Abstract
The elevated presence of opioid receptors and their ligands throughout the developing brain points to the existence of maturational functions of the endogenous opioid system that still remain poorly understood. The alarmingly increasing rates of opioid use and abuse underscore the urgent need for clear identification of those functions and the cellular bases and molecular mechanisms underlying their physiological roles under normal and pathological conditions. This review is focused on current knowledge on the direct and indirect regulatory roles that opioids may have on oligodendrocyte development and their generation of myelin, a complex insulating membrane that not only facilitates rapid impulse conduction but also participates in mechanisms of brain plasticity and adaptation. Information is examined in relation to the importance of endogenous opioid function, as well as direct and indirect effects of opioid analogues, which like methadone and buprenorphine are used in medication-assisted therapies for opioid addiction during pregnancy and pharmacotherapy in neonatal abstinence syndrome. Potential opioid effects are also discussed regarding late myelination of the brain prefrontal cortex in adolescents and young adults. Such knowledge is fundamental for the design of safer pharmacological interventions for opioid abuse, minimizing deleterious effects in the developing nervous system.
Keywords: oligodendrocyte development, brain myelination, opioid signaling, nociceptin, perinatal opioid exposure, opioid pharmacotherapy treatments
Introduction
Opioid abuse and misuse continues to represent a problem of major epidemic proportions. This is particularly alarming when considering the large number of babies exposed to opioids during pregnancy, and in addition, the significant percentage of these infants that require opioids after birth for the pharmacological treatment of neonatal abstinence syndrome (NAS)1. Newborns affected by NAS exhibit different symptoms of variable magnitude that may include central nervous system dysfunction reflected in tremors and seizures, inconsolable crying, excessive irritability, poor sleep, and elevated muscle tone; as well as autonomic nervous system effects resulting in various digestive and respiratory problems and altered temperature regulation2–6. Opioids and their metabolites have the capacity of crossing the placenta7–9 and blood-brain barrier10,11. Thus, NAS symptoms are logically considered to be the result of abrupt discontinuation of maternal opioid supply after birth. Current successful and necessary medication-assisted therapies for opioid addiction during pregnancy involve the administration of the synthetic long-lasting opioid analogue and full mu-opioid receptor agonist methadone; and more recently, buprenorphine, a partial mu-opioid agonist and kappa-opioid receptor antagonist that not only successfully prevents the maternal abuse of opioids but also exhibits higher efficacy than methadone in reducing the incidence and severity of NAS12–16. However, an increasing number of reports suggest that some of these opioid-based therapeutic approaches may also exert neurodevelopmental effects. While much is known about opioids and their role in pain regulation, the high expression levels of different opioid receptors and their endogenous opioid ligands throughout the developing brain point to the existence of maturational functions that still remain poorly understood. This raises the question of whether interference with the endogenous opioid system by exogenous opioids, including those used in pharmacotherapy treatments, could also alter important developmental brain processes. A recent large prospective study in which potential effects of other drug co-exposures and compounding factors were carefully controlled and periodically monitored, concluded that gestational opioid exposure sufficient to result in NAS also increased the proportion of neonates with reduced head circumference17. Notably, the great majority of those infants were born from mothers that were maintained under methadone or buprenorphine treatment. The mechanisms behind these effects remain poorly understood but different findings suggest the possibility of opioid actions on different neural cell types. For example, animal models showed that perinatal methadone exposure alters the function of dopaminergic, noradrenergic and serotonergic neurons in the neonatal and early postnatal period18. Furthermore, while human effects are difficult to evaluate, studies using cultured human cortical organoids indicated methadone suppressive actions on neuronal function and maturation19. Different lines of evidence also point to potential opioid roles on various glial cell populations20. This review is focused on the neurodevelopmental effects that opioids may have on brain oligodendrocytes and their synthesis of myelin, the remarkably complex multilamellar structure that not only facilitates the rapid “saltatory conduction” of nerve impulses21 but is also now recognized as a crucial player in brain plasticity and in active bidirectional neuron-glial communications22–24. As such, oligodendrocyte generation and myelin formation are among the most critical and vulnerable processes that take place during brain development.
Developmental oligodendrocyte generation and brain myelin formation as direct targets of endogenous and exogenous opioids
Oligodendrocytes are generated from bipolar highly proliferative and migratory progenitor cells that experience several distinct stages of differentiation prior to their transformation into quiescent and morphologically complex multipolar cells capable of myelin formation25. Importantly, each of these mature oligodendrocytes has the remarkable capacity of generating multiple extensive membrane extensions that contact numerous neurons and concentrically wrap around their axons generating multiple myelin internodes. This well-defined progression along the oligodendroglial lineage assumes the orchestration of both extrinsic and intrinsic factors that regulate gene expression by a variety of mechanisms that are still the center of active investigation26–30. The presence of opioid receptors in stem cells and the different stages of oligodendrocyte differentiation, support the notion that the endogenous opioid system plays crucial roles in controlling oligodendrocyte maturation and suggest that interference by exogenous opioids could alter developmental brain myelination31–34.
This represents a problem of significant developmental importance because it is now known that myelin functions expand well beyond of that as an insulator facilitating the rapid “saltatory conduction” of nerve impulses. Myelin plays a crucial role in regulating axonal extension and radial growth35, and its presence is required for both the induction and preservation of specific localization of Na+ and K+ channel domains at nodal and paranodal axonal regions36. Importantly, oligodendrocytes and myelin are critical players in mechanisms of neuronal survival and axonal function and integrity37,38. Oligodendrocytes also actively participate in electrical coupling to astrocytes39; and together with myelin, are capable of bidirectional glial-neuronal signaling and communication22. Furthermore, both oligodendrocytes and myelin are actively implicated in plastic memory and learning23,24,40–43, setting oligodendrocyte generation and myelin formation among the most critical and vulnerable processes that take place during brain development. Thus, it is not surprising that negative effects on myelin structure and stability are known to occur with the abuse of various drugs; including cocaine44, cannabinoids45, alcohol45,46 and methamphetamines47.
As discussed above, the dramatic increase in opioid use and abuse also triggered an alarming rising number of newborns that are exposed in utero to maternal pharmacotherapy treatments48, being methadone and buprenorphine the most successful and current recommended opioid analogues for these therapies12–16. Yet, reports on short- and long-term neurodevelopmental effects of prenatal exposure to these drugs and other opioids are conflicting and difficult to assess as multiple interacting factors such as maternal poly-drug use or social and educational environment can profoundly influence cognitive development (recently reviewed by49). The complexity of these compounding factors is further emphasized by studies in rodent models indicating that adult offspring of dams chronically exposed to morphine during puberty exhibit anxiety-like behaviors and enhanced morphine sensitization50.
