Abstract
Many persons infected with HIV-1 (PWH) and opioid-dependent individuals experience deficits in sociability that interfere with daily living. Sociability is regulated by the prefrontal cortico-hippocampal-amygdalar circuit. Within this circuit HIV-1 trans-activator of transcription (HIV-1 Tat) and opioids can increase dendritic pathology and alter neuronal firing. Changes in sociability are also associated with dysregulation of hypothalamic neuropeptides such as oxytocin or corticotropin releasing factor (CRF) in the prefrontal cortico-hippocampal-amygdalar circuit. Accordingly, we hypothesized that the interaction of Tat and morphine would impair inter-male social interactions and disrupt oxytocin and CRF within the PFC and associated circuitry. Male mice were exposed to HIV-1 Tat for 8 weeks and administered saline or escalating doses of morphine twice daily (s.c.) during the last 2 weeks of HIV-1 Tat exposure. HIV-Tat attenuated aggressive interactions with an unknown intruder, whereas morphine decreased both non-aggressive and aggressive social interactions in the resident-intruder test. However, there was no effect of Tat or morphine on non-reciprocal interactions in the social interaction and novelty tests. Tat, but not morphine, decreased oxytocin levels in the PFC and amygdala, whereas both Tat and morphine decreased the percentage of oxytocin-immunoreactive neurons in the hypothalamic paraventricular nucleus (PVN). In Tat(+) or morphine-exposed mice, regional levels of CRF and oxytocin correlated with alterations in behavior in the social interaction and novelty tests. Overall, decreased expression of oxytocin in the prefrontal cortico-hippocampal-amygdalar circuit is associated with morphine- and HIV-Tat-induced deficits in social behavior.
Keywords: Social interaction, social anxiety, aggression, resident-intruder, corticotropin releasing factor, HIV-associated neurocognitive disorders, opiates, opioids, hippocampus, paraventricular nucleus of the hypothalamus
1. Introduction
Social cognition — the process of perceiving, processing, and responding to dynamically changing social interactions is an under-recognized neurocognitive domain (Sachdev et al., 2014) that is impaired in persons infected with HIV-1 (PWH) (Homer et al., 2013). Deficits in recognizing facial emotions in others are exhibited in PWH (Gonzalez-Baeza et al., 2014; Grabyan et al., 2018) and are correlated with a decreased ability to communicate socially (Grabyan et al., 2018). In particular, PWH have difficulty recognizing negative facial emotions (e.g., fear, sadness, anger) (Baldonero et al., 2013; Clark et al., 2010; Clark et al., 2015; Gonzalez-Baeza et al., 2014), correlating with decreased memory (Baldonero et al., 2013) and anterior cingulate cortex volume (Clark et al., 2015). Furthermore, PWH with cognitive impairments exhibit decreased quality of life, including deficits in social functioning (Tozzi et al., 2003), and higher levels of social anxiety compared to the seronegative population (Brandt et al., 2017). In PWH, psychological variables such as social functioning and anxiety are stronger predictors of successful cognitive aging than HIV disease severity or treatment (Malaspina et al., 2011; Moore et al., 2014).
Substance use disorders, including opioid dependence, increase the likelihood that PWH will also experience difficulties engaging in mental and physical activities of daily living (Christensen et al., 2017). Opioid abuse exacerbates neuropsychological deficits and central nervous system (CNS) damage in PWH, including patients maintained on combination antiretroviral therapy (cART) (Anthony et al., 2005; Bell et al., 1998; Byrd et al., 2012; Byrd et al., 2011; Paydary et al., 2016). Opioid dependence in seronegative individuals is also associated with social anxiety (Shand et al., 2010) and impairments in recognizing facial emotions in others (Kornreich et al., 2003). Patients on opioid maintenance therapy have smaller gray matter volume in multiple frontal cortex areas, including the insula, which is correlated with increased social anxiety and feelings of social rejection (Bach et al., 2019). In both diseases, there is a complex interaction between feedback responses to social rejection from others and changes in social behavior and emotionality (Frischknecht et al., 2011; Smith et al., 2008). PWH on methadone maintenance are more likely to reinitiate heroin-taking and have social anxiety than non-infected patients (Applebaum et al., 2010), and PWH that use intravenous drugs are more likely to have increased anxiety and depression (Korthuis et al., 2008).
Endocrine dysregulation may play a role in opioid- and HIV-induced social anxiety and decreased social cognition. The neuropeptide oxytocin is highly conserved across animal species, synthesized in the paraventricular (PVN) and supraoptic (SON) nuclei of the mammalian hypothalamus, and plays an important role in social cognition and anxiety, as well as in parturition. In mammals, oxytocinergic axon terminals and oxytocin receptors are present in the prefrontal cortico-hippocampal-amygdalar circuit and other regions associated with social bonding, social reward, social recognition, and aggression (Marlin and Froemke, 2017). Oxytocin administration increases sociability in human patients (Hollander et al., 2007; Pedersen et al., 2011) and in preclinical rodent models (Ferguson et al., 2001; Lee et al., 2005; Teng et al., 2013) of autism, schizophrenia, and social anxiety disorder. In HIV+ women, oxytocin moderates the interactions among stress and CD4+ cell count, and social support and quality of sleep (Fekete et al., 2011; Fekete etal., 2014). Further, patients with AIDS, Huntington’s disease, or Parkinson’s disease exhibit decreased expression of hypothalamic oxytocin (Purba et al., 1993; Purba et al., 1994; Vercruysse et al., 2018). Chronic exposure to opioids in humans and rodents decreases oxytocin synthesis and release (Vuong et al., 2010).
Corticotropin releasing factor (CRF) is a neuropeptide, synthesized in the PVN of the hypothalamus and released in brain regions associated with stress and sociability, including the PFC, amygdala, and hippocampus (Backstrom and Winberg, 2013; Hostetler and Ryabinin, 2013). CRF mRNA expression is increased in the PVN of depressed patients and acutely socially isolated adult prairie voles — although hypothalamic CRF mRNA expression is not altered in adult male prairie voles isolated for 4 weeks (Grippo et al., 2007; Pournajafi-Nazarloo et al., 2011; Wang et al., 2008). CRF administered into the CNS of rodents decreases social interactions (Bagosi et al., 2017; Elkabir et al., 1990; Mele et al., 1987). Impairments in the hypothalamic pituitary adrenal (HPA) axis of PWH include increased basal plasma cortisol levels despite cART treatment (Bons et al., 2013), decreased HPA response to CRF (Biglino et al., 1995), and glucocorticoid resistance (Norbiato et al., 1997; Norbiato et al., 1994). Opioid abuse blunts the HPA axis response to CRF, adrenocorticotropic hormone, cortisol, and psychosocial stressors (Fountas et al., 2018).
The viral protein, HIV-trans-activator of transcription (HIV-Tat), is present in cerebrospinal fluid of cART treated patients (Henderson et al., 2019; Johnson et al., 2013). HIV-Tat is secreted by macrophages, microglia, and latently-infected astroglia within the CNS (Chopard et al., 2018; Debaisieux et al., 2012; Rayne et al., 2010), and induces neuroinflammation (El-Hage et al., 2008; El-Hage et al., 2005; Fitting et al., 2010b). HIV-Tat disrupts the morphology and function of bystander neurons (Chopard et al., 2018; Hategan et al., 2017), and this can be exacerbated by co-exposure to opiates (Fitting et al., 2014; Kim et al., 2018). These data suggest that Tat could be a major contributor to the social deficits and endocrine dysfunction seen in PWH.
Tat transgenic (Tat-tg) mice conditionally express Tat mRNA under the glial fibrillary acidic protein (GFAP) promoter, and exhibit CNS neuroinflammation, gliosis, morphological and functional changes in neurons (Bruce-Keller et al., 2008; Cirino et al., 2020; Fitting et al., 2010a; Gonek et al., 2018), and many of the neurocognitive deficits seen in HIV-associated neurocognitive disorder (HAND) (Fitting et al., 2013; Hahn et al., 2016; Hahn et al., 2015; Kesby et al., 2018; McLaughlin et al., 2017; Paris et al., 2014; Paris et al., 2016). The Tat-tg line used in the present study resembles a slower onset, chronic pathology typically exhibited by PWH (Dickens et al., 2017) that is worsened by opiate administration (Fitting et al., 2010a; Gonek et al., 2018; Salahuddin et al., 2020a; Salahuddin et al., 2020b). The effects of Tat on the oxytocin system are unknown. However, Tat decreases the level of the glucocorticoid precursor deoxycorticosterone in the brain (Paris et al., 2020), increases plasma corticosterone (Salahuddin et al., 2020a; Salahuddin et al., 2020b), induces glucocorticoid splenocyte resistance in male mice (Paris et al., 2020), and increases hypothalamic CRF levels in proestrus females (Salahuddin et al., 2020b), suggesting that Tat induces endocrine dysfunction similar to HIV-1 infection.
The objective of the present study was to investigate the interaction of long-term HIV-Tat exposure and repeated morphine administration on inter-male sociability, and changes in associated neuroendocrine and stress factors in the prefrontal cortico-hippocampal-amygdalar circuit that might underlie those observations. Correlation analyses were used to assess the role that CNS expression of oxytocin and CRF might play in Tat- or morphine-induced social interactions. We hypothesized that alterations in oxytocin and CRF expression in one or more components of prefrontal cortico-hippocampal-amygdalar circuitry coincide with Tat- and/or morphine-induced impaired sociability.
2. Materials and methods
The use of mice in these studies was approved by the Virginia Commonwealth University Animal Care and Use Committee and all experiments were conducted in accordance with the National Institutes of Health (NIH Publication No. 85–23) ethical guidelines.
2.1. Subjects and treatment
Adult (3-5-months-old) male doxycycline (DOX)-inducible HIV-Tat-tg mice (n = 79) expressing HIV-1IIIB Tat1-86 controlled by an rtTA-driven, GFAP promoter were generated in the vivarium of Virginia Commonwealth University as previously described (Bruce-Keller et al., 2008; Nass et al., 2020). Doxycycline (DOX)-inducible Tat-tg mice (Bruce-Keller et al., 2008; Fitting et al., 2010a) are a well characterized model of neuroHIV and express Tat mRNA and/or protein within 48 h of DOX administration throughout the CNS (e.g. cortex, hippocampus, striatum, and spinal cord) (Bruce-Keller et al., 2008; Carey et al., 2012; Dickens et al., 2017; Fitting et al., 2012; Fitting et al., 2010a). Mice were housed 3-4 per cage in a temperature- and humidity-controlled, AAALAC-accredited facility, with ad libitum access to food and water, on a 12:12 light:dark cycle. To establish territorial behavior for the social aggression tests (resident-intruder and social dominance tube tests) a cohort of mice (n = 39) were single-housed for 3 weeks before behavioral testing (Fig. 1) (Koolhaas et al., 2013; Miczek et al., 2001).
Fig. 1. Experimental design depicted on a timeline.
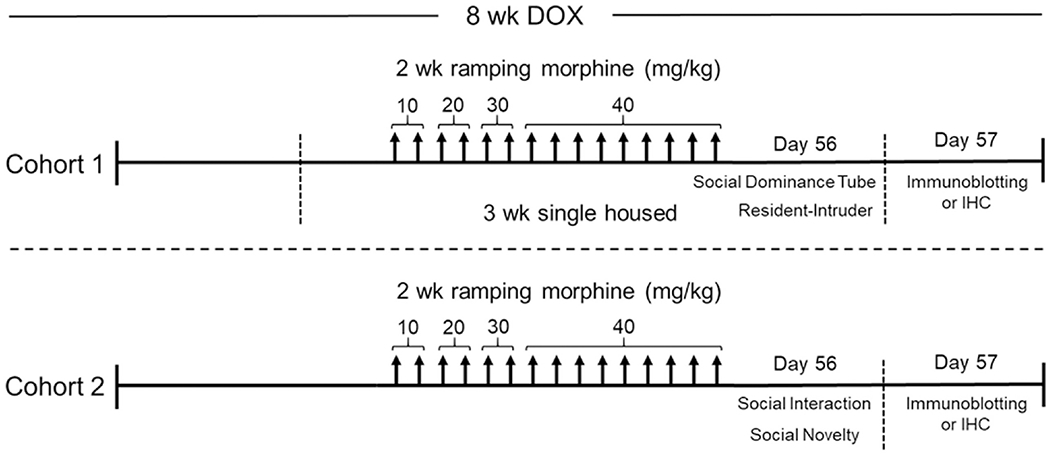
All Tat transgenic mice received DOX-containing chow for 8 weeks and were repeatedly injected with morphine (10 – 40 mg/kg, increasing by 10 mg/kg/2 day, s.c., b.i.d.) or saline for the last 2 weeks. Mice in cohort 1 were single housed starting at week 5 of DOX administration to establish territorial behavior. Then on day 14 of repeated morphine injections cohort 1 mice were tested in assays of aggressive sociability, the social dominance tube and resident-intruder tests. On day 14 of repeated morphine injections mice in cohort 2 were tested in the novel environment non-reciprocal social interaction and social novelty assays. The day following behavioral testing, mice in both cohorts were euthanized and tissues were randomly assigned to immunoblotting and ELISA or immunohistochemistry assays.
