Abstract
Aims
Despite statin and antihypertensive therapies, older Americans have high atherosclerotic cardiovascular disease (ASCVD) risk. Novel measures of triglyceride-rich lipoproteins, low-density lipoprotein triglycerides (LDL-TG), and remnant-like particle cholesterol (RLP-C), are associated with ASCVD in middle-aged adults. Polymorphisms in genes encoding angiopoietin-related protein 3 (ANGPTL3) and apolipoprotein C-III (apoC-III), two proteins involved in triglyceride catabolism, are associated with increased risk for hypertriglyceridaemia and ASCVD and are potential therapeutic targets. We examined associations of LDL-TG, RLP-C, apoC-III, and ANGPTL3 levels with ASCVD events in older adults in the Atherosclerosis Risk in Communities (ARIC) study.
Methods and results
In 6359 participants (mean age 75.8 ± 5.3 years) followed for ASCVD events [coronary heart disease (CHD) or ischaemic stroke] up to 6 years, associations between LDL-TG, RLP-C, apoC-III, and ANGPTL3 and ASCVD events were assessed using Cox regression. With adjustment for age, sex, and race, RLP-C, LDL-TG, apoC-III, and ANGPTL3 (as continuous variables) were significantly associated with CHD. However, after adjustment for traditional risk factors and lipid-lowering medications, only LDL-TG and ANGPTL3 were significantly associated with ASCVD events [hazard ratio (HR) 1.72, 95% confidence interval (CI) 1.25–2.37 per log unit increase in LDL-TG; HR 1.63, 95% CI 1.17–2.28 per log unit increase in ANGPTL3].
Conclusions
In older adults, LDL-TG, RLP-C, apoC-III, and ANGPTL3 were associated with CHD events in minimally adjusted models; LDL-TG and ANGPTL3 remained independent predictors of ASCVD events with further adjustment. Future studies should assess potential benefit of lowering hepatic apoC-III or ANGPTL3 expression in patients with elevated triglyceride-rich lipoproteins.
Keywords: Triglyceride-rich lipoprotein, Atherosclerotic cardiovascular disease, Low-density lipoprotein triglyceride, Remnant-like particle cholesterol, Apolipoprotein C-III, Angiopoietin-like 3
Introduction
Hypercholesterolaemia is an important risk factor for cardiovascular morbidity and mortality.1 Current guidelines target low-density lipoprotein cholesterol (LDL-C) levels to reduce atherosclerotic cardiovascular disease (ASCVD) risk.2,3 However, numerous statin, non-statin, and combination therapy trials clearly demonstrate that despite aggressive LDL-C lowering, significant residual ASCVD risk persists.4–6 Therefore, it is imperative to identify determinants of residual ASCVD risk, particularly in older adults, who have the highest ASCVD risk.7 The relationship between non-LDL-C lipid parameters, notably triglycerides (TG) and triglyceride-rich lipoproteins (TGRL), and residual cardiovascular risk is increasingly recognized,8 especially in patients on optimal statin therapy.
TGRL include chylomicrons, very-low-density lipoprotein (VLDL), and their remnant particles created during lipolysis. The cholesterol content of remnant particles, remnant-like particle cholesterol (RLP-C), is highly atherogenic.9,10 Furthermore, large epidemiological11,12 and genetic13,14 studies have established the role of elevated TG and RLP-C levels in the development of ASCVD.
In hypertriglyceridaemia, exchange of TG and cholesteryl esters between VLDL and LDL results in LDL enriched with TG and raises low-density lipoprotein triglycerides (LDL-TG) level. A previous analysis from the Atherosclerosis Risk in Communities (ARIC) cohort demonstrated significant associations of LDL-TG with incident coronary heart disease (CHD) and ischaemic stroke after adjustment for traditional risk factors in middle-aged adults (mean age 63 years).15 Although total cholesterol and LDL-C levels have weaker associations with incident ASCVD in older age,16 information is limited on associations of RLP-C and LDL-TG levels with ASCVD events in older adults receiving contemporary medical therapy.
Apolipoprotein C-III (apoC-III) and angiopoietin-related protein 3 (ANGPTL3) are inhibitors of lipoprotein lipase, a key enzyme in TG hydrolysis. Loss-of-function mutations in genes encoding for apoC-III (APOC3)13 and ANGPTL3 (ANGPTL3)17 are associated with favourable lipid profiles and reduced ASCVD risk. Novel therapies designed to reduce apoC-III or ANGPTL3 level, and consequently TG/TGRL, are in early-phase trials.18,19
To provide epidemiological data on whether these novel therapies may be of potential benefit to reduce ASCVD risk in older individuals with increased TGRL levels and high ASCVD risk, we investigated associations of LDL-TG, RLP-C, apoC-III, and ANGPTL3 with clinical and metabolic measurements and with incident ASCVD events in older ARIC participants (aged 67–90 years) receiving contemporary medical therapy.
Methods
More detailed methods are available in the Supplementary material online.
Study population
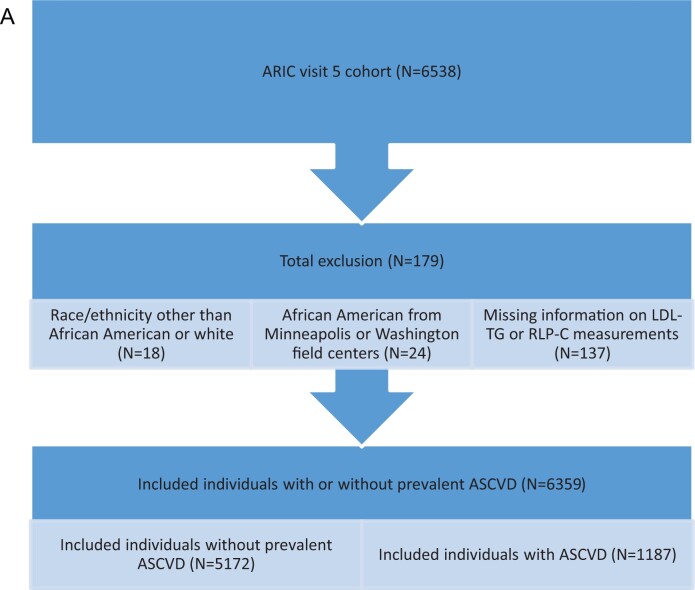
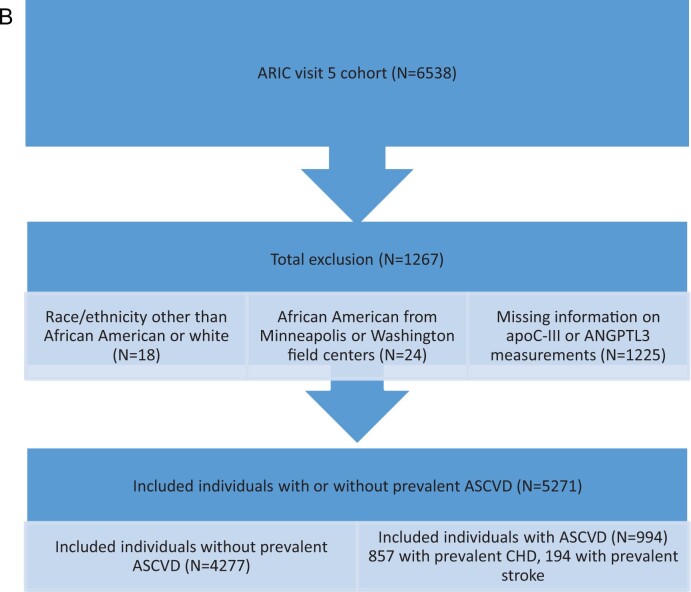
The design and objectives of the ARIC study have been published.20 Of 6538 participants at ARIC visit 5 (used as baseline; ages 67–90), after exclusions, 6359 (5172 without history of clinical ASCVD and 1187 with prevalent ASCVD) were included in event analyses for RLP-C and LDL-TG (Figure 1A), and 5271 (4277 without prevalent ASCVD and 857 with prevalent ASCVD) were included in time-to-event analyses for apoC-III and ANGPTL3 (Figure 1B).
