To the editor:
Patients undergoing maintenance dialysis (DP) have a high risk of fatal coronavirus disease 2019 (COVID-19).1 Recent epidemiological data raise apprehension with respect to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOC) for DP.2 , 3 Therefore, ensuring cellular and humoral immunity directed to SARS-CoV-2 including VOC isolates is essential for this population. There are no data on vaccine-induced nor on natural SARS-CoV-2 infection–induced long-term immunity and its responsiveness to VOC isolates in DP.
Here, we assessed cellular and humoral immunity to SARS-CoV-2 reference strain and alpha as well as beta VOC in 18 patients convalescing from mild or moderate COVID-19, which were compared to 22 age- and sex-matched DP after prime boost BNT162b2 vaccination (Supplementary Table S1). The great majority of infections occurred in November 2020 in Germany; therefore, contact of the convalescent subcohort with VOCs is unlikely. Appearance of the alpha and beta variants in Great Britain und South Africa, respectively, was first reported in December 2020.
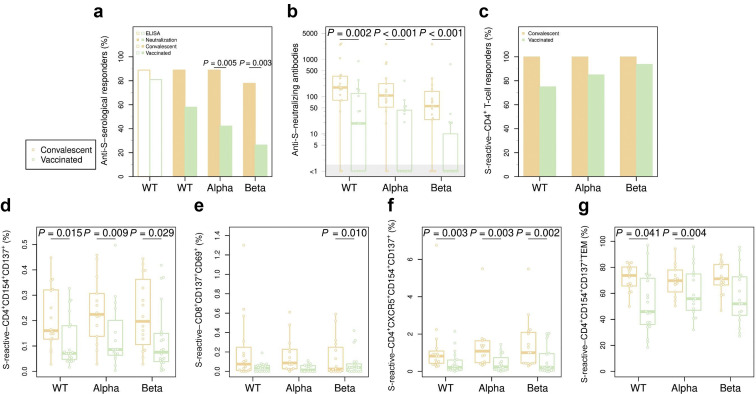
Our data demonstrate a significantly higher number of humoral responders to VOCs and titers of neutralizing antibodies to both SARS-CoV-2 and VOCs in convalescent compared with vaccinated DPs (Figure 1 a and b). Accordingly, cellular immune response also demonstrated significantly higher levels and functionality of T cells directed to the Spike (S)-protein of SARS-CoV-2 and VOCs in convalescent compared with vaccinated DPs. Frequencies of S-protein–reactive CD4+ T cells (Figure 1c) including effector molecule-producing T cells (Supplementary Figure S1) as mono- or polyfunctional cells were significantly higher in convalescent DPs. Furthermore, frequencies of S-protein–reactive CXCR5+ follicular T helper cells and effector memory T cells—phenotypes associated with T-cell functionality—were also significantly higher in convalescent patients (Figure 1f and g). For the employed gating strategy, see Supplementary Figure S2; representative dot plots of cytokine expression are shown in Supplementary Figure S3.
Figure 1.
A stronger humoral and cellular immune response in dialysis patients who were convalescent for coronavirus disease 2019 compared with vaccinated dialysis patients. Humoral response was assessed by neutralization assay for the reference strain and for variants of concern alpha and beta strains; and by conventional enzyme-linked immunosorbent assay (ELISA) for the reference strain; T-cell response was evaluated after stimulation with S-protein overlapping peptide pools of corresponding severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) strains and subtracting background activation levels (dimethylsulfoxide). (a) Percentage of patients with a detectable (neutralization) serological response, for Spike (S) protein wild type (WT), alpha, and beta. (b) Neutralization antibody titers for the 3 SARS-CoV-2 strains. The gray area shows donors with a titer below the detection limit. (c) Percentage of patients with a CD4+ T-cell response for reference, alpha, and beta SARS-CoV-2 strains, as defined by a stimulation index >3. (d–f) Percentage of activated CD4+ T cells (d), CD8+ T cells (e), and CD4+CXCR5+ T cells (f) for each of the 3 SARS-CoV-2 strain–derived S-protein overlapping peptide pools. (g) Percentage of effector memory T cells among S-protein–reactive CD4+ T cells.