2.2. HIV-1 Tat induction and morphine treatment
To induce the expression of HIV-1 Tat (or control for off target effects), Tat(+) and Tat(−) mice were fed a standard chow supplemented with DOX (6 g/kg, Harlan Laboratories Madison, WI) for 8 weeks. Starting at 6 weeks of DOX administration mice were also administered escalating doses of morphine (10 – 40 mg/kg, increasing by 10 mg/kg/2 day, s.c.; National Institute on Drug Abuse, Bethesda, MD) twice daily (b.i.d.) or saline vehicle (Fig. 1). Morphine was prepared in sterile saline and all solutions were administered at room temperature at a volume of 10 μl/g body weight. Previously published papers show that the DOX chow induces tat gene, mRNA and/or protein expression throughout the CNS in our model (e.g. cortex, hippocampus, striatum, hypothalamus, and spinal cord) (Bruce-Keller et al., 2008; Dickens et al., 2017; Duncan et al., 2008; Fitting et al., 2012; Fitting et al., 2010a) and in GFAP-expressing glia in the peripheral (Wodarski et al., 2018) and enteric (Ngwainmbi et al., 2014) nervous systems. Although Tat expression levels between morphine and saline administered mice have not been compared, based on the differences in mechanism of action and metabolism of DOX and morphine (De Gregori et al., 2012; Saivin and Houin, 1988) they do not appear to interact. Dox effects appear to be cumulative and tat expression appears to peak after about 2 weeks and is stable thereafter (Fitting et al., 2012) suggesting that any morphine-induced alterations in feeding behavior in the last 2 weeks of the 8 weeks of DOX administration should not interfere with Tat efficacy. Further, DOX does not alter the efficacy of morphine in the tail immersion and hot plate tests (Fitting et al., 2012; Fitting et al., 2016).
2.2. Behavioral assays
After 8 weeks of Tat exposure and 2 weeks of escalated morphine administration, mice were tested in multiple assays of social interaction. On testing day, mice were habituated to the testing room and received morphine (40 mg/kg, s.c.) or saline at least 4 h prior to behavioral testing, to decrease the confound of morphine-induced hypermobility (Babbini and Davis, 1972; Hecht and Schiorring, 1979). To minimize animal numbers, mice were subjected to two behavioral assays, either: (1) reciprocal social interaction, social dominance tube and resident-intruder, or (2) non-reciprocal social interaction and social novelty (Fig. 1). Testing was conducted in ascending order of presumed stress to reduce carry-over effects. Experimenters were blinded to treatment conditions throughout. The day after behavioral testing, mice were humanely euthanized, and brain tissues and serum were collected to assess neuropeptide levels in discrete regions of the prefrontal cortico-hippocampal-amygdalar circuitry and correlate alterations with behavioral changes. To avoid potential confounding effects of housing or prior behavioral test experiences, mice from each behavioral cohort were divided and randomly assigned to immunoblotting or immunohistochemistry experiments.
2.2.1. Social Dominance Tube Test
Mice were tested in assays of reciprocal social aggression, the social dominance tube, and resident-intruder tests, after being single-housed for 3 weeks to establish territorial behavior (Koolhaas et al., 2013; Miczek et al., 2001; Nishimura et al., 2004). The intruder mice in both the social dominance tube and resident intruder tests were group-housed male C57BL/6 mice that were weight-matched with test mice and allowed to freely interact with the test mouse. Older, group-housed mice were used as intruders to increase the likelihood that test mice would be aggressive, and not socially defeated during testing, which can increase serum corticosterone levels and induced glucocorticoid insensitivity (Avitsur et al., 2001; Miczek et al., 2001). Separate, comparable group-housed mouse cohorts were used in the social dominance tube test and were treated uniformly across assays. In the social dominance tube test, Tat(+) and Tat(−) mice entered the opening of a tube (28.5 cm long, 3 cm diameter) with a diameter large enough for only one mouse to pass. At the same time, a C57BL/6 mouse entered the opposite end of the tube. Mice could interact in the tube for up to 5 min. A mouse was declared dominant and the test was ended when it’s opponent retreated out of the tube on the same side it entered, indicating the dominant mouse forced the non-dominant mouse out of the tube (Nishimura et al., 2004).
2.2.2. Resident-Intruder Test
Mice were tested in the resident-intruder assay as previously described (Koolhaas et al., 2013). Briefly, an intruder mouse was placed in the home cage of a test mouse for 10 min and the social interactions initiated by the test mouse were video recorded by ANY-maze software (Stoelting Co., Wood Dale, IL, USA). A blinded experimenter used ANY-maze software to record non-aggressive (i.e., following, touching, sniffing, investigating) and aggressive (i.e., attacking, keep down, rearing, biting) social interactions initiated by the test mouse.
2.2.3. Non-Reciprocal Social Interaction Test
A separate group of mice were tested in assays of non-reciprocal social interaction with unfamiliar, same-sex conspecifics that were restrained from interacting with the test mouse as previously described (Morris et al., 2016; Moy et al., 2004; Nass et al., 2020). The unfamiliar, non-test mouse was placed underneath a mesh cup (~8 cm diameter) to allow the test mouse to smell, see, hear, approach, and interact with the non-test mouse without the non-test mouse being able to reciprocate the approach or interaction. Mice were individually habituated to a Plexiglas test chamber (40 × 40 × 35 cm; Stoelting Co.) for 10 min with 2 mesh cups (~8 cm diameter) at opposite ends of the chamber. Mice were removed and then immediately placed back in the chamber with an unfamiliar mouse that was restrained under one of the mesh cups for 5 min. Percentage of time spent interacting with the mesh cup with the mouse underneath versus the empty cup was video-recorded and coded by a blinded experimenter using ANY-maze software (Stoelting Co.). Distance traveled was also digitally recorded and coded by ANY-maze software (Stoelting Co.).
2.2.4. Non-Reciprocal Social Novelty Test
The social novelty test immediately followed the social interaction test. Test mice were removed from the chamber after social interaction testing and a novel unfamiliar mouse was restrained under the previously empty cup. The test mice were promptly placed back into the chamber for 5 min (Moy et al., 2004). ANY-maze software (Stoelting Co.) was used to digitally record and code distance traveled, and to allow a blinded experimenter to video-record and code the percentage of time spent interacting with the mesh cup with the novel unfamiliar mouse underneath versus the cup with the previously unfamiliar mouse.
2.3. Oxytocin and CRF Assessments
2.3.1. Immunoblotting
Immunoblotting was performed as previously described (Fitting et al., 2013; Nass et al., 2020) to assess the oxytocin and CRF levels in the PFC, hippocampus, and amygdala of Tat(+) and Tat(−) mice with or without morphine. The day after behavioral testing, PFC, hippocampus, and amygdala tissue were grossly dissected from whole brains, snap-frozen in liquid nitrogen, and stored at −80 °C until assay. Tissue samples were homogenized in Pierce IP lysis buffer (Thermo Fisher Scientific, Waltham, MA), containing Halt phosphatase inhibitor cocktail (Thermo Fisher Scientific). The protein concentration in each tissue lysate was quantified using the bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL). Lysates were boiled in 4× Laemmli buffer for 5 min, 40 μg per well were loaded into 4-20% Criterion TGX Stain-Free gels (Bio-Rad, Hercules, CA), and transferred to Immuno-Blot PVDF membranes (Bio-Rad). To probe the blots, antibodies to oxytocin (rabbit monoclonal ab212193, Abeam, Cambridge, MA; 1:1000) and CRF (rabbit monoclonal ab184238, Abeam; 1:1000) were used. Membranes were incubated with HRP-conjugated secondary antibodies (Southern Biotech, Birmingham, AL; 1:10000) and Alexa 488 (goat-anti-rabbit, Invitrogen, Carlsbad, CA; 1:500), respectively, visualized on a ChemiDoc MD imaging system, and analyzed using Image Lab 6.0.1 (Bio-Rad). Protein levels were normalized to GAPDH (mouse 6C5 ab8245, Abeam; 1:2500).
2.3.2. Histological processing
After 8 weeks of DOX and 2 weeks of morphine, mice were deeply anesthetized with isoflurane (Baxter, Deerfield, IL, USA) and perfused with 4 % paraformaldehyde (PFA, pH 7.4; Sigma-Aldrich Co., St. Louis, MO) in phosphate-buffered saline (PBS). Brains were immediately removed and post-fixed in fresh PFA overnight at 4 °C, washed in PBS, incubated in 10% sucrose followed by 20% sucrose for at least 24 h. Brains were then embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA), and stored at −80 °C. Coronal sections were cut at a thickness of 16 μm on a Leica CM1850 cryostat and mounted on SuperFrost Plus Gold slides (Thermo Fisher Scientific). Slides were dried for 5 min, incubated with citrate-based antigen unmasking solution (pH 6.0; Vector Laboratories, Burlingame, CA) in a rice cooker for 20 min, allowed to cool, rinsed in DI H20, and then rinsed in PBS for 5 min. Sections were incubated for 30 min in permeability solution (0.1% Triton X-100 in PBS) followed by blocking solution (5% normal goat serum in PBS) for 2 h, and primary antibodies against oxytocin (rabbit monoclonal ab184238, Abeam; 1:500), CRF (sheep polyclonal cat. no. NB110-81721, Novus Biologicals Littleton, CO; 1:1000), and NeuN (Mouse monoclonal cat. no. MAB377, Millipore, Burlington, MA; 1:200) diluted in blocking buffer overnight at 4 °C. Sections were rinsed in PBS and solutions containing appropriate, fluorescently labeled secondary antibodies Alexa 594 (donkey anti-sheep, Invitrogen; 1:100), Alexa 488 (goat anti-rabbit, Invitrogen; 1:500), and Alexa 647 (goat anti-mouse, Invitrogen; 1:100) were applied to sections for 1 h at room temperature. Tissue sections were again rinsed in PBS, incubated with Hoechst (Invitrogen; 1:20,000) for 10 min, repeatedly rinsed, and mounted in ProLong Gold Antifade reagent (Invitrogen). The hypothalamic PVN in the tissue sections were imaged using a Keyence VHX-7000 digital microscope at 40× magnification (Keyence, Itasca, IL). Images were stitched together (11x15) using the BZ-X800 Analyzer (Keyence).
2.3.2.1. Stereology
Cavalieri’s principle was used to stereologically assess the volume of the hypothalamic PVN (Gundersen et al., 1988; Mouton, 2002). Starting at a random point, images from histologically processed sections were sampled throughout the hypothalamic PVN based on The Mouse Brain in Stereotaxic Coordinates atlas (3rd edition; Franklin and Paxinos) and Allen Institute Mouse Brain Reference Atlas, Version 3. Non-uniform systematic sampling was used due to the variability in shape across the sagittal plane and small size of the PVN (Dorph-Petersen et al., 2000; Gardi et al., 2008). A standardized grid was randomly laid over PVN images and the number of points that hit the PVN were counted by a blinded experimenter. Then the volume was estimated by multiplying the point sum, average interval, section thickness, and grid point area (Gundersen et al., 1988; Mouton, 2002).
