Abstract
Recent drug safety concerns described fluoroquinolone (FQ)-induced serious musculoskeletal reactions. The objective of this study was to characterize reports with FQ-associated disabling musculoskeletal disorders, from VigiBase. The analysis included all FQ-induced musculoskeletal and connective tissue disorders adverse drug reaction (ADR) reports (up to July-2019), (disabling/incapacitating, or recovered/resolved with sequelae or fatal). We described aspects like reporter, suspected FQs, ADRs, associated corticosteroid therapy. We also looked into the disproportionality data in terms of proportional reporting ratio (PRR) and information component (IC) values. A total of 5355 reports with 13,563 ADRs and 5558 FQs were reported. The majority of reports were for patients aged 18–64 (62.67%), and the female gender prevailed (61.76%). Consumers reported almost half (45.99%), with a peak in reporting rates in 2017. Top reported ADRs were arthralgia (16.34%), tendonitis (11.04%), pain in extremity (9.98%), tendon pain (7.63%), and myalgia (7.17%). Top suspected FQs were levofloxacin (50.04%), ciprofloxacin (38.41%), moxifloxacin (5.16%), ofloxacin (3.17%) and norfloxacin (1.01%). For these, FQs-ADR association was supported by the disproportionality analysis. Corticosteroids were associated with about 7% of tendon related reports. The results augment the existing data on FQs safety concerns, specifically their potential effect on the musculoskeletal system.
Subject terms: Risk factors, Signs and symptoms
Introduction
The burden of adverse drug reactions (ADRs) on public health is high1. It represents an important medical issue resulting in 3–7% of all hospital admissions, being associated with a marked increase in morbidity and mortality2. Muscle disorders may be less promptly recognized as compared to the skin or allergic reactions; however, the musculoskeletal system can be a target for ADRs. Musculoskeletal ADRs can be temporarily disabling (e.g., muscle cramps), but can also be serious and life-threatening (e.g., rhabdomyolysis). Increased awareness of drug-induced adverse effects on the musculoskeletal system has been recognized during the last few years. Recent drug safety issues pointed out to serious or uncommon musculoskeletal reactions, including tendon rupture associated with fluoroquinolones (FQs), especially in older patients, renal failure and concomitant corticosteroid therapy1–3.
Concern regarding the impact of FQs on the musculoskeletal safety exists from preclinical studies, indicating cartilage injury in weight-bearing joints, dependent on dose and duration of therapy4,5. A class of antibiotics with broad-spectrum antimicrobial activity6, FQs have been used in clinical practice for up to 40 years, frequently prescribed due to their good tolerability7. The newer generation agents have demonstrated effectiveness in the treatment of respiratory tract infections8. While the efficacy of FQs has improved from the first (e.g., nalidixic acid) to the third generation (e.g., moxifloxacin), careful evaluation of their benefit-risk profile still needs to be in focus. Fluoroquinolones are increasingly being recognized as a cause of disabling ADRs such as tendinitis and tendon rupture9,10. Ciprofloxacin and levofloxacin, although generally safe for therapeutic use, may induce tendinopathic complications such as tendinitis11. When compared to other antibiotics, the relative risk of FQ-induced tendinitis was 3.7, as reported by a post-authorization cohort study12, while a different study observed a relative risk of 8.0 for tendinitis and tendon rupture in lung transplant patients prescribed ciprofloxacin10.
A safety review of disabling and potentially permanent serious side effects of systemic FQs involving the peripheral and central nervous system as well as tendons, muscles, and joints, was finalized by the U.S. Food and Drug Administration (FDA) in 2016. This evaluation resulted in the restriction of use and FQs being reserved for patients with no other treatment alternative13. In 2017, the European Medicines Agency (EMA) initiated an evaluation of FQ-associated safety concerns, aiming to assess the impact of these concerns on the benefit-risk balance of quinolone and FQ-containing medicinal products. As a result, the marketing authorization of medicines containing cinoxacin, flumequine, nalidixic acid, and pipemidic acid was suspended, and the use of the remaining FQs was restricted in the European Union3.
The objective of this retrospective study was to characterize individual case safety reports (ICSRs) with an FQ as the suspected drug, resulting in disabling musculoskeletal and connective tissue disorders, from VigiBase, the unique World Health Organization (WHO) global database of ICSRs.
Methods
Data source
VigiBase, a database maintained by the Uppsala Monitoring Centre (UMC), with more than 19 million ICSRs submitted by national pharmacovigilance centers from 1967 up to May 2019, was used as the data source for this study. ICSRs include information on patient characteristics, ADRs, suspected drugs, and additional information relevant to the report, such as seriousness, reporter type, reporting year, and region. The reports in VigiBase originate from multiple sources: different countries and different types of reporters (e.g., physicians, pharmacists, other health care professionals (HCPs), patients), and are generally notified post-marketing. As a result of the source diversity, a variation exists regarding the amount of information in each report. Also, the probability that the suspected adverse effect is drug-related is not the same in all cases.
All musculoskeletal and connective tissue disorders ADR reports (i.e., Medical Dictionary for Regulatory Activities [MedDRA] Preferred terms [PTs] grouped under the System Organ Class [SOC] Musculoskeletal and connective tissue disorders [further referred to as "musculoskeletal"]) associated with an FQ (ATC code: J01M) until 2019–07-01, were extracted from the UMC global database and were received deduplicated. In VigiBase, reactions are allocated codes according to the latest versions of the hierarchical structures of MedDRA (i.e., version 22.0 at the time of the search)14.
Data selection and analysis of case reports
Our analysis included all cases where an FQ was suspect (i.e., “WHO Drug preferred base name”), with seriousness criteria as disabling/incapacitating, or outcome recovered/resolved with sequelae or fatal. We excluded all reports that had additional drugs suspected to have caused an ADR of the evaluated SOC (i.e., other drugs with basis suspect in relation to a PT grouped under the upper mentioned SOC). In the absence of the respective narratives, we also excluded cases with seriousness criteria congenital anomaly/birth defect in addition to the seriousness criteria of interest. Lastly, we also excluded duplicates of the cases numbered twice due to having both the seriousness and outcome criteria of interest. For the selected reports we described aspects like reporter, region, year of the report, seriousness, suspected FQs, and route of administration, ADRs and dechallenge/re-challenge outcome.
