ABSTRACT
The Arctic is warming at approximately twice the global rate, with well-documented indirect effects on wildlife. However, few studies have examined the direct effects of warming temperatures on Arctic wildlife, leaving the importance of heat stress unclear. Here, we assessed the direct effects of increasing air temperatures on the physiology of thick-billed murres (Uria lomvia), an Arctic seabird with reported mortalities due to heat stress while nesting on sun-exposed cliffs. We used flow-through respirometry to measure the response of body temperature, resting metabolic rate, evaporative water loss and evaporative cooling efficiency (the ratio of evaporative heat loss to metabolic heat production) in murres while experimentally increasing air temperature. Murres had limited heat tolerance, exhibiting: (1) a low maximum body temperature (43.3°C); (2) a moderate increase in resting metabolic rate relative that within their thermoneutral zone (1.57 times); (3) a small increase in evaporative water loss rate relative that within their thermoneutral zone (1.26 times); and (4) a low maximum evaporative cooling efficiency (0.33). Moreover, evaporative cooling efficiency decreased with increasing air temperature, suggesting murres were producing heat at a faster rate than they were dissipating it. Larger murres also had a higher rate of increase in resting metabolic rate and a lower rate of increase in evaporative water loss than smaller murres; therefore, evaporative cooling efficiency declined with increasing body mass. As a cold-adapted bird, murres' limited heat tolerance likely explains their mortality on warm days. Direct effects of overheating on Arctic wildlife may be an important but under-reported impact of climate change.
KEY WORDS: Arctic climate change, Evaporative cooling efficiency, Evaporative water loss, Thick-billed murres, Heat stress, Seabirds
Summary: Thick-billed murres have limited heat tolerance, displaying low evaporative cooling efficiencies and increases in physiological traits associated with heat stress at low air temperatures relative to desert birds.
INTRODUCTION
Climate change is warming the Arctic at approximately twice the global rate (Anisimov et al., 2007; McBean et al., 2005). In the Canadian Arctic, mean annual air temperature has increased by ∼2.3°C from 1948 to 2016, and could increase by an additional 7.8°C by 2100 under high-emission scenarios (Zhang et al., 2019). This warming may have severe effects on cold-adapted homeothermic endotherms (i.e. organisms that actively maintain relatively constant body temperatures through metabolic heat production). Several studies have highlighted the indirect effects of warming on Arctic wildlife, such as compositional shifts in the prey base (Gaston and Hipfner, 1998; Gaston et al., 2005; Yurkowski et al., 2018), earlier breeding phenology and shifts in the timing of migration (Chmura et al., 2020; Clairbaux et al., 2019; Le Corre et al., 2017). In contrast, the direct effects of warming on the physiology and behaviour of Arctic endotherms has been observed but less studied (Gaston et al., 2002).
There is growing evidence that the heat tolerance limits of endotherms have ecological consequences (Rezende and Bacigalupe, 2015). In birds, which maintain their core body temperature at levels higher than in mammals (41°C versus 37°C), heat waves have caused mass mortality events (McKechnie et al., 2012) and reproductive failures (Bolger et al., 2005; Boersma and Rebstock, 2014). The forecasted increase in heat wave frequency is predicted to cause declines in select avian populations (Conradie et al., 2019; McKechnie and Wolf, 2010). However, as most avian heat tolerance studies have focused on desert birds (Gerson et al., 2014; Smit and Mckechnie, 2015; Whitfield et al., 2015), less is known about heat tolerance in Arctic birds. Recent evidence suggests that an Arctic passerine may be limited in its capacity to withstand even moderately high air temperatures (O'Connor et al., 2021). As larger birds have proportionally less surface area to volume ratios, and therefore less surface to dissipate heat, they may be even more sensitive to heat stress.
Diving Arctic seabirds are exposed to a range of environmental temperatures, resulting in an additional thermoregulatory challenge; they must avoid heat stress during nesting, yet must minimize heat loss while diving under icy waters for their prey. One such species is the thick-billed murre (Uria lomvia; hereafter ‘murres’). Murres have a circumpolar distribution (Frederiksen et al., 2016) and forage in waters typically colder than 8.0°C (Gaston et al., 2005).
Murres face several indirect impacts of climate change (Gaston et al., 2003, 2005, 2009), but mounting evidence suggests they will also be directly affected by warming during the breeding season (Gaston and Elliott, 2013; Gaston et al., 2002). Murres have been observed displaying heat dissipation behaviours (heavy panting and wing spreading), and have died on their nests as a result of heat stress and mosquito parasitism during calm days with maximum air temperatures as low as 22°C (Gaston and Elliott, 2013; Gaston et al., 2002). Murres' black back feathers have also been reported to reach temperatures as high as 46°C in full sun at ambient temperatures no greater than 23°C (Gaston et al., 2002). Despite evidence that murres are sensitive to heat, there has been no research that has linked increasing air temperatures to heat stress, which is important for predicting the effects of forecasted Arctic warming.
To examine physiological responses to heat stress in murres, we exposed birds to increasing air temperatures (Ta) and measured responses in: (1) body temperature (Tb); (2) resting metabolic rate (RMR); (3) evaporative water loss (EWL); and (4) evaporative cooling efficiency (the ratio of evaporative heat loss to metabolic heat production: EHL/MHP; Lasiewski et al., 1966). To ascertain the onset of heat stress, for each measurement we identified inflection points, which represent a sudden change in the trait due to increasing Ta. We predicted that as a large (∼1 kg) seabird with high metabolic rates relative to its size (Gabrielsen et al., 1988) that is adapted for foraging in cold waters, murres would exhibit inflection points at low Ta and tolerate low maximum Ta relative to smaller and more heat tolerant species. Therefore, we predicted murres would also have higher costs of thermoregulation and display large increases in RMR relative to birds of the same body size from lower latitudes.
MATERIALS AND METHODS
Study site, temperature and gas exchange measurements
All bird handling was approved by the animal care committee at McGill University and conducted under scientific permits from Environment and Climate Change Canada (Banding Permit 10892, Scientific Permit NUN-SCI-16-03) and the Government of Nunavut (2019-021). We studied thick-billed murres, Uria lomvia (Linnaeus 1758), between 26 June and 7 August 2019, from the ‘West colony’ on Coats Island in northern Hudson Bay, NU, Canada (62°57'N, 82°00'W). The West colony has approximately 30,000 breeding pairs of murres nesting on cliffs. Birds lay eggs around mid-June, and chicks fledge by early to mid-August (Gaston et al., 1994, 2012). We captured incubating adult murres (n=10) using noose polls. We were restricted in the number of murres we could capture by logistical constraints associated with the challenges of working in a remote Arctic location (no electricity, limited supplies, poor weather, etc.) and were unable to return in 2020 because of travel restrictions into Nunavut in response to COVID-19. We measured body mass (Mb) using a CS Series Ohaus portable scale (2000±1 g), by placing individual birds head-first into a tared cylinder (which secured their wings and held them still) on the scale and waited until the mass stabilized.
To determine RMR and rates of EWL of individual murres, we measured oxygen consumption rate (V̇O2; ml min−1) and water vapour pressure (WVP; kPa), using flow-throw respirometry. Immediately following capture, individual murres were placed in a sealed Plexiglas metabolic chamber (42×42×41 cm) at the Coats Island field station. The chamber was fitted with a mesh base; guano fell through and into approximately 1 cm of mineral oil covering the base of the chamber, which prevented evaporation from guano affecting WVP measurements. We placed the chamber inside a temperature-controlled and insulated box fitted with a Peltier heating unit (model T35 DC-S, Mobicool International, Zhuhai, China) and a Watercarbon Tech Era carbon fibre seat heater (Henan, China). We monitored and regulated Ta inside the box using a digital thermostat (ITC-1000F, Shenzhen Inkbird Technology Co., Shenzhen, China). We measured Ta inside the metabolic chamber using a thermistor probe (Sable Systems, Las Vegas, NV, USA) inserted through a small hole in the chamber and sealed with putty (Gorilla all-purpose epoxy) and connected to a Field Metabolic System (Sable Systems). Tb was measured using a thermocouple probe (TC-1000 Type-T Thermometer) inserted approximately 3 cm in the cloaca and secured with electrical tape. The thermocouple was connected to a Sable Systems thermocouple meter (model TC-1000) that measured Tb every second. Although the thermocouple probe fell out prematurely at Ta=22.3°C in one murre, we were able to obtain complete Tb measurements on 9 birds.
