (Kawamoto & Kawamoto, 2021) to preserve tissue morphology. Tissues for RNAscope were processed for paraffin sectioning. Embryonic and newborn tissues were treated as above for fixation and then dehydrated, embedded in paraffin. Decalcification on postnatal tissues for RNAscope was performed using Morse’s solution as previously described for RNA preservation in adult bone and cartilage tissues (de Charleroy et al., 2021). All sections were cut at 6 μm thick.
Immunofluorescent Staining.
Immunofluorescent staining for GFP was performed to preserve fluorescence due to rapid photobleaching of the HoxalleGFP reporter. Frozen sections were permeabilized for 20 minutes in 1X PBS with 0.1% triton. 1X PBS with 0.1% tween was used for all following washes and incubations. Sections were blocked in 5% donkey serum for 1 hour at room temperature, incubated with rabbit anti-GFP primary antibody (Invitrogen, cat. no. A11122 ) at 37°C for 1 hour and with secondary antibody alexafluor 488-conjugated donkey anti-rabbit secondary antibody (Jackson Immunoresearch, cat. no. 711–545-152) at room temperature for 1.5 hour in the dark. Sections were counterstained with DAPI (1mg/mL stock per manufacturer guidelines, Roche, cat. no. 10236276001) diluted 1:10,000 in wash buffer for 10 minutes and cover slipped using ProLong Gold (Invitrogen, cat no. P36930).
Tissue preparation for wholemount imaging of the tibial plateau.
Knees were harvested and disarticulated to expose the tibial plateau and meniscus was removed. Tissue was fixed overnight in 4% PFA. To stain nuclei, tissue was incubated in 1:2000 Hoechst (Invitrogen, cat. no. H3570) overnight at 4°C and then washed in 1XPBS or water prior to imaging. Imaging was performed within 2 weeks of collection and tissues were stored in 1% PFA long-term as needed. For imaging with second harmonic generation (SHG), knees were processed as described above without Hoechst.
RNAscope.
RNAscope in situ hybridization was carried out using RNAscope2.5 HD Detection reagent-RED (Advanced Cell Diagnostics, Newark, CA, USA) to visualize the spatio-temporal expression of Hoxa11 (cat. no. 495561), Hoxc11 (cat. No. 1049161-C1) and Hoxd11 (cat. No. 580621). Briefly, sections were pretreated with a custom reagent and the probe was hybridized for 2 hours at 40°C in a custom oven. Signal was amplified with multiple reagents as per manufacturer’s protocols and final signal was detected and visualized by reaction with Fast Red substrate for 10–20 minutes (dependent on age) at room temperature. Companion sections were hybridized with positive (Cat No. 313911) or negative control probes (Cat. No. 310043) to assure signaling specificity. Sections imaged with brightfield were counterstained with hematoxylin, dried and sealed with Permount. Sections imaged by confocal microscopy were counterstained with Hoescht and cover slipped with Prolong Gold.
Imaging and 3D reconstruction.
A Nikon Eclipse Ci-L upright microscope with attached DS-Fi3 camera was used to capture brightfield images for RNAscope. A Leica DMi8CEL Advanced inverted SP8 confocal was used to collect fluorescent and DIC images from tissue sections and for wholemount imaging of the postnatal tibial plateau. Images of entire limb sections were tile- scanned in Leica LAS software and stitched manually in Photoshop. For wholemount imaging of the entire postnatal tibial plateau, 8-well chambers were detached from culture slides (Corning, cat. no. 354118) and superglued to a glass coverslip. Once dry, water was placed in the wells and the prepared knee sample was placed tibial plateau surface side down in one of the wells. Using the SP8 confocal, 5 μm section images were obtained in several overlapping regions to cover the entire tibial plateau (medial, lateral, anterior and posterior), projected to 2-D images in ImageJ and tiled manually in Photoshop. A fluorescent stereoscope (Leica) was used to image live Hoxa11eGFP+ embryos. Several overlapping images were captured to cover the entire embryo for manual stitching in photoshop. For all 2-D images, photoshop was also used for channel overlay and brightness/contrast adjustments. For whole mount imaging and 3-D reconstruction throughout the depth of postnatal cartilage, processed samples were partially embedded in 2% agarose, leaving the tibial surface exposed and immersed in water. An upright laser scanning confocal microscope with 2-photon capability (SP5, Leica) was used to obtain 1 μm section images. A 3-D reconstruction of stacked images and movie were created using Lecia LAS software.
Results
Hox11 genes are expressed in synovial joint progenitors during mouse embryo development
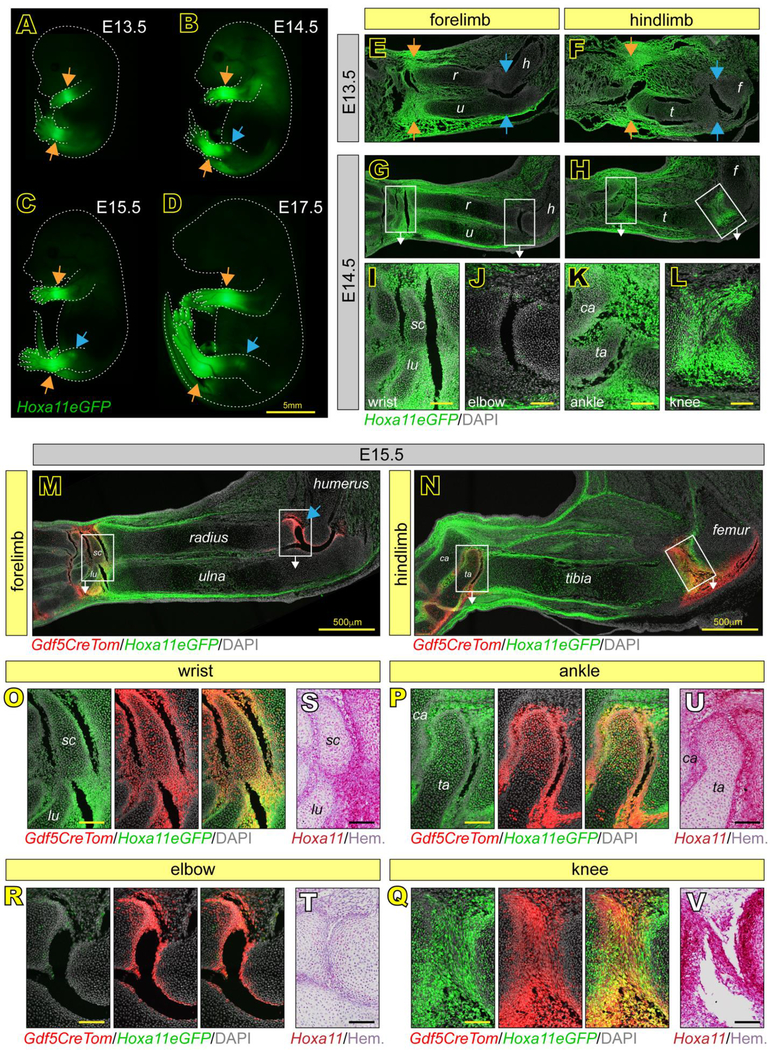
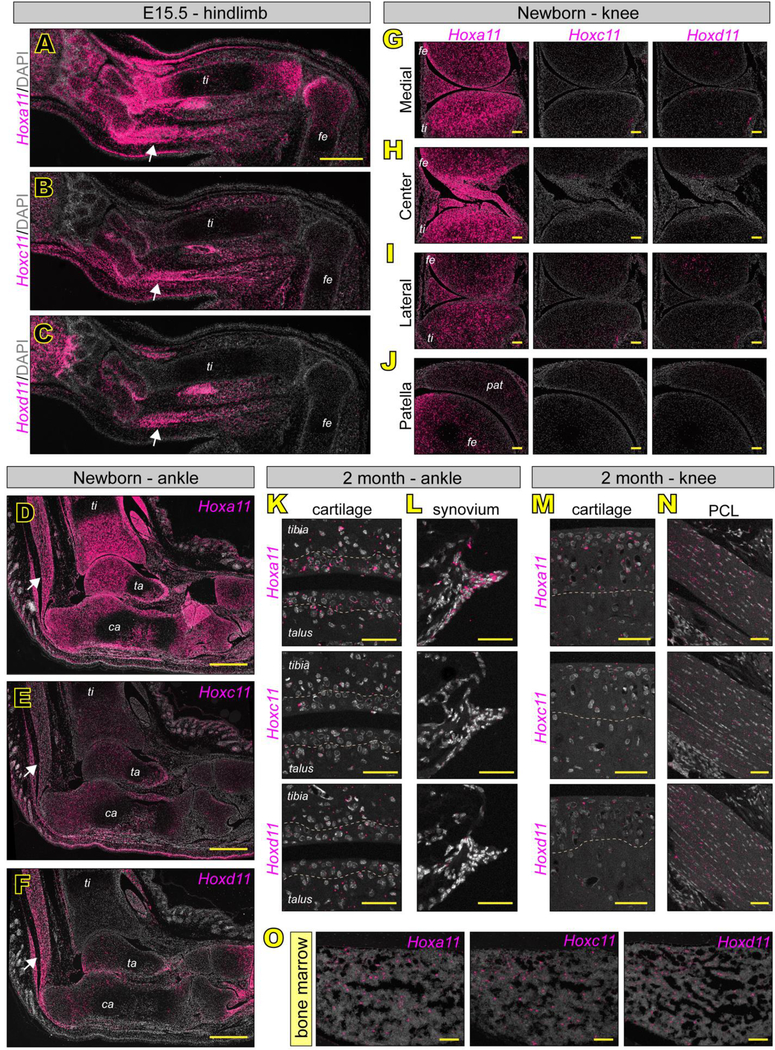
In our studies here, we first focused on the initial stages of limb joint determination and formation from E13.5 to E18.5. Cells expressing Hoxa11eGFP during embryonic zeugopod limb development were previously identified and characterized as mesenchymal progenitors in bone and muscle by Swinehart et al., but the study did not consider cells of the developing synovial joints (Swinehart et al., 2013). Thus, we sought to define them further here. Whole-embryo imaging showed that Hoxa11eGFP was highly restricted to the zeugopod at each stage, as previously reported (Nelson et al., 2008; Swinehart et al., 2013) (Fig. 1A–D). Particularly strong expression in the incipient E13.5 wrist and ankle joints persisted through E18.5 (Fig. 1A–D, orange arrows), and expression became apparent in incipient knee -but not elbow- starting around E14.5 (Fig. 1B–D, blue arrows). To enhance reporter detection, increase sensitivity and avoid problems of photobleaching, we used an antibody against GFP for examination of tissue sections. Parasagittal sections of Hoxa11eGFP forelimbs and hindlimbs in combination with confocal microscopy showed that Hoxa11eGFP was highly expressed in the distal most region of zeugopods of both forelimbs and hindlimbs including joint progenitor interzone cells and neighboring tissues in developing wrists and ankles by E13.5 (Fig. 1E–F, orange arrows and Fig. S1A–D‘) and maintained through E14.5 (Fig. 1G–I, K and Fig. S1G–J‘). In contrast, Hoxa11eGFP was not expressed in either proximal zeugopod joint at E13.5 (Fig. 1E–F, blue arrows and Fig. S1E–F‘), but became highly expressed in knees by E14.5 (Fig. 1H and L, and Fig. S1L–L’) while the elbow joints remained reporter negative (Fig. 1G and J and Fig. S1E–E’). Notably, expression was highest in the small joint skeletal elements juxtaposed to long bone anlage: the scaphoid (sc) and lunate (lu) in wrists (Fig. 1G and I), and the talus (ta) and calcaneous (ca) in ankles (Fig. 1H and K) (note that nomenclature of wrist and ankle bones differ among mouse studies).
