Abstract
The purpose of this study was to identify the mechanisms underlying effects of coffee on cognition in the context of brain networks. Here we investigated functional connectivity before and after drinking coffee using graph-theoretic analysis of electroencephalography (EEG). Twenty-one healthy adults voluntarily participated in this study. The resting-state EEG data and results of neuropsychological tests were consecutively acquired before and 30 min after coffee consumption. Graph analyses were performed and compared before and after coffee consumption. Correlation analyses were conducted to assess the relationship between changes in graph measures and those in cognitive function tests. Functional connectivity (FC) was reorganized toward more efficient network properties after coffee consumption. Performance in Digit Span tests and Trail Making Test Part B improved after coffee consumption, and the improved performance in executive function was correlated with changes in graph measures, reflecting a shift toward efficient network properties. The beneficial effects of coffee on cognitive function might be attributed to the reorganization of FC toward more efficient network properties. Based on our findings, the patterns of network reorganization could be used as quantitative markers to elucidate the mechanisms underlying the beneficial effects of coffee on cognition, especially executive function.
Subject terms: Cognitive neuroscience, Computational neuroscience
Introduction
Coffee is a widely used caffeinated beverage (International Coffee Organization, http://www.ico.org/prices/new-consumption-table.pdf.), with more than 165 million 60-kg bags consumed globally per year1. Potential beneficial health effects of coffee consumption have been reported, including prevention of cancer, cardiovascular disorders, diabetes, and Parkinson's disease2. Furthermore, given the expectation that coffee increases alertness and enhances psychomotor functioning, many people seek coffee to counteract fatigue, stay alert by warding off sleepiness, increase cognitive performance, and increase work efficiency3.
The stimulatory effects of coffee are mainly attributed to caffeine’s roles in antagonizing adenosine A1 and A2A receptors, leading to disinhibition of excitatory neurotransmitter release and enhancement of dopamine transmission via D2 receptor, respectively4. Although it is agreed that the acute effects of caffeine are due to its action as a central stimulant, inconsistent findings have been reported regarding the effects of coffee on higher cognitive functions, including working memory and executive functioning5. Some studies have shown beneficial effects of caffeine on cognitive functioning, including reaction times to cognitive tasks6, attention7, working memory8, and executive control9,10, whereas others reported no change after the use of caffeine11,12. Moreover, the expectancy of the stimulant effects of caffeine itself may have a role in the cognitive responses to caffeine12. Therefore, whether the beneficial effects of caffeine are derived from the direct enhancement of specific cognitive functions is unclear.
Integration of neural activities between different brain regions is required for physiological brain functioning. Therefore, analyzing functional connectivity (FC) between brain regions may provide more information than investigating activities of individual brain regions13,14. While most of the previous studies have evaluated the cognitive effects of caffeine mainly based on the results of neurocognitive tests, such as the Stroop test5, little attention has been paid to investigating the effects of caffeine on neurocognitive function in the context of FC. Recent advances in graph-theoretic network analysis allow for the assessment of important information regarding the topological architecture of complex human brain networks15,16. Therefore, graph-theoretic analysis could be an optimal framework for quantitatively characterizing network properties after coffee consumption and determining its effects on cognition.
To the best of our knowledge, no graph-theoretic analysis has applied electroencephalography (EEG) data to explore the effects of caffeine on FC. Here, we compared the properties of FC before and after coffee consumption to analyze the acute effects of caffeine on the brain network and its impact on neurocognitive function using graph-theoretic analysis of EEG data. We hypothesized that caffeine might improve neurocognitive function by shifting the FC of the brain to a more efficient state.
Methods and materials
Participants
Twenty-one healthy volunteers (11 women; 31.4 ± 3.9 years; 17.0 ± 1.4 years of education) who had no neurologic, psychiatric, chronic systemic disorders, or medical conditions that could affect the EEG results were included in this study. All participants were requested to abstain from drinking beverages containing caffeine and from the use of any psychoactive substances or medication for at least 24 h prior to the EEG and neurocognitive studies17. All subjects were fully informed of the nature and possible risks of this study. Written informed consent was obtained from all subjects prior to study enrollment. The study followed the ethical guidelines of the Declaration of Helsinki and was approved by the local ethics committee at Korea University Anam Hospital (No. 2019AN0418).
Neurocognitive function tests and caffeine intake
Global neurocognitive function was assessed using the Mini-Mental State Examination (MMSE) at least 24 h after cessation of coffee consumption in all participants. The neuropsychological tests were selected to evaluate the acute effects of caffeine on performance in multiple neurocognitive domains. The assessed domains and the tests were as follows: (1) attention and working memory—Digit Span Forward (up to nine digits) and Backward (up to eight digits) tests18, Target Detection Task using tapping; (2) executive function—Trail Making Test Part B (time to complete the tests)19; and (3) memory—Short-term memory recall task (two learning trials of five words) and delayed recall (after 5 min). All neuropsychological tests were performed at baseline EEG recording and 30 min after consumption of canned coffee19 using the same tests with a different set of contents. A commercial canned coffee (Let’s Be, Lotte Chilsung Beverage), which has the largest market share in Korea, was used for caffeine intake. One can of the canned coffee contains 160 mL and 67 mg of caffeine.
Electroencephalography recording
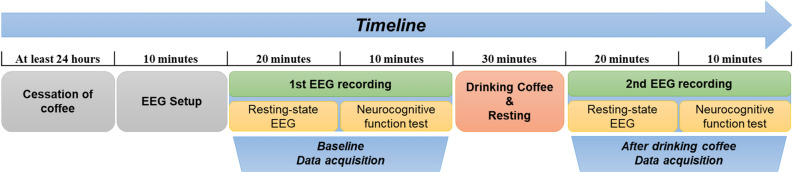
The EEG examination was performed twice, once at baseline and then again 30 min after the participants drank the canned coffee treatment20, using a 32-channel recording system (Comet-PLUS, Grass Technologies Inc., West Warwick, RI, USA) with electrodes placed according to the international 10–20 system. The EEG was recorded for 1 h in the waking-relaxed and eyes-closed conditions. EEG data were sampled at 200 Hz, and the bandpass filter was set between 0.1 and 70 Hz. A diagram of the study protocol is presented in Fig. 1.