2.3.2.2. Immunohistochemistry
Immunohistochemistry was performed to assess the percentage of neurons, as measured by NeuN immunoreactivity, that were also immunoreactive for oxytocin and/or CRF in the PVN of the hypothalamus. Images from histologically processed sections were exported to Image J and counted by a blinded experimenter using the cell counter plugin software. At least 200 neurons were counted per animal in corresponding coronal planes based on The Mouse Brain in Stereotaxic Coordinates atlas (3rd edition; Franklin and Paxinos). All cell count data are presented as a percent of the total NeuN-immunoreactive population in the PVN.
2.4. Statistical Analyses
All data met the parametric assumption of homogeneity of variance as measured by Levene’s test. Data were also assessed for parametric assumption of normality of variance by the Kolmogorov-Smirnov test. If the data were not normally distributed, they were analyzed by the non-parametric Kruskal-Wallis test. Data that met the assumptions for parametric analysis were analyzed by two-way analysis of variance (genotype x drug treatment), followed by the Tukey’s HSD post hoc test using Prism version 9.0.0 (GraphPad Software, Inc.). The social dominance tube test behavioral data was analyzed by the Chi-square test for independence (treatment x dominant/subordinate). Pearson’s correlation analyses were performed to assess the relationship between oxytocin or CRF levels and behavior in Tat(+) or morphine treated mice. All data are presented as the mean ± the S.E.M. Differences were considered statistically significant if p < 0.05.
3. Results
3.1. HIV-1 Tat and morphine decreased social interactions in the resident-intruder test
Tat(+) and Tat(−) mice were administered DOX for 8 weeks and escalated morphine (10 – 40 mg/kg, s.c., b.i.d.) or saline for 2 weeks to assess the effects of HIV-1 Tat and repeated morphine injections on aggressive-like behavior in reciprocal social interaction environments. In the social dominance tube test, there was no difference in Tat(+) and Tat(−) mice with or without morphine exposure [X2(1, N = 39) = 0.19, p = 0.66; n.s.; Fig. 2A]. When measuring the total time interacting with the unknown intruder in the resident-intruder test there was a main effect of Tat [F(1,35) = 4.20, p < 0.05; η2= 0.08; Fig. 2B] and morphine [F(1,35) = 12.62, p < 0.01; η2= 0.24; Fig. 2B], but no interaction (p = 0.28) indicating that Tat and morphine independently decreased the total amount of time spent interacting with the intruder mouse. We next broke the data down into the percentage of time engaging in non-aggressive and aggressive interactions with the unknown intruder. The latency to interact in a non-aggressive (p = 0.31; Fig. 2C) or aggressive (p = 0.67 Fig. 2E) manner was not significantly different across groups, as measured by the non-parametric Kruskal-Wallis test. However, morphine (main effect [F(1,35) = 6.25, p < 0.05; η2= 0.15; Fig. 2D]), decreased the percentage of non-aggressive social interactions with no significant interaction (p = 0.57) or main effect of Tat (p = 0.65). Both Tat (main effect [F(1,35) = 9.70, p < 0.01; η2= 0.19; Fig. 2F]) and morphine (main effect [F(1,35) = 5.30, p < 0.05; η2 = 0.10; Fig. 2F]) but not the interaction of Tat and morphine (p = 0.38), decreased the percentage of aggressive interactions with the intruder mouse.
Fig. 2. HIV-1 and morphine decreased aggressive, while morphine decreased non-aggressive interactions in the resident-intruder test.
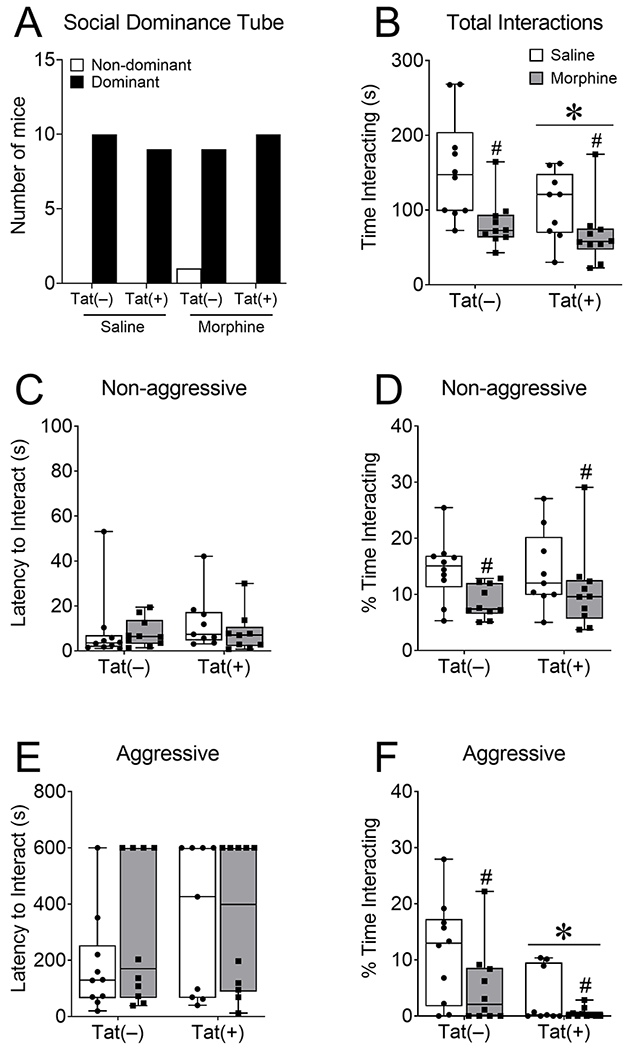
Tat(+) and Tat(−) mice were fed DOX chow for 8 weeks and administered escalated morphine (10 – 40 mg/kg, s.c., b.i.d.) for 2 weeks before being tested in aggressive measures of reciprocal social interaction. Tat and morphine did not affect dominance in the social dominance tube test (A), but exposure to both decreased total time spent interacting with the unfamiliar mouse in the resident-intruder test (B). Tat and morphine did not alter the latency to interact non-aggressively with the unfamiliar mouse in the test mouse’s home cage (C), but morphine decreased the percentage of time spent interacting non-aggressively (D). Tat and morphine also did not alter the latency of the test mouse to interact aggressively with the novel mouse (E), but both Tat and morphine decreased the percentage of time the test mouse interacted aggressively (F). Data are presented as mean ± SEM; n = 9-10 mice per group. Main effect of Tat, *p < 0.05 vs Tat(−) mice. Main effect of morphine, #p < 0.05 vs saline treated mice.
3.2. HIV-1 Tat and morphine did not significantly alter non-reciprocal social interactions in a novel environment
To assess the influence of HIV-1 Tat and repeated morphine on sociability in a non-reciprocal environment, where the non-test mouse was restrained from approaching the test mouse, a separate group of Tat(+) and Tat(−) mice were administered DOX for 8 weeks and escalated morphine (10 – 40 mg/kg, s.c.) or saline for 2 weeks and then tested in the non-reciprocal social interaction and novelty assays in a novel environment. In the social interaction test, neither Tat nor morphine exposure altered the latency to approach the mesh cup with an unfamiliar mouse (p = 0.75; Fig. 3A) or the amount of time the test mice spent with the unfamiliar mouse (p = 0.36; Fig. 3B) as measured by the Kruskal-Wallis test. Distance traveled was also digital coded by ANY-maze software (Stoelting Co.) in the social interaction and novelty tests to assess potential morphine-induced psychomotor confounds. In the social interaction test, there was a significant interaction in the distance traveled [F(1,36) = 6.64, p < 0.05; η2 = 0.15; Fig. 3C], but no main effect of Tat (p = 0.24) or morphine (p = 0.67). Post hoc tests reveal a non-significant increase in distance traveled in Tat(+)/saline mice compared to Tat(−)/saline (p = 0.054; d = 1.47) mice.
Fig. 3. HIV-1 Tat and morphine did not alter sociability in the non-reciprocal social interaction or social novelty tests.
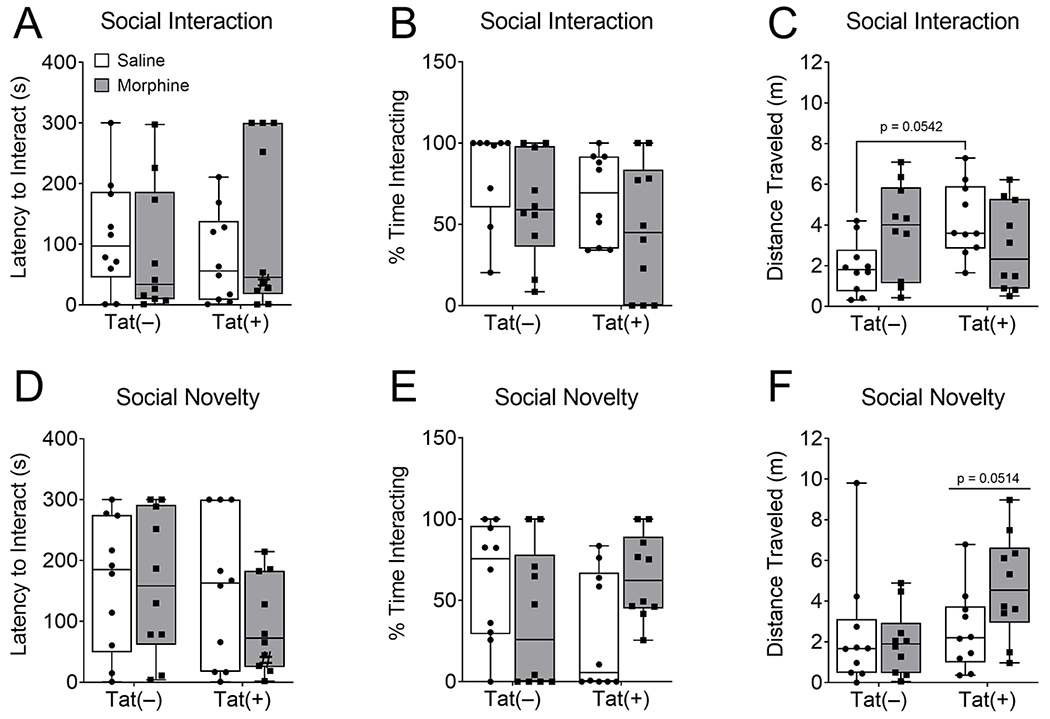
Tat exposure for 8 weeks and escalated morphine (10 – 40 mg/kg, s.c., b.i.d.) administration for 2 weeks did not significantly affect the latency to interact (A) or percentage of time spent interacting (B) with the unfamiliar mouse, or distance traveled (C) in the social interaction test performed in a novel environment. Similarly, in the social novelty test Tat and morphine did not affect the latency to interact (D) or percentage of time spent interacting with the novel unfamiliar mouse (E), or distance traveled (F). Data are presented as mean ± SEM; n = 9-10 mice per group.
In the social novelty test, when the empty cup was replaced by a novel unfamiliar mouse, HIV-1 Tat, morphine, or the interaction did not change the latency of the test mouse to approach the mesh cup with the novel unfamiliar mouse (p = 0.25, p = 0.42, p = 0.42, respectively; Fig. 3D). Similarly, Tat and morphine did not alter the amount of time spent with the novel mouse (p = 0.11; Fig. 3E) as measured by the Kruskal-Wallis test. Tat tended to increase distance traveled (main effect [F(1,36) = 4.06, p = 0.0514; η2= 0.09; Fig. 3F]), but there was no significant effect of morphine (p = 0.23) or the interaction of both (p = 0.09) during the social novelty test.
