Abstract
Immune system hypersensitivity is believed to contribute to mental frailty in the elderly. Solid evidence indicates NOD-like receptor pyrin domain containing-3 (NLRP3)-inflammasome activation intimately connects aging-associated chronic inflammation (inflammaging) to senile cognitive decline. Thioredoxin interacting protein (TXNIP), an inducible protein involved in oxidative stress, is essential for NLRP3 inflammasome activity. This study aims to find whether TXNIP/NLRP3 inflammasome pathway is involved in senile dementia. According to our studies on sex-matched mice, TXNIP was significantly upregulated in aged animals, paralleled by the NLRP3-inflammasome over-activity leading to enhanced caspase-1 cleavage and IL-1β maturation, in both sexes. This was closely associated with depletion of the anti-aging and cognition enhancing protein klotho, in aged males. Txnip knockout reversed age-related NLRP3-hyperactivity and enhanced thioredoxin (TRX) levels. Further, TXNIP inhibition with verapamil replicated TXNIP/NLRP3-inflammasome downregulation in aged animals, with FOXO-1 and mTOR upregulation. These alterations concurred with substantial improvements in both cognitive and sensorimotor abilities. Together, these findings substantiate the pivotal role of TXNIP to drive inflammaging in parallel with klotho depletion and functional decline, and delineate thioredoxin system as a potential target to decelerate senile dementia.
Keywords: Brain Ageing, TXNIP, NLRP3-inflmmasome, Oxidative Stress, Functional Decline
1. INTRODUCTION
While life expectancy has dramatically increased, owing to improved life style, the aging debilitating morbidities have inarguably remained inevitable. Strong evidence supports the hypothesis that degenerative alterations in aging brains stem from the state of subclinical innate immune hyperresponsiveness. This might be best characterized by high levels of circulatory pro-inflammatory markers (i.e IL-6, IL-1β and IL-8) in the majority of healthy older individuals (Ferrucci and Fabbri, 2018; Singh and Newman, 2011). Although the underlying causes of age-related neuroinflammation are not fully understood, the cytosolic pattern recognition receptor nucleotide binding and oligomerization domain (NOD)-like receptor protein-3 (NLRP3)-inflammasome is postulated to play a pivotal role. There is a hypothesis that “inflammaging” at least partly stems from defects in immune hemostasis and cellular senescence or impaired in removal of cellular debris. Accordingly, “inflammaging” is characterized by buildup of the cumulative immunogens, many of which may directly instigate NLRP3 inflammasome oligomerization. Upon sensing damage signals in the “sensitized” aged cells, the NLRP3-inflammasome readily recruits the adapter protein apoptosisassociated speck-like (ASC) and then pro-caspase-1, to drive massive caspase-1 production and subsequent interleukin-1β (IL-1β) and interleukin-8 (IL-8) maturation and release (Ferrucci and Fabbri, 2018; Latz and Duewell, 2018). With a report showing that the NLRP3-inflammasome is upregulated in naturally aged animals (Wang et al., 2018), our understanding of NLRP3-inflammasome involvement in senile cognitive decline is utterly limited. It has been suggested that NLRP3-inflammasome might work as the main gate linking low grade systemic inflammation to age-associated functional decline (Goldberg and Dixit, 2015; Youm et al., 2013).
We and others have recently demonstrated that thioredoxin-interacting protein (TXNIP), an endogenous inhibitor of the thioredoxin (TRX) pathway, is required for NLRP3-inflammasome assembly (Ishrat et al., 2015; Zhou et al., 2018). This implicate TXNIP as an upstream activator to aggravate inflammaging. Besides redox regulation, TXNIP is a key component in glucose metabolism, insulin resistance and inflammation (A PediatriaAbais et al., 2015; Lane et al., 2013; Yoshihara et al., 2014; Zhou et al., 2010). TXNIP’s role in aging is already supported by demonstration of enhanced TXNIP transcripts in aged postmortem human brains (Cribbs et al., 2012) and TXNIP protein in various aged human tissues, which appears to reduce resistance to oxidative challenge, a conserved role in humans and flies (Oberacker et al., 2018). Importantly, klotho, an anti-aging and cognition enhancing protein, has recently been shown to suppress NLRP3-inflammasome activity through TXNIP ablation. Along with aging, klotho is depleted in choroid plexus while Akt is activated throughout many cerebral regions (Song et al., 2007) and might activate NLRP3 inflammasome (Zhao et al., 2020). Such evidence may underline TXNIP/NLRP3 significance as the interface between the brain and immune system in the elderly (Zhu et al., 2018).
The present study mainly focused to evaluate cerebral TXNIP in tuning brain inflammaging will demonstrates sex-dependent TXNIP alterations and associations with cerebral NLRP3-inflammasome activity at different ages. Then we will illustrate the effect of genetic ablation of TXNIP on neuroinflammation in aged animals. As a translational implementation, we will further delineate the effect of verapamil, a recently defined repressor of TXNIP activity (Melone et al., 2018). The presented promising findings, suggest more investigations to validate potential off-label use of the drug to decelerate inflammaging.
2. MATERIALS AND METHODS
2.1. Animals
Male and female young (2 mos, 22-24 gm), middle aged (12 mos, 30-32 gm) and aged (18 mos, 32-35 gm) C57BL/6 (Jackson Laboratory, Bar Harbor, ME, USA and NIA, NIH), as well as Txnip knockout (Txnip−/−) mice were housed in a group (3-4 animals /cage) with standard humidity (45–50%) and temperature (21–25 °C) and 12-h light/dark cycle with food and water ad libitum. All experiments were conducted in accordance with Institutional Animal Care and Use Committee (IACUC) at UTHSC, Memphis TN and ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines (Kilkenny et al., 2010).
2.2. Experimental Design
Three main experimental designs were implemented to address the specific hypotheses. (1) There is a potential association between cerebral TXNIP/NLRP3 inflammasome and aging in both sexes: Brain samples of male and female young (2 mos), middle aged (12 mos) and aged (18 mos) mice (N=5/group) were subjected to immunoblotting, real-time PCR analysis or cell-specific immunostaining for TXNIP and different components of NLRP3 inflammasome assembly. (2) TXNIP mediates aging associated neuroinflammation: wild-type and Txnip−/− aged male mice brains (N=5/group) were compared in age-induced TXNIP/ NLRP3 inflammasome activation by immunoblotting (3) Verapamil, the calcium channel blocker and TXNIP suppressor; may reverse neuroinflammation and cognitive decline with aging: male aged + verapamil (1mg/kg/day, for 1 month) and young and aged mice (N=5/group) were compared in behavioral scores, followed by cerebral protein extraction and TXNIP/NLRP3 inflammasome analysis. Verapamil (Sigma, USA) was incorporated into animals’ drinking water by a blinded investigator; and adjusted to dose them with 1 mg/kg/day verapamil for 1 month. Verapamil dosage was selected based on the lowest required for TXNIP suppression (Melone et al., 2018), as addressed in earlier reports without affecting blood pressure (Suppl. Fig. 3). Using a battery of neurological tests including novel object recognition (NOR), morris water maze (MWM) and catwalk analysis we addressed cognitive and sensorimotor function. Gait abnormalities are typical features of sensorimotor decline in healthy aged animals. Since the gait control indices are a predictor of functional decline in aging humans (Studenski et al., 2011; Van Kan et al., 2009), we used a wide array of gait control indices to provide novel information on either aging or verapamil as a presumptive promising remedy (see Supl. Mat. For complementary information).
2.3. Novel Object Recognition Test
Based on the spontaneous tendency of rodents to explore and interact with a novel object, novel object recognition test (NOR) was utilized to evaluate animals’ non-spatial short-term working memory (Ishrat et al., 2006). Through a standard test procedure, animals were allowed to explore two identical objects (acquisition trial), one of which was replaced in next phase (preference trial). In the habituation phase (10 min) conducted one day before the start of the test, animals were allowed to acclimate to an empty standard size box. On the designated test day, animals were first presented with 2 identical objects and for 10 min allowed to explore the objects that were placed equidistant from the walls of the box, in the center and spaced 20 cm apart (acquisition trial). Following sample object exposure, the animals were returned to their home cage for a 90 min retention period. The 2nd preference/test trial (5 min) was conducted in the same manner as the 1st trial, except that a new/novel object replaced one of the familiar/sample objects (preference trial). The interval between sample and test trials was adjusted to 5 min for selective testing of short-term working memory. The time animals spent exploring familiar and new objects were recorded to calculate recognition index (RI) as indicators of working memory.
Where TF and TN are the times spent interacting with the familiar and novel object, respectively.
2.4. Water maze test
The Morris water maze was used to assess animals’ spatial learning and memory through a combination of training and probe trials (Ahmed et al., 2018). The initial learning/training phase consisted of 28 total trials. These were conducted as single daily sessions of 4 trials, 60 s each and a trial interval of approximately 30 s, for 7 consecutive days. In brief, training trial animals were allowed to leam the location of the hidden escape platform submerged 2 cm below the surface of the water (25 ± 2 °C) in a circular water tank (132 cm diameter and 60 cm height), divided into four virtual quadrants. On each trial day, animals were allowed to swim freely on four trials (once from each starting position). If the animal failed to reach the escape platform within the maximal allowed time of 60 s, it was gently placed on the platform and allowed to remain there for 30s. Fixed flags on particular quadrants were used as spatial cues for animals. The time to reach the hidden platform underneath the water (escape latency) was recorded as the index of learning. Spatial reference memory and consolidation were assessed with a standard place task/ probe test, conducted 24 h (on day 8) after the last daily training session. The probe test was conducted by removing the platform and animals were allowed to swim freely in the pool for 60 s. The time spent and distance moved in the target quadrant indicated the degree of memory consolidation that had taken place 24 h after learning.
