Abstract
Development of sperm requires microtubule-based movements that drive assembly of a compact head and flagellated tails. Much is known about how flagella are built given their shared molecular core with motile cilia, but less is known about the mechanisms that shape the sperm head. The Kinesin Superfamily Protein 3A (KIF3A) pairs off with a second motor protein (KIF3B) and the Kinesin Associated Protein 3 (KAP3) to form Heterotrimeric Kinesin II. This complex drives intraflagellar transport (IFT) along microtubules during ciliary assembly. We show that KIF3A and KAP3 orthologs in Schmidtea mediterranea are required for axonemal assembly and nuclear elongation during spermiogenesis. Expression of Smed-KAP3 is enriched during planarian spermatogenesis with transcript abundance peaking in spermatocyte and spermatid cells. Disruption of Smed-kif3A or Smed-KAP3 expression by RNA-interference results in loss of spermatozoa and accumulation of unelongated spermatids. Confocal microscopy of planarian testis lobes stained with alpha-tubulin antibodies revealed that spermatids with disrupted Kinesin II function fail to assemble flagella, and visualization with 4’,6-diamidino-2-phenylindole (DAPI) revealed reduced nuclear elongation. Disruption of Smed-kif3A or Smed-KAP3 expression also resulted in edema, reduced locomotion, and loss of epidermal cilia, which corroborates with somatic phenotypes previously reported for Smed-kif3B. These findings demonstrate that heterotrimeric Kinesin II drives assembly of cilia and flagella, as well as rearrangements of nuclear morphology in developing sperm. Prolonged activity of heterotrimeric Kinesin II in manchette-like structures with extended presence during spermiogenesis is hypothesized to result in the exaggerated nuclear elongation observed in sperm of turbellarians and other lophotrochozoans.
Keywords: Spermiogenesis, Spermatogenesis, Planarian, Heterotrimeric Kinesin II, cilia, flagella, manchette
Graphical Abstract

INTRODUCTION
Microtubules are essential components of every eukaryotic cell. They act structurally in the cytoskeleton, facilitate intracellular transport, and mediate chromosome distribution during cell division (Desai and Mitchison, 1997). Microtubules are also the main structural component of cilia and flagella, which are highly conserved organelles that propel cell motility, move fluids across surfaces, and participate in extracellular signaling (Goetz and Anderson, 2010; Ishikawa and Marshall, 2011; King, 2016; Malicki and Johnson, 2017). In humans, motile cilia are found on a variety of cell types and are essential for processes such as clearance of airways, circulation of cerebral spinal fluid, and embryonic development (Mitchison and Valente, 2017; Nonaka et al., 1998). Non-motile cilia (also known as primary cilia) support essential sensory mechanisms during embryonic development and in adult tissues. Ciliary dysfunction can result in infertility, embryonic aberrations, and a growing number of human genetic conditions collectively known as ciliopathies (Hildebrandt et al., 2011). Given their relevance to basic cellular functions and human disease, as well as high evolutionary conservation of their molecular components, cilia have been at the forefront of research for decades.
Sexual reproduction in animals is facilitated by ciliary structures that include sperm flagella and motile cilia of cells in the oviduct, which drive transport of gametes prior to fertilization (Inaba, 2003; Koyama et al., 2019). The post-meiotic events of sperm development involve dramatic transformations in cellular architecture in a process known as spermiogenesis. During spermiogenesis, spermatids develop a flagellum or multiple flagella (depending on species) and undergo cytoskeletal rearrangements that pack the cell nucleus into a compact head structure. Flagellar assembly in mammals is thought to involve many of the same molecular processes present during formation of their smaller counterparts (i.e. motile cilia), including Kinesin II-driven intraflagellar transport (IFT; Lehti et al., 2013; San Agustin et al., 2015; Scholey, 1996). Less is known about the mechanisms that drive structural changes during formation of the sperm head. In vertebrates, spermatid head formation and elongation are thought to require contributions from a transient microtubular structure known as the manchette (Kierszenbaum, 2002; Lehti and Sironen, 2016). The manchette forms as a ring of microtubules around the spermatid nucleus that extends towards the developing tail, and contains dynein and kinesin (Hall et al., 1992). The Kinesin II motor protein KIF3A, as well as intraflagellar transport proteins IFT20, IFT27, and IFT88, are all known to localize to the manchette (Lehti and Sironen, 2016). Therefore, it is hypothesized that in addition to contributing to flagellar assembly, as would be expected from fundamental roles of Kinesin II in axonemal formation, KIF3A interacts with proteins of the nuclear envelope and the manchette to shape sperm heads (Lehti et al., 2013). It remains to be determined whether KIF3A contributes to head elongation as part of Kinesin II or through association with independent partners.
We present results from studies of spermatogenesis in the planarian flatworm Schmidtea mediterranea that provide evidence of Kinesin II contributions in both flagellar axoneme assembly and spermatid head formation. Planarians have been used as an effective model for the analysis of proteins involved in cilia formation and function, given several aspects of their unique biology (Rompolas et al., 2013; Rompolas et al., 2009). Proliferative cells in planarians lack centrosomes, and centrioles are formed during terminal differentiation of ciliated cells (Azimzadeh et al., 2012). Disruption of essential ciliary activity has no direct immediate effect in planarian viability but does result in behavioral and physiological phenotypes that are visible to the naked eye, such as abnormal motility and bloating (Lesko and Rouhana, 2020; Rink et al., 2009; Scimone et al., 2011; Thi-Kim Vu et al., 2015). In addition, planarian spermiogenesis involves drastic rearrangements of sperm head and nuclear morphology that are readily susceptible to RNA-interference (RNAi), making this a good model to uncover molecular events required for spermatid elongation (Magley and Rouhana, 2019; Rouhana et al., 2017; Wang et al., 2010b; Wang et al., 2007). Using this model, we disrupted the expression of orthologs of two Kinesin II components, Smed-KAP3 and Smed-kif3A (the prefix “Smed” is used in S. mediterranea gene nomenclature as per (Reddien et al., 2008)). RNAi-mediated knockdown of either Smed-kif3A or Smed-KAP3 led to somatic defects in ciliary function and assembly that corroborate with those previously described for Smed-kif3B (Rink et al., 2009). Deficiencies in formation of sperm flagella were also observed, and planarians subjected to either Smed-KAP3 RNAi or Smed-kif3A RNAi displayed defects in sperm head elongation indicative of a role for heterotrimeric Kinesin II in manchette-like functions during planarian spermiogenesis.
MATERIALS AND METHODS
Propagation and maintenance of clonal lines of Schmidtea mediterranea
A hermaphroditic sexual strain of Schmidtea mediterranea (Zayas et al., 2005) was used for the experiments described in this study except for where use of an asexual strain (Sanchez Alvarado et al., 2002) is specifically noted. Clonal lines of the hermaphroditic strain were expanded by amputation and maintained at 18°C in 0.75x Montjuïc salts (Cebria and Newmark, 2005). Asexual planarians were expanded by natural fission and maintained at 21°C in modified Montjuïc salts as per Pearson and Gurley (http://lab.research.sickkids.ca/pearson/wpcontent/uploads/sites/54/2015/10/Planarian_careNfeedingNliverprep_Pearson.pdf). Cultures were only exposed to light during weekly/bi-weekly feedings of Golden Forest organic calf liver (Fremont Beef Company, Fremont, NE). Planarians were not fed a week prior to use in analyses or experimentation.
Identification and cloning of kinesin components from Schmidtea mediterranea
Primers designed based on cDNA sequence (uc_smed_v2_Contig44520; Rouhana et al., 2012) available through PlanMine (Brandl et al., 2016; Rozanski et al., 2019) were used to amplify approximately a third [5’-GAGCATTGGCACGAGTTCTTCGAGAAGA-3’ (forward) and 5’-CCTTCACTGATAACCGACGCAATCTCTG-3’ (reverse)] and most [5’-GAACTGGAACTATTGATGTACATCCAACTG-3’ (forward) and 5’-CTCCTCTTGACTTTCTAGCATATTGGC-3’ (reverse)] of the Smed-KAP3 ORF (Supplementary Figure S1). Primers designed to amplify Smed-kif3A (uc_smed_v2_Contig36153) [5’-GATTGCTCCAAGATTCCCTTGGAGG-3’ (forward) and 5’-CGGTTATTGGAGAAATCGGCAAAGG-3’ (reverse)]; Smed-KIF17-like (uc_smed_v2_Contig34848) [5’-CAAGGCACTGAATGCTCTCGGAG-3’ (forward) and 5’-CTGATGACCACCGGTTCACCTCG-3’ (reverse)]; Smed-kifC3 (uc_smed_v2_Contig5308) [5’-GCGAATGTGGGATCAGCACATTTC-3’ (forward) and 5’-TCAGTTGACATGGCAGTTTGGCC-3’ (reverse)]; and Smed-ARMC4 (uc_smed_v2_Contig40424) [5’-GGAGGCATACAACCTCTCATTG-3’ (forward) and 5’-CCAAAGTCCGAGTATCAGCGTC-3’ (reverse)] were used to clone partial cDNA sequences from sexual strain cDNA templates. Amplicons were ligated into pGEM-T using the T/A cloning protocol as per the manufacturer (Promega, Madison, WI) and the sequences of individual clones were verified by Sanger sequencing (Retrogen, San Diego, CA). Consensus sequences for partial cDNA clones were deposited into NCBI’s GenBank (accession numbers MT891311–MT891316).
Riboprobe and double-stranded RNA (dsRNA) synthesis by in vitro transcription
Templates for in vitro transcription of riboprobes and dsRNA were generated by PCR using pGEMT3T7v2 [5’-GAATTAATTAACCCTCACTAAAGGGAGAATTTAATACGACTCACTATAGGGCGAATTGG-3’] and pGEMT7SP6v2 [5’-GAATTTAATACGACTCACTATAGGGCGCCAAGCTATTTAGGTGACACTATAG AATACTC-3’] primers. These primers contain the sequence of T7 and SP6 promoters that flank the multiple cloning site of pGEM-T and add upstream T3 and T7 promoters, respectively, to amplicons. PCR products were cleaned using DNA Clean & Concentrator-5 columns (Zymo Research, Irvine, CA), eluted in equal volumes (20 μl) of RNase-free water, and subsequently used for in vitro transcription. SP6 RNA Polymerase was used to generate antisense riboprobes, while T7 RNA Polymerase was used to generate dsRNA. Digoxigenin-UTP-labeled riboprobes were synthesized as described by (King and Newmark, 2013), ethanol-precipitated using LiCl after DNase-treatment, and dissolved in 100 μl hybridization buffer. DsRNA was synthesized using T7 RNA polymerase as described by (Rouhana et al., 2013).