3.3. HIV-1 Tat, but not morphine, decreased oxytocin levels within discrete regions of the prefrontal cortico-hippocampal-amygdalar circuit
We first assessed oxytocin levels in the PFC, hippocampus, and amygdala as a possible underlying neural substrate of the Tat- and morphine-induced decreases in aggressive social interactions in the resident-intruder test. Oxytocin is released centrally and peripherally in response to stress (Babygirija et al., 2012) and is important in different types of social behaviors (Duarte-Guterman et al., 2020; Lim and Young, 2006; Resendez et al., 2020). Western blots showed that Tat reduced the levels of oxytocin in the PFC (main effect [F(119) = 7.99, p < 0.05; η2 = 0.29; Fig. 4A]) and amygdala (main effect [F(1,18) = 4.62, p < 0.05; η2= 0.19; Fig. 4B]). However, morphine or the interaction of Tat and morphine did not alter oxytocin levels in the PFC (p = 0.64, p = 0.50, respectively) or amygdala (p = 0.93, p = 0.21, respectively). There was no effect of Tat, morphine, or their interaction on hippocampal oxytocin levels (p = 0.38, p = 0.93, p = 0.54, respectively; Fig. 4C).
Fig. 4. HIV-1 Tat, but not morphine decreased PFC and amygdalar, but not hippocampal oxytocin levels.
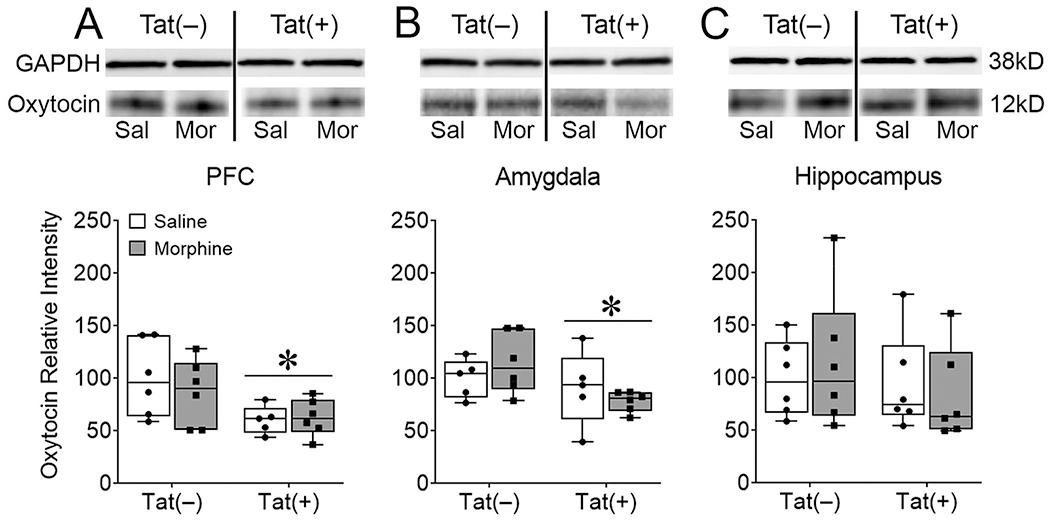
After 8 weeks of Tat, 2 weeks of morphine exposure, and behavioral testing in assays of sociability Tat(+) mice, regardless of morphine exposure, exhibited lower expression of oxytocin in the PFC (A) and amygdala (B), but not the hippocampus (C) as measured by western blotting. Representative blots show decreased oxytocin in Tat(+) compared to Tat(−) mice in the PFC (A, top) and amygdala (B, top). All oxytocin western blots are represented as relative intensity to GAPDH normalized to Tat(−)/Saline. Data are presented as mean ± SEM; n = 9-10 mice per group. Main effect of Tat, *p < 0.05 vs Tat(−) mice.
3.4. HIV-1 Tat and morphine do not alter CRF levels within the prefrontal cortico-hippocampal-amygdalar circuit or serum corticosterone
Previous research indicates HIV-1 Tat increases anxiety-like behavior (Hahn et al., 2016; Hahn et al., 2015; Paris et al., 2014; Paris et al., 2016), and Tat and morphine alter the HPA axis (Paris et al., 2020; Salahuddin et al., 2020a; Salahuddin et al., 2020b). Therefore, we investigated CRF levels in the PFC, hippocampus, and amygdala and circulating serum corticosterone. Western blots showed that Tat, morphine, and the interaction did not alter CRF levels in the PFC (p = 0.96, p = 0.96, p = 0.96, respectively; Fig. 5A), amygdala (p = 0.93, p = 0.47, p = 0.26, respectively; Fig. 5B), or hippocampus (p = 0.63, p = 0.37, p = 0.84, respectively; Fig. 5C). Similarly, serum corticosterone levels were not affected by Tat, morphine, nor the interaction (p = 0.65, p = 0.90, p = 0.88, respectively; Fig. 5D).
Fig. 5. HIV-1 Tat and morphine did not alter serum corticosterone or PFC, hippocampus, or amygdala corticotropin releasing factor (CRF) expression.
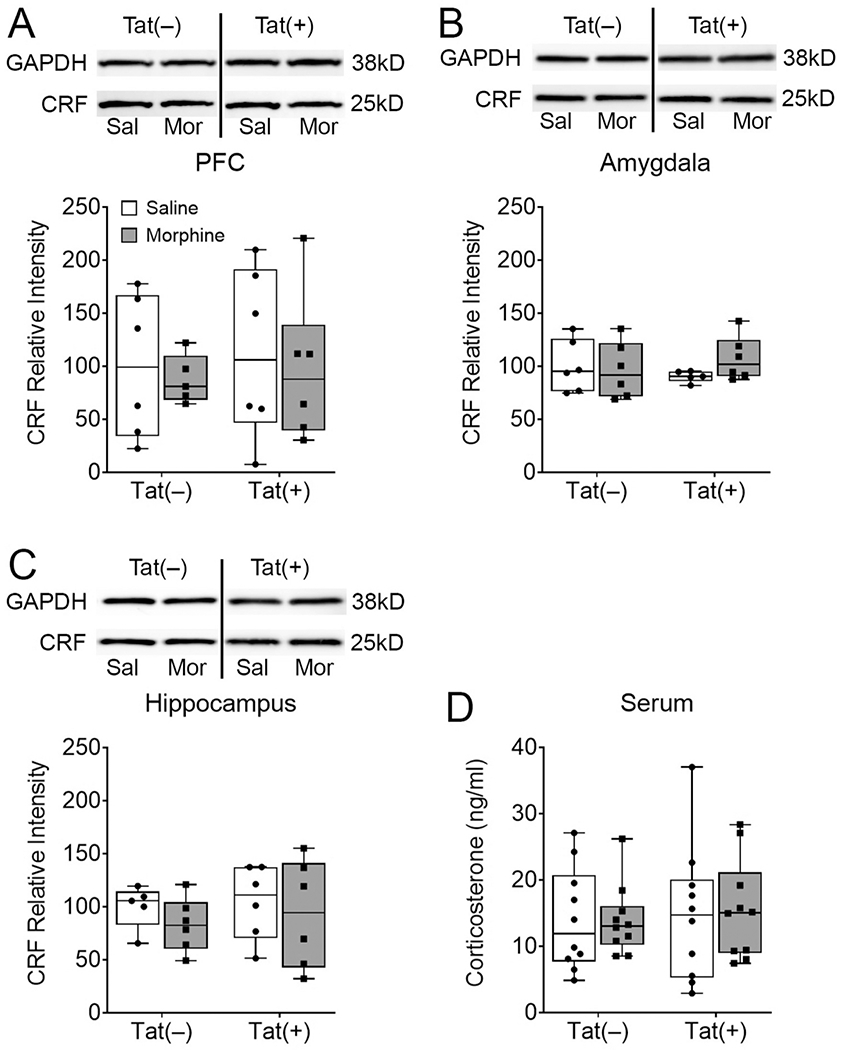
Mice that were behaviorally tested in assays of sociability after 8 weeks of Tat and 2 weeks of morphine exposure did not exhibit altered levels of CRF in the PFC (A), amygdala (B), or hippocampus (C) of behaviorally-tested mice as measured by western blotting. Tat and morphine also did not alter serum corticosterone levels (D). All CRF western blots are represented as relative intensity to GAPDH normalized to Tat(−)/Saline. Data are presented as mean ± SEM; n = 9-10 mice per group.
3.5. HIV-1 Tat and morphine decrease the percentage of oxytocin, but not CRF-expressing neurons in the hypothalamic paraventricular nucleus (PVN)
Since oxytocin and CRF are synthesized in the PVN of the hypothalamus, we assessed the nuclei volume and the vulnerability of oxytocin and CRF neurons in PVN sections immunolabeled for oxytocin, CRF, and NeuN (Fig. 6-7). Point grid stereology assessment showed HIV-1 Tat (p = 0.90), morphine (p = 0.85), nor the interaction (p = 0.27) altered the volume of the PVN (Fig. 6A-E). The quantification of PVN neuron subtypes showed Tat (main effect [F(1,17) = 4.81, p < 0.05; η2= 0.15]) and morphine (main effect [F(1,17) = 7.35, p < 0.05; η2 = 0.23]), decreased the percentage of oxytocin-immunoreactive neurons (Fig. 6A-D, F), without a significant interaction (p = 0.07). However, neither Tat, morphine, nor the interaction affected the percentage of CRF-positive cells (p = 0.23, p = 0.16, p = 0.56, respectively Fig. 6A-D, G). Similar to previous reports, the percentage of NeuN positive cells co-expressing oxytocin and CRF was low (Dabrowska et al., 2013). Further, neither Tat, morphine, nor the interaction altered the percentage of CRF- and oxytocin-positive colocalized neurons (p = 0.85, p = 0.43, p = 0.53, respectively Fig. 6A-D, H).
Fig. 6. HIV-1 Tat and morphine decrease hypothalamic paraventricular nucleus (PVN) oxytocin expression.
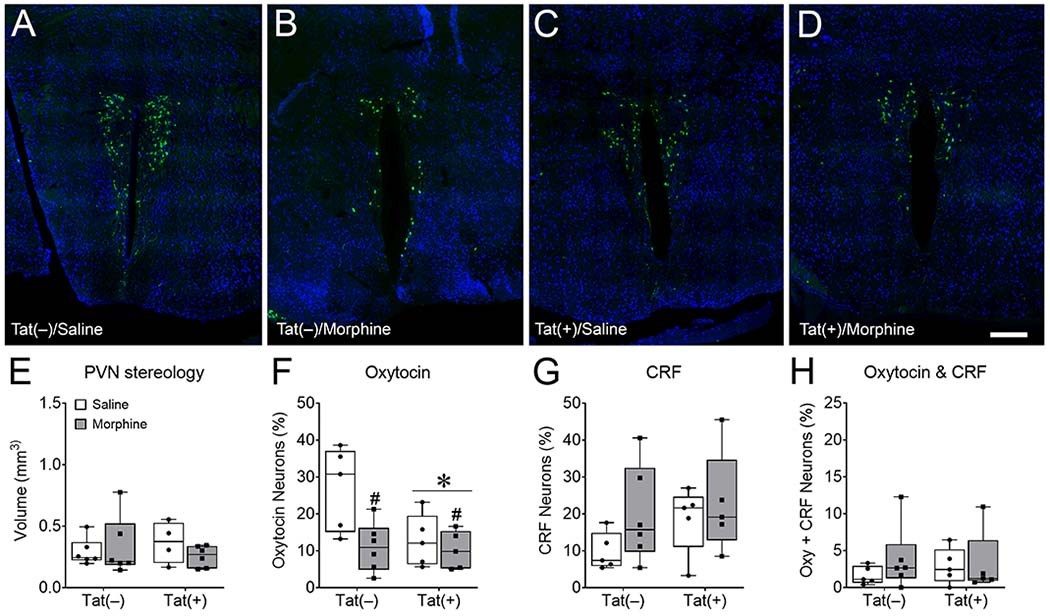
Representative images of cells immunoreactive for both oxytocin (green) and the neuronal marker NeuN (blue) in the PVN (A-D) imaged with Keyence VHX-7000 digital microscope at 40× magnification and stitched together. Mice exposed to Tat for 8 weeks, administered 2 weeks of morphine, and tested in social interaction assays did not demonstrate changes in hypothalamic PVN volume (E). Tat and morphine decreased oxytocin- (F), but not corticotropin releasing factor (CRF)- (G) or oxytocin- and CRF-immunoreactive colocalized (H) neurons in the PVN of the hypothalamus. These data suggest that oxytocin-expressing neurons in the PVN are selectively vulnerable to morphine and Tat, but do not show if this vulnerability is due to changes in the metabolism or release of oxytocin, or the loss of the oxytocin-expressing neuron subpopulation. Data are presented as mean ± SEM; n = 4-6 mice per group. Main effect of Tat, *p < 0.05 vs Tat(−) mice. Main effect of morphine, #p < 0.05 vs saline treated mice. Scale bar = 200 μm.