Figure 1.
(A) Study population for LDL-TG and RLP-C analyses. (B) Study population for apoC-III and ANGPTL3 analyses.
Figure 1.
Continued
The study complies with the Declaration of Helsinki. The protocol was approved by each field centre’s institutional review board, and all participants provided written informed consent.
Biochemical assessments
Lipid measurements for the ARIC study were performed on plasma samples collected after overnight fast and stored at −80°C prior to analysis as previously described.21 Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and TG were quantified using enzymatic measures; RLP-C22 and LDL-TG23 were quantified using fully automated detergent-based homogenous methods (Denka Seiken, Tokyo, Japan). ApoC-III and ANGPTL3 were measured using the SOMAscan assay (SomaLogic, Boulder, CO, USA), a proprietary multiplexed assay to detect >5000 proteins.24
Cardiovascular event assessment
The primary outcome was time to the first ASCVD event after visit 5 (2011–2013) through 31 December 2017. ASCVD was a composite of CHD25 (definite or probable myocardial infarction, definite coronary death, or coronary revascularization) and ischaemic stroke26 (validated definite or probable stroke) determined from hospital discharge codes or death certificates and adjudicated by the ARIC investigators.
Statistical analysis
Associations of LDL-TG, RLP-C, apoC-III, and ANGPTL3 with time to first cardiovascular event after visit 5 was determined with Cox proportional hazard models. Sensitivity analyses stratified participants by baseline ASCVD prevalence. Cardiovascular event analyses accounted for competing risk of non-cardiovascular death.27
LDL-TG, RLP-C, apoC-III, and ANGPTL3 were analysed as categorical (quartiles) and continuous (natural log-transformed) variables. For categorical analyses, the lowest quartile was used as reference. P-values for linear trend of hazard ratio (HR) were determined using Wald chi-square test on linearity hypothesis using ordered quartiles. HRs for continuous analyses were reported per natural log-unit increment of the log-transformed variable. Model 1 was adjusted for age, sex, and race. Model 2 included traditional risk factors—age, race, sex, total cholesterol, HDL-C, current smoking, systolic blood pressure, antihypertensive medication use, and diabetes status—along with prevalent ASCVD and lipid-lowering medication use.
Restricted cubic splines with knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles were used to explore the shape of the association of each lipid (RLP-C, LDL-TG, TG) or protein (apoC-III, ANGPTL3) with time to first CHD, ischaemic stroke, or ASCVD event after visit 5, using Cox proportional hazard model. For the Cox regression analysis, lipids or proteins were modelled as continuous variables, with the median value used as reference, and adjusted for age, race, and sex (Model 1). The incremental value in risk prediction of adding LDL-TG, RLP-C, or TG separately (as a natural log-transformed continuous variable) to Model 2 was assessed by area under the curve (AUC), net reclassification index (NRI), continuous NRI, and integrated discrimination index (IDI). Associations of apoC-III and ANGPTL3 levels with levels of RLP-C, LDL-TG, and TG individually were evaluated in linear regression models. P-value <0.05 was considered significant.
Results
Baseline characteristics and correlations
The study cohort (n = 6359) had mean ± standard deviation age 75.8 ± 5.3 years, 3721 (58.2%) women, 1441 (22.7%) African Americans, and 2046 (33.2%) individuals with diabetes. The median (25th percentile, 75th percentile) RLP-C level was 3.5 (2.5, 5.5) mg/dL and LDL-TG level was 14.6 (12.3, 17.9) mg/dL.
Individuals with RLP-C and LDL-TG in the highest quartile were more likely to be women and Caucasian-Americans and to have higher systolic and diastolic blood pressure, increased high-sensitivity C-reactive protein, and a more atherogenic lipid profile (higher TG and LDL-C and lower HDL-C) and less likely to use lipid-lowering medications (Supplementary material online, Tables S1 and S2).
Individuals with apoC-III and ANGPTL3 in the highest quartile were more likely to be women and Caucasian-Americans and to have lower body mass index and estimated glomerular filtration rate and higher total cholesterol, HDL-C, LDL-C, non-HDL-C, TG, small dense LDL-C, LDL-TG, and RLP-C, and less likely to have prevalent ASCVD. Individuals with higher ANGPTL3 levels were more likely to be older and less likely to have hypertension and diabetes and use lipid-lowering medications, whereas those with higher apoC-III were more likely to use lipid-lowering medications (Supplementary material online, Tables S3 and S4).
RLP-C and LDL-TG were positively correlated with TG (r = 0.73 and r = 0.40, respectively), with RLP-C showing a stronger association (Supplementary material online, Table S5). Both apoC-III and ANGPTL3 were positively correlated with RLP-C (r = 0.52 and 0.07, respectively) and LDL-TG (r = 0.22 and 0.13, respectively).
RLP-C, but not LDL-TG, was positively correlated with body mass index, fasting glucose, and haemoglobin A1C and inversely correlated with adiponectin. Both RLP-C and LDL-TG were positively correlated with high-sensitivity C-reactive protein.
In multivariate analysis, plasma apoC-III levels were positively and significantly associated with LDL-TG [β 0.03, 95% confidence interval (CI) 0.02–0.05], RLP-C (β 0.57, 95% CI 0.54–0.60), and TG (β 0.41, 95% CI 0.40–0.43) (Supplementary material online, Table S6). ANGPTL3 was positively and significantly associated with LDL-TG (β 0.04, 95% CI 0.01–0.07) but not with RLP-C or TG (Supplementary material online, Table S6).
Association of RLP-C and LDL-TG with cardiovascular events
Over median (25th percentile, 75th percentile) follow-up of 5.5 (4.8, 5.9) years, 514 first CHD, 194 first ischaemic stroke, and 675 first ASCVD (CHD or ischaemic stroke) events occurred. For RLP-C in categorical analyses, risk for first CHD, ischaemic stroke, and ASCVD event was significantly higher across increasing quartiles in model 1 (age, sex, race), driven primarily by the highest quartile. The associations were attenuated after adjustments for traditional risk factors and lipid-lowering medication use (Model 2) (Table 1).