Whole-mount colorimetric and fluorescent in situ hybridization
Sexual planarians of 1.0 to 1.5 cm in length were processed for in situ hybridization as described by King and Newmark (2013), with alterations as described below. Sexual animals were washed two times with 0.75x Monjuïc salts and euthanized using a solution of 10% N-acetyl cysteine (NAC) dissolved in phosphate-buffer saline (PBS). The samples were placed on a rocker at room temperature for 8–12 minutes in increments proportional with animal size. The NAC solution was exchanged with a fixative solution composed of PBS containing 0.3% Triton-X (PBSTx) and 4% formaldehyde and incubated for 1.5 hours at 4°C. Samples were gradually dehydrated through exchanges into a 1:1 methanol:PBSTx solution and 100% methanol, before storing samples at −20°C. After gradual rehydration using PBSTx, samples were bleached under fluorescent bright white light for 2 hours in a formamide-hydrogen peroxide solution formulated as per King and Newmark (2013), treated for 12 minutes at room temperature with 10 μg/ml Proteinase K (Roche, Mannheim, Germany) in PBSTx supplemented with 0.1% SDS, and post-fixed for 10 minutes in PBSTx containing 4% formaldehyde. For colorimetric in situ hybridization analysis, antibody incubation and signal development were performed as per (Pearson et al., 2009). Samples were mounted in a 4:1 glycerol:PBSTx solution and imaged using a Zeiss V.16 SteREO microscope equipped with a Canon EOS Rebel T3 camera (colorimetric) or with a Nikon C2+ confocal microscope equipped with NIS elements C software under 60x oil immersion magnification (fluorescence). Brightness was adjusted on FISH images to improve presentation without influencing interpretation of the results.
RNA-interference
RNA-interference was performed as described by Rouhana et al. (2013). Groups of six to ten sexual planarians or 10–15 asexual planarians were fed to satiation with liver puree containing gene-specific dsRNA at a concentration of ~100 ng/μl, twice a week for three or more weeks. Animals were assessed for movement defects, bloating, and light avoidance, before being fixed for anatomical analysis by immunofluorescence and DAPI staining. DsRNA of sequence corresponding to firefly luciferase was used for negative control groups. Smed-KAP3 RNAi was performed with sequences that targeted three different regions of Smed-KAP3 mRNA, all of which resulted in the same phenotype (Supplementary Figure S1). One of these dsRNAs corresponds to a 478 nt. fragment in the 5’-end of Smed-KAP3 mRNA, and was generated from a GeneArt Gene Synthesis construct template (Invitrogen, Carlsbad, CA). The other dsRNAs correspond to cloned cDNA fragments described above, one of 724 nts in the middle of the Smed-KAP3 ORF and another of 2622 nts covering almost the entire ORF. Templates for dsRNA synthesis targeting Smed-kif3A, Smed-kif17-like, Smed-kifC3, and Smed-ARMC4 were generated from amplicons of partial cDNA constructs in the pGEM-T backbone (see GenBank accession records, above) using T7 RNA Polymerase. Smed-DAW1 dsRNA was generated as previously described (Counts et al., 2017). Efficacy of RNAi was assessed from groups of three planarians a week after the first two dsRNA feedings by extracting total RNA using TRIzol (Invitrogen, Carlsbard, CA) and using the GoTaq 2-Step RT-qPCR system (Promega, Madison WI) as per the manufacturer’s instructions using only oligo(dT)15 primers during reverse transcription. Abundance of mRNA was normalized to ubiquitously expressed β-tubulin as per Miller and Newmark (2012). Primers and annealing temperatures for RT-qPCR are provided in Supplementary Table S2.
Light Avoidance Assay
Light avoidance assays were performed as described by Magley and Rouhana (2019). Briefly, groups Smed-KAP3 and luciferase RNAi sexual planarians were collected in groups of six and rinsed with fresh 0.75x Montjuïc salts in 50 ml conical tubes. Planarians were then decanted into an illuminated end of a 150 × 15 mm Petri dish filled with 50 ml 0.75x Montjuïc salts. A separate Petri dish was used per group. Planarian movement was recorded for 6 minutes and time spent within each section of the Petri dish (see Supplementary Figure S4A) was measured per animal. Mean was calculated for each group, along with standard deviation and two-tailed t-test for statistical significance.
Analysis of ciliated, nuclear, and mitotic structures by immunofluorescence and DAPI staining
Analysis of cilia by immunofluorescence on whole-mount samples was performed using anti-α-tubulin monoclonal antibody clone B-5-1-2 (1:1000 dilution; Cat. No. T5168, Sigma Aldrich, St. Louis, MO) and goat-anti-mouse IgG AlexaFluor-488 conjugated secondary (1:500 dilution; A11029, ThermoFisher, Waltham, MA) as per Magley and Rouhana (2019). Analysis of nuclear structures stained with DAPI during sperm development and mitotic clusters in testis lobes of whole-mount samples were performed as per Wang et al., (2010) after preparation and bleaching of samples using N-acetyl cysteine for mucus removal, formaldehyde fixation, and H202/formamide bleaching as per King and Newmark (2013). Anti-phospho-Histone H3 (PH3) Ser10 polyclonal antibody (Item no. 44-1190G; Invitrogen, Carlsbad, CA) was used at 1:500 dilution in combination with AlexaFluor-568 conjugated goat anti-rabbit secondaries (1:500 dilution; A11011, ThermoFisher, Waltham, MA). High-magnification analysis of sperm development was performed on testis lobes obtained by maceration of planarians (Baguñà and Romero, 1981) in a solution composed of 150 μl of methanol, 10 μl of acetic acid, 100 μl of glycerol, and 740 μl of water, with gentle agitation for 1–2 hours. Testis lobes were collected from the maceration solution and washed in a 1.5 ml centrifuge tube with PBS, separated by centrifugation at 1500 × g, and fixed in PBS solution containing 4% formaldehyde for 15 minutes. Fixed lobes were washed with PBS, placed in blocking solution (0.6% BSA and 0.45% Fish Gelatin PBSTx) overnight at 4°C, incubated in 100 μl blocking solution with anti-acetylated-α-tubulin (Lys40) monoclonal antibodies (clone 6-11B-1; Thermo Fisher, Waltham, MA; Catalog No. 32-2700; 1:400 dilution) for 2 hours at 4°C the following day. Lobes were incubated in primary antibody with PBSTx for 15 minutes and incubated in blocking solution supplemented with DAPI and Alexa Fluor 488-conjugated goat-anti-mouse IgG (Cat. No. A11029, ThermoFisher, Waltham, MA; 1:400 dilution) at 4°C overnight, before being mounted in a 1:1 glycerol:PBS solution and imaged. Samples were imaged on a Nikon C2+ confocal microscope equipped with NIS elements C software under 20x or 60x oil objective magnification.
Electron microscopy imaging of external cilia
Sexual juveniles of ~0.8 cm length were subjected to 2.5 weeks of RNAi feedings (6 feedings) and fixed a week after the last feeding by overnight incubation at room temperature in a 9:1 solution of PBS:glutaraldehyde. Fixed samples were submitted to the Campus Microscopy and Imaging Facility at the Ohio State University, where these were further processed by incubations in 2.5% Glutaraldehyde for 2 hrs., 0.1M phosphate buffer for 10 min., 0.1M phosphate buffer for 10 min., osmium tetroxide for 2 hrs., two 5 min. water washes, 2% uranyl acetate for 1 hr., gradual dehydration with two 10-min. incubations in each of 50%, 70%, 80%, and 95% ethanol, and two 15-min. incubations in 100% ethanol. Then, dehydrated samples were treated twice for 15-min. in acetone, and gradually transitioned into resin (2-hr. incubation in 3:7 acetone:resin, 3 hrs. in 1:9 acetone:resin, and two 3-hr. incubations in resin). The resin was made with 116 g of EMbed 812 (Electron Microscopy Sciences, Hatfield, PA), 64 g of Dodecenyl Succinic Anhydride, and 57.2 g of Methyl Nadic Anhydride (Ted Pella, Inc., Redding, CA), and supplemented with N-Benzyldimethylamine [30 mL/L] before use. Embedded samples were sectioned at 90 nm using a Leica ultramicrotome and imaged on a BioTwin transmission electron microscope at 80 kV.
RESULTS
Identification of a KAP3 ortholog required for spermatid elongation in Schmidtea mediterranea
Transcriptional analyses revealed enriched expression in the planarian reproductive system for genes categorized as cytoskeletal proteins (Chong et al., 2011; Wang et al., 2010b) and proteins involved in microtubule-based movement (Rouhana et al., 2017). Amongst these, preferential expression in planarian testis lobes has been validated by in situ hybridization for tektins, alpha- and beta-tubulin, kinesin light chain, and dynein assembly factors (Chong et al., 2011; Lesko and Rouhana, 2020; Rouhana et al., 2017; Wang et al., 2010b). However, functional assessment failed to reveal specific roles for these genes during planarian sperm development. To better understand the contributions of factors involved in microtubule-based movement during sperm development, we identified kinesin components whose expression in the reproductive system of S. mediterranea was suggested by a previous RNAseq study (Rouhana et al., 2017). Ten homologs of kinesin components were identified in contigs with reduced representation in transcriptomes of planarians with disrupted reproductive system development (≥ 4-fold, p-value < 0.05, in CPEB2(RNAi) vs. control sexual planarians; Supplementary Table S1). From these, partial cDNA clones were generated for homologs of Kinesin Family 17 (KIF17) (Conting34848; dd_Smes_v1_37026_2_1), KIFC3 (Contig5308; dd_Smes_v1_90546_1_1), and Kinesin Associated Protein 3 (KAP3; Contig29497; dd_Smes_v1_44359_1_1) and used to generate double-stranded RNA for functional analysis by RNAi.