Fig. 7. Cellular localization of oxytocin, corticotropin releasing factor (CRF), and NeuN immunoreactivity in the hypothalamic paraventricular nucleus (PVN).
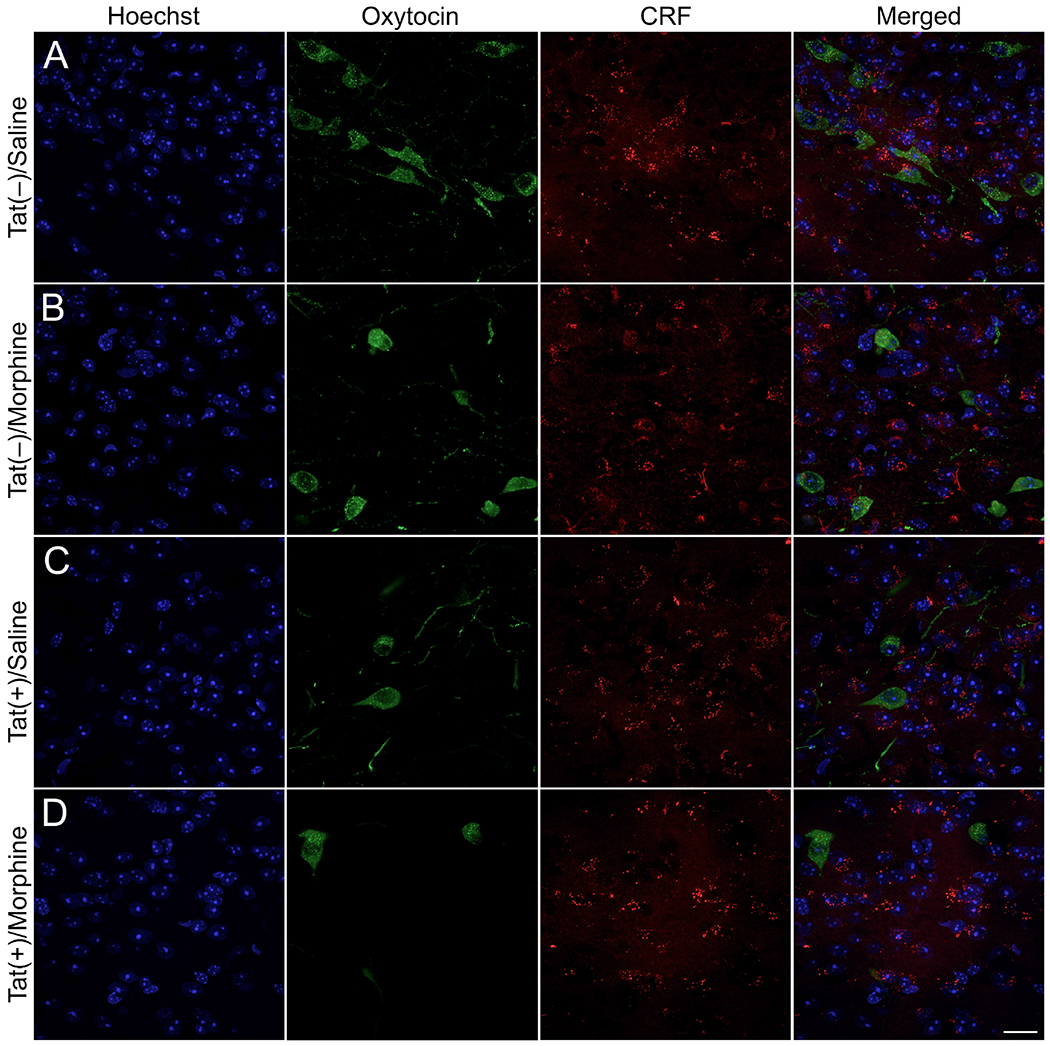
Representative images of oxytocin (green), CRF (red), and Hoechst (blue) positive cells were taken using a Zeiss LSM 700 microscope at 63× magnification (Zeiss, Oberkochen, Germany). Tat(−)/morphine (B), Tat(+)/saline (C), and Tat(+)/morphine (D) PVN tissue sections had less oxytocin immunoreactive-cells compared to Tat(−)/Saline (A) control sections. Scale bar = 20 μm.
3.6. Regional oxytocin or CRF levels in Tat(+)- or morphine-treated mice correlate with non-reciprocal social interaction and novelty behavior
We used correlation analyses to investigate the relationship between oxytocin or CRF levels within the PFC, amygdala, hippocampus, or hypothalamic PVN and social behaviors after Tat or morphine exposure. In morphine-exposed mice, the time spent with the unknown mouse in the social interaction test inversely correlated with oxytocin-immunoreactive cells in the PVN [r(6) = −0.824, p < 0.01; Fig. 8A]. Despite a lack of Tat-induced changes in the social interaction and novelty tests or in neurohormone levels, the latency to interact with the novel mouse in the social novelty test inversely correlated with hippocampal oxytocin levels in Tat(+) mice [r(6) = 0.931, p < 0.01; Fig. 8B]. Similarly, in the social interaction test, the latency to interact [r(6) = 0.726, p < 0.05; Fig. 8C] and time spent [r(6) = −0.73, p < 0.05; Fig. 8D] with the unknown mouse positively and negatively correlated, respectively, with amygdala CRF levels in Tat(+) mice. Additionally, in Tat(+) mice, the latency to interact [r(6) = 0.726, , p < 0.05; Fig. 8E] and time spent [r(6) = 0.719, p < 0.05; Fig. 8F] with the unknown mouse in the social interaction test also positively correlated with PFC and hippocampal CRF levels, respectively.
Fig. 8. In HIV-Tat- or morphine-exposed mice, oxytocin or corticotropin releasing factor (CRF) expression correlates with social behaviors.
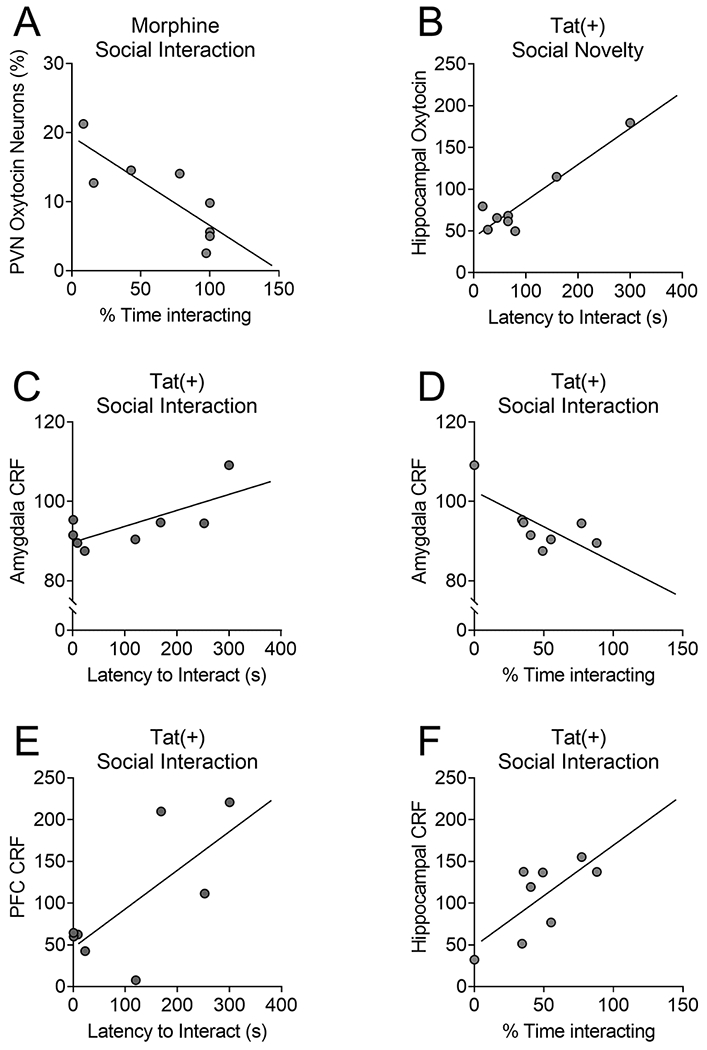
Despite the lack of morphine-induced changes in the social interaction test, a higher percentage of oxytocin-immunoreactive neurons within the hypothalamic paraventricular nucleus of morphine-exposed mice correlated with decreased time spent interacting with the unknown mouse in the social interaction test (A). Although Tat also did not change the overall oxytocin or CRF levels or social behaviors, in Tat(+) mice, hippocampal oxytocin levels positively correlated with time spent interacting with the novel unknown mouse in the social novelty test (B). In the social interaction test, higher amygdalar CRF levels in Tat(+) mice correlated with an increased latency to interact (C) and decreased time spent interacting (D) with the unknown mouse. The latency to interact and time spent interacting with the unknown mouse also positively correlated with PFC (E) and hippocampal (F) CRF levels, respectively. n = 8 mice per group.
4. Discussion
The ability to properly engage in social interactions with others is an important aspect of everyday life that is impaired in PWH and opioid-dependent individuals (Homer et al., 2013). The findings in the present study indicate that Tat and morphine differentially alter distinct aspects of social interaction with an unknown con-specific in a reciprocal familiar environment (as measured by the resident-intruder test), but not in a non-reciprocal novel environment. In Tat-expressing mice there was a modest decline in overall social interactions (η2= 0.08) that appears to be driven by a decrease in aggressive interactions (η2= 0.19), suggesting that the decrease in sociability seen in Tat(+) mice in the resident-intruder test might be overridden in a novel environment by the increased motivation to explore. In support, we have previously shown that Tat increases novelty exploration in response to a novel environment, food, or flavor, but did not alter non-reciprocal or reciprocal social exploration in a novel environment (Nass et al., 2020). Together these results suggest that Tat increases the motivation to explore, but this effect is potentially diminished in social situations. Alternatively, deficits in olfaction that are essential for social behavior in male mice (Liebenauer and Slotnick, 1996; Ryan et al., 2008) may also play a role. Although olfaction has not been directly tested in Tat-tg mice, HIV-tg rats that express 7 of the 9 viral proteins, including Tat, have intact olfaction (Vigorito et al., 2007). Further, PWH performed similar to controls in tests of olfaction (Jackson et al., 2017). While these data suggest that the decreased social interaction is not due to altered olfaction, future studies should nevertheless address whether olfactory deficits contribute to Tat-dependent decreases in social behavior. In preclinical models of schizophrenia, autism, and early life stress, male mice exhibit decreased reciprocal social interactions (Hara et al., 2017; Mohn et al., 1999; Veenema et al., 2007), suggesting other neuropsychological diseases and stressors may also decrease normal social behaviors.
The opiate system is important in modulating social motivation and reward. We found that repeated administration of morphine decreased non-aggressive and aggressive interactions in the resident-intruder test. One possible explanation is that administering μ-opioid receptor (MOR) agonists decreases sociability, whereas antagonists increase sociability in isolated adult rodents, but not in grouped-housed mice (Hol et al., 1996; Puglisi-Allegra et al., 1982; Slamberova et al., 2016). These data suggest that MOR agonists may replace the need for social interaction, although in a few studies β-endorphin increased sociability in previously isolated rats (Niesink and van Ree, 1984; van Ree and Niesink, 1983). These contradictory results may be due to opioids differentially affecting behavior based on behavioral characteristics present before opioid use. Repeated opioid administration decreases social interactions in previously sociable mice, but not in mice that previously avoided socializing (Madison et al., 2020). In preclinical studies, opioid-induced hypermobility can be a potential confound in behavioral testing (Babbini and Davis, 1972). Although morphine (4h after administration) did not alter distance travelled in the social interaction and novelty tests, we cannot exclude the possibility that interacting with the unknown mouse or empty cups influenced locomotor distance. Previously, Tat-induction for 4 weeks was found to reduce the distance traveled in the open field test (Hahn et al., 2016), but in the present study, despite no Tat-induced changes in the social interaction test after 8 weeks of exposure, there was a trend for saline-treated Tat(+) mice to travel further than Tat(−) mice. These data suggest that separate studies examining the effects of 8 weeks of Tat exposure and repeated morphine on locomotor activity independent of other tests are warranted.