Table 1.
Association of RLP-C quartiles with first cardiovascular event
| RLP-C quartiles (mg/dL) |
P-trend | |||||
|---|---|---|---|---|---|---|
| Q1 (0.6–2.4; N = 1616) | Q2 (2.5–3.5; N = 1629) | Q3 (3.6–5.5; N = 1560) | Q4 (5.6–111.6; N = 1554) | |||
| ASCVD | Event, n (%) | 180 (11.14) | 133 (8.16) | 157 (10.06) | 205 (13.19) | <0.001 |
| Model 1 | Ref | 0.80 (0.64–1.00) | 1.04 (0.84–1.29) | 1.37 (1.12–1.68) | <0.0001 | |
| Model 2 | Ref | 0.80 (0.63–1.02) | 1.00 (0.79–1.27) | 1.05 (0.82–1.34) | 0.13 | |
| CHD | Event, n (%) | 146 (9.03) | 97 (5.95) | 118 (7.56) | 153 (9.85) | <0.001 |
| Model 1 | Ref | 0.74 (0.57–0.96) | 0.99 (0.77–1.27) | 1.26 (1.00–1.59) | 0.0007 | |
| Model 2 | Ref | 0.77 (0.59–1.02) | 1.02 (0.78–1.33) | 1.00 (0.76–1.32) | 0.18 | |
| Ischaemic stroke | Event, n (%) | 40 (2.48) | 47 (2.89) | 46 (2.95) | 61 (3.93) | 0.11 |
| Model 1 | Ref | 1.19 (0.78–1.82) | 1.27 (0.83–1.94) | 1.77 (1.19–2.65) | 0.03 | |
| Model 2 | Ref | 1.05 (0.67–1.64) | 0.98 (0.61–1.55) | 1.11 (0.69–1.78) | 0.94 | |
Data are presented as n (%) or HR (95% CI). Model 1 is adjusted by age, sex, and race; Model 2 is model 1 plus total cholesterol, HDL-C, current smoking, systolic blood pressure, antihypertensive medication use, diabetes status, and lipid-lowering medication use. P-trend for linearity of hazard ratio of proportional hazard regression model is calculated based on the results of Wald chi-square test on linearity hypothesis of ordered RLP-C quartiles.
Similarly, RLP-C analysed as a continuous variable showed significant association with first CHD (HR 1.31, 95% CI 1.16–1.48), ischaemic stroke (HR 1.29, 95% CI 1.05–1.58), and ASCVD (HR 1.32, 95% CI 1.18–1.46) event in model 1. However, after adjusting for Model 2, the associations were not significant (Table 2). Stratification by prevalent ASCVD yielded a similar pattern of associations (Supplementary material online, Table S7).
Table 2.
Association of RLP-C and LDL-TG (in natural-log transformation) with first cardiovascular event
| RLP-C |
LDL-TG |
||||
|---|---|---|---|---|---|
| Model | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| ASCVD | 1 | 1.32 (1.18–1.46) | <0.001 | 2.30 (1.79–2.96) | <0.001 |
| 2 | 1.10 (0.97–1.25) | 0.13 | 1.72 (1.25–2.37) | 0.001 | |
| CHD | 1 | 1.31 (1.16–1.48) | <0.001 | 2.36 (1.77–3.14) | <0.001 |
| 2 | 1.13 (0.98–1.31) | 0.09 | 1.74 (1.20–2.51) | 0.003 | |
| Ischaemic stroke | 1 | 1.29 (1.05–1.58) | 0.01 | 2.41 (1.52–3.81) | <0.001 |
| 2 | 0.94 (0.74–1.21) | 0.64 | 1.65 (0.92–2.96) | 0.10 | |
Models as in Table 2.
