ABSTRACT
Danio rerio (zebrafish) are a powerful experimental model for genetic and developmental studies. Adaptation of zebrafish to study seizures was initially established using the common convulsant agent pentylenetetrazole (PTZ). Larval PTZ-exposed zebrafish exhibit clear behavioral convulsions and abnormal electrographic activity, reminiscent of interictal and ictal epileptiform discharge. By using this model, our laboratory developed simple locomotion-based and electrophysiological assays to monitor and quantify seizures in larval zebrafish. Zebrafish also offer multiple advantages for rapid genetic manipulation and high-throughput phenotype-based drug screening. Combining these seizure assays with genetically modified zebrafish that represent Dravet syndrome, a rare genetic epilepsy, ultimately contributed to a phenotype-based screen of over 3500 drugs. Several drugs identified in these zebrafish screens are currently in clinical or compassionate-use trials. The emergence of this ‘aquarium-to-bedside’ approach suggests that broader efforts to adapt and improve upon this zebrafish-centric strategy can drive a variety of exciting new discoveries.
KEY WORDS: Epilepsy, Perspective, Zebrafish
Summary: Screening programs using zebrafish could reshape traditional approaches to antiepileptic drug discovery. Here we describe steps to develop zebrafish models that represent human epilepsies, with an emphasis on a zebrafish model of Dravet syndrome.
Introduction
Epilepsy is a common neurological disorder with a variety of underlying etiologies. In adults, acquired epilepsies – resulting from traumatic brain injury (Annegers and Coan, 2000), a history of febrile seizures (Offringa et al., 1992) or infection (van Baalen et al., 2017) – are most common. Although exceptions exist, many adult epilepsy patients achieve adequate seizure control with antiepileptic drugs (AEDs) (Camfield and Camfield, 2008), vagal nerve stimulation (Nemeroff et al., 2006) or surgical resection (Barba et al., 2021). The success of AEDs in this population is likely to stem from the availability of 28 Federal Drug Administration (FDA)-approved drugs discovered in preclinical, primarily acute, rodent seizure models (Bourgeois, 1994; White, 1997; Loscher, 2002). Indeed, it can be argued that rodent seizure models, unlike those for Alzheimer's disease, stroke or spinal cord injury, are reasonably good preclinical predictors of AED activity. Nonetheless, consternation persists within the epilepsy community as the overall percentage of intractable epilepsy patients has remained in the 20–30% range for decades. Upon further reflection, maybe this is not surprising as none of the available rodent models commonly used to identify AEDs had been explicitly designed to represent pediatric forms of epilepsy, which specifically include a large number of rare genetic epilepsies associated with de novo single-gene mutations (Epi, 2012; Epi et al., 2013). Thus, FDA-approved AEDs for this population remain a significant unmet need, requiring alternative preclinical animal models.
“Zebrafish disease models can be used to evaluate a rapidly growing number of candidate genes for neurological disorders, including rare genetic epilepsies apparent in children.”
Aquarium-to-bedside in epilepsy
Approaching this problem from a different perspective, we focused our laboratory efforts on pediatric epilepsies. At that time (in the early 2000s), existing animal models for pediatric epilepsies were primarily limited to rodent models of cortical malformation; for example undercut (Hoffman et al., 1994), freeze-lesion (Jacobs et al., 1996) or in utero methylazoxymethanol exposure (Baraban et al., 2000). Relatively little was known about genetic causes for epilepsies, grouped as ‘idiopathic’, as this predated widespread identification of gene mutations in humans and accompanying mutagenesis studies in mice. As most AED discovery efforts rely heavily on seizure thresholds in acute or invoked rodent models (Alachkar et al., 2020; Loscher, 2017), we sought preclinical models that feature a more clinically relevant phenotype of spontaneous unprovoked seizures (epilepsy) and amenability to high-throughput drug screening. Larval zebrafish, a small vertebrate widely used for neurodevelopmental research, featuring genetic homology with humans as high as 82% (Howe et al., 2013), fit this overall goal. However, although the concept of using zebrafish appeared attractive, experimental techniques to study seizures – at behavioral or electrophysiological levels – were lacking. To address this problem, with assistance from experienced zebrafish collaborator Herwig Baier, we established simple locomotion-based and extracellular electrophysiological recording techniques to monitor seizure events elicited by application of a well-established convulsant drug, pentylenetetrazole (PTZ) (Baraban et al., 2005). Early PTZ studies described progressive seizure-like behavioral stages in larval zebrafish and provided a clear demonstration of abnormal ictal- and interictal-like electrographic activity, both of which are cardinal features in defining epileptic conditions. Published in 2005 and presented to the epilepsy research community at national meetings, initial responses from more-senior epilepsy investigators and clinicians were not entirely supportive. Undaunted, we continued to publish manuscripts describing molecular, behavioral, electrophysiological and pharmacological features of larval zebrafish seizures for another decade (Baraban et al., 2007; Chege et al., 2012; Hortopan and Baraban, 2011; Hortopan et al., 2010; Hunt et al., 2012; Ramirez et al., 2012). Eventually, much of our early work with acute larval PTZ seizures was replicated in laboratories worldwide (Afrikanova et al., 2013; Baxendale et al., 2012; Orellana-Paucar et al., 2013; Siebel et al., 2015; Turrini et al., 2017; Copmans et al., 2018; Fuller et al., 2018; Tanwar et al., 2019; Bandara et al., 2020; Hoffman et al., 2016; Berghmans et al., 2007; Zheng et al., 2018; Pisera-Fuster et al., 2017), providing independent validation of this experimental model. Now, with more than 325 PubMed entries for “zebrafish+seizure”, acute zebrafish seizure models are well-established to study networks and mechanisms that result in the generation or propagation of seizures. From a pharmacological perspective, zebrafish PTZ seizures are similar to those in rodent models (Loscher, 2011; Alachkar et al., 2020) and identify the same AED candidates. As the database of drugs screened in rodent PTZ models dates to the 1940s, opportunities for new discoveries, in any species, using this convulsant agent may be limited.
