Abstract
Background:
The pathological hallmarks of Parkinson’s disease include intraneuronal Lewy bodies, neuronal loss, and gliosis. We aim to correlate Parkinson’s disease neuropsychiatric symptoms, (e.g., depression, psychosis, and anxiety) with the severity of neuropathology in the substantia nigra and locus coeruleus.
Methods:
The brains of 175 participants with a primary pathologic diagnosis of Parkinson’s disease were analyzed semi-quantitatively to ascertain the burden of neuronal loss and gliosis and Lewy body pathology within the locus coeruleus and substantia nigra. Participants’ history of anxiety, depression, and psychosis were determined using a chart-extracted medical history or record of formal psychiatric evaluation.
Results:
Of the sample, 56% (n=98), 50% (n=88), and 31.25% (n=55) of subjects had a diagnosis of psychosis, depression, and anxiety, respectively. Psychosis (χ2=7.1, P=0.008, df=1) and depression (χ2=7.2, P=0.007, df=1) were associated with severe neuronal loss and gliosis in the substantia nigra but not in the locus coeruleus. No association was observed between anxiety and neuronal loss and gliosis in either region. No neuropsychiatric symptoms were associated with Lewy body score. After controlling for disease duration and dementia, psychosis (OR 3.1, 95% CI 1.5–6.4, χ2=9.4, P=0.012, df=1) and depression (OR 2.6, 95% CI 1.3–5.0, χ2=7.9, P=0.005, df=1) remained associated with severe neuronal loss and gliosis in the substantia nigra.
Conclusions:
These results suggest that psychosis and depression in Parkinson’s disease are associated with the underlying neurodegenerative process and demonstrate that cell loss and gliosis may be a better marker of neuropsychiatric symptoms than Lewy body pathology.
Keywords: Parkinson’s disease, neuropathology, depression, psychosis
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease, affecting 1–2% of the population older than 60.1 PD is characterized by progressive motor symptoms as well as resting tremor, and rigidity,2 and is often comorbid with neuropsychiatric features, the most common including depression, psychosis, and anxiety.3 Neuropsychiatric symptoms in PD negatively impact quality of life, reduce daily functioning, and increase caregiver burden even in early stages of the disease.4 The neuropathology of PD is hallmarked by two significant findings: 1) selective dopaminergic neuron loss in the substantia nigra pars compacta (SN), and 2) intracellular aggregates of misfolded alpha-synuclein and other proteins, termed Lewy bodies.5
Prior research has linked motor subtypes, tremor-dominant and akinetic-rigid subtypes of PD, to neuronal loss/gliosis within the locus coeruleus (LC) and SN.6,7 Similarly, a higher degree of gliosis in the SN has been linked to depression in PD.8 There also has been growing evidence from pathology and imaging studies suggesting that loss of dopamine and noradrenaline neurons of the LC is involved in the clinical expression of depression in PD.9,10 A histopathological analysis of the LC and SN, therefore, could build upon these results and further solidify the role of these structures in PD depression. Post-mortem studies have investigated potential links between visual hallucinations and pathology within the LC and SN, yet have found no such associations.11 Despite the disproportionately high prevalence of anxiety in PD and its adverse effects on quality of life, caregiver dependency, and distress,12,13 no prior research has explored its pathological correlates.
Braak et al. hypothesized that the spread of Lewy bodies through the brain might predate the progression of clinical symptoms in PD.14 However, several studies have shown intracellular Lewy bodies correlate poorly with nigral neuronal density and in-vivo clinical symptoms such as rigidity, akinesia, motor function, and disease duration.15–19 In contrast, close correlations were observed between SN neuronal density and severity of bradykinesia and rigidity.18,19 Due to the demonstrated significance of neuronal loss in the pathogenesis of some clinical symptoms in PD, we predict neuronal loss, rather than Lewy body pathology may be a more reliable marker of disease impact.
Evaluating the associations between regional Lewy body scores, neuronal loss/gliosis and the most impactful neuropsychiatric symptoms of PD would contribute to the understanding of their pathophysiologic mechanisms and allow further exploration of the involved neurochemical substrates and networks. The goal of this analysis is to evaluate associations between depression, psychosis, and anxiety and the severity of Lewy body score and neuronal loss in the SN and LC in a large cohort of PD patients.