Fig 1. Hox11 is expressed throughout zeugopod embryonic joint development.
(A-D) Whole mount imaging of Hoxa11eGFP embryos at E13.5, E14.5, E15.5 and E17.5 show regional restriction to distal zeugopod elements of the limbs at all stages (A-D, orange arrows) and in the knee starting at E14.5 (B-D, blue arrows). (E-L) Tissue sections of Hoxa11eGFP embryos at E13.5 show Hox11 expression only in the wrist and ankle joints (E-F, orange arrows) and not in elbow or knee joints (E-F, blue arrows). At E14.5, expression in distal joints is maintained and Hox11 is additionally expressed in the knee (G-H). High magnification images from boxed areas in G and H show particularly high Hox11 expression in the scaphoid (sc) and lunate (lu) of the wrist (I) and talus (ta) and calcaneous (ca) of the ankle (K) and adjacent zeugopod long bones. Expression is also high in the E14.5 knee (L), but is largely absent in the elbow (J). (M-R) Tissue sections at E15.5 of the entire limb zeugopod show continuous Hox11 expression in the wrist (M), ankle and knee (N), but not elbow (M, blue arrow) and overlap with Gdf5Cre;tdTomato (Gdf5CreTom) joint progenitors (yellow). High magnification images of boxed regions in M and N show further restriction of Hoxa11eGFP to outer cells of the scaphoid (sc) and lunate (lu) in the wrist (O) and talus (ta) and calcaneous (ca) in the ankle (P) and also the high degree of overlap with Gdf5-lineage cells (Gdf5CreTom). Hoxa11eGFP also overlaps strongly with Gdf5CreTom in the knee (Q), but not in elbow (R). (S-V) RNAscope for Hoxa11 confirms expression patterns of Hoxa11eGFP in the wrist (S), elbow (T), ankle (U) and knee (V). r, radius; u, ulna; h, humerus; t, tibia; f, femur. scale bar = 100 μm (if not otherwise noted).
To further assess these differential expression patterns in the developing joints, we crossed Hoxa11eGFP mice with Gdf5Cre;Rosa-tdTomato mice. Gdf5 is a unique marker of joint interzone progenitors (Rountree et al., 2004), and the Gdf5Cre allele was previously used to genetically trace those cells and show that their progenies (Gdf5-lineage cells) give rise to most, if not all, joint tissues over time (Decker et al., 2017; Koyama et al., 2008). There was a high degree of overlap between Gdf5-lineage (red) cells and Hoxa11eGFP-positive (green) cells in E15.5 wrists, ankles and knees as depicted by overall yellow fluorescence signal (Fig. 1M–N). Still, elbows displayed only Gdf5-lineage (red) cells (Fig. 1M, blue arrow). Higher magnification images showed that Gdf5/Hoxa11eGFP co-expressing cells were particularly evident along the perimeter of the scaphoid (sc) and lunate (lu) in wrists (Fig. 1O) and the talus (ta) and calcaneous (ca) in ankles (Fig. 1P) as well as along the opposing articular surfaces of the distal zeugopod long bones in these joints and the knee (Fig. 1O–Q). High magnification images verified that the E15.5 elbow joints only displayed Gdf5-positive cells (Fig. 1R).
Because the patterns depicted by the Hoxa11eGFP reporter were so diverse and dynamic, we utilized RNAscope with a Hoxa11 probe to confirm them and did indeed find that Hoxa11 mRNA expression fully matched the live reporter (Fig. 1S–V). Importantly, the RNAscope approach confirmed the broader regional gene expression specificity of the Hoxa11eGFP reporter, with strong Hoxa11 mRNA expression in wrist, ankle and knee (Fig. S2A–B, arrows) but relatively undetectable expression in shoulders (Fig. S2C) and hips (Fig. S2D). Sacral axial skeletal elements were strongly positive (Fig. S2E) as expected based on previous studies (Wellik & Capecchi, 2003).
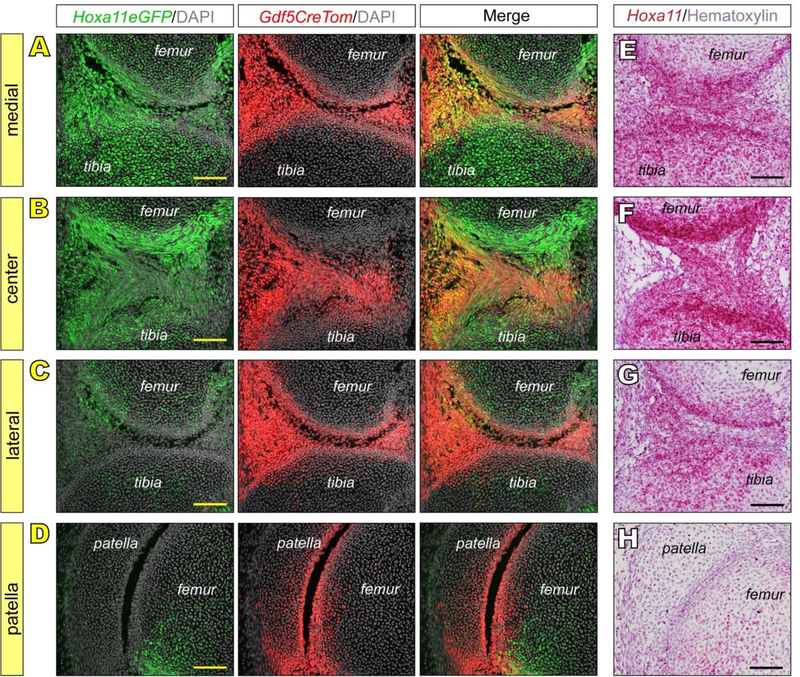
Intriguingly, when we examined serial sections throughout the depth of knees in the E15.5 Hoxa11eGFP;Gdf5CreTomato reporter mice, there were major regional differences in Hoxa11 expression versus distribution of Gdf5-lineage cells. As indicated above (Fig. 1N and Q), there was obvious overlap of Hoxa11eGFP-positive and Gdf5-lineage cells, but this overlap was only present in the medial and central portions of these joints, prominently characterizing also the incipient intrajoint anterior and posterior cruciate ligaments (ACL and PCL) (Fig. 2A–B). The lateral portion of the joints displayed abundant Gfd5-lineage cells, but there was minimal if any expression of Hoxa11eGFP (Fig. 2C). The same was seen in the patella (Fig. 2D). As above, Hoxa11 expression detected by RNAscope confirmed the reporter patterns (Fig. 2E–H). It should be noted that even the epiphyseal cartilaginous regions of long bone anlage contained many Hoxa11 -expressing cells in their medial and central, but not lateral, regions. These regional observations were unique to the knee as the wrist and ankle expression patterns described were consistent throughout all joint tissue sections.
Fig 2. Hox11 expression is restricted in the embryonic knee to medial and central components.
(A-D) Hoxa11eGFP is highly expressed and overlapping with Gdf5CreTom in nascent medial and central components of knees at E15.5, including epiphyseal chondrocytes and intra joint ligaments (A-B). In contrast, while Gdf5CreTom is highly expressed in lateral components and in patella, little Hoxa11eGFP is observed (C-D). (E-H) RNAscope for Hoxa11 confirms Hoxa11eGFP live reporter patterns in E15.5 knees. Scale bar = 100 μm.
