Abstract
Paraquat, a superoxide generator, can damage the cochlea causing an ototoxic hearing loss. The purpose of the study was to determine if deletion of Bak, a pro-apoptotic gene, would reduce paraquat ototoxicity or if deletion of Sirt3, which delays age-related hearing loss under caloric restriction, would increase paraquat ototoxicity. We tested these two hypotheses by treating postnatal day 3 cochlear cultures from Bak+/−, Bak−/−, Sirt3+/−, Sirt3−/− and WT mice with paraquat and compared the results to a standard rat model of paraquat ototoxicity. Paraquat damaged nerve fibers and dose-dependently destroyed rat outer hair cells (OHCs) and inner hair cells (IHCs). Rat hair cell loss began in the base of the cochlea with a 10 μM dose and as the dose increased from 50 to 500 μM, the hair cell loss increased near the base of the cochlea and spread towards the apex of the cochlea. Rat OHC losses were consistently greater than IHC losses. Unexpectedly, in all mouse genotypes, paraquat-induced hair cell lesions were maximal near the apex of the cochlea and minimal near the base. This unusual damage gradient is opposite to that seen in paraquat-treated rats and in mice and rats treated with other ototoxic drugs. However, paraquat always induced greater OHC loss than IHC loss in all mouse strains. Contrary to our hypothesis, Bak deficient mice were more vulnerable to paraquat ototoxicity than WT mice (Bak−/−>Bak+/−>WT), suggesting that Bak plays a protective role against hair cell stress. Also, contrary to expectation, Sirt3 deficient mice did not differ significantly from WT mice, possibly due to the fact that Sirt3 was not experimentally upregulated in Sirt3-expressing mice prior to paraquat treatment. Our results show for the first time a gradient of ototoxic damage in mice that is greater in the apex than the base of the cochlea.
Keywords: superoxide, ototoxicity, hair cells, auditory nerve, paraquat
1. Introduction
Hearing losses resulting from intense noise, ototoxic drugs and aging are believed to converge on a final common pathway linked to oxidative stress caused by the formation of excessive levels of highly toxic reactive oxygen species (ROS) such as the superoxide radical, hydroxyl radical, hydrogen peroxide as well as other peroxides (Chen et al., 2020, Kopke et al., 1999, McFadden et al., 2001, Schacht et al., 2012, Someya and Prolla, 2010). ROS levels are normally kept in check by a well-orchestrated panoply of antioxidant enzymes that reduce and eliminate most of these toxic molecules. However, when ROS levels exceed the body’s antioxidant defense systems these highly reactive compounds can damage virtually all cellular components including proteins, DNA and lipids. The superoxide radical, generated as a normal byproduct of oxygen metabolism, is one of the first molecules produced in a chain reaction that leads to the production of the highly toxic hydroxyl radical and hydrogen peroxide (McCord and Day, 1978). To prevent the buildup of superoxide, mammalian cells possess a family of superoxide dismutases, catalytic enzymes that convert superoxide into ordinary oxygen and hydrogen peroxide, which is subsequently converted by catalase into water.
Genetic studies have helped elucidate the biological importance of various members of the SOD antioxidant family. SOD1 (Cu/Zn SOD) knockout mice developed on a 129/CD-background suffer from significantly greater age-related hearing loss and noise-induced hearing loss than WT mice (Keithley et al., 2005, McFadden et al., 1999, Ohlemiller et al., 1999). The negative effects of SOD1 deficiency are more severe in mice that have the Cdh23 age-related hearing loss (ahl) mutation indicative of a digenic interaction (Johnson et al., 2010). Mn-SOD, expressed in mitochondria, also plays an important role in eliminating O2(−). Because deletion of both copies of the Mn-SOD gene is lethal (Li et al., 1995), the functional significance of Mn-SOD gene deletion has only been studied in heterozygous mice that express half the normal amount of Mn-SOD. In heterozygous Mn-SOD mice, the hearing loss and cochlear damage caused by intense noise exposure is more severe than in WT mice indicating that this mitochondrial enzyme protects against noise-induced oxidative stress (Tuerdi et al., 2017). Mitochondrial oxidative stress induced by superoxide is counteracted by Mn-SOD (Huang et al., 2016).
Excessive oxidative stress upregulates the expression of Bak, a mitochondrial pro-apoptotic gene (Fei et al., 2008). Blocking the expression of Bak with a small-interfering RNA reduces cell death induced by oxidative stress (Wilson et al., 2005). Mice lacking the mitochondrial pro-apoptotic Bak gene are also resistant to age-related hearing loss and exhibit less age-related loss of cochlear hair cells and spiral ganglion neurons than normal controls (Someya et al., 2009). In contrast, nutritional supplements, that upregulate mitochondrial antioxidant enzymes, suppress Bak expression and slow the progression of age-related hearing loss and hair cell loss (Someya et al., 2009). Caloric restriction, which decreases the expression of Bak, also slows the progression of age-related hearing loss and cochlear degeneration (Someya et al., 2007).
Sirtuins, a family of NAD+ dependent deacetylases, have been reported to slow the progression of many age-related diseases (Bell and Guarente, 2011). Caloric restriction, which increases the expression of Sirt3, reduces oxidative stress and slows the progression of age-related hearing loss and cochlear degeneration in WT mice, but failed to do so in Sirt3 deficient mice (Hallows et al., 2011, Han and Someya, 2013, Someya et al., 2007, Someya et al., 2010, Sweet et al., 1988). Sirt3 appears to mediate this protective effect by enhancing glutathione antioxidant defenses under starving conditions (Han and Someya, 2013).
These results suggest that Bak−/− mice lacking the pro-apoptotic Bak protein would exhibit enhanced resistance to oxidative stress-induced cell death. Similarly, Sirt3−/− mice deficient in the mitochondrial deacetylase sirtuin3 would be expected to be more vulnerable to oxidative stress and/or starvation. To test these two hypotheses, we treated postnatal day 3 (P3) cochlear cultures from Bak−/− and Sirt3−/− mice with paraquat, a potent superoxide generator that destroys cochlear hair cells in the rat cochlea beginning in the base and progressing towards the apex with increasing drug dose (Nicotera et al., 2004, Zhang et al., 2018) consistent with other ototoxic drugs (Forge and Schacht, 2000). This base to apex ototoxic gradient is believed to arise from the fact that antioxidant enzyme levels are lower in the base than the apex (Sha et al., 2001, Ying and Balaban, 2009). Therefore, we predicted that paraquat would cause the greatest hair cell loss in the basal turn of Bak−/− and Sirt3−/− mice.
2. Methods
2.1. Subjects:
To gauge the relative toxicity of different doses of paraquat on cochlear hair cell, we first conducted a dose-response study in Sprague-Dawley rats in which we quantified the degree of outer hair cell (OHC) and inner hair cell (IHC) damage along the length of the cochlea using paraquat doses ranging from 0 (Control) to 500 μM. Using the dose-response data as a guide, we compared the relative amount of hair cell damage that 50 μM of paraquat would produce in wild type (WT), heterozygous and homozygous Bak mice and Sirt3 mice. The paraquat dose-response study was carried out using 36 cochlear explants from postnatal day-3 (P3) Sprague-Dawley rat pups (Charles Rivers). The paraquat doses were 0 (Control), 10, 50, 100, 250 and 500 μM (n=6/per dose). Thirty-six P3 cochlear explants from 10 Sirt3 WT, 10 Sirt3−/− homozygous and 10 Sirt3+/− heterozygous mice were used to compare the ototoxic effects of 50 μM paraquat on the three Sirt3 genotypes. Another 24 cochlear explants from 8 Bak WT, 8 Bak−/− and 8 Bak+/− mice were used to compare the ototoxic effects of 50 μM paraquat on the three Bak genotypes.