We pushed atmospheric air into the metabolic chamber using an air pump (model ECOair 7, EcoPlus Commercial Air Pump). Air was scrubbed of water vapour and CO2 by passing the air stream through columns of Drierite (W.A. Hammond, Drierite Co. Ltd, Xenia, OH, USA) and soda lime connected in series. Once scrubbed, the airstream was split into a baseline channel that went directly to the analysers and a second channel that went to the chamber. Baseline flow rates were controlled using a needle valve (AS4200F, SMC, Tokyo, Japan), whereas chamber flow rates were controlled with an OMEGA mass flow controller (calibrated 18 January 2019; model FMI-100-MKC-2C, Norwalk, CT, USA). We maintained flow rates at 2630 ml min−1. These flow rates produced a mean maximum chamber dew point of 14.3°C (range 3.6–18.0°C). The maximum absolute humidity was 14.36 g m−3 at Ta=38.4°C.
We subsampled incurrent air from the baseline channel and excurrent air from the metabolic chamber by manually switching between them using the Field Metabolic System, which pulled air and first measured WVP. Within the same system, the airstream was then scrubbed of water vapor and CO2 for the measurement of O2 consumption. All tubing connecting the system was Bev-A-Line (Thermoplastic Processes Inc., Warren, NJ, USA). We digitized voltage outputs from all the analysers using a Sable Systems Universal Interface (model UI-2) and logged analyser outputs at a sampling rate of 5 s (0.2 Hz) with Expedata software (v.1.9.14, Sable Systems).
Experimental protocol
We placed murres in the chamber at approximately 21:00 h, within 5 min of capture, at a temperature within their thermoneutral zone (mean±s.e.m.: 18.6±0.1°C; range: 15.7 to 20.4°C; Gabrielsen et al., 1988; Elliott et al., 2013a) and held overnight for 14.65±1.4 h. All heat tolerance measurements took place immediately the following day between 06:00 h and 18:00 h (note however that previous studies have found no diurnal rhythm in the RMR of murres; Elliott et al., 2013a: from 04:30 h to 20:30 h on Coats Island; Gabrielsen et al., 1988: from 09:00 h to 03:00 h at NyÅlesund, Svalbard). Starting in the thermoneutral zone at Ta≈18.8°C, we exposed individual murres to a ramped profile of increasing Ta by approximately 2°C increments. Once the chamber Ta stabilized at ±1°C for approximately 2 min, we recorded data (V̇O2, WVP, Tb) for approximately 30 min before increasing Ta. A 10–25 min baseline was recorded at the beginning and end of each run, and in between measurements while adjusting the chamber temperature. To observe whether birds remained calm while in the chamber, we monitored their behaviour using a SmotecQ dome infrared camera (model DF-3500-AHD 1080P) and video capture software (ArcSoft ShowBiz, v.3.5.15.68). Following the protocol of Whitfield et al. (2015), we ceased experiments if birds exhibited continuous erratic behaviour such as pacing, flapping or escape behaviour, or an uncontrolled increase in Tb to 45°C. As the maximum Tb of murres was 43.3°C, we monitored continuous escape behaviour, which typically lasted for more than 2 min at the maximum Ta measured. After the run was terminated, birds were re-weighed and brought immediately outside to cool down. Once their behaviour and breathing appeared normal, murres were brought back to their nesting site and released into the wild. No adverse effects were observed and all birds were witnessed to have returned to their nesting site.
Data analysis
To examine the physiological responses to increasing Ta, we analysed V̇O2 and WVP data with Expedata 1.9.14. We first corrected for the time lag in the O2 and water vapour traces by using the lag correction function in Expedata. Next, we used a z-transformation (Bartholomew et al., 1981) to correct for chamber volume relative to the flow rate. We used a Catmull–Rom spline correction applied to baselines to correct for drift in the O2 and water vapour traces. Oxygen consumption was calculated using eqn 10.1 of Lighton (2019):
| (1) |
where FR is the incurrent flow rate (ml min−1), FiO2 is the incurrent fractional O2 concentration (0.2095) and FeO2 is the excurrent fractional O2 concentration. At each Ta, we measured resting values of V̇O2, WVP and Tb using the mean of the most stable 5 min period of V̇O2. For each Ta, we excluded data from any bird that did not remain calm based on our observations of their behaviour, which we later verified using our video recordings of the experiments. Birds were assumed to be post-absorptive as murres digest food within 1–2 h (Gaston and Noble, 1985) and had fasted in the chamber for 14.65±1.4 h prior to starting our heat tolerance runs. Therefore, to transform V̇O2 to RMR (W), we used eqn 9.13 of Lighton (2019) to derive energy equivalents (J ml−1 O2), assuming a respiratory quotient of 0.71 (Walsberg and Wolf, 1995). We calculated rates of EWL (mg min−1) by converting WVP (kPa) into water vapor density (mg ml−1) using the following equation from the Sable Systems water vapor analyser manual, then multiplying by the incurrent flow rate:
| (2) |
where 461.5 (J kg K−1) is the individual gas constant for water vapor. To determine how efficient an individual murre was at dissipating heat, we calculated their evaporative cooling efficiency by converting rates of EWL into EHL (W) assuming 2.406 J mg−1 H2O, and dividing by MHP, with low EHL/MHP values assumed to indicate a lower ability to dissipate heat (Lasiewski et al., 1966).
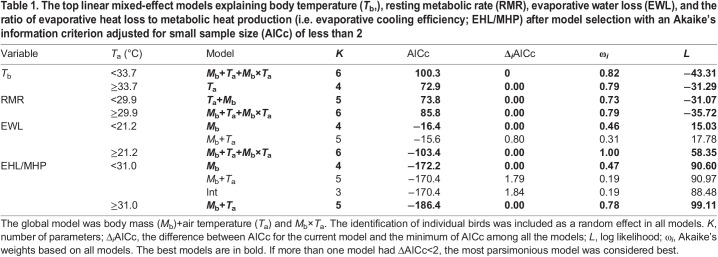
We characterized the onset of heat stress as the Ta inflection point for Tb, RMR, EWL and EHL/MHP in murres, obtained by fitting broken-stick regressions to identify significant changes in slope, using the R package SiZer (https://cran.r-project.org/package=SiZer). To examine the effect of body mass (Mb, measured before heat tolerance runs) and Ta on Tb, RMR, EWL and EHL:MHP, we took a subset of the data at the inflection points and fitted linear mixed effect models on the data below and above the inflection points using the lme4 package in R (Bates et al., 2015). We built a global model with Mb, Ta and their two-way interaction as predictors. To account for repeated measurements on the same individual, we included individual bird identification as a random factor. We then performed model selection using the dredge function in the MuMIn package (https://CRAN.R-project.org/package=MuMIn) based on Akaike's information criterion adjusted for small sample size (AICc). The minimum adequate model within a ΔAICc<2 was considered the best model (Burnham and Anderson, 2002). We calculated AICc weights based on all available models. We used paired t-tests to compare the Mb of birds before and after our heat tolerance runs.
Each of the top models met assumptions for normality, linearity and homogeneity of variance. We tested for outliers by calculating Cook's distance values for each bird using the influence.ME package (Nieuwenhuis et al., 2012). As the model for EWL above the inflection point for Ta had one individual with a Cook's distance value >1 (Logan, 2010), we fitted a robust-mixed effect model to the data for this particular model using the robustlmm package (Koller, 2016). All analyses were run using R 3.6.3 (http://www.R-project.org/) and significance was judged at α=0.05. We made all figures using ggplot2 (Wickham, 2016). Data are reported as means±1 s.e.m. All raw data are tabulated in Table S1.