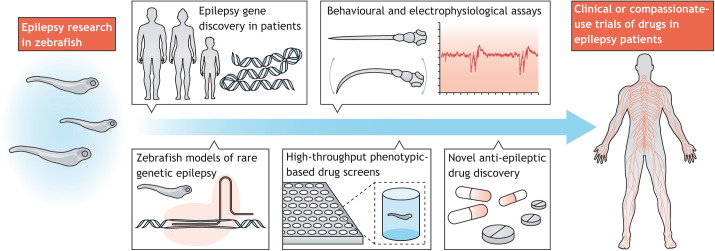
Fig. 1.
Aquarium-to-bedside drug development for epilepsy. Genes associated with rare genetic epilepsies, such as Dravet syndrome (DS), have been identified in patients. Genetically modified zebrafish models generated using ENU-mutagenesis or CRISPR-Cas9 technology can recapitulate these rare genetic epilepsies. Locomotion-based and local field potential (LFP) recording techniques adapted to larval zebrafish can then be used to study these rare genetic epilepsy models. Sensitive phenotype-based screening of hundreds to thousands of drugs can be achieved using a combination of these behavioral and electrophysiological assays in genetically modified zebrafish models, and use of a DS zebrafish model identified several drugs that progressed directly into clinical or compassionate-use trials.
Zebrafish disease models can be used to evaluate a rapidly growing number of candidate genes for neurological disorders, including rare genetic epilepsies apparent in children. These discoveries benefit from several advantages that are unique to this simple vertebrate system, including – but not limited to – a fully sequenced genome, rapid neurodevelopment and large clutches of transparent embryos that develop externally. Most relevant to neurological disorders, the main central nervous system subdivisions are similar to those in mammals, i.e. forebrain, midbrain, hindbrain and spinal cord, as are the common neurotransmitter systems, i.e. γ–aminobutyric acid (GABA), glutamate, dopamine, norepinephrine, serotonin and acetylcholine (Choi et al., 2021). A decades long history of generating mutants by using the chemical mutagenesis agent N-ethyl-N-nitrosourea (ENU) combined with advancements in transposons, transcription activator-like effector nuclease (TALEN) and Zinc-finger nuclease (ZFN) genome editing resulted in hundreds of zebrafish lines (Huang et al., 2012; Auer and Del Bene, 2014; Ata et al., 2016; Thyme et al., 2019). More recently, the CRISPR/Cas9 system was shown to be several-fold more efficient at generating germline mutations, resulting in several clinically relevant zebrafish lines that mimic known human epilepsy mutations (Grone et al., 2017; Pena et al., 2017; Hoffman et al., 2016). As CRISPR technology was unavailable until recently, our initial drug program utilized an ENU-generated mutant for the voltage-activated sodium channel SCN1A that is associated with Dravet syndrome (DS), one of the most-severe forms of genetic epilepsy. The didys552/scn1lab larval zebrafish mutants mimic a haploinsufficient SCN1A loss-of-function mutation seen in most DS patients. Following identification by Herwig Baier's laboratory in a large-scale screen for saccade deficits (Schoonheim et al., 2010), we determined that scn1lab mutant larvae exhibit frequent and recurrent spontaneous seizures early in development, are not associated with genetic compensation by other scn1 genes, die prematurely between 10 and 14 days post fertilization (Baraban et al., 2013), and experience sleep-wake cycle disturbances (Grone et al., 2017) as well as metabolic deficit (Banerji et al., 2021; Kumar et al., 2016). Seizures in scn1lab mutant larvae respond favorably to DS ‘standard of care’ AEDs valproate, benzodiazepines and stiripentol but are pharmaco-resistant to most other AEDs (Baraban et al., 2013; Griffin et al., 2018; Griffin et al., 2019; Banerji et al., 2021; Dinday and Baraban, 2015; Griffin et al., 2017; Hong et al., 2016). Many of these phenotypes, including spontaneous epileptic phenotypes at behavioral and electrophysiological levels, were subsequently replicated by other laboratories using didys552/scn1lab, CRISPR-generated scn1lab or morpholino antisense scn1lab knockdown larval zebrafish (Sourbron et al., 2017; Sourbron et al., 2016; Sourbron et al., 2019; Eimon et al., 2018; Weuring et al., 2020). These unique features recapitulate key clinical phenotypes and allow for high-throughput drug screening in larval zebrafish.
“By using a two-stage locomotion and electrophysiology phenotype-based screening strategy, and highlighting the predictive value of our DS zebrafish mutant, we identified fenfluramine […], synthetic cannabinoids […], structural analogs of clemizole and – as repurposed drugs – trazodone and lorcaserin.”