In restricted cubic spline regression analyses adjusted for Model 1, RLP-C as a continuous variable showed a generally linear association with CHD events but not ischaemic stroke (Figure 2A). The association with ASCVD events, though linear, was not as strong as with CHD, probably because ASCVD was a composite of CHD and ischaemic stroke.
Figure 2.
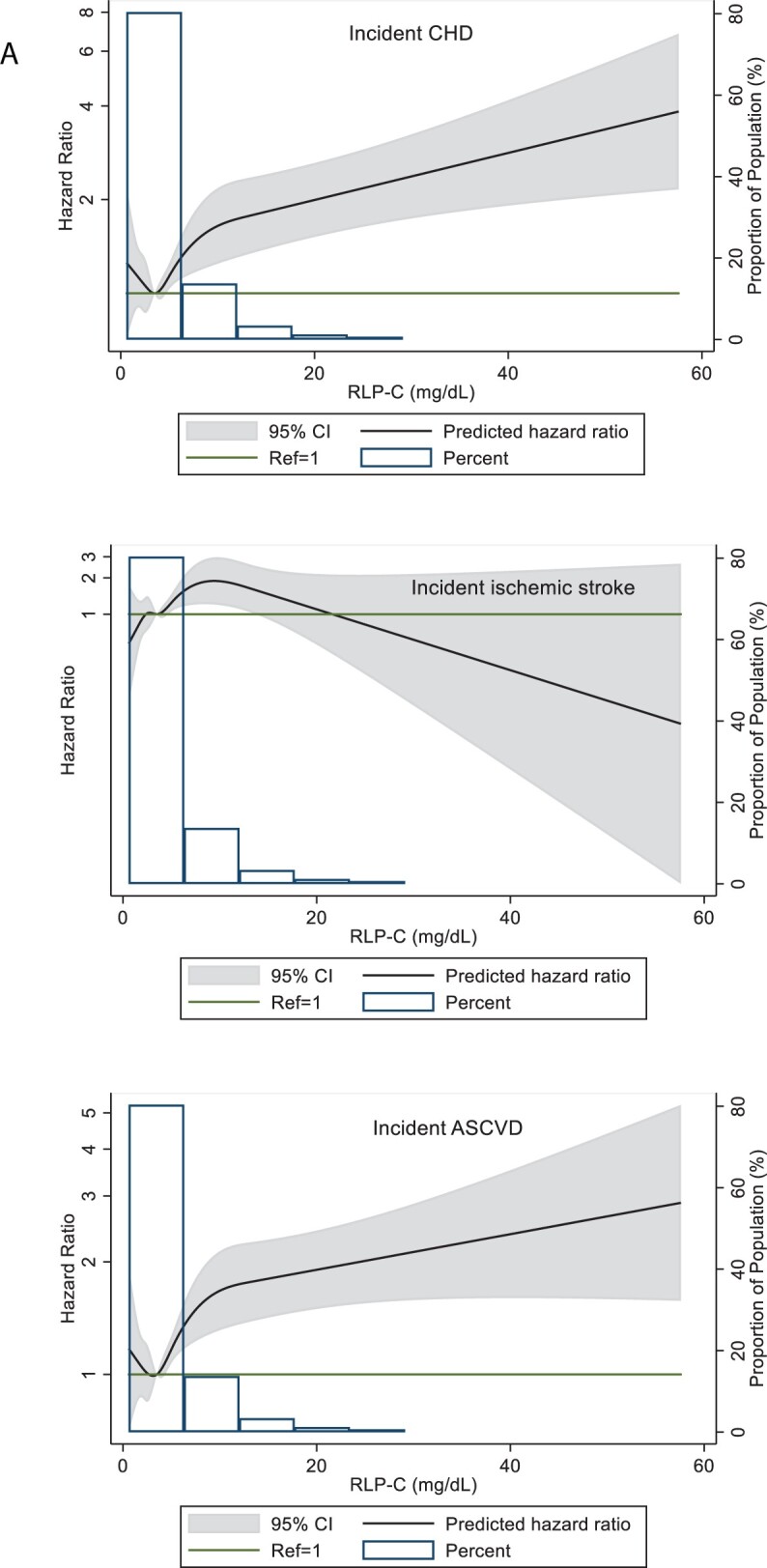
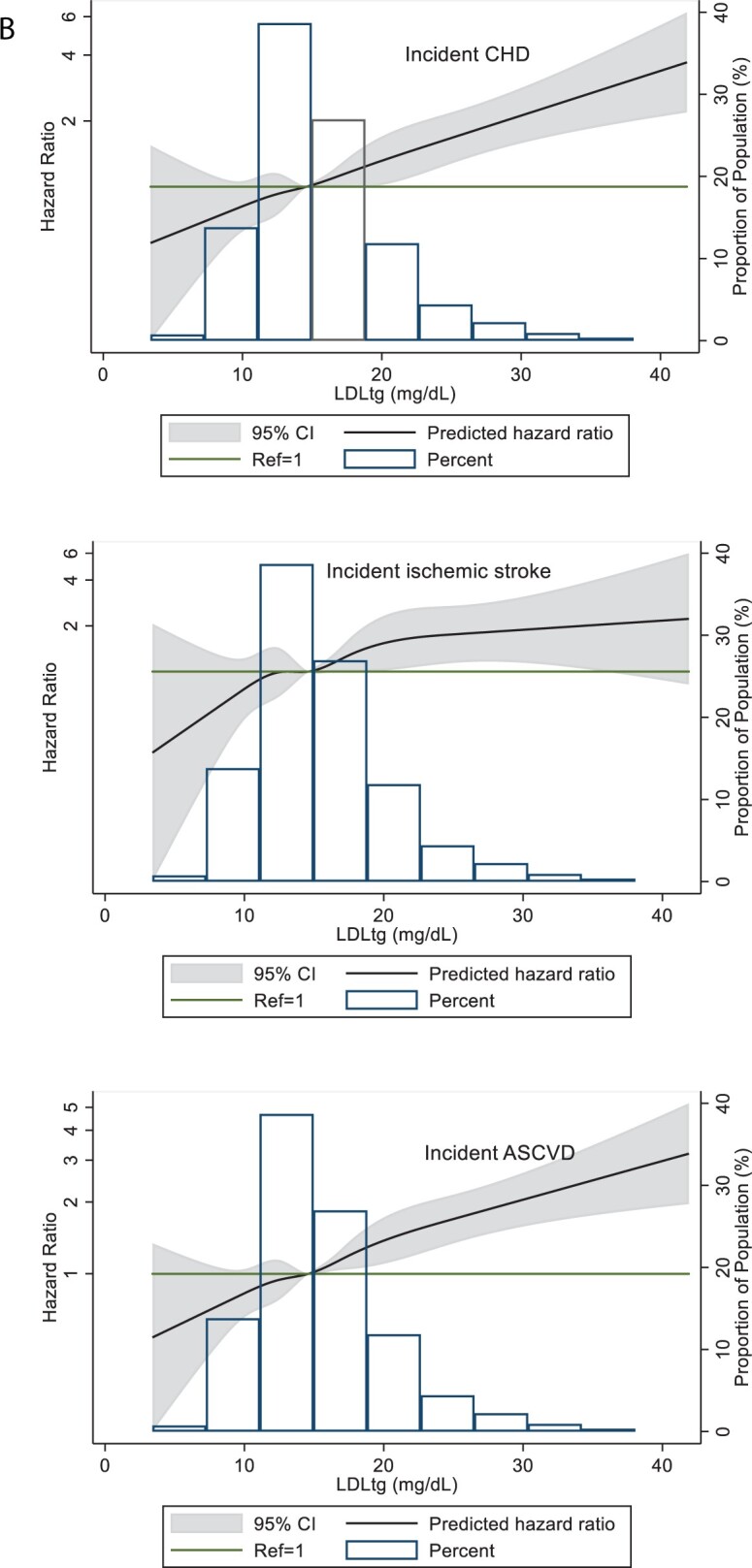
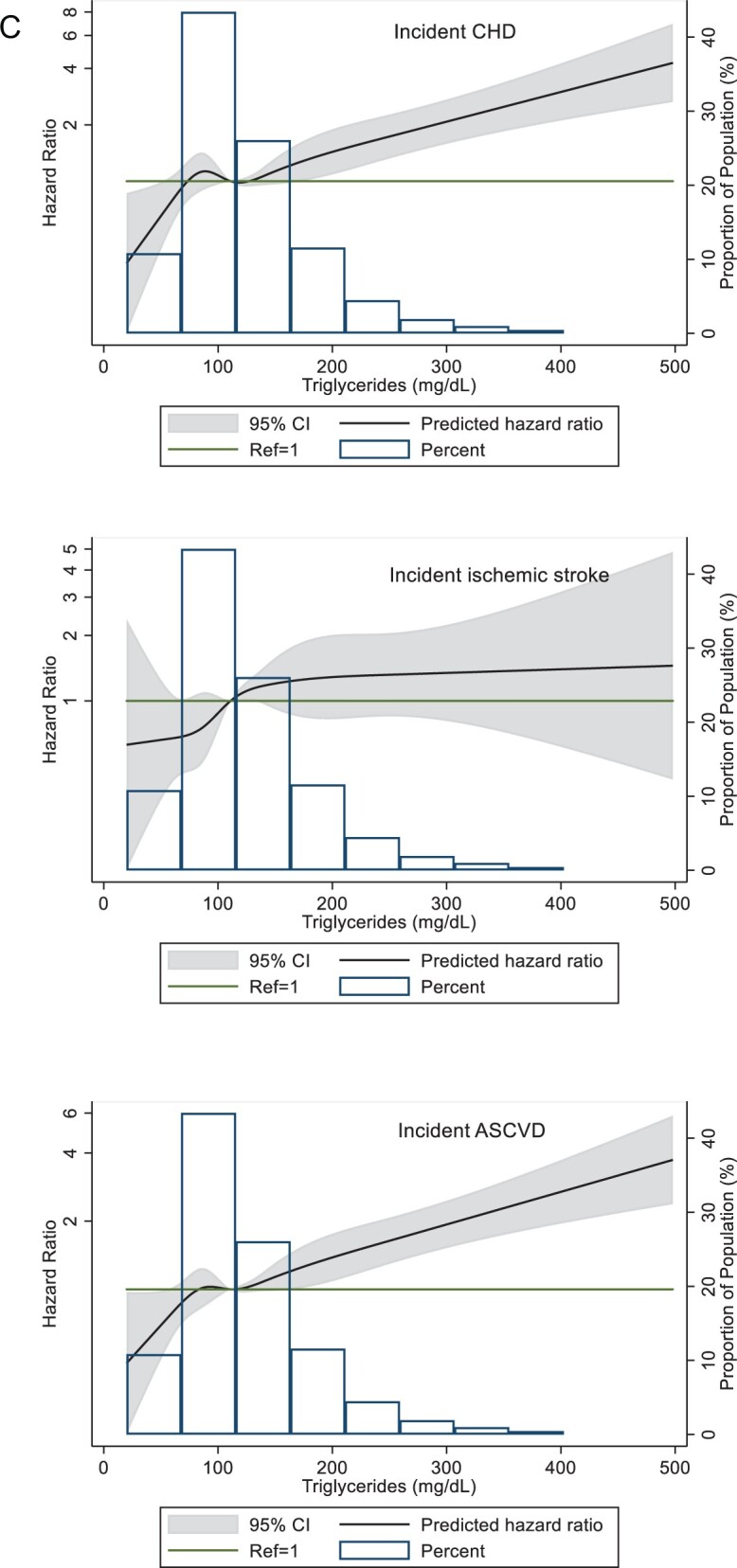
(A) Restricted cubic splines of Cox regression showing hazard ratios of coronary heart disease (CHD), ischaemic stroke, and atherosclerotic cardiovascular disease (ASCVD) events by remnant-like particle cholesterol (RLP-C) as a continuous variable, ARIC visit 5 (2011–2013). The median value (3.5 mg/dL) was used as reference in a Cox proportional hazard model adjusted for age, sex, and race (Model 1). The knots were placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. Extreme values of RLP-C > 60 mg/dL (n = 9) were excluded from the analysis. (B) Restricted cubic splines of Cox regression showing hazard ratios of CHD, ischaemic stroke, and ASCVD events by low-density lipoprotein triglycerides (LDL-TG) as a continuous variable, ARIC visit 5 (2011–2013). The median value (14.6 mg/dL) was used as reference in a Cox proportional hazard model adjusted for age, sex, and race (model 1). The knots were placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. Extreme values of LDL-TG >42 mg/dL (n = 7) were excluded from the analysis. (C) Restricted cubic splines of Cox regression showing hazard ratios of CHD, ischaemic stroke, and ASCVD events by triglycerides as a continuous variable, ARIC visit 5 (2011–2013). The median value (111 mg/dL) was used as reference in a Cox proportional hazard model adjusted for age, sex, and race (Model 1). The knots were placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. Extreme values of triglyceride >500 mg/dL (n = 14) were excluded from the analysis.
Figure 2.
Continued
Figure 2.
Continued
For LDL-TG in categorical analyses, risk for first CHD, ischaemic stroke, and ASCVD event was significantly higher across increasing quartiles in model 1, driven primarily by the highest quartile. After adjustment for Model 2, LDL-TG in the highest quartile remained significantly associated with CHD and ASCVD but not ischaemic stroke (Table 3). Compared with the lowest quartile, HRs for the highest LDL-TG quartile were 1.44 (95% CI 1.08–1.94) for CHD events and 1.45 (95% CI 1.12–1.88) for ASCVD events when adjusted for Model 2.
Table 3.
Association of LDL-TG quartiles with first cardiovascular event
| LDL-TG quartiles (mg/dL) |
P-trend | |||||
|---|---|---|---|---|---|---|
| Q1 (3.4–12.3; N = 1649) |
Q2 (12.4–14.6; N = 1541) |
Q3 (14.7–17.9; N = 1594) |
Q4 (18.0–66.4; N = 1575) |
|||