RESULTS
Mb
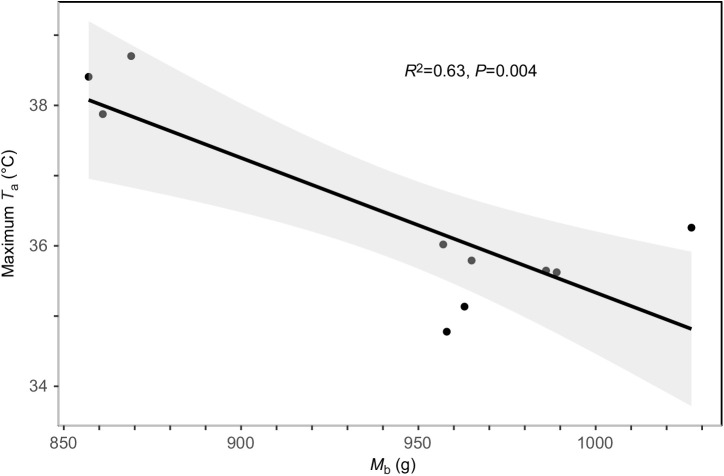
Murres weighed 999.3±20.2 g at capture (Mb range 906–1079 g, n=10). Mb decreased (paired t-test, t9=−12.30, P<0.0001) after overnight fasting in the thermoneutral zone (mean 14.65 h) prior to heat tolerance runs. Mean Mb prior to heat tolerance trials was 943.2±18.8 g and declined significantly during runs (post-heat tolerance Mb=900.7±18.0 g; paired t-test, t9=−21.91, P<0.0001), with a mean loss of 42.5±1.9 g (i.e. approximately 4.5% loss). When we compared the maximum Ta tolerated by murres with Mb prior to heat tolerance trials, larger birds had lower heat tolerance limits, with maximum tolerated Ta decreasing with increasing Mb (Fig. 1; r2=0.63, F1,8=16.5, P=0.0036).
Fig. 1.
Linear regression of the relationship between maximum tolerated air temperature (Ta) and total body mass (Mb) prior to heat tolerance runs. Data are for 10 murres. The shaded area represents the 95% confidence interval (CI) around the predicted values.
Tb
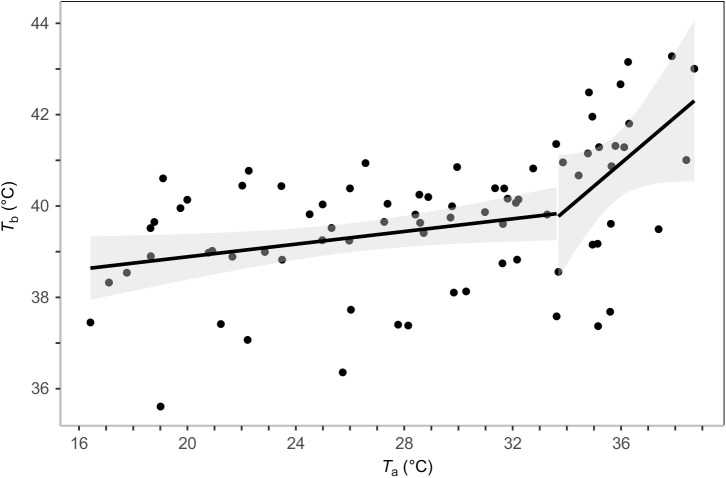
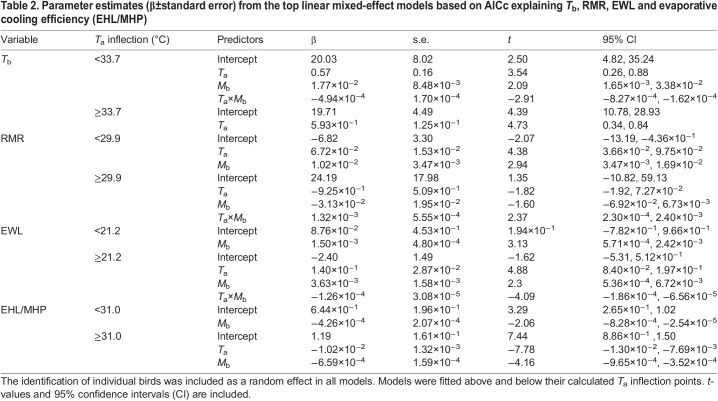
During heat tolerance trials, Tb of murres ranged from 35.6°C at Ta=19.0°C to 43.3°C at Ta=37.9°C. Murres demonstrated a significant inflection point in Tb at Ta=33.7°C (Fig. 2; 95% confidence interval CI=22.2–37.0°C). Below the inflection point, the minimum adequate model explaining Tb included Ta, Mb and their interaction (Table 1). Above the inflection point, the minimum adequate model included Ta only, with Tb increasing with Ta (Table 2; Fig. 2). Tb increased from 38.6±0.5°C at Ta=18.8±0.4°C to a mean maximum of 41.4±0.5°C at Ta=36.5±0.5°C.
Fig. 2.
Linear regression of the relationship between body temperature (Tb) and Ta. Tb data are for 10 murres (n=78). A significant inflection point in Tb was identified at Ta=33.7°C. The shaded area represents the 95% CI around the predicted values.
Table 1.
The top linear mixed-effect models explaining body temperature (Tb,), resting metabolic rate (RMR), evaporative water loss (EWL), and the ratio of evaporative heat loss to metabolic heat production (i.e. evaporative cooling efficiency; EHL/MHP) after model selection with an Akaike's information criterion adjusted for small sample size (AICc) of less than 2
Table 2.
Parameter estimates (β±standard error) from the top linear mixed-effect models based on AICc explaining Tb, RMR, EWL and evaporative cooling efficiency (EHL/MHP)
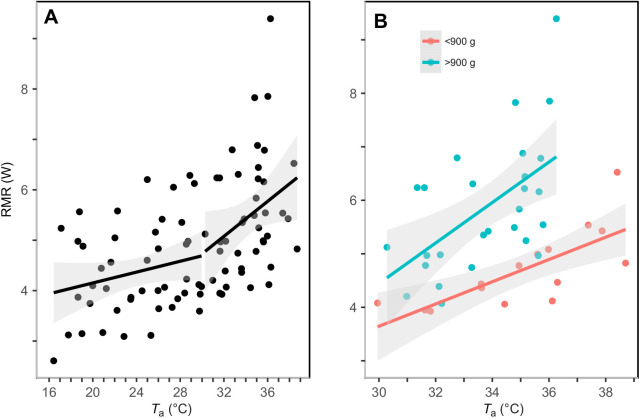
RMR
RMR ranged from 2.6 W at Ta=16.4°C to 9.4 W at Ta=36.3°C. A significant inflection point in RMR occurred at Ta=29.9°C (Fig. 3A; 95% CI=18.8–34.1°C). Below the inflection point, the minimum adequate model explaining RMR included Ta and Mb (Table 1), and RMR increased with both Ta (Table 2; 0.07±0.02 W °C−1) and Mb (0.01±0.003 W g−1). Above the inflection point, the minimum adequate model included Ta, Mb and the interaction between Ta and Mb. To control for the interaction, we divided individual birds into two Mb categories based on Fig. 1 (above and below 900 g), and found RMR increased with Ta at a faster rate (Fig. 3B; 0.35±0.06 W °C−1, 95% CI=0.24–0.47) in murres that were larger than 900 g, relative to smaller birds (0.22±0.04 W °C−1, 95% CI=0.15–0.29). Across all birds, RMR increased from 3.97±0.3 W at Ta=18.8±0.4°C to a mean maximum of 6.24±0.4 W at Ta=36.4±0.4°C.
Fig. 3.
Linear regression of the relationship between resting metabolic rate (RMR) and Ta. RMR data are for 10 murres. (A) A significant inflection point in RMR was identified at Ta=29.9°C (n=85). (B) Data for birds with Mb greater (n=27) or lower (n=15) than 900 g at Ta≥29.9°C. The shaded area represents the 95% CI around the predicted values.