In support of potential opioid effects on developmental myelination, recent imaging studies revealed white matter injury and abnormal myelin structure in the brain of infants prenatally exposed to opioids51. In this regard, examination of potential effects of perinatal exposure to buprenorphine and methadone indicated complex responses with significant alterations in the timing of rat brain myelination52,53. In these studies, pregnant rats (gestation day 7) were implanted with minipumps to deliver buprenorphine at doses of 0.3 (therapeutic) or 1 (supra-therapeutic) mg/kg/day. By using this experimental paradigm, pups were first exposed to the drug through the placenta immediately prior to brain development; and then through lactation, during a neurodevelopmental period equivalent to the third trimester in human pregnancy. Analysis at postnatal days 12, 19, and 26 (ages that respectively correspond to the beginning, peak, and end of the rapid period of myelin formation in rat brain), demonstrated that perinatal exposure to buprenorphine significantly alters their brain content of myelin basic proteins (MBPs)52; important myelin components that comprise about 30% of the total myelin protein and are required for the formation, compaction, and stability of myelin multilamellar structure54. Interestingly, these buprenorphine-induced effects were specifically dependent on the age of the pups at the time of tissue collection and the dose of administered drug. Myelin formation and growth in the mammalian brain is accompanied by a progressive increase in the expression of four major MBP isoforms generated by alternative splicing of a single developmentally regulated gene55. Unexpectedly, the accumulation of all MBP isoforms was accelerated and increased by exposure of the pups to the therapeutic buprenorphine dose of 0.3mg/kg/day. In contrast, supra-therapeutic levels delayed MBP expression. Furthermore, although MBP isoforms in pups exposed to elevated doses of buprenorphine finally reached control values by day 19, histological analysis of the corpus callosum fibers at 26 days of age still indicated a reduced number of axons that were myelinated52. Because MBPs are only synthesized when oligodendrocytes reach maturity, those findings suggested direct dose-specific effects of buprenorphine on oligodendrocyte development, a possibility supported by studies in which cultured cells directly isolated from the postnatal brain were treated with different drug concentrations34. Buprenorphine indeed exerts direct dose-dependent effects on oligodendrocyte differentiation, with low concentrations (0.5 μM) accelerating the transformation of immature pre-oligodendrocytes into morphologically complex MBP-making multipolar mature cells, an effect found to be mediated by mu-opioid receptor activation. In remarkable contrast, elevated drug concentrations (3 μM) block oligodendrocyte maturation, an inhibitory effect mediated by concomitant buprenorphine-dependent activation of the nociceptin/orphanin FQ receptor (NOR). Also known as opioid receptor like-1 (ORL-1), NOR is the most recently discovered member of the opioid receptor family, and while this G-protein coupled receptor shares a high degree of homology with the classical opioid receptors, it does not bind any of the endogenous opioid peptides and it is only specifically activated by the endogenous heptadecapeptide nociceptin56. Antagonist effects of the mu-opioid receptor and NOR were originally identified as responsible for the characteristic bell-shaped dose-response of buprenorphine effects on pain regulation57–59. Importantly, a number of recent publications point to the involvement of NOR and its ligand nociceptin as regulators in a variety of important processes that span from behavior, learning and memory60–62 to drug addiction control63,64. Together with the in vivo findings, buprenorphine effects on cultured cells pointed to a model in which this drug induces direct effects by binding to two receptors with different drug affinities and opposing roles on oligodendrocyte development. Activation of the high affinity mu-opioid receptor by low buprenorphine concentrations results in stimulation of oligodendrocyte maturation, while this positive action is counteracted by an inhibitory effect induced by high drug doses and simultaneous signaling through the low affinity NOR. Consistent with this idea, oligodendrocyte maturation is also stimulated by the mu-opioid receptor agonist methadone53. As observed for the low concentrations of buprenorphine, direct exposure of post-mitotic but still immature pre-oligodendrocytes to methadone results in a significant increase in the expression of different myelin specific proteins and morphological complexity. [3H]Thymidine incorporation into DNA, showed that methadone also stimulates the proliferation of cultured oligodendrocyte progenitor cells53, a finding that is in agreement with earlier reports indicating that mu-receptor activation can increase the mitogenic capacity of these still immature cells31,65. In agreement with those findings, electron microscopic analysis of the corpus callosum in 2-week-old pups perinatally exposed to therapeutic doses of methadone indicated accelerated myelination with abnormally elevated number of axons with already highly compacted myelin sheaths53. These effects of methadone and buprenorphine support an important role of the endogenous mu-opioid and NOR signaling systems in the control of oligodendrocyte maturation and the precise timing of brain myelination. In support of physiological developmental functions of the mu-opioid receptor and NOR, male and female rat brain expression levels of their respective endogenous ligands, endomorphin-1 and nociceptin are also developmentally regulated66. Nociceptin concentrations exhibit a significant and progressive decrease from postnatal day 9 and thereafter, reaching background levels of detection by one month of age, a pattern of expression that inversely correlates with the progression of rat brain myelination. Endomorphin-1 levels are still elevated at postnatal day 9 but gradually decrease from day 13. Just like methadone and low buprenorphine concentrations, endomorphin-1 stimulates oligodendrocyte maturation and morphological complexity. Interestingly, this stimulation is abolished by co-incubation of the cultures with nociceptin. An inhibitory effect of nociceptin in oligodendrocyte maturation and myelinating activity is further supported by the observation that in vivo inhibition of NOR signaling results in accelerated myelination. Surprisingly, these effects of endomorphin-1 and nociceptin are most significantly observed for the female rat oligodendrocytes and the female brain66.
Altogether, these findings suggest that a complex balance between opposing functions of mu-opioid receptor and NOR signaling may play a crucial role in controlling the timing of brain myelination. Importantly, nociceptin also appears to have an important stimulatory role on neuronal development, as this peptide was shown to exert a supportive effect on rat cerebellar granule neurons67 as well as positive actions on neurite outgrowth in mouse hippocampal cells68. Thus, it is possible that nociceptin may play a crucial double function stimulating on one side neuronal maturation while on the other hand deterring premature myelination, a situation that could interfere with early axonal elongation and neuronal connectivity. Such a situation may in part underlie the still puzzling finding from earlier studies indicating that, regardless of the dose, the brain of rat pups perinatally exposed to buprenorphine exhibited increased caliber of myelinated axons with disproportionally thinner myelin sheaths52. Since no differences could be found for nonmyelinated axons, that observation suggested that buprenorphine could perhaps interfere with the mechanisms coordinating axonal outgrowth with myelin formation. Methadone-induced enhanced proliferation of oligodendrocyte progenitors and acceleration of cell differentiation and myelination may also derail the delicate balance between opposing functions of mu-opioid receptor and NOR signaling, thus altering the proper timing of brain myelination and neuronal connectivity (Figure 1).
Figure 1.