Male and female Bak−/−mice, developed on the C57BL/6 background, were purchased from the Jackson Laboratory. Because C57BL/6 mice develop hearing loss as early as 3-months of age, the Bak−/− mice were backcrossed in the Prolla lab at the University of Wisconsin for five or more generations on to B6.CAST-Cdh23ahl+ mice (Jackson Laboratory). The B6.CAST-Cdh23ahl+ mice carry the wild type allele of Cdh23 and retain normal hearing; therefore, backcrossing the Bak−/− mice with this strain produced offspring did not develop early age-related hearing loss. Breed pairs of Bak+/− mice, on the B6.CAST-Cdh23ahl+ background, were shipped to the Salvi lab at the University of Buffalo and used to generate postnatal day 3 (P3) pups (males; females) that were used to prepare cochlear organotypic cultures. Cochlear organotypic cultures were prepared from P3 Bak−/−; B6.CAST-Cdh23ahl+ (n=10), Bak+/−; B6.CAST-Cdh23ahl+(n=10) and Bak+/+; B6.CAST-Cdh23ahl+(n=10) mice.. Tissue samples obtained from each P3 pup in the culture study was used for genotyping (described below) to identity cultures from Bak+/+, Bak+/− and Bak−/− mice.
Male and female Sirt3−/− mice were purchased from the Jackson Laboratory and backcrossed in the Someya lab at the University of Florida for more than five generations onto CBA/CaJ mice (Jackson Laboratory) that do not carry the early onset hearing loss susceptibility allele (Cdh23ahl). Breeding pairs of Sirt3+/− mice on the CBA/CaJ background were shipped to the Salvi lab at the University of Buffalo and used to generate postnatal day 3 (P3) pups (males; females) that were used to prepare cochlear organotypic cultures. Cochlear organotypic cultures were prepared from postnatal day 3 (P3) Sirt3−/−; CBA/CaJ (n=10), Sirt3+/−; CBA/CaJ (n=10), and Sirt3+/+; CBA/CaJ (n=10) mice to determine if reducing the sirtuin3 mitochondrial deacetylase would make the cochlea more vulnerable to paraquat-induced oxidative stress. A tail snip obtained from each P3 mouse used in the culture studies was used to genotype the WT, Sirt3−/− and Sirt3+/− mice.
2.2. Cochlear organotypic cultures:
Our procedures for preparing mouse and rat cochlear organotypic cultures have been described previously (Ding et al., 2002, McFadden et al., 2003, Prakash Krishnan Muthaiah et al., 2017, Zhang et al., 2018). Briefly, a mixture of rat-tail collagen (9:1:1 ratio of Type 1 collagen, BD Biosciences, #4236, Bedford, MA), 10X basal medium eagle (Sigma B9638) and 2% sodium carbonate) was prepared and 10 μl of the mixture placed in the center of a 35 mm diameter culture dish (Falcon 1008, Becton Dickinson) for 30 minutes until the solution had gelled. Then 1.3 ml of serum-free medium [2 g bovine serum albumin (BSA, Sigma A-4919), 2 ml Serum-Free Supplement (Sigma I-1884), 4.8 ml of 20% glucose (Sigma G-2020), 0.4 ml penicillin G (Sigma P-3414), 2 ml of 200 mM glutamine (Sigma G-6392), and 190.8 ml of 1×BME (Sigma B-1522)] was added to the culture dish. The P3 mouse and rat pups were decapitated, the temporal bones removed and the entire cochlear basilar membrane carefully dissected out in Hank’s Balanced Salt Solution and placed on the surface of collagen gel in the culture dish. Both ears from each rat pup were used, but a different condition was applied to the genetically identical ear-pairs (e.g., 0 μM paraquat control vs. 200 μM paraquat). Each experiment was repeated with ears from different litters of mouse and rat pups. Afterwards, the samples were placed in an incubator (Forma Scientific, #3029) and maintained at 37 °C in 5% CO2 for 1 h after which an additional 0.7 ml of serum-free medium was added to the culture dish sufficient to cover the cochlear explants. Samples were maintained in the incubator overnight
2.3. Mouse genotyping:
A tail snip was obtained from each mouse used to prepare a cochlear culture and the tissue was used to identify the Sirt3 or Bak genotype. DNA was isolated from the tissue using QIAGEN DNeasy (Cat no: 69506, Blood/Tissue isolation kit) and 1 μl of purified DNA was used to perform the PCR assay using Choice-Taq™ DNA Polymerase (Cat no: CB4050–2, Denville Scientific Inc.). The PCR amplification conditions for Bak mice were: first cycle of 3 min at 94 °C, 35 cycles of 30 s each at 94 °C, 1 min at 65 °C, 1 min at 72 °C and a final cycle of 2 min at 72 °C with primers (Thermo Fisher Scientific) S1(5’ GGC TCT TCA CCC CTT ACA TCA G 3’), S2 (5’ GTT TAG CGG GCC TGG CAA CG 3’) and S3 (5’ GCA GCG CAT CGC CTT CTA TC 3’) for the amplification. Amplified fragments were resolved in 2% agarose gel. A gel with a single band at 540-bp was considered a WT, one with two bands, one at 540-bp and the other at 400-bp, was classified as Bak+/− and a gel with one band at 400-bp was labeled as Bak−/−.
The PCR amplification conditions for Sirt3 mice were: first cycle of 5 min at 94 °C, 33 cycles of 30 s at 94 °C, 30 s at 60 °C, 45 s at 72 °C and a final cycle of 7 min at 72 °C with primers (Thermo Fisher Scientific) S1(5’- CAG AGC ATC ATG GCG CTA A −3’), S2 (5’- TGT TCC TTG GGA GGG TCT −3’), S3 (5’- GCT AGC TTG CCA AAC CTA CA −3’) and S4 (5’- AGG CTG TCT ACT GAG AAT TTG TG −3’) for the amplification. Amplified fragments were resolved in 1.5 % agarose gel. A gel with a single band at 404-bp was considered a WT, a gel with three bands, one at 404-bp, a second at 291-bp and a third at 207-bp, was classified as Sir3 +/− and a gel with two bands, one at 291-bp and the other at 207-bp was considered as Sirt3−/−.
2.4. Paraquat Treatments:
A paraquat dose-response function was first established for rat cochlear explants by culturing the specimens for 24 h in serum-free medium containing 0, 10, 50, 100, 200, or 500 μM of paraquat (n = 6/concentration). Based on these results, a moderate 50 μM dose of paraquat was applied to cochlear organotypic cultures from Bak−/−, Bak+/− and WT mice and Sirt3−/−, Sirt3+/− and WT mice (n=6/group). Some Control cochlear cultures (0 μM) were evaluated from Bak−/−, Bak+/− and WT mice (n=2/group) and Sirt3−/−, Sirt3+/− and WT mice (n=4/group).