Figure 1.
A diagram of the study protocol. The study protocol according to the timeline is presented schematically.
Since the neuropsychological tests evaluating different domains were performed in succession, resting-state EEG data were used for analysis to avoid mixed effects of different domain-specific functional networks in this study. Ten non-consecutive resting-state 2-s epochs for each participant were carefully reviewed and selected by two board-certified neurologists according to the following protocol: (1) presence of continuous physiological alpha activity with voltage maximum in posterior regions; (2) absence of artifacts, epileptiform discharges, and other nonstationary elements; and (3) absence of patterns indicating drowsiness or arousal.
Graph-theoretic and statistical analyses
Resting-state FC was evaluated by coherence, which reflects the level of functional signal communication between different regions of the brain21. The coherence is defined as
where Sxy(f) is the cross-spectral density between x and y, and Sxx(f) and Syy(f) are the auto-spectral densities of x and y, respectively. K represents the coherency function. |S| denotes the modulus of S. The coherence value ranges between 0 and 1 with 0 denoting no statistical relationship and 1 being full coherence21. In addition, the phase lag index (PLI) was used to measure phase synchronization between all pairs of 19 EEG channels22,23. The PLI is defined as PLI = |< sign[Δφ(tk)] >|, where Δφ(tk) is the phase difference of time series tk (k = 1, …, N). It ranges between 0 and 1—0 indicates either no coupling or phase difference centered around 0 mod π, while 1 indicates perfect phase synchrony as a value of Δφ different from 0 mod π. Epochs were then bandpass filtered into the following frequency bands: delta (0.1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–50 Hz). Subsequent analyses were performed separately for each band. Network properties were characterized using a weighted undirected network model of graph-theoretic analysis in order to avoid the arbitrariness of threshold selection for producing an adjacency matrix and to preserve the continuous nature of the correlated information24. Graph measures (average degree, average strength, radius, diameter, characteristic path length, global and local efficiency, clustering coefficient, transitivity, modularity, assortativity, and small-worldness) were computed using the Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net) and BRAPH toolbox (http://braph.org) working on MATLAB R2019b (MathWorks, Natick, MA, USA)24,25. PLI analysis and visualization were performed using tailored Python scripts and the MNE-Python package (version 0.22.0)26.
Graph measures were compared before and after consumption of canned coffee using non-parametric tests with 1,000 permutations. Statistical significance was set at P < 0.05 and corrected for multiple comparisons using false discovery rate (FDR). Differences in the results of neuropsychological tests before and after consumption of canned coffee were compared using paired t-test. Since the ranges of the graph measures’ values differed from each other, the degree of change was normalized, and then the correlation between the score changes of Trail Making Test Part B and the graph measures was analyzed using the normalized changed values (Pearson’s correlation, P < 0.05). The purpose of the statistical tests is to determine whether each of the graph measures is correlated with the score change of Trail Making Test Part B. Accordingly, the statistical test was performed independently for each graph measure with respect to the neuropsychological test.
Results
Neuropsychological tests
The results of the neuropsychological tests are detailed in Table 1. All participants had an MMSE score of 30. Performance in the Digit Span Forward (8.5 ± 0.8 digits vs. 8.9 ± 0.2 digits, P = 0.025) and Backward tests (6.2 ± 1.8 digits vs. 7.3 ± 1.1 digits, P = 0.001) improved after coffee consumption relative to baseline. There were no errors in the target detection task using the tapping test, Trail Making Test Part B, and short-term and delayed memory recall tests before and after coffee consumption. Compared to baseline, performance in the Trail Making Test Part B improved after coffee consumption (5.8 ± 1.4 s vs. 4.9 ± 1.2 s, P = 0.002). Individual changes in performance in the Trail Making Test Part B after coffee drinking are presented in Fig. 2.
Table 1.
Results of neuropsychological tests.
| Baseline | After coffee consumption | P value | |
|---|---|---|---|
| <Global function> | |||
| Mini-mental status examination (correct/30 items) | 30 | 30 | – |
| <Attention and working memory> | |||
| Digit Span Test forward (correct/9 digits) | 8.52 ± 0.75 | 8.95 ± 0.22 | 0.025 |
| Digit Span Test backward (correct/8 digits) | 6.24 ± 1.76 | 7.29 ± 1.10 | 0.001 |
| Target Detection Task (correct/11 syllable targets) | 11 | 11 | – |
| <Executive function> | |||
| Trail Making Test Part B (s) | 5.80 ± 1.41 | 4.87 ± 1.17 | 0.002 |
| <Memory> | |||
| Short-term memory recall (correct/5 words) | 5 | 5 | – |
| Delayed recall (correct/5 words) | 4.57 ± 0.68 | 4.81 ± 0.40 | 0.135 |
Values represent mean ± standard deviation.
Figure 2.
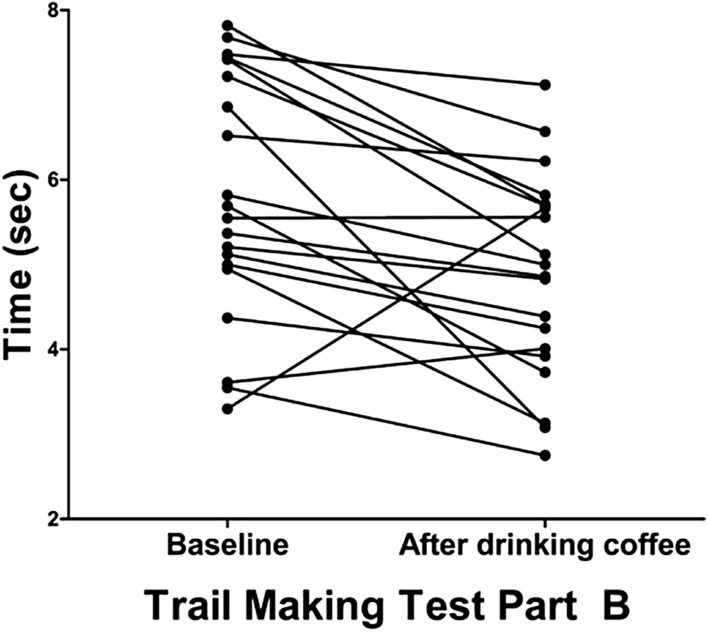
Acute effects of coffee consumption on executive function. Individual changes in time (s) to complete Trail Making Test Part B are presented.