| ASCVD | Event, n (%) | 155 (9.40) | 153 (9.93) | 150 (9.41) | 217 (13.78) | <0.001 |
| Model 1 | Ref | 1.19 (0.95–1.49) | 1.17 (0.93–1.47) | 1.86 (1.50–2.29) | <0.0001 | |
| Model 2 | Ref | 1.11 (0.88–1.41) | 1.08 (0.84–1.38) | 1.45 (1.12–1.88) | 0.02 | |
| CHD | Event, n, (%) | 122 (7.40) | 111 (7.20) | 116 (7.28) | 165 (10.48) | 0.001 |
| Model 1 | Ref | 1.11 (0.85–1.43) | 1.18 (0.91–1.53) | 1.83 (1.44–2.33) | <0.0001 | |
| Model 2 | Ref | 1.04 (0.79–1.37) | 1.11 (0.84–1.47) | 1.44 (1.08–1.94) | 0.05 | |
| Ischaemic stroke | Event, n, (%) | 37 (2.24) | 49 (3.18) | 44 (2.76) | 64 (4.06) | 0.02 |
| Model 1 | Ref | 1.53 (1.00–2.36) | 1.36 (0.87–2.11) | 2.07 (1.37–3.13) | 0.005 | |
| Model 2 | Ref | 1.41 (0.89–2.24) | 1.19 (0.73–1.93) | 1.49 (0.90–2.47) | 0.35 | |
Data are presented as n (%) or HR (95% CI). Models as in Table1. P-trend for linearity of hazard ratio of proportional hazard regression model is calculated based on the results of Wald chi-square test on linearity hypothesis of ordered LDL-TG quartiles.
Similarly, LDL-TG analysed as a continuous variable and adjusted for Model 2 was significantly associated with CHD events (HR 1.74, 95% CI 1.20–2.51) and ASCVD events (HR 1.72, 95% CI 1.25–2.37) but not ischaemic stroke (Table 2). In sensitivity analyses, association of LDL-TG with ASCVD events remained unchanged in individuals with prevalent ASCVD, whereas the association with CHD events was slightly attenuated (Model 2) (Supplementary material online, Table S7).
In restricted cubic spline regression analyses adjusted for Model 1, LDL-TG as a continuous variable showed linear and significant associations with CHD and ASCVD at levels higher than the median value of LDL-TG, but the association was not linear for ischaemic stroke (Figure 2B). A similar relationship was seen for TG, with moderate elevations >150 mg/dL significantly associated with CHD and ASCVD events (Figure 2C).
We assessed the incremental value in ASCVD risk prediction of RLP-C, LDL-TG, and TG by adding each individually to a model containing traditional risk factors along with prevalent ASCVD and use of lipid-lowering medications (Model 2) and determining their impact on AUC, NRI, and IDI. Adding LDL-TG to model 2 significantly increased AUC by 0.002, thereby demonstrating the incremental value of LDL-TG to ASCVD risk prediction. Furthermore, adding LDL-TG to Model 2 significantly improved IDI (i.e. net improvement in the discrimination of the risk prediction model) and resulted in an estimated net improvement in risk reclassification of 5.3% of individuals, although NRI was not statistically significant. Adding RLP-C or TG to Model 2 did not improve risk prediction (Supplementary material online, Table S8).