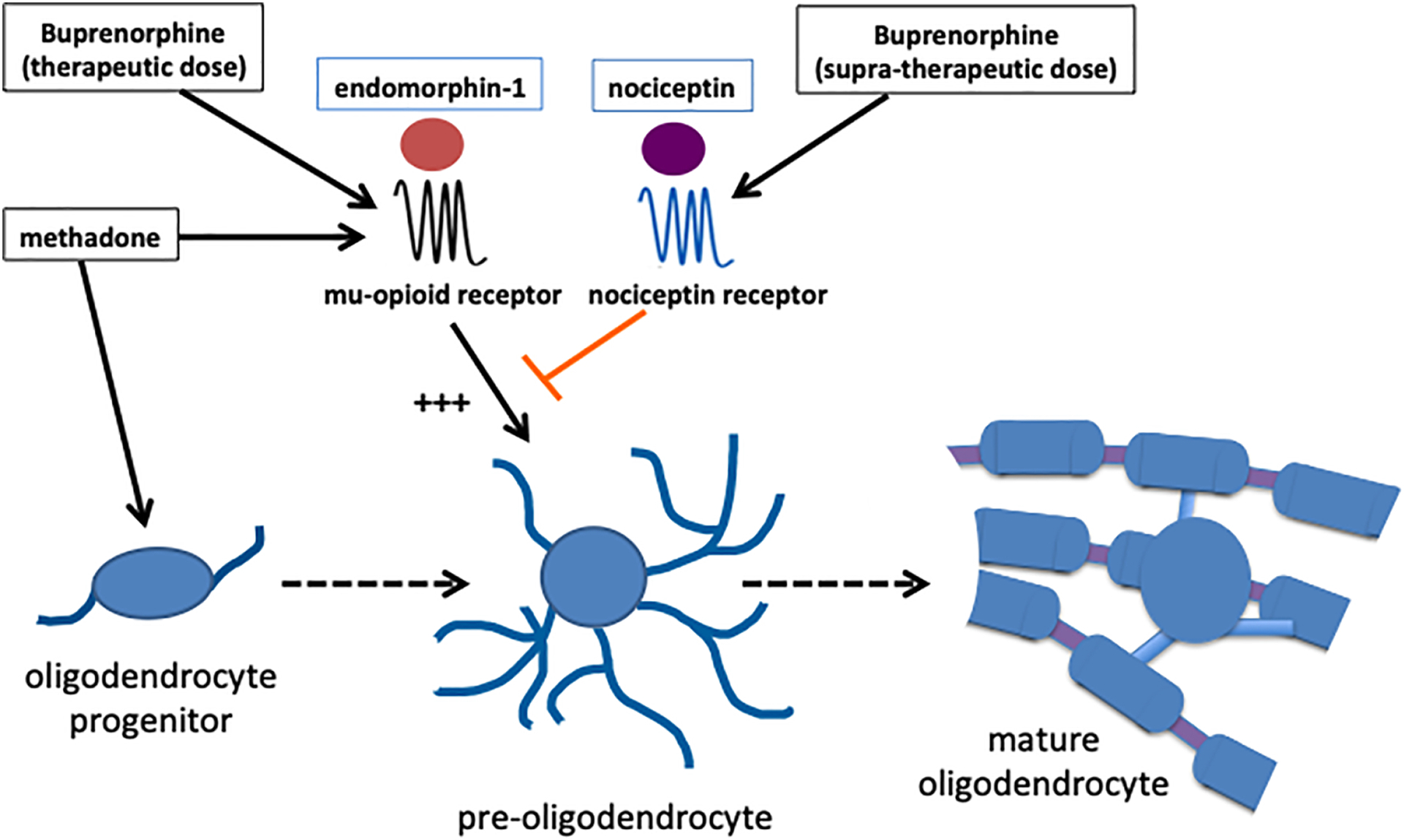
A delicate balance between opposing effects of the mu-opioid- and nociceptin receptor activities appears to control the precise timing of oligodendrocyte maturation preventing precocious myelinating activity. Exogenous opioids like methadone and buprenorphine may alter this balance, a situation that could affect the time-dependent coordination of myelination with axonal outgrowth and connectivity.
Precocious oligodendrocyte maturation may also ultimately result in a long-term reduced pool of differentiated cells, a possibility that could at least in part explain the decreased amount of myelin protein recently reported in a different model of perinatal methadone exposure69.
Oligodendrocyte maturation and developmental myelination as secondary targets of opioid effects
The previous section of this review focused on physiological functions of endogenous opioid systems in oligodendrocyte development and the consequences of their direct interference by exogenous opioids. However, as a logical reflection of the multiple roles and integrative functions of oligodendrocytes and myelin, important consideration should also be given to potential indirect effects mediated by primary opioid actions on other diverse cell targets. Understanding of such secondary effects could be of critical importance in the design of treatments for opioid pharmacotherapy during pregnancy and NAS. It is conceivable that effects of methadone, buprenorphine, and morphine on oligodendrocyte and myelination may also be mediated through their actions on the other two major glial cell types, astrocytes and microglia. Although this possibility remains to be examined, several lines of evidence indicate that both of these cells exhibit functional opioid receptors70–74. Studies with cultured cells demonstrated that morphine inhibits astrocyte proliferation75, thus a reduction in the size of astrocyte pools at developmental times of active myelination may decrease the availability of multiple astrocyte-secreted factors which as platelet-derived growth factor (PDGF)76–78 and neurotrophins79–82 are known to exert modulatory effects on the oligodendroglial lineage and myelination. A decreased number of astrocytes could also limit the concentration of leukemia inhibitory factor (LIF), a molecule that controls the self-renewal and proliferation of neural stem cells and subsequent generation of oligodendrocyte progenitors83. Moreover, studies in neuronal-glial co-cultures showed that astrocyte-secreted LIF could directly stimulate oligodendrocytes to support myelination in response to neuronal electrical impulses84. It is also possible to speculate that reduced pools of astrocytes would result in decreased brain concentrations of nociceptin85, an endogenous peptide that as described in the previous section was shown to play a function counteracting the stimulatory mu-opioid signaling effect on oligodendrocytes and thus precluding untimely precocious brain myelination34,66
Importantly, microglial cells could mediate negative effects of morphine on myelination. Studies in cultured cells showed that mu-opioid receptor activation by morphine stimulates the microglial secretion of interleukin-1β72, a pro-inflammatory cytokine that disrupts developmental myelination86. Strongly supporting the importance of neuroinflammatory effects, recent studies in which pups were exposed to methadone (8–16 mg/kg) from embryonic day 16 to postnatal day 21, showed a dose-dependent increased in serum inflammatory biomarkers and microglia activation, accompanied by decreased myelin protein expression87. This may represent a mechanism of critical importance as microglial activation and neuroinflammation have been linked to the pathogenesis of neurodevelopmental diseases such as schizophrenia88 and autism89.
Last but not least, there is also the important possibility that secondary opioid effects on myelination could be mediated by various primary neuronal effects. Developing oligodendrocytes express neurotransmitter receptors and are responsive to different neurotransmitter signals90,91, therefore opioid addiction treatments could affect cell maturation and myelination through the disruption of oligodendroglial-neuronal signaling. Pioneer studies demonstrated that perinatal exposure to methadone delays the expression of the cholinergic phenotype in the striatum92–95, reducing striatal acetylcholine (Ach) levels in neonatal rats regardless of whether or not drug exposure continues into the early postnatal period95. Perinatal methadone exposure also alters the function of dopaminergic, noradrenergic and serotonergic neurons in the neonatal and early postnatal period with some of these changes even persisting into adulthood18. Abnormal neuronal signaling to developing oligodendrocytes may also result from exposure to buprenorphine. Similar to methadone, therapeutic doses of buprenorphine were shown to accelerate the development of righting reflex96 and prenatal drug exposures reduce striatal Ach content during the first week of life. Such altered cholinergic development may reflect methadone or buprenorphine effects on the expression of nerve growth factor (NGF), a neurotrophin that stimulates expression of the cholinergic phenotype in striatal neurons97.