2.5. CatWalk Test
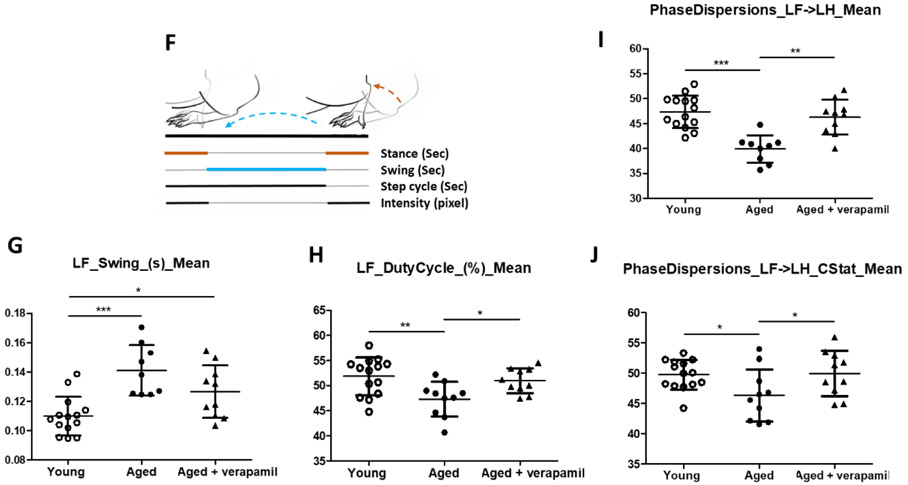
The Catwalk test was performed to get a detailed view of gait control parameters in aged animals, which is intimately affected by sensorimotor frailty (Herbin et al., 2007). The Catwalk XT system records animals’ footprint images and foot force profiles, captured by a camera mounted underneath a glass walkway (Lemieux et al., 2016). These initial recordings work as inputs into the CatWalk XT software package (Noldus Information Technology, Wageningen, The Netherlands) to calculate the specified indices. As shown in figure 5 F, basic measures of a gait cycles were obtained by the Catwalk system, to automatically calculate several variables including “Gait/Step cycle”, “Paw intensity” and “Inter-paw coordination”, each detailed in the supplementary materials. In brief, step cycle indicates the time needed to complete a single cycle of a step, with the duty cycle being the percentage of the whole step cycle that is covered by the stance phase. Paw intensity reflects the maximal weight over the paws and mirrors any gait shift during the gait cycle. Finally, “Inter-paw coordination”, mostly addressed by phase dispersion, reflects gait shift derangement, galloping, or limping, by comparing anchor paws’ step cycles.
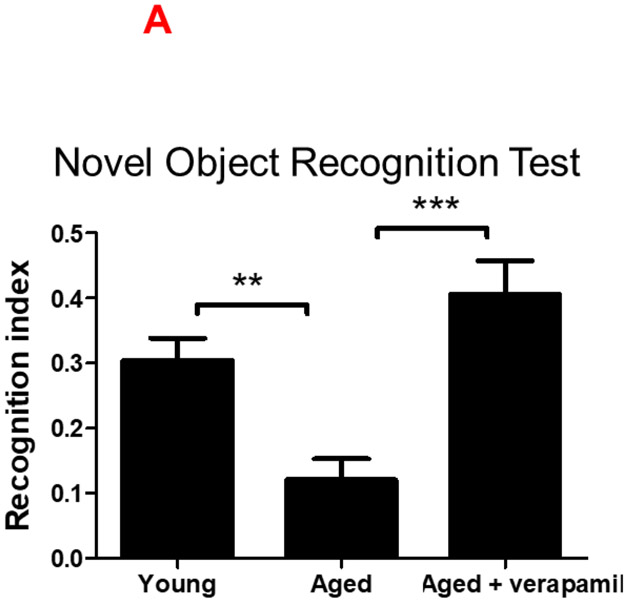
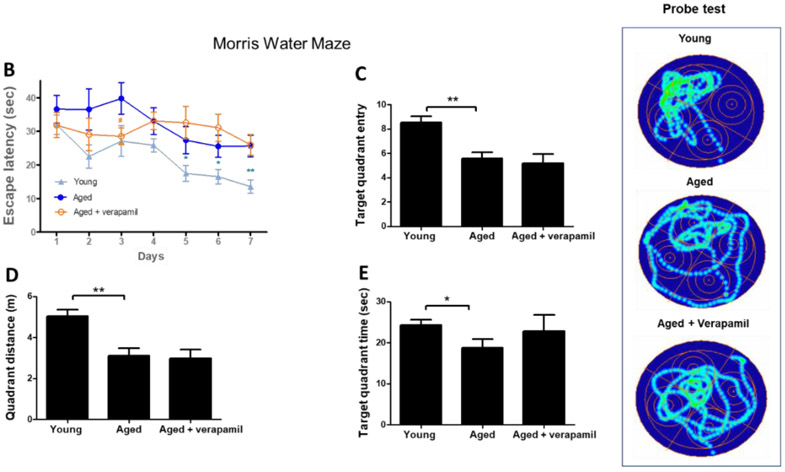
Figure 5: Effect of verapamil on cognitive decline in aged animals.
Aged (18 months) male C57BL/6 mice treated with verapamil (1 mg/kg/day, in drinking water, 1 months) were subjected to NOR and MWM tests and compared to aged and young (2 months) animals. In NOR test examining non-spatial working memory, verapamil treatment improved aged animals’ cognitive function assessed by recognition index (A). In the MWM test of spatial memory, aged animals demonstrated poor learning and memory as they failed to reduce escape latencies in training trials (B), correctly remember the target quadrant place (C), or have sufficient distance (D) or time (E) swimming in the target quadrant in MWM probe test. Our MWM did not real an effect of verapamil on aged animals’ spatial memory. The CatWalk XT system was used to calculate gait control indices based on basic measures of animals’ footprints on the walkway (F), as detailed in the methods section. Accordingly, aged animals demonstrated an upward shift from normal swing time (G), leading to reduced step cycle and thus less duty cycle (%) (H). Aged animals also showed degrees of limping and poor inter-paw coordination as determined by the phase dispersion mean (I) and the due cyclic statistic (J) values. Verapamil treatment improved age-related gait abnormalities and restore gait control indices to values comparable to those of young animals (G-J). Values are expressed as mean ±SEM. *p<0.05, **p<0.01, ***p<0.001; NOR, novel object recognition; MWM, Morris water maze; LF, Left Fore-paw; LH, Left Hind-paw.
2.6. Real-time PCR
Following RNA isolation, genomic DNA was removed and single-strand complementary DNA was synthesized using QuantiNova™ Reverse Transcription Kit (Cat.#205413, Qiagen, Germany). As described in the supplementary materials (Suppl. Mat. Tab. 1), specific oligonucleotide primer pairs for β-actin, Txnip, Nlrp3, Caspase1, Il-1β and Trx1 were selected for qPCR amplification (synthesized by Integrated DNA Technologies, IA) followed by real-time quantitative PCR performed using PowerUp™ SYBR™ Green Master Mix (Applied Biosystems) and Bio-Rad CFX connect™ Real-Time system (Serial#BR003568, Bio-Rad Laboratories, CA). The thermal cycler parameters were as follows: UDG activation at 50°C (2 min), and Dual-Lock™ DNA polymerase activation at 95°C (2 min), followed by amplification of cDNA for 40 cycles with denaturation at 95°C (15 sec) and annealing/extension at 60°C (1 min). All values were normalized using mouse β-actin mRNA level as an endogenous internal standard (synthesized by Integrated DNA Technologies, IA).
2.7. Western blotting
Standardized samples, prepared from mice (30-μg protein); were electrophoresed and transferred to PVDF membranes. They were then blocked with 5% skimmed milk and incubated with the specific primary antibodies, specified in supplementary file (Suppl. Mat. Tab. 2) at 4 °C overnight. The membranes were then incubated with a horseradish peroxidase–conjugated secondary antibody (1: 10,000, Sigma). The bands were then visualized by means of an enhanced chemiluminescent substrate system (Thermo Fisher Scientific) and analyzed by Image J software, normalized to loading controls, and expressed as fold change.
2.8. Immunofluorescence staining
For immunofluorescence staining, the animals were anesthetized with ketamine/xylazine and transcardially perfused with ice cold PBS (30 ml) followed by 50ml of 10% buffered formalin (Fischer Scientific). Brains were then removed and post-fixed in 10% buffered formalin overnight at 4 °C and then sequentially immersed in 30 % sucrose in PBS solution for 72 h. Brains were embedded in blocks of optimal cutting temperature (OCT) by rapid freezing on dry ice for 10 min. The brains were sectioned in the coronal plane at a thickness of 12-μm thickness were cut along the coronal plane using Leica cryostat. Sections from each group were blocked with Serum-Free Protein Block (X0909, DAKO) followed by incubation with primary antibodies specific primary antibodies (Suppl. Mat. Tab. 2). The sections were then washed three times followed by incubation with the respective Alexa Fluor- 488 (042-03-18-06) and /or Alexa Fluor −594 (A-11012) tagged secondary antibodies (1:200) for 1h at room temperature. For double staining, the process was repeated with primary antibodies against Neu-N (1:250; ABN78, Millipore), Iba-1 (1:250; WDF6884; WAKO) or GFAP (1:500; 20334, Dako). After single or double-staining, sections were mounted with ProLong™ Diamond Antifade Mountant with DAPI (Invitrogen), and viewed using a Zeiss 710 confocal laserscanning microscope. Negative controls were prepared by omitting the primary antibodies.
2.9. Statistical Analysis
Statistical analyses were performed using one-way ANOVA with GraphPad Prism Instat 7.0 software. Quantitative data in all graphs were presented using means ± SD (standard deviation of mean). Differences between animals of different age groups were evaluated by one-way ANOVA followed by Tukey’s post-hoc tests. Two-way ANOVA was used to analyze sex- and age-specific effects and interactions, followed by Bonferroni’s multi-comparison when required. Adjusted P values were reported to specify significant differences.
2.10. Online supplemental material
Fig S1. Comparing male and female mice brain for activity of NLRP3 inflammasome as well as TXNIP and TRX expression. Fig S2. Alterations in Akt signaling activity in aging mice brain. Fig S3. Effect of verapamil on BP measurements (A) and complementary catwalk outputs (B-D) in aged WT mice.