With respect to serious criteria, (i.e., “yes” or “no”), a positively serious criterion is based on patient/event outcome or action posing a threat to a patient's life or functioning. This information becomes of critical importance since it can indicate if due to the nature of the reaction (“serious”) or due to the significant, unexpected information the report provides, expedited reporting is justified in terms of regulatory actions15. In VigiBase, whether or not the report was “serious” is a given unique criterion applicable to the overall report. Seriousness on the other hand, although linked with a specific ADR, is treated in bulk per report. Reports considered not “serious” had seriousness either unknown or “disabling/incapacitating”—but presumably not representing a threat to the patient’s life or functioning.
Further descriptive analysis was performed for the topmost reported FQs and ADRs for which we looked into time to onset and duration of the reaction. For the top reported FQs with oral or parenteral administration we selected the musculoskeletal disorders related to the tendon (i.e., PTs: tendon discomfort, tendon disorder, tendon pain, and tendonitis), for which we looked into potential risk factors such as age, gender, dose, concomitant/interacting corticosteroids (i.e., a search performed for all corticosteroids for systemic use, within ATC code: H02A, that were concomitant or interacting), treatment duration. We considered a low/high dose any amount below/above WHO's Defined Daily Doses (DDD) for each FQ16 and a long duration of therapy to be more than 7 days.
The present analysis refers to ADRs in terms of MedDRA PTs. More than one FQ and/or ADR could be reported per ICSR.
Descriptive statistics were used to summarize the baseline characteristics of the reports and reactions.
Disproportionality data analysis
Disproportionality data analysis was performed overall, for all musculoskeletal FQ-related ADRs. Proportional Reporting Ratio (PRR) and information component (IC) (specifically developed and validated by UMC as a flexible, automated indicator value for disproportionate reporting) with their 95% confidence intervals (CIs) were used as provided by the UMC. A PRR025 (lower end of the 95% CI for PRR) value > 1 associated with ≥ 5 cases was considered a positive association between the FQ and the ADR17,18. An IC025 value (lower end of the CI for IC) ≥ 0 was considered a positive FQ-ADR association. Details on the IC and IC025 calculations were previously described19.
Results
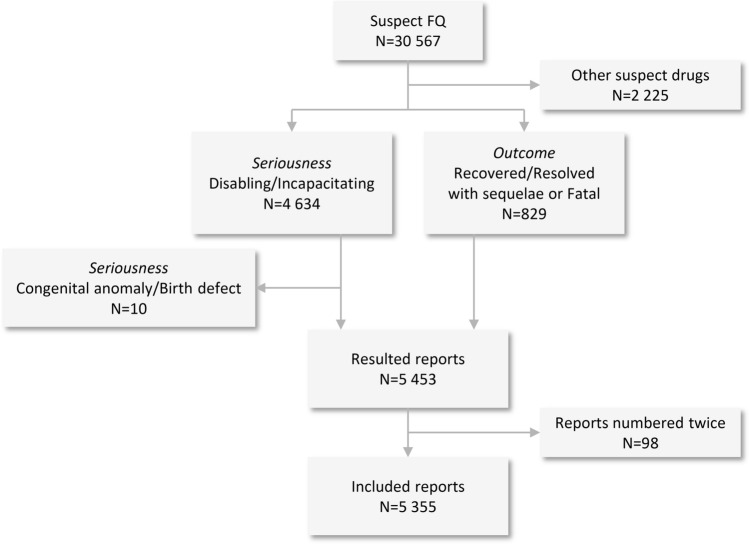
Out of a total number of 30,567 reports with an FQ suspect of causing musculoskeletal disorders, a total of 5355 were included in the analysis based on the inclusion/exclusion criteria. (Fig. 1).
Figure 1.
Overview of inclusion and exclusion criteria.
The majority of reports were for patients between 18 and 64 years of age (62.67%), and the female gender prevailed (61.76%). Most of them originated from America (74.21%) and were generally reported by consumers/non-HCPs (45.99%) (Table 1). A peak in reporting rates was observed in 2017. The year trends of reporting are presented in Fig. 2.
Table 1.
Characteristics of musculoskeletal and connective tissue disorders reports for fluoroquinolones.
| Number of reports N = 5355, n (%) | n (%) | ||
|---|---|---|---|
| Sex | Reporter* | n = 5512 (100.00) | |
| Male | 1957 (36.54) | HCP | 1428 (25.91) |
| Female | 3307 (61.76) | Consumer | 2535 (45.99) |
| Unknown | 91 (1.70) | Other | 35 (0.63) |
| Unknown | 1514 (27.47) | ||
| Age, years | ADR† | n = 13,563 (100.00) | |
| < 18 | 51 (0.95) | Arthralgia | 2216 (16.34) |
| 18–64 | 3356 (62.67) | Tendonitis | 1498 (11.04) |
| ≥ 65 | 1251 (23.36) | Pain in extremity | 1353 (9.98) |
| Unknown | 697 (13.02) | Tendon pain | 1035 (7.63) |
| UN Continent | Myalgia | 973 (7.17) | |
| America | 3974 (74.21) | Tendon disorder | 776 (5.72) |
| Europe | 1350 (25.21) | Muscular weakness | 541 (3.99) |
| Asia | 22 (0.41) | Musculoskeletal pain | 479 (3.53) |
| Africa | 6 (0.11) | Muscle spasms | 438 (3.23) |
| Oceania | 3 (0.06) | Back pain | 358 (2.64) |
| Reporting years | FQ suspect‡ | n = 5558 (100.00) | |
| 1987–1994 | 32 (0.60) | Levofloxacin | 2781 (50.04) |
| 1995–1999 | 60 (1.12) | Ciprofloxacin | 2135 (38.41) |
| 2000–2004 | 427 (7.97) | Moxifloxacin | 287 (5.16) |
| 2005–2009 | 755 (14.10) | Ofloxacin | 176 (3.17) |
| 2010–2014 | 1296 (24.20) | Norfloxacin | 56 (1.01) |
| 2015–2019 | 2785 (52.01) |
FQ fluoroquinolone; HCP healthcare professional; N total number of reports; n: number of reports in a given category; UN Continent: Continent of the primary source, according to the United nations.