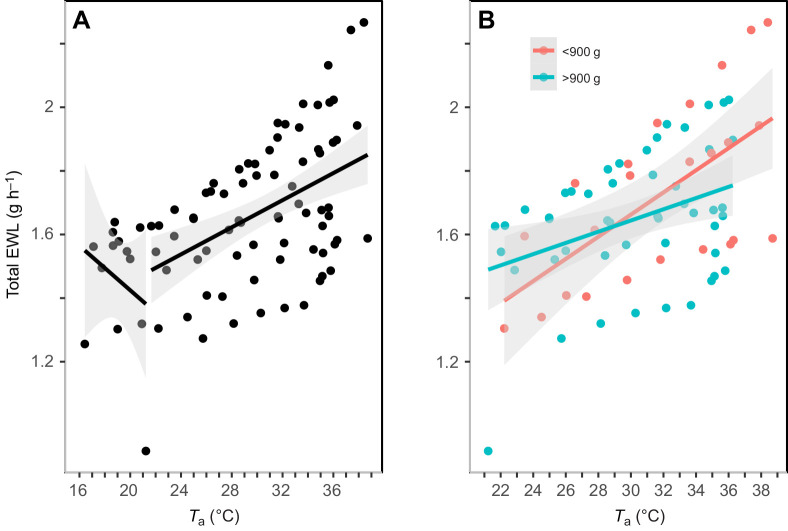
EWL
EWL rates ranged from 0.92 g h−1 at Ta=21.2°C to 2.27 g h−1 at Ta=38.4°C. Murres began panting at Ta=25.9±0.5°C and Tb=39.3±0.5°C. We detected a significant inflection point in rates of EWL at 21.2°C (Fig. 4A; 95% CI=20.9–36.6°C). However, one bird displayed noticeably lower rates of EWL relative to the others and the removal of this bird increased the EWL inflection point to Ta=24.5°C (95% CI=17.7–36.8), a value closely aligning with the mean Ta when panting started. Below the first inflection point, EWL rates were best predicted by Mb (Table 1). Above the inflection point, the minimum adequate model explaining rates of EWL included Ta, Mb and the interaction between Ta and Mb (Table 2; Fig. 4B). To control for the interaction, we divided individual birds into two Mb categories based on Fig. 1 (above and below 900 g). EWL increased with Ta at a faster rate (Fig. 4B; 0.04±0.005 g h °C−1, 95% CI=0.03–0.05) in murres that were smaller than 900 g relative to larger birds (0.02±0.002 g h °C−1, 95% CI=0.01–0.02). Murres increased their rate of EWL 1.26-fold relative to baseline rates measured at Ta=18.8±0.4°C, increasing from a mean of 1.44±0.1 g h−1 to 1.80±0.1 g h−1 at Ta=36.4±0.4°C.
Fig. 4.
Linear regression of the relationship between evaporative water loss (EWL) rate and Ta. EWL data are for 10 murres. (A) A significant inflection point in EWL was identified at Ta=21.2°C (n=85). (B) Data for birds with Mb greater (n=49) or lower (n=24) than 900 g at Ta≥21.2°C. The shaded area represents the 95% CI around the predicted values.
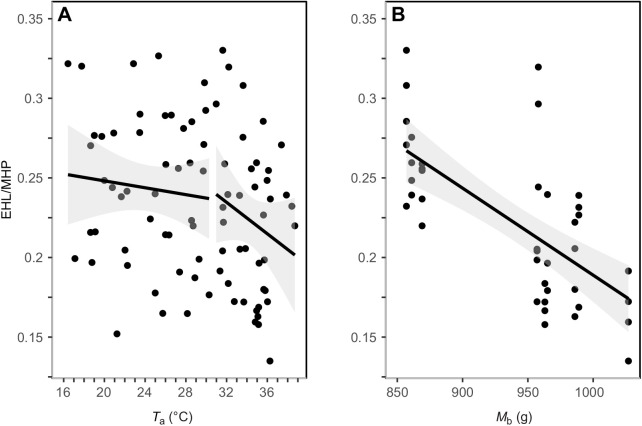
Evaporative cooling efficiency (EHL/MHP)
Evaporative cooling efficiency (EHL/MHP) ranged from 0.33 at Ta=31.6°C to 0.13 at Ta=36.3°C (mean±s.e.m.=0.23±0.01). A significant inflection point was detected at Ta=31.0°C (Fig. 5A; 95% CI=18.6–35.2°C). Below the inflection point, EHL/MHP was best predicted by Mb (Table 1). Above the inflection point, the minimum adequate model explaining EHL/MHP included Ta and Mb (Fig. 5B). EHL/MHP decreased significantly with both Ta (Table 2; −0.01±0.001 g h °C−1, 95% CI=−0.013 to −0.008) and Mb (Table 2; −0.0007±0.0002 g h g−1, 95% CI=−0.001 to −0.0003).
Fig. 5.
Linear regression of the relationship between evaporative cooling efficiency and Ta. Evaporative cooling efficiency data (the ratio of evaporative heat loss to metabolic heat production, EHL/MHP) are for 10 murres. (A) A significant inflection point in EHL/MHP was identified at Ta=31.0°C (n=85). (B) EHL/MHP versus Mb at Ta≥31.0°C. The shaded area represents the 95% CI around the predicted values.
DISCUSSION
Murres displayed markedly reduced heat tolerance relative to birds originating from hot and arid climates, with individuals showing signs of active physiological responses to evaporate heat at much milder temperatures. Murres, to our knowledge, also have the lowest maximum evaporative cooling efficiency ever recorded in birds, including Cape rockjumpers (Chaetops frenatus, ∼0.75; Oswald et al., 2018) and the juniper titmouse (Baeolophus ridgwayi, ∼0.75; Weathers and Greene, 1998). Below, we outline how murre physiological responses to increasing Ta demonstrate their limited capacity to tolerate even moderately elevated temperatures, making them vulnerable to Arctic warming when incubating on sun-exposed cliffs.
Tb
When Tb exceeds Ta, facultative hyperthermia (Gerson et al., 2019; Tieleman and Williams, 1999) aids birds in conserving water by increasing the thermal gradient between Tb and Ta (Weathers, 1981; Tieleman and Williams, 1999; Gerson et al., 2019). Murres displayed hyperthermia and increased their Tb at Ta=33.7°C (95% CI=22.2–37.0°C). The inflection point at Ta=33.7°C is similar to that in snow buntings (Plectrophenax nivalis, 32.6°C, 95% CI=31.0–34.4°C; O'Connor et al., 2021), the only other Arctic bird for which heat tolerance measurements are available, and is also within the Ta range of desert and non-desert avian species (Tieleman and Williams, 1999). Murres' maximum Tb (43.3°C) was similar to the mean Tb of birds from non-desert (43.3°C, range 41.1–45.8°C) and desert (43.6°C, range 41.5–45.4°C) environments at Ta=45.0°C (Tieleman and Williams, 1999). While the mean increase in Tb across 23 desert and non-desert avian species from their lower critical temperatures to 45.0°C was similar to that of murres (3.3 versus 2.8°C), the maximum Ta endured by any murre was much lower (38.7 versus 45.0°C; Tieleman and Williams, 1999). The regulation of a low Tb will result in a narrowing thermal gradient as Ta increases, impeding murres' capacity for dry heat loss and forcing them to increasingly rely on evaporative cooling.