3. RESULTS
3.1. Aging Upregulates TXNIP in Male and Female Mice
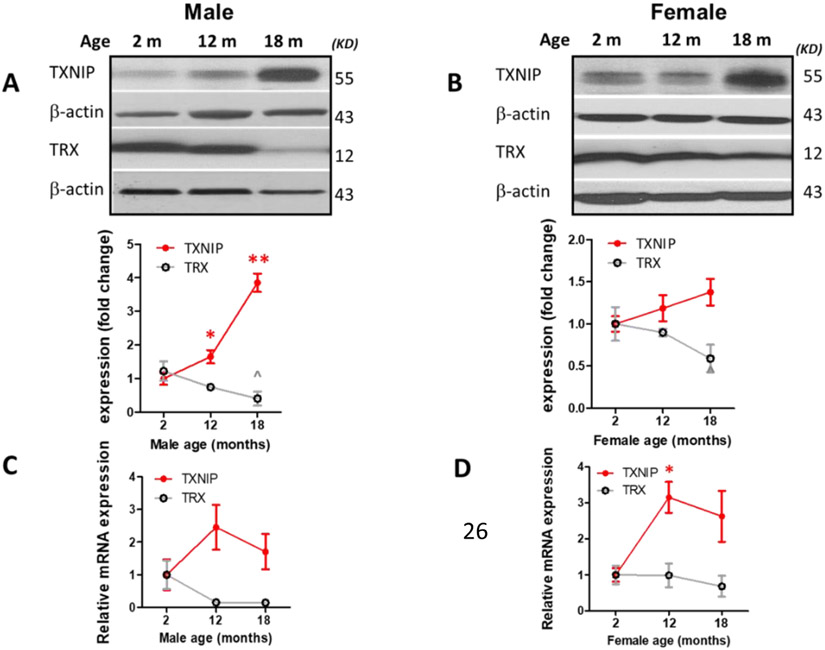
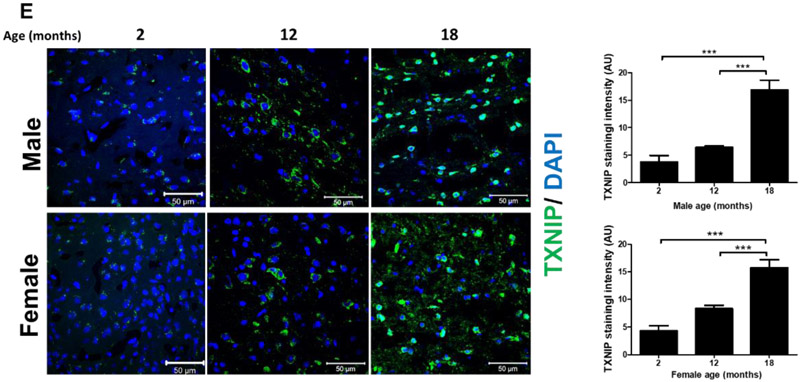
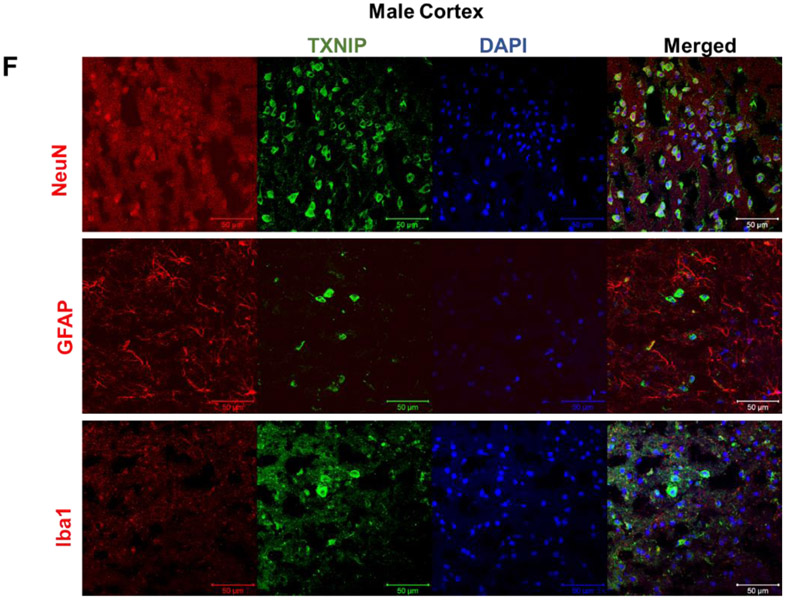
Based on whole brain immunoblot one-way ANOVA, indicating remarkable TXNIP alterations with aging [F (2, 11) = 46.78, P<0.0001], TXNIP expression showed a drastic increase in 18-month-old (aged) males (P<0.0001) (Fig. 1, A), with a similar, albeit nonsignificant trend in females (Fig. 1, B). This concurred with a significant change in TRX expression [F (2, 10) = 4.02, P=0.052], showing significant upregulation in aged compared to young animals (P=0.042). Nevertheless, our real-time PCR data indicates a non-significant trend toward enhanced TXNIP transcripts with aging, suggesting aging is more likely to impact thioredoxin system at post-transcriptional levels (Fig 1. C, D). In cerebral-cortical immunostaining examinations, profound TXNIP alterations [F (2, 13) = 38.12, P<0.0001] showed a remarkable increasing trend in aged males (P<0.0001) and aged females (P<0.0001 vs 12 month; P=0.0009 vs 2 month) (Fig. 1. E). Cell specific expression of TXNIP was further examined either in aging male or female cortical sections. Accordingly, age associated TXNIP upregulation mainly occurs in neural cytosols but also engages some glial cells (Fig 1. F).
Figure 1: Effect of aging on cerebral thioredoxin system components.
Male (A, C) and female (B, D) C57BL/6 mouse whole-brain homogenates were subjected to either immunoblotting or real-time PCR analysis at defined life stages: 2 months (young), 12 months (middle age) and 18 months (aged). Cerebral TXNIP showed a drastic upregulation, paralleled by TRX downregulation, particularly in aged male mice (A) compared to females (B). Along with protein upregulation, TXNIP transcripts showed an incremental trend with aging (C, D). The regional immunoreactivity of TXNIP similarly showed a remarkable upregulation in cortices of both sexes (E). Our double-stained cortical sections showed TXNIP is upregulated predominantly in vicinity of cells positive for NeuN (neurons) and in a lesser extent Iba-1 (microglial cell), but not GFAP (astrocytes) in aged males (F). Magnification 40×.Scale bar = 50 μm. Values are expressed as mean ± SEM. *p<0.05; ***p<0.001, ^p<0.05 vs younger counterparts. GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei; TXNIP, thioredoxin interacting protein; TRX, thioredoxin; DAPI, diamidino phenylindole.
3.2. NLRP3-inflammasome Activity Increases with TXNIP Upregulation and Aging
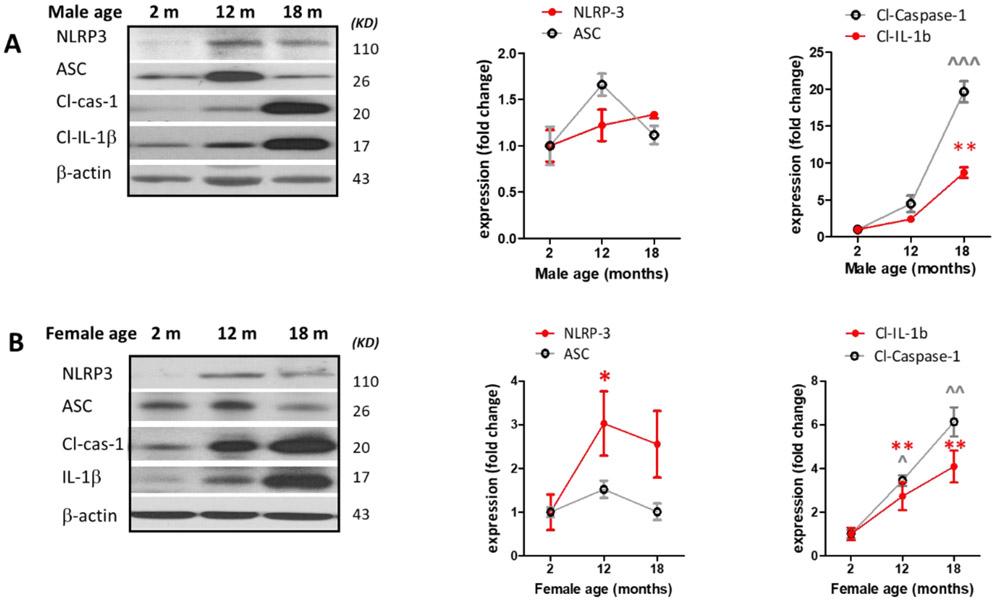
Our findings on NLRP3 inflammasome components (Fig.2. A, B) indicated a significant but transient ASC upregulation in middle-aged males (P=0.021), remarkable cleaved caspase-1 elevation in middle aged males (0.0017) as well as aged males (P<0.0001) and females (P=0.0013). This was paralleled with substantial rise in cleaved-IL-1β in aged males (P<0.0001) and females (P=0.0097). Although NLRP3 and ASC alterations were subtle in our aged animal brains, the remarkable rise in their prominent pro-inflammatory products, cleaved IL-β and cleaved caspase-1, substantiates increasing NLRP3-inflammasome cleavage activity with aging.
Figure 2: Effect of aging on NLRP3 inflammasome activity in mice brain.