Association of apoC-III and ANGPTL3 with cardiovascular events
ApoC-III analysed as a continuous variable was significantly associated with ASCVD events (HR 1.24, 95% CI 1.07–1.44), mostly driven by CHD events (HR 1.34, 95% CI 1.13–1.58), when adjusted for Model 1, but the associations were not significant when adjusted for Model 2 (Table 4). In categorical analyses, individuals in the highest quartile of apoC-III had significantly higher risk for CHD but not stroke or ASCVD event in Model 1; the association was not significant when adjusted for Model 2 (Supplementary material online, Table S9).
Table 4.
Associations of apoC-III and ANGPTL3 (natural log transformed) with cardiovascular events
| Model | apoC-III |
ANGPTL3 |
|||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| ASCVD | 1 | 1.24 (1.07–1.44) | 0.004 | 1.33 (0.97–1.83) | 0.08 |
| 2 | 1.10 (0.93–1.29) | 0.26 | 1.63 (1.17–2.28) | 0.004 | |
| CHD | 1 | 1.34 (1.13–1.58) | 0.001 | 1.44 (1.00–2.07) | 0.05 |
| 2 | 1.15 (0.96–1.38) | 0.13 | 1.82 (1.25–2.66) | 0.002 | |
| Ischaemic stroke | 1 | 1.04 (0.79–1.38) | 0.76 | 1.05 (0.58–1.91) | 0.87 |
| 2 | 0.90 (0.67–1.23) | 0.52 | 1.10 (0.58–2.09) | 0.76 | |
Data are presented as n (%) or HR (95% CI). Models as in Table 1.
ANGPTL3 analysed as a continuous variable was significantly associated with CHD (HR 1.82, 95% CI 1.24–2.66) and ASCVD (HR 1.63, 95% CI 1.17–2.28) events even after adjusting for traditional risk factors and lipid-lowering medication use (Model 2) (Table 4). Moreover, in categorical analyses, individuals in the highest quartile of ANGPTL3 had significantly increased risk for CHD and ASCVD events, but not stroke, after adjustment for Model 2 (Supplementary material online, Table S10).
Discussion
Our study adds to accumulating evidence for the role of TG and TGRL in ASCVD risk11,28 including residual risk in statin-treated patients.4 We found that (i) LDL-TG was independently associated with increased risk for CHD and ASCVD events in older adults (mean age 76 ± 5 years) notwithstanding relatively short follow-up of ∼5.5 years; (ii) LDL-TG was an independent predictor of subsequent ASCVD events in older adults with known ASCVD, despite the use of lipid-lowering medication in ∼78% of this subgroup; (iii) LDL-TG level was positively associated with apoC-III and ANGPTL3 levels, whereas RLP-C level was associated with apoC-III but not ANGPTL3 level; and (iv) ANGPTL3 level was predictive of increased risk for CHD and ASCVD events independent of traditional risk factors.
A previous study from ARIC was the first to demonstrate significant associations of LDL-TG levels with incident CHD and stroke in middle-aged adults (mean age 63 ± 5 years) followed up to 16 years.15 Similarly, in the Ludwigshafen Risk and Cardiovascular Health study (mean age 63 ± 10 years), high LDL-TG levels were associated with increased risk for cardiovascular mortality, independent of LDL-C, during ∼10-year follow-up.29 Furthermore, recently published data from two Chinese population studies (mean age 64 ± 8 years and 58 ± 10 years, respectively) showed that LDL-TG levels were predictive of future major adverse cardiovascular events.30,31
Our results support and extend these findings to an older population (mean age 76 ± 5 years) with increased lipid-lowering medication use (∼56% vs. ∼7% in previous ARIC report15). Individuals with higher LDL-TG and RLP-C levels—markers of dysfunctional TG metabolism—had significantly higher cardiovascular event risk; LDL-TG, but not RLP-C, remained significantly associated with CHD and ASCVD events after adjustment for traditional risk factors, and LDL-TG levels predicted subsequent cardiovascular events in individuals with prevalent ASCVD, despite considerable statin use (∼75%) in this subgroup. TGRL may therefore be clinically important biomarkers to identify residual cardiovascular risk in statin-treated older adults, who may benefit from therapies targeting TGRL metabolism. The cholesterol content of remnant lipoproteins is highly atherogenic, and although remnant particles have a relatively high TG content, it is not fully understood whether or to what extent TGs in remnant lipoproteins contribute to ASCVD risk. As in our previous study,15 LDL-TG remained an independent predictor of ASCVD events whereas RLP-C did not. Although earlier studies showed that elevated remnant cholesterol level predicted cardiovascular disease risk independent of other cardiovascular risk factors,14,32 besides differences in study populations, RLP-C was quantified directly using a detergent-based homogenous assay in our study, unlike in these earlier studies. Furthermore, RLP-C did predict ASCVD events in our study, but did not add to future ASCVD risk prediction in multivariate analysis including variables from the Pooled Cohort Equation. Because the Pooled Cohort Equation model includes both total cholesterol and HDL-C (and thus indirectly non-HDL-C), it may not be surprising that RLP-C does not add to ASCVD risk prediction independent of these lipid measures. Indeed, RLP-C was not significantly associated with future ASCVD events when non-HDL-C was added to our multivariate model, whereas LDL-TG remained an independent predictor of ASCVD events (data not shown). The significant independent association of LDL-TG with increased ASCVD risk in our study suggests that LDL-TG may be an important marker of atherogenic altered remnant/LDL metabolism not detected by routine lipid measures. The strong positive correlations of LDL-TG with apoC-III and ANGPTL-3 levels suggest that inhibiting the activity of key enzymes involved in the lipolytic conversion of TGRLs (lipoprotein lipase, endothelial lipase, hepatic lipase) and lipoprotein remodelling (cholesteryl ester transfer protein) may lead to increased LDL-TG levels and increased ASCVD risk. In a secondary analysis of the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) trial,33 LDL-TG failed to predict ASCVD events. Although this study used a detergent-based homogenous assay as in our study, AIM-HIGH was a secondary-prevention trial in 3094 patients on statin therapy randomized to high-dose or low-dose niacin, predominantly white men, with a mean 3-year follow-up. By comparison, the ARIC cohort is larger and biracial, with a longer follow-up of up to 6 years.
Both LDL-TG and RLP-C, but not LDL-C, are related to systemic low-grade inflammation and vascular damage.34,35 In our study, individuals with elevated LDL-TG or RLP-C level also had increased levels of the inflammatory marker high-sensitivity C-reactive protein.
TGRL levels are associated with diabetes, obesity, and metabolic syndrome.29,36,37 We found that LDL-TG and RLP-C had significant associations with TG, and individuals with higher LDL-TG and RLP-C levels were more likely to have increased systolic blood pressure and lower HDL-C levels. RLP-C level was also significantly association with other markers of cardiometabolic risk, including higher body mass index, fasting glucose level, and haemoglobin A1C and lower adiponectin level.