We hypothesized that the function of kinesin components with enriched expression in the reproductive system would be required for sperm development in S. mediterranea. To test this, juvenile sexual planarians were fed twice per week for four weeks with liver mixed with dsRNA generated from the constructs mentioned above. Juvenile sexual planarians can be identified by the absence of a gonopore and possess underdeveloped reproductive structures that mature as they grow in response to frequent nutritional intake. A control group was fed liver containing dsRNA of firefly luciferase sequence known not to interfere with planarian physiology (Figure 1, A and B; Lesko and Rouhana, 2020; Magley and Rouhana, 2019). Abundace of targeted mRNAs was measured by reverse transcription quantitative PCR (RT-qPCR) relative to the luciferase control (Figure 1C). Reduction of tarteted mRNA levels by 85% or more was observed for each group except for Smed-kif17-like(RNAi), which was therefore excluded from further discussion. Planarians were fixed a week after the last RNAi feeding and stained with DAPI, which allows easy visualization of testes due to their dorsolateral position, structure, and cellular density (Figure 1A), as well as analysis of changes in nuclear morphology of developing sperm. Comparable distribution and morphology of testis lobes was observed between planarians of the control group (luciferase(RNAi); Figure 1B) and planarians subjected to Smed-kifC3-like knockdown (Figure 1D). However, planarians subjected to Smed-KAP3 RNAi (Smed-KAP3(RNAi)) displayed differences in nuclear morphology of developing sperm in the inner lumen of testes lobes (Figure 1E). Rather than elongated nuclear structures of spermatozoa observed in control samples (Figure 1B, inset), the inner-most region of Smed-KAP3(RNAi) testis lobes contained only round or partially elongated nuclear structures indicative of defects in the inwards progression of spermatogenesis (Figure 1E, inset). This phenotype was validated by experiments using dsRNA targeting different exons of Smed-KAP3, as well as most of the predicted ORFs encoded by this gene, all of which yielded identical phenotypes (Supplementary Figure S1).
Figure 1. Smed-KAP3 is required for sperm development in S. mediterranea.

(A) Whole-mount DAPI-stained control sexual planarian viewed by fluorescence stereomicroscopy reveals areas of high cellular density, such as the pharynx and dorso-lateral testis lobes. Sperm accumulated in the copulatory apparatus is also visible in this sample. (B) Single confocal section reveals normal anatomy of developing cells in testis lobes of DAPI-stained planarians after four weeks of luciferase dsRNA feedings. Inset shows 2-fold magnified view of round spermatids (r.spd), elongating spermatids (e.spd), and spermatozoa (spz) in the inner lumen of a testis lobe. (C) Reverse transcription quantitative PCR results showing effective reduction of targeted mRNA levels in planarians subjected to Smed-kifC3-like, Smed-KAP3, or Smed-ARMC4-like dsRNA, but not in planarians fed Smed-kif17-like dsRNA. (D-F) Nuclear morphology comparable to control animals (B) is observed in testis lobes of Smed-kifC3-like (D) and Smed-ARMC4-like (F) knockdowns, including filamentous nuclei in spermatozoa that are largely absent in testis lobes of Smed-KAP3 RNAi samples (E). The fraction of biological replicates that displayed the phenotype represented by each image is shown in parenthesis. Scale bars = 1 mm in A, 50 μm in B, D-F.
Sequence analysis of Smed-KAP3 revealed this to be a KAP3/KIFAP3 ortholog: the top match in reciprocal BLAST searches between human (NCBI Reference Sequence: NP_001191443.1) and S. mediterranea sequences (E-value = 0.00). Differently spliced transcripts of Smed-KAP3 are predicted to contain three to four Armadillo Repeat Motifs (ARMs; Supplementary Figure S2) and to share 52% to 49.5% identical sequence with human KAP3 protein (Supplementary Figure S3). A search for proteins with enriched expression in the planarian reproductive system that also possess ARMs revealed a homolog of Armadillo Repeat-Containing Protein 4 (Smed-ARMC4-like; dd_Smes_v1_38309_1_1) with 11.46-fold reduced expression in planarians with disrupted sexual maturation (Supplementary Table S1). Knockdown of Smed-ARMC-like did not result in disruption of testis lobe morphology, sperm development, or any other detectable phenotypes (Figure 1F), which served as further evidence for the specificity of the phenotype observed by Smed-KAP3 RNAi. Altogether these results demonstrate that Smed-KAP3 is required for progression of planarian sperm development. Given that potential paralogs of Smed-KAP3 were not identified in the genome of S. mediterranea (Grohme et al., 2018), the Smed-KAP3(RNAi) phenotype is hypothesized to reflect loss of heterotrimeric Kinesin II activity.
Distribution of expression of Smed-KAP3 was assessed by whole-mount in situ hybridization (WMISH). Riboprobes designed to hybridize to Smed-KAP3 mRNAs, inclusive of the different splicing patterns identified, detected enriched abundance of these transcripts in testis lobes of sexual planarians (Figure 2A). Specificity of Smed-KAP3 WMISH signal was determined by parallel analysis using luciferase riboprobes, which revealed non-specific background signals in the planarian gut (Figure 2B). To determine the distribution of Smed-KAP3 expression in testis at the cellular level, double fluorescent in situ hybridization (dFISH) was performed using riboprobes against markers of specific stages of spermatogenesis (Chong et al., 2011; Wang et al., 2010b). dFISH analyses confirmed WMISH observations, as it revealed Smed-KAP3 expression in testes and more specifically during latter stages of spermatogenesis (Figure 2, C–E). Smed-KAP3 expression overlapped heavily with that of tektin-1 (tkn-1; Figure 2, D–D”) and with that of protein kinase A (pka; Figure 2, E–E”), which correspond to spermatocyte and spermatid cell stages, respectively (Chong et al., 2011). Smed-KAP3 expression was also detected in cells expressing the spermatogonia and germline stem cell marker germinal histone H4 (gh4; Wang et al., 2010b), although with qualitativaly less intensity than in spermatocytes and spermatids (Figure 2, C–C”). These results indicate that Smed-KAP3 is expressed in during spermatogenesis in planarians. More specifically, Smed-KAP3 seems to be initially transcribed in spermatogonia and peak in mRNA abundance at the spermatocyte and spermatid stages. The timing of Smed-KAP3 expression correlates with its role in late stages of spermatogenesis, potentially spermiogenesis, as suggested by RNAi functional analysis (Figure 1E).
Figure 2. Smed-KAP3 expression is enriched during sperm development.
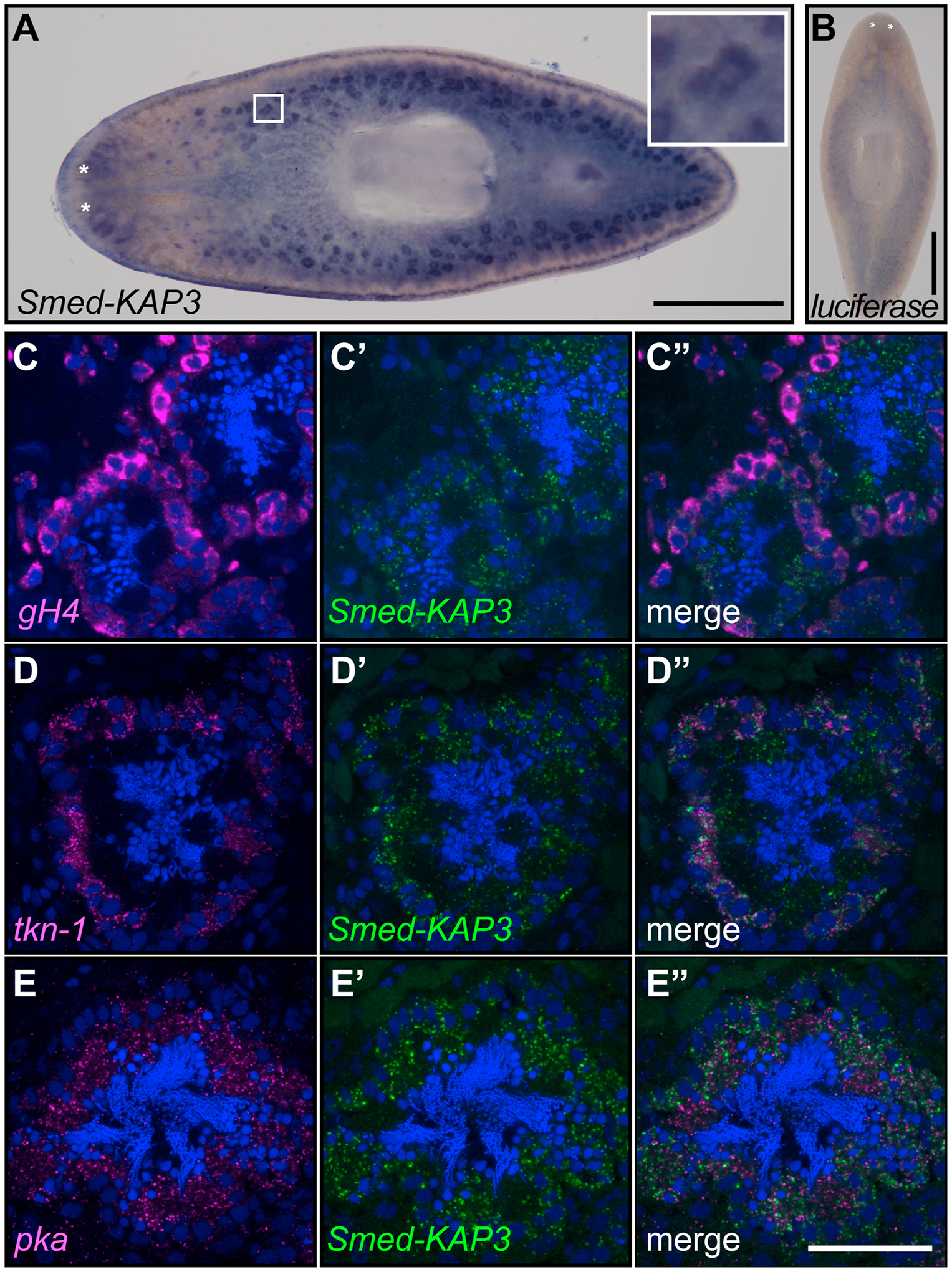
(A) Whole-mount in situ hybridization analysis in sexual planarian shows enriched Smed-KAP3 expression in testis lobes (5x magnified view inset). (B) Parallel analysis using luciferase sequence riboprobe shows background signal. (C-E) Confocal sections of samples analyzed by double fluorescence in situ hybridization using Smed-KAP3 (green; C’-C”; D’-D”; and E’-E”) riboprobes with markers (magenta) of spermatogonia (gH4; C and C”), spermatocytes (tkn-1; D and D”), and spermatids (pka, E and E”). DAPI staining is shown in blue. Scale bar = 1 mm in A-B, and 50 μm in C-E.