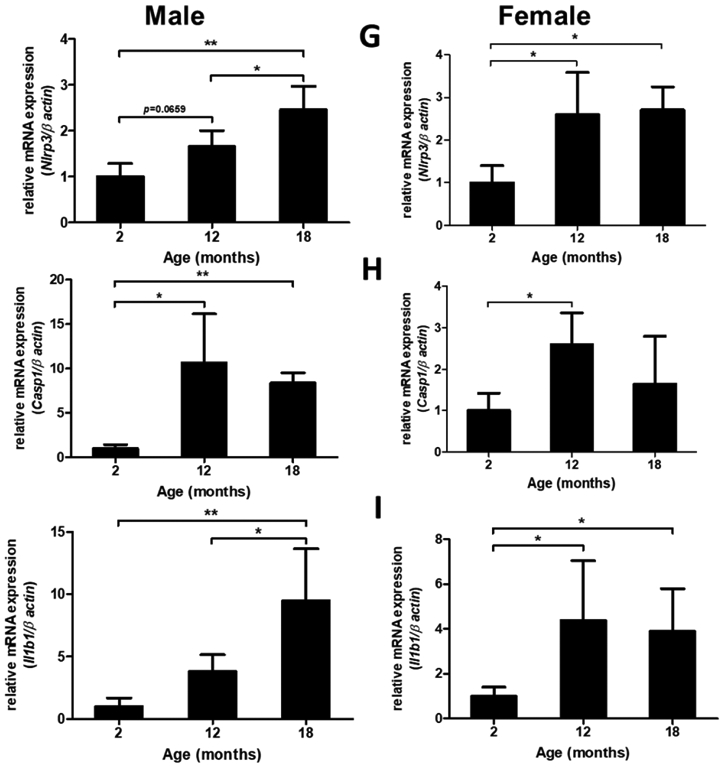
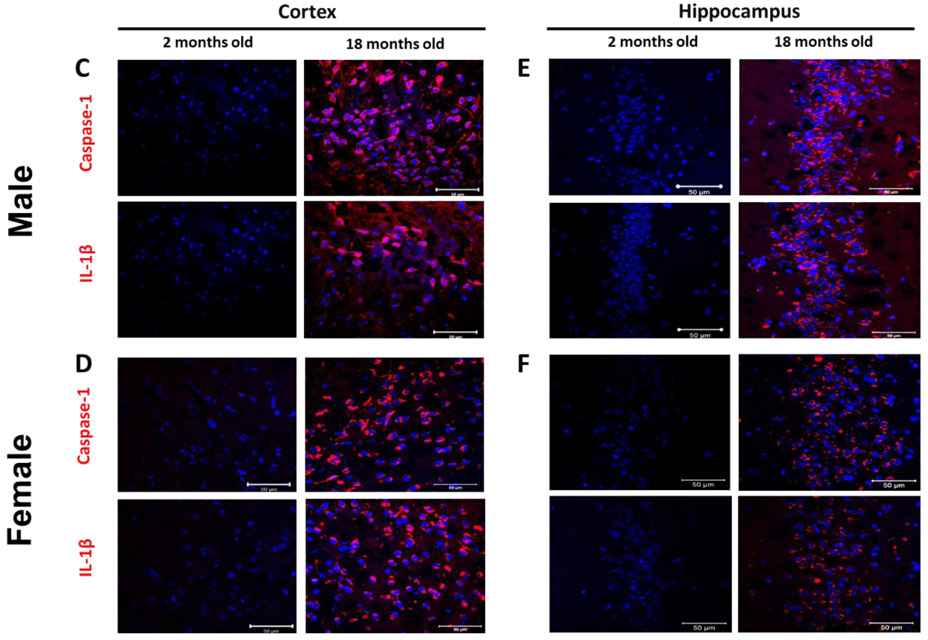
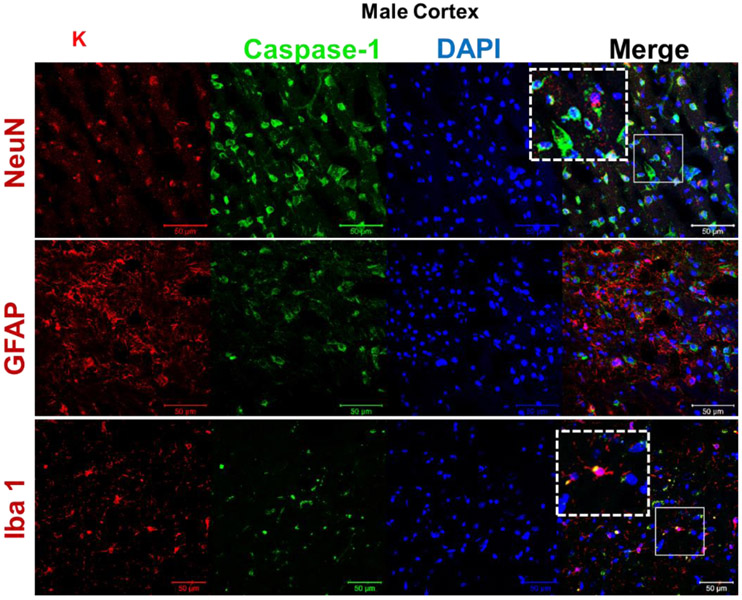
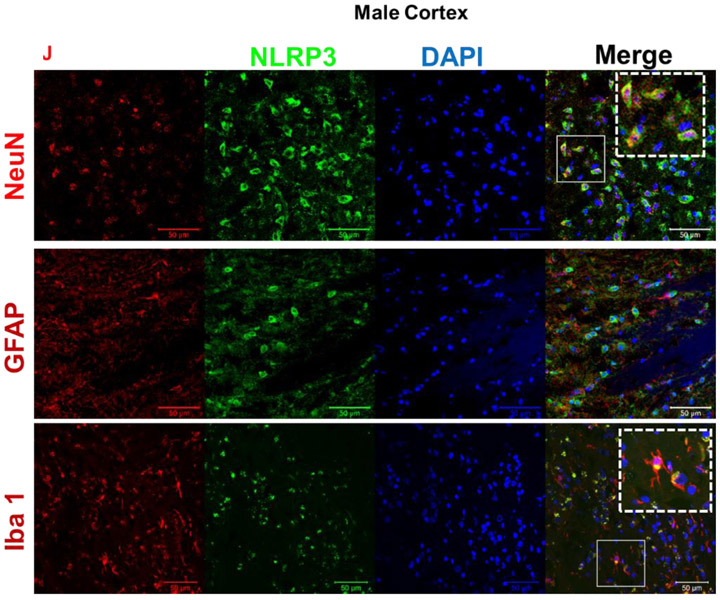
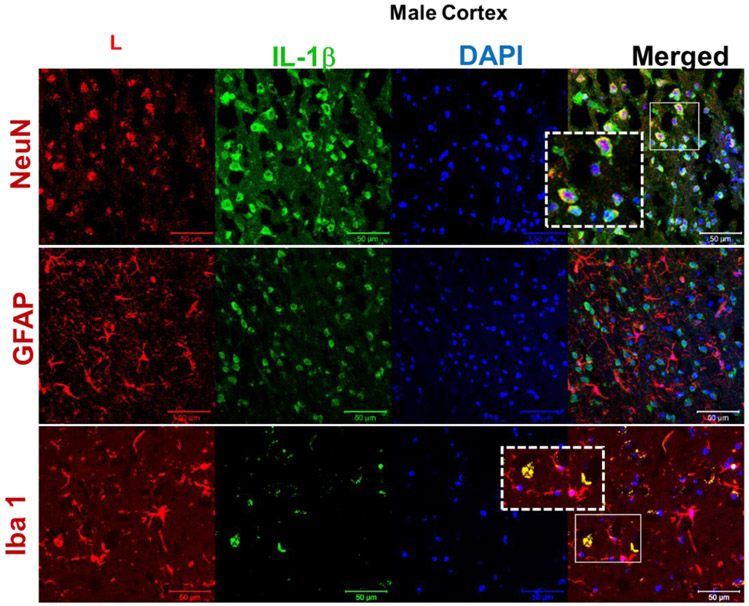
Male (A) and female (B) C57BL/6 mice whole brains homogenates were subjected to immunoblotting at defined ages: 2 months (young), 12 months (middle age) and 18 months (aged). Expression of inflammasome components NLRP3 and ASC showed a modest incremental trend in middle-aged mice. However, the inflammasome activity kept rising in aged brains showing high levels of Cl-caspase-1 and Cl-IL-1β. Immunostaining of males (C, D), females (E, F) cortical, and hippocampal sections confirmed the profound rise in Cl-caspase-1 and Cl-IL-1β in aged animals. While transcripts of NLRP3 (G) showed a discernible rise in both aged males and females compared to young animals, elevation in caspase-1 (H) and IL-1β (I) age-associated priming was more pronounced in male brains. Our double-stained cortical sections indicated in the same manner with TXNIP (see figure 1), NLRP3 (J) caspase-1 (K) and IL-1β (L) are upregulated predominantly in the vicinity of cells positive for NeuN (neurons) and to lesser extent Iba-1 (microglial cells), but not GFAP (astrocytes) in aged males. Magnification 40×. Scale bar = 50 μm. Values are expressed as mean ±SEM. *p<0.05, **p<0.01 and ^p<0.05, ^^p<0.01, ^^^p<0.001, vs younger counterparts. NLRP3, nucleotide oligomerization domain (NOD)-like receptor protein-3; ASC, apoptosis associated speck-like; Cl-Cas-1, cleaved caspase-1; Cl-IL-1β, cleaved interleukin-1 beta; GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei; DAPI, diamidino phenylindole.
These alterations were confirmed, in both sexes, by immunofluorescent staining of cortical (Fig. 2. C, D) and hippocampal (Fig. 2. E, F) sections. Based on our mRNA analysis data (Fig. 2. G-I), along with aging the transcripts of NLRP3 infammasome components increase with more remarkable changes in aged males, showing a significant rise in NLRP3 (P=0.0037) and IL-1β (P=0.008) transcripts. Two-tailed T test could also specify a significant rise in caspase-1 transcripts (P= 0.0005) in aged males’ whole brain homogenates supporting western blot observations showing a sharp increase of IL-1β in aged male brains, compared with female ones. NLRP3, caspase-1 and IL-1β immunostaining signals were mostly detectable in neurons and to a less extent in microglial cells (Fig. 2. J-K).