This study confirmed our previous findings that Tat does not induce changes in social interaction time in the non-reciprocal social interaction test in a novel environment (Nass et al., 2020), while additionally exploring the consequences of morphine coadministration. However, these data do not coincide with previous data in a slightly different Tat-tg mouse model (Paris et al., 2014) that has more copies of tat (Kim et al., 2003) than the present model (Bruce-Keller et al., 2008), or with studies in which mice are exposed to a single intracerebroventricular (i.c.v.) injection of Tat (Lawson et al., 2011). Both previous studies showed a Tat-induced decrease in social interactions in a reciprocal novel environment. The disagreement may result from differences in (i) the duration of Tat exposure, (ii) the level of Tat expression, (iii) the route of exposure, or (iv) the ability of the unfamiliar con-specific to reciprocally interact or not with the test mouse. In addition, we previously postulated that the initial decrease in social interaction time seen in these studies might be due to sickness behavior resulting from transient increases in proinflammatory cytokines (e.g. TNFα, IL-1β, and IL-6) within the CNS after acute administration of Tat that are not necessarily present after 2 months of exposure (Gonek et al., 2018; Nass et al., 2020).
The neuropeptide oxytocin is implicated in stress and anxiety and is a well-known regulator of social interactions. HIV+ women with high levels of self-reported stress and low plasma oxytocin are more likely to have lower CD4+ cell counts (Fekete et al., 2011). Therefore, we assessed oxytocin as a possible underlying neural substrate for the reduction in aggressive interactions after Tat and morphine administration, and non-aggressive interactions after morphine administration. Since oxytocin has differential effects on social behaviors, including aggression, in males and females, we limited our study to males. Most rodent inter-male aggression studies have been performed in healthy wild-type rodents, and have shown a tendency for oxytocin-receptor agonists to decrease aggressive and increase pro-social behavior in reciprocal social interactions (Calcagnoli et al., 2014; Calcagnoli et al., 2015; Ferguson et al., 2001; Tan et al., 2019a). However, our study and others suggest that pathological states may alter the behavioral responses to oxytocin (Harony-Nicolas et al., 2017; Resendez et al., 2020; Wang et al., 2019). Indeed, Tat exposure decreased oxytocin levels in the PFC and amygdala, while decreasing aggressive social interactions. However, social isolation can influence CNS oxytocin levels, although the effects in males separated as adults from same-sex littermates appear to be time and activity dependent (Bosch et al., 2016; Grippo et al., 2007), suggesting further research is needed.
In humans with social anxiety disorder, oxytocin increases the functional connectivity between the amygdala and a variety of PFC subregions when observing fearful faces (Gorka et al., 2015). In the PFC, optical activation of oxytocin receptor-expressing projection neurons increases BOLD activation in the amygdala and impairs identification of novel conspecifics, but not overall social interactions or anxiety-like behavior (Tan et al., 2019b). These data suggest that Tat-induced decline in aggressive interactions in the resident-intruder test may result from decreases in oxytocin in both the PFC and amygdala. This may be related to the finding that male rats exposed to a single, prolonged stressor in a model of PTSD exhibited decreased social interactions accompanied by decreased oxytocin receptor levels in the PFC and amygdala, but not the hippocampus. Social deficits in that model were restored by administering oxytocin (Wang et al., 2019). Further, higher hippocampal oxytocin levels in Tat(+) mice correlated with a decreased latency to interact with the novel mouse in the social novelty test, despite the absence of Tat-induced changes in overall levels. This is in line with previous studies showing that increased oxytocin in the hippocampus is associated with increased social recognition (Cilz et al., 2019). Alternatively, oxytocin agonists are anxiolytic in male mice (Ring et al., 2006); whereas male oxytocin receptor-knockout mice display decreased anxiety-like behavior in the elevated plus maze (Mantella et al., 2003). Since most social behaviors in the present study did not correlate with oxytocin levels in the Tat(+) mice, and we have previously shown that Tat expression is anxiogenic (Hahn et al., 2016; Hahn et al., 2015; Paris et al., 2014; Paris et al., 2016), it is also plausible that the decreased oxytocin levels in the PFC and amygdala may alter anxiety-like behavior in Tat(+) mice. Future studies will investigate the influence of oxytocin administration on social and anxiety-like behaviors in Tat- and morphine-exposed mice.
HIV-1 Tat and morphine decreased the percentage of oxytocin-immunoreactive neurons in the PVN of the hypothalamus. Further, the latency to interact with the unknown mouse in the social interaction test negatively correlated with oxytocin-immunoreactive cells in the PVN of morphine-exposed mice. The oxytocinergic system plays a role in the emotional and rewarding effects of opiate addiction (Zanos et al., 2018). The PVN of the hypothalamus has a high expression of μ-opioid receptors (Atweh and Kuhar, 1983) and 7 days of escalated morphine decreases oxytocin expression in the hypothalamus of male mice (Zanos et al., 2014). Shank3b is a postsynaptic density (PSD) protein at glutamatergic synapses. Similar to the findings in the present study, male Shank3b KO mice, a preclinical model of autism, exhibit decreased oxytocin-positive neurons within the PVN of the hypothalamus. Furthermore, acute administration of the oxytocin agonist Way267464 or chemogenetic activation of oxytocin neurons in the PVN increases male Shank3b KO mice non-reciprocal social interactions; whereas chemogenetic inactivation decreases interaction time (Resendez et al., 2020). These data indicate that the oxytocin system within the PVN influences social behavior and suggest that the Tat- and morphine-induced decreases of PVN oxytocin-immunoreactive neurons may mediate the decreased social interactions in the resident-intruder test.
We also explored alterations in the HPA axis as a possible explanation for the Tat and morphine-induced changes in sociability in the resident-intruder test. Despite cART treatment many HIV patients have increased basal peripheral cortisol levels and glucocorticoid resistance (Bons et al., 2013). Our previous work indicates that glucocorticoid resistance develops following 8 weeks of Tat induction in male transgenic mice regardless of morphine treatment (Paris et al., 2020), which aligns with reports in PWH (Norbiato et al., 1997; Norbiato et al., 1994). Although changes in serum corticosterone levels were not evident after Tat or morphine exposure in the present study, this might result from length of time after behavioral assays (24 h) or injections (5 h) were conducted, or time of day serum was extracted. After one week of Tat induction, male transgenic mice administered saline or oxycodone, and Tat(+) proestrous female mice exhibit elevated plasma corticosterone compared to Tat(−) control mice when extracted 30 min after injections and subsequent behavioral testing, but not at 2 h after injections (Salahuddin et al., 2020a; Salahuddin et al., 2020b).
CRF decreases social interactions and aggression in rodents (Bagosi et al., 2017; Elkabir et al., 1990; Mele et al., 1987). In line with these prior findings, high CRF levels in the hippocampus and amygdala of Tat(+) mice correlated with more and less time spent with an unknown mouse, respectively, in the social interaction test. In Tat(+) mice, the levels of CRF in the amygdala and PFC also positively correlated with latency to approach in the social interaction test. However, we did not see any differences in overall PFC, hippocampal, or amygdalar CRF levels or percentage of CRF positive neurons in the hypothalamic PVN of Tat(+) or Tat(−) male mice irrespective of morphine. Administration of the CRF type 1 receptor antagonist antalarmin attenuated anxiety-like behavior in male Tat(+) mice measured 15 min after administration, and behavior was diminished by oxycodone. Interestingly, antalarmin did not alter plasma corticosterone levels (Salahuddin et al., 2020a) suggesting that Tat and opioid-induced changes in CRF are transient and time dependent. Alternatively, CRF levels may have been altered by the social isolation experienced by the subset of mice used for aggressive behavioral assays (Hostetler and Ryabinin, 2013). However, the two cohorts that experienced different housing conditions and behavioral assays did not significantly differ in CRF levels within each treatment group.
This study provides the first empirical evidence that HIV-1 Tat alters oxytocin expression in males and expands on prior findings that Tat-induced decreases in social interactions might be time and environment dependent (Lawson et al., 2011; Nass et al., 2020; Paris et al., 2014). Our findings suggest that decreases in aggressive behavior are associated with Tat-induced reductions in oxytocin protein expression in the PFC, amygdala, and hypothalamic PVN. Thus, Tat per se may have a role in mediating the alterations in oxytocin levels and associated social deficits seen in PWH. We also found that morphine decreased non-aggressive and aggressive social interactions in the resident-intruder test were accompanied by decreases in oxytocin-immunoreactive neurons in the hypothalamic PVN. Importantly, the present study does not reveal whether the loss of oxytocin antigenicity results from morphine- and/or Tat-dependent dynamic changes in oxytocin biosynthesis, degradation, or release, or the loss of a subpopulation of oxytocin-expressing neurons—although the latter scenario seems less likely. Surprisingly, despite findings that CRF levels in the hippocampus, amygdala, and PFC of Tat(+) mice correlated with social behavior in the non-reciprocal social interaction test, plasma corticosterone levels and CRF protein expression throughout prefrontal cortico-hippocampal-amygdalar circuit were unaffected by Tat or morphine. We speculate that Tat or morphine may affect corticosterone or CRF function, respectively, by influencing glucocorticoid receptor or corticotropin-releasing hormone receptor 1 levels or function. Overall, the data suggest that oxytocin is a potential therapeutic target for social deficits in PWH.
Highlights.