Graph-theoretic analyses
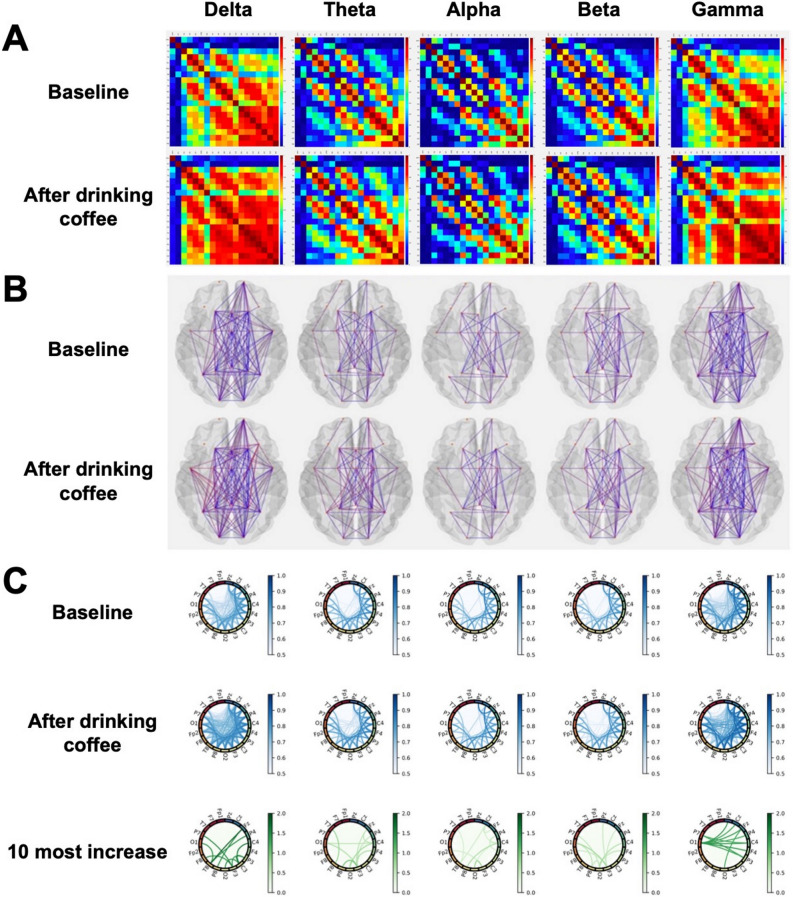
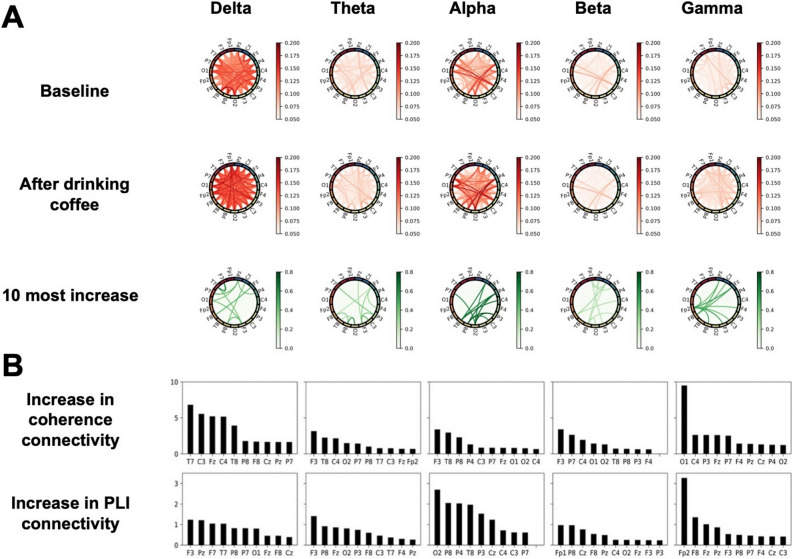
FC in terms of coherence is represented with adjacent matrices, connectivity circles, and brain topologies in Fig. 3A–C. PLIs are presented using the connectivity circles in Fig. 4A. FC was enhanced in all frequency bands for both methods, and similar patterns were found—the delta and gamma bands exhibited relatively large increases in both coherence (Fig. 3C) and PLI (Fig. 4A); for coherence, the theta, alpha, and beta bands increase less than those of delta and gamma; the alpha band also largely increased for PLI. The ten most highly increased connectivities are displayed in Fig. 4B; this indicates the relevant brain regions responsible for the increase of FC. Comparisons of global graph measures between the conditions are detailed in Table 2.
Figure 3.
Coherence averaged across all subjects. (A) The plots show the coherence between 19 pairs of scalp electroencephalography electrodes in each frequency band at baseline (upper) and after coffee consumption (low). (B) Brain topologies of functional connectivity at baseline (upper) and after coffee consumption (low) are presented. (C) Each column corresponds to a frequency band as indicated on the top of the figure. Coherence matrices corresponding to the resting-state EEG before and after coffee consumption are plotted on the first and second rows, respectively; to avoid confusion from too many lines, those of coherence less than 0.5 are not shown. The third row displays the top 10 most increased lines.