3.3. TXNIP/NLRP3-Inflammasome Demonstrate Differential Upregulation in Aged Male and Female Mice
Using two-way ANOVA we compared quantified data obtained from age-matched immunoblots to detect sex-specific effects in age-linked cerebral NLRP3-inflammasome hyperactivity. Age and sex did not show any interaction in NLRP3 [F (1, 6) = 4.427, P=0.08], ASC [F (1, 13) = 0.02268, P=0.88], Cl-caspase-1 [F (1, 6) = 5.955, P=0.0505], IL-1β [F (1, 8) = 3.933, P=0.082], Age was the main factor to induce the changes. In ASC expression however, sex was exceptionally the main source of variation. Accordingly, Bonferonni’s post-hoc multi-comparison showed a significant rise in both young (P=0.012) and aged (P=0.013) females compared to age-matched males. In contrast, there was a male-specific upregulation in cleaved caspase-1 (P=0.014), with a remarkable increase in cleaved IL-1β suggesting higher NLRP3-inflammasome activity (Suppl. Fig. 1. A, B). This was consistent with higher age-dependent TXNIP overexpression in males (P=0.0002; young vs aged) and might explain the relatively higher TRX in females (youth P=0.0006, aging P=0.475) compared to age-matched males. Both male and female aged animals showed remarkable TRX reduction with aging (Suppl. Fig. 1 C). Statistics on the mRNA analysis did not show any significant changes between males and females, suggesting sex-specific TXNIP/NLRP3-inflammasome activation mainly relies on post-transcriptional modifications with aging (Suppl. Fig. 1 D).
3.4. Klotho Depletion Associates TXNIP Upregulation in Aged Brains
To postulate any potential link between TXNP upregulation with aging, levels of Akt signaling and Klotho protein were analyzed for effects of age and sex factors. Akt signaling is intimately involved in insulin effects and glucose consumption, at least partly tough tuning TXNIP level. Restraining TXNIP pro-oxidant and pro-inflammatory activity appears to be an emerging effector of Akt prosurvival signaling (Huy et al., 2018; Nasoohi et al., 2018; Waldhart et al., 2017). As analyzed by two-way ANOVA, age and sex did not show any interaction in Akt/p-Akt ratio [F (1, 10) = 2.387, P=0.1534], indicating parallel changes. Aging appeared as the main source of difference rather than sex, causing drastic TXNIP upregulation (P=0.0176) in aged males (Fig. 3 A). Noteworthy, the moderate Akt activation with aging (P=0.046) did not precede TXNIP upregulation in examined life span points, (Suppl. Fig. 2), suggesting the rise in TXNIP is likely a consequence of other late-age associated traits. In contrast, we found profound Klotho protein reduction in aged brains in parallel with higher TXNIP levels (Fig. 3. B). Two-way ANOVA did not show any interaction between the effects of age and sex on Klotho depletion [F (1, 12) = 10.64, P=0.0068], due to reducing trend in both sexes. Klotho age-related depletion was prominent (P=0.036) merely in aged males based on the Bonferroni’s multi-comparison output. However, T-test comparison showed a milder reduction in females’ klotho with aging (P=0.08). The “antiaging hormone” Klotho has an established impact to boost cellular antioxidant capacity which has been partly ascribed to limiting TXNIP expression and activity, in particular cell types (Zhu et al., 2018). Thereby the pronounced TXNIP/NLRP3 inflammasome hyperactivity could be rationally linked to Klotho downregulation, particularly in males.
Figure 3: Klotho depletion parallels upregulation of Akt activity with aging.
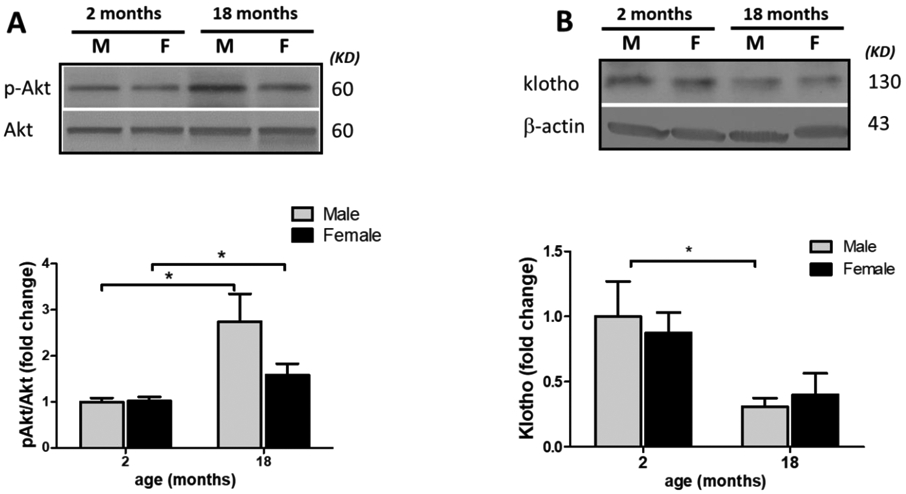
To explain the remarkable rise in TXNP/NLRP3 inflammasome signaling, wild type aged (18 months) and young (2 months) animals, whole brain were probed for Klotho and Akt signaling as presumptive TXNIP regulators. As determined by p-Akt/Akt ratio both males and females showed an increasing trend in Akt activation with aging, which was more eye-catching in males (A). Interestingly, male brain immunoblots demonstrated more significant Klotho depletion with aging (B). Values are expressed as mean ± SEM. *p<0.05 vs younger counterparts.
3.5. Genetic and Pharmacological inhibition of TXNIP Prevents Age-associated NLRP3-inflammasome Upregulation
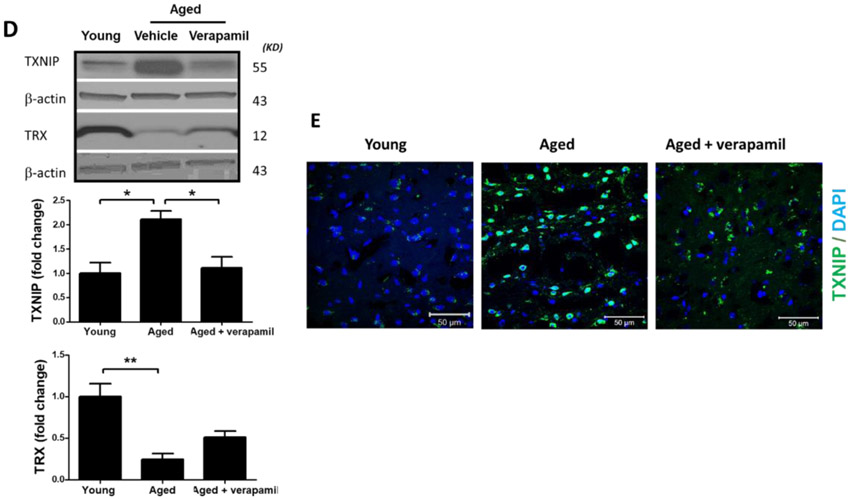
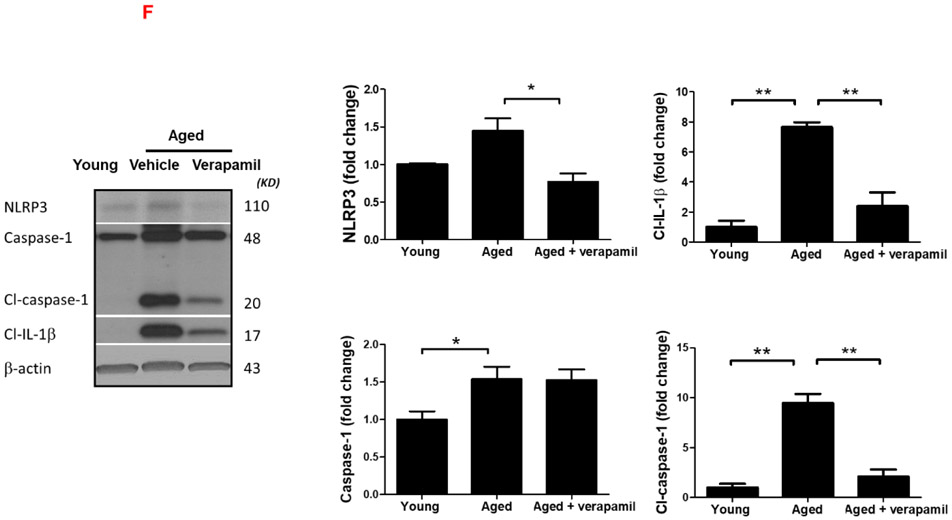
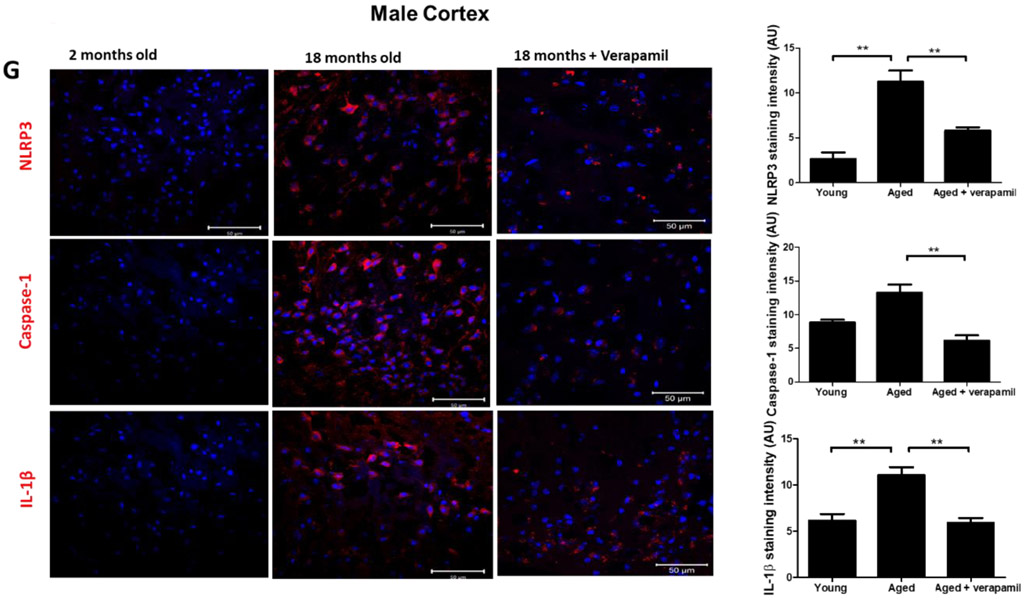
Aged animals’ Txnip−/− brains demonstrated a significant attenuation in age-related cleaved caspase-1 and cleaved IL-β upregulation, indicative of repressed NLRP3 activity, which reversed TRX downregulation in aged brains (Fig. 4. A-C). Tukey’s post-hoc following one-way ANOVA showed Txnip knockout reduces cleaved caspase-1 (P<0.0001) and IL-1β (P=0.0001), in aged male brains. Using T-test, TRX showed also tangible upregulation in Txnip−/− brains compared to wild type aged ones (P=0.0007). For pharmacological modulation of TXNIP, we dosed aged animals with verapamil, an L-type Ca++ channel blocker, which is recently identified to suppress TXNIP expression (Melone et al., 2018). Verapamil (1mg/kg/day, 1 month) strongly suppressed TXNIP expression as demonstrated by Tukey’s multi-comparison (P=0.017) (Fig. 4. D). This was further confirmed by our immunostaining findings which also verified TXNIP downregulation in treated animals (Fig. 4. E). Based on one-way ANOVA, verapamil treatment also caused significant decrease in NLRP3 (P=0.0092), cleaved caspase-1 (P<0.0001) and IL-1β (P=0.007), compared to not-treated aged mice (Fig. 4. F). Consistently signal density analysis confirmed a significant repression in NLRP3 (P=0.004), caspase-1 (P=0.0002) and IL-1β (P<0.0001) in aged animals dosed with verapamil (Fig. 4. G).