Detailed analysis of testis cell morphology was performed to uncover the processes in which Smed-KAP3 function is most crucial during spermatogenesis. Testis lobes isolated from control and Smed-KAP3(RNAi) planarians were visualized by confocal microscopy after nuclear staining with DAPI DNA dye and flagella labeling with acetylated-alpha-tubulin (AcTub) antibodies (Figure 3A). The lumens of testis lobes from control planarians were filled with filamentous nuclear (Figure 3B and B”) and flagellar (Figure 3B’ and 3B”) structures, as expected for normal sperm development in S. mediterranea. In contrast, the lumen of testis lobes from Smed-KAP3(RNAi) planarians were filled with round and partially or abnormally elongated nuclear structures (Figure 3C and 3C”). Furthermore, flagellar structures in testis lobes of Smed-KAP3(RNAi) (Figure 3C’ and C”) were shorter and less prominent that those observed in controls (Figure 3B’ and B”). These results validated the initial observation that Smed-KAP3 is required for spermatogenesis. More specifically, the presence of spermatids with some degree of flagellar assembly in Smed-KAP3(RNAi) testis lobes indicates that Smed-KAP3 is crucial during the process of spermiogenesis (Figure 3D). Surprisingly, not only were flagellar structures compromised, as would be expected from known Kinesin II function is axoneme formation, but nuclear structures of spermatids also failed to elongate properly. This last observation suggests that Smed-KAP3 plays a conserved role in shaping sperm head architecture as part of the Kinesin II complex, which corroborates with the previously identified role of the KIF3A motor protein in manchette function during spermiogenesis in mice (Lehti et al., 2013).
Figure 3. Smed-KAP3 is required for spermatid elongation and flagellar assembly.

(A) Illustration of processing steps for high-magnification analysis of spermatogenesis by DAPI and acetylated alpha-Tubulin (AcTub) staining of isolated testis lobes. (B-C) Representative image of filamentous structures of spermatozoa nuclei (B and B”; arrows) and flagella (B’ and B”; asterisks) observed in testis lobes of luciferase(RNAi) planarians stained with DAPI (blue in B” and C”) and anti-acetylated alpha-tubulin (green in B” and C”), whereas only partially elongated nuclear structures (C and C”; arrowheads) and short flagella (C’ and C”) were observed in lobes of Smed-KAP3(RNAi) planarians after 4-weeks of RNAi. Insets show 3x-magnified views. Scale bar = 10 μm. (D) Depiction of normal steps in progression of planarian spermiogenesis and failure to progress through spermatid elongation upon Smed-KAP3 RNAi.
Smed-KAP3 is necessary for motile cilia development and function in the planarian soma
Inch-worming movements became noticeable in planarians subjected to 4 weeks of Smed-KAP3 RNAi (Supplementary Video 1), which is a phenotype known to represent epidermal cilia malfunction (Reddien et al., 2005; Rink et al., 2009; Rompolas et al., 2009; Rompolas et al., 2010). This inch-worming movement was not observed in control planarians, which were fed luciferase dsRNA (Supplementary Video 2). To quantify the differences in movement, control and Smed-KAP3(RNAi) planarians were subjected to a motility assay based on planarian light avoidance behavior (Inoue et al., 2015; Inoue et al., 2004). Planarian RNAi groups were placed directly into an illuminated region of a 150 mm Petri dish (region I) and their migration to less-illuminated regions was recorded for five minutes (Supplementary Figure S4, A–C). Control planarians moved out of the illuminated regions much faster (average of 49 seconds) than Smed-KAP3(RNAi) planarians (average of 226.9; Supplementary Figure S4C). All control planarians traveled to the region of the dish furthest from the light source (region IV), which is where they spent the most time (Supplementary Figure S4, A–A” and C). Conversely, Smed-KAP3(RNAi) planarians rarely reached region IV and remained in region I for most of the assay (Supplementary Figure S4, B–B” and C). These results suggested that Smed-KAP3 is required for normal planarian motility and may contribute to formation of epidermal cilia that drive planarian gliding.
In addition to inch-worming, planarians subjected to Smed-KAP3 RNAi exhibited edema (Supplementary Video 1), which is a phenotype that is indicative of cilia dysfunction in the protonephridial system (i.e. ciliated structures responsible for fluid homeostasis in planarians). This phenotype was further analyzed in asexual planarians, which lack reproductive structures and are routinely used for studies of protonephridial cell function (Rink et al., 2011; Scimone et al., 2011; Thi-Kim Vu et al., 2015). As the RNAi feedings progressed, the posterior region of Smed-KAP3 knockdowns bloated in ways not observed in control planarians (Supplementary Figure S4, D and E). As RNAi treatment continued, Smed-KAP3(RNAi) planarians developed lesions and ultimately died by lysis (Supplementary Figure S4, E and F). By contrast, control planarians did not develop edema, lesions, or lysis (Supplementary Figure S4D). Instead, control planarians increased in number as a product of nutritional growth and transverse fission, as expected of healthy asexual planarians under sufficient feeding regimen (Supplementary Figure S4F).
Multiciliated epithelial cells were analyzed by immunofluorescence (Supplementary Figure S5, A–D) and Transmission Electron Microscopy (Supplementary Figure S5, E–H) to determine whether the integrity of cilia in the soma was compromised upon Smed-KAP3 RNAi. Asexual planarians subjected to luciferase and Smed-KAP3 dsRNA feedings were processed for analysis 2.5 weeks into the RNAi treatment, once the inch-worming manifested in Smed-KAP3(RNAi) (this phenotypic manifestation progresses faster in asexual planarians than in their larger sexual counterparts). First, cilia of ventral (Supplementary Figure S5, A and B) and lateral (Supplementary Figure S5, C and D) epithelial cells were visualized by confocal microscopy after anti-α-tubulin antibody staining as previously performed by others (Glazer et al., 2010; Rompolas et al., 2009; Sanchez Alvarado and Newmark, 1999). Anti-α-tubulin signal was detected in ventral epithelia of both groups (Supplementary Figure S5, A and B), but was less abundant in Smed-KAP3(RNAi) (Supplementary Figure S5B). The ventral epidermis of luciferase(RNAi) planarians displayed dense swaths of α-tubulin (Supplementary Figure S5, A and A’) and showed apical extensions of ciliary axonemes in lateral epithelial cells (Supplementary Figure S5C). Structures labeled by anti-α-tubulin were noticeably less abundant in ventral and lateral epithelial cells of Smed-KAP3(RNAi). Maximum projection of confocal z-stack images revealed small anti-α-tubulin foci in ventral epithelial cells of Smed-KAP3(RNAi) (Supplementary Figure S5, B and B’), which we interpret to be partially-formed cilia or basal bodies. Axonemal structures were also not observed during analysis of lateral epithelial cells in Smed-KAP3(RNAi), which only displayed faint α-tubulin signal in their distal ends (Supplementary Figure S5D). Analysis of luciferase(RNAi) by Transmission Electron Microscopy revealed ciliary projections away from the cytoplasm of epithelial cells that carried the characteristic 9+2 microtubular axonemal structure (Supplementary Figure S5E). In contrast, comparable ciliary structures were not observed in Smed-KAP3(RNAi) samples (Supplementary Figure S5F). Only shorter structures of smaller diameter were observed projecting from the distal end of Smed-KAP3(RNAi) epithelial cells (Supplementary Figure S5F), which did not display the diameter or 9+2 arrangement of microtubules observed in controls (Supplementary Figure S5E). Basal bodies were observed attached to the cell membrane in sections of both control (Supplementary Figure S5G) and Smed-KAP3(RNAi) (Supplementary Figure S5H). These results demonstrate that Smed-KAP3 is required for assembly of axonemal structures in epithelial cells of S. mediterranea, but suggest that its function may be dispensable for basal body assembly.
Spermiogenesis defects in Smed-KAP3 RNAi are not due to ciliary failure in the soma or mitotic errors during spermatogenesis
Given the changes in motility and protonephridial function observed upon Smed-KAP3 RNAi, it seemed plausible that defects in sperm development resulted from non-autonomous processes impacting the planarian soma. To test this, we performed parallel analyses of Smed-KAP3(RNAi) with knockdowns of Dynein Assembly factor with WD repeat domains 1 (DAW1). DAW1 is a conserved contributor to ciliary function recently shown to be required for planarian motility and protonephridial function, but dispensable for spermatid elongation in S. mediterranea (Lesko and Rouhana, 2020). Juvenile sexual planarians were fed twice per week for 3 weeks with liver containing luciferase, Smed-KAP3, or Smed-DAW1 dsRNA, and scored for motility defects (Figure 4, A–C). As expected, luciferase(RNAi) motility was normal throughout the experiment (Figure 4A). Planarians in the Smed-DAW1(RNAi) group began to display mild motility defects (slow movement) on day 11 (D11) of treatment, which progressed into the inchworm motility phenotype starting on D18 of the experiment (Figure 4B). Motility defects progressed with a slight delay in Smed-KAP3(RNAi) when compared to Smed-DAW1(RNAi), with slow motility starting at D18 and inchworm motility at D22 (Figure 4C). Planarians were fixed and stained with DAPI on D26, at which point edema was not detectable. Analysis of sperm development amongst planarians that reached sexual maturity (as judged by the presence of the copulatory structures) revealed normal sperm head elongation in testis lobes of luciferase(RNAi) and Smed-DAW1(RNAi) (Figure 4D and 4E). In contrast, sperm was absent in testis lobes of Smed-KAP3(RNAi) (Figure 4F). These results corroborate with previously published results for Smed-DAW1(RNAi) (Lesko and Rouhana, 2020) and support the notion that sperm development defects observed in Smed-KAP3(RNAi) are not due to ciliary dysfunction outside of the reproductive system.