With the establishment of this genetic zebrafish model, and in combination with optimized simple locomotion-based and electrophysiological recording assays, we initiated our first phenotype-based drug-screening program using a commercially available 312 compound drug library spiked with FDA-approved drugs (Baraban et al., 2013). As FDA-approved treatments for DS were unavailable when we started this research in 2012, we reasoned that a repurposed drug library could yield a lead candidate(s) permissive to rapid clinical translation. In the initial phenotype-based screen, now expanded to over 3500 drugs (Griffin et al., 2020, 2017, 2019; Dinday and Baraban, 2015; Banerji et al., 2021), we identified the first-generation antihistamine clemizole (EPX-100) to be a powerful inhibitor of spontaneous recurrent seizures. We also learned that, by itself, a simple locomotion-based assay yielded ‘false positive’ results of drugs that reduced seizure-like behavior but did not change electrographic events because they are sedatives or muscle-relaxants. It is worth a strong note of caution that many subsequent screening efforts that have adapted our larval zebrafish seizure models largely relied upon locomotion-based outcomes but lack any electrophysiological confirmation of anti-seizure activity, thereby adding putative ‘hit’ compounds to the literature that are likely to be false positives (Sharma et al., 2020; Shen et al., 2020; Gong et al., 2020; Baxendale et al., 2012; Jones et al., 2020; Thornton et al., 2020; Brillatz et al., 2020; Li et al., 2020; Decui et al., 2020; Plate et al., 2019; Brueggeman et al., 2019; Aourz et al., 2019; Bertoncello et al., 2018; Moradi-Afrapoli et al., 2017; Li et al., 2015; Siebel et al., 2015; Rahn et al., 2014; Buenafe et al., 2013). Specifically, acute larval PTZ-induced seizure assays coupled with electrophysiological confirmation can accurately predict AED efficacy (Baraban et al., 2005; Afrikanova et al., 2013; Berghmans et al., 2007), whereas behavior-only discoveries lacking this crucial electrophysiology assay are difficult to interpret. By using a two-stage locomotion and electrophysiology phenotype-based screening strategy, and highlighting the predictive value of our DS zebrafish mutant, we identified fenfluramine (now FDA-approved as Fintepla®) (Dinday and Baraban, 2015), synthetic cannabinoids (similar to the FDA-approved cannabadiol Epidiolex®) (Griffin et al., 2020), structural analogs of clemizole and – as repurposed drugs – trazodone (Desyrel®) and lorcaserin (Belviq®) (Griffin et al., 2017). Interestingly, and suggesting an additional advantage of a zebrafish model, we combined medicinal chemistry, receptor binding assays and scn1lab mutant assays to identify a potentially unifying mechanism of action of these drugs, which is linked to the modulation of serotonin receptors (Griffin et al., 2019). Although innovative from a preclinical model perspective and accomplished in less than a decade, the fact likely to have brought this zebrafish model to the attention and acceptance of the epilepsy community is that, during compassionate-use trials, lorcaserin and, to a lesser extent, trazodone have both shown efficacy against seizures in children suffering from DS (Griffin et al., 2017; Tolete et al., 2018; Brigo et al., 2018). Moreover, clemizole (EPX-100), which exhibited a broad safety profile in recent phase I clinical studies is now under investigation as an ‘add-on treatment’ in a pivotal phase II clinical trial (https://clinicaltrials.gov/ct2/show/NCT04462770).
Conclusion
Our success with a larval zebrafish model for DS is but one small example of what may be possible. Although this system may not easily adapt to modeling forms of epilepsy that are acquired in adulthood (as many advantages inherent in using larvae are lost in adult zebrafish) or, indeed, all genetic forms of epilepsy (as zebrafish orthologs for every human gene may not be available), zebrafish models could offer similar routes to drug discovery for personalized medicine. In line with this, we recently used CRISPR/Cas9 genome editing to generate 37 stable mutant zebrafish lines that represent a wide spectrum gene mutations important regarding human epilepsy (https://zebrafishproject.ucsf.edu/) (Griffin et al., 2020). We envision these zebrafish models, which are based on loss-of-function mutations found in humans, as a starting point for future studies aiming to better understand how a single-gene mutation leads to altered network function and, ultimately, intractable forms of epilepsy. For example, mechanistic insights into seizure generation and/or propagation may be possible when using cutting-edge imaging techniques (Dana et al., 2016; Marvin et al., 2019; Shemesh et al., 2020; Liu et al., 2021) combined with opto- or chemogenetic manipulation of neuronal sub-populations. In addition to drug discoveries as described above, genetic zebrafish lines also offer opportunities to design novel phenotype-based screening strategies for preclinical development of targeted therapies based on antisense-oligonucleotide, CRISPR-Cas9 or adeno-associated viral vector gene manipulation technologies (Han et al., 2020; Colasante et al., 2020; Niibori et al., 2020). More broadly, as a challenge for the zebrafish community, our epilepsy-specific strategy might be adaptable to a wider variety of neurological disorders. In consideration of these challenges and our experience, we suggest that scientists seeking alternative approaches to rodent preclinical models should seriously consider zebrafish – as zebrafish offer many advantages for the potential identification of new treatments for genetic diseases.
Acknowledgements
I would like to thank Colleen Carpenter, Aliesha Griffin, Tully, Paige Whyte-Fagundes and Robert Hunt for insightful comments and inspiration.
Footnotes
Competing interests
S.C.B. is a co-Founder and Scientific Advisor for EpyGenix Therapeutics
Funding
This work was supported by NIH/NINDS awards R01-NS103139, R01 NS096976, and U54-NS11710; and NIH/NICHD R01 HD102071 to S.C.B.