Hoxa11 gene expression is maintained through postnatal development of distal zeugopod joints
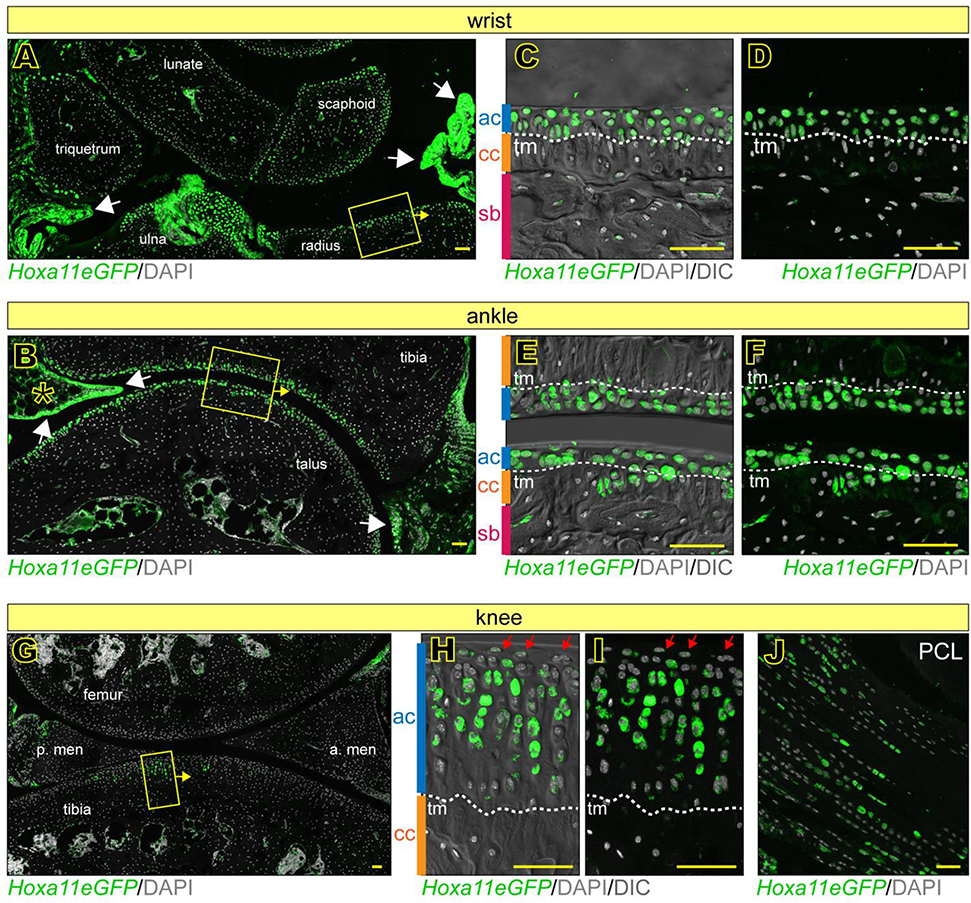
As we reported previously, joint tissues and structures are defined by birth, but continue their development and maturation over postnatal life (Decker et al., 2017; Koyama et al., 2008; Rux et al., 2019). Thus, it became important to monitor and define Hox11 expression in postnatal joints. We first examined distal zeugopod joints where Hoxa11 expression was highest during embryonic development. Hoxa11eGFP reporter analysis indicated that Hoxa11 was broadly expressed in neonatal wrists and ankles and characterized also neighboring synovial fibroblasts (Fig. 3A–B, arrows). Higher magnification images showed that nascent articular chondrocytes juxtaposed to the emerging synovial joint space strongly expressed Hoxa11eGFP (Fig. 3C–F, ac) in contrast to much lower expression levels in flanking centrally-located chondrocytes of the ossification center within the small skeletal elements, visualized with and without DIC (Fig. 3C–F, oc). In contrast, we found that Hoxa11 was highly expressed in the chondrocytes that contribute to secondary ossification in the epiphyseal cartilage beneath nascent articular cartilage of the long bones (radius/ulna and tibia) (Fig. 3A–B, asterisks and 3C–F, ec). These distinct patterns were fully verified by RNAscope for Hoxa11 (Fig. 3G–H). When we examined adult (2 months old) reporter mice, we found that quite interestingly, Hoxa11eGFP expression had not only persisted but was still strong and much more restricted within wrist and ankle joints (Fig. 3I–J). In particular, it prominently characterized mature articular chondrocytes that were present above the tidemark (tm) recognized by DIC imaging (Fig. 3M–L, ac). There were additional sites of prominent Hoxa11 expression such as the synovial capsule and its lining cells (Fig. 3I–J, white arrows) and fat pad tissues in ankles (Fig. 3J, asterisk). In line with recent studies, clear Hoxa11eGFP-expressing cells were also present in marrow/woven bone, likely representing skeletal progenitors (Fig. 3I–J, red arrowheads) (Pineault et al., 2019; Rux et al., 2016). Notably, Hoxa11 was not expressed in the elbow (Fig. S3A–F) or in more proximal limb joints such as the hip (Fig. S3G–I) at any time during postnatal development.
Fig 3. Hox11 expression is maintained through postnatal development in distal zeugopod joints.
(A-H) Hoxa11eGFP is broadly expressed at newborn stages in wrists and ankles, including synovial lining (white arrows). Higher magnification images of boxed regions in A and B shown with (C-D) or without (E-F) DIC imaging reveal details of cell/tissue morphology. They also show high Hoxa11eGFP in nascent articular cartilage (ac) and secondary ossification center/epiphyseal cartilage (ec) of radius/tibia, but low expression in ossification centers (oc) of smaller joint elements (C-F). RNAscope for Hoxa11 confirms these observations (G-H). (I-L) Hoxa11eGFP is maintained in adult (2 months old) wrist (I) and ankle (J) joints including synovial lining (I-J, white arrows) and fat pad of the ankle (J, asterisk). Higher magnification images (M-L) of boxed regions in I and J show Hoxa11eGFP with (M-N) and without (K-L) DIC imaging. Hoxa11eGFP is restricted to the non-calcified articular cartilage (ac) above the tidemark (tm), calcified cartilage (cc) and subchondral bone (sb). Hoxa11eGFP is also present in woven bone regions and likely represent bone marrow stromal cells (I and J, red arrow heads). Dashed lines denote boundaries of cartilage zones. ha; hamate; cap, capitate; tr, trapezoid; lu, lunate; sc, scaphoid; ta, talus; ca, calcaneous. scale bar = l00 μm.
Hoxa11 expression is regionally restricted and dynamic during postnatal development of knees
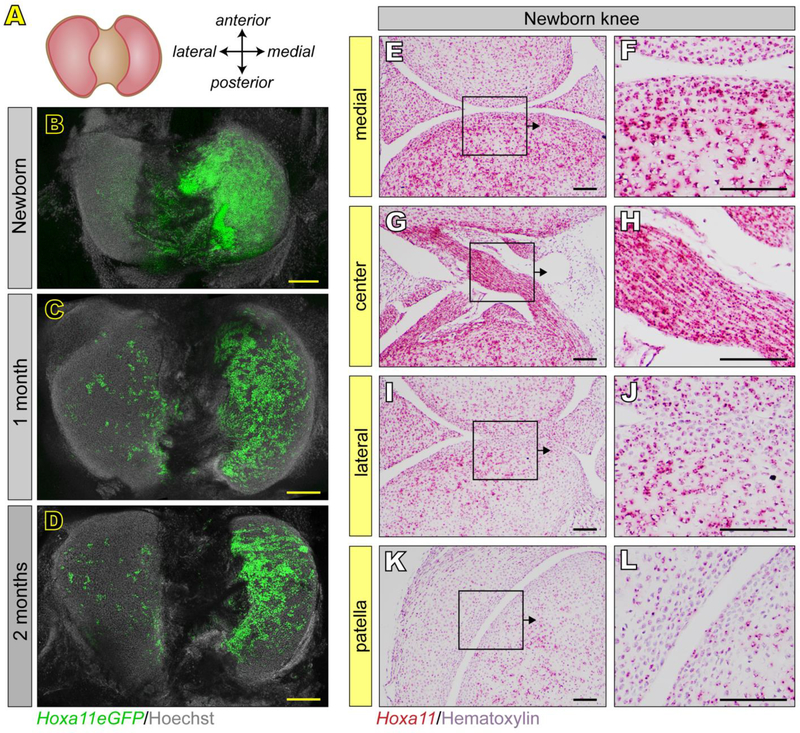
Knees are of particular biomedical relevance as they are prone to disease given their routine mechanical challenges involving primarily the tibial plateau, and also contain unique and complex components such as meniscus and patella (Archer et al., 1999; Mow & Sugalski, 2001). Indeed, our embryonic data above already showed that Hoxa11 expression was differential and regionally restricted to the medial and central regions of E14.5-E15.5 knees, including intra joint ligaments (see Fig. 2). In order to image postnatal knee joints and capture the morphological complexities of the plateau panoramically, we developed a whole mount imaging technique. Briefly, knees were bisected to expose the entire tibial plateau (Fig. 4A), paraformaldehyde- fixed, stained with Hoechst and imaged with a SP8 confocal microscope, capturing a tissue depth of about 3 cell layers. In line with our embryonic data, whole mount images from neonatal Hoxa11eGFP mice showed that Hoxa11 expression remained largely confined to the medial portion of the plateau (Fig. 4B). Strikingly, such regionalization of Hoxa11 expression persisted in a nearly identical pattern in 1 month- and 2 month-old plateaus (Fig. 4C–D). In comparison, the lateral portion of the plateau exhibited only a few scattered Hoxa11eGFP-expressing cells, and the anterior and posterior contours were essentially negative (Fig. 4C–D). RNAscope confirmed that Hoxa11 expression was indeed prominent in both medial regions and centrally-located intra joint ligaments (Fig. 4E–H), but minimal in lateral portions and patella (Fig. 4I–L).
Fig 4. Restriction of Hox11 expression to the medial knee is maintained postnatally.