Additional support for a disruption of glial-neuronal communication by perinatal buprenorphine exposure is the observation that the corpus callosum of rat pups perinatally exposed to buprenorphine exhibited an increased proportion of high caliber axons with disproportionally thinner myelin sheaths. As discussed before, such a situation may be in part mediated by interference with the normal function of nociceptin. Interestingly, this abnormal axonal diameter/myelin thickness ratio was accompanied by increased levels of the myelin associated glycoprotein (MAG)52, a protein that is majorly localized in the periaxonal myelin layer and may play a crucial function as a mediator of glial-axonal communication98. In addition, the previously described acceleration of myelination in pups exposed to therapeutic doses of buprenorphine was accompanied by increased interaction of MAG with the Src-family tyrosine kinase Fyn, a signaling molecule that mediates axonal-oligodendroglial interactions leading to myelination52. Thus, it is clear that much remains to be investigated to fully understand the direct and indirect effects that endogenous and exogenous opioids may have on developmental brain myelination.
Discussion
The findings described in this review point to significant opioid modulatory effects on perinatal oligodendrocyte differentiation and brain myelination. Cell culture studies and animal models of perinatal opioid exposure suggest that a complex balance between opposing effects of the mu-opioid- and nociceptin receptor activities control the precise timing of oligodendrocyte maturation and myelinating activity34,66. Such balance may play and important function preventing precocious myelination, a situation that could interfere with early axonal elongation. Exogenous opioids like methadone and buprenorphine may alter this balance, a situation that would affect the time-dependent coordination of myelination with axonal outgrowth and connectivity. Furthermore, recent imaging studies determined the presence of white matter injury and abnormal myelin structure in the brain of infants that were prenatally exposed to opioids51, supporting potential effects of these drugs on developmental human myelination.
While most of the review included evidence regarding the effects of methadone and buprenorphine, it is also particularly concerning the administration of morphine for sedative purposes in preterm neonates99. While little is still known about opioid effects at such young age in the human brain, studies investigating the consequences of morphine administration in the developing rat brain demonstrated about 30% reduced myelin basic protein mRNA expression induced by daily morphine administration during the first postnatal week100. Equally important is to consider the potential effects that chronic opioid abuse may have at later ages of brain development. Early histological analyses and imaging demonstrated that heroin and morphine abuse in adult humans can result in severe myelin damage and spongiform leukoencephalopathy101–106. Importantly, similar myelin damage in the adult brain could also result from acute overdose of prescription opioid painkillers. Severe leukoencephalopathy was observed in cases of acute Oxycodone intoxication107 and Fentanyl overdose108,109. While these effects may potentially involve the overlooked actions of multi-drug use and other compounding factors, these observations also raise the need for studies on the effects of some of these “newer” drugs in child brain myelination as Fentanyl is used within the neonatal or pediatric intensive care settings110. More recently, the importance of oligodendrocytes as targets of opioid addiction at later stages of brain maturation is further supported by recent studies in which validated single-cell RNA-sequencing was used to profile cell-type-specific changes in the nucleus accumbens of adult mice four hours after acute morphine administration111. While both neurons and glial cells exhibited significant changes in gene expression, a remarkably strong transcriptional response was observed in the oligodendrocytes. In these particular cells, upregulation of multiple glucocorticoid receptor signaling related genes was accompanied by decreased expression of genes encoding heat shock- and endoplasmic reticulum (ER) chaperone proteins that are critical for ER quality control and the unfolded protein response (UPR). This is particularly significant as different studies showed that UPR control plays a very important role in oligodendrocyte cell survival and myelin maintenance112,113. Particularly disturbing in this regard are the potential deleterious effects that these drugs could have in teenagers and young adults, a population that represents the largest group for the use and abuse of prescription and non-prescription opioids114–117. This concern stems from the fact that a very active and extensive late wave of myelination in humans takes place in the adolescent and young adult prefrontal cortex (PFC)118–120, a brain region that is highly interconnected with other cortical and subcortical areas and it is crucially involved in complex cognitive control and behavior121–124. Furthermore, myelin pathology at this age bracket has been observed in an array of psychiatric conditions, including bipolar depression125,126 and schizophrenia127. Importantly, a variety of processes associated with PFC function, including among others learning and memory, motivation, and self-control, are characteristically altered in individuals affected by drug addiction (reviewed by Goldstein and Volkow128).
In conclusion, the information summarized in this review supports an important role of the endogenous opioid system in controlling the development of oligodendrocytes and their myelinating activity. Studies in animal models and cultured oligodendrocytes indicate that interference with mu-opioid and nociceptin-receptor signaling systems by exogenous opioids used in drug maintenance treatments during pregnancy alters the timing of brain myelination and may therefore disrupt its crucial coordination with axonal outgrowth and synaptic connectivity. While the precise neurodevelopmental functions of endogenous opioid peptides in the developing human brain remain poorly understood, such possibilities deserve further investigation as longitudinal studies along childhood have shown a significant correlation between general cognitive ability and the precise timing and pattern of neurodevelopmental brain myelination129. Important in this regard are recent observations indicating abnormal microstructure of major white matter tracts in newborns exposed in utero to methadone130, and the existence of persistent neurocognitive alterations in teenagers and young adults that were prenatally exposed to opioids131. Recent information on sex-specific responses to endogenous opioid and opioid-related peptides in oligodendrocytes and myelination of the female rat brain66, stresses the need for further understanding of the molecular events mediating these functions and future studies addressing potential sex-related differences in the neurodevelopmental effects of both therapeutic opioid painkillers and opioid-pharmacotherapy treatments.
The elevated presence of opioid receptors and their ligands throughout the developing brain points to the existence of maturational functions of the endogenous opioid system that still remain poorly understood.
The alarmingly increasing rates of opioid use and abuse underscore the urgent need for clear identification of those functions and the cellular bases and molecular mechanisms underlying their physiological roles under normal and pathological conditions
The findings described in this review point to significant opioid modulatory effects on perinatal oligodendrocyte differentiation and brain myelination
Studies in animal models and cultured oligodendrocytes indicate that interference with mu-opioid and nociceptin-receptor signaling systems by exogenous opioids used in pharmacological treatments during pregnancy alters the timing of brain myelination and may therefore disrupt its crucial coordination with axonal outgrowth and synaptic connectivity
ACKNOWLEDGEMENTS.
Some of the studies reported in this review were supported by grants R21DA027099-01 from the National Institutes of Health, RG 1501-2891 from the National Multiple Sclerosis Society, and a sub-award from NIH CTSA grant UL1TR00058 from the Virginia Commonwealth University (VCU) Center for Clinical and Translational Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: The authors state no conflict of interest.