Figure 4: TXNIP ablation reverses age associated NLRP3-inflammasome upregulation.
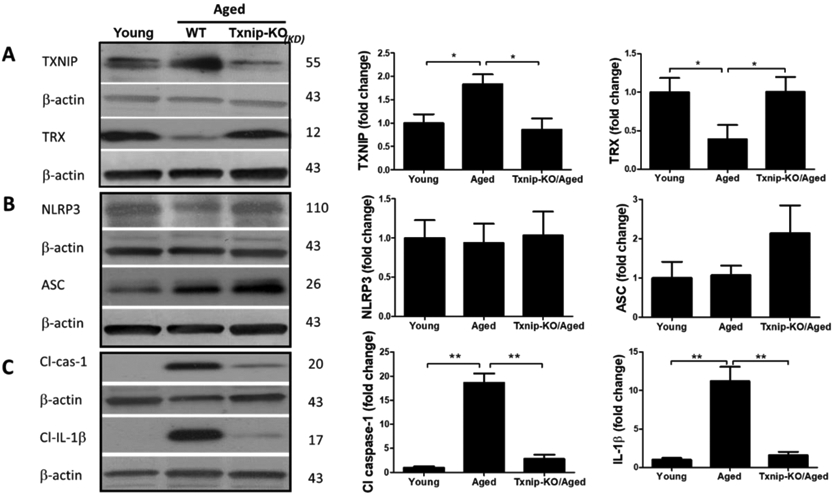
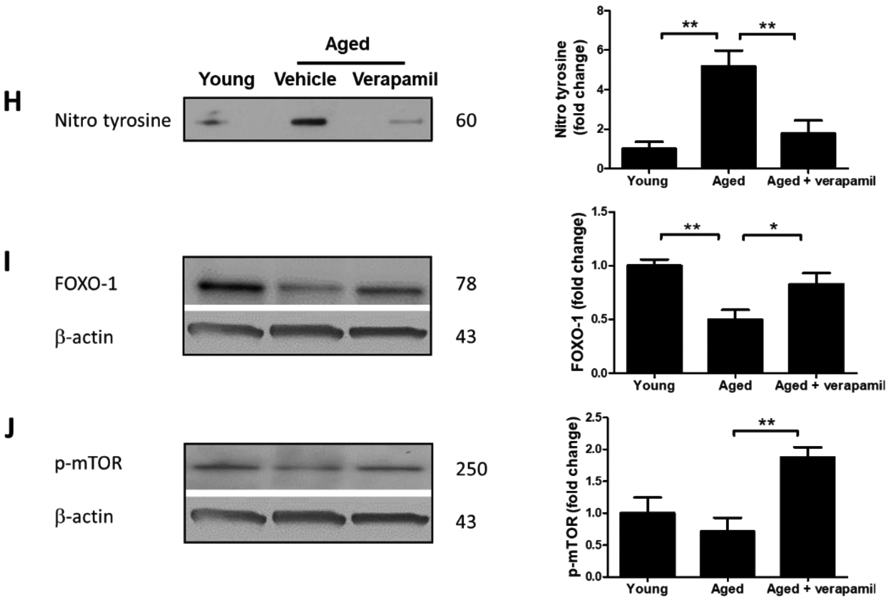
TXNIP-KO aged (18 months) male C57BL/6 mice brains were probed for components of NRLP3-inflammasomes and compared to wild-type aged and young (2 months) animals. As shown in representative blots, Txnip knock out in aged brains efficiently reduced TXNIP and increased TRX (A) to levels comparable to young wild-type animals. With no difference in NLRP3 protein, TXNIP-KO brains showed a trend toward a higher expression of ASC (B). The cleavage activity of NLRP3 was dramatically repressed in KO aged mice as determined by far lower levels of Cl-Cas-1 and IL-1β (C) compared to wild-type counterparts. Treatment with verapamil (1 mg/kg/day, p.o., 1 months) produced similar suppressive effects on TXNIP as demonstrated by immunoblotting (D) and immunostaining data (E). This concurred with substantial inhibition of the NLRP3-inflammasome, Cl-Cas-1 and IL-1β in aged male brains as identified by immunoblotting (F) and confirmed by cortical immunostaining examination in aged males (G). According to densitometric analysis of the immunoblots, verapamil treatment significantly reduced nitro tyrosine levels (H), reflecting peroxynitrile level and oxidative stress, which might be explained partly for restoring FOXO-1 levels (I) in aged brains. This was consistent with improved mTOR activity (J). Magnification 40×. Scale bar = 50 μm. Values are expressed as mean±SEM. *p<0.05, **p<0.01; ***p<0.001. TRX, Thioredoxin; TXNIP, TRX interacting protein; NLRP3, nucleotide oligomerization domain (NOD)-like receptor protein-; ASC, apoptosis associated speck-like; Cl. Caspase-1, cleaved caspase-1; IL-1β, cleaved interleukin-1 beta; FOXO, Forkhead box protein O; mTOR, mammalian target of rapamycin.
Verapamil was also efficient enough to reduce nitro tyrosine (P=0.008), an indicator of superoxide–dependent peroxynitrite formation (Fig. 4. H), as well as FOXO-1 protein level (P=0.059) (Fig. 4. I), which has an established role in superoxide dismutase and catalase gene expression and protection against oxidative stress (Burgering and Medema, 2003). Notably, verapamil also boosted mTOR activity in aged animals, to levels higher than we observed in their young counterparts (P=0.0055) (Fig. 4. J).
3.6. Verapamil Improves Functional Decline along with TXNIP Downregulation in Aged Animals
To assess spatial and non-spatial working memory, we compared our MWM and NOR observations in aged animals receiving verapamil at the 1 mg/kg/day (p.o., 1 month) dosage which did not affect systemic blood pressure (Suppl. Fig. 3.A). Tukey’s post-hoc comparison following ANOVA [F (2, 22) = 12.86, P=0.0002], indicated verapamil consumption produced a strong rejuvenating effect on aged animals’ cognitive abilities, as determined by the improved recognition index (P=0.0002) (Fig. 5. A). However MWM analysis did not show any protective effect of verapamil on spatial memory, efficiently differentiated aged animals from young counterparts. Accordingly, aged mice demonstrated increased escape latency time in days 5 afterwards (Fig. 5 B), and reduced entries to the target quadrant (P= 0.0048), as well as less distance and time spent moving in the quadrant (Fig. 5 C-E). Although the likely age-associated low locomotion may shadow our conclusions MWM conclusions, our main comparisons between vehicle and verapamil treatment could not get affected.
Verapamil oral administration in aged animals could also improve gait control indices, which are simplified in the pictorial description of figure 5. F. Based on ANOVA statistics, our aged mice showed longer swing time (P=0.0002) and lower duty cycle values (P=0.0068), indicating proportionally reduced stance phase compared to swing phase (Fig. 5 G, H). Interestingly, verapamil treatment restored gait cycle, as indicated by elevated duty cycle (P=0.050) and partially improved swing time (T-test, P=0.089) in aged animals. Verapamil also improved paw force in aged mice, as determined by right hind paw (RH) maximal intensity values (P=0.0217) (Suppl. Fig. 3. B), probably as a consequence of the elevated duty cycle and higher shift in weight between paws during stance. According to the phase dispersion of LH -> RH, no difference was found between aged and young animals, indicating that girdle hind paws showed similar coordination patterns (Suppl. Fig. 3. C). Nevertheless, lateral pairs (LF -> LH) showed a discernible upwards shift compared in aged verapamil treated animals, both in phase dispersion mean (P=0.0004) and phase dispersion Cstat mean (P=0.066) (Fig. 5. I, J), with no significant change in the coupling values (Suppl. Fig. 3. D).
4. DISCUSSION
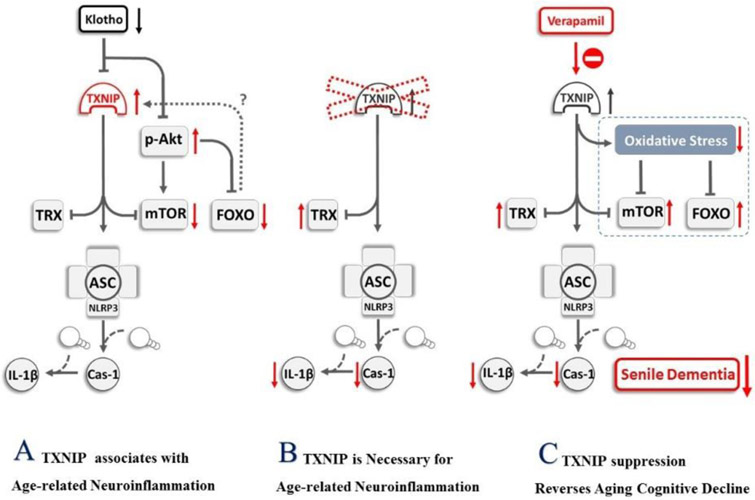
Recent evidence from human studies suggests that the thioredoxin system has a pivotal role in preserving brain integrity and cognitive function (Aydın et al., 2018; Bas et al., 2017; Glade, 2010). Our findings provide new insights into the requirement for TXNIP in establishment of age-linked neuroinflammation via its activation of the NLRP3 inflammasome. We demonstrated that as levels of TXNIP and the components of the NLRP3 inflammasome increased, the anti-aging protein klotho was depleted, suggesting a model in which klotho may drive inflammaging through regulation of TXNIP expression (Fig. 6). Our results showed induction of the NLRP3 inflammasome could be reversed when TXNFP is repressed, either via stable Txnip KO or by treatment of elderly mice with verapamil, leading to gain-of-function phenotypes in learning and memory and in mobility (gait). Ultimately, these findings will lead to a greater understanding of how reversal of TXNIP/NLRP3 inflammasome induction might help preserve neurological health in elderly human populations.