We studied two proteins involved in TG metabolism, ANGPTL3 and apoC-III, as these are targets of novel therapies currently in clinical trials38 and polymorphisms in ANGPTL3 and APOC3 are associated with ASCVD risk.13,17,39 We found positive significant associations of apoC-III with both RLP-C and LDL-TG and of ANGPTL3 with LDL-TG. ApoC-III promotes accumulation of TG and atherogenic remnants by inhibiting both lipolysis via lipoprotein lipase and apoE- and apoB-induced lipoprotein clearance; the latter also promotes formation of dense LDL.40 ANGPTL3 is an endogenous inhibitor of lipoprotein lipase and endothelial lipase, and rare loss-of-function variants in ANGPTL3 are associated with lower TG, HDL-C, and LDL-C levels and decreased risk for ASCVD.41 A recent study in hyperlipidaemic patients showed that ANGPTL3 controls VLDL catabolism upstream of LDL and that ANGPTL3 inhibition lowered LDL-C levels by limiting LDL particle production.42 ANGPTL3-mediated delayed VLDL catabolism and increased LDL-C (due to increased LDL particle production and delayed clearance) may lead to enhanced exchange of TG and cholesteryl esters between VLDL and LDL and correlate with increased levels of LDL-TG as seen in our study.
Associations of circulating apoC-III and ANGPTL3 levels with long-term ASCVD outcomes had previously been reported only in smaller observational studies, mostly limited to patients with CHD.41,43,44 To the best of our knowledge, ours is the largest prospective, population-based cohort study showing significant associations of plasma apoC-III and ANGPTL3 levels with CHD and ASCVD events. Plasma ANGPTL3 level remained an independent predictor of ASCVD risk in models adjusted for traditional risk factors and lipid-lowering medication use.
To provide further data on these novel findings, we analysed apoC-III and ANGPTL3 levels measured at an earlier ARIC visit (visit 3; mean age 57.9 ± 5.1 years), when statins were seldom used, in participants without prevalent ASCVD (n = 10 373; 4.2% on statin). ApoC-III and ANGPTL3 levels were significantly associated with CHD, ischaemic stroke, and ASCVD events in analyses adjusted for age, sex, and race, and ANGPTL3 level remained an independent predictor of ASCVD risk in younger adults without clinical ASCVD even after adjusting for traditional risk factors (data not shown). Taken together, these findings support evidence from prior genetic and observational studies.
Gene-silencing strategies that target APOC3 and ANGPTL3 provide a new avenue to treat hypertriglyceridaemia.38 Our study provides clinical data on the relationships among plasma TGRL, apoC-III, ANGPTL3, and ASCVD risk. RLP-C or apoC-III level did not add risk prediction value beyond traditional risk factors; however, increased LDL-TG and ANGPTL3 may help identify individuals with higher ASCVD risk who might therefore derive greater benefit from therapies targeting apoC-III or ANGPTL3, or other therapies for persistent hypertriglyceridaemia despite statin therapy. A recent double-blind, placebo-controlled, phase 3 trial in patients with homozygous familial hypercholesterolaemia showed that pharmacologic inhibition of ANGPTL3 by evinacumab significantly reduced plasma LDL-C levels independently of LDL-receptor activity, in addition to significant reductions in TG and apoC-III levels.45 Therefore, evinacumab may be clinically useful for patients with a variety of dyslipidaemias resulting from altered TGRL metabolism; future studies should investigate its impact on reductions of LDL-TG and ASCVD risk.
The strengths of this study include the large and well-characterized ARIC population with appropriate follow-up to examine ASCVD incidence. RLP-C and LDL-TG were measured directly using homogenous and validated assays. Limitations of this study include: (i) measuring total plasma apoC-III levels and therefore no information on apoC-III levels in individual lipoproteins (VLDL, LDL, HDL), which could have provided a more precise ASCVD risk estimation; and (ii) despite rigorous measurement of traditional risk factors, possible residual confounding as with any observational study.
In summary, in a large population of older adults (half on statins), LDL-TG and RLP-C were associated with TG, and higher LDL-TG level was associated with CHD and ASCVD risk independent of traditional risk factors. Plasma apoC-III and ANGPTL3 levels were also associated with CHD and ASCVD events. TG-related lipid traits, especially LDL-TG, are important determinants of residual cardiovascular risk in individuals on lipid-lowering therapy and may prove to be important targets for ASCVD risk reduction. Ongoing clinical trials of novel therapies will provide needed information on the role of TGRL in cardiovascular risk.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Data availability
Requests for access to Atherosclerosis Risk In Communities (ARIC) study data should be submitted online at: https://sites.cscc.unc.edu/aric/distribution-agreements (URL verified; last access data: January 4, 2021)
Funding
This work was supported by the National Institutes of Health [contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I; grant numbers K24-DK106414 to E.S., R01-DK089174 to E.S., R01-HL134320 to E.S., C.M.B.]; and Department of Veteran Affairs [Merit Award to V.N.].
Conflict of interest: A.H., C.S., J.C., and X.J. declare that they have no conflict of interest. E.S. has received honoraria from Novo Nordisk. V.N. is site principal investigator for a study sponsored by Merck. C.M.B. has received grants or research support (to his institution) from Abbott Diagnostic, Akcea, Amgen, Esperion, Novartis, Regeneron, and Roche Diagnostic and is a consultant for Abbott Diagnostics, Akcea, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Corvidia, Denka Seiken, Esperion, Intercept, Janssen, Matinas BioPharma Inc., Merck, Novartis, Novo Nordisk, Regeneron, Roche Diagnostic, and Sanofi-Synthelabo. R.C.H. has received research grants (to his institution) from Denka Seiken and is a consultant for Denka Seiken.