References
- Afrikanova, T., Serruys, A. S., Buenafe, O. E., Clinckers, R., Smolders, I., De Witte, P. A., Crawford, A. D. and Esguerra, C. V. (2013). Validation of the zebrafish pentylenetetrazol seizure model: locomotor versus electrographic responses to antiepileptic drugs. PLoS ONE 8, e54166. 10.1371/journal.pone.0054166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alachkar, A., Ojha, S. K., Sadeq, A., Adem, A., Frank, A., Stark, H. and Sadek, B. (2020). Experimental models for the discovery of novel anticonvulsant drugs: focus on pentylenetetrazole-induced seizures and associated memory deficits. Curr. Pharm. Des. 26, 1693-1711. 10.2174/1381612826666200131105324 [DOI] [PubMed] [Google Scholar]
- Annegers, J. F. and Coan, S. P. (2000). The risks of epilepsy after traumatic brain injury. Seizure 9, 453-457. 10.1053/seiz.2000.0458 [DOI] [PubMed] [Google Scholar]
- Aourz, N., Serruys, A. K., Chabwine, J. N., Balegamire, P. B., Afrikanova, T., Edrada-Ebel, R., Grey, A. I., Kamuhabwa, A. R., Walrave, L., Esguerra, C. V.et al. (2019). Identification of GSK-3 as a potential therapeutic entry point for epilepsy. ACS Chem. Neurosci. 10, 1992-2003. 10.1021/acschemneuro.8b00281 [DOI] [PubMed] [Google Scholar]
- Ata, H., Clark, K. J. and Ekker, S. C. (2016). The zebrafish genome editing toolkit. Methods Cell Biol. 135, 149-170. 10.1016/bs.mcb.2016.04.023 [DOI] [PubMed] [Google Scholar]
- Auer, T. O. and Del Bene, F. (2014). CRISPR/Cas9 and TALEN-mediated knock-in approaches in Zebrafish. Methods 69, 142-150. 10.1016/j.ymeth.2014.03.027 [DOI] [PubMed] [Google Scholar]
- Bandara, S. B., Carty, D. R., Singh, V., Harvey, D. J., Vasylieva, N., Pressly, B., Wulff, H. and Lein, P. J. (2020). Susceptibility of larval zebrafish to the seizurogenic activity of GABA type A receptor antagonists. Neurotoxicology 76, 220-234. 10.1016/j.neuro.2019.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji, R., Huynh, C., Figueroa, F., Dinday, M. T., Baraban, S. C. and Patel, M. (2021). Enhancing glucose metabolism via gluconeogenesis is therapeutic in a Zebrafish model of Dravet syndrome. Brain Commun. 3, fcab004. 10.1093/braincomms/fcab004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban, S. C., Wenzel, H. J., Hochman, D. W. and Schwartzkroin, P. A. (2000). Characterization of heterotopic cell clusters in the hippocampus of rats exposed to methylazoxymethanol in utero. Epilepsy Res. 39, 87-102. 10.1016/S0920-1211(99)00104-7 [DOI] [PubMed] [Google Scholar]
- Baraban, S. C., Taylor, M. R., Castro, P. A. and Baier, H. (2005). Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 131, 759-768. 10.1016/j.neuroscience.2004.11.031 [DOI] [PubMed] [Google Scholar]
- Baraban, S. C., Dinday, M. T., Castro, P. A., Chege, S., Guyenet, S. and Taylor, M. R. (2007). A large-scale mutagenesis screen to identify seizure-resistant Zebrafish. Epilepsia, 48, 1151-1157. 10.1111/j.1528-1167.2007.01075.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban, S. C., Dinday, M. T. and Hortopan, G. A. (2013). Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat. Commun. 4, 2410. 10.1038/ncomms3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba, C., Cossu, M., Guerrini, R., Di Gennaro, G., Villani, F., De Palma, L., Grisotto, L., Consales, A., Battaglia, D., Zamponi, N.et al. (2021). Temporal lobe epilepsy surgery in children and adults: a multicenter study. Epilepsia 62, 128-142. 10.1111/epi.16772 [DOI] [PubMed] [Google Scholar]
- Baxendale, S., Holdsworth, C. J., Meza Santoscoy, P. L., Harrison, M. R., Fox, J., Parkin, C. A., Ingham, P. W. and Cunliffe, V. T. (2012). Identification of compounds with anti-convulsant properties in a zebrafish model of epileptic seizures. Dis. Model. Mech. 5, 773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghmans, S., Hunt, J., Roach, A. and Goldsmith, P. (2007). Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res. 75, 18-28. 10.1016/j.eplepsyres.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Bertoncello, K. T., Aguiar, G. P. S., Oliveira, J. V. and Siebel, A. M. (2018). Micronization potentiates curcumin's anti-seizure effect and brings an important advance in epilepsy treatment. Sci. Rep. 8, 2645. 10.1038/s41598-018-20897-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois, B. F. (1994). Pharmacologic intervention and treatment of childhood seizure disorders: relative efficacy and safety of antiepileptic drugs. Epilepsia 35 Suppl. 2, S18-S23. 10.1111/j.1528-1157.1994.tb05933.x [DOI] [PubMed] [Google Scholar]
- Brigo, F., Striano, P., Balagura, G. and Belcastro, V. (2018). Emerging drugs for the treatment of Dravet syndrome. Expert Opin. Emerg. Drugs 23, 261-269. 10.1080/14728214.2018.1552937 [DOI] [PubMed] [Google Scholar]