The data obtained from DPs (who were convalescent for >5 months), compared with data from vaccinated DPs, suggest superiority of adaptive immunity directed to SARS-CoV-2 and VOCs. This is remarkable, due to the longer time since infection compared with time from vaccination. The data on humoral immunity are in contrast to most available data in healthy cohorts.4, 5, 6, 7, 8 Thus, mRNA vaccines have been repeatedly found to elicit stronger humoral neutralizing responses against the reference strain and VOCs.4, 5, 6, 7, 8 The results are even more striking when considering that a drop of >50% in neutralizing antibody titers has been observed after the third month after infection.6 We are not aware of any study directly comparing cellular immunity in the general population, although robust T-cell responses in convalescent and mRNA-vaccinated immunocompetent patients have already been reported.9 , 10
The reason for a significantly stronger humoral and cellular immune response found in DP after natural COVID-19 infection is unknown. We hypothesize that the high inflammation level observed in DPs during COVID-19 contributes to a stronger antigenic challenge and lymphocyte recruitment, generating superior cellular and humoral immunity as compared to prime boost vaccination in DPs. Although further studies are required, our preliminary data might have important implications for vaccination recommendation in patients who are convalescing.
Acknowledgments
We thank Thorsten Wolff, Jessica Schulz, and Christian Mache from the Robert-Koch Institute (Fachbereich 17) and Martin Beer from the Friedrich Loeffler Institute for providing SARS-CoV-2 VOC alpha and beta isolates.
Footnotes
Table S1. Characteristics of convalescent and vaccinated hemodialysis patients.
Figure S1. Frequency of IFN-γ–, IL-2–, and TNF-α–producing cells among S-protein reactive CD4+CD154+CD137+ T cells in convalescent and vaccinated hemodialysis patients.
Figure S2. Gating strategy to identify S-protein–reactive T cells among CD4+ T cells, CD4+ CXCR5+ T cells, and CD8+ T cells.
Figure S3. Representative dot plots illustrating expression of IL-2, TNF-α, and IFN-γ by activated CD4+CD154+CD137+ T cells.
Supplementary Methods.
Supplementary Material
References
- 1.ERA-EDTA Council, ERACODA Working Group Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36:87–94. doi: 10.1093/ndt/gfaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V., Iyengar K., Garg R., Vaishya R. Elucidating reasons of COVID-19 re-infection and its management strategies. Diabetes Metab Syndr. 2021;15:1001–1006. doi: 10.1016/j.dsx.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geers D., Immunol S., Geers D. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Röltgen K, Nielsen SCA, Arunachalam PS, et al. mRNA vaccination compared to infection elicits an IgG-predominant response with greater SARS-CoV-2 specificity and similar decrease in variant spike recognition [preprint]. medRxiv. 10.1101/2021.04.05.21254952. Accessed June 30, 2021. [DOI]
- 5.Supasa P., Zhou D., Dejnirattisai W. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201–2211. doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edara VV, Norwood C, Floyd K, et al. Reduced binding and neutralization of infection- and vaccine-induced antibodies to the B.1.351 (South African) SARS-CoV-2 variant [preprint]. bioRxiv. 10.1101/2021.02.20.432046. Accessed June 30, 2021. [DOI]
- 7.Khoury D.S., Cromer D., Reynaldi A. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 8.Mulligan M.J., Lyke K.E., Kitchin N. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 9.Anft M., Blazquez-Navarro A., Paniskaki K. SARS-CoV-2–reactive cellular and humoral immunity in hemodialysis population. Kidney Int. 2021;99:1489–1490. doi: 10.1016/j.kint.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westhoff TH, Seibert FS, Anft M, et al. Correspondence on ‘SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response' [e-pub ahead of print]. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2021-220756. Accessed July 18, 2021. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.