RMR
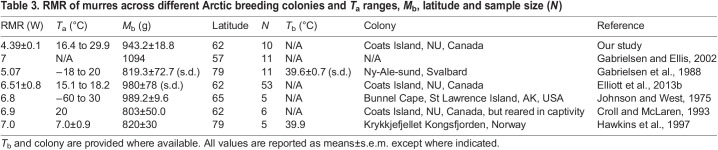
Murres increased their RMR at an upper critical temperature (Tuc) of 29.9°C (95% CI=18.8–34.1°C), which is similar to the Tuc of other cold-region birds, including snow buntings (29.8°C, 95% CI=27.9–42.2°C; O'Connor et al., 2021), little penguins (Eudyptula minor, 30.0°C; Stahel and Nicol, 1982), Peruvian penguins (Spheniscus humboldti, 30.0°C; Drent and Stonehouse, 1971) and Cassin's auklet (Ptychoramphus aleuticus, ∼25.0°C; Richman and Lovvorn, 2011) and lower than those of arid and semi-arid birds (33.9–46.5°C; McKechnie et al., 2017; Smith et al., 2015; Smith et al., 2017). RMR increased by 1.57-fold from a mean Ta=18.8°C to 36.4°C in murres, which is higher than the mean fractional increase in snow buntings (1.4; O'Connor et al., 2021) and some desert birds (1.26–2.66; McKechnie et al., 2016, 2017, Smith et al., 2017, McWhorter et al., 2018, Talbot et al., 2018, Czenze et al., 2020). When we compared mass-specific RMRs in murres above their Tuc, the slope was approximately twice as steep as that predicted from their mean Mb (0.298 versus 0.146 mW g °C−1; Weathers, 1981). In addition, post-absorptive RMRs of murres (Table 3) are approximately 1.1–2.3 times higher than predicted by allometric equations for non-passerines and seabirds (Aschoff and Pohl, 1970; Croll and McLaren, 1993; Ellis, 1984; Gabrielsen and Ellis, 2002; Lasiewski and Dawson, 1967). Murres' high RMRs at thermoneutral temperatures are proposed to be an adaptive response to higher energy requirements in cold climates, diving foraging strategies and high activity levels (Croll and McLaren, 1993; Ellis, 1984; Gabrielsen and Ellis, 2002; Gabrielsen et al., 1988). However, high RMRs will likely become disadvantageous at higher air temperatures because they will lead to murres experiencing greater total heat loads, which must ultimately be dissipated.
Table 3.
RMR of murres across different Arctic breeding colonies and Ta ranges, Mb, latitude and sample size (N)
EWL
Murres began to show signs of heat stress, such as panting and increased rates of EWL, at relatively low air temperatures. In comparison, the onset of panting and increased EWL rate occurred at higher air temperatures in snow buntings (33.2°C and 34.6°C, 95% CI=31.1–36.2°C, respectively; O'Connor et al., 2021) and arid-zone birds (panting: 38.0–42.3°C, increase in EWL: 36–46.5°C; Smith et al., 2015, 2017, McKechnie et al., 2017, O'Connor et al., 2017, Czenze et al., 2020). It is worth noting that a Ta of 21.2°C is ecologically relevant, and corresponds to the maximum Ta at Coats Island during years in which murres displayed noticeable heat dissipation behaviours and experienced higher rates of mortality and egg loss due to the combination of heat stress and mosquito parasitism (22°C in 1998: Gaston et al., 2002; 21.2 and 21.3°C in 2011: Gaston and Elliott, 2013).
The maximum Ta tolerated was correlated with higher evaporative scope (maximum EWL/minimum EWL) in birds (Czenze et al., 2020). The evaporative scope of murres was low relative to that of snow buntings (O'Connor et al., 2021) and birds from desert environments (range 4.73–15.33; McKechnie et al., 2017, Smith et al., 2017, McWhorter et al., 2018, Czenze et al., 2020). The murres' low evaporative scope presumably corresponded with their low mean maximum Ta (36.4°C) relative to that of other birds (snow buntings: 43.0°C: O'Connor et al., 2021; desert birds: 46.0–54°C: McKechnie et al., 2017; Smith et al., 2017; McWhorter et al., 2018; Czenze et al., 2020). At 25°C, the mean EWL in murres was 1.54 g h−1 or approximately 37.0 ml day−1, higher than the allometric prediction (31.2 ml day−1; see eqn 2 of Williams, 1996). Similarly, the EWL rate of murres at 25°C was higher than that (25.8 ml day−1) of Houbara bustards (Chlamydotis macqueenii), a similar-sized (1245 g) desert bird (Tieleman et al., 2002). Furthermore, based on equations from McKechnie and Wolf (2010), the predicted EWL inflection point was higher (33.6°C; but within the 95% CI=20.9–36.6°C), and the predicted mass-specific slope in the rates of EWL with Ta was 16.6-fold steeper than observed in murres (0.479 versus 0.029 mg H2O g h °C−1). The minimal increase in EWL rate of murres suggests they are restricted in their capacity to increase evaporative heat dissipation with increasing temperatures.
Evaporative cooling efficiency (EHL/MHP)
To our knowledge, murres displayed the lowest maximum evaporative cooling efficiency ever reported in a bird. However, we acknowledge that our dew points, and resulting absolute humidity levels, exceed those of previous heat tolerance investigations (e.g. dew points <5°C; Smith et al., 2017). Consequently, the maximum evaporative cooling capacity of thick-billed murres may have been negatively impacted given the interactive effects of humidity and Ta on EHL/MHP (Lasiewski et al., 1966). Unfortunately, available data on the interaction between humidity and Ta in birds at high Ta is scarce and suggests substantial variation among taxa (e.g. Gerson et al., 2014; van Dyk et al., 2019), and we therefore at present cannot definitively say whether our dew points markedly influenced murres' cooling efficiency. At best, murres could only dissipate one-third of the heat they produced metabolically. Ratios of EHL/MHP<1 indicate an organism is unable to dissipate all of its metabolic heat through evaporative heat loss (Lasiewski et al., 1966). While snow buntings also showed low evaporative cooling efficiencies, the inflection point for Ta in murres was lower than in buntings (31°C, 95% CI=18.6–35.2°C versus 36.7°C, 95% CI=31.0–42.3°C; O'Connor et al., 2021). More importantly, evaporative cooling efficiency decreased with increasing Ta in murres, suggesting they were producing heat at a faster rate than they were dissipating it. Murres depend on metabolic heat production to maintain their core Tb (Johnson and West, 1975) and increase their metabolic rate with decreasing water temperatures (Croll and McLaren, 1993). High RMRs and low increases in EWL likely resulted in very low EHL/MHP values and heat tolerance capacities in murres.
Mb and heat tolerance
Larger murres were more vulnerable to heat stress as a result of higher RMRs and lower rates of EWL. Foraging strategies of murres vary with body size, with larger murres spending the most time at deeper and colder depths (Orben et al., 2015). While a larger body size may convey an advantage for minimizing heat loss in murres when diving in cold water, it may also result in an increased risk of overheating while sitting on their nesting ledges, as evaporative cooling efficiency and heat tolerance limits both declined with increasing Mb. To our knowledge, this is the first heat tolerance study on a diving seabird, or any large polar bird, and the adaptations for diving in icy waters may conflict with murres' ability to tolerate heat. In contrast, heat tolerance limits increased with Mb in Australian passerines (McKechnie et al., 2017), but there was no clear relationship in Sonoran passerines (Smith et al., 2017). Body mass was the most important predictor of EWL across 174 bird species, with higher EWL rates in larger birds (Song and Beissinger, 2020); however, smaller passerines experience higher rates of mass-specific EWL rates and have a greater risk of dehydration than larger birds (Albright et al., 2017; McKechnie and Wolf, 2010). Larger murres demonstrated steeper increases in RMR and shallower increases in EWL, which clearly influenced EHL/MHP. In contrast, maximum EHL/MHP increased with increasing Mb in Australian passerines (McKechnie et al., 2017); however, other studies have not found a clear relationship between Mb and EHL/MHP (Smith et al., 2017; Whitfield et al., 2015).
Conclusions and ecological implications
Recent heat waves in the Gulf of Alaska were associated with the mass mortality and reproductive failures of several colonies of common murres (Uria aalge) (Piatt et al., 2020). While these mortalities were hypothesized to be due to an ‘ectothermic vice’ on forage fish where birds faced increased foraging competition and reduced prey quality and quantity (Piatt et al., 2020), direct effects of heat stress may have also contributed. We demonstrated that thick-billed murres have limited heat tolerance. As a dark-plumage bird with 12–24 h incubation shifts, low heat tolerance may explain their heat dissipating behaviours, reproductive failures and mortalities at a maximum Ta as low as 16–22°C (Gaston and Elliott, 2013; Gaston et al., 2002). Importantly, when incubating in full sun, murre surface temperatures can reach 46°C (Gaston et al., 2002); therefore, the operative temperatures of the birds are likely much higher than the maximum Ta measured at Coats Island. In addition, the maximum EWL rate (2.27 g h−1) recorded here would only result in a loss of 2.9% of the mean Mb (943 g) of murres over the period of an incubation shift (12 h, most of which may not be in direct sunlight because of cloud cover and nest orientation on the cliff), and is likely unable to cause dehydration. Thus, we argue that murre mortality is likely due to tissue stress associated with high body temperatures coupled with blood loss from mosquito parasitism (Gaston and Elliott, 2013; Gaston et al., 2002).