2.5. Labeling of hair cells and nerve fibers:
Some cochlear explants from P3 rat and mouse pups were single-labeled with fluorescently labeled phalloidin to visualize the hair cells. After fixation with 10% formalin, specimens were rinsed with PBS, rinsed three times in PBS, and stained with Alexa Fluor 488 conjugated phalloidin (Invitrogen A12379) for 30 min. After rinsing with PBS, specimens were mounted on glass slides as surface preparations in Fluromount and examined with a confocal microscope (Zeiss LSM-510) using appropriate filters.
Other explants from rat and mouse pups were double-labeled with fluorescently labeled phalloidin to visualize the hair cells and a neurofilament antibody to label the nerve fibers (NFs) (Ding et al., 2013, Ding et al., 2020). After fixation with 10% formalin, specimens were rinsed with PBS, and then immersed overnight (4 °C) in a solution containing 20 μl of mouse anti-neurofilament 200 antibody (Sigma p1951, 1:100) dissolved in a solution containing 20 μl Triton X-100 (10%), 6 μl normal goat serum, 154 μl of 0.1 M PBS. After rinsing, the specimen was immersed in a solution containing 20 μl of TRITC-labeled secondary antibody (goat anti-mouse IgG, Sigma T5393) in 12 μl normal goat serum, 40 μl Triton X-100 (10%) and 328 μl of 0.1 M PBS. Specimens were rinsed three times in PBS, and then stained with Alexa Fluor 488 conjugated phalloidin (Invitrogen A12379) for 30 min. After rinsing with PBS, specimens were mounted on glass slides as surface preparations in Fluromount and examined with a confocal microscope (Zeiss LSM-510) using appropriate filters. Confocal images were processed using Zeiss LSM image Examiner and Adobe Photoshop 5.5 software as described previously (Ding et al., 2011, Ding et al., 2018, Ding et al., 2013, Ding et al., 2020). Using the Zeiss LSM Image Examiner software, a single horizontal x–y image plane was derived by merging multiple horizontal images into a single plane.
2.6. Cochleograms:
The cochlea explants were mounted on glass slides in glycerin as flat surface preparations, cover slipped, and examined with an epifluorescence-equipped microscope (Zeiss Axioskop, 400X) with appropriate filters to visualize the phalloidin-labeled (Alexa 488) hair cells. The numbers of IHCs and OHCs were counted over 0.24 mm intervals along the entire length of the cochlea. Hair cells were counted as missing if the stereocilia and cuticular plate were absent. Counts were entered into a custom computer program that computed a cochleogram showing the percent missing IHCs and OHCs as function of percent distance from the apex based lab hair cell norms for SASCO Sprague–Dawley rats (Ding et al., 2013, Li et al., 2015). Data from each individual cochleogram were averaged to generate a mean cochleogram for each experimental condition.
2.7. Statistics:
Data were plotted and analyzed using GraphPad Prism (Version 5.01) software as described in more detail in the Results.
2.8. Animal subject approval:
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University at Buffalo, University of Wisconsin-Madison and the University of Florida and conform to the National Institutes of Health guide for the care and use of Laboratory animals.
3. Results
3.1. Paraquat-induced hair cell and nerve fiber damage in rat cochlear explants:
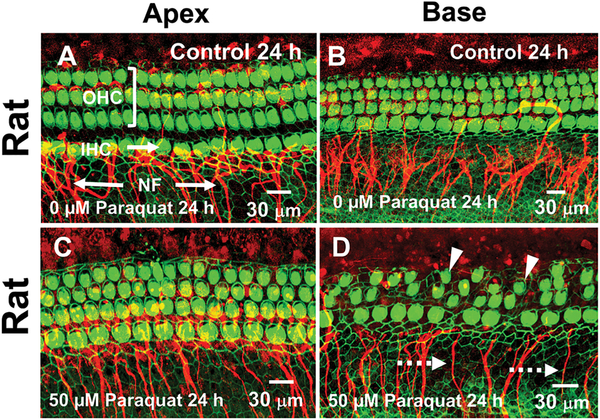
To estimate the relative ototoxic potential of different doses of paraquat, we carried out a preliminary dose-response study in our standard Sprague-Dawley rat model that did not require selective breeding or genotyping. Sprague-Dawley rat cochlear explants were cultured for 24 h in serum-free medium containing 0 (Control), 10, 50, 100, 200, or 500 μM of paraquat. Figure 1 shows a series of representative photomicrographs from the low frequency apical region and high frequency basal region of the rat cochlea in the Control group (0 μM paraquat) and the group treated with 50 μM of paraquat for 24 h. The OHCs in the Control cultures are arranged in three orderly parallel rows adjacent to a single row of IHCs in both the apex (Fig. 1A) and base (Fig. 1B) of the cochlear explant. Thick fascicles of NFs project out radially towards the IHCs and to a lesser extent the OHCs. In the cultures treated for 24 h with 50 μM of paraquat, there was little evidence of damage to the OHCs, IHCs or NFs in the apical region of the cochlea (Fig. 1C). In contrast, many OHCs and IHCs were missing or severely shrunken in the base of the cochlea and there was a reduced number of NFs (Fig. 1D).
Figure 1:
Paraquat preferentially destroys outer hair cells (OHCs), inner hair cells (IHCs) and nerve fibers (NFs) in the base of the rat cochlea. Representative confocal images of cochlear surface preparations cultured for 24 h and then labeled with Alexa Fluor 488 conjugated phalloidin (green) that labels the stereocilia and cuticular plate of OHCs and IHCs and an antibody against neurofilament 200 conjugated to a secondary antibody labeled with Alexa Fluor 555 (red) that binds to NFs. Photomicrographs of Control (0 μM paraquat) cochlear surface preparations from the apex (A) and base (B) of the rat cochlea showing three parallel rows OHCs, a single row of IHCs and fascicles of NFs projecting out radially to the IHCs and OHCs. After 24 h treatment with 50 μM paraquat, OHCs, IHCs and NFs (arrows) were largely intact in the apex of the cochlea (C) whereas many OHCs and IHCs (arrowhead) were shrunken or missing in the base of the cochlea and many NFs were missing (dashed arrow).
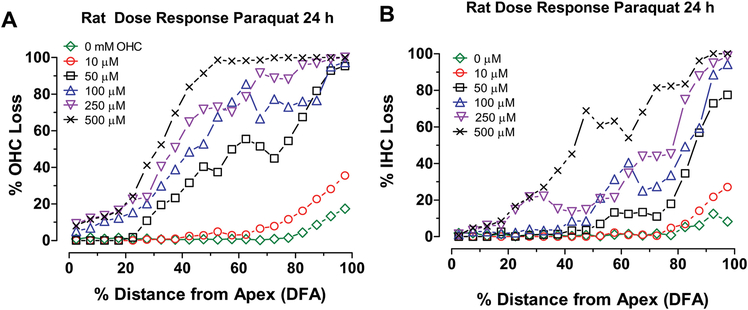
Mean cochleograms were prepared showing the average loss of OHCs and IHCs for each paraquat dose. The OHCs and IHCs were largely intact except for a small percentage of missing OHCs (Fig. 2A) and to lesser extent IHCs (Fig. 2B) near the base of the cochlea likely the result of preparations artifacts. As the dose of paraquat increased from 10 μM to 500 μM, the OHC and IHC lesions increased in an orderly manner and expanded from the base towards the apex. There was only a slight increase in the OHC and IHC lesion when the dose was increased from 0 to 10 μM, but a large increase in the OHC and IHC lesion occurred when dose was raised from 10 μM to 50 μM. For each dose, the OHC lesion (Fig. 1A) was consistently greater than the IHC lesion. These results are consistent with two well-known ototoxic trends, namely that hair cell lesions begin in the base of the cochlea and spread towards the apex with increasing drug dose and that OHCs are more susceptible to ototoxic drugs than IHCs (Hawkins Jr., 1976, Huang and Schacht, 1989, Rybak and Ramkumar, 2007).