REFERENCES
- 1.Honein MA, Boyle C & Redfield RR Public Health Surveillance of Prenatal Opioid Exposure in Mothers and Infants. Pediatrics 143, doi: 10.1542/peds.2018-3801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finnegan LP, Kron RE, Connaughton JF & Emich JP Assessment and treatment of abstinence in the infant of the drug-dependent mother. Int J Clin Pharmacol Biopharm 12, 19–32 (1975). [PubMed] [Google Scholar]
- 3.Desai RJ, Huybrechts KF, Hernandez-Diaz S, Mogun H, Patorno E, Kaltenbach K, Kerzner LS & Bateman BT Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ 350, h2102, doi: 10.1136/bmj.h2102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoegerman G, Wilson CA, Thurmond E & Schnoll SH Drug-exposed neonates. West J Med 152, 559–564 (1990). [PMC free article] [PubMed] [Google Scholar]
- 5.Jones HE, Kaltenbach K, Johnson E, Seashore C, Freeman E & Malloy E Neonatal Abstinence Syndrome: Presentation and Treatment Considerations. J Addict Med 10, 224–228, doi: 10.1097/ADM.0000000000000222 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Pomar E & Finnegan LP The Epidemic of Neonatal Abstinence Syndrome, Historical References of Its’ Origins, Assessment, and Management. Front Pediatr 6, 33, doi: 10.3389/fped.2018.00033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nekhayeva IA, Nanovskaya TN, Deshmukh SV, Zharikova OL, Hankins GD & Ahmed MS Bidirectional transfer of methadone across human placenta. Biochem Pharmacol 69, 187–197, doi: 10.1016/j.bcp.2004.09.008 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Krishna BR, Zakowski MI & Grant GJ Sufentanil transfer in the human placenta during in vitro perfusion. Can J Anaesth 44, 996–1001, doi: 10.1007/BF03011972 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Malek A & Mattison DR Drugs and medicines in pregnancy: the placental disposition of opioids. Curr Pharm Biotechnol 12, 797–803, doi: 10.2174/138920111795470859 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Bostrom E, Hammarlund-Udenaes M & Simonsson US Blood-brain barrier transport helps to explain discrepancies in in vivo potency between oxycodone and morphine. Anesthesiology 108, 495–505, doi: 10.1097/ALN.0b013e318164cf9e (2008). [DOI] [PubMed] [Google Scholar]
- 11.Bourasset F, Cisternino S, Temsamani J & Scherrmann JM Evidence for an active transport of morphine-6-beta-d-glucuronide but not P-glycoprotein-mediated at the blood-brain barrier. J Neurochem 86, 1564–1567, doi: 10.1046/j.1471-4159.2003.01990.x (2003). [DOI] [PubMed] [Google Scholar]
- 12.Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M & Aschauer H Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction 101, 275–281, doi: 10.1111/j.1360-0443.2006.01321.x (2006). [DOI] [PubMed] [Google Scholar]
- 13.Lejeune C, Simmat-Durand L, Gourarier L, Aubisson S & Groupe d’Etudes Grossesse et, A. Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenophine substitution. Drug and alcohol dependence 82, 250–257, doi: 10.1016/j.drugalcdep.2005.10.001 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Welle-Strand GK, Skurtveit S, Jones HE, Waal H, Bakstad B, Bjarko L & Ravndal E Neonatal outcomes following in utero exposure to methadone or buprenorphine: a National Cohort Study of opioid-agonist treatment of Pregnant Women in Norway from 1996 to 2009. Drug and alcohol dependence 127, 200–206, doi: 10.1016/j.drugalcdep.2012.07.001 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Minozzi S, Amato L, Bellisario C, Ferri M & Davoli M Maintenance agonist treatments for opiate-dependent pregnant women. Cochrane Database Syst Rev 12, CD006318, doi: 10.1002/14651858.CD006318.pub3 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR & Fischer G Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med 363, 2320–2331, doi: 10.1056/NEJMoa1005359 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Towers CV, Hyatt BW, Visconti KC, Chernicky L, Chattin K & Fortner KB Neonatal Head Circumference in Newborns With Neonatal Abstinence Syndrome. Pediatrics 143, doi: 10.1542/peds.2018-0541 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Rech RH, Lomuscio G & Algeri S Methadone exposure in utero: effects on brain biogenic amines and behavior. Neurobehavioral toxicology 2, 75–78 (1980). [PubMed] [Google Scholar]
- 19.Wu W, Yao H, Dwivedi I, Negraes PD, Zhao HW, Wang J, Trujillo CA, Muotri AR & Haddad GG Methadone Suppresses Neuronal Function and Maturation in Human Cortical Organoids. Front Neurosci 14, 593248, doi: 10.3389/fnins.2020.593248 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser KF & Knapp PE Opiate Drugs with Abuse Liability Hijack the Endogenous Opioid System to Disrupt Neuronal and Glial Maturation in the Central Nervous System. Front Pediatr 5, 294, doi: 10.3389/fped.2017.00294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castelfranco AM & Hartline DK The evolution of vertebrate and invertebrate myelin: a theoretical computational study. J Comput Neurosci 38, 521–538, doi: 10.1007/s10827-015-0552-x (2015). [DOI] [PubMed] [Google Scholar]
- 22.Fields RD Oligodendrocytes changing the rules: action potentials in glia and oligodendrocytes controlling action potentials. Neuroscientist 14, 540–543, doi: 10.1177/1073858408320294 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasan M, Kanna MS, Jun W, Ramkrishnan AS, Iqbal Z, Lee Y & Li Y Schema-like learning and memory consolidation acting through myelination. FASEB J 33, 11758–11775, doi: 10.1096/fj.201900910R (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H & Richardson WD Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci 19, 1210–1217, doi: 10.1038/nn.4351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumann N & Pham-Dinh D Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81, 871–927, doi: 10.1152/physrev.2001.81.2.871 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Sock E & Wegner M Transcriptional control of myelination and remyelination. Glia 67, 2153–2165, doi: 10.1002/glia.23636 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi QS, Xin M, Wang F, Appel B & Lu QR MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65, 612–626, doi: 10.1016/j.neuron.2010.02.018 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Moyon S, Hernandez M & Casaccia P Epigenetic control of oligodendrocyte development: adding new players to old keepers. Curr Opin Neurobiol 39, 133–138, doi: 10.1016/j.conb.2016.06.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elbaz B & Popko B Molecular Control of Oligodendrocyte Development. Trends Neurosci 42, 263–277, doi: 10.1016/j.tins.2019.01.002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bercury KK & Macklin WB Dynamics and mechanisms of CNS myelination. Dev Cell 32, 447–458, doi: 10.1016/j.devcel.2015.01.016 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knapp PE, Maderspach K & Hauser KF Endogenous opioid system in developing normal and jimpy oligodendrocytes: mu and kappa opioid receptors mediate differential mitogenic and growth responses. Glia 22, 189–201 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Hahn JW, Jagwani S, Kim E, Rendell VR, He J, Ezerskiy LA, Wesselschmidt R, Coscia CJ & Belcheva MM Mu and kappa opioids modulate mouse embryonic stem cell-derived neural progenitor differentiation via MAP kinases. Journal of neurochemistry 112, 1431–1441, doi: 10.1111/j.1471-4159.2009.06479.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persson AI, Bull C & Eriksson PS Requirement for Id1 in opioid-induced oligodendrogenesis in cultured adult rat hippocampal progenitors. Eur J Neurosci 23, 2277–2288, doi: 10.1111/j.1460-9568.2006.04764.x (2006). [DOI] [PubMed] [Google Scholar]