While we used multiple measures, we acknowledge that our low sample size of individual murres (n=10) due to logistical constraints makes it difficult for us to draw solid conclusions. Our confidence intervals surrounding our inflection point temperatures were large (approximately 15°C), which may be the result of our small sample size and large range of Mb (857–1027 g) among individuals. While we measured the effects of warmer temperatures on resting murres, further investigation should consider the impacts of heat stress during high energy behaviours, as endotherms are limited in the maximal energy they can expend by their ability to dissipate heat (Speakman and Król, 2010). For example, common eiders (Somateria mollissima) experience hyperthermia during flight and must stop to cool down, which is a major cost of migration (Guillemette et al., 2016, 2017). As murres are large seabirds with high energetic costs of flight (Elliott et al., 2013b) and high daily energy requirements (Elliott and Gaston, 2014), their low heat tolerance may lead to energetic trade-offs to support their high costs of thermoregulation, which may impact their behaviour, reproductive success and, ultimately, survival.
Supplementary Material
Acknowledgements
Field and technical assistance as well as equipment were provided by Jupie Angootealuk, Alyssa Eby, Bronwyn Harkness, Sébastien Harvey, Holly Hennin, Josiah Nakoolak, Douglas Noblet, Allison Patterson, Sarah Poole, Samuel Richard, Russell Turner, Shannon Whelan and the laboratory of Dr Vincent Careau at the University of Ottawa. We would also like to thank Dr Tony Gaston for reviewing our manuscript prior to submission. We thank the two anonymous reviewers for their insightful feedback which greatly improved the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.S.C., R.S.O., A.L.H., O.P.L., F.V., K.H.E.; Methodology: E.S.C.; Formal analysis: E.S.C.; Investigation: E.S.C.; Resources: R.S.O., H.G.G., K.H.E.; Data curation: E.S.C.; Writing - original draft: E.S.C.; Writing - review & editing: E.S.C., R.S.O., H.G.G., A.L.H., O.P.L., F.V., K.H.E.; Visualization: E.S.C.; Supervision: H.G.G., K.H.E.; Funding acquisition: E.S.C., H.G.G., A.L.H., O.P.L., F.V., K.H.E.
Funding
Project support was provided by a Fonds de Recherche du Quebec Nature et Technologies Team research project grant provided to F.V., A.L.H., O.P.L. and K.H.E. Field work at Coats Island was supported by Discovery grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) to K.H.E. and O.P.L. Logistical and financial support was provided by Environment and Climate Change Canada, the Polar Continental Shelf Program (PCSP), the Nunavut Research Institute (NRI), and the Northern Scientific Training Program (NSTP). The W. Garfield Weston Foundation Award for Northern Research, Fonds de Recherche du Quebec Nature et Technologies, L'Oréal-UNESCO Women in Science Research Excellence and Natural Sciences and Engineering Research Council of Canada (NSERC) postdoctoral research fellowships were provided to E.S.C., along with an Earth Rangers grant and an Arctic Institute of North America Grant-in-Aid. Open access funding provided by McGill University. Deposited in PMC for immediate release.
References
- Albright, T. P., Mutiibwa, D., Gerson, A. R., Smith, E. K., Talbot, W. A., O'Neill, J. J., McKechnie, A. E. and Wolf, B. O. (2017). Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc. Natl. Acad. Sci. USA 114, 2283-2288. 10.1073/pnas.1613625114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov, O. A., Vaughan, D. G., Callaghan, T. V., Furgal, C., Marchant, H., Prowse, T. D., Vilhjálmsson, H. and Walsh, J. E. (2007). Polar regions (Arctic and Antarctic). In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (ed. Parry M. L., Canziani O. F., Palutikof J. P., van der Linden P. J. and Hanson C. E.), pp. 653-685. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Aschoff, J. and Pohl, H. (1970). Der ruheumsatz von vögeln als funktion der tageszeit und der körpergröße. J. Ornithol. 111, 38-47. 10.1007/BF01668180 [DOI] [Google Scholar]
- Bartholomew, G. A., Vleck, D. and Vleck, C. M. (1981). Instantaneous measurements of oxygen consumption during pre-flight warm-up and post-flight cooling in sphingid and saturniid moths. J. Exp. Biol. 90, 17-32. 10.1242/jeb.90.1.17 [DOI] [Google Scholar]
- Bates, D., Mächler, M., Bolker, B. and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Boersma, P. D. and Rebstock, G. A. (2014). Climate change increases reproductive failure in magellanic penguins. PLoS ONE 9, e85602. 10.1371/journal.pone.0085602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger, D. T., Patten, M. A. and Bostock, D. C. (2005). Avian reproductive failure in response to an extreme climatic event. Oecologia 142, 398-406. 10.1007/s00442-004-1734-9 [DOI] [PubMed] [Google Scholar]
- Burnham, K. P. and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd edn. New York: Spring-Verlag New York, Inc. [Google Scholar]
- Chmura, H. E., Krause, J. S., Pérez, J. H., Ramenofsky, M. and Wingfield, J. C. (2020). Autumn migratory departure is influenced by reproductive timing and weather in an Arctic passerine. J. Ornithol. 161, 779-791. 10.1007/s10336-020-01754-z [DOI] [Google Scholar]
- Clairbaux, M., Fort, J., Mathewson, P., Porter, W., Strøm, H. and Grémillet, D. (2019). Climate change could overturn bird migration: Transarctic flights and high-latitude residency in a sea ice free Arctic. Sci. Rep. 9, 1-13. 10.1038/s41598-019-54228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradie, S. R., Woodborne, S. M., Cunningham, S. J. and McKechnie, A. E. (2019). Chronic, sublethal effects of high temperatures will cause severe declines in southern African arid-zone birds during the 21st century. Proc. Natl. Acad. Sci. USA 116, 14065-14070. 10.1073/pnas.1821312116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll, D. A. and McLaren, E. (1993). Diving metabolism and thermoregulation in common and thick-billed murres. J. Comp. Physiol. B 163, 160-166. 10.1007/BF00263602 [DOI] [PubMed] [Google Scholar]
- Czenze, Z. J., Kemp, R., van Jaarsveld, B., Freeman, M. T., Smit, B., Wolf, B. O. and McKechnie, A. E. (2020). Regularly drinking desert birds have greater evaporative cooling capacity and higher heat tolerance limits than non-drinking species. Funct. Ecol. 34, 1589-1600. 10.1111/1365-2435.13573 [DOI] [Google Scholar]
- Drent, R. H. and Stonehouse, B. (1971). Thermoregulatory responses of the peruvian penguin, Spheniscus humboldti. Comp. Biochem. Physiol. A Physiol. 40, 689-710. 10.1016/0300-9629(71)90254-4 [DOI] [PubMed] [Google Scholar]
- Elliott, K. H. and Gaston, A. J. (2014). Dive behaviour and daily energy expenditure in thick-billed murres Uria Lomvia after leaving the breeding colony. Mar. Ornithol. 42, 183-189. [Google Scholar]