Figure 4. Spermatid elongation defects of Smed-KAP3(RNAi) are not upon knockdown of Smed-DAW1, a regulator of ciliary function in the planarian soma.

(A-C) Assessment of planarian motility in luciferase(RNAi) (A), Smed-DAW1(RNAi) (B), and Smed-KAP3(RNAi) (C), reveals progression from normal (green) to slower (yellow) and inchworm (red) phenotype develop first in Smed-DAW1(RNAi) (B) and later in Smed-KAP3(RNAi) (C). The y-axis denotes the percent (%) of animals (n = 13 to 14) per group scored under the different phenotypes. The x-axis represents days into the RNAi treatment starting with the day of the first feeding (1). (D-F) Single confocal section reveals normal nuclear anatomy of developing cells in testis lobes of DAPI-stained luciferase(RNAi) (D) and Smed-DAW1(RNAi) (E) planarians, but loss of elongated structures in Smed-KAP3(RNAi) (F). Parentheses in (D-F) show the fraction of sexually mature animals (defined as per presence of copulatory structures) from the groups analyzed in (A-C) upon fixation on day 26 from the initial dsRNA feeding that show the phenotype displayed. Scale bar = 50 μm.
Over-expression of a KIF3B mutant unable to associate with KAP3 leads to mitotic defects in a mouse cell line derived from embryonic fibroblasts (Haraguchi et al., 2006). Spermatogenesis in planarians involves three rounds of mitotic divisions with incomplete cytokinesis, which generate eight primary spermatocytes that undergo meiosis to produce 32 spermatids (Franquinet and Lender, 1973; Newmark et al., 2008). To address the possibility that spermatid elongation defects observed upon Smed-KAP3 RNAi could be due to errors in mitosis, we compared mitotic structures in testis lobes of luciferase(RNAi) and Smed-KAP3(RNAi) (Figure 5). Testis lobes of planarians fixed after four and six weeks of dsRNA feedings were analyzed for the presence of cysts composed of 1, 2, 4, 8, and 16 mitotic cells labeled by phospho-Histone H3 (Ser10) antibodies (as per Wang et al., 2010b). At the 4-week timepoint, there was no significant difference in the presence or distribution of mitotic cell cysts of in testis lobes of control and Smed-KAP3(RNAi) animals (Figure 5, A–B and D). This was regardless of the fact that elongated spermatozoa were abundant in testis lobes of luciferase(RNAi) (Figure 5A), whereas those of Smed-KAP3(RNAi) were filled with round and partially elongated spermatids (Figure 5B). Upon prolonged RNAi and progression of noticeable edema, the presence of mitotic structures and spermatids became significantly reduced in testis lobes of Smed-KAP3(RNAi) (Figure 5, C and E). At this timepoint (6-weeks of RNAi) cysts of mitotic cells in lobes of Smed-KAP3(RNAi) were composed mainly of 1 or 2 cells, whereas lobes of luciferase(RNAi) contained cysts of comparable distribution to those observed at the 4-week timepoint, as well as those reported by Wang et al. (2010b). Given the clear manifestation of spermatid elongation defects in Smed-KAP3(RNAi) at a timepoint when distribution of mitotic cells seems normal (4-weeks), we conclude that Smed-KAP3 plays a direct role in spermatid elongation. We hypothesize that the collapse in mitotic progression of spermatogenic cells observed after extended knockdown of Smed-KAP3 (i.e. 6-weeks) is due to severe changes in overall physiology by this timepoint.
Figure 5. Spermatid elongation defects in Smed-KAP3(RNAi) precede collapse of mitotic divisions in testis lobes.

(A-C) Maximal projection of partial z-stack confocal images of testis lobes from luciferase(RNAi) (A) and Smed-KAP3(RNAi) (B-C) stained with DAPI (blue) and anti-PH3 after 4 (A and B) and 6-week (C) RNAi treatments. (D-E) Quantification of PH3(+) cysts classified as composed of 1–2, 4, 8, or 16 cells testis lobes of luciferase(RNAi) and Smed-KAP3(RNAi) after four (D) and six (E) weeks of RNAi. Each timepoint includes analysis of 18 to 27 testis lobes visualized from each of at least four biological samples by confocal z-stack imaging throughout as much of the samples as technically feasible. Bars show the mean and error bars the standard deviation. Asterisks indicate p < 0.01, Student’s t-test).
Analysis of heterotrimeric Kinesin II complex motor proteins
The motility and bloating phenotypes observed upon Smed-KAP3 RNAi resemble those reported for the KIF3B motor protein ortholog Smed-kif3B (GenBank ID: GQ337489, Rink et al., 2009; also characterized as “Smed-kif3a” by Rompolas et al., 2010), which supports the hypothesis that phenotypes caused by Smed-KAP3 RNAi are due to disruption of heterotrimeric Kinesin II function. To further test this hypothesis, we analyzed Smed-kif3A (GenBank IDs: GQ337490, Rink et al., 2009; and KT161944, Li and Yang, NCBI GenBank record), which is orthologous to the other motor protein of the heterotrimeric Kinesin II complex.
Coordinated expression of Smed-KAP3 with motor protein genes is expected for formation of functional kinesin complexes. Review of single cell transcriptome data from (Plass et al., 2018) confirmed that transcripts corresponding to Smed-KAP3 (dd_Smed_v6_4094_0), Smed-kif3A (dd_Smed_v6_4343_0; dd_Smed_v6_13424_0), and Smed-kif3B (dd_Smed_v6_4343_0; dd_Smed_v6_13424_0), are all detected in epidermis and protonephridial cells (Supplementary Figure S6). In addition, expression of these heterotrimeric Kinesin II complex genes was also enriched in pharynx cells, as well as in cholinergic and GABAergic neurons (Supplementary Figure S6).
Knockdown of Smed-kif3A resulted in inch-worming motility as well as bloating (Supplementary Figure S7; Supplementary Video 3), and ultimately lysis and death (death rate ranged between 16% to 67% when experiments were stopped, as compared to 0% for luciferase(RNAi)). Analysis of ciliary structures in ventral and lateral epithelial cells revealed that axonemal structures labeled by anti-alpha-tubulin in control animals were absent in Smed-kif3A(RNAi) (Supplementary Figure S7), which corroborates with the somatic phenotypes observed upon Smed-KAP3 RNAi (Supplementary Figures S4 and S5). These results indicate that the heterotrimeric Kinesin II complex is required for planarian osmoregulation, as well as gliding and ciliary assembly in epithelial cells of S. mediterranea.
Enrichment of Smed-KAP3 transcript abundance in sexual planarians with mature reproductive structures, as compared to CPEB2(RNAi) lacking mature reproductive structures (4.00-fold; p-value = 0.042), was not paralleled by either Smed-kif3A nor Smed-kif3B (1.03-fold increased and −1.17-fold decreased abundance, respectively; p-value above 0.05; Supplementary Table S1). Direct analysis of Smed-kif3A expression in sexual planarians was performed by whole-mount in situ hybridization. Colorimetric development of Smed-kif3A signal revealed broad distribution of expression in sexual planarians (Supplementary Figure S7), which complicated assessment of expression for this gene in the testes. To determine whether Smed-KAP3 and Smed-kif3A overlap in expression during spermatogenesis directly, we performed double fluorescence in situ hybridization (Figure 6A–A’“). Visualization by confocal microscopy revealed enriched Smed-kif3A expression in spermatocytes and spermatids (Figure 6A’ and 6A”’), which represent the stages of spermatogenesis with highest expression of Smed-KAP3 (Figure 2D and 2E; Figure 6A” and 6A”’).
Figure 6. Smed-kif3A(RNAi) phenocopies Smed-KAP3(RNAi).

(A) Confocal section of samples analyzed by double fluorescence in situ hybridization using Smed-kif3A (green; A’ and A”’) and Smed-KAP3 (magenta; A” and A”’) riboprobes. DAPI staining shows nuclear structures of developing sperm (white; A-A”’). Insets show 3-fold magnified views. (B-C) Single confocal sections of whole-mount samples stained with DAPI reveal morphology of developing sperm in testis lobes of luciferase(RNAi) (B) and Smed-kif3A(RNAi) (C) planarians fixed after 4-weeks of RNAi. Filamentous nuclear structures of spermatozoa (B, inset) are largely absent in testis lobes of Smed-kif3A(RNAi) (C, inset). Insets show 2-fold magnified views. The fraction of biological replicates showing the phenotype displayed is shown in parenthesis (B and C). (D) RT-qPCR measurement of Smed-kif3A mRNA abundance relative to levels in luciferase(RNAi) shows efficacy of Smed-kif3A RNAi. Scale bars = 50 μm.
Functional analysis by RNAi revealed that the accumulation of spermatozoa observed in control samples (Figure 6B) was severely reduced upon disruption of Smed-kif3A expression (Figure 6C). Instead of elongated spermatozoa (Figure 6B), the lumen of Smed-kif3A(RNAi) testis lobes mostly contained round and partially elongated spermatids (Figure 6C), which correlates with the spermatogenesis defects observed in Smed-KAP3(RNAi) (Figures 1E, 3C, 4F, and 5B). Altogether, these results support a model in which heterotrimeric Kinesin II activity is required for structural integrity and function of cilia in the soma of S. mediterranea, as well as for sperm development through contributions towards nuclear elongation and flagellar formation.