- Brillatz, T., Jacmin, M., Vougogiannopoulou, K., Petrakis, E. A., Kalpoutzakis, E., Houriet, J., Pellissier, L., Rutz, A., Marcourt, L., Queiroz, E. F.et al. (2020). Antiseizure potential of the ancient Greek medicinal plant Helleborus odorus subsp. cyclophyllus and identification of its main active principles. J. Ethnopharmacol. 259, 112954. 10.1016/j.jep.2020.112954 [DOI] [PubMed] [Google Scholar]
- Brueggeman, L., Sturgeon, M. L., Martin, R. M., Grossbach, A. J., Nagahama, Y., Zhang, A., Howard, M. A., III, Kawasaki, H., Wu, S., Cornell, R. A.et al. (2019). Drug repositioning in epilepsy reveals novel antiseizure candidates. Ann. Clin. Transl. Neurol. 6, 295-309. 10.1002/acn3.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenafe, O. E., Orellana-Paucar, A., Maes, J., Huang, H., Ying, X., De Borggraeve, W., Crawford, A. D., Luyten, W., Esguerra, C. V. and De Witte, P. (2013). Tanshinone IIA exhibits anticonvulsant activity in zebrafish and mouse seizure models. ACS Chem. Neurosci. 4, 1479-1487. 10.1021/cn400140e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield, P. and Camfield, C. (2008). When is it safe to discontinue AED treatment? Epilepsia 49 Suppl. 9, 25-28. 10.1111/j.1528-1167.2008.01923.x [DOI] [PubMed] [Google Scholar]
- Chege, S. W., Hortopan, G. A., matthew, T. D. and Baraban, S. C. (2012). Expression and function of KCNQ channels in larval zebrafish. Dev. Neurobiol. 72, 186-198. 10.1002/dneu.20937 [DOI] [PubMed] [Google Scholar]
- Choi, T. Y., Choi, T. I., Lee, Y. R., Choe, S. K. and Kim, C. H. (2021). Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 53, 310-317. 10.1038/s12276-021-00571-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasante, G., Lignani, G., Brusco, S., Di Berardino, C., Carpenter, J., Giannelli, S., Valassina, N., Bido, S., Ricci, R., Castoldi, V.et al. (2020). dCas9-based scn1a gene activation restores inhibitory interneuron excitability and attenuates seizures in dravet syndrome mice. Mol. Ther. 28, 235-253. 10.1016/j.ymthe.2019.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copmans, D., Orellana-Paucar, A. M., Steurs, G., Zhang, Y., Ny, A., Foubert, K., Exarchou, V., Siekierska, A., Kim, Y., De Borggraeve, W.et al. (2018). Methylated flavonoids as anti-seizure agents: Naringenin 4’,7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models. Neurochem. Int. 112, 124-133. 10.1016/j.neuint.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Dana, H., Mohar, B., Sun, Y., Narayan, S., Gordus, A., Hasseman, J. P., Tsegaye, G., Holt, G. T., Hu, A., Walpita, D.et al. (2016). Sensitive red protein calcium indicators for imaging neural activity. Elife 5, e12727. 10.7554/eLife.12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decui, L., Garbinato, C. L. L., Schneider, S. E., Mazon, S. C., Almeida, E. R., Aguiar, G. P. S., Muller, L. G., Oliveira, J. V. and Siebel, A. M. (2020). Micronized resveratrol shows promising effects in a seizure model in zebrafish and signalizes an important advance in epilepsy treatment. Epilepsy Res. 159, 106243. 10.1016/j.eplepsyres.2019.106243 [DOI] [PubMed] [Google Scholar]
- Dinday, M. T. and Baraban, S. C. (2015). Large-scale phenotype-based antiepileptic drug screening in a Zebrafish model of dravet syndrome. eNeuro 2, ENEURO.0068-15.2015. 10.1523/ENEURO.0068-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimon, P. M., Ghannad-Rezaie, M., De Rienzo, G., Allalou, A., Wu, Y., Gao, M., Roy, A., Skolnick, J. and Yanik, M. F. (2018). Brain activity patterns in high-throughput electrophysiology screen predict both drug efficacies and side effects. Nat. Commun. 9, 219. 10.1038/s41467-017-02404-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epi, K. C. (2012). Epi4K: gene discovery in 4,000 genomes. Epilepsia 53, 1457-1467. 10.1111/j.1528-1167.2012.03511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epi, K. C., Epilepsy Phenome/Genome, P., Allen, A. S., Berkovic, S. F., Cossette, P., Delanty, N., Dlugos, D., Eichler, E. E., Epstein, M. P., Glauser, T.et al. (2013). De novo mutations in epileptic encephalopathies. Nature 501, 217-221. 10.1038/nature12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, T. D., Westfall, T. A., Das, T., Dawson, D. V. and Slusarski, D. C. (2018). High-throughput behavioral assay to investigate seizure sensitivity in zebrafish implicates ZFHX3 in epilepsy. J. Neurogenet. 32, 92-105. 10.1080/01677063.2018.1445247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, G., Chen, H., Kam, H., Chan, G., Tang, Y. X., Wu, M., Tan, H., Tse, Y. C., Xu, H. X. and Lee, S. M. (2020). In Vivo screening of Xanthones from garcinia oligantha identified oliganthin H as a novel natural inhibitor of convulsions. J. Nat. Prod. 83, 3706-3716. 10.1021/acs.jnatprod.0c00963 [DOI] [PubMed] [Google Scholar]