- Elliott, K. H., Welcker, J., Gaston, A. J., Hatch, S. A., Palace, V., Hare, J. F., Speakman, J. R. and Anderson, W. G. (2013a). Thyroid hormones correlate with resting metabolic rate, not daily energy expenditure, in two charadriiform seabirds. Biol. Open 2, 580-586. 10.1242/bio.20134358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, K. H., Ricklefs, R. E., Gaston, A. J., Hatch, S. A., Speakman, J. R. and Davoren, G. K. (2013b). High flight costs, but low dive costs, in auks support the biomechanical hypothesis for flightlessness in penguins. Proc. Natl. Acad. Sci. USA 110, 9380-9384. 10.1073/pnas.1304838110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, H. I. (1984). Energetics of free-ranging seabirds. In Seabird Energetics (ed. Whittow G. C.), pp. 203-234. New York: Plenum Press. [Google Scholar]
- Frederiksen, M., Descamps, S., Erikstad, K. E., Gaston, A. J., Gilchrist, H. G., Grémillet, D., Johansen, K. L., Kolbeinsson, Y., Linnebjerg, J. F., Mallory, M. L.et al. (2016). Migration and wintering of a declining seabird, the thick-billed murre Uria lomvia, on an ocean basin scale: Conservation implications. Biol. Conserv. 200, 26-35. 10.1016/j.biocon.2016.05.011 [DOI] [Google Scholar]
- Gabrielsen, G., Ellis, H. (2002). Energetics of free-ranging seabirds. In Biology of Marine Birds (ed. Schreiber B. A. and Burger J.), pp. 359-407. Boca Raton: CRC Press. [Google Scholar]
- Gabrielsen, G. W., Mehlum, F. and Karlsen, H. E. (1988). Thermoregulation in 4 species of Arctic Seabirds. J. Comp. Physiol. B 157, 703-708. 10.1007/BF00691000 [DOI] [Google Scholar]
- Gaston, A. J. and Elliott, K. H. (2013). Effects of climate-induced changes in parasitism, predation and predator-predator interactions on reproduction and survival of an Arctic marine bird. Arctic 66, 43-51. 10.14430/arctic4265 [DOI] [Google Scholar]
- Gaston, A. J. and Hipfner, M. (1998). The effect of ice conditions in northern Hudson Bay on breeding by thick-billed murres (Uria lomvia). Can. J. Zool. 76, 480-492. 10.1139/z97-222 [DOI] [Google Scholar]
- Gaston, A. J. and Noble, D. G. (1985). The diet of thick-billed murres (Uria lomvia) in west Hudson Strait and north-east Hudson Bay. Can. J. Zool. 63, 1148-1160. 10.1139/z85-173 [DOI] [Google Scholar]
- Gaston, A. J., de Forest, L. N., Donaldson, G. and Noble, D. G. (1994). Population parameters of thick-billed murres at Coats Island, Northwest Territories, Canada. Condor 96, 935-948. 10.2307/1369103 [DOI] [Google Scholar]
- Gaston, A. J., Hipfner, J. M. and Campbell, D. (2002). Heat and mosquitoes cause breeding failures and adult mortality in an arctic-nesting seabird. Ibis (Lond. 1859). 144, 185-191. 10.1046/j.1474-919X.2002.00038.x [DOI] [Google Scholar]
- Gaston, A. J., Woo, K. and Hipfner, J. M. (2003). Trends in forage fish populations in northern Hudson Bay since 1981, as determined from the diet of nestling thick-billed murres Uria lomvia. Arctic 56, 227-233. 10.14430/arctic618 [DOI] [Google Scholar]
- Gaston, A. J., Gilchrist, H. G. and Hipfner, J. M. (2005). Climate change, ice conditions and reproduction in an Arctic nesting marine bird: Brunnich's guillemot (Uria lomvia L.). J. Anim. Ecol. 74, 832-841. 10.1111/j.1365-2656.2005.00982.x [DOI] [Google Scholar]
- Gaston, A. J., Gilchrist, H. G., Mallory, M. L. and Smith, P. A. (2009). Changes in seasonal events, peak food availability, and consequent breeding adjustment in a marine bird: a case of progressive mismatching. Condor 111, 111-119. 10.1525/cond.2009.080077 [DOI] [Google Scholar]
- Gaston, A. J., Mallory, M. L. and Gilchrist, H. G. (2012). Populations and trends of Canadian Arctic seabirds. Polar Biol. 35, 1221-1232. 10.1007/s00300-012-1168-5 [DOI] [Google Scholar]
- Gerson, A. R., Smith, E. K., Smit, B., McKechnie, A. E. and Wolf, B. O. (2014). The impact of humidity on evaporative cooling in small desert birds exposed to high air temperatures. Physiol. Biochem. Zool. 87, 782-795. 10.1086/678956 [DOI] [PubMed] [Google Scholar]
- Gerson, A. R., McKechnie, A. E., Smit, B., Whitfield, M. C., Smith, E. K., Talbot, W. A., McWhorter, T. J. and Wolf, B. O. (2019). The functional significance of facultative hyperthermia varies with body size and phylogeny in birds. Funct. Ecol. 33, 597-607. 10.1111/1365-2435.13274 [DOI] [Google Scholar]
- Guillemette, M., Woakes, A. J., Larochelle, J., Polymeropoulos, E. T., Granbois, J.-M., Butler, P. J., Pelletier, D., Frappell, P. B. and Portugal, S. J. (2016). Does hyperthermia constrain flight duration in a short-distance migrant? Philos. Trans. R. Soc. B Biol. Sci. 371, 20150386. 10.1098/rstb.2015.0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette, M., Polymeropoulos, E. T., Portugal, S. J. and Pelletier, D. (2017). It takes time to be cool: On the relationship between hyperthermia and body cooling in a migrating seaduck. Front. Physiol. 8, 1-10. 10.3389/fphys.2017.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins, P. A. J., Butler, P. J., Woakes, A. J. and Gabrielsen, G. W. (1997). Heat increment of feeding in Brunnich's guillemot Uria lomvia. J. Exp. Biol. 200, 1757-1763. 10.1242/jeb.200.12.1757 [DOI] [PubMed] [Google Scholar]
- Johnson, S. R. and West, G. C. (1975). Growth and development of heat regulation in nestlings, and metabolism of adult common and thick-billed murres. Ornis Scand. 6, 109-115. 10.2307/3676282 [DOI] [Google Scholar]
- Koller, M. (2016). robustlmm: an R package for robust estimation of linear mixed-effects models. J. Stat. Softw. 75, 1-24. 10.18637/jss.v075.i06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiewski, R. C. and Dawson, W. R. (1967). A re-examination of the relation between standard metabolic rate and body weight in birds. Condor 69, 13-23. 10.2307/1366368 [DOI] [Google Scholar]
- Lasiewski, R. C., Acosta, A. L. and Bernstein, M. H. (1966). Evaporative water loss in birds-I. Characteristics of the open flow method of determination, and their relation to estimates of thermoregulatory ability. Comp. Biochem. Physiol. 19, 445-457. 10.1016/0010-406X(66)90153-8 [DOI] [Google Scholar]
- Le Corre, M., Dussault, C. and Côté, S. D. (2017). Weather conditions and variation in timing of spring and fall migrations of migratory caribou. J. Mammal. 98, 260-271. 10.1093/jmammal/gyw177 [DOI] [Google Scholar]
- Lighton, J. R. B. (2019). Measuring Metabolic Rates: A Manual for Scientists, 2nd edn. Oxford: Oxford University Press. [Google Scholar]
- Logan, M. (2010). Biostatistical Design and Analysis using R: A Practical Guide. Chichester: Wiley-Blackwell. [Google Scholar]
- McBean, G., Alekseev, G., Chen, D., Førland, E., Fyfe, J., Groisman, P. Y. P., King, R., Melling, H., Vose, R. and Whitfield, P. P. H. (2005). Arctic climate: past and present. In Arctic Climate Impact Assessment (eds. Symon C., Arris L. and Heal B.), pp. 21-60. Cambridge, UK: Cambridge University Press. [Google Scholar]
- McKechnie, A. E. and Wolf, B. O. (2010). Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253-256. 10.1098/rsbl.2009.0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKechnie, A. E., Hockey, P. A. R. and Wolf, B. O. (2012). Feeling the heat: Australian landbirds and climate change. Emu 112, i-vii. 10.1071/MUv112n2_ED [DOI] [Google Scholar]
