Abstract
Mummified remains have long attracted interest as a potential source of ancient DNA. However, mummification is a rare process that requires an anhydrous environment to rapidly dehydrate and preserve tissue before complete decomposition occurs. We present the whole-genome sequences (3.94 X) of an approximately 1600-year-old naturally mummified sheep recovered from Chehrābād, a salt mine in northwestern Iran. Comparative analyses of published ancient sequences revealed the remarkable DNA integrity of this mummy. Hallmarks of postmortem damage, fragmentation and hydrolytic deamination are substantially reduced, likely owing to the high salinity of this taphonomic environment. Metagenomic analyses reflect the profound influence of high-salt content on decomposition; its microbial profile is predominated by halophilic archaea and bacteria, possibly contributing to the remarkable preservation of the sample. Applying population genomic analyses, we find clustering of this sheep with Southwest Asian modern breeds, suggesting ancestry continuity. Genotyping of a locus influencing the woolly phenotype showed the presence of an ancestral ‘hairy’ allele, consistent with hair fibre imaging. This, along with derived alleles associated with the fat-tail phenotype, provides genetic evidence that Sasanian-period Iranians maintained specialized sheep flocks for different uses, with the ‘hairy’, ‘fat-tailed’-genotyped sheep likely kept by the rural community of Chehrābād's miners.
Keywords: ancient DNA, sheep, mummy
1. Introduction
In 1993, a remarkably preserved human body dating to approximately 1700 years Before Present (BP) was discovered in the Douzlākh salt mine near Chehrābād village in the Zanjan Province of northwest Iran [1–3]. A total of eight ‘Salt Men’ have been identified at the mine [4,5], several retaining keratinous tissues such as skin, hair and both endo- and exoparasites, despite dating to the Achaemenid (550–330 BCE, 2500–2280 BP) and Sasanian (224–651 CE, approximately 1700–1300 BP) periods. The mine, also known as Chehrābād, was active in various periods, and its archaeological refilling layers represent an extraction history that ranged from the sixth century BCE to twentieth-century CE. In addition to the ‘Salt Men’, textiles, leather objects and animal remains have been discovered [6,7], likely preserved by the high salinity and low moisture content of the mine. Isotopic, genetic and lipid analyses have been reported for this material [1], and studies have been carried out to characterize genomic DNA survival [8]. These human and animal remains are examples of natural mummification—the spontaneous desiccation of soft tissue by a dry environment that rapidly dehydrates soft tissue before decay begins [9].
Mummification has been suggested as a mechanism that may sufficiently preserve keratinized tissue for ancient DNA (aDNA) sequencing [9]. The effects of age-related damage in aDNA are well documented and include base misincorporation at strand overhangs, fragmentation and low endogenous content [10]. Both deamination and depurination, associated with postmortem transition error and DNA fragmentation, respectively, require water as a substrate [10]. Ancient DNA from Chehrābād, a highly saline, anhydrous environment, presents an opportunity to investigate potential differences in nucleotide degradation resulting from this unusual taphonomic context.
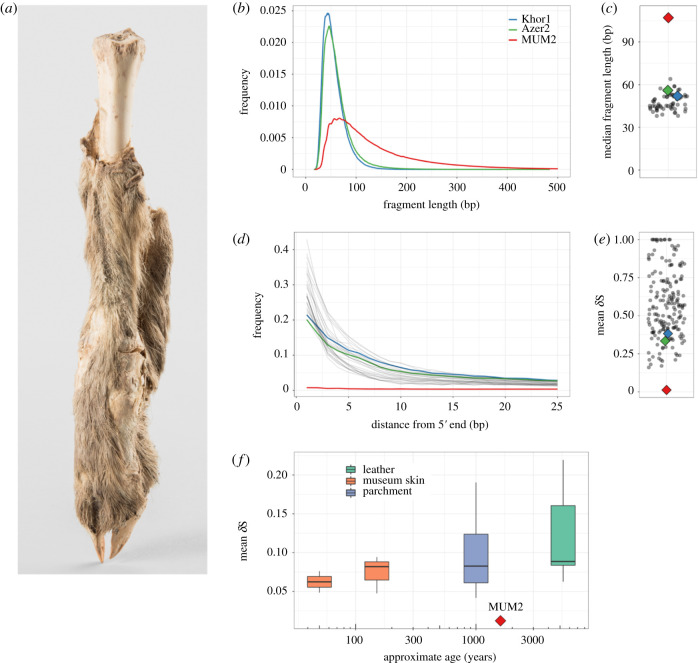
In this study, we sequenced DNA from the approximately 1600-year-old (Sasanian period) mummified sheep leg 4305, recently discovered in a large mining gallery in the northwestern edge of the Douzlākh saltmine of Chehrābād by Iranian–German researchers during archaeological excavations (figure 1a) [2]. The specimen was likely deposited during refilling activities in the fourth–fifth centuries CE after the gallery's reopening in the Early Sasanian period (second–third centuries CE) and following its initial collapse between 405 and 380 BCE. The leg was possibly discarded during food preparation activities, as both sheep and goat were likely used as provisioning for Sasanian-period miners; equines may have been used as beasts of burden [16]. By this time, sheep were an established commodity for their meat and secondary products such as wool fibre, which was widespread by the fourth millennium BCE and showed regional specialization by the third millennium BCE [17].