*More than one reporter could be mentioned per individual case;
†More than one ADR could be reported per individual case; Data presented for the first 10 ADRs.
‡More than one FQ could be reported per individual case; Data presented for the first 5 FQs.
Figure 2.
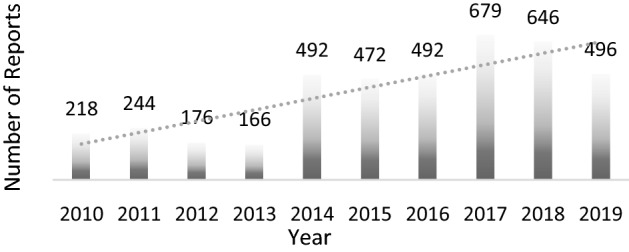
Year trends of reporting.
The most frequently reported indication for women was urinary tract infection (N = 594, 18.32%) followed by sinusitis (N = 457, 14.09%), while for men prostatitis was the most reported indication (N = 218, 12.08%) followed by urinary tract infection (N = 171, 9.47%); a total of 1.55% FQs were indicated for prophylaxis. Of note is the fact that more than one indication could have been reported per ICSR.
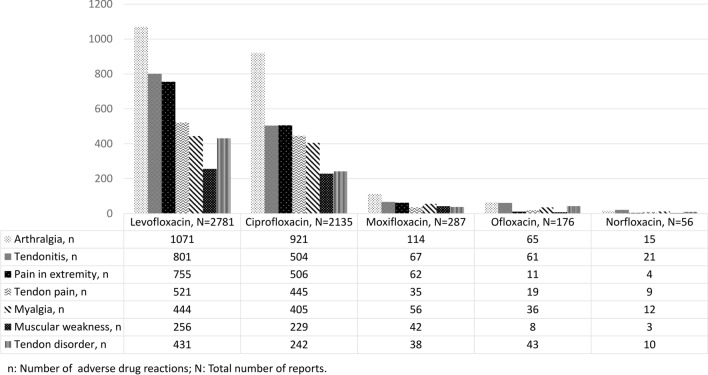
A total of 13,563 musculoskeletal ADRs (2.53 ADRs per case) were reported in the 5355 included reports with seriousness criteria as disabling/incapacitating, or outcome recovered/resolved with sequelae or fatal. A total of 4750 (88.70%) reports were serious, while 106 (1.98%) were not. The most frequently reported ADRs were arthralgia (N = 2216, 16.34%), tendonitis (N = 1498, 11.04%), pain in extremity (N = 1353, 9.98%), tendon pain (N = 1035, 7.63%) and myalgia (N = 973, 7.17) (Table 1).
Among the total number of 5355 reports included in our analysis, 583 reports had tendon ruptures (N = 534) and/or tendon injuries (N = 107), ADRs reported as PTs included in Injury, poisoning and procedural complications SOC.
The topmost reported FQs were levofloxacin (N = 2781), ciprofloxacin (N = 2135), moxifloxacin (N = 287), ofloxacin (N = 176), and norfloxacin (N = 56) and represented 97% of the total reported FQ-musculoskeletal reports (Table 1, Fig. 3). With respect to the route of administration, the majority of the FQs were administered orally (N = 4202, 57.34%), followed by intravenous administration (N = 193, 2.63%), while a total of 2755 (37.60%) administration routes were unknown.
Figure 3.
Top most frequent FQs suspected in the reports with musculoskeletal ADRs. ADRs adverse drug reactions; FQ fluoroquinolone. More than one ADR could be reported per FQ.
Dechallenge/re-challenge actions and their outcomes, where available, are presented in Table 2.
Table 2.
ADRs dechallenge and re-challenge actions and outcomes.
| ADRs overall (N = 13,563) | |||
|---|---|---|---|
| De-challenge | Re-challenge | ||
| Action N (%) | Outcome n (%) | Action n (%) | Outcome n (%) |
|
Drug withdrawn 3947 (29.10) |
No effect observed 2616 (66.28) |
Re-challenge 1401 (10.33) |
No recurrence 283 (20.20) |
|
Reaction abated N = 923 (23.38) |
Reaction recurred 74 (5.28) |
||
|
Fatal 2 (0.05) |
Effect unknown 1044 (74.51) |
||
|
Effect unknown 406 (10.29) | |||
|
Dose not changed 326 (2.40) |
No effect observed 115 (35.28) |
No Re-challenge 377 (2.78) |
Unknown 377 (100) |
|
Reaction abated 72 (22.09) | |||
|
Fatal 1 (0.31) | |||
|
Effect unknown 138 (42.33) | |||
|
Dose reduced 8 (0.06) |
No effect observed 3 (37.50) |
||
|
Reaction abated 2 (25.00) | |||
|
Effect unknown 3 (37.50) | |||
ADRs adverse drug reactions; N number of ADRs; n number of ADRs in a given category.
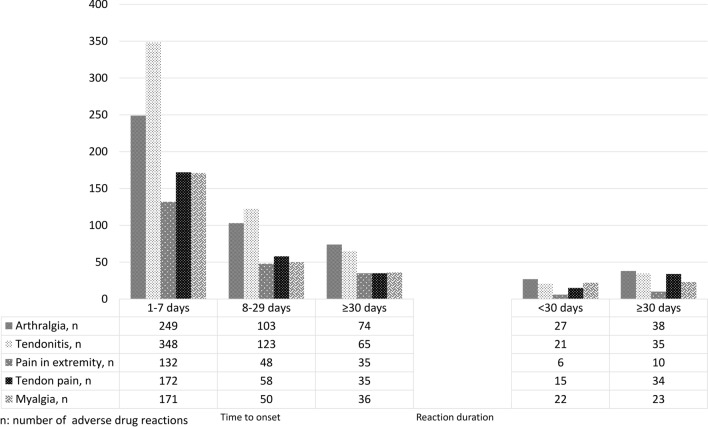
Overall, the time to onset for the majority of ADRs was between one and seven days (N = 1751, 61.50% from the total N = 2847 ADRs with this data available), and reaction duration exceeded 30 days for most (N = 265, 65.27% from the total N = 406 ADRs with this data available). Figure 4 presents the time to onset and duration of reaction for the top five most reported ADRs, individually.