(A-D) Using whole mount confocal imaging, the entire surface of the tibial plateau is visualized (A). Hoxa11eGFP is restricted to the medial tibial plateau at birth (B), 1 month (C) and 2 months (D) of age. (B) RNAscope for Hoxa11 in tissue sections through the knee at newborn stages confirms restriction to medial and intra joint elements (E-H), reduced expression in lateral elements (I-J) and relatively little expression in patella (K-L). Higher magnification images (F, H, J, L) are from boxed regions in E, G, I and K. scale bar for B-D = 200 μm. scale bar for E-L = 100 μm.
To extend the above analyses of articular cartilage, we closely assessed Hoxa11eGFP expression starting with incipient neonatal tissue -rich in cells but poor in matrix and structure- and continuing postnatally until 2 months of age when the tissue becomes fully mature and zonal (Decker et al., 2017). At birth, Hoxa11eGFP expression characterized about 30–40% of nascent articular chondrocytes within the 6–7 cell layers of prospective articular tissue (Fig. 5A–B, ac) defined by differential gene marker expression and Gdf5-lineage fate tracing (Decker et al., 2017). There was also very strong expression in underlying non-articular chondrocytes within the epiphyseal cartilage (Fig. 5A–B, ec), consistent with what we detected in ossification- destined chondrocytes beneath articular cartilage in the long bones of wrists and ankles at this stage (Fig. 3A–F, ec). By 2 weeks, Hoxa11eGFP expression was strong and more uniform throughout articular cartilage that had by now thickened but was still largely composed of scattered chondrocytes (Fig. 5C–D, ac). The hypertrophying chondrocytes within the underlying secondary ossification center/epiphyseal cartilage also continued to express Hoxa11 (Fig. 5D, ec). Of particular interest was the finding that Hoxa11eGFP expression was maintained in maturing and mature articular chondrocytes in 1 month- and 2 month-old tissue, in close temporal-spatial conjunction with their repositioning and stacking into functional columns within the intermediate and deep zones (Fig. 5E–H). High magnification images (Fig. 5F and H) show the equally intriguing observation that both the superficial zone (red arrows) and the calcified cartilage (cc) beneath the tidemark (tm) contained fewer and scattered Hoxa11eGFP-expressing cells at these stages compared to the bulk of articular cartilage between these zones (ac). This lack of expression in calcified cartilage in the knees is consistent with our observations above in the wrists and ankles (Fig. 3M–L). A similar trend in Hoxa11eGFP expression was seen for femoral articular cartilage through 1 month of age, but was dramatically reduced in 2 months old animals (Fig. 5A, C, E, G). To visualize the spatial relationships between chondrocyte columns and collagen matrix components at 2 months, we utilized multi-photon microscopy to image and produce 3D reconstructions of the live Hoxa11eGFP reporter in combination with second harmonic generation (SHG) to visualize collagen. Top and side views showed that Hoxa11eGFP expression characterized the entire chondrocyte columns flanked by similarly-oriented collagen fibers (Fig. 5I–J and Supp. movie 1).
Fig 5. Hox11 is dynamically expressed during postnatal development of knee joint tissues.
(A-J) Hoxa11eGFP in knee articular cartilage. In nascent articular cartilage (ac) at birth, Hoxa11eGFP is relatively low (A-B) and increases rapidly by 2 weeks (C-D) above the secondary ossification center/epiphyseal cartilage (ec) of the tibia where it is highly expressed (B and D). In 1 month and 2 months old animals (E-H), Hoxa11eGFP is maintained at high levels in articular cartilage (ac) above the tidemark (tm) and calcified cartilage (cc) (F and H). Only a few Hoxa11eGFP+ cells are present in the superficial zone (F and H, red arrows). Higher magnification images (B, D, F, H) are from boxed regions in A, C, E and G. Volume rendered 2-photon confocal images of the live reporter from the medial tibial plateau of 2 months old animals confirm Hoxa11eGFP in articular cartilage (I-J). (K-P) Hoxa11eGFP in meniscus and intra joint ligaments (PCL, posterior collateral ligament) is high at birth (K-M) and declines through 2 months of age (N-P). Dashed lines denote boundaries of cartilage zones. scale bar = 50 μm.
A clear demonstration of uniqueness and selectivity of the above patterns in cartilage was provided by examining meniscus and intrajoint knee ligaments at the above stages. Hoxa11eGFP was highly expressed in these tissues at birth (Fig. 5K–M) and then gradually declined, being maintained in only a few cells in adult tissues (Fig. 5N–P and Fig. S4). These trends were thus opposite to the gradual increase in expression in knee articular cartilage over the same time period.
Hoxa11 expression persists in zeugopod joints through adult life
Articular cartilage and its cells are long lived along with collagen II fibers that are functionally stable (Heinemeier et al., 2016). This raised the question as to whether once fully established into distinct zones by 2 months of age, articular cartilage would continue to express, and possibly rely on, Hox11 genes for long-term maintenance. Thus, we examined 6 months-old Hoxa11eGFP reporter mice that approximately correspond to a human age of 35 years. We found that Hoxa11 was still strongly expressed in the wrist and ankle and in patterns resembling those observed at 2 months of age (Fig. 6A–F). Expression was strong throughout articular cartilage in elements including scaphoid, lunate, radius and ulna in the wrist (Fig. 6A, C–D, ac) and the talus and tibia (Fig. 6B, E–F, ac) and consistently above the tidemark (Fig. 6C–F, tm). Expression was also obvious in synovial fibroblasts and the fat pad (Fig. 6A–B, white arrows and asterisk, respectively). In the 6 months-old knee however, Hoxa11eGFP expression patterns had undergone significant changes in comparison to those in 2 months-old animals. Expression was no longer detectable over much of articular cartilage in tibia and femur as well as meniscus (Fig. 6G), but had been maintained in medial tibial plateau that receives the most compressive loads during daily activities (Fig. 6G) (Park et al., 2019). Within this restricted region (Fig. 6H–I), Hoxa11eGFP expression still characterized only the intermediate and deep articular cartilage (ac) zones above the tidemark (tm), being essentially excluded from the top superficial (red arrows) and bottom calcified cartilage (cc). Persistent Hoxa11eGFP expression characterized intrajoint ligaments in patterns and levels resembling those at 2 months of age (Fig. 6J).
Fig 6. Hoxa11eGFP is maintained in 6 month old synovial joints.
(A-F) Hoxa11eGFP is maintained in the wrist and ankle synovial joints at high levels including articular cartilage and synovial lining (A-B, white arrows), and the fat pad of the ankle (B, asterisk). Higher magnification images of boxed regions in A and B with (C and E) and without (D and F) DIC imaging reveal details of cell/tissue morphology and restriction of Hoxa11eGFP to articular cartilage (ac) above the tidemark (tm) and subchondral bone (sb) in wrist (C-D) and in ankle (E-F). (G-J) Hoxa11eGFP is dramatically reduced in 6-month old knee joints. Some Hoxa11eGFP-expressing cells are present in articular cartilage (boxed region), but are absent from meniscus and femoral cartilage. Higher magnification images of boxed region in G show that Hoxa11eGFP expression is still strong in articular cartilage (ac) but is largely restricted to deep zone columnar chondrocytes (H-I) above the tidemark (tm) and calcified cartilage (cc). Superficial zone cells (H-I, red arrows) mostly do not express Hoxa11eGFP. Hoxa11eGFP-expressing cells are also maintained in intra joint ligaments (J, PCL). Dashed lines denote boundaries of cartilage zones. anterior meniscus, a. men.; posterior meniscus, p. men; posterior collateral ligament, PCL. scale bar = 50 μm.
Hox11 paralogous group genes display non-overlapping expression profiles in developing and postnatal zeugopod joints
Finally, we sought to define whether the expression dynamics of Hoxa11 overlap with those of Hoxc11 and Hoxd11. To this end, serial sections of forelimb and hindlimb joints at E15.5, newborn and 2 months were simultaneously processed for RNAscope RED2.5 in situ hybridization as above. To provide maximal contrast and details, we imaged the data using confocal microscopy, taking advantage of the fluorescent nature of fast red detection. In E15.5 forelimbs, both Hox11 and Hoxd11 were highly expressed, but with notable and striking differences in pattern. Hoxa11 displayed the broadest and dynamic expression, with the highest expression restricted to distal end of both zeugopod bones and most proximal wrist bones (scaphoid and lunate) (Fig. 7A). Hoxd11 displayed a pattern of expression that was more specific and restricted to the posterior regions of the limb and extended more prominently into the proximal zeugopod to include the olecranon (Fig. 7B). Hoxc11 was absent from the forelimb consistent with previous findings (Fig. 7C). These patterns of expression extended to the postnatal joints. Newborn wrists displayed broad levels of Hoxa11 expression while Hoxd11 was lower and restricted to the posterior side (Fig. 7D–E). Expression of both genes was low in newborn elbow, albeit with slightly higher Hoxd11 expression in olecranon (Fig. 7F–G). Adult expression of these Hox11 genes as revealed by RNAscope was very low in general due, at least in part, to the necessity of decalcification, but could be visualized at high magnification. We found that in adult wrist cartilage and synovial lining cells, Hoxa11 expression was maintained (Fig. 7H and J) consistent with Hoxa11eGFP reporter data, whereas Hoxd11 expression was absent from articular cartilage (Fig. 7I) and displayed low expression in synovial lining (Fig. 7K).
Fig 7. RNAscope reveals Hoxa11 as the dominant Hox11 parlog in developing forelimb zeugopod joints.