Figure 4.
Phase lag index (PLI) averaged across all subjects and ten most highly increased nodes in each band. (A) Each column corresponds to a frequency band as indicated on the top of the figure. PLI matrices corresponding to the resting-state EEG before and after coffee consumption are plotted on the first and second rows, respectively; lines that have PLI values under 0.05 are not displayed. The third row plots the top 10 most increased lines. (B) The ten channels (nodes) with the highest increase in connectivity in terms of coherence (top row) and PLI (bottom row) are shown for each frequency band (corresponding to each column). Node connectivity was determined by taking the ten most highly increased links after coffee consumption from the averaged functional connectivity matrix (as in the third rows of Figs. 3C and A) and summing the weights of those edges connected to each node.
Table 2.
Comparisons of global graph measures between baseline and after coffee consumption.
| Graph measures | Baseline | Coffee | P value | Baseline | Coffee | P value | Baseline | Coffee | P value | Baseline | Coffee | P value | Baseline | Coffee | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta | Theta | Alpha | Beta | Gamma | |||||||||||
| Average degree | 13.920 | 15.058 | 0.001 | 12.005 | 12.988 | 0.013 | 10.782 | 11.378 | 0.043 | 12.145 | 13.033 | 0.027 | 14.852 | 15.649 | 0.061 |
| Average strength | 9.253 | 11.333 | 0.001 | 6.175 | 7.338 | 0.010 | 5.037 | 5.434 | 0.021 | 5.924 | 6.376 | 0.125 | 9.453 | 10.685 | 0.012 |
| Radius | 5.885 | 6.120 | 0.810 | 13.820 | 6.191 | 0.923 | 5.481 | 5.760 | 0.218 | 7.162 | 5.194 | 0.112 | 4.414 | 4.336 | 0.915 |
| Diameter | 10.702 | 9.697 | 0.586 | 17.776 | 10.809 | 0.948 | 9.598 | 9.773 | 0.720 | 11.061 | 9.066 | 0.459 | 7.952 | 7.544 | 0.510 |
| Characteristic path length | 2.799 | 2.377 | 0.218 | 4.071 | 3.056 | 0.183 | 3.520 | 3.377 | 0.228 | 3.598 | 3.116 | 0.054 | 2.449 | 2.182 | 0.066 |
| Global efficiency | 0.568 | 0.659 | 0.004 | 0.444 | 0.488 | 0.015 | 0.397 | 0.412 | 0.054 | 0.428 | 0.445 | 0.108 | 0.580 | 0.640 | 0.029 |
| Local efficiency | 1.718 | 2.255 | 0.001 | 1.057 | 1.267 | 0.019 | 0.826 | 0.884 | 0.026 | 0.945 | 1.004 | 0.208 | 1.696 | 1.958 | 0.049 |
| Clustering coefficient | 0.560 | 0.670 | 0.002 | 0.380 | 0.439 | 0.035 | 0.328 | 0.341 | 0.197 | 0.357 | 0.372 | 0.277 | 0.542 | 0.605 | 0.052 |
| Transitivity | 0.913 | 1.083 | 0.002 | 0.595 | 0.699 | 0.022 | 0.500 | 0.525 | 0.140 | 0.554 | 0.581 | 0.208 | 0.858 | 0.951 | 0.046 |
| Modularity | 0.063 | 0.026 | 0.010 | 0.188 | 0.137 | 0.001 | 0.258 | 0.241 | 0.329 | 0.195 | 0.171 | 0.101 | 0.070 | 0.036 | 0.011 |
| Assortativity | 0.111 | 0.050 | 0.188 | 0.160 | 0.077 | 0.105 | 0.156 | 0.123 | 0.223 | 0.122 | 0.080 | 0.116 | 0.025 | 0.019 | 0.202 |
| Small-worldness | 1.042 | 1.233 | 0.612 | 0.855 | 1.250 | 0.289 | 0.891 | 1.054 | 0.460 | 0.868 | 0.945 | 0.364 | 0.927 | 0.901 | 0.166 |
Values represent the mean.
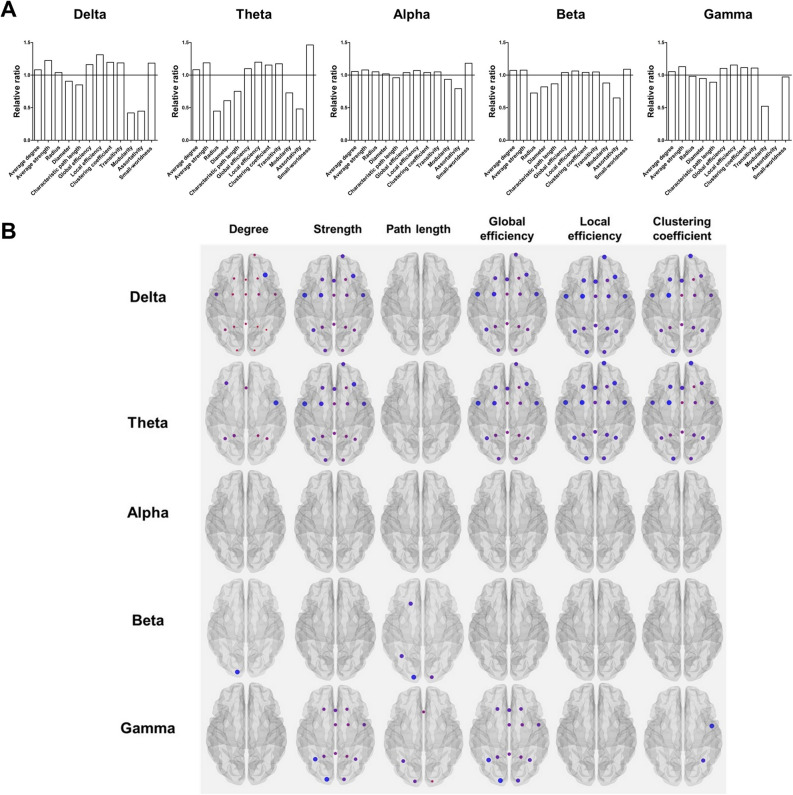
Compared to baseline, the relative ratio of global graph measures after coffee consumption is presented in Fig. 5A. Average degree (except in gamma band), average strength (except in beta band), global efficiency (except in alpha and beta bands), and local efficiency (except in beta band) increased after coffee consumption relative to baseline in most frequency bands (FDR-corrected P < 0.05). The clustering coefficient in delta and theta bands, as well as transitivity in the delta, theta, and gamma bands, increased after coffee consumption relative to baseline (FDR-corrected P < 0.05). Compared to baseline, modularity in the delta, theta, and gamma bands decreased after coffee consumption (FDR-corrected P < 0.05). Significant differences in the nodal measures (degree, strength, global efficiency, local efficiency, and clustering coefficient) between the conditions were prominent mainly in the fronto-centro-parietal regions, especially in the delta and theta bands (Fig. 5B).