Figure 1.
(a) Mummified sheep leg (4305) after cleaning. Photography: N. Tehrani. (b) Read length distributions of MUM2, Khor1 and Azer2, calculated from PE data. MUM2 shows a reduced rate of fragmentation. The median read length of MUM2 (107 bp) exceeds the median read length of Khor1 and Azer2 (52 bp and 56 bp, respectively). (c) Median read lengths of 61 published ancient Ovicaprid samples [11]. The median read length of MUM2 (107 bp; 90 bp among collapsed reads only) exceeds the longest among published ovicaprid genomes (64 bp). (d) Deamination patterns of MUM2, Khor1, Azer2 and other ancient ovicaprids for non UDG-treated libraries. Low levels of base misincorporation at the 5′ ends of reads were observed for MUM2 compared to Khor1 and Azer2. (e) Mean δS of 182 published ancient bone samples [11,12]. The mean δS of MUM2 (0.012) is unusual in its low levels of deamination. (f) Comparison of mean δS of published ancient skins [13–15]. Lower damage rates are recorded compared to all samples, including some of approximately 50 years old.
We find unusual survival patterns of endogenous DNA given its distance from the equator, implying that exceptional preservation of nucleic acid integrity was afforded by the unique salt-rich environment. This enables characterization of the mummy skin metagenome and population genomic profiling of this sheep in the context of modern breeds. We also genotype 51 SNPs within the first intron of the platelet-derived growth factor D (PDGFD) that are highly differentiated between fat-tailed and thin-tailed breeds [18]. Finally we genotype the antisense EIF2S2 retrogene insertion within the 3′ UTR of the IRF2BP2 gene that influences the woolly phenotype and is derived relative to the ancestral coarse ‘hairy’ coat [19], in tandem with fibre analysis using scanning electron microscopy (SEM).
2. Material and methods
A sample of the mummified sheep skin (table 1, MUM2) from sheep leg 4305 was directly radiocarbon dated at the 14CHRONO Centre (Queen's University Belfast). OxCal 4.3.2 [20] was used to calibrate its age (95.4% confidence interval) using [21].
Table 1.
Summary information of samples sequenced in this study.
| name | tissue | origin | period | age | endogenous DNA % | coverage (X) |
|---|---|---|---|---|---|---|
| MUM2 | mummified skin | Chehrābād, Iran | Sasanian Empire period | 399–539 cal CEa | 31.01 | 3.94 |
| Khor1 | petrous bone | Nishapur, Iran | Sasanian–Islamic periods | 600–1200 CE | 58.44 | 0.04 |
| Azer2 | petrous bone | Tepe Hasanlu, Iran | Iron Age III | 800–600 BCE | 31.32 | 0.07 |
adirectly dated.
Sample preparation, extraction and library preparation were performed in a dedicated aDNA laboratory in the Smurfit Institute of Genetics, Trinity College Dublin according to standard protocols (see electronic supplementary material). Sequencing of MUM2 and two Iranian sheep bone samples (Khor1 and Azer2) of approximately similar ages (table 1) for comparison was performed on Illumina MiSeq (50 bp SE) and HiSeq 2500 platforms (100 bp SE and 100 bp paired-end (PE)).
Sequencing reads were aligned to OviAri3.1 and filtered to produce bam files following standard aDNA sequencing pipelines (electronic supplementary material). Damage patterns were assessed using mapDamage2.0 [22].
Filtered reads not aligned to either sheep or human genomes were taxonomically assigned using the metagenomic classifier Kraken 2 [23]. Microbial sources were estimated using SourceTracker2 [24] with a custom metagenomic database [25–29] (electronic supplementary material). Bacterial species abundances were generated using MIDAS [30].
Mitochondrial sequences were produced using ANGSD [31] and a maximum-likelihood phylogenetic tree was generated using SeaView and PhyML [32–34] with the HKY85 substitution model, selected using jModelTest2 [35,36] and 100 bootstrap repeats.
A SNP dataset of modern breeds [37] was used to investigate genomic affinities (electronic supplementary material). LASER (v. 2.03) principal components analysis (PCA) [38], outgroup f3 statistics [39], TreeMix [40] and ADMIXTURE [41] analyses were completed (electronic supplementary material, table S5).