RNAscope was performed using the RED2.5 detection system, imaged with confocal microscopy and pseudo-colored pink to provide maximal contrast. (A-C) Low magnification images of full forelimb zeugopod at E15.5 and expression of Hox11 paralogous group genes. Hoxa11 (A) displays the broadest expression profile and is highly expressed in the distal zeugopod surrounding and including the developing distal growth plates. Hoxd11 (B) displays a more posteriorly restricted profile surrounding but excluded from the developing distal growth plates and extending further into the proximal olecranon. Hoxc11 (C) is not expressed in the forelimb. (D-G) Expression of Hoxa11 and Hoxd11 in newborn zeugopod joints. Hoxa11 (D) is highly and broadly expressed in all elements of the newborn wrist while Hoxd11 (E) is restricted to the posterior wrist elements. Hoxa11 and Hoxd11 are both minimally expressed in the elbow at newborn stages (F-G). (H-K) In the adult wrist joint, Hoxa11 is maintained in both the cartilage (H) and synovial lining (J). Hoxd11 is not expressed in articular cartilage (I) and displays low expression in the synovial lining (K). Dashed lines denote approximate boundaries of the tidemark. r, radius; u, ulna; h, humerus; ha; hamate; cap, capitate; tr, trapezoid; lu, lunate; sc, scaphoid; ole, olecrenon. scale bars: A-G = 500 μm; H-K = 50 μm.
There were notable differences in expression of the three paralogs in hindlimbs. At E15.5, Hoxa11 once again displayed the broadest expression profile, with notable high expression in distal tibia and most proximal ankle bones (talus and calcaneous). At variance with forelimbs, Hoxa11 was also highly expressed in the proximal zeugopod joint (the knee) (Fig. 8A). Hoxc11 and Hoxd11 both displayed much more specific and restricted expression profiles, both being largely restricted to the posterior region of zeugopod and both displaying much lower expression in zeugopod joints (Fig. 8B–C). These expression patterns carried over into newborn joints where Hoxa11 was predominantly expressed. In the ankle, Hoxa11 was highly and broadly expressed (Fig. 8D) as described, while Hoxc11 and Hoxd11 were both quite low and non-overlapping (Fig. 8E–F). In newborn knees, only Hoxa11 was expressed in the pattern previously described (Fig. 8G–J). Again, adult Hox11 expression was low in general, but could be visualized at high magnification. Hoxa11 was predominantly expressed in adult ankle articular cartilage (Fig. 8K), ankle synovial cells (Fig. 8L) and knee articular cartilage (Fig. 1M). Interestingly, the adult PCL seemed to display relatively equal, albeit scattered expression of all three paralogs (Fig. 8N). Other regions showing high overlapping expression of Hoxa11, Hoxc11 and Hoxd11 included the zeugopod bone marrow (Fig. 8O) and Achilles tendon (Fig. 8D–F, white arrows). Importantly, we found that Hox11 and Hoxd11 expression patterns in regions outside of the zeugopod matched those already described for Hoxa11 (Fig. S2). Little to no expression of the three paralogs was detectable noted in shoulder (Fig. S5A–C) or hip (Fig. S5D–F) joints while high expression of all three genes was displayed by the sacrum (Fig. S5G–H).
Fig 8. RNAscope reveals Hoxa11 as the dominant Hox11 parlog in developing hindlimb zeugopod joints.
RNAscope was performed using the RED2.5 detection system, imaged with confocal microscopy and pseudo-colored pink to provide maximal contrast. (A-C) Low magnification images of full hindlimb zeugopod at E15.5 and expression of Hox11 paralogous group genes. Hoxa11 (A) displays the broadest expression profile and is highly expressed in the distal zeugopod surrounding and including the developing distal growth plates as well as in the developing knee. Hoxc11 (B) and Hoxd11 (C) display more specific and posteriorly-restricted profiles. A notable region of paralog overlap was the developing achilles tendon (A-C, arrows). (D-J) Hox11 expression at newborn stages mirrors patterns established at E15.5. In the ankle, Hoxa11 (D) is the highest and most broadly expressed. Hoxc11 (E) and Hoxd11 (F) are posteriorly-restricted. Overlap in the achilles tendon is maintained (D-F, arrows). Hoxa11 is the only Hox11 paralog expressed in the neonatal knee displaying high expression in the medial (G) and central (H) regions and largely absent from the lateral regions (I) and the patella (J). (K-O) Hox11 paralogous group gene expression in the adult zeugopod. In articular cartilage (K) and synovial fibroblasts (L) of the ankle Hoxa11 is the predominantly expressed Hox11 paralog. In the knee, Hoxa11 is predominantly expressed in cartilage (M) and all 3 paralogs are expressed in the adult PCL (N). Hoxa11, Hoxc11 and Hoxd11 are all highly expressed in adult tibia bone marrow (O), potentially overlapping in adult skeletal progenitor cells. Dashed lines denote approximate boundaries of the tidemark. ti, tibia; fe, femur; ta, talus; ca, calcareous; pat, patella. scale bars: A-F = 500 μm; G-J and O = 100 μm; K-N = 50 μm.
Discussion
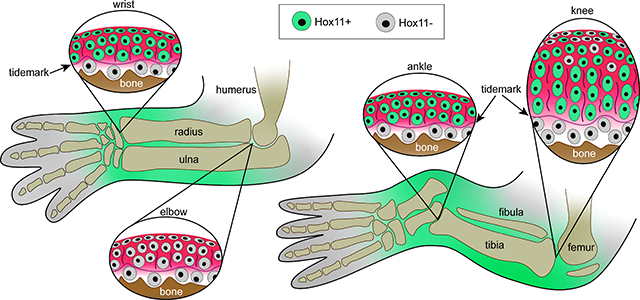
Our data reveal that the topographical map of Hox11 expression in prenatal and postnatal zeugopods is quite distinct from the anatomical map of resident skeletal elements. We find that Hox11 expression is remarkably concentrated to incipient wrist and ankle joints and immediately surrounding tissues in E13.5 mouse embryo limbs, with a tapering of expression proximally. By E14.5, Hox11 expression becomes apparent in delimited regions of developing knees but remains dormant in elbow joints. Notably, these differential expression patterns are broadly maintained through the reminder of fetal development and well into adulthood, though they become more restricted and specific within certain joint tissues including articular cartilage and ligaments over postnatal time. Data and insights here complement well recent studies supporting the overall key notion that rather than being only embryonic patterning regulators as long assumed, Hox genes are expressed postnatally and are likely to be continuously needed for function of specific cell populations, maintaining a topographic code that mirrors embryonic patterning in mice and humans (Ackema & Charité, 2008; Kulebyakina & Makarevich, 2020; Rux et al., 2016; Rux & Wellik, 2017). Such studies have revealed that Hox genes are expressed quite broadly in adult mouse cell types including skin fibroblasts (Chang et al., 2002; Rinn et al., 2006, 2008), mesenchymal dermal papilla (Yu et al., 2018), human synovial fibroblasts (Frank-Bertoncelj et al., 2017) and bone marrow and periosteal mesenchymal progenitors (Leucht et al., 2008; Pineault et al., 2019; Rux et al., 2016, 2017; Song et al., 2020). The latter studies also revealed a functional requirement for Hox11 genes for local bone formation as well as fracture repair of zeugopod elements, without affecting repair in stylopod elements (Rux et al., 2016, 2017). The data in the present study now demonstrate that Hox11 genes intimately and dynamically characterize the diverse tissues of developing and maturing synovial joints, clearly pointing to the possibility that the genes are needed to generate and dictate structure, composition and function of joint tissues prenatally and postnatally.
These enticing conclusions are sustained in particular by our data on persistent Hox11 expression in articular cartilage over time. As pointed out above, this tissue undergoes a remarkable process of initiation, growth and morphogenesis from embryonic life into adulthood, being a nondescript cell-rich and matrix-poor tissue at neonatal stages to becoming a thick, zonal, anisotropic, highly structured and mechanically capable tissue in adults (Decker, 2017; Decker et al., 2017). Our data show that Hox11 expression is closely and dynamically coupled to these sequential steps in mouse tissue development, reorganization and morphogenesis. Nascent articular cartilage in wrists and ankles and parts of knees strongly expresses Hox11 genes, and expression is maintained into adulthood up to at least 6 months of age. In wrist and ankle articular cartilage, expression encompasses the entire thickness of the non-calcified tissue at each stage examined, strongly indicating that Hox11 genes are continuously needed to set phenotypic character and stability pre- and postnatally. In tibial plateau articular cartilage however, expression is initially uniform in medial/central locations at neonatal stages but then becomes restricted to chondrocytes in the intermediate and deep zones in adults, with minimal expression in superficial and calcified zones. The sequential and differential spatio-temporal expression patterns of Hox11 genes may have morphogenetic value and allow the different zones to acquire their distinct cell phenotypic characters, and possibly promoting chondrocyte translocation and repositioning into columnar stacks within the intermediate and deep zones (Decker et al., 2017) able to withstand locally-concentrated mechanical demands (Paranjape et al., 2019). We should underline here that this is the first demonstration we know of that Hoxa11 remains actively expressed in fully differentiated and mature cells as adult articular chondrocytes where embryonic positioning is retained. While it has been previously reported that Hox genes are expressed in human nasal and articular chondrocytes (Pelttari et al., 2014), the expression profiles in this study did not reflect positional patterning characteristic of the Hox genes. The strong enduring Hox11 expression we observe in mature intra joint ligaments and other specific joint structures falls into the same paradigm. It should be noted that the lateral portion of adult tibial plateau also displays a zonal organization with chondrocyte columns and yet, there appears to be minimal to no Hox11 expression in that region, even at earlier stages. These intriguing data suggest that other Hox gene groups –and most likely Hox9 and Hox10 genes- would be active in the lateral knee region and provide analogous supervisory action to local articular cartilage for cell columnar growth and zonal morphogenesis. If so, this would suggest that the specific boundaries of expression and action by Hox genes are far subtler and regionalized than those indicated by the overall subdivision of limbs into stylopod, zeugopod and autopod. This is in line with single-allele loss-of-function mutations for Hox13 and Hox11 genes indicating that Hox13 genes function to pattern the distal small skeletal elements of wrists and ankles, while Hox11 genes pattern proximal ones (Reno et al., 2016). Studies focused on chromosomal cluster loss-of- function mutations (such as Hoxd11–13 genes) have also provided evidence that the joints are specified by the combined input of multiple paralogous groups (Albrecht et al., 2002; Villavicencio-Lorini et al., 2010).