Figure 6: Pictorial description of txnip role in brain inflammaging and cognitive decline.
According to our findings and others, (A) aging-induced klotho depletion upregulates TXNIP in close association with NLRP3-inflammasome hyperactivity. This might be ascribed to enhanced Akt signaling to prevent more FOXO-1 from its transcriptional activity (B) Profound repression of NLRP3-inflammasome activity in our TXNIP-KO aged animals provides solid evidence that age-related neuroinflammation and thioredoxin insufficiency is mediated by TXNIP upregulation. (C) Administration of verapamil as a non-specific TXNIP inhibitor reduced NLRP3-inflammasome activity in aged brains and mitigated oxidative stress, concurring with FOXO-1 and mTOR upregulation and improved function in aged mice. Verapamil efficiently restored cognitive decline and gait abnormality in aged mice, suggesting TXNIP involvement in age-related functional decline. TRX, Thioredoxin; TXNIP, TRX interacting protein; NLRP3, nucleotide oligomerization domain (NOD)-like receptor protein-3; ASC, apoptosis associated speck-like; Cl. Caspase-1, cleaved caspase-1; IL-1β, cleaved interleukin-1 beta; FOXO, Forkhead box protein O; mTOR, mammalian target of rapamycin.
The age-associated TXNFP increasing trend in our aged animal brains concurred with TRX downregulation and was consistent with earlier reports of TXNIP upregulation in aged retina (Bartoli et al., 2007) and the brain of a D-galactose amine-induced aging model (Sun et al., 2015). More recently, age-dependent upregulation of TXNIP, has been speculated to perturb intracellular redox equilibrium in T-cells (Oberacker et al., 2018) and platelets (Sverdlov et al., 2013). Our understanding of naturally aging brains has been confined to a microarray study demonstrating that rise TXNIP transcripts in both aged men and women (Cribbs et al., 2012). This is in agreement with a more recent study indicating an obvious elevation in TXNIP transcripts in Alzheimer’s disease (AD) brains (Hokama et al., 2013). In a cell-specific manner, TXNIP is differentially regulated at either transcriptional and translational levels (Nasoohi et al., 2018). Akt signaling as an important regulator of TXNIP, shows a region-specific change within aging brain (Jackson et al., 2009; Orellana et al., 2015; Song et al., 2007), and is affected by TXNIP itself (Huy et al., 2018). Whereas post-translational phosphorylation of TXNIP provides an immediate way for Akt to restrain TXNIP activity, Akt phosphorylated FoxO remains in the cytoplasm and unable to enhance TXNIP transcription. Consistently, our aged animal brains showed a significant fall in un-phosphorylated FOXO-1 along with Akt hyperactivity with aging. Still however, given both TXNIP and Akt/mTOR showed parallel elevation in aged male and female mice, probably they are not likely regulated by the other. Intriguingly, although with some discrepancies (Zeldich et al., 2014), klotho has been also shown to inhibit Akt activity to restrain transcriptional activity of FOXO-1 (Lim et al., 2017; Yamamoto et al., 2005). Our findings on age-associated alteration in Klotho, Akt and FOXO-1 is well in line with such hypothesis. Still however, any causative link between FOXO-1 and TXNIP upregulation in our aged brains requires further verification, given the effect of FOXO-1 to induce (Li et al., 2009; Yamaguchi et al., 2013) or suppress (Kibbe et al., 2013) TXNIP transcription appears to be context sensitive. In this connection, age-associated klotho depletion in our samples showed a reciprocal correlation with TXNIP upregulation. Given the solid evidence linking klotho to TXNIP/NLRP3-inflammasome suppression (Zhu et al., 2018), this may suggest that age-related TXNIP upregulation is a consequence of profound klotho depletion. This might be further affirmed with higher TXNIP levels in aged male brains demonstrating strong klotho reduction. Since FOXO-1 has been mostly described to enhance TXNIP transcription, the presumptive Klotho/Akt/FOXO-1 link does not seem to work as a mechanism; however, the accumulative ROS injury consequent to Klotho depletion appears enough for TXNIP buildup with aging.
In agreement with earlier findings (Lee et al., 2015; Moreno-Ulloa et al., 2014), our aged animal brains manifested marginal TRX depletion at mRNA and protein levels, hypothetically because of TXNIP upregulation. This hypothesis was later confirmed in additional experiments showing that aged Txnip knockout (Txnip−−) mice completely retain TRX levels.
Beyond the regulatory effect on TRX, TXNIP upregulation paralleled with NLRP3-inflammasome activation rather than its priming in aging brains suggests a causative link. Further comparisons between Txnip−/− and wild-type aged male mice showed that Txnip knock out is sufficient to reduce NLRP3-inflammasome activity to the normal juvenile levels. Although these finding provides compelling evidence supporting a triggering role of TXNIP in brain inflammaging, TXNIP governing role appears to be context-sensitive. In fact recent findings indicate TXNIP deletion does not always completely reverse caspase-1 and IL-1β activation (Yang et al., 2019), and further NLRP3 inflammasome-independent mechanisms are involved in caspase-1 activation (Tschopp et al., 2010). Interestingly, our observations with caspase-1 and IL-1β were replicated in aged wild-type animals receiving verapamil, a non-specific TXNIP inhibitor. In comparison however, verapamil could not restore TRX levels to the similar degrees in aged Txnip−/−animals.
Although we sought verapamil treatment as a pharmacological tool to modulate TXNIP, one cannot ascribe its benefits merely to TXNIP modulation. In fact, a number of studies have shown that treatment with calcium channel antagonists can ameliorate learning and memory impairments in aged animals, mostly ascribed to reversal of age-associated disturbances in calcium metabolism (Thompson et al., 1992; Yamamoto et al., 1995) and protection against oxidative stress and excitotoxicity (Jackson et al., 2018; Nimmrich and Eckert, 2013). Verapamil prevents the binding of carbohydrate response element binding protein (ChREB) to the promotor region of the TXNIP, thereby it regulates the transcription of TXNIP (Xu et al., 2012). ChREBP is a glucose-activated transcription factor that regulates glucose and lipid metabolism (Iizuka, 2017). Here however, our data provides the first mechanistic evidence indicating benefits of verapamil concurs with strong TXNIP inhibition, plausibly leading to remarkable repression of oxidative stress, in parallel with FOXO-1 and mTOR upregulation. Presumably, while reduced oxidative stress may explain mTOR hyperactivity (Xi et al., 2018), verapamil-induced TXNIP repression may also contribute to the drastic rise in mTOR activity through Redd1 modulation (Jin et al., 2011). TXNIP downregulation could reasonably end with more glucose influx and energy availability, decreasing AMPK control over mTOR signaling (Nasoohi et al., 2018).
Either in age-related cognitive or gait control decline, our data highlight the association between verapamil-induced TXNIP repression and functional retention. Given the presumptive impact of animals’ weight and locomotion, memory indices in MWM might be affected by, conclusions on comparisons between big age gaps should be made conservatively. Comparing aged animals however, we did not detect a significant benefit from verapamil in MWM test. This might be ascribed to the particularly stressful MWM conditions, lowering its inter-individual sensitivity for aged animals (Harrison et al., 2009; NancyEllen et al., 2006). Analyzing NOR test and CatWalk indices as well, we could detect substantial benefit of verapamil in memory impairement of aged animals. Corresponding changes in young and aged mice CatWalk indices, also provide an extended view and further information to the existing knowledge on senile sensorimotor abnormalities in preclinical rodent models (Mock et al., 2018).
5. CONCLUSION
Supporting the free radical theory of aging, TXNIP deletion has been found to increase lifespan (Oberacker et al., 2018). Beyond its pro-oxidant activity, TXNIP bridges age-associated neuroinflammation to resistance in insulin receptors highly concentrated in cerebral regions involved in cognition (Reagan et al., 2001; Unger et al., 1991). Regarding the role of the NLRP3-inflammasome in linking systemic inflammation to functional decline in aging (Youm et al., 2013), our findings highlight TXNIP’s role in age-associated dementia and support promising rejuvenating effects of verapamil (Fig. 6). Data from tandem mass analysis (Ping et al., 2018) and functional human brain imaging have unveiled intriguing knowledge of a spatial correlation between genes implicated in glucose metabolism and AD pathology, as the typical senile dementia (Mullins et al., 2017). This supports the hypothesis that enhanced TXNIP transcription may perturb brain metabolism, stimulate NLRP3-inflammasome and contribute to senile dementia. To offset the physiological anti-tumorigenic effects of TXMIP further investigations are required to selectively target its deteriorating pro-oxidant activity, to approach promising translational implementation.
Supplementary Material
Highlights:
Aging upregulates TXNIP/NLRP3 inflammasome in the brain.
TXNIP knock-out prevents age-related NLRP3-hyperactivity and neuroinflammation.
TXNIP modulation with verapamil mitigates senile neuroinflammation and dementia.
ACKNOWLEDGEMENTS
This work was supported by startup fund, Department of Anatomy and Neurobiology, UTHSC Memphis TN (TI); National Institute of Health: R01-NS097800 (TI) and R01- AG058467 (FFL).