Figure 4.
Time to onset and reaction duration of the topmost frequent FQ-musculoskeletal ADRs with available information. ADRs adverse drug reactions; FQ fluoroquinolone. Data presented for the ADRs that had this information available; More than one ADR could be reported per report.
Among the topmost reported FQs, a total of 2226 unique reports contained 2685 tendon related ADRs, (i.e., tendon discomfort, tendon disorder, tendon pain and tendonitis) linked with N = 2269 FQs administered orally or parenterally.
Among the 2269 FQs, most were administered in a low dose FQ (27.90%), as compared with reports with high doses (5.64%), and this was independent of treatment duration. Overall, the male gender prevailed in ≥ 65 years old age group (N = 311 vs. N = 278), as opposed to female gender being predominant among the 18–64 year-olds (N = 878 vs. N = 552). Corticosteroids were associated with about 7% of FQs, and mostly in the elderly (N = 75 vs. N = 63) (Table 3).
Table 3.
Tendon related reports with associated risk factors.
| Levofloxacin N = 1208 |
Ciprofloxacin N = 856 |
Moxifloxacin N = 82 |
Ofloxacin N = 91 |
Norfloxacin N = 32 |
Overall N = 2269, n (%) |
|
|---|---|---|---|---|---|---|
| Dose | ||||||
| High dose | 107 | 16 | 0 | 5 | 0 | 128 (5.64) |
| Low dose | 296 | 253 | 35 | 30 | 19 | 633 (27.90) |
| Unknown | 805 | 587 | 47 | 56 | 13 | 1508 (66.46) |
| Treatment duration ≤ 7 days | ||||||
| N: | 656 | 448 | 43 | 39 | 21 | 1207 (53.20) |
| Dose: (high/low/unknown) | (67/194/395) | (10/126/312) | (0/23/20) | (3/15/21) | (0/12/9) | (80/370/757) |
| Treatment duration > 7 days | ||||||
| N: | 381 | 248 | 23 | 35 | 9 | 696 (30.67) |
| Dose: (high/low/unknown) | (30/70/281) | (7/78/163) | (0/5/18) | (2/13/20) | (0/6/3) | (39/172/485) |
| Treatment duration unknown | ||||||
| 171 | 160 | 16 | 17 | 2 | 366 (16.13) | |
| Age ≥ 65 | ||||||
| N: | 405 | 145 | 5 | 32 | 10 | 597 (26.31) |
| Gender: (male/female/unknown) | (212/189/4) | (69/72/4) | (4/1/0) | (20/12/0) | (6/4/0) | (311/278/8) |
| Age 18–64 | ||||||
| N: | 668 | 639 | 67 | 46 | 21 | 1441 (63.50) |
| Gender: (male/female/unknown) | (225/439/4) | (269/363/7) | 25/42/0) | (27/19/0) | (6/15/0) | (552/878/11) |
| Corticosteroids treatment | ||||||
| N: | 88 | 49 | 2 | 12 | 1 | 152 (6.70) |
| Age (y.o) | ||||||
| ≥ 65/18–64 | 43/34 | 25/21 | 0/2 | 6/6 | 1/0 | 75/63 |
N number of FQs numbered once per report; n number of FQs numbered once per report, in a given category; NA not applicable; y.o years old.
For the top reported FQs, we also looked for disproportionality for the most reported ADRs and for tendon related ADRs in patients who received FQs compared to the reporting of the same ADRs in the full database. Although a positive association for all evaluated FQ-ADR was observed (Table 4), tendonitis, tendon disorder, tendon pain, and tendon discomfort had the highest IC and PRR values for all top FQs.
Table 4.
Disproportionality data analysis.