Methods
2.1. Participants
The Morris K. Udall Parkinson’s Disease Research Center at the Johns Hopkins School of Medicine includes an ongoing longitudinal research program, initiated in 1998, which follows patients with PD, healthy controls, and individuals with related movement disorders. These studies in the program are approved by the Johns Hopkins Institutional Review Board (NA_00032761). Participants are recruited from tertiary care clinics, community practices, and area support groups. Written informed consent is obtained from all participants or their legally authorized representatives. Included in these studies is a brain donation registry for individuals wishing to donate their brain for PD research. The program includes two separate arms: one in which participants are evaluated in person every two years, and one in which participants are followed remotely via telephone and mail every two to three years. Both study arms include the collection of medical records and the eventual donation of participants’ brains upon their deaths. For participants evaluated during in-person visits (n=51), movement disorders-trained study physicians perform serial clinical assessments to ascertain the presence of depression, psychosis, anxiety, and dementia at baseline and longitudinally every two years using formal Diagnostic and Statistical Manual of Mental Disorders-IV-TR (DSM-IV-TR) criteria. Participants followed remotely (n=124) were assessed for the presence of psychiatric symptomology (depression, anxiety and psychosis) via completed questionnaires and/or histories extracted from neurology, primary care, or nursing facility notes when available.
The analysis presented here is a retrospective investigation of a histopathologic database, and includes 175 autopsied participants with a primary pathological diagnosis of idiopathic PD. Those with comorbid secondary diagnoses such as Alzheimer’s disease, cerebrovascular disease, and progressive supranuclear palsy were also included (Table 1).
Table 1: Primary and Associated Diagnoses.
The primary clinical-pathological and associated pathological diagnoses of the participants.
| Primary Pathological Diagnosis | Primary Clinical-Pathological Diagnosis | Associated Pathological Findings | n |
|---|---|---|---|
| Lewy Body Disease (n = 175) | Parkinson’s Disease (n = 62, 35%) | None | 42 |
| Primary Age-Related Tauopathy | 2 | ||
| Low-level Alzheimer Disease pathology | 13 | ||
| Non-Alzheimer Disease Tauopathy | 1 | ||
| Progressive Supranuclear Palsy and Low-level Alzheimer Disease pathology | 1 | ||
| Neurofibrillary Degeneration | 3 | ||
| Parkinson’s Disease with Dementia (n = 109, 63%) |
None | 34 | |
| Primary Age-Related Tauopathy | 2 | ||
| Low-level Alzheimer Disease pathology | 16 | ||
| Intermediate-level Alzheimer Disease pathology | 31 | ||
| High-level Alzheimer Disease pathology | 14 | ||
| Progressive Supranuclear Palsy | 1 | ||
| Frontotemporal lobar degeneration | 1 | ||
| Neurofibrillary Degeneration | 1 | ||
| Cerebrovascular Disease | 5 | ||
| Cerebrovascular Disease, Intermediate-level Alzheimer Disease pathology | 1 | ||
| Cerebrovascular Disease, High-level Alzheimer Disease pathology | 2 | ||
| Low-level Multiple System Atrophy pathology, Intermediate-level Alzheimer Disease pathology | 1 | ||
| Dementia with Lewy Bodies (n = 4, 2%) | None | 1 | |
| Intermediate-level Alzheimer Disease pathology | 2 | ||
| High-level Alzheimer Disease pathology | 1 |
2.2. Pathology
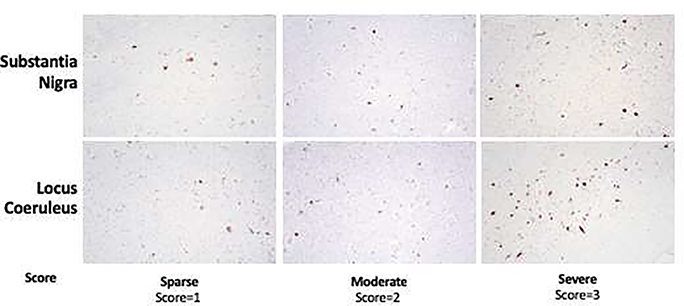
Autopsies were conducted by the Johns Hopkins Division of Neuropathology. The procedure for specimen preparation and staining has been previously described.20 The diagnostic formulation and neuropathological assessment followed the recommendations of the third report of the Dementia with Lewy Bodies consortium.21 A standardized dissection protocol was employed to examine the same anatomical regions within the LC and SN in all participants. Lewy bodies are identified by Hematoxylin & Eosin staining and immunohistochemistry for alpha-synuclein to reveal a Lewy body score, which was quantified according to consensus criteria.22 We semiquantitatively assessed for severity of Lewy body pathology (range 0–3) and neuronal loss/gliosis (“none”, “mild to moderate”, or “severe”) in the LC and SN. Scores of 1,2, and 3 signified a sparse, moderate, and severe degree of Lewy bodies, respectively (Figure 1). If Lewy bodies were absent in the first slice on H&E staining, subsequent slices (up to three) were analyzed for the presence of Lewy bodies and their density using anti-alpha synuclein stained slices.