- McKechnie, A. E., Smit, B., Whitfield, M. C., Noakes, M. J., Talbot, W. A., Garcia, M., Gerson, A. R., Wolf, B. O., Mckechnie, A. E., Smit, B.et al. (2016). Avian thermoregulation in the heat: evaporative cooling capacity in an archetypal desert specialist, Burchell's sandgrouse (Pterocles burchelli). J. Exp. Biol. 219, 2137-2144. 10.1242/jeb.146563 [DOI] [PubMed] [Google Scholar]
- McKechnie, A. E., Gerson, A. R., McWhorter, T. J., Smith, E. K., Talbot, W. A. and Wolf, B. O. (2017). Avian thermoregulation in the heat: evaporative cooling in five Australian passerines reveals within-order biogeographic variation in heat tolerance. J. Exp. Biol. 220, 2436-2444. 10.1242/jeb.155507 [DOI] [PubMed] [Google Scholar]
- McWhorter, T. J., Gerson, A. R., Talbot, W. A., McKechnie, A. E., Smith, E. K. and Wolf, B. O. (2018). Avian thermoregulation in the heat: evaporative cooling capacity and thermal tolerance in two Australian parrots. J. Exp. Biol. 221, jeb168930. 10.1242/jeb.168930 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis, R., te Grotenhuis, M. and Pelzer, B. (2012). influence.ME: tools for detecting influential data in mixed effects models. R J. 4, 38-47. 10.32614/RJ-2012-011 [DOI] [Google Scholar]
- O'Connor, R. S., Wolf, B. O., Brigham, R. M. and McKechnie, A. E. (2017). Avian thermoregulation in the heat: efficient evaporative cooling in two southern African nightjars. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 187, 477-491. 10.1007/s00360-016-1047-4 [DOI] [PubMed] [Google Scholar]
- O'Connor, R. S., Le Pogam, A., Young, K. G., Robitaille, F., Choy, E. S., Love, O. P., Elliott, K. H., Hargreaves, A. L., Berteaux, D., Tam, A.et al. (2021). Limited heat tolerance in an Arctic passerine: thermoregulatory implications for cold-specialized birds in a rapidly warming world. Ecol. Evol. 11, 1609-1619. 10.1002/ece3.7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orben, R. A., Paredes, R., Roby, D. D., Irons, D. B. and Shaffer, S. A. (2015). Body size affects individual winter foraging strategies of thick-billed murres in the Bering Sea. J. Anim. Ecol. 84, 1589-1599. 10.1111/1365-2656.12410 [DOI] [PubMed] [Google Scholar]
- Oswald, K. N., Lee, A. T. K. and Smit, B. (2018). Seasonal physiological responses to heat in an alpine range-restricted bird: the Cape Rockjumper (Chaetops frenatus). J. Ornithol. 159, 1063-1072. 10.1007/s10336-018-1582-8 [DOI] [Google Scholar]
- Piatt, J. F., Parrish, J. K., Renner, H. M., Schoen, S. K., Jones, T. T., Arimitsu, M. L., Kuletz, K. J., Bodenstein, B., García-Reyes, M., Duerr, R. S.et al. (2020). Extreme mortality and reproductive failure of common murres resulting from the northeast Pacific marine heatwave of 2014-2016. PLoS One 15, 1-32. 10.1371/journal.pone.0226087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende, E. L. and Bacigalupe, L. D. (2015). Thermoregulation in endotherms: physiological principles and ecological consequences. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 185, 709-727. 10.1007/s00360-015-0909-5 [DOI] [PubMed] [Google Scholar]
- Richman, S. E. and Lovvorn, J. R. (2011). Effects of air and water temperatures on resting metabolism of auklets and other diving birds. Physiol. Biochem. Zool. 84, 316-332. 10.1086/660008 [DOI] [PubMed] [Google Scholar]
- Smit, B. and Mckechnie, A. E. (2015). Water and energy fluxes during summer in an arid-zone passerine bird. Ibis (Lond. 1859). 157, 774-786. 10.1111/ibi.12284 [DOI] [Google Scholar]
- Smith, E. K., O'Neill, J., Gerson, A. R. and Wolf, B. O. (2015). Avian thermoregulation in the heat: Resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert doves and quail. J. Exp. Biol. 218, 3636-3646. 10.1242/jeb.128645 [DOI] [PubMed] [Google Scholar]
- Smith, E. K., O'Neill, J. J., Gerson, A. R., McKechnie, A. E. and Wolf, B. O. (2017). Avian thermoregulation in the heat: resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert songbirds. J. Exp. Biol. 220, 3290-3300. 10.1242/jeb.161141 [DOI] [PubMed] [Google Scholar]
- Song, S. and Beissinger, S. R. (2020). Environmental determinants of total evaporative water loss in birds at multiple temperatures. Auk 137, 1-12. 10.1093/auk/ukz069 [DOI] [Google Scholar]
- Speakman, J. R. and Król, E. (2010). Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726-746. 10.1111/j.1365-2656.2010.01689.x [DOI] [PubMed] [Google Scholar]
- Stahel, C. D. and Nicol, S. C. (1982). Temperature regulation in the little penguin, Eudyptula minor, in air and water. J. Comp. Physiol. B 148, 93-100. 10.1007/BF00688892 [DOI] [Google Scholar]
- Talbot, W. A., Gerson, A. R., Smith, E. K., McKechnie, A. E. and Wolf, B. O. (2018). Avian thermoregulation in the heat: metabolism, evaporative cooling and gular flutter in two small owls. J. Exp. Biol. 221, jeb171108. 10.1242/jeb.171108 [DOI] [PubMed] [Google Scholar]
- Tieleman, B. I. and Williams, J. B. (1999). The role of hyperthermia in the water economy of desert birds. Physiol. Biochem. Zool. 72, 87-100. 10.1086/316640 [DOI] [PubMed] [Google Scholar]
- Tieleman, B. I., Williams, J. B., LaCroix, F. and Paillat, P. (2002). Physiological responses of Houbara bustards to high ambient temperatures. J. Exp. Biol. 205, 503-511. 10.1242/jeb.205.4.503 [DOI] [PubMed] [Google Scholar]
- van Dyk, M., Noakes, M. J. and McKechnie, A. E. (2019). Interactions between humidity and evaporative heat dissipation in a passerine bird. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 189, 299-308. 10.1007/s00360-019-01210-2 [DOI] [PubMed] [Google Scholar]
- Walsberg, G. E. and Wolf, B. O. (1995). Variation in the respiratory quotient of birds and implications for indirect calorimetry using measurements of carbon dioxide production. J. Exp. Biol. 198, 213-219. 10.1242/jeb.198.1.213 [DOI] [PubMed] [Google Scholar]
- Weathers, W. W. (1981). Physiological thermoregulation in heat-stressed birds: consequences of body size. Physiol. Zool. 54, 345-361. 10.1086/physzool.54.3.30159949 [DOI] [Google Scholar]
- Weathers, W. W. and Greene, E. (1998). Thermoregulatory responses of bridled and juniper titmice to high temperature. Condor 100, 365-372. 10.2307/1370278 [DOI] [Google Scholar]
- Whitfield, M. C., Smit, B., McKechnie, A. E. and Wolf, B. O. (2015). Avian thermoregulation in the heat: scaling of heat tolerance and evaporative cooling capacity in three southern African arid-zone passerines. J. Exp. Biol. 218, 1705-1714. 10.1242/jeb.121749 [DOI] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. [Google Scholar]
- Williams, B. (1996). A phylogenetic perspective of evaporative water loss in birds. Auk 113, 457-472. 10.2307/4088912 [DOI] [Google Scholar]
- Yurkowski, D. J., Hussey, N. E., Ferguson, S. H. and Fisk, A. T. (2018). A temporal shift in trophic diversity among a predator assemblage in a warming Arctic. R. Soc. Open Sci. 5, 180259. 10.1098/rsos.180259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Flato, G., Kirchmeier-Young, M., Vincent, L., Wan, H., Wang, X., Rong, R., Fyfe, J., Li, G. and Kharin, V. (2019). Changes in temperature and precipitation across Canada. In Canada's Changing Climate Report, pp. 112-193 (ed. Bush E. and Lemmen D.). https://changingclimate.ca/CCCR2019/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.