| N | IC (95% CI) | PRR (95% CI) | |
|---|---|---|---|
| Levofloxacin | |||
| Arthralgia | 4231 | 1.75 (1.71–1.80) | 3.41 (3.31–3.51) |
| Pain in extremity | 2865 | 1.52 (1.47–1.58) | 2.90 (2.79–3.00) |
| Tendonitis | 4742 | 6.09 (6.06–6.13) | 96.65 (93.50–99.80) |
| Tendon pain | 1847 | 6.29 (6.22–6.36) | 120.88 (114.32–127.43) |
| Myalgia | 2373 | 1.23 (1.17–1.28) | 2.35 (2.26–2.44) |
| Tendon disconfort | 137 | 5.74 (5.49–5.99) | 91.45 (75.07–107.83) |
| Tendon disorder | 1995 | 6.27 (6.20–6.34) | 117.35 (111.25–123.45)130 |
| Ciprofloxacin | |||
| Arthralgia | 3504 | 1.26 (1.22–1.31) | 2.42 (2.34–2.50) |
| Tendonitis | 2714 | 5.07 (5.01–5.12) | 40.38 (38.77–41.99) |
| Pain in extremity | 2054 | 0.82 (0.76–0.89) | 1.78 (1.70–1.85) |
| Myalgia | 2021 | 0.78 (0.71–0.84) | 1.72 (1.65–1.79) |
| Muscular weakness | 880 | 0.97 (0.87–1.06) | 1.96 (1.84–2.09) |
| Tendon discomfort | 130 | 5.48 (5.23–5.73) | 73.09 (59.80–86.38) |
| Tendon disorder | 920 | 4.94 (4.84–5.04) | 36.59 (34.12–39.06) |
| Tendon pain | 1475 | 5.75 (5.68–5.82) | 75.11 (70.77–79.45) |
| Moxifloxacin | |||
| Arthralgia | 865 | 0.70 (0.61–0.80) | 1.63 (1.53–1.74) |
| Tendonitis | 514 | 4.11 (3.99–4.24) | 18.14 (16.62–19.66) |
| Tendon disorder | 195 | 4.12 (3.91–4.33) | 18.71 (16.23–21.19) |
| Myalgia | 569 | 0.41 (0.29–0.53) | 1.33 (1.22–1.43) |
| Tendon pain | 285 | 4.8 (4.63–4.97) | 30.75 (27.31–34.20) |
| Tendon discomfort | 13 | 3.29 (2.40–4.18) | 15.19 (8.75–21.63) |
| Ofloxacin | |||
| Arthralgia | 521 | 0.60 (0.48–0.73) | 1.52 (1.40–1.64) |
| Tendonitis | 674 | 5.12 (5.01–5.23) | 37.23 (34.51–39.96) |
| Tendon disorder | 260 | 5.13 (4.95–5.31) | 39.09 (34.54–43.63) |
| Myalgia | 422 | 0.61 (0.46–0.75) | 1.52 (1.39–1.66) |
| Tendon pain | 113 | 4.06 (3.78–4.34) | 18.28 (15.18–21.38) |
| Tendon discomfort | 12 | 3.55 (2.62–4.48) | 21.67 (12.22–31.12) |
| Norfloxacin | |||
| Tendonitis | 369 | 5.04 (4.89–5.19) | 35.18 (31.78–38.59) |
| Arthralgia | 235 | 0.27 (0.08–0.46) | 1.21 (1.06–1.35) |
| Myalgia | 209 | 0.41 (0.20–0.61) | 1.33 (1.16–1.49) |
| Tendon disorder | 54 | 3.61 (3.20–4.03) | 13.79 (10.55–17.02) |
| Tendon pain | 55 | 3.76 (3.36–4.17) | 15.49 (11.88–19.09) |
| Tendon discomfort | 11 | 3.81 (2.83–4.79) | 34.89 (19.20–50.58) |
Bolded text represents IC025 and PRR025, the lower ends of the 95% respective confidence intervals.
CI confidence interval; IC information component; N number of reports; PRR proportional reporting ratio.
Discussion
The present study is, to the best of our knowledge, the first to evaluate the case reports with FQ-associated disabling musculoskeletal ADRs, as reported in VigiBase, the unique WHO global database of ICSRs. The study augments the review performed by EMA and FDA. Generally, VigiBase includes more data when compared to those of one or more countries.
The majority of the cases included in our analysis were reported in America and Europe, while a notably lower number was reported in other UN continents. An increase in the frequency of reporting has been observed, with a peak reached in 2017, followed by a slow decrease until 2019. This could be associated with a Weber-like effect triggered by the regulatory activities raising awareness on safety issues, such as the safety communication made by the FDA in December 2016 and the start of the referral procedure in EU early 2017, thus resulting in a peak in reporting followed by a continuous decline thereafter20.
Similar to a summary of reports in the FDA’s adverse event reporting system which found that 3% of the FQ-induced tendon-rupture reports were for FQs used for prophylaxis21, we too observed that 1.55% of the FQs reported in VigiBase were indicated in prophylaxis. This aspect could in fact hypothesise a pattern of unnecessary overuse in the context of uncertain indication of use or prophylaxis.
Consumers were the most frequent reporters, followed by physicians and pharmacists. A recent analysis of voluntary reports of ADRs in the United States (US) noted an increase in those notified by consumers since 201322. Our analysis showed an increase in consumers/ non-HCPs reports, and overall, these represented the majority of the cases, reflecting the impact these ADRs have on the consumer's quality of life (QoL)23–25, in addition to musculoskeletal ADRs being more easily identifiable23. Patient reports usually provide more information on the severity of the experienced events as well as the impact on their QoL24 and tend to report disabilities more often than HCPs23. Based on this, our selection criteria (i.e., disabling/incapacitating ADRs) would in fact favor patient-reported cases.
Several case reports have revealed an association between FQ use and tendinopathies (tendinitis, tendinosis)12,26, with more than 40 cases reported already between 1992 and 199427. As a result of their expanded use, an increase in the reports of FQ-associated tendinitis with and without rupture has been observed26.
Arthralgia, tendonitis, pain in extremity, and tendon pain were among the topmost frequently reported ADRs in our analysis, in accordance with the latest literature claims26,28–30. FQ-induced tendon damage is thought to be linked to oxidative stress and mitochondrial toxicity31, and although the exact pathophysiology of FQ-induced tendon injury is not entirely understood, the negative effect of FQs on tendons is likely multifactorial32. Due to their chelating proprieties, FQs can interact with regulating proteins of tendinocites, thus damaging the tendon structure. In addition to this, the final event in the pathogenetic mechanism has been suggested to be apoptosis26. Direct toxicity and degenerative changes in collagen fibers as a potential mechanism for FQ-induced tendon disorders have also been described29.
FQs most frequently linked with ADRs were ciprofloxacin, levofloxacin, moxifloxacin, and ofloxacin. Most of the reports originated from the US; therefore, prescription and utilization patterns of specific quinolone antibacterials might impact the type of FQ most frequently reported. A study on trends in utilization in the US found that more than 80% of quinolone antibacterials prescriptions reimbursed by Medicaid were for ciprofloxacin and levofloxacin, followed by ofloxacin33. Also, for example, in the UK, ciprofloxacin, levofloxacin, ofloxacin, and moxifloxacin are the only FQs licensed for use34.
Animal models revealed that each quinolone possesses a different potential to cause cartilage toxicity; however, when given a sufficiently high exposure, cartilage changes will occur with all quinolones35. For tendon rupture risk, a study found that it was not apparently different among the FQs36. Among the top FQs in our analysis, no significant differences were observed. Of note is the fact that our data is based on a spontaneous reporting system (SRS), therefore, a direct comparison among the FQs was not possible nor intended.