Figure 1. Representative Micrographs of Lewy Bodies in the Substantia Nigra and Locus Coeruleus.
Lewy bodies are identified by Hematoxylin & Eosin staining and immunohistochemistry for alpha-synuclein to reveal a Lewy body score. Scores of 1, 2, and 3 signified a “sparse”, “moderate”, and “severe” degree of Lewy bodies, respectively. Representative images for the scoring system are displayed here.
2.3. Statistical methods
Lewy body scores and neuronal loss/gliosis severity were compared between participants with and without depression, psychosis and anxiety using Chi-squared analyses (Tables 2 and 3). Multiple logistic regression was used to control for disease duration and dementia. (Table 4). P-values were adjusted to correct for multiple comparisons using the Holm method.23
Table 2: Neuronal loss/gliosis (NLG) and their association with neuropsychiatric symptoms (NPS) and Lewy body score.
Psychosis was associated with neuronal loss/gliosis in the locus coeruleus and the substantia nigra. Depression was associated with neuronal loss/gliosis in the substantia nigra.
| Locus coeruleus | ||||||
| NPS (n=120) | Mild to moderate NLG (n=75) | Severe NLG (n=45) | χ2 | df | P | Adjusted P |
| Depression | 30 (40.0%) | 26 (57.8%) | 2.89 | 1 | 0.089† | 0.267a |
| Anxiety | 23 (30.7%) | 17 (37.8%) | 0.36 | 1 | 0.549† | 1.000a |
| Psychosis | 31 (41.3%) | 30 (66.7%) | 6.24 | 1 | 0.012† | 0.048a |
| Lewy Body score (n=86) | Mild to moderate NLG (n=55) | Severe NLG (n=31) | ||||
| 1 (sparse) | 9 (16.4%) | 5 (16.1%) | ||||
| 2 (moderate) | 22 40.0%) | 13 (41.9%) | 0.03 | 2 | 0.984† | 0.984a |
| 3 (severe) | 24 (43.6%) | 13 (41.9%) | ||||
|
| ||||||
| Substantia nigra | ||||||
| NPS (n=153) | Mild to moderate NLG (n=76) | Severe NLG (n=77) | χ2 | df | P | Adjusted P |
| Depression | 27 (35.5%) | 45 (58.4%) | 7.17 | 1 | 0.007† | 0.042a |
| Anxiety | 22 (28.9%) | 25 (32.5%) | 0.09 | 1 | 0.767† | 1.000a |
| Psychosis | 33 (43.4%) | 51 (66.2%) | 7.14 | 1 | 0.008† | 0.042a |
| Lewy Body score (n=105) | Mild to moderate NLG (n=56) | Severe NLG (n=49) | ||||
| 1 (sparse) | 16 (28.6%) | 11 (22.4%) | ||||
| 2 (moderate) | 14 (25.0%) | 18 (36.7%) | 1.76 | 2 | 0.417† | 0.834a |
| 3 (severe) | 27 (46.4%) | 20 (40.8%) | ||||
|
| ||||||
Differences between groups were compared using Chi-Squared analysis
P-values adjusted to correct for multiple comparisons using the Holm method.
Table 3: Neuropsychiatric symptoms do not correlate with Lewy body score.