Figure 5.
Results of graph-theoretic analyses and correlation analysis. (A) By setting the values of graph measures at baseline to 1.0 (shown as a line at the value of 1.0), the relative ratio of global graph measures after coffee consumption are presented. (B) Changes after coffee consumption in the nodal measures of graph-theoretic analyses are presented (FDR-corrected P < 0.05). Larger nodes indicate greater differences between the conditions. The regions showing higher significance of differences are colored red, whereas the regions showing lower significance of difference are colored blue.
Correlation analyses
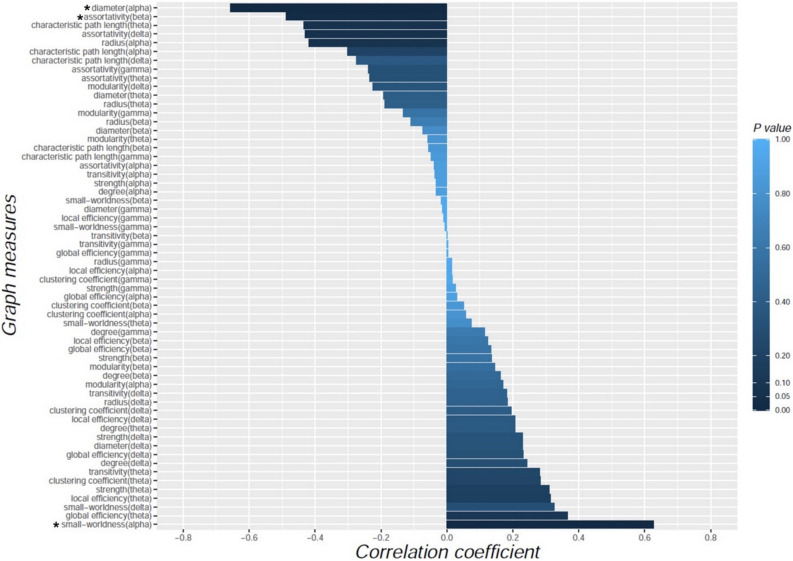
The degree of improved performance in Trail Making Test Part B after coffee consumption was negatively correlated with diameter in the alpha band (r = ‒ 0.657, P = 0.002) and assortativity in the beta band (r = ‒ 0.488, P = 0.029), whereas it was positively correlated with small-worldness in the alpha band (r = 0.627, P = 0.004; Fig. 6). There was no relationship between the results of other neuropsychological tests and changes in graph measures.
Figure 6.
Correlations between the changes in global graph measures and the degree of improved performance on Trail Making Test Part B after coffee consumption. The horizontal bars represent the values of correlation coefficient. The color bar represents statistical significance (P value). Asterisks (*) represent statistical significance (P < 0.05).
Discussion
We investigated the acute effects of caffeine on neurocognition and EEG FC in healthy adults. The major findings were as follows: (1) the property of EEG FC was reorganized toward a more efficient network after coffee consumption relative to baseline, (2) Performance in the Digit Span tests and Trail Making Test Part B was improved after coffee consumption, and (3) improved performance in the Trail Making Test Part B after coffee consumption was correlated with changes in graph measures reflecting a shift toward efficient network property.
The human brain is considered to be a large-scale complex network and has properties of efficient small-world networks that refer to locally well-connected clusters and efficient global connections24,27. The properties of small-world networks are known to enable higher rates of information processing and learning with a lower cost than those of random networks28. In terms of these network properties, changes in cognitive functional status or cognitive capacity might be associated with changes in the configuration of brain functional networks27. Indeed, there are several lines of evidence suggesting that loss of the small-world configuration might be implicated in the cognitive deficits observed in various brain disorders, such as Alzheimer’s disease, schizophrenia, and brain tumors29–31. Based on the aforementioned notion, our findings of changes in graph measures to high clustering and short path length after coffee consumption suggest that functional reorganization toward more efficient network properties might be a mechanism underlying the enhancement of cognitive function observed after coffee consumption.
The mechanism underlying the shift in FC toward efficient network properties after coffee consumption remains to be determined. It is believed that caffeine’s effect on cognition is associated with the blockade of the inhibitory properties of endogenous adenosine (particularly at A1 and A2A receptors), resulting in increased dopamine, norepinephrine, and glutamate release4. In addition, the cardiostimulatory effects of caffeine are considered to result from interactions with both adenosine and phosphodiesterase32. The caffeine-induced increases in dopamine and glutamate concentrations, coupled with phosphodiesterase inhibition, could be considered as a crucial mechanism underlying the net increase in the central nervous system and cardiovascular activity. Based on the actions of caffeine, it is plausible that the stimulatory effects of caffeine might directly lead to the reorganization of network properties toward a state of increased efficiency. A recent fMRI study showed that habitual coffee drinkers had distinct brain FC properties from non-coffee drinkers, which could support our speculation33. Further studies are needed to unveil the mechanisms underlying the changes in network properties after coffee consumption.