In terms of age, even though pain incidence37 and myopathies are more likely to occur in the elderly population38, we found that patients between 18 and 64 years old were subject of over half of the case reports, while patients aged 65 or older, were involved in just about a quarter. The relatively important percentage of younger subjects might be explained by the fact the elders might be less likely to report ADRs23. The use of FQs in elderly and younger populations has been evaluated in comparative studies, and even though age per se did not seem to negatively impact the safety of these antibacterials, special consideration must be given to specific ADRs when bacterial infections therapy is chosen39. Renal function declines consistently with age and consequently, doses of renally excreted quinolones (e.g., ofloxacin, levofloxacin) need to be adjusted in case a clinically relevant reduction of creatinine clearance is identified39. Moreover, age older than 60, aside from male gender, renal dysfunction, or transplant, were described in literature among the predisposing factors involved in FQ-induced tendinopathy40. In terms of gender, similar to a UK study reporting that the detrimental effects of quinolones, such as tendon disorders, are more pronounced among women41, in our analysis, the female gender prevailed as well. This might be a real association, or might be linked to a higher likelihood of reporting of ADRs by the female gender23.
FQ-induced tendon disorders were reported to have occurred soon after the exposure, with tendon ruptures within one week of administration10 and tendinopathies within the first month32. Similarly, we too, observed that the time to onset for the topmost frequent FQ-musculoskeletal ADRs was less than 7 days, with the majority in the first month, while the duration of reaction exceeded 30 days for most cases. However, it should be mentioned that a very limited number of reports had this information available. Also, our initial selection of disabling/incapacitating or recovered/resolved with sequelae might explain the slightly higher number of long-lasting or prolonged (≥ 30 days) ADRs.
With regards to de/re-challenge actions, a low number of reports had this information available. In almost a quarter of the ADRs with drug subsequently withdrawn, the reaction abated, and in more than half, no effect was observed. Similarly, from the ADRs with dose kept the same, generally, no effect was observed. No recurrence of the reaction was observed in the majority of the ADRs where re-challenge was performed. However, the scarce data available regarding de/re-challenge actions (60%/ 80% of the total number of ADRs did not have this information available) may weaken the possibility of generating any hypothesis based on this data. Moreover, through the prism of the selection criteria, cases with a poor outcome have been favored for inclusion in the analysis, while positive outcomes might have been missed (i.e., reaction abated), thus explaining the prevalence of the former.
Most of the literature evidence points to a tendon related risk associated with FQs, with different potential associated risk factors. Consequently, we limited the number of reports for which we looked for these risk factors strictly to reports of tendon related disorders (2226 unique reports).
A notable risk of tendon disorders was found to be associated with the use of systemic corticosteroids36, especially in combination with current exposure to FQs, when the risk of tendon rupture/injury was found to be strongly increased11,40. For the top FQ reports with tendon related ADRs, a low number had concomitant corticosteroid use reported (6.70%). Among these, a slightly increased corticosteroid exposure was observed in patients older than 65 years old, as compared to younger patients (49.34 vs. 41.45).
A systematic review of observational studies noted that advanced age might constitute a risk factor for FQ-induced tendon disorders32, and a critical review of literature observed a ratio of men to women of 1.9:1 for tendinopathies secondary to FQs42. In our analysis, although only a few cases with both advanced age and male gender were identified, we did notice a predominance for male gender among the ≥ 65 years old age group, possibly suggesting that after the age of 65, men could be more predisposed to tendon disorders. The same systematic review pointed out that higher FQ dosages may be associated with higher risk of tendon disorders32. From the reports evaluated for risk factors, we did not find any indication pointing to high doses being a contributing factor. Moreover, treatment duration, where available, was less than 7 days in most reports, suggesting that a prolonged FQ treatment duration might not contribute to risk potentiation.
An added greater risk for tendon disorders could also be linked with obesity in the context of mechanical forces exerted on body structures during activity41 as well as diseases like diabetes, renal impairment32,41, or organ transplant41. Unfortunately, in the context of the dataset we based our analysis on, comorbidities could not be thoroughly depicted nor described.
With regards to disproportionality data analysis performed overall, on the total number of musculoskeletal ADRs, for the top FQs, all evaluated ADRs had PRR025 value ≥ 1, associated with ≥ 5 case reports, and were therefore considered positive associations. Similarly, the IC025 value (the lower end of the CI for IC) was ≥ 0 in all cases, indicating a positive FQ-ADR association. Tendonitis, tendon disorder, and tendon pain had the highest IC and PRR values.
Limitations
A variation between reports and submitting countries exists with regards to the amount of information reported. Causality can also vary from report to report. Some countries collect only suspected ADRs with at least a possibility of a causal relationship between the drug and reported event, whilst for example, the US (contributing almost half of the reports in VigiBase) collects "any adverse event associated with the use of a drug in humans, whether or not considered drug related"14. As with all SRSs, limitations are mainly related to under-reporting and variable quantity and quality of the reported data43. Another notable limitation of SRS, for our study was the low availability of data regarding concomitant medication. Therefore, this did not allow to evaluate the contributory role of corticosteroids use described in literature as potential potentiator of tendon disorder risk among patients with concomitant FQ and corticosteroid use.
Through the prism of selecting reports pertaining exclusively to musculoskeletal SOC, we might have missed ADRs in terms of PTs, such as tendon rupture and injury, included in other SOCs (e.g., Injury, poisoning and procedural complications etc.). However, we aimed to evaluate ADRs strictly related to FQ induced musculoskeletal system disorders, and avoid possible confounding factors like for example situations where injuries, procedural, or device complication factors could be significant in the medical event being reported (as is the case for Injury, poisoning and procedural complications SOC).
The present analysis might also be limited by the selection of disabling/incapacitating or recovered/resolved with sequelae or fatal ADR reports, which could have impacted the results in terms of proportion of long-lasting ADRs, de/rechallenge outcomes, and may have even added bias with regards to predominant notifier type. However, since the safety concerns targeted long-lasting, disabling, and potentially irreversible ADRs, we considered these to be of primary interest.