- 34.Eschenroeder AC, Vestal-Laborde AA, Sanchez ES, Robinson SE & Sato-Bigbee C Oligodendrocyte responses to buprenorphine uncover novel and opposing roles of mu-opioid- and nociceptin/orphanin FQ receptors in cell development: implications for drug addiction treatment during pregnancy. Glia 60, 125–136, doi: 10.1002/glia.21253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin X, Crawford TO, Griffin JW, Tu P, Lee VM, Li C, Roder J & Trapp BD Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci 18, 1953–1962 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasband MN & Trimmer JS Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol 236, 5–16, doi: 10.1006/dbio.2001.0326 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Saab AS, Tzvetanova ID & Nave KA The role of myelin and oligodendrocytes in axonal energy metabolism. Curr Opin Neurobiol 23, 1065–1072, doi: 10.1016/j.conb.2013.09.008 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Morrison BM, Lee Y & Rothstein JD Oligodendroglia: metabolic supporters of axons. Trends Cell Biol 23, 644–651, doi: 10.1016/j.tcb.2013.07.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ransom BR & Kettenmann H Electrical coupling, without dye coupling, between mammalian astrocytes and oligodendrocytes in cell culture. Glia 3, 258–266, doi: 10.1002/glia.440030405 (1990). [DOI] [PubMed] [Google Scholar]
- 40.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H & Ullen F Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8, 1148–1150, doi: 10.1038/nn1516 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Scholz J, Klein MC, Behrens TE & Johansen-Berg H Training induces changes in white-matter architecture. Nat Neurosci 12, 1370–1371, doi: 10.1038/nn.2412 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K & Richardson WD Motor skill learning requires active central myelination. Science 346, 318–322, doi: 10.1126/science.1254960 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato D, Wake H, Lee PR, Tachibana Y, Ono R, Sugio S, Tsuji Y, Tanaka YH, Tanaka YR, Masamizu Y, Hira R, Moorhouse AJ, Tamamaki N, Ikenaka K, Matsukawa N, Fields RD, Nabekura J & Matsuzaki M Motor learning requires myelination to reduce asynchrony and spontaneity in neural activity. Glia 68, 193–210, doi: 10.1002/glia.23713 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P & Mintz J Brain maturation may be arrested in chronic cocaine addicts. Biological psychiatry 51, 605–611 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD & Tapert SF Altered white matter microstructure in adolescent substance users. Psychiatry research 173, 228–237, doi: 10.1016/j.pscychresns.2009.04.005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR & Tapert SF Altered white matter integrity in adolescent binge drinkers. Alcoholism, clinical and experimental research 33, 1278–1285, doi: 10.1111/j.1530-0277.2009.00953.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W & London ED Structural abnormalities in the brains of human subjects who use methamphetamine. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 6028–6036, doi: 10.1523/JNEUROSCI.0713-04.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobon AL, Habecker E & Forray A Opioid Use in Pregnancy. Curr Psychiatry Rep 21, 118, doi: 10.1007/s11920-019-1110-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conradt E, Flannery T, Aschner JL, Annett RD, Croen LA, Duarte CS, Friedman AM, Guille C, Hedderson MM, Hofheimer JA, Jones MR, Ladd-Acosta C, McGrath M, Moreland A, Neiderhiser JM, Nguyen RHN, Posner J, Ross JL, Savitz DA, Ondersma SJ & Lester BM Prenatal Opioid Exposure: Neurodevelopmental Consequences and Future Research Priorities. Pediatrics 144, doi: 10.1542/peds.2019-0128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byrnes EM Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 182, 537–544, doi: 10.1007/s00213-005-0122-4 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Merhar SL, Parikh NA, Braimah A, Poindexter BB, Tkach J & Kline-Fath B White Matter Injury and Structural Anomalies in Infants with Prenatal Opioid Exposure. AJNR Am J Neuroradiol 40, 2161–2165, doi: 10.3174/ajnr.A6282 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez ES, Bigbee JW, Fobbs W, Robinson SE & Sato-Bigbee C Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia 56, 1017–1027, doi: 10.1002/glia.20675 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE & Sato-Bigbee C The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci 36, 409–421, doi: 10.1159/000365074 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Readhead C, Takasashi N, Shine HD, Saavedra R, Sidman R & Hood L Role of myelin basic protein in the formation of central nervous system myelin. Ann N Y Acad Sci 605, 280–285 (1990). [DOI] [PubMed] [Google Scholar]
- 55.de Ferra F, Engh H, Hudson L, Kamholz J, Puckett C, Molineaux S & Lazzarini RA Alternative splicing accounts for the four forms of myelin basic protein. Cell 43, 721–727 (1985). [DOI] [PubMed] [Google Scholar]
- 56.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B & et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377, 532–535, doi: 10.1038/377532a0 (1995). [DOI] [PubMed] [Google Scholar]
- 57.Dum JE & Herz A In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. British journal of pharmacology 74, 627–633 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lizasoain I, Leza JC & Lorenzo P Buprenorphine: bell-shaped dose-response curve for its antagonist effects. General pharmacology 22, 297–300 (1991). [DOI] [PubMed] [Google Scholar]
- 59.Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT & Evans CJ Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci 23, 10331–10337 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor RM, Jeong IH, May MD, Bergman EM, Capaldi VF, Moore NLT, Matson LM & Lowery-Gionta EG Fear expression is reduced after acute and repeated nociceptin/orphanin FQ (NOP) receptor antagonism in rats: therapeutic implications for traumatic stress exposure. Psychopharmacology (Berl) 237, 2943–2958, doi: 10.1007/s00213-020-05582-0 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Adem A, Madjid N, Kahl U, Holst S, Sadek B, Sandin J, Terenius L & Ogren SO Nociceptin and the NOP receptor in aversive learning in mice. Eur Neuropsychopharmacol 27, 1298–1307, doi: 10.1016/j.euroneuro.2017.09.005 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Rekik K, Faria Da Silva R, Colom M, Pacifico S, Zaveri NT, Calo G, Rampon C, Frances B & Mouledous L Activation of nociceptin/orphanin FQ receptors inhibits contextual fear memory reconsolidation. Neuropharmacology 125, 39–49, doi: 10.1016/j.neuropharm.2017.07.006 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Tollefson S, Himes M & Narendran R Imaging corticotropin-releasing-factor and nociceptin in addiction and PTSD models. Int Rev Psychiatry 29, 567–579, doi: 10.1080/09540261.2017.1404445 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Bellia F, Fernandez MS, Fabio MC, Pucci M, Pautassi RM & D’Addario C Selective alterations in endogenous opioid system genes expression in rats selected for high ethanol intake during adolescence. Drug Alcohol Depend 212, 108025, doi: 10.1016/j.drugalcdep.2020.108025 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Knapp PE & Hauser KF mu-Opioid receptor activation enhances DNA synthesis in immature oligodendrocytes. Brain Res 743, 341–345 (1996). [DOI] [PubMed] [Google Scholar]