Our findings of improved performance in the Digit Span Forward test suggest that attentional function could be enhanced by coffee consumption, which is in line with previous observations that coffee consumption has beneficial effects on attention7,34–36. In addition, our findings of greater performance in the Digit Span Backward test18 after coffee consumption may support findings from previous studies that have shown the role of coffee in improving working memory37–39. A recent functional magnetic resonance imaging (fMRI) study found that the alerting network, known as being responsible for maintaining an alert state throughout task performance, recruited a distributed network of brain regions, primarily the thalamus and bilateral fronto-parietal regions40,41. Based on these fMRI findings, our results that FC changes after coffee consumption are mainly observed in the fronto-centro-parietal regions imply that improvement of attentional function might be derived from activation of the alerting network.
We also found that performance in the Trail Making Test Part B was improved after coffee consumption and that the degree of improvement of the test was correlated with the changes in graph measures reflecting a shift toward more efficient network properties. It is well known that the Trail Making Test Part B is a representative tool for evaluating the ability of executive function responsible for psychomotor speed, visuospatial searching, target-directed motor tracking, and set-shifting42. Therefore, our findings further support previous studies that showed the beneficial effects of caffeine on executive function and psychomotor speed4,5,43. Performance of executive controls requires activation of widespread prefrontal regions in concert with the anterior cingulate cortex4,44,45. These brain areas have been shown to be upregulated by caffeine39,46, supporting the stimulatory effects of caffeine on executive function. Moreover, dopamine was found to be a critical neurotransmitter for supporting executive function in these areas47. Given that dopamine concentrations can be increased by caffeine through blockade of the inhibitory properties of adenosine, caffeine may enhance executive function through the interaction of dopaminergic pathways with anterior cingulate and prefrontal cortical regions.
Our findings of the relationship between improved executive function and graph measures suggest that changing network topology toward more efficient network properties might be a crucial mechanism underlying the beneficial effects of coffee on executive function. Our speculation is supported by prior studies using fMRI that found increases in FC in multiple brain regions during the performance of the Trail Making Test Part B47–51. In addition, the aforementioned relationships were mainly observed in the alpha band, which is in accordance with a recent study showing that executive functions have a positive relationship with alpha coherence between regions of the right and left hemispheres52. Taken together, our findings support those of previous studies that coffee may enhance the FC responsible for performance on executive function, especially in the alpha band. Meanwhile, we did not find any changes in nodal graph measures after coffee consumption in the alpha band. The changes in global network properties without any region-specific changes in the alpha band suggest that coffee consumption might further enhance the improvement of physiological network efficiency responsible for activating cognitive function across the whole brain, rather than causing changes in the network properties of specific localized areas. Given the involvement of the dopaminergic pathways in executive function47, another plausible explanation is that our findings of changes in cortico-cortical network properties may not fully reflect the interactions of subcortical dopaminergic pathways with the cortical areas responsible for executive function.
We did not find any relationship between performance in Digit Span tests and graph measures. It is not fully understood why the results of the Digit Span tests, which reflect the function of attention/working memory, were not correlated with graph measures. However, it is plausible that there was a ceiling effect in the performance of the Digit Span tests in our cognitively normal population. In addition, given that attention/working memory is associated with not only cortical function but also various subcortical neurotransmitter systems (e.g., basal forebrain cholinergic systems and dopaminergic systems), a FC analysis evaluating the cortico-cortical network using EEG might not be sufficient to reveal the mechanism underlying the attention/working memory function.
There are several limitations of the present study that should be considered when interpreting our results. First, our study population was relatively small, and was only composed of highly educated young adults. Therefore, our results could not be generalized to the overall population, especially to the elderly. Second, we did not measure individual differences in biological susceptibility to caffeine or expectancy for coffee drinking to stimulate cognitive function12. Further studies incorporating measurements of caffeine blood level and investigation of a subjective expectation of coffee drinking as a cognitive enhancer may clarify the dose–response relationship and main contributor of the FC changes. Third, the results of the neuropsychological tests after coffee consumption may be biased due to learning effects. However, learning effects were likely mitigated by the use of different sets of contents in the repetition of the same tests. Finally, since canned coffee contains various ingredients other than caffeine, it is unclear whether our results were due to the effect of caffeine or the combined effects with other ingredients. Nevertheless, our study is the first EEG network analysis investigating the effects of canned coffee, containing a precisely controlled content of caffeine, on neurocognitive function.
The strength of our study is that FC was evaluated using two methods, coherence and PLI, which were compared to mitigate the limitations of scalp-level EEG analysis. We used two representative building blocks for characterizing brain FC in sensor space, coherence, and PLI, and obtained consistent results. Coherence is the most common method used to quantify the correlation between signals from different brain regions in terms of both amplitude and phase. In contrast, PLI measures the stability of the phase differences of short- and long-range neuronal activities over time independent of the amplitude of oscillations. This method is designed to reliably estimate phase synchronization against the presence of common sources such as volume conduction and active reference electrodes. In brief, it can be accomplished by discarding 0 and π phase differences between two time series22.
While not reported with the results, we performed classification of functional connectivities before and after coffee consumption using machine learning/deep learning algorithms. Eight global graph-theoretic measures of the PLI networks were used for classification using 70% and 30% training-test data split. We used supervised machine learning methods including support vector machine (SVM), k-nearest neighbor (kNN), decision tree, naïve Bayes, linear discriminant analysis (LDA), and logistic regression. Among the algorithms tested, the kNN (k = 4) exhibited the highest classification accuracy of 63.7%. This limited accuracy obtained may be because those machine learning techniques do not properly reflect geometric information based on channel locations. Observing the changes in functional connectivity between specific channels (Figs. 2 and 3) may be more informative for assessing the effects of drinking coffee. We note that several methods have been used to distinguish the EEG of patients with depression from control53–56. In particular, some methods had been proposed that detect depression with good accuracy using three-electrode EEG devices54,55. This suggests that performing channel selection with methods such as kernel-target alignment56 may provide additional insights into coffee consumption by identifying the key channels. We leave this to a future study.