DISCUSSION
Characterization of Kinesin II complexes in S. mediterranea
In this study we characterized components of the heterotrimeric Kinesin II complex in the planarian flatworm S. mediterranea. Evidence of expression and function of orthologs of the accessory protein KAP3 and the motor protein KIF3A were observed in the ciliated epidermis and protonephridia of S. mediterranea. Our results corroborate and complement those described for the ortholog of KIF3A’s partner motor protein in this complex, KIF3B, which was been characterized in detail by others (Rink et al., 2009; Rompolas et al., 2010). Together, these studies suggest that the composition and function of the heterotrimeric Kinesin II complex in intraflagellar transport during ciliogenesis and sperm tail formation are conserved in planarians. Accessing single cell RNA sequencing data from Plass et al. (2018) also revealed that expression of members of the heterotrimeric Kinesin II are highly enriched in subsets of neurons in S. mediterranea (i.e. GABA neurons and ChAT neurons; Supplementary Figure S6), which suggests that known roles for this complex in axonal elongation (Aizawa et al., 1992; Kondo et al., 1994) may also be present in planarians, although our experiments did not test this hypothesis.
Alternative versions of heterotrimeric Kinesin II in other organisms contain the motor protein KIF3C in place of KIF3B (Muresan et al., 1998). We did not find a clear ortholog of KIF3C in S. mediterranea, which corroborates with studies in mice that show KIF3C to be dispensable for development (Yang et al., 2001). This implies that the heterotrimeric Kinesin II complex in planarians is composed of Smed-KIF3A, Smed-KIF3B, and Smed-KAP3, the latter of which may be present in alternatively spliced isoforms (Supplementary Figures S1 and S2). Another conserved Kinesin II family motor is the homodimeric complex composed of two KIF17 units. KIF17 is involved in microtubule-based transport of protein and mRNA in rat hippocampal neurons and in germ cells of mouse testis (Chennathukuzhi et al., 2003), and has also been postulated to contribute to epithelial morphogenesis and brain development in mammals (Jaulin and Kreitzer, 2010; Irla et al., 2007; reviewed by Wong-Riley and Besharse, 2012). Several KIF17 homologs were identified in the genome of S. mediterranea, including two that are predicted to have preferential expression in the reproductive system (Supplementary Table S1). Disruption of expression of one of these was unsuccessful (Smed-kif17-like; Figure 1C). However, the gene with highest degree of identical sequence with vertebrate KIF17 protein (dd_Smes_v1_99042_1_1; dd_Smed_v6_13424_0_1; E-value = 0.00) was not tested. Interestingly, single cell RNAseq data indicates that the distribution of expression of this potential KIF17 ortholog is very similar to that of heterotrimeric Kinesin II components (i.e. enriched in cells of the epidermis, pharynx, and protonephridia, as well as GABA and ChAT neurons) and decreases in planarians upon disrupted development of reproductive structures (Supplementary Table S1). It will be interesting to see whether any redundancies exist between different Kinesin II complexes in planarians, as KIF17 and KIF3 complexes are known to function cooperatively during sensory cilia development in nematodes (Snow et al., 2004).
Multiple roles for heterotrimeric Kinesin II during spermiogenesis
Defects in spermatid elongation were observed upon disruption of heterotrimeric Kinesin II activity via knockdown of either Smed-KAP3 or Smed-kif3A. The least surprising aspect of this phenotype is the loss of complete flagella formation, as transport of proteins required for ciliogenesis is a well-documented conserved role of Kinesin II (Cole et al., 1998; Scholey, 2013). Expression of heterotrimeric Kinesin II motor proteins in developing sperm has been observed in a wide range of animals, including octopus, fish, and newts (Dang et al., 2012; Hu et al., 2012; Wang et al., 2019; Wang et al., 2010a). One can predict that heterotrimeric Kinesin II plays a conserved role in development of sperm flagella, although notable exceptions including nematodes (whose sperm lack flagella; Ellis and Stanfield, 2014) and flies (in which KAP3 mutation has no effect on development of sperm flagella; Sarpal et al., 2003). A less established, yet potentially ancestral role of kinesins during spermatogenesis, relates to their contribution in shaping the sperm head and nucleus. Studies in mice have shown that KIF3A is required for organization of a perinuclear ring of microtubules known as the manchette, which is transiently present in elongating spermatids (Lehti et al., 2013). KIF1-binding protein (KBP) and meiosis-specific nuclear structural protein 1 (MNS1) bind KIF3A and colocalize to the manchette (Lehti et al., 2013; Lehti et al., 2015). The interaction between KIF3A and MNS1 may contribute to transport of MNS1 to the developing sperm flagella, whereas the relevance of interaction with KBP remains to be determined. Whether KIF3A in mice functions as part of the Kinesin II complex in the manchette remains to be determined, but our results suggest that it does. Further research using planarians may reveal partners that facilitate Kinesin II functions in the nuclear elongation process.
Contributions of the sperm head, flagella, and ciliated somatic structures to planarian sperm motility
Planarian fertilization occurs through internal cross-fertilization and requires that sperm travel substantial distances before and after insemination. Sperm must first travel through sperm ducts from the dorsolateral position of the testes to seminal vesicles of the copulatory apparatus, which is positioned along the midline in the posterior end of these animals (Fig. 1A). After ejaculation, sperm must travel from the bursa in the copulatory apparatus to the end of the oviducts that is closer to the ovaries (which are located in the anterior end of the animal; see pink dots in Figure 3A). We looked for differences in accumulation of sperm in seminal vesicles between luciferase(RNAi) and Smed-KAP3(RNAi), but the fraction of animals with (and without) visible sperm accumulated in the seminal vesicles was relatively equal (Supplementary Figure S8). Qualitatively, six of fourteen Smed-KAP3 animals with visible sperm in their ducts appeared to have abnormally high accumulation extending towards the anterior of the anatomy (Supplementary figure S8). It is possible that developing sperm with partial KAP3 function are able to progress far enough into spermiogenesis to be funneled into the sperm duct, but fail to travel normally before or after copulation. Any conclusions regarding the role of kinesin II in sperm motility would require further investigation, particularly when taking into consideration that oviducts and sperm ducts are also ciliated structures. Perhaps future studies with dissected parts of the planarian reproductive system may shed light into the mechanisms that propel sperm motility at different stages of the reproductive process.
Extent of manchette presence as a function of sperm shape evolution
Sperm shapes come in a remarkable range of forms that reflect the rapid evolution of this cell type in metazoans (Avidor-Reiss, 2018). Styles range from the popularly known ancestral morphology, which includes a compact nuclear region (i.e. “head”) and single flagellum, to the long filamentous head and double flagella seen in planarians. There are even instances in which flagellar structures are absent in sperm that is nevertheless motile, as is the case in Allostoma and Diplozoon species of flatworms (Lanfranchi, 1998; Justine et al., 1985). The diversity of sperm morphology in flatworms is so vast that it is used as a tool to make predictions about phylogenetic relationships in Platyhelminthes (Justine, 1998; Justine et al., 1985; Justine and Poddubnaya, 2018), but also provides a valuable resource for understanding the molecular mechanisms that drive spermiogenesis and sperm function.
The transient appearance of cortical microtubules along the axis of nuclear elongation in spermatids (i.e., the manchette) has been reported in a broad range of animals. The manchette has been best studied in mice, but has been observed in vertebrates ranging from birds (Soley, 1997; Asano and Tajima, 2017), to toads (Burgos and Fawcett, 1956) and cats (Burgos and Fawcett, 1955). Manchette-like structures with extended presence as cortical microtubules in spermatozoa are seen in a wide range of Platyhelminthes, not only in freshwater planarians, but also in marine and parasitic flatworms (Bakhoum et al., 2017; Liana and Litvaitis; 2007; Silveira and Porter, 1964). Amongst Lophotrochozoa, the formation of cortical microtubules during spermatogenesis is not limited to platyhelminthes, for these are observed in spermatids of some polychaetes (Rouse, 1995) and the bryozoan Cristatella mucedo (Lützen et al., 2009). Amongst Ecdysozoa, a microtubular structure known as “the dense body” that aligns to one side of the nucleus during spermiogenesis has been described as analogous to the manchette because of its structural composition, as well as its functional roles in intracellular transport and nuclear shape (Tokuyaso, 1974; Fabian and Brill, 2012). Insects (Jamieson, 1987) and other arthropods also exhibit transient manchette (or manchette-like) structures during spermatid elongation (e.g., Chelicerata, reviewed by Alberti, 1995), and in some cases cortical microtubules surround the nucleus of elongated sperm cells as in planaria (e.g., Nymphon, van Deurs, 1974). Finally, manchette formation has also been reported in spermatids of in non-bilaterian phyla, including Hydra (West, 1978) and other Cnidaria (Rouse and Pitt, 2000; reviewed in Hinsch, 1974), but no such structures are reported in analyses of sperm development in Porifera (Barthel and Detmer, 1990).
Reports of manchette formation in multiple taxa within four of the major animal lineages (Deuterostomia, Lophotrochozoa, Ecdysozoa, and Cnidaria), which encompass more than 99% of all animal species, suggests that this structure dates back to the last common ancestor of Cnidaria and Bilateria (>600 million years ago). However, only a small number of taxa have been analyzed relative to the level necessary to establish whether the manchette is truly present or absent ancestrally. Furthermore, information regarding the potential molecular mechanisms that drive spermatid elongation are limited to a handful of species (e.g., mice, flies, planarians in this study). It is therefore possible that this structure arose independently multiple times in evolution.
The elongated head is a characteristic feature of spermatozoa in Platyhelminthes (Hendelberg, 1986). Ultrastructural analyses reveal the presence of approximately 30 to 40 microtubule filaments surrounding the nucleus and mitochondrion in parallel arrangement along the longitudinal axis of flatworm spermatozoon heads (Harrath et al., 2012; Silveira and Porter, 1964). We hypothesize that these tubular structures derive from structures homologous to the manchette. However, unlike the rapid transition between assembly and dismantling of the manchette seen in murine spermatogenesis, the ring of microtubules that forms around the nucleus of elongating spermatids outlasts spermiogenesis and remains present in spermatozoa of many Lophotrochozoans. It is conceivable that the extended presence of this structure is directly associated with prolonged Kinesin II activity that drives the extremely elongated nuclear regions of spermatozoa in Platyhelminthes.