While we cannot as yet ascribe specific joint tissue functions to the Hox11 paralogous group based on expression, it is important to underline one of the more surprising findings we report here, that is the relative lack of overlapping expression of Hoxa11, Hoxc11 and Hoxd11 beginning at E15.5. In one regard, these data underscore the synovial joint defects associated with single loss-of-function mutations already mentioned (Reno et al., 2016) but they also raise new questions about how Hox paralogous group genes function together for broader patterning functions in specific limb regions. For example, we provide evidence here that while Hoxd11 is highly expressed in E15.5 olecranon, the expression of Hoxa11 is minimal. However, loss of function of both genes is required to produce the ectopic patella-like structure in the global loss- of-function mouse models (Koyama et al., 2010a). One explanation for this could be that Hoxa11 and Hoxd11 may display a higher degree of overlap earlier in limb development that is responsible for the coordinated patterning of the olecrenon before E15.5. When one paralogous member is ablated, the other member could be up-regulated, providing compensatory action and preventing the emergence of a skeletal phenotype in global single mutant embryos. Along these lines, it would also be interesting to clarify whether compensatory Hox11 paralogous expression is responsive not only to mutations but also tissue injury.
Lastly, it is important to point out additional aspects of the potential pathogenic relevance of Hox genes in joints as our study highlights novel expression of Hox11 genes in adult articular chondrocytes and possible function in their maintenance throughout life. Indeed, previous studies reported the expression of Hox genes in osteoarthritic tissues. One study suggested deregulation of Hox gene patterning in osteoarthritis pathogenesis (den Hollander et al., 2014). It was found that methylation marks in human knee versus hip cartilage showed lack of typical Hox gene collinearity, suggesting that regional chondrocyte identity may have been affected and possibly contributed to cellular destabilization and disease progression (den Hollander et al., 2014). In addition, increasing evidence shows that subchondral bone maintenance is critical to the function of articular cartilage (Castañeda et al., 2012; Goldring & Goldring, 2010; G. Li et al., 2013; Mazur et al., 2019). Another study of differential hyper/hypomethylation of Hox genes and OCT4 pathways in subchondral bone showed an association with osteoarthritis, leading authors to conclude that pathways of tissue repair were being upregulated in disease (Zhang et al., 2016). While both of these studies highlight the intriguing possibility that Hox genes function in joint maintenance and are dysregulated in human disease, it remains to be clarified what these role(s) are. Further genetic murine studies could thus test whether deregulation of specific Hox genes contributes to osteoarthritis pathogenesis and whether the genes could also be used to create new therapeutic strategies for restoration of articular cartilage structural organization and function.
Supplementary Material
Fig S1. High magnification images of Hoxa11eGFP in E13.5 and E14.5 zeugopod joints. (AF’) Hoxa11eGFP in developing limbs at E13.5. Low magnification images of Hoxa11eGFP overlaid with DIC imaging at E13.5 from figure 1 in the forelimb (A) and hindlimb (B) provide broad Hoxa11eGFP expression patterns. Higher magnification images of boxed regions provide cellular detail using DIC imaging alone (C-D) and overlaid with Hoxa11eGFP (C’-D’). At E13.5, Hoxa11eGFP is highly expressed in the distal joints (C-D’) and largely excluded from the proximal joints (E-F’). (G-L’) Hoxa11eGFP in developing limbs at E14.5. Low magnification images of Hoxa11eGFP overlaid with DIC imaging at E14.5 from figure 1 in the forelimb (G) and hindlimb (H) provide broad Hoxa11eGFP expression patterns. Higher magnification images of boxed regions provide cellular detail using DIC imaging alone (I-L) and overlaid with Hoxa11eGFP (I’-L’). At E14.5, Hoxa11eGFP is highly expressed in the distal joints (I-J’) and in the knee (L-L’) but still largely excluded from the elbow (K-K’). Dashed lines denote approximate boundaries of embryonic skeletal elements. iz, interzone/developing joint; r, radius; u, ulna; h, humerus; ti, tibia; ta, talus; fe, femur. scale bar = 100 μm.
Fig S2. RNAscope for Hoxa11 at E15.5. (A-B) Low magnification images of full limb tissue sections at E15.5 show RNAscope for Hoxa11 in forelimbs (A) and hindlimbs (B). Hoxa11 expression is highest in wrist, ankle and knee (A-B, black arrows). (C-E) Higher magnification of boxed regions in A and B show absence of Hoxa11 from the shoulder joint (C) and hip joint (D) and high expression in the sacral region of the axial skeleton (E). A-B; scale bar = 1mm. C-D; scale bar = 100 μm.
Fig S3. Hox11 exclusion from elbow and hip joints during postnatal development. (A-F) Hoxa11eGFP is largely excluded from elbows in postnatal life. Few Hoxa11eGFP-expressing cells are present at birth (A, C, E) and are absent by 2 months of age (B, D, F). Higher magnification images (C-F) are from boxed regions in A and B and shown with (C-D) and without (E-F) DIC imaging to reveal cell/tissue morphology. (G-I) Hoxa11eGFP is not detectable in hip joint. Higher magnification images (H-I) are from the boxed region in G and shown with (H) and without (I) DIC imaging to reveal cell/tissue morphology. Dashed lines denote boundaries of cartilage zones. scale bar = 100 μm.
Fig S4. Hoxa11eGFP in meniscus and PCL during postnatal development. Hoxa11eGFP-expressing cells gradually decrease over postnatal time but are still appreciable at 2 weeks (A-C) and 1 month (D-F) in meniscus (A-B, D-E) and intra joint ligaments (C and F). Dashed lines denote boundaries of the meniscal horns. PCL; posterior collateral ligament. scale bar = 50 μm.
Fig S5. RNAscope for Hox11 paralogous genes expression at E15.5. (A-F) Hoxa11, Hoxc11 and Hoxd11 are all largely excluded from the proximal joints of the stylopod forelimb (A-C, shoulder) and hindlimb (D-F, hip/femoral head) elements consistent with regionalized function characterized previously. (G-I) In contrast, sacral elements of the axial skeleton express high levels of all three Hox11 paralogous genes where Hox11 function has been previously characterized. Dashed lines denote approximate boundaries of embryonic skeletal elements. sc, scapula; h, humerus; fh, femoral head. scale bar = 100μm.
Video 1. Three dimensional fluorescence imaging of medial tibia plateau articular cartilage in the knee from 2 month-old Hoxa11eGFP mice. The three panels show second harmonic generation alone (left), Hoxa11eGFP (center) and merged (right). The movie begins with a view of the top of the articular cartilage (the surface zone) and completes a full rotation to also visualize from the bottom (as if looking from beneath the tidemark). Note the presence of Hoxa11eGFP+ positive cells (green) throughout the entire thickness of non-calcified articular cartilage in the center panel. Superimposed on the fluorescent cells is the collagen matrix captured with Second Harmonic Generation imaging (white).
Highlights.
Hox11 is expressed in articular chondrocytes throughout development and adulthood.
Hox11 is restricted to joints in the zeugopod region (except the elbow).
Hox11 is excluded from chondrocyes beneath the tidemark in adult articular cartilage.
Hox11 paralogs exhibit divergent expression patterns through joint development
Hoxa11 is the dominant Hox11 paralog in zeugopod synovial joints.
Acknowledgements
We thank Dr. Hyun-Duck Nah for kindly providing use of the fluorescent stereoscope (Leica) for whole mount imaging of live Hoxa11eGFP reporter embryos. We also thank the Penn Vet Imaging Core and Dr. Gordon Ruthel for his assistance with whole mount confocal imaging using the Leica confocal SP8 with 2-photon capability.
Funding
This study was supported by NIH-NIAMS Grant F32AR074227 awarded to D.R. and NIH-NIAMS Grant R01AR062908 awarded to M.P..