None of the neuropsychiatric symptoms correlated with Lewy body pathology in the locus coeruleus or the substantia nigra.
|
Lewy Body Regional Score
|
|||
| Neuropsychiatric symptom | Substantia nigra (n = 106) | Locus coeruleus (n = 86) | |
| 1 (n = 27) | 1 (n = 14) | ||
| 2 (n = 35) | 2 (n = 32) | ||
| 3 (n = 37) | 3 (n = 47) | ||
|
| |||
| Depression | N (%) | 11 (40.7%) | 7 (50%) |
| 13 (37.1%) | 13 (40.7%) | ||
| 22 (47.8%) | 19 (51.4%) | ||
|
| |||
| χ2 | 0.537 | 1.61 | |
| (df) | (2) | (2) | |
| P | 0.765† | 0.447† | |
|
| |||
| Adjusted P | 1.000a | 1.000a | |
|
| |||
| Anxiety | N (%) | 4 (14.8%) | 4 (28.6%) |
| 17 (53.1%) | 12 (34.3%) | ||
| 14 (30.4%) | 13 (35.1%) | ||
|
| |||
| χ2 | 9.98 | 0.204 | |
| (df) | (2) | (2) | |
| P | 0.007† | 0.903† | |
|
| |||
| Adjusted P | 0.042a | 1.000a | |
|
| |||
| Psychosis | N (%) | 14 (51.9%) | 6 (42.9%) |
| 18 (56.3%) | 17 (48.6%) | ||
| 20 (43.5%) | 15 (40.5%) | ||
|
| |||
| χ2 | 1.31 | 0.482 | |
| (df) | (2) | (2) | |
| P | 0.519† | 0.786† | |
| Adjusted P | 1.000a | 1.000a | |
|
| |||
Differences between groups were compared using Chi-Squared analysis
P-values adjusted to correct for multiple comparisons using the Holm method.
Table 4: Neuropsychiatric Symptoms (NPS) and Neuronal Loss and Gliosis (NLG) After Adjusting for Disease Duration and Dementia.
Psychosis remained associated with neuronal loss/gliosis in the substantia nigra, but not the locus coeruleus, after adjusting for disease duration and dementia diagnosis. Depression remained associated with neuronal loss/gliosis in the substantia nigra after adjusting for disease duration and dementia diagnosis.
| Severe NLG in Locus coeruleus | ||||
| NPS (dependent variable) | Odds ratio (95% CI) | χ 2 (df) | P | Adjusted P |
| Depression | 2.06 (0.97 – 4.39) | 3.53 | 0.060† | 0.180 a |
| Anxiety | 1.30 (0.59 – 2.87) | 0.43 | 0.511† | 1.000 a |
| Psychosis | 2.91 (1.25 – 6.76) | 6.14 | 0.013† | 0.052 a |
|
| ||||
| Severe NLG in Substantia nigra | ||||
| NPS (dependent variable) | Odds ratio (95% CI) | χ 2 (df) | P | Adjusted P |
| Depression | 2.58 (1.33 – 5.01) | 7.85 (1) | 0.005† | 0.025 a |
| Anxiety | 1.20 (0.59 – 2.45) | 0.26 (1) | 0.608† | 1.000 a |
| Psychosis | 3.10 (1.50 – 6.40) | 9.35 (1) | 0.002† | 0.012 a |
|
| ||||
P-values for multiple logistic regression analyses
P-values adjusted to correct for 6 comparisons using the Holm method
Results
The majority of the sample (n=175) was male (67.4%) and Caucasian (97.8%). The mean age of PD onset was 62.4 years (SD = 10.57, range= 28–87). The mean duration of illness was 16.0 years (SD= 6.8, range=3–34 years). The average age at death was 78.0 (SD= 7.78, range= 55–95). Hoehn and Yahr (H&Y) staging was not standardly assessed; however, based on available data, the proportion of participants with a H&Y score of ≤ 3 was 57.6% (n=34) and the proportion of subjects with a score > 3 was 42.4% (n=25). The mean duration of follow-up for participants in our cohort was 7.1 years (SD = 7.72, range = 0–17.8 years). The mean elapsed time between the last evaluation and autopsy was approximately 2 years and 9 months. Of the sample, 50% of subjects (n=88) had an antemortem diagnosis of depression, 31.25% (n=55) with anxiety, and 56.0% (n=98) with psychosis. Of the 175 participants in the study, our final sample for analysis of LC pathology included the 120 participants with data for the degree of neuronal loss/gliosis in the LC. Similarly, analysis of the SN pathology included 153 participants with data for the degree of neuronal loss/gliosis within the SN. Similarly, the 105 participants with Lewy body pathology data in the SN were used to investigate the association between SN Lewy body pathology and neuropsychiatric symptoms, while the 86 participants with information regarding Lewy body pathology in the LC were used to investigate potential clinicopathological correlates to neuropsychiatric symptoms. Regarding neuropsychiatric symptoms, 88 patients in our sample were classified as depressed using DSM-IV-TR criteria (n=31) and retrospective chart review (n=57). A total of 55 patients were considered anxious (n=53) by DSM-IV-TR criteria (n=13) and chart review (n=42). Finally, 98 patients were deemed to have psychosis by DSM-IV-criteria (n=30) and by chart review (n=68).