CONCLUSIONS
Planarian spermiogenesis requires the function of heterotrimeric Kinesin II components for both flagellar formation and nuclear/head elongation. These findings support a model in which the extended presence of manchette-like structures prolongs Kinesin II-driven rearrangements that give rise to long filamentous heads in spermatozoa of Platyhelminthes.
Supplementary Material
HIGHLIGHTS.
Heterotrimeric Kinesin II components are required for spermatid elongation.
Dramatic sperm head elongation in planarians facilitates studies of spermiogenesis.
Evolution of manchette-like structures may contribute to sperm shape diversity.
ACKNOWLEDGEMENTS
The authors thank Dr. Tracy Chong and Dr. Phil Newmark for sharing details on testis lobe maceration and immunostaining protocols, Dr. John Brubacher for insight regarding transmission electron studies of sperm from Lophotrochozoa, and Dr. Joseph F. Ryan for feedback on the manuscript. DC was supported by a Graduate Teaching Assistantship from Wright State University. This work was supported by NICHD award R15 HD082754 to LR.
Abbreviations:
- KIF
Kinesin Superfamily Proteins
- KAP
Kinesin Associated Protein
- WMISH
whole-mount in situ hybridization
- FISH
fluorescent in situ hybridization
- RNAi
RNA-interference
- DAPI
4’,6-diamidine-2’-phenylindole dihydrochloride
- Smed
Schmidtea mediterranea
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aizawa H, Sekine Y, Takemura R, Zhang Z, Nangaku M and Hirokawa N (1992). Kinesin family in murine central nervous system. J Cell Biol 119, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti G (1995). Comparative spermatology of Chelicerata: review and perspective. In: Jamieson BGM, Ausio J and JUSTINE JL (eds), Advances in Spermatozoal Phylogeny and Taxonomy. Mem. Mus. natn. Hist. nat 166, 203–230. Paris. [Google Scholar]
- Asano A and Tajima A (2017). Development and Preservation of Avian Sperm. In: Sasanami T (eds) Avian Reproduction. Advances in Experimental Medicine and Biology, vol 1001. Springer, Singapore. [DOI] [PubMed] [Google Scholar]
- Avidor-Reiss T (2018). Rapid Evolution of Sperm Produces Diverse Centriole Structures that Reveal the Most Rudimentary Structure Needed for Function. Cells 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J, Wong ML, Downhour DM, Sanchez Alvarado A and Marshall WF (2012). Centrosome loss in the evolution of planarians. Science 335, 461–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguñà J and Romero R (1981). Quantitative analysis of cell types during growth, degrowth and regeneration in the planarians Dugesia mediterranea and Dugesia tigrina. Hydrobiologia 84, 181–194. [Google Scholar]
- Bakhoum AJS, Miquel J, Ndiaye PI, Justine JL, Falchi A, Bâ CT, Marchand B and Quilichini Y (2017). Advances in Spermatological Characters in the Digenea: Review and Proposal of Spermatozoa Models and Their Phylogenetic Importance. Adv Parasitol. 98,111–165. [DOI] [PubMed] [Google Scholar]
- Barthel D and Detmer A (1990). The spermatogenesis of Halichondria panicea (Porifera, Demospongiae). Zoomorphology 110, 9–15. [Google Scholar]
- Brandl H, Moon H, Vila-Farre M, Liu SY, Henry I and Rink JC (2016). PlanMine--a mineable resource of planarian biology and biodiversity. Nucleic Acids Res 44, D764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos MH and Fawcett DW (1956). Studies on the fine structure of the mammalian testis. I. Differentiation of the spermatids in the cat (Felis domestica). J Biophys Biochem Cytol. 1, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos MH and Fawcett DW (1956). An electron microscope study of spermatid differentiation in the toad, Bufo arenarum Hensel. The Journal of Cell Biology, 2, 223–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebria F and Newmark PA (2005). Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development 132, 3691–3703. [DOI] [PubMed] [Google Scholar]
- Chennathukuzhi V, Morales CR, El-Alfy M and Hecht NB (2003). The kinesin KIF17b and RNA-binding protein TB-RBP transport specific cAMP-responsive element modulator-regulated mRNAs in male germ cells. Proc Natl Acad Sci U S A 100, 15566–15571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T, Stary JM, Wang Y and Newmark PA (2011). Molecular markers to characterize the hermaphroditic reproductive system of the planarian Schmidtea mediterranea. BMC Dev Biol 11, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC and Rosenbaum JL (1998). Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 141, 993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang R, Zhu JQ, Tan FQ, Wang W, Zhou H and Yang WX (2012). Molecular characterization of a KIF3B-like kinesin gene in the testis of Octopus tankahkeei (Cephalopoda, Octopus). Mol Biol Rep 39, 5589–5598. [DOI] [PubMed] [Google Scholar]
- Desai A and Mitchison TJ (1997). Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 13, 83–117. [DOI] [PubMed] [Google Scholar]
- Ellis RE and Stanfield GM (2014). The regulation of spermatogenesis and sperm function in nematodes. Seminars in cell & developmental biology 29, 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian L and Brill JA (2012). Drosophila spermiogenesis: Big things come from little packages. Spermatogenesis 2, 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franquinet R and Lender T (1973). Étude ultrastructurale des testicules de Polycelis tenuis et Polycelis nigra (Planaires). Evolution des cellules germinales mâles avant la spermiogenèse. Z Mikrosk Anat Forsch 87, 4–22. [PubMed] [Google Scholar]
- Glazer AM, Wilkinson AW, Backer CB, Lapan SW, Gutzman JH, Cheeseman IM and Reddien PW (2010). The Zn finger protein Iguana impacts Hedgehog signaling by promoting ciliogenesis. Dev Biol 337, 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC and Anderson KV (2010). The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11, 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohme MA, Schloissnig S, Rozanski A, Pippel M, Young GR, Winkler S, Brandl H, Henry I, Dahl A, Powell S, et al. (2018). The genome of Schmidtea mediterranea and the evolution of core cellular mechanisms. Nature 554, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ES, Eveleth J, Jiang C, Redenbach DM and Boekelheide K (1992). Distribution of the microtubule-dependent motors cytoplasmic dynein and kinesin in rat testis. Biol Reprod 46, 817–828. [DOI] [PubMed] [Google Scholar]
- Haraguchi K, Hayashi T, Jimbo T, Yamamoto T, Akiyama T (2006). Role of the kinesin-2 family protein, KIF3, during mitosis. J Biol Chem. 281, 4094–9. [DOI] [PubMed] [Google Scholar]
- Harrath AH, Alwasel S, Zghal F and Tekaya S (2012). Ultrastructure of spermatogenesis and mature spermatozoon of the freshwater planarian Schmidtea mediterranea (Platyhelminthes, Paludicola). C R Biol 335, 87–95. [DOI] [PubMed] [Google Scholar]
- Hendelberg J (1986). The phylogenetic significance of sperm morphology in the Platyhelminthes. Hydrobiologia 132, 53–58. [Google Scholar]
- Hildebrandt F, Benzing T and Katsanis N (2011). Ciliopathies. N Engl J Med 364, 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsch GW (1974). Comparative Ultrastructure of Cnidarian Sperm, American Zoologist, 14, 457–465. [Google Scholar]
- Hu JR, Xu N, Tan FQ, Wang DH, Liu M and Yang WX (2012). Molecular characterization of a KIF3A-like kinesin gene in the testis of the Chinese fire-bellied newt Cynops orientalis. Mol Biol Rep 39, 4207–4214. [DOI] [PubMed] [Google Scholar]
- Inaba K (2003). Molecular architecture of the sperm flagella: molecules for motility and signaling. Zoolog Sci 20, 1043–1056. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hoshino H, Yamashita T, Shimoyama S and Agata K (2015). Planarian shows decision-making behavior in response to multiple stimuli by integrative brain function. Zoological Lett 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Kumamoto H, Okamoto K, Umesono Y, Sakai M, Sanchez Alvarado A and Agata K (2004). Morphological and functional recovery of the planarian photosensing system during head regeneration. Zoolog Sci 21, 275–283. [DOI] [PubMed] [Google Scholar]
- Irla M, Saade M, Fernandez C, Chasson L, Victorero G, Dahmane N, Chazal G, and Nguyen C (2007). Neuronal distribution of spatial in the developing cerebellum and hippocampus and its somatodendritic association with the kinesin motor KIF17. Exp Cell Res 313, 4107–4119. [DOI] [PubMed] [Google Scholar]
- Ishikawa H and Marshall WF (2011). Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 12, 222–234. [DOI] [PubMed] [Google Scholar]
- Jamieson BGM (1987). The Ultrastructure and Phylogeny of Insect Spermatozoa. Cambridge, Cambridge University Press: 1–320 [Google Scholar]
- Jaulin F and Kreitzer G (2010). KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol 190, 443–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justine JL (1998). Spermatozoa as phylogenetic characters for the Eucestoda. J Parasitol 84, 385–408. [PubMed] [Google Scholar]
- Justine JL, Lambert A and Mattei X (1985). Spermatozoon ultrastructure and phylogenetic relationships in the monogeneans (Platyhelminthes). Int J Parasitol 15, 601–608. [DOI] [PubMed] [Google Scholar]
- Justine JL, Le Brun N, and Mattei X (1985). The aflagellate spermatozoon of Diplozoon (Platyhelminthes: Monogenea: Polyopisthocotylea). A demonstrative case of relationship between sperm ultrastructure and biology of reproduction. J. Ultr. Res 92, 47–54. [DOI] [PubMed] [Google Scholar]
- Justine JL and Poddubnaya LG (2018). Spermiogenesis and spermatozoon ultrastructure in basal polyopisthocotylean monogeneans, Hexabothriidae and Chimaericolidae, and their significance for the phylogeny of the Monogenea. Parasite 25, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum AL (2002). Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail. Mol Reprod Dev 63, 1–4. [DOI] [PubMed] [Google Scholar]