- 66.Mohamed E, Paisley CE, Meyer LC, Bigbee JW & Sato-Bigbee C Endogenous opioid peptides and brain development: Endomorphin-1 and Nociceptin play a sex-specific role in the control of oligodendrocyte maturation and brain myelination. Glia 68, 1513–1530, doi: 10.1002/glia.23799 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corbiere A, Walet-Balieu ML, Chan P, Basille-Dugay M, Hardouin J & Vaudry D A Peptidomic Approach to Characterize Peptides Involved in Cerebellar Cortex Development Leads to the Identification of the Neurotrophic Effects of Nociceptin. Mol Cell Proteomics 17, 1737–1749, doi: 10.1074/mcp.RA117.000184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ring RH, Alder J, Fennell M, Kouranova E, Black IB & Thakker-Varia S Transcriptional profiling of brain-derived-neurotrophic factor-induced neuronal plasticity: a novel role for nociceptin in hippocampal neurite outgrowth. J Neurobiol 66, 361–377, doi: 10.1002/neu.20223 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Oberoi R, Chu T, Mellen N, Jagadapillai R, Ouyang H, Devlin LA & Cai J Diverse changes in myelin protein expression in rat brain after perinatal methadone exposure. Acta Neurobiol Exp (Wars) 79, 367–373 (2019). [PubMed] [Google Scholar]
- 70.Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu DF, Archer S, Bidlack JM & Peterson PK kappa opioid receptors in human microglia downregulate human immunodeficiency virus 1 expression. Proceedings of the National Academy of Sciences of the United States of America 93, 8051–8056 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takayama N & Ueda H Morphine-induced chemotaxis and brain-derived neurotrophic factor expression in microglia. The Journal of neuroscience : the official journal of the Society for Neuroscience 25, 430–435, doi: 10.1523/JNEUROSCI.3170-04.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merighi S, Gessi S, Varani K, Fazzi D, Stefanelli A & Borea PA Morphine mediates a proinflammatory phenotype via mu-opioid receptor-PKCvarepsilon-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochemical pharmacology 86, 487–496, doi: 10.1016/j.bcp.2013.05.027 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF & Knapp PE Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at mu-opioid receptor-expressing glia. Brain : a journal of neurology 134, 3616–3631, doi: 10.1093/brain/awr281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eriksson PS, Hansson E & Ronnback L Delta and kappa opiate receptors in primary astroglial cultures from rat cerebral cortex. Neurochemical research 15, 1123–1126 (1990). [DOI] [PubMed] [Google Scholar]
- 75.Stiene-Martin A, Gurwell JA & Hauser KF Morphine alters astrocyte growth in primary cultures of mouse glial cells: evidence for a direct effect of opiates on neural maturation. Brain research. Developmental brain research 60, 1–7 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill RA, Patel KD, Medved J, Reiss AM & Nishiyama A NG2 cells in white matter but not gray matter proliferate in response to PDGF. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 14558–14566, doi: 10.1523/JNEUROSCI.2001-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Richardson WD, Pringle N, Mosley MJ, Westermark B & Dubois-Dalcq M A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell 53, 309–319 (1988). [DOI] [PubMed] [Google Scholar]
- 78.Raff MC, Lillien LE, Richardson WD, Burne JF & Noble MD Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature 333, 562–565, doi: 10.1038/333562a0 (1988). [DOI] [PubMed] [Google Scholar]
- 79.Barres BA, Raff MC, Gaese F, Bartke I, Dechant G & Barde YA A crucial role for neurotrophin-3 in oligodendrocyte development. Nature 367, 371–375, doi: 10.1038/367371a0 (1994). [DOI] [PubMed] [Google Scholar]
- 80.D’Souza SD, Alinauskas KA & Antel JP Ciliary neurotrophic factor selectively protects human oligodendrocytes from tumor necrosis factor-mediated injury. Journal of neuroscience research 43, 289–298, doi: (1996). [DOI] [PubMed] [Google Scholar]
- 81.McTigue DM, Horner PJ, Stokes BT & Gage FH Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience 18, 5354–5365 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coelho RP, Yuelling LM, Fuss B & Sato-Bigbee C Neurotrophin-3 targets the translational initiation machinery in oligodendrocytes. Glia 57, 1754–1764, doi: 10.1002/glia.20888 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buono KD, Vadlamuri D, Gan Q & Levison SW Leukemia inhibitory factor is essential for subventricular zone neural stem cell and progenitor homeostasis as revealed by a novel flow cytometric analysis. Developmental neuroscience 34, 449–462, doi: 10.1159/000345155 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL & Fields RD Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832, doi: 10.1016/j.neuron.2006.02.006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyer LC, Paisley CE, Mohamed E, Bigbee JW, Kordula T, Richard H, Lutfy K & Sato-Bigbee C Novel role of the nociceptin system as a regulator of glutamate transporter expression in developing astrocytes. Glia 65, 2003–2023, doi: 10.1002/glia.23210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, Lacaud A, Saliba E, Dammann O, Gallego J, Sizonenko S, Hagberg H, Lelievre V & Gressens P Systemic inflammation disrupts the developmental program of white matter. Annals of neurology 70, 550–565, doi: 10.1002/ana.22489 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Jantzie LL, Maxwell JR, Newville JC, Yellowhair TR, Kitase Y, Madurai N, Ramachandra S, Bakhireva LN, Northington FJ, Gerner G, Tekes A, Milio LA, Brigman JL, Robinson S & Allan A Prenatal opioid exposure: The next neonatal neuroinflammatory disease. Brain Behav Immun 84, 45–58, doi: 10.1016/j.bbi.2019.11.007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chew LJ, Fusar-Poli P & Schmitz T Oligodendroglial alterations and the role of microglia in white matter injury: relevance to schizophrenia. Developmental neuroscience 35, 102–129, doi: 10.1159/000346157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morgan JT, Barger N, Amaral DG & Schumann CM Stereological study of amygdala glial populations in adolescents and adults with autism spectrum disorder. PloS one 9, e110356, doi: 10.1371/journal.pone.0110356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sato-Bigbee C, Pal S & Chu AK Different neuroligands and signal transduction pathways stimulate CREB phosphorylation at specific developmental stages along oligodendrocyte differentiation. Journal of neurochemistry 72, 139–147 (1999). [DOI] [PubMed] [Google Scholar]
- 91.Yuan X, Eisen AM, McBain CJ & Gallo V A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development 125, 2901–2914 (1998). [DOI] [PubMed] [Google Scholar]
- 92.Guo HZ, Enters EK, McDowell KP & Robinson SE The effect of prenatal exposure to methadone on neurotransmitters in neonatal rats. 57, 296–298 (1990). [DOI] [PubMed] [Google Scholar]
- 93.Robinson SE, Guo H & Spencer RF Prenatal exposure to methadone delays the development of striatal cholinergic neurons. Brain research. Developmental brain research 76, 239–248 (1993). [DOI] [PubMed] [Google Scholar]
- 94.Robinson SE, Guo H, Maher JR, McDowell KP & Kunko PM Postnatal methadone exposure does not prevent prenatal methadone-induced changes in striatal cholinergic neurons. Brain research. Developmental brain research 95, 118–121 (1996). [DOI] [PubMed] [Google Scholar]
- 95.Robinson SE, Mo Q, Maher JR, Wallace MJ & Kunko PM Perinatal exposure to methadone affects central cholinergic activity in the weanling rat. Drug and alcohol dependence 41, 119–126 (1996). [DOI] [PubMed] [Google Scholar]