On chi-square analysis, a history of psychosis was significantly associated with severe neuronal loss/gliosis in the LC (χ2=6.2, P=0.048, degrees of freedom (df) = 1) and SN (χ2=7.1, P=0.042, df = 1). Depression was also linked to severe neuronal loss/gliosis in the SN (χ2=7.2, P=0.042, df = 1) but not in the LC. Finally, the analysis comparing anxious to non-anxious participants found no significant differences between neuronal loss/gliosis severity in either region (Table 2). Based on available autopsy data, we performed secondary analyses using Lewy body density in place of neuronal loss/gliosis. We had Lewy body density scores for the LC in 86 of 120 participants anlyzed for LC neuronal loss/gliosis and in 105 of the 153 SN neuronal loss/gliosis participants. Lewy body score was not associated with any neuropsychiatric symptom (Table 3). Furthemore, neuronal loss/gliosis severity did not correlate with Lewy body score. When multiple logistic regression was used to control for disease duration and dementia presence, psychosis remained significantly associated with severe neuronal loss/gliosis in the SN (OR 3.1, 95% CI 1.5–6.4, χ2=9.4, P=0.012, df = 1), but not in the LC (OR 2.9, 95% CI 1.3–6.8, χ2=6.1, P=0.052, df = 1). Depression also remained significantly associated with severe neuronal loss/gliosis in the SN on multiple logistic regression (OR=2.6, 95% CI=1.3–5.0, χ2=7.85, P=0.025, df =1) (Table 4).
Discussion
This clinicopathological investigation, conducted in a markedly large brain autopsy cohort, found patients with PD and a history of psychosis were more likely to have severe neuronal loss/gliosis in the SN than did those without a history of psychosis. Depression was also linked to severe neuronal loss/gliosis in the SN. These findings remained significant after controlling for disease duration and dementia diagnosis. Anxiety was not associated with neuronal loss/gliosis severity in either region. No neuropsychiatric symptoms were associated with Lewy body score in either the SN or the LC.
Psychosis, most commonly represented by visual hallucinations, is a common neuropsychiatric symptom of PD that classically manifests later in the disease and is likely due to a complex interplay of medication effects (e.g. dopaminergic compounds) and disease-related factors.24,25 An integrated model has been proposed that includes impaired visual input and central visual processing, impaired brainstem regulation of sleep–wake cycle with fluctuating vigilance, intrusion of rapid eye movement dream imagery into wakefulness and emergence of internally generated imagery, cognitive dysfunction and influence of dopaminergic drugs.26 The lack of clear clinical causality makes the pathology of PD psychosis an area of keen interest. An early investigation linked elevated Lewy body density in the basolateral nucleus of the amygdala to formed visual hallucinations in PD (n=18).27 A more recent investigation using autopsy data from 94 participants did not find Lewy body pathology in the SN or LC to be associated with visual hallucinations, but did report associations in the middle frontal, temporal, transentorhinal, and anterior cingulate cortices.11 An analysis comparing 18 PD patients with a history of psychosis to 27 PD patients without such history found no significant difference in neuronal loss in the SN or LC between the two cohorts.6 In contrast, our much larger neuropathological study does demonstrate a link between SN pathology and psychosis in PD. It remains unclear why our findings are discordant with those of prior reports. However. evidence from structural imaging studies26,28 and pathology case reports29 support the hypothesis that abnormality and dysfunction of the SN contribute to the development of visual hallucinations in PD. Our findings contribute to this literature and further support the role of brainstem pathology in the onset of visual hallucinations in PD.