Figure 2:
Paraquat dose-dependently and preferentially destroys hair cells in the base of the rat cochlea. Mean (+/− SEM, n=6) cochleograms showing percent (A) OHC loss and (B) IHC loss as a function of percent distance from apex of the cochlea after 24 h treatment with 0, 10, 50, 100, 250 and 500 μM of paraquat.
3.2. Greater paraquat damage in Bak deficient mice:
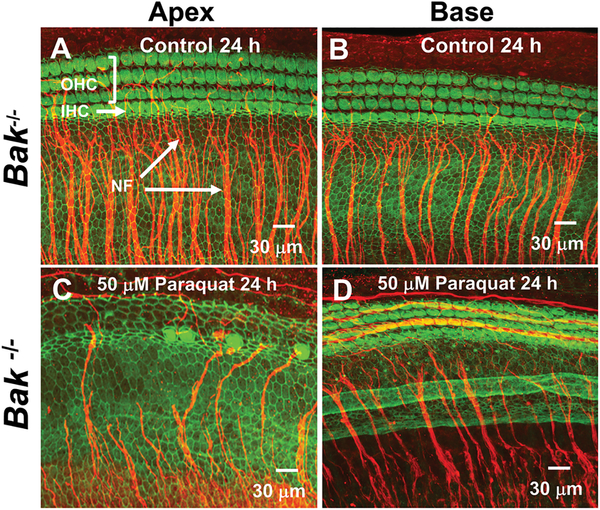
Paraquat was expected to cause less cochlear damage in Bak-deficient mice because of reduced levels of apoptosis. To test this hypothesis, P3 cochlear cultures from Bak−/−, Bak+/− and WT mice were treated with 50 μM of paraquat for 24 h and compared to those from Controls (0 μM). Representative photomicrograph of cochlear cultures from Bak homozygous mice in the Control group and the group treated with 50 μM paraquat for 24 h are shown in Figure 3. The OHCs in Control cultures are arranged in three parallel rows adjacent to a single row of IHCs as illustrated by the images in the apex (Fig. 3A) and base of the cochlea (Fig. 3B). Numerous NF fascicles project out radially towards the IHCs in both the apex and base of the cochlea. When 50 μM of paraquat was applied to cochlear cultures from Bak−/− mice, nearly all the OHCs, IHCs and NFs in the apex of the cochlea were destroyed (Fig. 3C) whereas virtually all the OHCs, IHCs and NFs in the base of the cochlea were intact (Fig. 3D). Thus, paraquat unexpectedly caused more damage in the apex of the cochlea than in the base in contrast to the drug’s effects in rat cochlea cultures and most other ototoxic drugs.
Figure 3:
Paraquat destroys hair cells and nerve fibers in the apical turn of Bak homozygous (Homo) mice. Representative confocal photomicrographs of cochlear organotypic cultures from the apex and base of the cochlea from a Bak−/− mouse double-labeled with phalloidin conjugated to Alexa 488 (green) to label the three parallel rows of outer hair cells (OHCs) and a single row of inner hair cells (IHCs) plus a primary antibody against neurofilament 200 conjugated to a secondary antibody (red) to label the nerve fibers (NFs) projecting out radially towards the hair cells. Note orderly rows of hair cells and large fascicles of nerve fibers in (A) apical turn and (B) basal turn of Control cochleae (0 μM paraquat) cultured for 24 h. After 24 h treatment with 50 μM of paraquat, nearly all OHCs, IHCs and NFs were missing in the (C) apex of the cochlea whereas (D) most hair cells and nerve fibers were present in the base of the cochlea.
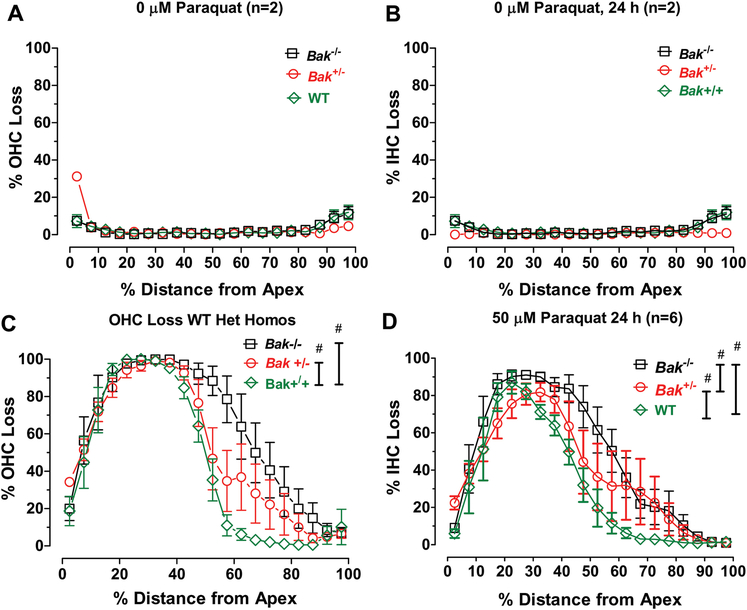
To quantify the effects of paraquat on Bak−/− mice with different genetic backgrounds, we prepared mean (+/−SEM) cochleograms showing the percent OHC and IHC loss versus percent distance from the apex in Bak−/−, Bak+/− and WT mice in the Control (0 μM paraquat) group (n=2/genotype) and Bak−/− mice treated for 24 h with 50 μM paraquat (n=6/genotype). In the Control groups, there was relatively little OHC (Fig. 4A) or IHC loss (Fig. 4B) among the Bak−/−, Bak+/− and WT genotypes. Thus, differences in Bak gene expression had essentially no effect on hair cell survival under normal culture conditions. However, when the cochlear cultures were treated with 50 μM paraquat for 24 h, there was a massive loss of OHCs (Fig. 4A) and IHCs (Fig. 4D) in the apical half of the cochlea. The peak hair cell loss occurred ~30% distance from the apex (DFA) and decreased to less than 10% near the base of the cochlea. Careful inspection reveals clear trends among the three genotypes. OHC and IHC losses were greatest in Bak−/− mice, least in WT mice and intermediate in Bak+/− mice (Fig. 4A–B). There was a significant difference in OHC loss among the three Bak genotypes (Two-way repeated measure ANOVA, F (2, 38) =28.06, p <0.0001). Bonferroni post-hoc analysis revealed a significant difference in OHC loss between WT mice and Bak+/− genotypes (p<0.05) and significant difference between Bak+/− and Bak−/− genotypes (p<0.05). There was also a significant difference in IHC loss among the three genotypes (Two-way repeated measure ANOVA, F (2, 38) =38.75, p <0.0001). Bonferroni post-hoc analysis revealed a significant difference in IHC loss between WT mice and Bak+/− genotypes (p<0.05), a significant difference between Bak+/− and Bak−/− genotypes (p<0.05) and a significant difference between WT and Bak−/− genotypes (p<0.05). Thus, eliminating the Bak gene that codes for the pro-apoptotic Bak protein unexpectedly made mouse P3 cochlear explants more vulnerable to paraquat damage.