HIV-1 Tat reduces aggressive inter-male interactions in the resident-intruder test
Morphine decreases inter-male social interactions in the resident-intruder test
HIV-1 Tat decreases oxytocin levels in the PFC and amygdala, but not hippocampus
Regional oxytocin levels correlate with behavior in Tat(+) or morphine exposed mice
Morphine and Tat decrease the percentage of oxytocin cells in the hypothalamic PVN
Acknowledgments
This work was supported by the National Institute on Drug Abuse (NIDA) R01 DA034231 (PEK and KFH), K02 DA027374 (KFH), and R01 DA045588 (KFH). We thank Nabeel Elhaj for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE, 2005. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol.Appl.Neurobiol. 31, 325–338. [DOI] [PubMed] [Google Scholar]
- Applebaum AJ, Bullis JR, Traeger LN, O’Cleirigh C, Otto MW, Pollack MH, Safren SA, 2010. Rates of mood and anxiety disorders and contributors to continued heroin use in methadone maintenance patients: A comparison by HIV status. Neurobehav HIV Med 2010, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atweh SF, Kuhar MJ, 1983. Distribution and physiological significance of opioid receptors in the brain. Br Med Bull 39, 47–52. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Sheridan JF, 2001. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav 39, 247–257. [DOI] [PubMed] [Google Scholar]
- Babbini M, Davis WM, 1972. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. British journal of pharmacology 46, 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babygirija R, Bulbul M, Yoshimoto S, Ludwig K, Takahashi T, 2012. Central and peripheral release of oxytocin following chronic homotypic stress in rats. Auton Neurosci 167, 56–60. [DOI] [PubMed] [Google Scholar]
- Bach P, Frischknecht U, Bungert M, Karl D, Vollmert C, Vollstadt-Klein S, Lis S, Kiefer F, Hermann D, 2019. Effects of social exclusion and physical pain in chronic opioid maintenance treatment: fMRI correlates. Eur Neuropsychopharmacol 29, 291–305. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Winberg S, 2013. Central corticotropin releasing factor and social stress. Front Neurosci 7, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagosi Z, Karasz G, Czebely-Lenart A, Csabafi K, Jaszberenyi M, Telegdy G, 2017. The effects of CRF and urocortins on the sociability of mice. Brain Res 1663, 114–122. [DOI] [PubMed] [Google Scholar]
- Baldonero E, Ciccarelli N, Fabbiani M, Colafigli M, Improta E, D’Avino A, Mondi A, Cauda R, Di Giambenedetto S, Silveri MC, 2013. Evaluation of emotion processing in HIV-infected patients and correlation with cognitive performance. BMC Psychol 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P, 1998. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain 121 ( Pt 11), 2043–2052. [DOI] [PubMed] [Google Scholar]
- Biglino A, Limone P, Forno B, Pollono A, Cariti G, Molinatti GM, Gioannini P, 1995. Altered adrenocorticotropin and cortisol response to corticotropin-releasing hormone in HIV-1 infection. Eur J Endocrinol 133, 173–179. [DOI] [PubMed] [Google Scholar]
- Bons J, Moreau L, Lefebvre H, 2013. Adrenal disorders in human immunodeficiency virus (HIV) infected patients. Ann Endocrinol (Paris) 74, 508–514. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, Neumann ID, Young LJ, 2016. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, O’Cleirigh CM, 2017. Anxiety symptoms and disorders among adults living with HIV and AIDS: A critical review and integrative synthesis of the empirical literature. Clin Psychol Rev 51, 164–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF, 2008. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia 56, 1414–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd D, Murray J, Safdieh G, Morgello S, 2012. Impact of opiate addiction on neuroinflammation in HIV. J Neurovirol 18, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, Atkinson JH, Clifford DB, Collier AC, Marra CM, Gelman B, McCutchan JA, Duarte NA, Simpson DM, McArthur J, Grant I, Group C, 2011. Neurocognitive impact of substance use in HIV infection. Jaids-J Acq Imm Def 58, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnoli F, Meyer N, de Boer SF, Althaus M, Koolhaas JM, 2014. Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Horm Behav 65, 427–433. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, Stubbendorff C, Meyer N, de Boer SF, Althaus M, Koolhaas JM, 2015. Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology 90, 74–81. [DOI] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP, 2012. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res 229, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopard C, Tong PBV, Toth P, Schatz M, Yezid H, Debaisieux S, Mettling C, Gross A, Pugniere M, Tu A, Strub JM, Mesnard JM, Vitale N, Beaumelle B, 2018. Cyclophilin A enables specific HIV-1 Tat palmitoylation and accumulation in uninfected cells. Nature communications 9, 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B, Qin Z, Byrd DA, Yu F, Morgello S, Gelman BB, Moore DJ, Grant I, Singer EJ, Fox HS, Baccaglini L, 2017. Measures of Physical and Mental Independence Among HIV-Positive Individuals: Impact of Substance Use Disorder. AIDS Res Hum Retroviruses 33, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilz NI, Cymerblit-Sabba A, Young WS, 2019. Oxytocin and vasopressin in the rodent hippocampus. Genes Brain Behav 18, e12535. [DOI] [PubMed] [Google Scholar]
- Cirino TJ, Harden SW, McLaughlin JP, Frazier CJ, 2020. Region-specific effects of HIV-1 Tat on intrinsic electrophysiological properties of pyramidal neurons in mouse prefrontal cortex and hippocampus. J Neurophysiol 123, 1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Cohen RA, Westbrook ML, Devlin KN, Tashima KT, 2010. Facial emotion recognition impairments in individuals with HIV. J Int Neuropsychol Soc 16, 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark US, Walker KA, Cohen RA, Devlin KN, Folkers AM, Pina MJ, Tashima KT, 2015. Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia 70, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG, 2013. Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 7, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregori S, De Gregori M, Ranzani GN, Allegri M, Minella C, Regazzi M, 2012. Morphine metabolism, transport and brain disposition. Metab Brain Dis 27, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaisieux S, Rayne F, Yezid H, Beaumelle B, 2012. The ins and outs of HIV-1 Tat. Traffic (Copenhagen, Denmark) 13, 355–363. [DOI] [PubMed] [Google Scholar]
- Dickens AM, Yoo SW, Chin AC, Xu J, Johnson TP, Trout AL, Hauser KF, Haughey NJ, 2017. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Scientific reports 7, 7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Gundersen HJ, Jensen EB, 2000. Non-uniform systematic sampling in stereology. J Microsc 200, 148–157. [DOI] [PubMed] [Google Scholar]
- Duarte-Guterman P, Lieblich SE, Qiu W, Splinter JEJ, Go KA, Casanueva-Reimon L, Galea LAM, 2020. Oxytocin has sex-specific effects on social behaviour and hypothalamic oxytocin immunoreactive cells but not hippocampal neurogenesis in adult rats. Horm Behav 122, 104734. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Bruce-Keller AJ, Conner C, Knapp PE, Xu R, Nath A, Hauser KF, 2008. Effects of chronic expression of the HIV-induced protein, transactivator of transcription, on circadian activity rhythms in mice, with or without morphine. Am J Physiol Regul Integr Comp Physiol 295, R1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Yakovleva T, Bakalkin G, Knapp PE, Hauser KF, 2008. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca2+]i, NF-kB trafficking and transcription. PloS one 3, e4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF, 2005. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia 50, 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkabir DR, Wyatt ME, Vellucci SV, Herbert J, 1990. The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul Pept 28, 199–214. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Antoni MH, Lopez C, Mendez AJ, Szeto A, Fletcher MA, Klimas N, Kumar M, Schneiderman N, 2011. Stress buffering effects of oxytocin on HIV status in low-income ethnic minority women. Psychoneuroendocrinology 36, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, Seay J, Antoni MH, Mendez AJ, Fletcher MA, Szeto A, Schneiderman N, 2014. Oxytocin, social support, and sleep quality in low-income minority women living with HIV. Behav Sleep Med 12, 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ, 2001. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21, 8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF, 2013. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry 73, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Scoggins KL, Xu R, Dever SM, Knapp PE, Dewey WL, Hauser KF, 2012. Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur J Pharmacol 689, 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Stevens DL, Khan FA, Scoggins KL, Enga RM, Beardsley PM, Knapp PE, Dewey WL, Hauser KF, 2016. Morphine Tolerance and Physical Dependence Are Altered in Conditional HIV-1 Tat Transgenic Mice. J Pharmacol Exp Ther 356, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Xu R, Bull C, Buch SK, El-Hage N, Nath A, Knapp PE, Hauser KF, 2010a. Interactive comorbidity between opioid drug abuse and HIV-1 Tat: chronic exposure augments spine loss and sublethal dendritic pathology in striatal neurons. Am J Pathol 177, 1397–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Zou S, Chen W, Vo P, Hauser KF, Knapp PE, 2010b. Regional heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV-1 Tat, gp120, and morphine revealed by multiplex analysis. J Proteome Res 9, 1795–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Zou S, El-Hage N, Suzuki M, Paris JJ, Schier CJ, Rodriguez JW, Rodriguez M, Knapp PE, Hauser KF, 2014. Opiate addiction therapies and HIV-1 Tat: interactive effects on glial [Ca(2)(+)]i, oxyradical and neuroinflammatory chemokine production and correlative neurotoxicity. Curr Hiv Res 12, 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountas A, Chai ST, Kourkouti C, Karavitaki N, 2018. MECHANISMS OF ENDOCRINOLOGY: Endocrinology of opioids. Eur J Endocrinol 179, R183–R196. [DOI] [PubMed] [Google Scholar]
- Frischknecht U, Beckmann B, Heinrich M, Kniest A, Nakovics H, Kiefer F, Mann K, Hermann D, 2011. The vicious circle of perceived stigmatization, depressiveness, anxiety, and low quality of life in substituted heroin addicts. Eur Addict Res 17, 241–249. [DOI] [PubMed] [Google Scholar]
- Gardi JE, Nyengaard JR, Gundersen HJ, 2008. The proportionator: unbiased stereological estimation using biased automatic image analysis and non-uniform probability proportional to size sampling. Comput Biol Med 38, 313–328. [DOI] [PubMed] [Google Scholar]
- Gonek M, McLane VD, Stevens DL, Lippold K, Akbarali HI, Knapp PE, Dewey WL, Hauser KF, Paris JJ, 2018. CCR5 mediates HIV-1 Tat-induced neuroinflammation and influences morphine tolerance, dependence, and reward. Brain, behavior, and immunity 69, 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Baeza A, Perez-Valero I, Carvajal-Molina F, Bayon C, Montes-Ramirez M, Bernardino JI, Arribas JR, 2014. Facial emotional processing deficits in long-term HIV-suppressed patients. J Int AIDS Soc 17, 19664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, Labuschagne I, Hosanagar A, Wood AG, Nathan PJ, Phan KL, 2015. Oxytocin modulation of amygdala functional connectivity to fearful faces in generalized social anxiety disorder. Neuropsychopharmacology 40, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabyan JM, Morgan EE, Cameron MV, Villalobos J, Grant I, Paul Woods S, Group HIVNRP, 2018. Deficient Emotion Processing is Associated with Everyday Functioning Capacity in HIV-associated Neurocognitive Disorder. Arch Clin Neuropsychol 33, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS, 2007. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology 32, 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. , 1988. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96, 379–394. [DOI] [PubMed] [Google Scholar]
- Hahn YK, Paris JJ, Lichtman AH, Hauser KF, Sim-Selley LJ, Selley DE, Knapp PE, 2016. Central HIV-1 Tat exposure elevates anxiety and fear conditioned responses of male mice concurrent with altered mu-opioid receptor-mediated G-protein activation and beta-arrestin 2 activity in the forebrain. Neurobiology of disease 92, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn YK, Podhaizer EM, Farris SP, Miles MF, Hauser KF, Knapp PE, 2015. Effects of chronic HIV-1 Tat exposure in the CNS: heightened vulnerability of males versus females to changes in cell numbers, synaptic integrity, and behavior. Brain Struct Funct 220, 605–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Ago Y, Higuchi M, Hasebe S, Nakazawa T, Hashimoto H, Matsuda T, Takuma K , 2017. Oxytocin attenuates deficits in social interaction but not recognition memory in a prenatal valproic acid-induced mouse model of autism. Horm Behav 96, 130–136. [DOI] [PubMed] [Google Scholar]
- Harony-Nicolas H, Kay M, du Hoffmann J, Klein ME, Bozdagi-Gunal O, Riad M, Daskalakis NP, Sonar S, Castillo PE, Hof PR, Shapiro ML, Baxter MG, Wagner S, Buxbaum JD, 2017. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hategan A, Bianchet MA, Steiner J, Karnaukhova E, Masliah E, Fields A, Lee MH, Dickens AM, Haughey N, Dimitriadis EK, Nath A, 2017. HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity. Nat Struct Mol Biol 24, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Schiorring E, 1979. Behavioral effects of low and high acute doses of morphine in solitary mice. Psychopharmacology (Berl) 64, 73–79. [DOI] [PubMed] [Google Scholar]
- Henderson LJ, Johnson TP, Smith BR, Reoma LB, Santamaria UA, Bachani M, Demarino C, Barclay RA, Snow J, Sacktor N, McArthur J, Letendre S, Steiner J, Kashanchi F, Nath A, 2019. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 33 Suppl 2, S145–S157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol T, Ruven S, Van Ree JM, Spruijt BM, 1996. Chronic administration of Org2766 and morphine counteracts isolation-induced increase in social interest: implication of endogenous opioid systems. Neuropeptides 30, 283–291. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S, 2007. Oxytocin increases retention of social cognition in autism. Biol Psychiatry 61, 498–503. [DOI] [PubMed] [Google Scholar]