Our results are consistent with previous reports implicating the SN in PD depression. For example, previous studies have demonstrated depression in PD to increase the likelihood of SN gliosis at autopsy by three-fold.8 Case reports have also described instances in which SN stimulation during a deep brain stimulation procedure has induced acute depressive episodes in PD patients.30 Additionally, in the general population, it has been well established that cerebrovascular lesions within the basal ganglia, which receive dopaminergic input from the SN, are linked to the development of depression.31 These findings parallel the current study showing a correlation between pathology in the SN and the presence of depression.
To our knowledge, this is one of the first studies to attempt to characterize the correlation of disease-specific regional pathology with anxiety in PD. Our analysis did not find an association between anxiety in PD and pathology within the SN or LC. One explanation for our findings is that the pathophysiology of PD anxiety may be better explained by circuitry dysfunction rather than post-mortem pathology. For example, serotonergic neurons from the raphe nucleus provide input to limbic structures controlling anxiety, and there are several studies showing anxiety disorders may be caused by abnormalities in serotonin action.32 Further, Menza et al. found that PD patients who carried a short allele for serotonin transporter scored higher on anxiety rating scales.33 The raphe nucleus was not quantitatively assessed as part of our brain donation protocol. Our results suggest that anxiety in PD may not be explained by pathologic abnormalities within the LC or SN, and future research is warranted regarding the role of serotonergic circuitry dysfunction and raphe nucleus pathology in PD anxiety.
Despite some evidence correlating Lewy bodies to specific clinical symptoms, some have questioned their use as a marker for clinical symptoms of PD.17–19 Greffard et al. showed no associations between Lewy body pathology with motor symptoms or disease duration, yet demonstrated close correlations between SN neuronal density and severity of bradykinesia and rigidity.18 The pattern of distribution and degree of Lewy body accumulation in post-mortem brain tissue may not be an accurate marker of clinical symptoms in PD because the degree of cell loss may confound its presence. This is similar in principle to the debate surrounding the role of amyloid beta protein in a related neurodegenerative disorder, Alzheimer’s disease. Many researchers have challenged the notion that the accumulation of amyloid beta causes dysfunction in Alzheimer’s disease by demonstrating a poor correlation between amyloid plaque burden and degree of cognitive impairment.34
Our results, which did not find the severity of Lewy body pathology to be associated with psychosis, depression, or anxiety further support the notion that in-vivo clinical symptoms of PD are either not caused by Lewy body pathology or that the relationship is confounded by the time of autopsy. Numerous studies have demonstrated that the proportion of Lewy body-containing neurons remains relatively stable throughout the disease course.17,19 One hypothesis explaining this observation could be that because Lewy bodies are intracellular, their number could plateau as neuronal loss progresses and balances the rate of new Lewy body formation. Based on current knowledge, it is also unclear whether Lewy bodies are present before or after neuron death. Due to their presence in sites of neuronal loss, such as the SN and LC, they have been considered the cause of neuronal death, yet some have reported that most nigral neurons undergoing apoptosis do not contain Lewy body inclusions and, thus, may be dying before Lewy body formation occurs.35 Further, it remains unclear how long Lewy bodies survive in deceased neurons before degrading. Taken together, our ability to observe an association between clinical features such as neuropsychiatric symptoms and Lewy body pathology at autopsy remains limited. Our results demonstrated correlations between neuronal loss/gliosis and neuropsychiatric symptoms in PD, suggesting that cell loss may be a better marker of in-vivo clinical manifestations at autopsy.
The strengths of this study include its power to effectively explore the relationship between histopathology and neuropsychiatric symptoms in PD patients given its large and well-characterized sample, which is unusual in comparison to other post-mortem studies of its kind. We additionally controlled our analysis for the potentially confounding effects of disease duration and dementia presence.