References
- 1.Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J.. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 3.Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists' (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 6. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; for the FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 7. Yazdanyar A, Newman AB.. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med 2009;25:563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawler PR, Akinkuolie AO, Chu AY, Shah SH, Kraus WE, Craig D, Padmanabhan L, Glynn RJ, Ridker PM, Chasman DI, Mora S.. Atherogenic lipoprotein determinants of cardiovascular disease and residual risk among individuals with low low-density lipoprotein cholesterol. J Am Heart Assoc 2017;6:e005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maggi FM, Raselli S, Grigore L, Redaelli L, Fantappie S, Catapano AL.. Lipoprotein remnants and endothelial dysfunction in the postprandial phase. J Clin Endocrinol Metab 2004;89:2946–2950. [DOI] [PubMed] [Google Scholar]
- 10. Miller YI, Choi SH, Fang L, Tsimikas S.. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell Biochem 2010;51:229–251. [DOI] [PubMed] [Google Scholar]
- 11. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V.. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007;115:450–458. [DOI] [PubMed] [Google Scholar]
- 12. Varbo A, Freiberg JJ, Nordestgaard BG.. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin Chem 2015;61:533–543. [DOI] [PubMed] [Google Scholar]
- 13.TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG.. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol 2013;61:427–436. [DOI] [PubMed] [Google Scholar]
- 15. Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, Coresh J, Guild CS, Boerwinkle E, Ballantyne CM, Hoogeveen RC.. Remnant-like particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol 2018;72:156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krumholz HM, Seeman TE, Merrill SS, Mendes de Leon CF, Vaccarino V, Silverman DI, Tsukahara R, Ostfeld AM, Berkman LF.. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA 1994;272:1335–1340. [PubMed] [Google Scholar]
- 17. Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Habegger L, Lopez A, Penn J, Zhao A, Shao W, Stahl N, Murphy AJ, Hamon S, Bouzelmat A, Zhang R, Shumel B, Pordy R, Gipe D, Herman GA, Sheu WHH, Lee I-T, Liang K-W, Guo X, Rotter JI, Chen Y-DI, Kraus WE, Shah SH, Damrauer S, Small A, Rader DJ, Wulff AB, Nordestgaard BG, Tybjærg-Hansen A, van den Hoek AM, Princen HMG, Ledbetter DH, Carey DJ, Overton JD, Reid JG, Sasiela WJ, Banerjee P, Shuldiner AR, Borecki IB, Teslovich TM, Yancopoulos GD, Mellis SJ, Gromada J, Baras A.. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med 2017;377:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S.. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–232. [DOI] [PubMed] [Google Scholar]
- 19. Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, Yang Q, Hughes SG, Geary RS, Arca M, Stroes ESG, Bergeron J, Soran H, Civeira F, Hemphill L, Tsimikas S, Blom DJ, O’Dea L, Bruckert E.. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med 2019;381:531–542. [DOI] [PubMed] [Google Scholar]
- 20.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 21. Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE.. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb 1994;14:1098–1104. [DOI] [PubMed] [Google Scholar]
- 22. Hirao Y, Nakajima K, Machida T, Murakami M, Ito Y.. Development of a novel homogeneous assay for remnant lipoprotein particle cholesterol. J Appl Lab Med 2018;3:26–36. [DOI] [PubMed] [Google Scholar]
- 23. Ito Y, Ohta M, Ikezaki H, Hirao Y, Machida A, Schaefer EJ, Furusyo N.. Development and population results of a fully automated homogeneous assay for LDL triglyceride. J Appl Lab Med 2018;2:746–756. [DOI] [PubMed] [Google Scholar]
- 24. Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, Sterling DG, Williams SA.. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA 2016;315:2532–2541. [DOI] [PubMed] [Google Scholar]
- 25. Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang CH, Heiss G.. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med 1998;339:861–867. [DOI] [PubMed] [Google Scholar]
- 26. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E.. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, Zhang MJ, Fine J.. A proportional hazards regression model for the subdistribution with right-censored and left-truncated competing risks data. Stat Med 2011;30:1933–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A.. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 29. Silbernagel G, Scharnagl H, Kleber ME, Delgado G, Stojakovic T, Laaksonen R, Erdmann J, Rankinen T, Bouchard C, Landmesser U, Schunkert H, Marz W, Grammer TB.. LDL triglycerides, hepatic lipase activity, and coronary artery disease: an epidemiologic and Mendelian randomization study. Atherosclerosis 2019;282:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ding XH, Ye P, Wang XN, Cao RH, Yang X, Xiao WK, Zhang Y, Bai YY, Wu HM.. The predictive value of baseline LDL-TG level on major adverse cardiovascular events in a followed up cohort population. Eur Rev Med Pharmacol Sci 2017;21:1060–1064. [PubMed] [Google Scholar]
- 31. Jin JL, Zhang HW, Cao YX, Liu HH, Hua Q, Li YF, Zhang Y, Guo YL, Wu NQ, Zhu CG, Xu RX, Gao Y, Li XL, Cui CJ, Liu G, Sun J, Dong Q, Santos R, Li JJ.. Long-term prognostic utility of low-density lipoprotein (LDL) triglyceride in real-world patients with coronary artery disease and diabetes or prediabetes. Cardiovasc Diabetol 2020;19:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joshi PH, Khokhar AA, Massaro JM, Lirette ST, Griswold ME, Martin SS, Blaha MJ, Kulkarni KR, Correa A, D'Agostino RB Sr, Jones SR, Toth PP; Lipoprotein Investigators Collaborative Study Group. Remnant lipoprotein cholesterol and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. J Am Heart Assoc 2016;5:e002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Albers JJ, Slee A, O'Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO Jr, Xu P, Marcovina SM.. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol 2013;62:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. MäRz W, Scharnagl H, Winkler K, Tiran A, Nauck M, Boehm BO, Winkelmann BR.. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health study. Circulation 2004;110:3068–3074. [DOI] [PubMed] [Google Scholar]
- 35. Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG.. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013;128:1298–1309. [DOI] [PubMed] [Google Scholar]
- 36. Despres JP, Moorjani S, Tremblay A, Ferland M, Lupien PJ, Nadeau A, Bouchard C.. Relation of high plasma triglyceride levels associated with obesity and regional adipose tissue distribution to plasma lipoprotein-lipid composition in premenopausal women. Clin Invest Med 1989;12:374–380. [PubMed] [Google Scholar]
- 37. Satoh A, Adachi H, Tsuruta M, Hirai Y, Hiratsuka A, Enomoto M, Furuki K, Hino A, Takeuchi T, Imaizumi T.. High plasma level of remnant-like particle cholesterol in the metabolic syndrome. Diabetes Care 2005;28:2514–2518. [DOI] [PubMed] [Google Scholar]
- 38. Nordestgaard BG, Nicholls SJ, Langsted A, Ray KK, Tybjærg-Hansen A.. Advances in lipid-lowering therapy through gene-silencing technologies. Nat Rev Cardiol 2018;15:261–272. [DOI] [PubMed] [Google Scholar]
- 39. Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O'Connell JR, Shuldiner AR.. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 2008;322:1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng C, Khoo C, Furtado J, Sacks FM.. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation 2010;121:1722–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, Natarajan P, Klarin D, Emdin CA, Zekavat SM, Nomura A, Erdmann J, Schunkert H, Samani NJ, Kraus WE, Shah SH, Yu B, Boerwinkle E, Rader DJ, Gupta N, Frossard PM, Rasheed A, Danesh J, Lander ES, Gabriel S, Saleheen D, Musunuru K, Kathiresan S; for the PROMIS and Myocardial Infarction Genetics Consortium Investigators. ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol 2017;69:2054–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adam RC, Mintah IJ, Alexa-Braun CA, Shihanian LM, Lee JS, Banerjee P, Hamon SC, Kim HI, Cohen JC, Hobbs HH, Van Hout C, Gromada J, Murphy AJ, Yancopoulos GD, Sleeman MW, Gusarova V.. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J Lipid Res 2020;61:1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wyler von Ballmoos MC, Haring B, Sacks FM.. The risk of cardiovascular events with increased apolipoprotein CIII: a systematic review and meta-analysis. J Clin Lipidol 2015;9:498–510. [DOI] [PubMed] [Google Scholar]
- 44. Chen MC, Hsu BG, Lee CJ, Wang JH.. High-serum angiopoietin-like protein 3 levels associated with cardiovascular outcome in patients with coronary artery disease. Int J Hypertens 2020;2020:2980954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, Ali S, Banerjee P, Chan KC, Gipe DA, Khilla N, Pordy R, Weinreich DM, Yancopoulos GD, Zhang Y, Gaudet D; for the ELIPSE HoFH Investigators. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med 2020;383:711–720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to Atherosclerosis Risk In Communities (ARIC) study data should be submitted online at: https://sites.cscc.unc.edu/aric/distribution-agreements (URL verified; last access data: January 4, 2021)