Moreover, we only selected cases where an FQ was the suspect drug, thus excluding reports with interacting FQs. By doing so, we aimed to avoid reports where ADRs might have been induced by other drugs or that might have had other alternative causes. The objective was to analyze cases where a clear FQ-ADR association was assumed, with as little confounding factors as possible.
Conclusions
The present analysis of musculoskeletal disorders associated with FQs in VigiBase revealed that the majority of the cases were reported by consumers/non-HCPs, with an indicated ascending trend of reporting. The female gender and 18–64 years old age group prevailed. The topmost reported ADRs (arthralgia, tendonitis, pain in extremity, tendon pain, myalgia) and FQs (levofloxacin, ciprofloxacin, moxifloxacin, ofloxacin, norfloxacin) corroborate the musculoskeletal safety concerns recently evaluated by the authorities, also supported by the disproportionality data.
Acknowledgements
Data was provided by UMC but the study results and conclusions are those of the authors and not necessarily those of the UMC or WHO.
Author contributions
M.H. participated in research design, data acquisition, data analysis and interpretation, writing the article and approved the final version. A.F. participated in research design, data acquisition, data interpretation, revising the article and approved the final version. D.L. participated in data analysis and approved the final version. C.B. and M.S. participated in data interpretation, revising the article and approved the final version. C.M. participated in research design and supervision, revising the article and approved the final version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by the “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca (PCD 1529/32/18.01.19).
Data availability
The data that support the findings of this study are available from UMC but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of UMC.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Conforti A, et al. Musculoskeletal adverse drug reactions: A review of literature and data from adr spontaneous reporting databases. Curr. Drug Saf. 2008;2(1):47–63. doi: 10.2174/157488607779315516. [DOI] [PubMed] [Google Scholar]
- 2.Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br. J. Clin. Pharmacol. 2011;72(6):905–908. doi: 10.1111/j.1365-2125.2011.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Medicines Agency: EMA/818158/2018. Pharmacovigilance Risk Assessment Committee (PRAC), 16 October 2018. Assessment Report. Referral Under Article 31 of Directive 2001/83/EC Resulting from Pharmacovigilance Data. https://www.ema.europa.eu/en/documents/referral/quinolone-fluoroquinolone-article-31-referral-assessment-report_en.pdf (2018).
- 4.Sendzik J, Lode H, Stahlmann R. Quinolone-induced arthropathy: An update focusing on new mechanistic and clinical data. Int. J. Antimicrob. Agents. 2009;33(3):194–200. doi: 10.1016/j.ijantimicag.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Patterson DR. Quinolone toxicity: Methods of assessment. Am. J. Med. 1991;91(6A):35S–37S. doi: 10.1016/0002-9343(91)90308-K. [DOI] [PubMed] [Google Scholar]
- 6.Binz J, Adler CK, So TY. The risk of musculoskeletal adverse events with fluoroquinolones in children: What is the verdict now? Clin. Pediatr. (Phila). 2016;55(2):107–110. doi: 10.1177/0009922815599959. [DOI] [PubMed] [Google Scholar]
- 7.Almulhim AS, Aldayyen A, Yenina K, Chiappini A, Khan TM. Optimization of antibiotic selection in the emergency department for urine culture follow ups, a retrospective pre-post intervention study: Clinical pharmacist efforts. J. Pharm. Policy Pract. 2019;12(1):1–7. doi: 10.1186/s40545-019-0168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhanel GG, et al. A critical review of the fluoroquinolones: Focus on respiratory infections. Drugs. 2002;62(1):13–59. doi: 10.2165/00003495-200262010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Lapi F, et al. Safety profile of the fluoroquinolones. Drug Saf. 2010;33(9):789–799. doi: 10.2165/11536810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Akali AU, Niranjan NS. Management of bilateral Achilles tendon rupture associated with ciprofloxacin: A review and case presentation. J. Plast. Reconstr. Aesthetic Surg. 2008;61(7):830–834. doi: 10.1016/j.bjps.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Pantalone A, Abate M, D’ovido C, Carnevale A, Salini V. Diagnostic failure of ciprofloxacin-induced spontaneous bilateral Achilles tendon rupture: Case-report and medical-legal considerations. Int. J. Immunopathol. Pharmacol. 2011;24(2):519–522. doi: 10.1177/039463201102400227. [DOI] [PubMed] [Google Scholar]
- 12.Van der Linden PD, Van de Lei J, Nab HW, Knol A, Stricker BHC. Achilles tendinitis associated with fluoroquinolones. Br. J. Clin. Pharmacol. 1999;48(3):433–437. doi: 10.1046/j.1365-2125.1999.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA (US Food and Drug Administration). FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. Safety Announcement. https://www.fda.gov/media/99425/download (2016)
- 14.Lindquist M. VigiBase, the WHO Global ICSR Database System: Basic facts. Drug Inf. J. 2008;42(5):409–419. doi: 10.1177/009286150804200501. [DOI] [Google Scholar]
- 15.European Medicines Agency: CPMP/ICH/377/95. ICH Topic E 2 A Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. June 1995. https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-15.pdf.
- 16.WHO. Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index 2020. https://www.whocc.no/atc_ddd_index/ (2020).