- 96.Robinson SE & Wallace MJ Effect of perinatal buprenorphine exposure on development in the rat. The Journal of pharmacology and experimental therapeutics 298, 797–804 (2001). [PubMed] [Google Scholar]
- 97.Mobley WC, Rutkowski JL, Tennekoon GI, Buchanan K & Johnston MV Choline acetyltransferase activity in striatum of neonatal rats increased by nerve growth factor. Science 229, 284–287 (1985). [DOI] [PubMed] [Google Scholar]
- 98.Lopez PH Role of myelin-associated glycoprotein (siglec-4a) in the nervous system. Advances in neurobiology 9, 245–262, doi: 10.1007/978-1-4939-1154-7_11 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Fleishman R, Zhou C, Gleason C, Larison C, Myaing MT & Mangione-Smith R Standardizing morphine use for ventilated preterm neonates with a nursing-driven comfort protocol. J Perinatol 35, 46–51, doi: 10.1038/jp.2014.131 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Traudt CM, Tkac I, Ennis KM, Sutton LM, Mammel DM & Rao R Postnatal morphine administration alters hippocampal development in rats. J Neurosci Res 90, 307–314, doi: 10.1002/jnr.22750 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schiffer D, Brignolio F, Giordana MT, Mongini T, Migheli A & Palmucci L Spongiform encephalopathy in addicts inhaling pre-heated heroin. Clinical neuropathology 4, 174–180 (1985). [PubMed] [Google Scholar]
- 102.Rizzuto N, Morbin M, Ferrari S, Cavallaro T, Sparaco M, Boso G & Gaetti L Delayed spongiform leukoencephalopathy after heroin abuse. Acta neuropathologica 94, 87–90 (1997). [DOI] [PubMed] [Google Scholar]
- 103.Wolters EC, van Wijngaarden GK, Stam FC, Rengelink H, Lousberg RJ, Schipper ME & Verbeeten B Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet 2, 1233–1237, doi: 10.1016/s0140-6736(82)90101-5 (1982). [DOI] [PubMed] [Google Scholar]
- 104.Weber W, Henkes H, Moller P, Bade K & Kuhne D Toxic spongiform leucoencephalopathy after inhaling heroin vapour. Eur Radiol 8, 749–755, doi: 10.1007/s003300050467 (1998). [DOI] [PubMed] [Google Scholar]
- 105.Chen CY, Lee KW, Lee CC, Chin SC, Chung HW & Zimmerman RA Heroin-induced spongiform leukoencephalopathy: value of diffusion MR imaging. J Comput Assist Tomogr 24, 735–737, doi: 10.1097/00004728-200009000-00013 (2000). [DOI] [PubMed] [Google Scholar]
- 106.Nanan R, von Stockhausen HB, Petersen B, Solymosi L & Warmuth-Metz M Unusual pattern of leukoencephalopathy after morphine sulphate intoxication. Neuroradiology 42, 845–848, doi: 10.1007/s002340000442 (2000). [DOI] [PubMed] [Google Scholar]
- 107.Morales Odia Y, Jinka M & Ziai WC Severe leukoencephalopathy following acute oxycodone intoxication. Neurocrit Care 13, 93–97, doi: 10.1007/s12028-010-9373-y (2010). [DOI] [PubMed] [Google Scholar]
- 108.Kim J, Hyung SW, Seo J, Lee H, Yu HJ & Sunwoo MK Delayed Post-Hypoxic Leukoencephalopathy Caused by Fentanyl Intoxication in a Healthy Woman. Dement Neurocogn Disord 19, 170–172, doi: 10.12779/dnd.2020.19.4.170 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Foy L, Seeyave DM & Bradin SA Toxic leukoencephalopathy due to transdermal fentanyl overdose. Pediatr Emerg Care 27, 854–856, doi: 10.1097/PEC.0b013e31822c281f (2011). [DOI] [PubMed] [Google Scholar]
- 110.Schiller RM, Allegaert K, Hunfeld M, van den Bosch GE, van den Anker J & Tibboel D Analgesics and Sedatives in Critically Ill Newborns and Infants: The Impact on Long-Term Neurodevelopment. J Clin Pharmacol 58 Suppl 10, S140–S150, doi: 10.1002/jcph.1139 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Avey D, Sankararaman S, Yim AKY, Barve R, Milbrandt J & Mitra RD Single-Cell RNA-Seq Uncovers a Robust Transcriptional Response to Morphine by Glia. Cell Rep 24, 3619–3629 e3614, doi: 10.1016/j.celrep.2018.08.080 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin W & Popko B Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci 12, 379–385, doi: 10.1038/nn.2273 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu S, Stone S, Nave KA & Lin W The Integrated UPR and ERAD in Oligodendrocytes Maintain Myelin Thickness in Adults by Regulating Myelin Protein Translation. J Neurosci 40, 8214–8232, doi: 10.1523/JNEUROSCI.0604-20.2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Unick GJ, Rosenblum D, Mars S & Ciccarone D Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993–2009. PloS one 8, e54496, doi: 10.1371/journal.pone.0054496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calcaterra S, Glanz J & Binswanger IA National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug and alcohol dependence 131, 263–270, doi: 10.1016/j.drugalcdep.2012.11.018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Compton WM & Volkow ND Major increases in opioid analgesic abuse in the United States: concerns and strategies. 81, 103–107, doi: 10.1016/j.drugalcdep.2005.05.009 (2006). [DOI] [PubMed] [Google Scholar]
- 117.Voon P & Kerr T “Nonmedical” prescription opioid use in North America: a call for priority action. Subst Abuse Treat Prev Policy 8, 39, doi: 10.1186/1747-597X-8-39 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB & Lim KO A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 51, 874–887 (1994). [DOI] [PubMed] [Google Scholar]
- 119.Qiu D, Tan LH, Zhou K & Khong PL Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage 41, 223–232, doi: 10.1016/j.neuroimage.2008.02.023 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC & Reiss AL White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex 15, 1848–1854, doi: 10.1093/cercor/bhi062 (2005). [DOI] [PubMed] [Google Scholar]
- 121.Fuster JM The prefrontal cortex--an update: time is of the essence. Neuron 30, 319–333 (2001). [DOI] [PubMed] [Google Scholar]
- 122.Miller EK & Cohen JD An integrative theory of prefrontal cortex function. 24, 167–202, doi:Doi 10.1146/Annurev.Neuro.24.1.167 (2001). [DOI] [PubMed] [Google Scholar]
- 123.Wood JN & Grafman J Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci 4, 139–147, doi: 10.1038/nrn1033 (2003). [DOI] [PubMed] [Google Scholar]
- 124.Miller EK The prefrontal cortex and cognitive control. Nat Rev Neurosci 1, 59–65, doi: 10.1038/35036228 (2000). [DOI] [PubMed] [Google Scholar]
- 125.Brambilla P, Bellani M, Yeh PH, Soares JC & Tansella M White matter connectivity in bipolar disorder. Int Rev Psychiatry 21, 380–386, doi: 10.1080/09540260902962172 (2009). [DOI] [PubMed] [Google Scholar]
- 126.Mahon K, Burdick KE & Szeszko PR A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev 34, 533–554, doi: 10.1016/j.neubiorev.2009.10.012 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roussos P & Haroutunian V Schizophrenia: susceptibility genes and oligodendroglial and myelin related abnormalities. Front Cell Neurosci 8, 5, doi: 10.3389/fncel.2014.00005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goldstein RZ & Volkow ND Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12, 652–669, doi: 10.1038/nrn3119 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Deoni SC, O’Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N, Dirks H, Piryatinsky I, Dean DC 3rd & Jumbe NL White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct 221, 1189–1203, doi: 10.1007/s00429-014-0947-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Monnelly VJ, Anblagan D, Quigley A, Cabez MB, Cooper ES, Mactier H, Semple SI, Bastin ME & Boardman JP Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin 18, 9–14, doi: 10.1016/j.nicl.2017.12.033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nygaard E, Slinning K, Moe V, Due-Tonnessen P, Fjell A & Walhovd KB Neuroanatomical characteristics of youths with prenatal opioid and poly-drug exposure. Neurotoxicol Teratol 68, 13–26, doi: 10.1016/j.ntt.2018.04.004 (2018). [DOI] [PubMed] [Google Scholar]