Abbreviations
- TRX
Thioredoxin
- TXNIP
TRX interacting protein
- NLRP3
nucleotide oligomerization domain (NOD)-like receptor protein-3
- ASC
apoptosis associated speck-like
- Cl. Caspase-1
cleaved caspase-1
- IL-1β
interleukin-1 beta
- Cl. IL-1β
cleaved IL-1β
- FOXO
Forkhead box protein O
- mTOR
mammalian target of rapamycin
- MWM
Morris Water Maze
- NOR
Novel Object Recognition
Footnotes
Compliance with Ethical Standards Conflict of Interest:
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A PediatriaAbais JM, et al. , 2015. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 22, 1111–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed HA, et al. , 2018. Role of angiotensin system modulation on progression of cognitive impairment and brain MRI changes in aged hypertensive animals–A randomized double-blind pre-clinical study. Behavioural brain research. 346, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydın EP, et al. , 2018. Thioredoxin is not a marker for treatment-resistance depression but associated with cognitive function: An rTMS study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 80, 322–328. [DOI] [PubMed] [Google Scholar]
- Bartoli M, et al. , 2007. Decreased antioxidant capacity in the aged and in the diabetic retina correlates with altered regulation of the thioredoxin system. Investigative Ophthalmology & Visual Science. 48, 3643–3643. [Google Scholar]
- Bas A, et al. , 2017. Level of serum thioredoxin and correlation with neurocognitive functions in patients with schizophrenia using clozapine and other atypical antipsychotics. Psychiatry research. 247, 84–89. [DOI] [PubMed] [Google Scholar]
- Burgering BM, Medema RH, 2003. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. Journal of leukocyte biology. 73, 689–701. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, et al. , 2012. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. Journal of neuroinflammation. 9, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Fabbri E, 2018. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology. 15, 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glade MJ, 2010. Oxidative stress and cognitive longevity. Nutrition. 26, 595–603. [DOI] [PubMed] [Google Scholar]
- Goldberg EL, Dixit VD, 2015. Drivers of age-related inflammation and strategies for healthspan extension. Immunological reviews. 265, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flarrison F, et al. , 2009. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behavioural brain research. 198, 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flerbin M, et al. , 2007. Gait parameters of treadmill versus overground locomotion in mouse. Behavioural brain research. 181, 173–179. [DOI] [PubMed] [Google Scholar]
- Hokama M, et al. , 2013. Altered expression of diabetes-related genes in Alzheimer's disease brains: the Hisayama study. Cerebral cortex. 24, 2476–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huy H, et al. , 2018. TXNIP regulates AKT-mediated cellular senescence by direct interaction under glucose-mediated metabolic stress. Aging cell. 17, e12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lizuka K, 2017. The Role of Carbohydrate Response Element Binding Protein in Intestinal and Hepatic Fructose Metabolism. Nutrients. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, et al. , 2006. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behavioural brain research. 171, 9–16. [DOI] [PubMed] [Google Scholar]
- Ishrat T, et al. , 2015. Thioredoxin-interacting protein: a novel target for neuroprotection in experimental thromboembolic stroke in mice. Molecular neurobiology. 51, 766–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DA, et al. , 2018. Prevention of severe hypoglycemia-induced brain damage and cognitive impairment with verapamil. Diabetes. 67, 2107–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TC, et al. , 2009. Regional hippocampal differences in AKT survival signaling across the lifespan: implications for CA1 vulnerability with aging. Cell death and differentiation. 16, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, et al. , 2011. TXNIP potentiates Redd1-induced mTOR suppression through stabilization of Redd1. Oncogene. 30, 3792. [DOI] [PubMed] [Google Scholar]
- Kibbe C, et al. , 2013. FOXO1 competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic beta cells. Journal of Biological Chemistry. 288, 23194–23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, et al. , 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T, et al. , 2013. TXNIP shuttling: missing link between oxidative stress and inflammasome activation. Frontiers in Physiology. 4, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Duewell P, NLRP3 inflammasome activation in inflammaging. Seminars in immunology, Vol. 40. Elsevier, 2018, pp. 61–73. [DOI] [PubMed] [Google Scholar]
- Lee CH, et al. , 2015. Differences in the protein expression levels of Trx2 and Prx3 in the hippocampal CA1 region between adult and aged gerbils following transient global cerebral ischemia. Molecular medicine reports. 12, 2555–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M, et al. , 2016. Speed-dependent modulation of the locomotor behavior in adult mice reveals attractor and transitional gaits. Frontiers in neuroscience. 10, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. , 2009. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochemical and biophysical research communications. 381, 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SW, et al. , 2017. Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell death & disease. 8, e2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone MAB, et al. , 2018. Verapamil Inhibits Ser202/Thr205 Phosphorylation of Tau by Blocking TXNIP/ROS/p38 MAPK Pathway. Pharmaceutical research. 35, 44. [DOI] [PubMed] [Google Scholar]
- Mock JT, et al. , 2018. Gait Analyses in Mice: Effects of Age and Glutathione Deficiency. Aging and disease. 9, 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Ulloa A, et al. , 2014. Recovery of indicators of mitochondrial biogenesis, oxidative stress, and aging with (−)-epicatechin in senile mice. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 70, 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RJ, et al. , 2017. Insulin resistance as a link between Amyloid-Beta and Tau pathologies in Alzheimer’s disease. Frontiers in aging neuroscience. 9, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NancyEllen C, et al. , 2006. Spatial learning and psychomotor performance of C57BL/6 mice: age sensitivity and reliability of individual differences. Age. 28, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasoohi S, et al. , 2018. Thioredoxin-interacting protein (TXNIP) in cerebrovascular and neurodegenerative diseases: regulation and implication. Molecular neurobiology. 55, 7900–7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V, Eckert A, 2013. Calcium channel blockers and dementia. British journal of pharmacology. 169, 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberacker T, et al. , 2018. Enhanced expression of thioredoxin-interacting-protein regulates oxidative DNA damage and aging. FEBS letters. 592, 2297–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana AMM, et al. , 2015. Age-related neuroinflammation and changes in AKT-GSK-3β and WNT/β-CATENIN signaling in rat hippocampus. Aging (Albany NY). 7, 1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping L, et al. , 2018. Global quantitative analysis of the human brain proteome in Alzheimer’s and Parkinson’s Disease. Scientific data. 5, 180036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan LP, et al. , 2001. Localization and regulation of GLUTx1 glucose transporter in the hippocampus of streptozotocin diabetic rats. Proceedings of the National Academy of Sciences. 98, 2820–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Newman AB, 2011. Inflammatory markers in population studies of aging. Ageing research reviews. 10, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song GY, et al. , 2007. Region-specific reduction of Gβ4 expression and induction of the phosphorylation of PKB/Akt and ERK1/2 by aging in rat brain. Pharmacological research. 56, 295–302. [DOI] [PubMed] [Google Scholar]
- Studenski S, et al. , 2011. Gait speed and survival in older adults. Jama. 305, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, et al. , 2015. Age-related changes in mitochondrial antioxidant enzyme Trx2 and TXNIP–Trx2–ASK1 signal pathways in the auditory cortex of a mimetic aging rat model: changes to Trx2 in the auditory cortex. The FEBS journal. 282, 2758–2774. [DOI] [PubMed] [Google Scholar]
- Sverdlov AL, et al. , 2013. Reciprocal regulation of NO signaling and TXNIP expression in humans: impact of aging and ramipril therapy. International journal of cardiology. 168, 4624–4630. [DOI] [PubMed] [Google Scholar]
- Thompson L, et al. , Cellular mechanisms for nimodipine’s reduction of aging-related learning deficits. Treatment of dementias. Springer, 1992, pp. 241–256. [Google Scholar]
- Tschopp J, et al. , 2010. Schroder K NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature reviews immunology. 10, 210–215. [DOI] [PubMed] [Google Scholar]
- Unger JW, et al. , 1991. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Progress in neurobiology. 36, 343–362. [DOI] [PubMed] [Google Scholar]
- Van Kan GA, et al. , 2009. Gait speed at usual pace as a predictor of adverse outcomes in communitydwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The journal of nutrition, health & aging. 13, 881–889. [DOI] [PubMed] [Google Scholar]
- Waldhart AN, et al. , 2017. Phosphorylation of TXNIP by AKT mediates acute influx of glucose in response to insulin. Cell reports. 19, 2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, et al. , 2018. Critical role of NLRP3-caspase-1 pathway in age-dependent isoflurane-induced microglial inflammatory response and cognitive impairment. Journal of neuroinflammation. 15, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J.-s., et al. , 2018. Mangiferin Potentiates Neuroprotection by Isoflurane in Neonatal Hypoxic Brain Injury by Reducing Oxidative Stress and Activation of Phosphatidylinositol-3-Kinase/Akt/Mammalian Target of Rapamycin (PI3K/Akt/mTOR) Signaling. Medical science monitor: international medical journal of experimental and clinical research. 24, 7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, et al. , 2012. Preventing beta-cell loss and diabetes with calcium channel blockers. Diabetes. 61, 848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F, et al. , 2013. FOXO/TXNIP pathway is involved in the suppression of hepatocellular carcinoma growth by glutamate antagonist MK-801. BMC cancer. 13, 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, et al. , 2005. Regulation of oxidative stress by the anti-aging hormone klotho. Journal of Biological Chemistry. 280, 38029–38034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, et al. , 1995. Effects of calcium channel blockers on impairment of brain function in senescence-accelerated mice. Archives internationales de Pharmacodynamie et de Therapie. 330, 125–137. [PubMed] [Google Scholar]
- Yang Y, 2019. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell death & disease. 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara E, et al. , 2014. Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol. 4, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm Y-H, et al. , 2013. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell metabolism. 18, 519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, et al. , 2020. AKT Regulates NLRP3 Inflammasome Activation by Phosphorylating NLRP3 Serine 5. The Journal of Immunology. 205, 2255–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldich E, et al. , 2014. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. Journal of Biological Chemistry. 289, 24700–24715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, et al. , 2018. Verapamil ameliorates hepatic metaflammation by inhibiting thioredoxin-interacting protein/NLRP3 inflammasome activation. Frontiers in endocrinology. 9, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, et al. , 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Immunology. 11, 136–140. [DOI] [PubMed] [Google Scholar]
- Zhu L, et al. , 2018. Klotho controls the brain–immune system interface in the choroid plexus. Proceedings of the National Academy of Sciences. 115, E11388–E11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.