- 17.Baan EJ, et al. Exploratory study of signals for asthma drugs in children, using the eudravigilance database of spontaneous reports. Drug Saf. 2020;43(1):7–16. doi: 10.1007/s40264-019-00870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundmark B, Holmberg L, Garmo H, Zethelius B. Reducing the noise in signal detection of adverse drug reactions by standardizing the background: A pilot study on analyses of proportional reporting ratios-by-therapeutic area. Eur. J. Clin. Pharmacol. 2014;70(5):627–635. doi: 10.1007/s00228-014-1658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salem J, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;2045(18):1–11. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora A, Jalali RK, Vohora D. Relevance of the Weber effect in contemporary pharmacovigilance of oncology drugs. Ther. Clin. Risk Manag. 2017;13:1195–1203. doi: 10.2147/TCRM.S137144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arabyat RM, Raisch DW, McKoy JM, Bennett CL. Fluoroquinolone-associated tendon-rupture: A summary of reports in the Food and Drug Administration's adverse event reporting system. Expert Opin. Drug Saf. 2015;14(11):1653–1660. doi: 10.1517/14740338.2015.1085968. [DOI] [PubMed] [Google Scholar]
- 22.Toki T, Ono S. Spontaneous reporting on adverse events by consumers in the United States: An analysis of the food and drug administration adverse event reporting system database. Drugs. 2018;5(2):117–128. doi: 10.1007/s40801-018-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Langen J, van Hunsel F, Passier A, de Jong-van den Berg L, van Grootheest K. Adverse drug reaction reporting by patients in the Netherlands. Drug Saf. 2008;31(6):515–524. doi: 10.2165/00002018-200831060-00006. [DOI] [PubMed] [Google Scholar]
- 24.Watson S, et al. Safety concerns reported by patients identified in a collaborative signal detection workshop using VigiBase: Results and reflections from Lareb and Uppsala Monitoring Centre. Drug Saf. 2018;41:203–212. doi: 10.1007/s40264-017-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakkarainen KM, Sundell KA, Petzold M, Ha S. Prevalence and perceived preventability of self- reported adverse drug events: A population-based survey of 7099 adults. PLoS ONE. 2013;8(9):e73166. doi: 10.1371/journal.pone.0073166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corrao G, et al. Evidence of tendinitis provoked by fluoroquinolone treatment a case-control study. Drug Saf. 2006;29(10):889–896. doi: 10.2165/00002018-200629100-00006. [DOI] [PubMed] [Google Scholar]
- 27.Huston K. Achilles tendinitis and tendon rupture due to fluoroquinolone antibiotics. N. Engl. J. Med. 1994;331:748. doi: 10.1056/NEJM199409153311116. [DOI] [PubMed] [Google Scholar]
- 28.Baombe JP, Ford R. Do fluoroquinolones increase the incidence of tendinopathy? Emerg. Med. J. 2009;33(7):519–522. doi: 10.1136/emermed-2016-205965.2. [DOI] [PubMed] [Google Scholar]
- 29.Hori K, Yamakawa K, Yoshida N, Ohnishi K, Kawakami J. Detection of fluoroquinolone-induced tendon disorders using a hospital database in Japan. Pharmacoepidemiol. Drug Saf. 2012;21(8):886–889. doi: 10.1002/pds.3285. [DOI] [PubMed] [Google Scholar]
- 30.Sode J, Obel N, Hallas J, Lassen A. Use of fluroquinolone and risk of Achilles tendon rupture: A population-based cohort study. Eur. J. Clin. Pharmacol. 2007;63(5):499–503. doi: 10.1007/s00228-007-0265-9. [DOI] [PubMed] [Google Scholar]
- 31.Godoy-Santos AL, et al. Fluoroquinolones and the risk of achilles tendon disorders: Update on a neglected complication. Urology. 2018;113:20–25. doi: 10.1016/j.urology.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Stephenson AL, Wu W, Cortes D, Rochon PA. Tendon injury and fluoroquinolone use: A systematic review. Drug Saf. 2013;36(9):709–721. doi: 10.1007/s40264-013-0089-8. [DOI] [PubMed] [Google Scholar]
- 33.Almalki ZS, Yue X, Xia Y, Wigle PR, Guo JJ. Utilization, spending, and price trends for quinolones in the us medicaid programs: 25 years’ experience 1991–2015. PharmacoEconomics Open. 2017;1(2):123–131. doi: 10.1007/s41669-016-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NHS. North Central London Joint Formulary Committee. Safe prescribing of fluoroquinolones Position Statement. https://www.ncl-mon.nhs.uk/wp-content/uploads/Guidelines/5_Fluoroquinolones_Safe_Prescribing_P (2019).
- 35.Jackson MA, et al. The use of systemic and topical fluoroquinolones. Pediatrics. 2016;138(5):e1034. doi: 10.1542/peds.2016-2706. [DOI] [PubMed] [Google Scholar]
- 36.Seeger JD, et al. Achilles tendon rupture and its association with fluoroquinolone antibiotics and other potential risk factors in a managed care population. Pharmacoepidemiol. Drug Saf. 2006;15(11):784–792. doi: 10.1002/pds.1214. [DOI] [PubMed] [Google Scholar]
- 37.Kim YS, Park JM, Moon YS, Han SH. Assessment of pain in the elderly: A literature review. Natl. Med. J. India. 2017;30(4):203–207. doi: 10.4103/0970-258X.218673. [DOI] [PubMed] [Google Scholar]
- 38.Echaniz-Laguna A, Mohr M, Lannes B, Tranchant C. Myopathies in the elderly: A hospital-based study. Neuromuscul. Disord. 2010;20(7):443–447. doi: 10.1016/j.nmd.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Stahlmann R, Lode H. Safety considerations of fluoroquinolones in the elderly: An update. Drugs Aging. 2010;27(3):193–209. doi: 10.2165/11531490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Horn, J. R. & Hansten, P. D. Fluoroquinolones and steroids: An achilles heel interaction. Pharm. Times. 82(4), (2016).
- 41.Wise BL, Peloquin C, Choi H, Lane NE, Zhang Y. Impact of age, sex, obesity, and steroid use on quinolone-associated tendon disorders. Am. J. Med. 2012;125(12):1228.e23–1228.e28. doi: 10.1016/j.amjmed.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khaliq Y, Zhanel G. Fluoroquinolone-associated tendinopathy: A critical review of the literature. Clin. Infect. Dis. 2003;36(90):1404–1410. doi: 10.1086/375078. [DOI] [PubMed] [Google Scholar]
- 43.Palleria C, et al. Limitations and obstacles of the spontaneous adverse drugs reactions reporting: Two “challenging” case reports. J. Pharmacol. Pharmacother. 2013;4(SUPPL1):S66–72. doi: 10.4103/0976-500X.120955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from UMC but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of UMC.