Figure 4:
Paraquat-induced hair cell lesions are greater in Bak heterozygous (Het) and homozygous (Homo) mice than Bak WT mice. Mean cochleograms showing percent OHC loss and percent IHC loss as a function of percent distance from apex of the cochlea of Bak WT, Het and Homo mice. Mean (+/− SEM) cochleograms from Control cochlea (0 μM paraquat) exhibit little OHC (A) or IHC (B) loss in Bak WT (n=2), Het (n=2) and Homo (n=2) mice. Mean (n=6/group, +/− SEM) cochleograms showing (C) OHC loss and (D) IHC loss in Bak WT, Het and Homo mice. # indicates significant difference (p<0.05) between groups designated by the vertical bars.
3.3. No significant effect of Sirt3 deficiency on paraquat ototoxicity:
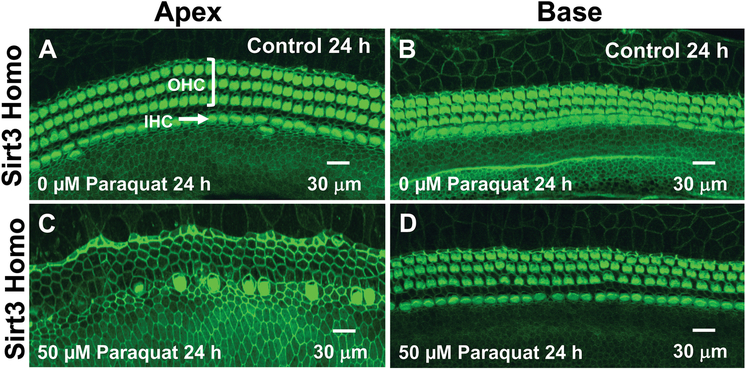
The genotype of each cochlear culture used in the paraquat ototoxicity study was determined from a gel prepared using a tail snip obtained from each Sirt3−/− mouse. Sirt3+/+ mice had a single band at 404-bp; Sirt3+/− mice had one band at 404-bp, a second at 291-bp and a third at 207-bp whereas Sirt3−/− mice had a single band at 207-bp. We hypothesized that Sirt3−/− mice deficient in mitochondrial deacetylase sirtuin3 would be more vulnerable to paraquat-induced oxidative stress. To test this hypothesis, P3 cochlear cultures from Sirt3−/−, Sirt3+/− and WT mice were treated with 50 μM of paraquat for 24 h and compared to those from Controls (0 μM). Representative photomicrograph of cochlear cultures from Sirt3 homozygous mice in the Control group and the group treated with 50 μM paraquat for 24 h are shown in Figure 5. OHCs in Controls formed three orderly parallel rows adjacent to a single row of IHCs in the apex (Fig. 5A) and base (Fig. 5B) of the cochlea. Treatment of the Sirt3−/− cultures with 50 μM of paraquat resulted in massive loss of OHCs and IHCs in the apex of the cochlea (Fig. 5D), but in contrast, the hair cells in the base of the cochlea were intact (Fig. 5D). Similar damage was seen in the apex of the cochlea of Sirt3+/− and WT mice (not shown). Thus, paraquat unexpectedly caused maximal hair cell damage in the apex of the cochlea and minimal damage in the base of all Sirt3 genotypes similar to the Bak mice. This apex to base damage gradient was opposite of that seen in the rat cochlea explants.
Figure 5:
Paraquat destroys hair cells in the apical turn of Sirt3 heterozygous mice. Representative confocal photomicrographs of cochlear organotypic cultures from the apex and base of the cochlea from Sirt3 heterozygous mouse cochlea labeled with phalloidin conjugated to Alexa 488 (green) to label the three parallel rows of outer hair cells (OHCs) and single row of inner hair cells (IHCs). Note orderly rows of hair cells in the (A) apex and (B) base of Control cochlea (0 μM paraquat) cultured for 24 h. Treatment of Sirt3 heterozygous mice with 50 μM of paraquat causes (C) massive loss of IHCs, and OHCs in the apex of the cochlea, but (D) little or no hair cell damage in the base of the cochlea.
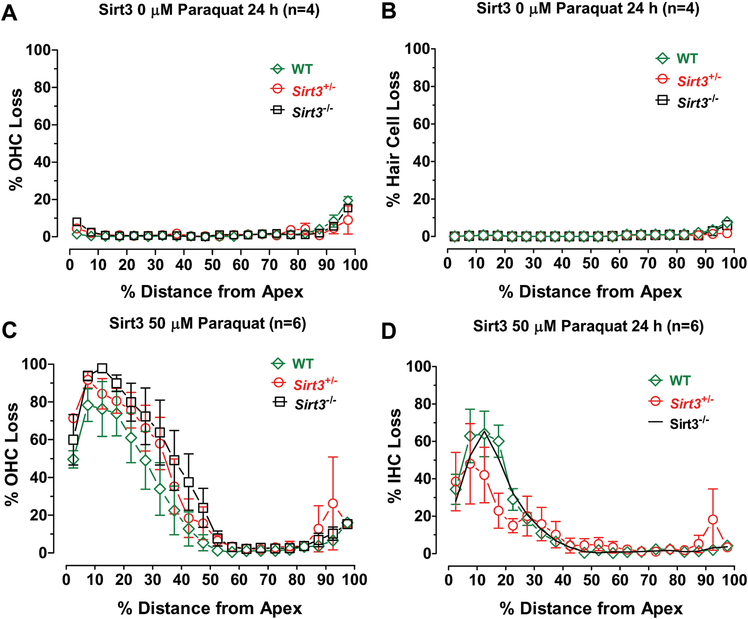
The effects of paraquat on the three Sirt3 genotypes were assessed by preparing mean (+/− SEM) cochleograms showing the percent OHC and IHC loss versus percent distance from the apex. In the Control condition (0 μM paraquat, n=4/group), there was little OHC (Fig. 6A) or IHC loss (Fig. 6B) among the three Sirt3 genotypes. Thus, the differences in Sirt3 expression had no effect on hair cells under normal culture conditions. However, 24 h treatment of P3 cultures with 50 μM resulted in considerable loss of OHCs (Fig. 6A) and IHCs (Fig. 6D) in the apex of the cochlea. The peak hair cell loss occurred roughly 15% of the distance from the apex and rapidly decreased to less than 10% loss over most of the basal half of the cochlea. Hair cell losses in Sirt3−/− and Sirt3+/− mice tended to be slightly greater than WT mice, but none of these differences were statistically significant. Thus, Sirt3 mice deficient in mitochondrial deacetylase sirtuin3 were not significantly more vulnerable to paraquat ototoxicity than their WT counterparts.