Footnotes
Competing Interests
The authors declare no competing or financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackema KB, & Charité J (2008). Mesenchymal stem cells from different organs are characterized by distinct topographic Hox codes. Stem Cells and Development, 17(5), 979–991. 10.1089/scd.2007.0220 [DOI] [PubMed] [Google Scholar]
- Albrecht AN, Schwabe GC, Stricker S, Böddrich A, Wanker EE, & Mundlos S (2002). The synpolydactyly homolog (spdh) mutation in the mouse—A defect in patterning and growth of limb cartilage elements. Mechanisms of Development, 112(1–2), 53–67. 10.1016/s0925-4773(01)00639-6 [DOI] [PubMed] [Google Scholar]
- Archer CW, Caterson B, Benjamin M, & Ralphs JR (1999). The biology of the synovial joint. Harwood Academics. [Google Scholar]
- Castañeda S, Roman-Blas JA, Largo R, & Herrero-Beaumont G (2012). Subchondral bone as a key target for osteoarthritis treatment. Biochemical Pharmacology, 83(3), 315–323. 10.1016/j.bcp.2011.09.018 [DOI] [PubMed] [Google Scholar]
- Chang HY, Chi J-T, Dudoit S, Bondre C, Rijn M. van de, Botstein D, & Brown PO (2002). Diversity, topographic differentiation, and positional memory in human fibroblasts. Proceedings of the National Academy of Sciences, 99(20), 12877–12882. 10.1073/pnas.162488599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisaka O, & Capecchi MR (1991). Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature, 350(6318), 473–479. 10.1038/350473a0 [DOI] [PubMed] [Google Scholar]
- Davis AP, Witte DP, Hsieh-Li HM, Potter SS, & Capecchi MR (1995). Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature, 375(6534), 791–795. 10.1038/375791a0 [DOI] [PubMed] [Google Scholar]
- de Charleroy C, Haseeb A, & Lefebvre V (2021). Preparation of Adult Mouse Skeletal Tissue Sections for RNA In Situ Hybridization. Methods in Molecular Biology (Clifton, N.J.), 2245, 85–92. 10.1007/978-1-0716-1119-7_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker RS (2017). Articular cartilage and joint development from embryogenesis to adulthood. Seminars in Cell & Developmental Biology, 62, 50–56. 10.1016/j.semcdb.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker RS, Um H-B, Dyment NA, Cottingham N, Usami Y, Enomoto-Iwamoto M, Kronenberg MS, Maye P, Rowe DW, Koyama E, & Pacifici M (2017). Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Developmental Biology, 426(1), 56–68. 10.1016/j.ydbio.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander W, Ramos YFM, Bos SD, Bomer N, van der Breggen R, Lakenberg N, de Dijcker WJ, Duijnisveld BJ, Slagboom PE, Nelissen RGHH, & Meulenbelt I (2014). Knee and hip articular cartilage have distinct epigenomic landscapes: Implications for future cartilage regeneration approaches. Annals of the Rheumatic Diseases, 75(12), 2208–2212. 10.1136/annrheumdis-2014-205980 [DOI] [PubMed] [Google Scholar]
- Duboule D (2007). The rise and fall of Hox gene clusters. Development (Cambridge, England), 134(14), 2549–2560. 10.1242/dev.001065 [DOI] [PubMed] [Google Scholar]
- Frank-Bertoncelj M, Trenkmann M, Klein K, Karouzakis E, Rehrauer H, Bratus A, Kolling C, Armaka M, Filer A, Michel BA, Gay RE, Buckley CD, Kollias G, Gay S, & Ospelt C (2017). Epigenetically-driven anatomical diversity of synovial fibroblasts guides joint-specific fibroblast functions. Nature Communications, 8. 10.1038/ncomms14852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromental-Ramain C, Warot X, Lakkaraju S, Favier B, Haack H, Birling C, Dierich A, Doll e P, & Chambon P (1996a). Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development (Cambridge, England), 122(2), 461–472. [DOI] [PubMed] [Google Scholar]
- Fromental-Ramain C, Warot X, Messadecq N, LeMeur M, Dollé P, & Chambon P (1996b). Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development (Cambridge, England), 122(10), 2997–3011. [DOI] [PubMed] [Google Scholar]
- Gannon AR, Nagel T, Bell AP, Avery NC, & Kelly DJ (2015). Postnatal changes to the mechanical properties of articular cartilage are driven by the evolution of its collagen network. European Cells & Materials, 29, 105–121; discussion 121–123. 10.22203/ecm.v029a09 [DOI] [PubMed] [Google Scholar]
- Goldring MB, & Goldring SR (2010). Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Annals of the New York Academy of Sciences, 1192, 230–237. 10.1111/j.1749-6632.2009.05240.x [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Schjerling P, Heinemeier J, Møller MB, Krogsgaard MR, Grum- Schwensen T, Petersen MM, & Kjaer M (2016). Radiocarbon dating reveals minimal collagen turnover in both healthy and osteoarthritic human cartilage. Science Translational Medicine, 8(346), 346ra90. 10.1126/scitranslmed.aad8335 [DOI] [PubMed] [Google Scholar]
- Holder N (1977). An experimental investigation into the early development of the chick elbow joint. Journal of Embryology and Experimental Morphology, 39, 115–127. [PubMed] [Google Scholar]
- Hunziker EB, Kapfinger E, & Geiss J (2007). The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis and Cartilage, 15(4), 403–413. 10.1016/jjoca.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Julkunen P, Harjula T, Iivarinen J, Marjanen J, Seppänen K, Närhi T, Arokoski J, Lammi MJ, Brama PA, Jurvelin JS, & Helminen HJ (2009). Biomechanical, biochemical and structural correlations in immature and mature rabbit articular cartilage. Osteoarthritis and Cartilage, 17(12), 1628–1638. 10.1016/j.joca.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Kawamoto T, & Kawamoto K (2021). Preparation of Thin Frozen Sections from Nonfixed and Undecalcified Hard Tissues Using Kawamoto’s Film Method. Methods in Molecular Biology (Clifton, N.J.), 2230, 259–281. 10.1007/978-1-0716-1028-2_15 [DOI] [PubMed] [Google Scholar]
- Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, Yuasa T, Okabe T, Ochiai T, Kamiya N, Rountree RB, Kingsley DM, Iwamoto M, Enomoto- Iwamoto M, & Pacifici M (2008). A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Developmental Biology, 316(1), 62–73. 10.1016/j.ydbio.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Yasuda T, Minugh-Purvis N, Kinumatsu T, Yallowitz AR, Wellik DM, & Pacifici M (2010a). Hox11 genes establish synovial joint organization and phylogenetic characteristics in developing mouse zeugopod skeletal elements. Development (Cambridge, England), 137(22), 3795–3800. 10.1242/dev.053447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Yasuda T, Wellik DM, & Pacifici M (2010b). Hox11 paralogous genes are required for formation of wrist and ankle joints and articular surface organization. Annals of the New York Academy of Sciences, 1192(1), 307–316. 10.1111/j.1749-6632.2009.05234.x [DOI] [PubMed] [Google Scholar]
- Kulebyakina M, & Makarevich P (2020). Hox-Positive Adult Mesenchymal Stromal Cells: Beyond Positional Identity. Frontiers in Cell and Developmental Biology, 8. 10.3389/fcell.2020.00624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht P, Kim J-B, Amasha R, James AW, Girod S, & Helms JA (2008). Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development (Cambridge, England), 135(17), 2845–2854. 10.1242/dev.023788 [DOI] [PubMed] [Google Scholar]
- Lewis EB (1978). A gene complex controlling segmentation in Drosophila. Nature, 276(5688), 565–570. 10.1038/276565a0 [DOI] [PubMed] [Google Scholar]
- Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, & Zheng MH (2013). Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Research & Therapy, 15(6), 223. 10.1186/ar4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Newton PT, Bouderlique T, Sejnohova M, Zikmund T, Kozhemyakina E, Xie M, Krivanek J, Kaiser J, Qian H, Dyachuk V, Lassar AB, Warman ML, Barenius B, Adameyko I, & Chagin AS (2017). Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 31(3), 1067–1084. 10.1096/fj.201600918R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi L, Li T, Tagliafierro L, Temple JD, Willcockson HH, Ye P, Esposito A, Xu F, & Spagnoli A (2015). Synovial joints: From development to homeostasis. Current Osteoporosis Reports, 13(1), 41–51. 10.1007/s11914-014-0247-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo M, Wellik DM, & Deschamps J (2010). Hox genes and regional patterning of the vertebrate body plan. Developmental Biology, 344(1), 7–15. 10.1016/j.ydbio.2010.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur CM, Woo JJ, Yee CS, Fields AJ, Acevedo C, Bailey KN, Kaya S, Fowler TW, Lotz JC, Dang A, Kuo AC, Vail TP, & Alliston T (2019). Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Research, 7(1), 1–17. 10.1038/s41413-019-0070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic D (1978). Development of the diarthrodial joints in the rat embryo. The American Journal of Anatomy, 151(4), 475–485. 10.1002/aja.1001510403 [DOI] [PubMed] [Google Scholar]
- Mizuhashi K, Ono W, Matsushita Y, Sakagami N, Takahashi A, Saunders TL, Nagasawa T, Kronenberg HM, & Ono N (2018). Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature, 563(7730), 254–258. 10.1038/s41586-018-0662-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow V, & Sugalski M (2001). Physiology of synovial joints and articular cartilage: Vol. E.G. Gonzales (Ed.) (3rd ed.). Butterwoth Heinemann Pubs. [Google Scholar]
- Nelson LT, Rakshit S, Sun H, & Wellik DM (2008). The generation and expression of a Hoxa11eGFP targeted allele in mice. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 237(11), 3410–3416. 10.1002/dvdy.21756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PT, Li L, Zhou B, Schweingruber C, Hovorakova M, Xie M, Sun X, Sandhow L, Artemov AV, Ivashkin E, Suter S, Dyachuk V, El Shahawy M, Gritli-Linde A, Bouderlique T, Petersen J, Mollbrink A, Lundeberg J, Enikolopov G, …Chagin AS (2019). A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature, 567(7747), 234–238. 10.1038/s41586-019-0989-6 [DOI] [PubMed] [Google Scholar]