- Griffin, A., Hamling, K. R., Knupp, K., Hong, S., Lee, L. P. and Baraban, S. C. (2017). Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain 140, 669-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, A., Hamling, K. R., Hong, S., Anvar, M., Lee, L. P. and Baraban, S. C. (2018). Preclinical animal models for dravet syndrome: seizure phenotypes, comorbidities and drug screening. Front. Pharmacol. 9, 573. 10.3389/fphar.2018.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, A. L., Jaishankar, P., Grandjean, J. M., Olson, S. H., Renslo, A. R. and Baraban, S. C. (2019). Zebrafish studies identify serotonin receptors mediating antiepileptic activity in Dravet syndrome. Brain Commun. 1, fcz008. 10.1093/braincomms/fcz008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, A., Anvar, M., Hamling, K. and Baraban, S. C. (2020). Phenotype-based screening of synthetic cannabinoids in a dravet syndrome Zebrafish model. Front. Pharmacol. 11, 464. 10.3389/fphar.2020.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone, B. P., Qu, T. and Baraban, S. C. (2017). Behavioral comorbidities and drug treatments in a Zebrafish scn1lab model of dravet syndrome. eNeuro 4, ENEURO.0066-17.2017. 10.1523/ENEURO.0066-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Z., Chen, C., Christiansen, A., Ji, S., Lin, Q., Anumonwo, C., Liu, C., Leiser, S. C., Meena, Aznarez, I.et al. (2020). Antisense oligonucleotides increase Scn1a expression and reduce seizures and SUDEP incidence in a mouse model of Dravet syndrome. Sci. Transl. Med. 12, eaaz6100. 10.1126/scitranslmed.aaz6100 [DOI] [PubMed] [Google Scholar]
- Hoffman, E. J., Turner, K. J., Fernandez, J. M., Cifuentes, D., Ghosh, M., Ijaz, S., Jain, R. A., Kubo, F., Bill, B. R., Baier, H.et al. (2016). Estrogens suppress a behavioral phenotype in Zebrafish mutants of the autism risk gene, CNTNAP2. Neuron. 89, 725-733. 10.1016/j.neuron.2015.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, S. N., Salin, P. A. and Prince, D. A. (1994). Chronic neocortical epileptogenesis in vitro. J. Neurophysiol. 71, 1762-1773. 10.1152/jn.1994.71.5.1762 [DOI] [PubMed] [Google Scholar]
- Hong, S., Lee, P., Baraban, S. C. and Lee, L. P. (2016). A novel long-term, multi-channel and non-invasive electrophysiology platform for Zebrafish. Sci. Rep. 6, 28248. 10.1038/srep28248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortopan, G. A. and Baraban, S. C. (2011). Aberrant expression of genes necessary for neuronal development and Notch signaling in an epileptic mind bomb zebrafish. Dev. Dyn. 240, 1964-1976. 10.1002/dvdy.22680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortopan, G. A., Dinday, M. T. and Baraban, S. C. (2010). Spontaneous seizures and altered gene expression in GABA signaling pathways in a mind bomb mutant zebrafish. J. Neurosci. 30, 13718-13728. 10.1523/JNEUROSCI.1887-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, K., Clark, M. D., Torroja, C. F., Torrance, J., Berthelot, C., Muffato, M., Collins, J. E., Humphray, S., Mclaren, K., Matthews, L.et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature. 496, 498-503. 10.1038/nature12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, P., Zhu, Z., Lin, S. and Zhang, B. (2012). Reverse genetic approaches in zebrafish. J. Genet. Genomics 39, 421-433. 10.1016/j.jgg.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Hunt, R. F., Hortopan, G. A., Gillespie, A. and Baraban, S. C. (2012). A novel zebrafish model of hyperthermia-induced seizures reveals a role for TRPV4 channels and NMDA-type glutamate receptors. Exp. Neurol. 237, 199-206. 10.1016/j.expneurol.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, K. M., Gutnick, M. J. and Prince, D. A. (1996). Hyperexcitability in a model of cortical maldevelopment. Cereb. Cortex 6, 514-523. 10.1093/cercor/6.3.514 [DOI] [PubMed] [Google Scholar]
- Jones, A., Barker-Haliski, M., Ilie, A. S., Herd, M. B., Baxendale, S., Holdsworth, C. J., Ashton, J. P., Placzek, M., Jayasekera, B. A. P., Cowie, C. J. A.et al. (2020). A multiorganism pipeline for antiseizure drug discovery: Identification of chlorothymol as a novel gamma-aminobutyric acidergic anticonvulsant. Epilepsia 61, 2106-2118. 10.1111/epi.16644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, M. G., Rowley, S., Fulton, R., Dinday, M. T., Baraban, S. C. and Patel, M. (2016). Altered glycolysis and mitochondrial respiration in a Zebrafish model of dravet syndrome. eNeuro 3, ENEURO.0008-16.2016. 10.1523/ENEURO.0008-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. L., Zhou, J., Chen, Z. H., Guo, S. Y., Li, C. Q. and Zhao, W. M. (2015). Bioactive C21 steroidal glycosides from the roots of cynanchum otophyllum that suppress the seizure-like locomotor activity of Zebrafish Caused by pentylenetetrazole. J. Nat. Prod. 78, 1548-1555. 10.1021/np501058b [DOI] [PubMed] [Google Scholar]