Figure 6:
Paraquat-induced apical-turn hair cell lesions are similar in Sirt3 heterozygous (Het,), homozygous (Homo,) and Sirt3 wild type (WT) mice. Mean cochleograms showing percent OHC loss and percent IHC loss as a function of percent distance from apex of the cochlea of Sirt3 WT, Het and Homo mice. Mean (+/− SEM) cochleograms from Control cochlea (0 μM paraquat) exhibit little OHC (A) or IHC (B) loss in Sirt3 WT (n=4), Het (n=4) and Homo (n=4) mice. Mean (n=6/group, +/− SEM) cochleograms showing (C) OHC loss and (D) IHC loss in Sirt3 WT, Het and Homo mice.
3.4. Bak mice are more vulnerable to paraquat than Sirt3 mice:
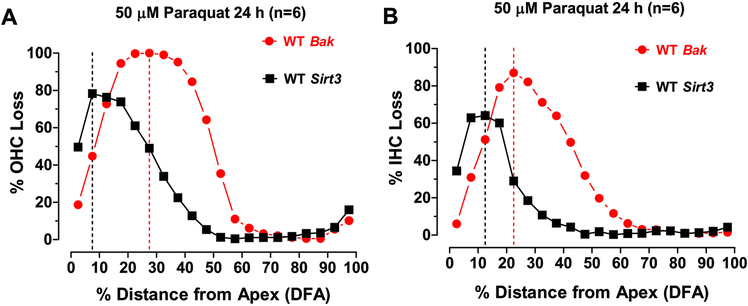
Bak deficient mice appeared to be more vulnerable to paraquat ototoxicity than the Sirt3 genotypes. OHC losses in WT mice occurred in a broad peak in the apical 60% of the cochlea; the maximum OHC loss reached 100% approximately 30% distance from the apex (Fig. 7A). OHC losses in Sirt3 deficient mice occurred in a narrower peak in the apical 40% of the cochlea; the maximum OHC loss approached 80% roughly 10% distance from the apex (Fig. 7A). The IHC lesions in the Bak and Sirt3 mice followed a similar pattern, but were less severe and less widespread than those for OHCs. The maximum IHC loss occurred approximately 22.5% distance from the apex in Bak mice versus 12.5% distance from the apex in Sirt3 mice. The IHC lesion in Bak mice extended over most of the apical half of the cochlea and the peak loss, approached 90%. In contrast, the IHC lesion in Sirt3 mice was restricted to a narrow range in the apical 30% of the cochlea and the maximum loss approached 80%. Thus, the hair cells lesions in the Bak deficient mice were broader, more severe and peaked slightly further from the apex than those in the Sirt3 deficient mice.
Figure 7:
Bak WT mice are more vulnerable to paraquat ototoxicity than Sirt3 WT mice. (A) Mean OHC loss versus percent distance from the apex (DFA) in Bak WT mice compared to Sirt3 WT mice. Maximum OHC loss occurred 27.5% DFA in Bak mice versus. 7.5% DFA in Sirt3 mice. (B) Mean IHC loss versus percent DFA in Bak WT mice compared to Sirt3 WT mice. Maximum IHC loss occurred approximately 22.5% DFA in Bak mice versus. 12.5 DFA in Sirt3 mice. The width of the IHC lesion in Bak mice extended over much of the apical half of the cochlea; the peak loss, which approached 90%, occurred roughly 25% from the apex. The IHC lesion in the Sirt3 group was restricted to a narrower range in the apical 30% of the cochlea and the maximum loss approached 80% and occurred roughly 10% from the apex. Thus, the hair cells lesions in the Bak mice were broader, more severe and peaked slightly further from the apex than those in the Sirt3 mice.
4. Discussion
Superoxide is the first free radical in a chain reaction that leads to the generation of other toxic molecules such as hydrogen peroxide, the hydroxyl radical and peroxides. When superoxide dismutase antioxidant defenses are overwhelmed, the cascade of ROS and reactive nitrogen species that are generated can damage the sensory hair cells, supports cells and neurons along the length of the cochlea (Bielefeld et al., 2005, Harris et al., 2006, Nicotera et al., 2004, Webber et al., 2005, Zhang et al., 2018).
4.1. Spatial gradient of paraquat ototoxicity is species dependent:
To put the paraquat data into perspective, the results from the Bak and Sirt3 deficient mice can be compared to data obtained from mouse cochlear cultures treated with other ototoxic drugs. When C57BL/10J mouse cochlear cultures were treated with gentamicin, we found that the hair cell lesions were greatest in the base and declined towards the apex of the cochlea and that OHC lesions were greater than IHC lesions (Hou et al., 2005). Similarly, when cisplatin ototoxicity was examined in cochlear cultures from 129SV and NMRI mice, hair cell losses were more severe in the base of the cochlea than in the apex and OHC losses were greater than IHC losses (Tropitzsch et al., 2014). Paraquat caused extensive damage to hair cells and nerve fibers in both rats and mice, but the damage occurred at different location. OHC damage occurred in the most basal region of the rat cochlea with the lowest dose of paraquat, 10 μM, and spread towards the apex when the dose was increased to 50 μM. However, even the highest dose of paraquat, 500 μM, destroyed less than 20% of the OHC in the apex of the cochlea.
Unlike other ototoxic drugs, the paraquat-induced lesions in all Bak and Sirt3 genotypes were greatest near the apex of the cochlea. Minimal hair cell loss occurred near the base of the cochlea even after treatment with the highest dose of paraquat, 500 μM. The location of maximum hair cell damage, however, was slightly different in the two mouse strains. Maximum damage occurred ~10% distance from the apex in Sirt3 mice, but was located ~30% distance from the apex in Bak mice. We previously investigated paraquat ototoxicity in cochlear cultures from the middle turn of C57BL/10J mice (Nicotera et al., 2004) and found that 50 μM paraquat destroyed roughly 37% of the OHCs, the magnitude of the OHC lesion in C57BL/10J mice was similar to the OHC lesions in the Sit3 and Bak mice. However, we were unable to test for a longitudinal gradient of hair cell loss in our previous study because we only evaluated the middle turn of the C57BL/10J mouse cochlea rather than the whole cochlea. Because of potential strain differences, it would be useful to test for longitudinal damage gradients in other strains of mouse cochlear cultures treated with paraquat.
It is unclear why apical turn hair cells in Bak and Sirt3 mouse cultures were more susceptible to paraquat ototoxicity than apical turn hair cells in the rat cochlea (Fig. 7) (Zhang et al., 2018) or why basal turn hair cells in Bak and Sirt3 mice were more resistant to paraquat ototoxicity than basal turn hair cells in the rat. It has been suggested that hair cell vulnerability is related to the level of antioxidant enzymes present at different locations along the length of the cochlea (Sha et al., 2001, Ying and Balaban, 2009). These differences could be related to variations in the amount of superoxide antioxidant enzyme expressed along the length of the mouse versus rat cochleae. Because the paraquat-induced lesions in mice were much greater in the apex than the base of the cochlea, one might predict that superoxide antioxidant levels in Bak and Sirt3 mice were much lower in the apex than the base of the cochlea. Conversely, because the paraquat-induced lesions in rats were greater in the base than the apex, the superoxide antioxidant enzyme levels would be expected to be lower in the base than the apex of the rat cochlea. This hypothesis could be tested by measuring the level the various types of superoxide antioxidant enzymes along the length of the rat and mouse cochleae (Tumminia et al., 1996, Yang et al., 2002). Because the postnatal cochlea is still developing, the superoxide antioxidant levels could change as the cochlea develops (Allen and Balin, 1988, Zelck et al., 1993).