- Pacifici M, Decker RS, & Koyama E (2018). Chapter 4 - Limb Synovial Joint Development From the Hips Down: Implications for Articular Cartilage Repair and Regeneration. In Stoddart MJ, Craft AM, Pattappa G, & Gardner OFW (Eds.), Developmental Biology and Musculoskeletal Tissue Engineering (pp. 67–101). Academic Press. 10.1016/B978-0-12-811467-4.00004-8 [DOI] [Google Scholar]
- Paranjape CS, Cutcliffe HC, Grambow SC, Utturkar GM, Collins AT, Garrett WE, Spritzer CE, & DeFrate LE (2019). A New Stress Test for Knee Joint Cartilage. Scientific Reports, 9(1), 2283. 10.1038/s41598-018-38104-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee S, Yoon J, & Chae S-W (2019). Finite element analysis of knee and ankle joint during gait based on motion analysis. Medical Engineering & Physics, 63, 33–41. 10.1016/j.medengphy.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Pelttari K, Pippenger B, Mumme M, Feliciano S, Scotti C, Mainil-Varlet P, Procino A, von Rechenberg B, Schwamborn T, Jakob M, Cillo C, Barbero A, & Martin I (2014). Adult human neural crest-derived cells for articular cartilage repair. Science Translational Medicine, 6(251), 251ra119. 10.1126/scitranslmed.3009688 [DOI] [PubMed] [Google Scholar]
- Pineault KM, Song JY, Kozloff KM, Lucas D, & Wellik DM (2019). Hox11 expressing regional skeletal stem cells are progenitors for osteoblasts, chondrocytes and adipocytes throughout life. Nature Communications, 10. 10.1038/s41467-019-11100-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineault KM, & Wellik DM (2014). Hox genes and limb musculoskeletal development. Current Osteoporosis Reports, 12(4), 420–427. 10.1007/s11914-014-0241-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines AM, Magella B, Adam M, & Potter SS (2015). Key pathways regulated by HoxA9,10,11/HoxD9,10,11 during limb development. BMC Developmental Biology, 15(1), 28. 10.1186/s12861-015-0078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno PL, Kjosness KM, & Hines JE (2016). The Role of Hox in Pisiform and Calcaneus Growth Plate Formation and the Nature of the Zeugopod/Autopod Boundary. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 326(5), 303–321. 10.1002/jez.b.22688 [DOI] [PubMed] [Google Scholar]
- Rinn JL, Bondre C, Gladstone HB, Brown PO, & Chang HY (2006). Anatomic Demarcation by Positional Variation in Fibroblast Gene Expression Programs. PLoS Genetics, 2(7). 10.1371/journal.pgen.0020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, Wang JK, Allen N, Brugmann SA, Mikels AJ, Liu H, Ridky TW, Stadler HS, Nusse R, Helms JA, & Chang HY (2008). A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes & Development, 22(3), 303–307. 10.1101/gad.1610508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, & Kingsley DM (2004). BMP Receptor Signaling Is Required for Postnatal Maintenance of Articular Cartilage. PLoS Biology, 2(11). 10.1371/journal.pbio.0020355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rux DR, Decker RS, Koyama E, & Pacifici M (2019). Joints in the appendicular skeleton: Developmental mechanisms and evolutionary influences. Current Topics in Developmental Biology, 133, 119–151. 10.1016/bs.ctdb.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rux DR, Song JY, Pineault KM, Mandair GS, Swinehart IT, Schlientz AJ, Garthus KN, Goldstein SA, Kozloff KM, & Wellik DM (2017). Hox11 Function Is Required for Region-Specific Fracture Repair. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 32(8), 1750–1760. 10.1002/jbmr.3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rux DR, Song JY, Swinehart IT, Pineault KM, Schlientz AJ, Trulik KG, Goldstein SA, Kozloff KM, Lucas D, & Wellik DM (2016). Regionally Restricted Hox Function in Adult Bone Marrow Multipotent Mesenchymal Stem/Stromal Cells. Developmental Cell, 39(6), 653–666. 10.1016/j.devcel.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rux DR, & Wellik DM (2017). Hox genes in the adult skeleton: Novel functions beyond embryonic development. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 246(4), 310–317. 10.1002/dvdy.24482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JY, Pineault KM, Dones JM, Raines RT, & Wellik DM (2020). Hox genes maintain critical roles in the adult skeleton. Proceedings of the National Academy of Sciences, 117(13), 7296–7304. 10.1073/pnas.1920860117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinehart IT, Schlientz AJ, Quintanilla CA, Mortlock DP, & Wellik DM (2013). Hox11 genes are required for regional patterning and integration of muscle, tendon and bone. Development, 140(22), 4574–4582. 10.1242/dev.096693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villavicencio-Lorini P, Kuss P, Friedrich J, Haupt J, Farooq M, Türkmen S, Duboule D, Hecht J, & Mundlos S (2010). Homeobox genes d11–d13 and a13 control mouse autopod cortical bone and joint formation. The Journal of Clinical Investigation, 120(6), 1994–2004. 10.1172/JCI41554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellik DM (2007). Hox patterning of the vertebrate axial skeleton. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 236(9), 2454–2463. 10.1002/dvdy.21286 [DOI] [PubMed] [Google Scholar]
- Wellik DM, & Capecchi MR (2003). Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science (New York, N.Y.), 301(5631), 363–367. 10.1126/science.1085672 [DOI] [PubMed] [Google Scholar]
- Yu Z, Jiang K, Xu Z, Huang H, Qian N, Lu Z, Chen D, Di R, Yuan T, Du Z, Xie W, Lu X, Li H, Chai R, Yang Y, Zhu B, Kunieda T, Wang F, & Chen T (2018). Hoxc-Dependent Mesenchymal Niche Heterogeneity Drives Regional Hair Follicle Regeneration. Cell Stem Cell, 23(4), 487–500.e6. 10.1016/_j.stem.2018.07.016 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fukui N, Yahata M, Katsuragawa Y, Tashiro T, Ikegawa S, & Lee MTM (2016). Identification of DNA methylation changes associated with disease progression in subchondral bone with site-matched cartilage in knee osteoarthritis. Scientific Reports, 6(1), 34460. 10.1038/srep34460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. High magnification images of Hoxa11eGFP in E13.5 and E14.5 zeugopod joints. (AF’) Hoxa11eGFP in developing limbs at E13.5. Low magnification images of Hoxa11eGFP overlaid with DIC imaging at E13.5 from figure 1 in the forelimb (A) and hindlimb (B) provide broad Hoxa11eGFP expression patterns. Higher magnification images of boxed regions provide cellular detail using DIC imaging alone (C-D) and overlaid with Hoxa11eGFP (C’-D’). At E13.5, Hoxa11eGFP is highly expressed in the distal joints (C-D’) and largely excluded from the proximal joints (E-F’). (G-L’) Hoxa11eGFP in developing limbs at E14.5. Low magnification images of Hoxa11eGFP overlaid with DIC imaging at E14.5 from figure 1 in the forelimb (G) and hindlimb (H) provide broad Hoxa11eGFP expression patterns. Higher magnification images of boxed regions provide cellular detail using DIC imaging alone (I-L) and overlaid with Hoxa11eGFP (I’-L’). At E14.5, Hoxa11eGFP is highly expressed in the distal joints (I-J’) and in the knee (L-L’) but still largely excluded from the elbow (K-K’). Dashed lines denote approximate boundaries of embryonic skeletal elements. iz, interzone/developing joint; r, radius; u, ulna; h, humerus; ti, tibia; ta, talus; fe, femur. scale bar = 100 μm.
Fig S2. RNAscope for Hoxa11 at E15.5. (A-B) Low magnification images of full limb tissue sections at E15.5 show RNAscope for Hoxa11 in forelimbs (A) and hindlimbs (B). Hoxa11 expression is highest in wrist, ankle and knee (A-B, black arrows). (C-E) Higher magnification of boxed regions in A and B show absence of Hoxa11 from the shoulder joint (C) and hip joint (D) and high expression in the sacral region of the axial skeleton (E). A-B; scale bar = 1mm. C-D; scale bar = 100 μm.
Fig S3. Hox11 exclusion from elbow and hip joints during postnatal development. (A-F) Hoxa11eGFP is largely excluded from elbows in postnatal life. Few Hoxa11eGFP-expressing cells are present at birth (A, C, E) and are absent by 2 months of age (B, D, F). Higher magnification images (C-F) are from boxed regions in A and B and shown with (C-D) and without (E-F) DIC imaging to reveal cell/tissue morphology. (G-I) Hoxa11eGFP is not detectable in hip joint. Higher magnification images (H-I) are from the boxed region in G and shown with (H) and without (I) DIC imaging to reveal cell/tissue morphology. Dashed lines denote boundaries of cartilage zones. scale bar = 100 μm.
Fig S4. Hoxa11eGFP in meniscus and PCL during postnatal development. Hoxa11eGFP-expressing cells gradually decrease over postnatal time but are still appreciable at 2 weeks (A-C) and 1 month (D-F) in meniscus (A-B, D-E) and intra joint ligaments (C and F). Dashed lines denote boundaries of the meniscal horns. PCL; posterior collateral ligament. scale bar = 50 μm.
Fig S5. RNAscope for Hox11 paralogous genes expression at E15.5. (A-F) Hoxa11, Hoxc11 and Hoxd11 are all largely excluded from the proximal joints of the stylopod forelimb (A-C, shoulder) and hindlimb (D-F, hip/femoral head) elements consistent with regionalized function characterized previously. (G-I) In contrast, sacral elements of the axial skeleton express high levels of all three Hox11 paralogous genes where Hox11 function has been previously characterized. Dashed lines denote approximate boundaries of embryonic skeletal elements. sc, scapula; h, humerus; fh, femoral head. scale bar = 100μm.
Video 1. Three dimensional fluorescence imaging of medial tibia plateau articular cartilage in the knee from 2 month-old Hoxa11eGFP mice. The three panels show second harmonic generation alone (left), Hoxa11eGFP (center) and merged (right). The movie begins with a view of the top of the articular cartilage (the surface zone) and completes a full rotation to also visualize from the bottom (as if looking from beneath the tidemark). Note the presence of Hoxa11eGFP+ positive cells (green) throughout the entire thickness of non-calcified articular cartilage in the center panel. Superimposed on the fluorescent cells is the collagen matrix captured with Second Harmonic Generation imaging (white).