- Li, X., Himes, R. A., Prosser, L. C., Christie, C. F., Watt, E., Edwards, S. F., Metcalf, C. S., West, P. J., Wilcox, K. S., Chan, S. S. L.et al. (2020). Discovery of the first vitamin k analogue as a potential treatment of pharmacoresistant seizures. J. Med. Chem. 63, 5865-5878. 10.1021/acs.jmedchem.0c00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Salvati, K. A. and Baraban, S. C. (2021) In vivo calcium imaging reveals disordered interictal network dynamics in epileptic stxbp1b zebrafish. iScience 24, 102558. 10.1016/j.isci.2021.102558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher, W. (2002). Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res. 50, 105-123. 10.1016/S0920-1211(02)00073-6 [DOI] [PubMed] [Google Scholar]
- Loscher, W. (2011). Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 20, 359-368. 10.1016/j.seizure.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Loscher, W. (2017). Animal models of seizures and epilepsy: past, present, and future role for the discovery of antiseizure drugs. Neurochem. Res. 42, 1873-1888. 10.1007/s11064-017-2222-z [DOI] [PubMed] [Google Scholar]
- Marvin, J. S., Shimoda, Y., Magloire, V., Leite, M., Kawashima, T., Jensen, T. P., Kolb, I., Knott, E. L., Novak, O., Podgorski, K.et al. (2019). A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nat. Methods 16, 763-770. 10.1038/s41592-019-0471-2 [DOI] [PubMed] [Google Scholar]
- Moradi-Afrapoli, F., Van Der Merwe, H., De Mieri, M., Wilhelm, A., Stadler, M., Zietsman, P. C., Hering, S., Swart, K. and Hamburger, M. (2017). HPLC-based activity profiling for GABAA receptor modulators in searsia pyroides using a larval Zebrafish locomotor assay. Planta Med. 83, 1169-1175. 10.1055/s-0043-110768 [DOI] [PubMed] [Google Scholar]
- Nemeroff, C. B., Mayberg, H. S., Krahl, S. E., Mcnamara, J., Frazer, A., Henry, T. R., George, M. S., Charney, D. S. and Brannan, S. K. (2006). VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 31, 1345-1355. 10.1038/sj.npp.1301082 [DOI] [PubMed] [Google Scholar]
- Niibori, Y., Lee, S. J., Minassian, B. A. and Hampson, D. R. (2020). Sexually divergent mortality and partial phenotypic rescue after gene therapy in a mouse model of dravet syndrome. Hum. Gene. Ther. 31, 339-351. 10.1089/hum.2019.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offringa, M., Derksen-Lubsen, G., Bossuyt, P. M. and Lubsen, J. (1992). Seizure recurrence after a first febrile seizure: a multivariate approach. Dev. Med. Child Neurol. 34, 15-24. 10.1111/j.1469-8749.1992.tb08559.x [DOI] [PubMed] [Google Scholar]
- Orellana-Paucar, A. M., Afrikanova, T., Thomas, J., Aibuldinov, Y. K., Dehaen, W., De Witte, P. A. and Esguerra, C. V. (2013). Insights from zebrafish and mouse models on the activity and safety of ar-turmerone as a potential drug candidate for the treatment of epilepsy. PLoS ONE 8, e81634. 10.1371/journal.pone.0081634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena, I. A., Roussel, Y., Daniel, K., Mongeon, K., Johnstone, D., Weinschutz Mendes, H., Bosma, M., Saxena, V., Lepage, N., Chakraborty, P.et al. (2017). Pyridoxine-dependent epilepsy in Zebrafish caused by Aldh7a1 deficiency. Genetics 207, 1501-1518. 10.1534/genetics.117.300137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisera-Fuster, A., Otero, S., Talevi, A., Bruno-Blanch, L. and Bernabeu, R. (2017). Anticonvulsant effect of sodium cyclamate and propylparaben on pentylenetetrazol-induced seizures in zebrafish. Synapse 71. 10.1002/syn.21961 [DOI] [PubMed] [Google Scholar]
- Plate, J., Sassen, W. A., Hassan, A. H., Lehne, F., Koster, R. W. and Kruse, T. (2019). S-Sulfocysteine induces seizure-like behaviors in Zebrafish. Front. Pharmacol. 10, 122. 10.3389/fphar.2019.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn, J. J., Bestman, J. E., Josey, B. J., Inks, E. S., Stackley, K. D., Rogers, C. E., Chou, C. J. and Chan, S. S. (2014). Novel Vitamin K analogs suppress seizures in zebrafish and mouse models of epilepsy. Neuroscience 259, 142-154. 10.1016/j.neuroscience.2013.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez, I. B., Pietka, G., Jones, D. R., Divecha, N., Alia, A., Baraban, S. C., Hurlstone, A. F. and Lowe, M. (2012). Impaired neural development in a zebrafish model for Lowe syndrome. Hum. Mol. Genet. 21, 1744-1759. 10.1093/hmg/ddr608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheim, P. J., Arrenberg, A. B., Del Bene, F. and Baier, H. (2010). Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. J Neurosci, 30, 7111-7120. 