4.2. Bak deficiency does not suppress paraquat ototoxicity:
Because mice lacking the pro-apoptotic Bak gene on the C57BL/6 background exhibit less age-related hearing loss and hair cell loss (Someya et al., 2009), we predicted that the Bak-deficient cochlear cultures on the B6.CAST-Cdh23ahl+ background would be more resistant to superoxide-mediated oxidative stress induced by paraquat. Contrary to expectation, OHC and IHC losses were significantly greater in Bak+/− and Bak−/− mice than in their WT counterparts for reasons that are unclear. While both Bax and Bak proteins are generally considered key regulators of apoptosis, programmed cell death has been shown to occur in cells in which both Bax and Bak genes have been deleted (Green and Reed, 1998, Lomonosova et al., 2009, Skulachev, 2002). This type of Bax/Bak-independent cell death is believed to involve BH3 proteins, p53 and mitochondrial permeabilization. Paraquat has been shown to increase nitric oxide levels which activate the novel NO/GAPDH/Siah cell death pathway leading to downstream activation of p53 (Ortiz-Ortiz et al., 2010). While these results suggest that paraquat-induced cochlear damage could occur largely independent of the Bak cell death pathway, the contribution of the Bak protein to some hair cell protective mechanisms seems likely because Bak deficient mice are more vulnerable than WT mice.
4.3. Sirt3 deficiency did not alter paraquat ototoxicity:
Because dietary restriction upregulates the expression of Sirt3 and slows the development of age-related hearing loss ostensibly by enhancing glutathione antioxidant defenses (Han and Someya, 2013, Someya et al., 2010), Sirt3 deficient mice were expected to be more vulnerable to paraquat-induced oxidative stress. However, cochlear lesions in Sirt3 deficient mice were not significantly different from WT mice. The lack of a paraquat treatment effect may be due to the fact that caloric restriction is needed to upregulate glutathione antioxidant levels in WT mice whereas caloric restriction has no effect on glutathione levels in Sirt3 deficient mice. Implementing a caloric restriction paradigm in vivo or treating WT and Sirt3 deficient mice in vivo with paraquat, other ototoxic drugs or noise exposure would appear to be a feasible way of testing this hypothesis.
4.4. Summary:
Paraquat induced diametrically opposed longitudinal gradients of hair cell and nerve fiber damage in mouse and rat P3 cochlear cultures. In rats, the lesions were greatest in the base of the cochlea and declined towards the apex, a pattern seen with other ototoxic drugs such as cisplatin and gentamicin. However, in all Bak and Sirt3 mouse genotypes, the lesions were greatest near the apex and declined towards the base, a longitudinal gradient opposite to that reported in mice using classical ototoxic drugs such as gentamicin and cisplatin (Hou et al., 2005, McFadden et al., 2003). The maximum damage from paraquat occurred ~30% distance from the apex in Bak mice whereas in Sirt3 mice, the maximum loss occurred ~10% distance from the apex. However, in both rats and mice, paraquat was more toxic to OHCs than IHCs. To our knowledge, this is the first time an ototoxic drug has been found to cause greater damage in the apex than the base of the cochlea. This unusual longitudinal gradient of paraquat-induced damage in mice could be related to the distribution and/or developmental expression of superoxide dismutase enzymes that inactivate or remove the toxic superoxide radical. In guinea pig, superoxide dismutase increased significantly from fetal age to P2 with further increase into adulthood (Zelck et al., 1993); however, no one to our knowledge has measured the developmental upregulation of superoxide dismutase along the length of the cochlea during development. Deletion of the proapoptotic Bak gene, which was expected to protect against paraquat ototoxicity actually made the hair cells more vulnerable suggesting that paraquat-induced ototoxicity may have occurred largely through a Bak/Bak independent cell death pathway (Green and Reed, 1998, Lomonosova et al., 2009, Ortiz-Ortiz et al., 2010). Alternatively, Bak may have unknown roles in the protection of cells against oxidative stress. Removal of the potentially protective Sirt3 gene, which was expected to make the cochlea more vulnerable to paraquat ototoxicity had no measureable effect, possibly because the protective effect of Sirt3 may only occur in the context of caloric restriction (Han and Someya, 2013).
Acknowledgement:
This research was supported by NIH grant to TP (R01DC014693) and SS (R03 DC011840, R01 DC012552, R01 DC014437)
List of abbreviations:
- BSA
bovine serum albumin
- DFA
distance from apes
- IHC
inner hair cell
- NF
nerve fiber
- OHC
outer hair cell
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SOD1
Cu/Zn SOD
- WT
Wild type
Footnotes
Competing Interests: The authors declare no competing interest related to this manuscript and research. Dr. Salvi is a consultant for Auris Medical and CilCare.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Allen RG, Balin AK (1988) Developmental changes in the superoxide dismutase activity of human skin fibroblasts are maintained in vitro and are not caused by oxygen. J Clin Invest;82:731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Guarente L (2011) The SirT3 divining rod points to oxidative stress. Mol Cell;42:561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld EC, Hu BH, Harris KC, Henderson D (2005) Damage and threshold shift resulting from cochlear exposure to paraquat-generated superoxide. Hear Res;207:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Daszynski DM, Ding D, Jiang H, Woolman T, Blessing K, et al. (2020) Novel oral multifunctional antioxidant prevents noise-induced hearing loss and hair cell loss. Hear Res;388:107880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, He J, Allman BL, Yu D, Jiang H, Seigel GM, et al. (2011) Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear Res;282:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Jiang H, Zhang J, Xu X, Qi W, Shi H, et al. (2018) Cisplatin-induced vestibular hair cell lesion-less damage at high doses. J Otol;13:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Qi W, Yu D, Jiang H, Han C, Kim MJ, et al. (2013) Addition of exogenous NAD+ prevents mefloquine-induced neuroaxonal and hair cell degeneration through reduction of caspase-3-mediated apoptosis in cochlear organotypic cultures. PLoS One;8:e79817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Stracher A, Salvi RJ (2002) Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res;164:115–26. [DOI] [PubMed] [Google Scholar]
- Ding D, Zhang J, Liu F, Li P, Qi W, Xing Y, et al. (2020) Antioxidative stress-induced damage in cochlear explants. J Otol;15:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Q, McCormack AL, Di Monte DA, Ethell DW (2008) Paraquat neurotoxicity is mediated by a Bak-dependent mechanism. J Biol Chem;283:3357–64. [DOI] [PubMed] [Google Scholar]
- Forge A, Schacht J (2000) Aminoglycoside antibiotics. Audiol Neurootol;5:3–22. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC (1998) Mitochondria and apoptosis. Science;281:1309–12. [DOI] [PubMed] [Google Scholar]
- Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, et al. (2011) Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell;41:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Someya S (2013) Maintaining good hearing: calorie restriction, Sirt3, and glutathione. Exp Gerontol;48:1091–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Bielefeld E, Hu BH, Henderson D (2006) Increased resistance to free radical damage induced by low-level sound conditioning. Hear Res;213:118–29. [DOI] [PubMed] [Google Scholar]
- Hawkins JE Jr 1976. Drug ototoxicity. In: Keidel WD, Neff WD, editors. Handbook of Sensory Physiology. . ed. Berlin: Springer Verlag. [Google Scholar]
- Hou QL, Ding D, Jiang H, Salvi R, Yin SW (2005) Gentamicin ototoxicity model in mouse cochlear organotypic cultures. Chin J Otol;3:191–3. [Google Scholar]
- Huang CL, Chao CC, Lee YC, Lu MK, Cheng JJ, Yang YC, et al. (2016) Paraquat Induces Cell Death Through Impairing Mitochondrial Membrane Permeability. Mol Neurobiol;53:2169–88. [DOI] [PubMed] [Google Scholar]
- Huang MY, Schacht J (1989) Drug-induced ototoxicity. Pathogenesis and prevention. Medical Toxicology and Adverse Drug Experience;4:452–67. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Yu H, Ding D, Jiang H, Gagnon LH, Salvi RJ (2010) Separate and combined effects of Sod1 and Cdh23 mutations on age-related hearing loss and cochlear pathology in C57BL/6J mice. Hear Res;268:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR (2005) Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res;209:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke R, Allen KA, Henderson D, Hoffer M, Frenz D, Van de Water T (1999) A radical demise. Toxins and trauma share common pathways in hair cell death. Ann N Y Acad Sci;884:171–91. [DOI] [PubMed] [Google Scholar]
- Li P, Ding D, Salvi R, Roth JA (2015) Cobalt-Induced Ototoxicity in Rat Postnatal Cochlear Organotypic Cultures. Neurotox Res;28:209–21. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, et al. (1995) Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet;11:376–81. [DOI] [PubMed] [Google Scholar]
- Lomonosova E, Ryerse J, Chinnadurai G (2009) BAX/BAK-independent mitoptosis during cell death induced by proteasome inhibition? Molecular Cancer Research;7:1268–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Day ED Jr. (1978) Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Letters;86:139–42. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, et al. (1999) Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129,CD-1 mice. J Comp Neurol;413:101–12. [PubMed] [Google Scholar]
- McFadden SL, Ding D, Ohlemiller K, Salvi RJ (2001) The role of superoxide dismutase in age-related and noise-induced hearing loss: Clues from SOD1 knockout mice. James F Willott Handbook of Mouse Anditory Research from Behavior to Molecular Biology;CRS Press. Florida. :489–504. [Google Scholar]
- McFadden SL, Ding D, Salvemini D, Salvi RJ (2003) M40403, a superoxide dismutase mimetic, protects cochlear hair cells from gentamicin, but not cisplatin toxicity. Toxicol Appl Pharmacol;186:46–54. [DOI] [PubMed] [Google Scholar]
- Nicotera TM, Ding D, McFadden SL, Salvemini D, Salvi R (2004) Paraquat-induced hair cell damage and protection with the superoxide dismutase mimetic m40403. Audiol Neurootol;9:353–62. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Flood DG, Reaume AG, Hoffman EK, et al. (1999) Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (Sod1) increases susceptibility to noise-induced hearing loss. Audiol Neurootol;4:237–46. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ortiz MA, Moran JM, Ruiz-Mesa LM, Bravo-San Pedro JM, Fuentes JM (2010) Paraquat exposure induces nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the activation of the nitric oxide-GAPDH-Siah cell death cascade. Toxicol Sci;116:614–22. [DOI] [PubMed] [Google Scholar]
- Prakash Krishnan Muthaiah V, Ding D, Salvi R, Roth JA (2017) Carbaryl-induced ototoxicity in rat postnatal cochlear organotypic cultures. Environ Toxicol;32:956–69. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Ramkumar V (2007) Ototoxicity. Kidney Int;72:931–5. [DOI] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, Rybak LP (2012) Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken);295:1837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J (2001) Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res;155:1–8. [DOI] [PubMed] [Google Scholar]
- Skulachev VP (2002) Programmed death phenomena: from organelle to organism. Ann N Y Acad Sci;959:214–37. [DOI] [PubMed] [Google Scholar]
- Someya S, Prolla TA (2010) Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev;131:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, et al. (2009) Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A;106:19432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Weindruch R, Prolla TA, Tanokura M (2007) Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol Aging;28:1613–22. [DOI] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, et al. (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell;143:802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RJ, Price JM, Henry KR (1988) Dietary restriction and presbyacusis: periods of restriction and auditory threshold losses in the CBA/J mouse. Audiology;27:305–12. [DOI] [PubMed] [Google Scholar]
- Tropitzsch A, Arnold H, Bassiouni M, Muller A, Eckhard A, Muller M, et al. (2014) Assessing cisplatin-induced ototoxicity and otoprotection in whole organ culture of the mouse inner ear in simulated microgravity. Toxicol Lett;227:203–12. [DOI] [PubMed] [Google Scholar]
- Tuerdi A, Kinoshita M, Kamogashira T, Fujimoto C, Iwasaki S, Shimizu T, et al. (2017) Manganese superoxide dismutase influences the extent of noise-induced hearing loss in mice. Neuroscience Letters;642:123–8. [DOI] [PubMed] [Google Scholar]
- Tumminia SJ, Chambers C, Qin C, Zigler JM Jr., Russell P(1996) A comparison of antioxidant enzyme activities in organ-cultured rhesus monkey lenses following peroxide challenge. Current Eye Research;15:845–51. [DOI] [PubMed] [Google Scholar]
- Webber DS, Lopez I, Korsak RA, Hirota S, Acuna D, Edmond J (2005) Limiting iron availability confers neuroprotection from chronic mild carbon monoxide exposure in the developing auditory system of the rat. J Neurosci Res;80:620–33. [DOI] [PubMed] [Google Scholar]
- Wilson C, Foster GH, Bitzan M (2005) Silencing of Bak ameliorates apoptosis of human proximal tubular epithelial cells by Escherichia coli-derived Shiga toxin 2. Infection;33:362–7. [DOI] [PubMed] [Google Scholar]
- Yang J, Lam EW, Hammad HM, Oberley TD, Oberley LW (2002) Antioxidant enzyme levels in oral squamous cell carcinoma and normal human oral epithelium. Journal of Oral Pathology and Medicine;31:71–7. [DOI] [PubMed] [Google Scholar]
- Ying YL, Balaban CD (2009) Regional distribution of manganese superoxide dismutase 2 (Mn SOD2) expression in rodent and primate spiral ganglion cells. Hear Res;253:116–24. [DOI] [PubMed] [Google Scholar]
- Zelck U, Nowak R, Karnstedt U, Koitschev A, Kacker N (1993) Specific activities of antioxidative enzymes in the cochlea of guinea pigs at different stages of development. Eur Arch Otorhinolaryngol;250:218–9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sun H, Salvi R, Ding D (2018) Paraquat initially damages cochlear support cells leading to anoikis-like hair cell death. Hear Res;364:129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]