- King RS and Newmark PA (2013). In situ hybridization protocol for enhanced detection of gene expression in the planarian Schmidtea mediterranea. BMC Dev Biol 13, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM (2016). Axonemal Dynein Arms. Cold Spring Harb Perspect Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y and Hirokawa N (1994). KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol 125, 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Shi D and Fujimori T (2019). Biophysics in oviduct: Planar cell polarity, cilia, epithelial fold and tube morphogenesis, egg dynamics. Biophys Physicobiol 16, 89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranchi A (1998). Ultrastructural observations on the development of male gametes in Allostoma sp. (Plathelminthes, Prolecithophora). Hydrobiologia 383, 227–233. [Google Scholar]
- Lehti MS, Kotaja N and Sironen A (2013). KIF3A is essential for sperm tail formation and manchette function. Mol Cell Endocrinol 377, 44–55. [DOI] [PubMed] [Google Scholar]
- Lehti MS, Kotaja N and Sironen A (2015). KIF1-binding protein interacts with KIF3A in haploid male germ cells. Reproduction 150, 209–216. [DOI] [PubMed] [Google Scholar]
- Lehti MS and Sironen A (2016). Formation and function of the manchette and flagellum during spermatogenesis. Reproduction 151, R43–54. [DOI] [PubMed] [Google Scholar]
- Lesko SL and Rouhana L (2020). Dynein assembly factor with WD repeat domains 1 (DAW1) is required for the function of motile cilia in the planarian Schmidtea mediterranea. Dev Growth Differ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liana MK, Litvaitis MK. (2007). Comparative spermatology of selected polyclad flatworms (platyhelminthes). J Morphol. 268, 891–897. [DOI] [PubMed] [Google Scholar]
- Lützen J, Jespersen Å and Nielsen C (2009). Ultrastructure of spermiogenesis in Cristatella mucedo Cuvier (Bryozoa: Phylactolaemata: Cristatellidae). Zoomorphology 128, 275–283. [Google Scholar]
- Magley RA and Rouhana L (2019). Tau Tubulin Kinase is required for spermatogenesis and development of motile cilia in planarian flatworms. Mol Biol Cell, mbcE18100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki JJ and Johnson CA (2017). The Cilium: Cellular Antenna and Central Processing Unit. Trends Cell Biol 27, 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CM and Newmark PA (2012). An insulin-like peptide regulates size and adult stem cells in planarians. Int J Dev Biol. 56, 75–82. [DOI] [PubMed] [Google Scholar]
- Mitchison HM and Valente EM (2017). Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol 241, 294–309. [DOI] [PubMed] [Google Scholar]
- Muresan V, Abramson T, Lyass A, Winter D, Porro E, Hong F, Chamberlin NL and Schnapp BJ (1998). KIF3C and KIF3A form a novel neuronal heteromeric kinesin that associates with membrane vesicles. Mol Biol Cell 9, 637–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M and Hirokawa N (1998). Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837. [DOI] [PubMed] [Google Scholar]
- Pearson BJ, Eisenhoffer GT, Gurley KA, Rink JC, Miller DE and Sanchez Alvarado A (2009). Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev Dyn 238, 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plass M, Solana J, Wolf FA, Ayoub S, Misios A, Glazar P, Obermayer B, Theis FJ, Kocks C and Rajewsky N (2018). Cell type atlas and lineage tree of a whole complex animal by single-cell transcriptomics. Science 360. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Bermange AL, Murfitt KJ, Jennings JR and Sanchez Alvarado A (2005). Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev Cell 8, 635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien PW, Newmark PA and Sanchez Alvarado A (2008). Gene nomenclature guidelines for the planarian Schmidtea mediterranea. Dev Dyn 237, 3099–3101. [DOI] [PubMed] [Google Scholar]
- Rink JC, Gurley KA, Elliott SA and Sanchez Alvarado A (2009). Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science 326, 1406–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink JC, Vu HT and Sanchez Alvarado A (2011). The maintenance and regeneration of the planarian excretory system are regulated by EGFR signaling. Development 138, 3769–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Azimzadeh J, Marshall WF and King SM (2013). Analysis of ciliary assembly and function in planaria. Methods Enzymol 525, 245–264. [DOI] [PubMed] [Google Scholar]
- Rompolas P, Patel-King RS and King SM (2009). Schmidtea mediterranea: a model system for analysis of motile cilia. Methods Cell Biol 93, 81–98. [DOI] [PubMed] [Google Scholar]
- Rompolas P, Patel-King RS and King SM (2010). An outer arm Dynein conformational switch is required for metachronal synchrony of motile cilia in planaria. Mol Biol Cell 21, 3669–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhana L, Tasaki J, Saberi A and Newmark PA (2017). Genetic dissection of the planarian reproductive system through characterization of Schmidtea mediterranea CPEB homologs. Dev Biol 426, 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhana L, Vieira AP, Roberts-Galbraith RH and Newmark PA (2012). PRMT5 and the role of symmetrical dimethylarginine in chromatoid bodies of planarian stem cells. Development 139, 1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhana L, Weiss JA, Forsthoefel DJ, Lee H, King RS, Inoue T, Shibata N, Agata K and Newmark PA (2013). RNA interference by feeding in vitro-synthesized double-stranded RNA to planarians: methodology and dynamics. Dev Dyn 242, 718–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse GW (1995). Is Sperm Ultrastructure Useful in Polychaete Systematics? An Example Using 20 Species of the Fabriciinae (Polychaeta: Sabellidae). Acta Zoologica 76, 57–74. [Google Scholar]
- Rouse GW and Pitt K (2000). Ultrastructure of the sperm of Catostylus mosaicus and Phyllorhiza punctata (Scyphozoa, Cnidaria): Implications for sperm terminology and the inference of reproductive mechanisms. Invertebrate Reproduction & Development 38, 23–34. [Google Scholar]
- Rozanski A, Moon H, Brandl H, Martin-Duran JM, Grohme MA, Huttner K, Bartscherer K, Henry I and Rink JC (2019). PlanMine 3.0-improvements to a mineable resource of flatworm biology and biodiversity. Nucleic Acids Res 47, D812–D820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Agustin JT, Pazour GJ and Witman GB (2015). Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol Biol Cell 26, 4358–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A and Newmark PA (1999). Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc Natl Acad Sci U S A 96, 5049–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Alvarado A, Newmark PA, Robb SM and Juste R (2002). The Schmidtea mediterranea database as a molecular resource for studying platyhelminthes, stem cells and regeneration. Development 129, 5659–5665. [DOI] [PubMed] [Google Scholar]
- Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, Erickson JW, Ray K and Eberl DF (2003). Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol 13, 1687–1696. [DOI] [PubMed] [Google Scholar]
- Scholey JM (1996). Kinesin-II, a membrane traffic motor in axons, axonemes, and spindles. J Cell Biol 133, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey JM (2013). Kinesin-2: a family of heterotrimeric and homodimeric motors with diverse intracellular transport functions. Annu Rev Cell Dev Biol 29, 443–469. [DOI] [PubMed] [Google Scholar]
- Scimone ML, Srivastava M, Bell GW and Reddien PW (2011). A regulatory program for excretory system regeneration in planarians. Development 138, 4387–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira M and Porter KR (1964). The spermatozoids of Flatworms and their microtubular systems. Protoplasma 59, 240–265. [Google Scholar]
- Snow JJ, Ou G, Gunnarson AL, Walker MR, Zhou HM, Brust-Mascher I and Scholey JM (2004). Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat Cell Biol 6, 1109–1113. [DOI] [PubMed] [Google Scholar]
- Soley JT (1997). Nuclear morphogenesis and the role of the manchette during spermiogenesis in the ostrich (Struthio camelus). Journal of Anatomy, 190, 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi-Kim Vu H, Rink JC, McKinney SA, McClain M, Lakshmanaperumal N, Alexander R and Sanchez Alvarado A (2015). Stem cells and fluid flow drive cyst formation in an invertebrate excretory organ. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu KT (1974). Dynamics of spermiogenesis in Drosophila melanogaster: IV. Nuclear transformation. Journal of Ultrastructure Research 48, 284–303. [DOI] [PubMed] [Google Scholar]
- van Deurs B (1974), Spermatology of some Pycnogonida (Arthropoda), with special reference to a microtubule-nuclear envelope complex. Acta Zoologica 55, 151–162. [Google Scholar]
- Wang J, Gao X, Zheng X, Hou C, Xie Q, Lou B and Zhu J (2019). Expression and potential functions of KIF3A/3B to promote nuclear reshaping and tail formation during Larimichthys polyactis spermiogenesis. Dev Genes Evol 229, 161–181. [DOI] [PubMed] [Google Scholar]
- Wang W, Dang R, Zhu JQ and Yang WX (2010a). Identification and dynamic transcription of KIF3A homologue gene in spermiogenesis of Octopus tankahkeei. Comp Biochem Physiol A Mol Integr Physiol 157, 237–245. [DOI] [PubMed] [Google Scholar]
- Wang Y, Stary JM, Wilhelm JE and Newmark PA (2010b). A functional genomic screen in planarians identifies novel regulators of germ cell development. Genes Dev 24, 2081–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zayas RM, Guo T and Newmark PA (2007). nanos function is essential for development and regeneration of planarian germ cells. Proc Natl Acad Sci U S A 104, 5901–5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DL (1978). Ultrastructural and cytochemical aspects of spermiogenesis in Hydra hymanae, with reference to factors involved in sperm head shaping. Dev Biol, 65, 139–154. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT and Besharse JC (2012). The kinesin superfamily protein KIF17: one protein with many functions. Biomol Concepts 3, 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Roberts EA and Goldstein LS (2001). Functional analysis of mouse kinesin motor Kif3C. Mol Cell Biol 21, 5306–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas RM, Hernandez A, Habermann B, Wang Y, Stary JM and Newmark PA (2005). The planarian Schmidtea mediterranea as a model for epigenetic germ cell specification: analysis of ESTs from the hermaphroditic strain. Proc Natl Acad Sci U S A 102, 18491–18496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