One limitation of our analysis is that pathology data in our anatomical structures of interest were only available in subsets of participants. Due to the retrospective nature of this analysis, we were unable to collect substantia nigra and locus coeruleus pathology information for all patients. Future investigations should strive to implement a standardized protocol in which identical pathological markers (Lewy body and neuronal loss/gliosis) are measured in the same structures (substantia nigra and locus coeruleus) in all patients enrolled. We also did not assess cell loss in the raphe nuclei, which may prevent us from formulating a more comprehensive hypothesis. Another limitation is that the collection of clinical data and the observation of pathology in brain tissue at autopsy were sometimes dissociated in time (average of 2 years and 9 months apart). Therefore, the presence of neuropsychiatric symptoms and dementia may not have been noted in cases in which a participant was autopsied several years after the last evaluation. Also, in a portion of the cases who were followed remotely, assessment of depression, psychosis, anxiety, and dementia was based on retrospective chart review, which carries the risk of underreporting. Furthermore, no standardized method to evaluate psychiatric disorders was employed for participants whose diagnoses were obtained through retrospective chart review. Hoehn & Yahr staging was not standardly assessed among brain donation participants, so we were unable to control for disease severity in this analysis. Similarly, we did not assess medication records of our subjects because this was not systematically collected in all participants. Lastly, scoring of Lewy bodies and neuronal loss were both subjective to interobserver variability. Future directions include further elucidating the neurobiology underlying neuropsychiatric symptoms in PD by looking at pathology in functional subregions and eventually by using new functional imaging techniques in-vivo. Improving knowledge of the association between pathological findings and clinical symptoms has the potential to inform future biomarkers, diagnoses, and therapies.
Highlights.
-
What is the primary question addressed by this study?
Do Parkinson’s disease neuropsychiatric symptoms (depression, anxiety, and psychosis) correlate with neuronal loss/gliosis and Lewy body pathology in the substantia nigra and locus coeruleus?
-
What is the main finding of this study?
Depression and psychosis, but not anxiety, were associated with neuronal loss/gliosis in the substantia nigra after controlling for disease duration and dementia. No neuropsychiatric symptoms were associated with Lewy body score.
-
What is the meaning of the finding?
Neuronal loss/gliosis may be a better histopathological marker for neuropsychiatric symptoms in Parkinson’s disease than Lewy body pathology.
Acknowledgements:
We thank all study participants and their families.
Disclosure/Conflicts of interest: The authors report no conflicts of interest. Dr. Rosenthal and Dr. Dawson disclose external funding for aspects of this work (NIH/NINDS P50 NS8377).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016; 46(4):292–300. [DOI] [PubMed] [Google Scholar]
- 2.Berardelli A, Wenning GK, Antonini A, et al. EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol 2013; 20(1):16–34. [DOI] [PubMed] [Google Scholar]
- 3.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci 2017; 18(7):435–450. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord 2009; 24(15):2175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H. The Lewy Body in Parkinson’s Disease and Related Neurodegenerative Disorders. Mol Neurobiol 2012; 47(2):495–508. [DOI] [PubMed] [Google Scholar]
- 6.Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of parkinson’s disease. J Neuropathol Exp Neurol 1991; 50(6):743–55. [DOI] [PubMed] [Google Scholar]
- 7.Jellinger KA. Post mortem studies in Parkinson’s disease--is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl 1999; 1–29. [DOI] [PubMed] [Google Scholar]
- 8.Frisina PG, Haroutunian V, Libow LS. The neuropathological basis for depression in Parkinson’s disease. Park Relat Disord 2009; 15(2):144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira D, Guerra A. Depression and Parkinson’s Disease: Role of the Locus Coeruleus. Eur Psychiatry 2015; 30(1):641.25758156 [Google Scholar]