Conclusion
Our results support the general belief and previous notion that coffee improves cognitive function. Moreover, our findings suggest that the beneficial effects of coffee might be attributed to reorganization of FC toward more efficient network properties. Our findings of changes in network properties may provide novel insights into the biological mechanisms underlying the beneficial effects of coffee on cognitive function. Furthermore, the patterns of network reorganization could be quantitative markers for elucidating the mechanisms underlying the beneficial effects of coffee on cognition, especially executive function.
Acknowledgements
This work was supported by grants of Korea University College of Medicine (JBK, K1922861, K2022991) and Korea Institute of Science and Technology (KIST) Institutional program with projects (KH, 2E30762, 2K02430). We would like to thank the participants themselves, all of whom contributed greatly to the successful completion of this study.
Author contributions
Conceptualization: H.K., J.B.K.; Formal Analysis: S.H.K. (Soon Ho), K.H., J.B.K.; Data curation: S.H.K. (Sung Hoon), S.H.K. (Seong Hwan), J.H., and J.G.K.; Writing—original draft preparation: H.K., K.H., J.B.K.; Writing—review and editing: H.K., J.B.K.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kyungreem Han, Email: khan@kist.re.kr.
Jung Bin Kim, Email: kjbin80@korea.ac.kr.
References
- 1.Butt MS, Sultan MT. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2011;51:363–373. doi: 10.1080/10408390903586412. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig IA, Clifford MN, Lean ME, Ashihara H, Crozier A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014;5:1695–1717. doi: 10.1039/c4fo00042k. [DOI] [PubMed] [Google Scholar]
- 3.Franke AG, Bagusat C, Rust S, Engel A, Lieb K. Substances used and prevalence rates of pharmacological cognitive enhancement among healthy subjects. Eur. Arch. Psychiatry Clin. Neurosci. 2014;264(Suppl 1):S83–90. doi: 10.1007/s00406-014-0537-1. [DOI] [PubMed] [Google Scholar]
- 4.Lorist MM, Tops M. Caffeine, fatigue, and cognition. Brain Cogn. 2003;53:82–94. doi: 10.1016/s0278-2626(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 5.McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine's effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016;71:294–312. doi: 10.1016/j.neubiorev.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Souissi Y, Souissi M, Chtourou H. Effects of caffeine ingestion on the diurnal variation of cognitive and repeated high-intensity performances. Pharmacol. Biochem. Behav. 2019;177:69–74. doi: 10.1016/j.pbb.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Einother SJ, Giesbrecht T. Caffeine as an attention enhancer: Reviewing existing assumptions. Psychopharmacology. 2013;225:251–274. doi: 10.1007/s00213-012-2917-4. [DOI] [PubMed] [Google Scholar]
- 8.Smith A, Kendrick A, Maben A, Salmon J. Effects of breakfast and caffeine on cognitive performance, mood and cardiovascular functioning. Appetite. 1994;22:39–55. doi: 10.1006/appe.1994.1004. [DOI] [PubMed] [Google Scholar]
- 9.Brunye TT, Mahoney CR, Lieberman HR, Giles GE, Taylor HA. Acute caffeine consumption enhances the executive control of visual attention in habitual consumers. Brain Cogn. 2010;74:186–192. doi: 10.1016/j.bandc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Dong X, Li S, Sun J, Li Y, Zhang G. Association of coffee, decaffeinated coffee and caffeine intake from coffee with cognitive performance in older adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients. 2020;12:840. doi: 10.3390/nu12030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards S, Brice C, Craig C, Penri-Jones R. Effects of caffeine, practice, and mode of presentation on Stroop task performance. Pharmacol. Biochem. Behav. 1996;54:309–315. doi: 10.1016/0091-3057(95)02116-7. [DOI] [PubMed] [Google Scholar]
- 12.Oei A, Hartley LR. The effects of caffeine and expectancy on attention and memory. Hum. Psychopharmacol. 2005;20:193–202. doi: 10.1002/hup.681. [DOI] [PubMed] [Google Scholar]
- 13.Varela F, Lachaux J-P, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 14.Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Hum. Brain Mapp. 1994;2:56–78. doi: 10.1002/hbm.460020107. [DOI] [Google Scholar]
- 15.Sporns O. Structure and function of complex brain networks. Dialogues Clin. Neurosci. 2013;15:247–262. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farahani FV, Karwowski W, Lighthall NR. Application of graph theory for identifying connectivity patterns in human brain networks: A systematic review. Front. Neurosci. 2019;13:585. doi: 10.3389/fnins.2019.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rogers PJ. Caffeine, mood and mental performance in everyday life. Nutr. Bull. 2007;32:84–89. doi: 10.1111/j.1467-3010.2007.00607.x. [DOI] [Google Scholar]
- 18.Baddeley A. The episodic buffer: A new component of working memory? Trends Cogn. Sci. 2000;4:417–423. doi: 10.1016/S1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 19.Kortte KB, Horner MD, Windham WK. The trail making test, part B: Cognitive flexibility or ability to maintain set? Appl. Neuropsychol. 2002;9:106–109. doi: 10.1207/S15324826AN0902_5. [DOI] [PubMed] [Google Scholar]
- 20.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 21.Thatcher RW, Krause PJ, Hrybyk M. Cortico-cortical associations and EEG coherence: A two-compartmental model. Electroencephalogr. Clin. Neurophysiol. 1986;64:123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- 22.Stam CJ, Nolte G, Daffertshofer A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007;28:1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraga Gonzalez G, et al. EEG resting state functional connectivity in adult dyslexics using phase lag index and graph analysis. Front. Hum. Neurosci. 2018;12:341. doi: 10.3389/fnhum.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Mijalkov M, et al. BRAPH: A graph theory software for the analysis of brain connectivity. PLoS One. 2017;12:e0178798. doi: 10.1371/journal.pone.0178798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gramfort A, et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. 2013;7:267. doi: 10.3389/fnins.2013.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- 28.Simard D, Nadeau L, Kröger H. Fastest learning in small-world neural networks. Phys. Lett. A. 2005;336:8–15. doi: 10.1016/j.physleta.2004.12.078. [DOI] [Google Scholar]