10.1523/JNEUROSCI.5193-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P., Kumari, S., Sharma, J., Purohit, R. and Singh, D. (2020). Hesperidin interacts with CREB-BDNF signaling pathway to suppress pentylenetetrazole-induced convulsions in Zebrafish. Front. Pharmacol. 11, 607797. 10.3389/fphar.2020.607797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh, O. A., Linghu, C., Piatkevich, K. D., Goodwin, D., Celiker, O. T., Gritton, H. J., Romano, M. F., Gao, R., Yu, C. J., Tseng, H. A.et al. (2020). Precision calcium imaging of dense neural populations via a cell-body-targeted calcium indicator. Neuron 107, 470-486.e11. 10.1016/j.neuron.2020.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, D., Chen, J., Liu, D., Shen, M., Wang, X., Wu, Y., Ke, S., Macdonald, R. L. and Zhang, Q. (2020). The GABRG2 F343L allele causes spontaneous seizures in a novel transgenic zebrafish model that can be treated with suberanilohydroxamic acid (SAHA). Ann. Transl. Med. 8, 1560. 10.21037/atm-20-3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebel, A. M., Menezes, F. P., Da Costa Schaefer, I., Petersen, B. D. and Bonan, C. D. (2015). Rapamycin suppresses PTZ-induced seizures at different developmental stages of zebrafish. Pharmacol. Biochem. Behav. 139, 163-168. 10.1016/j.pbb.2015.05.022 [DOI] [PubMed] [Google Scholar]
- Sourbron, J., Schneider, H., Kecskes, A., Liu, Y., Buening, E. M., Lagae, L., Smolders, I. and De Witte, P. (2016). Serotonergic modulation as effective treatment for dravet syndrome in a Zebrafish mutant model. ACS Chem. Neurosci. 7, 588-598. 10.1021/acschemneuro.5b00342 [DOI] [PubMed] [Google Scholar]
- Sourbron, J., Smolders, I., De Witte, P. and Lagae, L. (2017). Pharmacological analysis of the anti-epileptic mechanisms of fenfluramine in scn1a mutant Zebrafish. Front. Pharmacol. 8, 191. 10.3389/fphar.2017.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourbron, J., Partoens, M., Scheldeman, C., Zhang, Y., Lagae, L. and De Witte, P. (2019). Drug repurposing for Dravet syndrome in scn1Lab(−/−) mutant zebrafish. Epilepsia 60, e8-e13. 10.1111/epi.14647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanwar, G., Mazumder, A. G., Bhardwaj, V., Kumari, S., Bharti, R., Yamini, Singh, D., Das, P. and Purohit, R. (2019). Target identification, screening and in vivo evaluation of pyrrolone-fused benzosuberene compounds against human epilepsy using Zebrafish model of pentylenetetrazol-induced seizures. Sci. Rep. 9, 7904. 10.1038/s41598-019-44264-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton, C., Dickson, K. E., Carty, D. R., Ashpole, N. M. and Willett, K. L. (2020). Cannabis constituents reduce seizure behavior in chemically-induced and scn1a-mutant zebrafish. Epilepsy Behav. 110, 107152. 10.1016/j.yebeh.2020.107152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyme, S. B., Pieper, L. M., Li, E. H., Pandey, S., Wang, Y., Morris, N. S., Sha, C., Choi, J. W., Herrera, K. J., Soucy, E. R.et al. (2019). Phenotypic landscape of schizophrenia-associated genes defines candidates and their shared functions. Cell 177, 478-491.e20. 10.1016/j.cell.2019.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolete, P., Knupp, K., Karlovich, M., Decarlo, E., Bluvstein, J., Conway, E., Friedman, D., Dugan, P. and Devinsky, O. (2018). Lorcaserin therapy for severe epilepsy of childhood onset: a case series. Neurology 91, 837-839. 10.1212/WNL.0000000000006432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrini, L., Fornetto, C., Marchetto, G., Mullenbroich, M. C., Tiso, N., Vettori, A., Resta, F., Masi, A., Mannaioni, G., Pavone, F. S.et al. (2017). Optical mapping of neuronal activity during seizures in zebrafish. Sci. Rep. 7, 3025. 10.1038/s41598-017-03087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baalen, A., Vezzani, A., Hausler, M. and Kluger, G. (2017). Febrile infection-related epilepsy syndrome: clinical review and hypotheses of Epileptogenesis. Neuropediatrics 48, 5-18. [DOI] [PubMed] [Google Scholar]
- Weuring, W. J., Singh, S., Volkers, L., Rook, M. B., Van ‘T Slot, R. H., Bosma, M., Inserra, M., Vetter, I., Verhoeven-Duif, N. M., Braun, K. P. J.et al. (2020). NaV1.1 and NaV1.6 selective compounds reduce the behavior phenotype and epileptiform activity in a novel zebrafish model for Dravet Syndrome. PLoS ONE 15, e0219106. 10.1371/journal.pone.0219106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, H. S. (1997). Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia 38 Suppl. 1, S9-S17. 10.1111/j.1528-1157.1997.tb04523.x [DOI] [PubMed] [Google Scholar]
- Zheng, Y. M., Chen, B., Jiang, J. D. and Zhang, J. P. (2018). Syntaxin 1B mediates Berberine's roles in epilepsy-like behavior in a Pentylenetetrazole-induced seizure Zebrafish model. Front. Mol. Neurosci. 11, 378. 10.3389/fnmol.2018.00378 [DOI] [PMC free article] [PubMed] [Google Scholar]