- Homer BD, Halkitis PN, Moeller RW, Solomon TM, 2013. Methamphetamine use and HIV in relation to social cognition. J Health Psychol 18, 900–910. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE, 2013. The CRF system and social behavior: a review. Front Neurosci 7, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C, Rai N, McLean CK, Hipolito MMS, Hamilton FT, Kapetanovic S, Nwulia EA, 2017. Overlapping Risky Decision-Making and Olfactory Processing Ability in HIV-Infected Individuals. Clin Exp Psychol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A, 2013. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A 110, 13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Fields JA, Chang A, Coban H, Achim CL, Semenova S, Group T, 2018. Effects of HIV-1 TAT protein and methamphetamine exposure on visual discrimination and executive function in mice. Behav Brain Res 349, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ, 2003. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol 162, 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Hahn YK, Podhaizer EM, McLane VD, Zou S, Hauser KF, Knapp PE, 2018. A central role for glial CCR5 in directing the neuropathological interactions of HIV-1 Tat and opiates. J Neuroinflammation 15, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ, 2013. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp, e4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich C, Foisy ML, Philippot P, Dan B, Tecco J, Noel X, Hess U, Pelc I, Verbanck P, 2003. Impaired emotional facial expression recognition in alcoholics, opiate dependence subjects, methadone maintained subjects and mixed alcohol-opiate antecedents subjects compared with normal controls. Psychiatry Res 119, 251–260. [DOI] [PubMed] [Google Scholar]
- Korthuis PT, Zephyrin LC, Fleishman JA, Saha S, Josephs JS, McGrath MM, Hellinger J, Gebo KA, Network HIVR, 2008. Health-related quality of life in HIV-infected patients: the role of substance use. AIDS Patient Care STDS 22, 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Kelley KW, Dantzer R, 2011. Intracerebroventricular administration of HIV-1 Tat induces brain cytokine and indoleamine 2,3-dioxygenase expression: a possible mechanism for AIDS comorbid depression. Brain, behavior, and immunity 25, 1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI, 2005. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology 30, 1883–1894. [DOI] [PubMed] [Google Scholar]
- Liebenauer LL, Slotnick BM, 1996. Social organization and aggression in a group of olfactory bulbectomized male mice. Physiol Behav 60, 403–409. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ, 2006. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav 50, 506–517. [DOI] [PubMed] [Google Scholar]
- Madison CA, Wellman PJ, Eitan S, 2020. Response to opioids is dependent on sociability levels. Behav Pharmacol 31, 293–307. [DOI] [PubMed] [Google Scholar]
- Malaspina L, Woods SP, Moore DJ, Depp C, Letendre SL, Jeste D, Grant I, Group HIVNRP, 2011. Successful cognitive aging in persons living with HIV infection. J Neurovirol 17, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, Li X, Amico JA, 2003. Female oxytocin-deficient mice display enhanced anxiety-related behavior. Endocrinology 144, 2291–2296. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Froemke RC, 2017. Oxytocin modulation of neural circuits for social behavior. Dev Neurobiol 77, 169–189. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Paris JJ, Mintzopoulos D, Hymel KA, Kim JK, Cirino TJ, Gillis TE, Eans SO, Vitaliano GD, Medina JM, Krapf RC, Stacy HM, Kaufman MJ, 2017. Conditional Human Immunodeficiency Virus Transactivator of Transcription Protein Expression Induces Depression-like Effects and Oxidative Stress. Biol Psychiatry Cogn Neurosci Neuroimaging 2, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele A, Cabib S, Oliverio A, Melchiorri P, Puglisi-Allegra S, 1987. Effects of corticotropin releasing factor and sauvagine on social behavior of isolated mice. Peptides 8, 935–938. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S, 2001. Aggressive behavioral phenotypes in mice. Behav Brain Res 125, 167–181. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH, 1999. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98, 427–436. [DOI] [PubMed] [Google Scholar]
- Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, Woods SP, Group HIVNRP, 2014. Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS Behav 18, 1186–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Na ES, Autry AE, Monteggia LM, 2016. Impact of DNMT1 and DNMT3a forebrain knockout on depressive- and anxiety like behavior in mice. Neurobiol Learn Mem 135, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, 2002. Principles and Practices Of Unbiased Stereology: An Introduction For Bioscientists. The Johns Hopkins University Press. [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN, 2004. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3, 287–302. [DOI] [PubMed] [Google Scholar]
- Nass SR, Hahn YK, McLane VD, Varshneya NB, Damaj MI, Knapp PE, Hauser KF, 2020. Chronic HIV-1 Tat exposure alters anterior cingulate cortico-basal ganglia-thalamocortical synaptic circuitry, associated behavioral control, and immune regulation in male mice. Brain, Behavior, & Immunity - Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwainmbi J, De DD, Smith TH, El-Hage N, Fitting S, Kang M, Dewey WL, Hauser KF, Akbarali HI, 2014. Effects of HIV-1 Tat on enteric neuropathogenesis. J Neurosci 34, 14243–14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesink RJ, van Ree JM, 1984. Neuropeptides and social behavior of rats tested in dyadic encounters. Neuropeptides 4, 483–496. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, Carmi R, Stone EM, Sheffield VC, 2004. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A 101, 16588–16593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbiato G, Bevilacqua M, Vago T, 1997. Glucocorticoids and the immune system in AIDS. Psychoneuroendocrinology 22 Suppl 1, S19–25. [DOI] [PubMed] [Google Scholar]
- Norbiato G, Galli M, Righini V, Moroni M, 1994. The syndrome of acquired glucocorticoid resistance in HIV infection. Baillieres Clin Endocrinol Metab 8, 777–787. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Liere P, Kim S, Mahdi F, Buchanan ME, Nass SR, Qrareya AN, Salahuddin MF, Pianos A, Fernandez N, Shariat-Madar Z, Knapp PE, Schumacher M, Hauser KF, 2020. Pregnane steroidogenesis is altered by HIV-1 Tat and morphine: Physiological allopregnanolone is protective against neurotoxic and psychomotor effects. Neurobiol Stress 12, 100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Singh HD, Ganno ML, Jackson P, McLaughlin JP, 2014. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology (Berl) 231, 2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris JJ, Zou S, Hahn YK, Knapp PE, Hauser KF, 2016. 5alpha-reduced progestogens ameliorate mood-related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV-1 Tat. Brain, behavior, and immunity 55, 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paydary K, Mahin Torabi S, SeyedAlinaghi S, Noori M, Noroozi A, Ameri S, Ekhtiari H, 2016. Impulsivity, Sensation Seeking, and Risk-Taking Behaviors among HIV-Positive and HIV-Negative Heroin Dependent Persons. AIDS Res Treat 2016, 5323256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL, 2011. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res 132, 50–53. [DOI] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Partoo L, Yee J, Stevenson J, Sanzenbacher L, Kenkel W, Mohsenpour SR, Hashimoto K, Carter CS, 2011. Effects of social isolation on mRNA expression for corticotrophin-releasing hormone receptors in prairie voles. Psychoneuroendocrinology 36, 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Oliverio A, Mandel P, 1982. Effects of opiate antagonists on social and aggressive behavior of isolated mice. Pharmacol Biochem Behav 17, 691–694. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hofman MA, Portegies P, Troost D, Swaab DF, 1993. Decreased number of oxytocin neurons in the paraventricular nucleus of the human hypothalamus in AIDS. Brain 116 ( Pt 4), 795–809. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hofman MA, Swaab DF, 1994. Decreased number of oxytocin-immunoreactive neurons in the paraventricular nucleus of the hypothalamus in Parkinson’s disease. Neurology 44, 84–89. [DOI] [PubMed] [Google Scholar]
- Rayne F, Debaisieux S, Yezid H, Lin YL, Mettling C, Konate K, Chazal N, Arold ST, Pugniere M, Sanchez F, Bonhoure A, Briant L, Loret E, Roy C, Beaumelle B, 2010. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J 29, 1348–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Namboodiri VMK, Otis JM, Eckman LEH, Rodriguez-Romaguera J, Ung RL, Basiri ML, Kosyk O, Rossi MA, Dichter GS, Stuber GD, 2020. Social Stimuli Induce Activation of Oxytocin Neurons Within the Paraventricular Nucleus of the Hypothalamus to Promote Social Behavior in Male Mice. J Neurosci 40, 2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, Schechter LE, Rizzo S, Rahman Z, Rosenzweig-Lipson S, 2006. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 185, 218–225. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Moy SS, Crawley JN, 2008. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav Brain Res 193, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Blacker D, Blazer DG, Ganguli M, Jeste DV, Paulsen JS, Petersen RC, 2014. Classifying neurocognitive disorders: the DSM-5 approach. Nature reviews. Neurology 10, 634–642. [DOI] [PubMed] [Google Scholar]
- Saivin S, Houin G, 1988. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet 15, 355–366. [DOI] [PubMed] [Google Scholar]
- Salahuddin MF, Mahdi F, Paris JJ, 2020a. HIV-1 Tat Dysregulates the Hypothalamic-Pituitary-Adrenal Stress Axis and Potentiates Oxycodone-Mediated Psychomotor and Anxiety-Like Behavior of Male Mice. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin MF, Qrareya AN, Mahdi F, Jackson D, Foster M, Vujanovic T, Box JG, Paris JJ, 2020b. Combined HIV-1 Tat and oxycodone activate the hypothalamic-pituitary-adrenal and -gonadal axes and promote psychomotor, affective, and cognitive dysfunction in female mice. Horm Behav 119, 104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand FL, Degenhardt L, Nelson EC, Mattick RP, 2010. Predictors of social anxiety in an opioid dependent sample and a control sample. J Anxiety Disord 24, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberova R, Mikulecka A, Macuchova E, Hrebickova I, Sevcikova M, Nohejlova K, Pometlova M, 2016. Morphine decreases social interaction of adult male rats, while THC does not affect it. Physiol Res 65, S547–S555. [DOI] [PubMed] [Google Scholar]
- Smith R, Rossetto K, Peterson BL, 2008. A meta-analysis of disclosure of one’s HIV-positive status, stigma and social support. AIDS Care 20, 1266–1275. [DOI] [PubMed] [Google Scholar]
- Tan O, Musullulu H, Raymond JS, Wilson B, Langguth M, Bowen MT, 2019a. Oxytocin and vasopressin inhibit hyper-aggressive behaviour in socially isolated mice. Neuropharmacology 156, 107573. [DOI] [PubMed] [Google Scholar]
- Tan Y, Singhal SM, Harden SW, Cahill KM, Nguyen DM, Colon-Perez LM, Sahagian TJ, Thinschmidt JS, de Kloet AD, Febo M, Frazier CJ, Krause EG, 2019b. Oxytocin Receptors Are Expressed by Glutamatergic Prefrontal Cortical Neurons That Selectively Modulate Social Recognition. J Neurosci 39, 3249–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, Baker LK, Pedersen CA, Jarstfer MB, Moy SS, 2013. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology 72, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Galgani S, Murri R, Bellagamba R, Narciso P, Antinori A, Giulianelli M, Tosi G, Costa M, Sampaolesi A, Fantoni M, Noto P, Ippolito G, Wu AW, 2003. Neurocognitive performance and quality of life in patients with HIV infection. AIDS Res Hum Retroviruses 19, 643–652. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Niesink RJ, 1983. Low doses of beta-endorphin increase social contacts of rats tested in dyadic encounters. Life Sci 33 Suppl 1, 611–614. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID, 2007. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology 32, 437–450. [DOI] [PubMed] [Google Scholar]
- Vercruysse P, Vieau D, Blum D, Petersen A, Dupuis L, 2018. Hypothalamic Alterations in Neurodegenerative Diseases and Their Relation to Abnormal Energy Metabolism. Front Mol Neurosci 11, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL, 2007. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol 2, 319–328. [DOI] [PubMed] [Google Scholar]
- Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC, 2010. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev 31, 98–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Lin CC, Tzeng NS, Tung CS, Liu YP, 2019. Effects of oxytocin on prosocial behavior and the associated profiles of oxytocinergic and corticotropin-releasing hormone receptors in a rodent model of posttraumatic stress disorder. J Biomed Sci 26, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF, 2008. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry 13, 786–799, 741. [DOI] [PubMed] [Google Scholar]
- Wodarski R, Bagdas D, Paris JJ, Pheby T, Toma W, Xu R, Damaj MI, Knapp PE, Rice ASC, Hauser KF, 2018. Reduced intraepidermal nerve fibre density, glial activation, and sensory changes in HIV type-1 Tat-expressing female mice: involvement of Tat during early stages of HIV-associated painful sensory neuropathy. Pain Rep 3, e654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Weber C, Robinson F, Kouimtsidis C, Niforooshan R, Bailey A, 2018. Oxytocin and opioid addiction revisited: old drug, new applications. British journal of pharmacology 175, 2809–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, Bailey A, 2014. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology 39, 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]