- 29.Stam C, et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer's disease. Brain. 2009;132:213–224. doi: 10.1093/brain/awn262. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 31.Bosma I, et al. Disturbed functional brain networks and neurocognitive function in low-grade glioma patients: A graph theoretical analysis of resting-state MEG. Nonlinear Biomed. Phys. 2009;3:9. doi: 10.1186/1753-4631-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis JM, et al. Central nervous system effects of caffeine and adenosine on fatigue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R399–R404. doi: 10.1152/ajpregu.00386.2002. [DOI] [PubMed] [Google Scholar]
- 33.Magalhães R, et al. Habitual coffee drinkers display a distinct pattern of brain functional connectivity. Mol. Psychiatry. 2021 doi: 10.1038/s41380-021-01075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alharbi WDM, Azmat A, Ahmed M. Comparative effect of coffee robusta and coffee arabica (Qahwa) on memory and attention. Metab. Brain Dis. 2018;33:1203–1210. doi: 10.1007/s11011-018-0230-6. [DOI] [PubMed] [Google Scholar]
- 35.Haskell-Ramsay CF, et al. The acute effects of caffeinated black coffee on cognition and mood in healthy young and older adults. Nutrients. 2018 doi: 10.3390/nu10101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunye TT, Mahoney CR, Lieberman HR, Taylor HA. Caffeine modulates attention network function. Brain Cogn. 2010;72:181–188. doi: 10.1016/j.bandc.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Klaassen EB, et al. The effect of caffeine on working memory load-related brain activation in middle-aged males. Neuropharmacology. 2013;64:160–167. doi: 10.1016/j.neuropharm.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Nehlig A. Is caffeine a cognitive enhancer? J. Alzheimer's Dis. JAD. 2010;20(Suppl 1):S85–S94. doi: 10.3233/jad-2010-091315. [DOI] [PubMed] [Google Scholar]
- 39.Koppelstaetter F, et al. Does caffeine modulate verbal working memory processes? An fMRI study. Neuroimage. 2008;39:492–499. doi: 10.1016/j.neuroimage.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 40.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 41.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: Validation using a set-switching paradigm. J. Clin. Exp. Neuropsychol. 2000;22:518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- 43.Soar K, Chapman E, Lavan N, Jansari AS, Turner JJ. Investigating the effects of caffeine on executive functions using traditional Stroop and a new ecologically-valid virtual reality task, the Jansari assessment of Executive Functions (JEF((c))) Appetite. 2016;105:156–163. doi: 10.1016/j.appet.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 45.Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- 46.Koppelstaetter F, et al. Caffeine and cognition in functional magnetic resonance imaging. J. Alzheimers Dis. 2010;20(Suppl 1):S71–84. doi: 10.3233/JAD-2010-1417. [DOI] [PubMed] [Google Scholar]
- 47.Ko JH, et al. Increased dopamine release in the right anterior cingulate cortex during the performance of a sorting task: A [11C]FLB 457 PET study. Neuroimage. 2009;46:516–521. doi: 10.1016/j.neuroimage.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moll J, de Oliveira-Souza R, Moll FT, Bramati IE, Andreiuolo PA. The cerebral correlates of set-shifting: An fMRI study of the trail making test. Arq. Neuropsiquiatr. 2002;60:900–905. doi: 10.1590/s0004-282x2002000600002. [DOI] [PubMed] [Google Scholar]
- 49.Jacobson SC, Blanchard M, Connolly CC, Cannon M, Garavan H. An fMRI investigation of a novel analogue to the Trail-Making Test. Brain Cogn. 2011;77:60–70. doi: 10.1016/j.bandc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Allen MD, Owens TE, Fong AK, Richards DR. A functional neuroimaging analysis of the Trail Making Test-B: Implications for clinical application. Behav. Neurol. 2011;24:159–171. doi: 10.3233/ben-2011-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43:1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Basharpoor S, Heidari F, Molavi P. EEG coherence in theta, alpha, and beta bands in frontal regions and executive functions. Appl. Neuropsychol. Adult. 2019 doi: 10.1080/23279095.2019.1632860. [DOI] [PubMed] [Google Scholar]
- 53.Sun S, Li X, Zhu J, Wang Y, La R, Zhang X, Wei L, Hu B. Graph theory analysis of functional connectivity in major depression disorder with high-density resting state EEG data. IEEE Trans. Neural Syst. Rehabil. Eng. 2019;27:429–439. doi: 10.1109/TNSRE.2019.2894423. [DOI] [PubMed] [Google Scholar]
- 54.Shen, J., Zhao, S., Yao, Y., Wang, Y. & Feng, L. A novel depression detection method based on pervasive EEG and EEG splitting criterion. IEEE International Conference on Bioinformatics and Biomedicine (BIBM) 1879–1886. 10.1109/BIBM.2017.8217946 (2017).
- 55.Shen J, Zhang X, Hu B, Wang G, Ding Z, Hu B. An improved empirical mode decomposition of electroencephalogram signals for depression detection. IEEE Trans. Affect. Comput. 2019 doi: 10.1109/TAFFC.2019.2934412. [DOI] [Google Scholar]
- 56.Shen J, Zhang X, Huang X, Wu M, Gao J, Lu D, Ding Z, Hu B. An optimal channel selection for EEG-based depression detection via kernel-target alignment. IEEE J. Biomed. Health Inform. 2020 doi: 10.1109/JBHI.2020.3045718. [DOI] [PubMed] [Google Scholar]