- 10.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: Loss of dopamine and noradrenaline innervation in the limbic system. Brain 2005; 128(6):1314–22. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher DA, Parkkinen L, O’Sullivan SS, et al. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain 2011; 134(11):3299–309. [DOI] [PubMed] [Google Scholar]
- 12.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of Psychiatric and Other Nonmotor Symptoms on Disability in Parkinson’s Disease. J Am Geriatr Soc 2004; 52(5):784–8. [DOI] [PubMed] [Google Scholar]
- 13.Richard IH. Anxiety disorders in Parkinson’s disease. Adv Neurol 2005; 96:42–55 [PubMed] [Google Scholar]
- 14.Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 2003; 110(5):517–36. [DOI] [PubMed] [Google Scholar]
- 15.Parkkinen L, Kauppinen T, Pirttilä T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol 2005; 57(1):82–91. [DOI] [PubMed] [Google Scholar]
- 16.Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol 2009; 68(7):816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkkinen L, O’Sullivan SS, Collins C, et al. Disentangling the Relationship between Lewy bodies and nigral neuronal loss in Parkinson’s disease. J Parkinsons Dis 2011; 1(3):277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greffard S, Verny M, Bonnet AM, et al. Motor score of the unified Parkinson disease rating scale as a good predictor of lewy body-associated neuronal loss in the substantia nigra. Arch Neurol 2006; 63(4):584–8. [DOI] [PubMed] [Google Scholar]
- 19.Greffard S, Verny M, Bonnet AM, Seilhean D, Hauw JJ, Duyckaerts C. A stable proportion of Lewy body bearing neurons in the substantia nigra suggests a model in which the Lewy body causes neuronal death. Neurobiol Aging 2010; 63(4):584–8. [DOI] [PubMed] [Google Scholar]
- 20.Mills KA, Mari Z, Bakker C, et al. Gait function and locus coeruleus Lewy body pathology in 51 Parkinson’s disease patients. Park Relat Disord 2016; 33:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeith IG, Boeve BF, DIckson DW, et al. Diagnosis and management of dementia with Lewy bodies. Neurology 2017; 89(1):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology 2005; 65(12):1863–72. [DOI] [PubMed] [Google Scholar]
- 23.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6(2):65–70 [Google Scholar]
- 24.Barnes J, David AS. Visual hallucinations in Parkinson’s disease: A review and phenomenological survey. J Neurol Neurosurg Psychiatry 2001; 70(6):727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ffytche DH, Creese B, Politis M, et al. The psychosis spectrum in Parkinson disease. Nat Rev Neurol 2017; 13(2):81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenka A, Jhunjhunwala KR, Saini J, Pal PK. Structural and functional neuroimaging in patients with Parkinson’s disease and visual hallucinations: A critical review. Park Relat Disord 2015; 21(7):683–91. [DOI] [PubMed] [Google Scholar]
- 27.Harding AJ, Stimson E, Henderson JM, Halliday GM. Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 2002; 125(11):2431–45 [DOI] [PubMed] [Google Scholar]
- 28.Li T, Shi J, Qin B, et al. Increased substantia nigra echogenicity correlated with visual hallucinations in Parkinson’s disease: a Chinese population-based study. Neurol Sci 2020; 41:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai K, Ikeda T, Ishida C, Komai K, Yamada M. Delusions and visual hallucinations in a patient with Parkinson’s disease with dementia showing pronounced Lewy body pathology in the nucleus basalis of Meynert. Neuropathology 2019; 13(1):1–10. [DOI] [PubMed] [Google Scholar]
- 30.Blomstedt P, Hariz MI, Lees A, et al. Acute severe depression induced by intraoperative stimulation of the substantia nigra: A case report. Parkinsonism Relat Disord 2008; 14(3): 253–6. [DOI] [PubMed] [Google Scholar]
- 31.Fang J, Cheng Q. Etiological mechanisms of post-stroke depression: a review. Neurol Res 2009; 31(9):904–9. [DOI] [PubMed] [Google Scholar]
- 32.Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol 2003; 70(2):83–244. [DOI] [PubMed] [Google Scholar]
- 33.Menza M a, Palermo B, DiPaola R, Sage JI, Ricketts MH. Depression and anxiety in Parkinson’s disease: possible effect of genetic variation in the serotonin transporter. J Geriatr Psychiatry Neurol 1999; 12(2):49–52. [DOI] [PubMed] [Google Scholar]
- 34.Morris GP, Clark IA, Vissel B. Inconsistencies and Controversies Surrounding the Amyloid Hypothesis of Alzheimer’s Disease. Acta Neuropathol Commun 2014; 2(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tompkins MM, Hill WD. Contribution of somal Lewy bodies to neuronal death. Brain Res 1997; 775(1–2):24–9. [DOI] [PubMed] [Google Scholar]