We investigated the woolly locus located on chromosome 25 [19]. Two modified OviAri3.1 assemblies were produced, one representing the ancestral ‘hairy’ phenotype, the other representing the ‘woolly’ phenotype (electronic supplementary material). Final bam files were visualized using IGV [42]. Hair fibres were examined using scanning electron and light microscopes at USTEM, TU Wien and Austrian Archaeological Institute, respectively. We assessed 51 SNPs in the PDGFD gene associated with the derived fat-tail phenotype [18], using the genotype calls of modern fat and thin-tailed breeds to define the derived allele [43,44]. As the average genome coverage was too low for accurate diploid genotype calls, we report base calls for both alleles.
3. Results and discussion
(a) . DNA preservation and metagenomics
The Chehrābād mummy sample (MUM2) was directly dated to the fifth–sixth century CE (2 sigma 1621–1481 cal BP, uncalibrated 1600 ± 30 BP; electronic supplementary material, figure S3). This aligns with the Sasanian Empire period of Iran, a time when the mine was in active use [1]. Initial DNA screening indicated high endogenous DNA for MUM2, and also the comparative Iranian sheep samples from relatively close time periods (table 1).
Sequencing of the Chehrābād mummy produced a 3.94 X genome after quality filtering (electronic supplementary material, table S1), in addition to the low coverage comparative genomes (0.0 4 X and 0.07 X). MUM2 differs from the two comparative sheep samples in displaying longer fragment lengths (median 107 bp versus 52 bp and 56 bp; figure 1b; collapsed reads-only 90 bp versus 50 bp and 55 bp) and substantially lower rates of deamination (figure 1d) (δS, single-strand cytosine deamination probability, mean δS = 0.012 versus 0.382 and 0.334). Contrasting previously published ancient ovicaprid data from Southwest Asia and Europe (electronic supplementary material, table S3), MUM2 falls outside the ranges of both median fragment length and mean δS values (figure 1c,e), indicating remarkably low fragmentation and deamination of the Chehrābād sheep mummy genomic material given its latitude. Similar length distributions have been reported primarily from high latitude and permafrost environments [45–48]. A low level of thermal fluctuations may also contribute to DNA preservation [12], as comparable fragment lengths have been reported in a human sample from Wezmeh Cave, Iran [49].
Recent models of postmortem DNA fragmentation suggest rate-constant hydrolytic depurination over time [50], or age-independence, driven by environment-dependent biotic and abiotic factors [12]. The depurination rates of MUM2 are similar to the more-fragmented comparative samples (electronic supplementary material, figure S4), implying that other processes in the Chehrābād environment underlie the lower fragmentation rates. The highly alkaline, cool and anhydrous conditions may have contributed to the inhibition of cellular nucleases that would otherwise degrade and fragment endogenous DNA [9]. Postmortem DNA deamination via cytosine hydrolysis [51] is thought to be strongly correlated with age [52] and thermal age [12]. The substantially lower rates of deamination observed in MUM2 are likely owing to the scarcity of environmental free water, required for hydrolytic deamination. These results are consistent with Chehrābād providing a taphonomic environment conducive to genome preservation.
DNA preservation may also be influenced by its tissue-of-origin; for example, bone hydroxyapatite rather than keratin fractions is associated with smaller fragment size [53]. As hydrophobic keratinized tissue may provide resistance to environmental water [54], we compared MUM2 to published genomes of ancient skins (figure 1f) to determine if tissue providence was solely responsible for DNA preservation. The mean δS of MUM2 falls outside the range of other ancient skin genomes, including twentieth-century CE goat skins [13] and leather recovered from the Tyrolean Iceman [14]. While this does not discount keratinized tissue being specifically enriched with longer DNA fragments, the Chehrābād sheep mummy appears to be singular in its DNA integrity among published skin samples.
Given the distinctive geochemical composition of Chehrābād, we examined if its salt-rich environment was reflected in the metagenomic profile of MUM2. Taxonomic assignment and abundance estimation assigned 57.13% of classified reads to the halophilic Class of Archaea Halobacteria (electronic supplementary material, table S2). Similarly, SourceTracker2 predicted that 0.4725–0.7458 of the microbial community originated from a salt-rich environment (table 2; electronic supplementary material, figure S5). A complementary analysis using MIDAS identified 76 unique bacterial species in the mummified sheep (electronic supplementary material, table S4). The most abundant species is the halophilic bacterium Actinopolyspora halophila 58532, accounting for approximately 29% of identified reads. This signal of a dominant halophilic microbial community is not replicated in comparison samples or controls (table 2; electronic supplementary material). Rapid colonization by saprophytic microbial communities, with key decomposers being ubiquitous across soil types, is typical for mammalian corpses postmortem [55]. The halophilic metagenome profile observed in the Chehrābād sheep mummy skin indicates that the typical decomposers may be less abundant in this alkaline, salt-rich setting, which may have contributed to soft tissue and molecular preservation.
Table 2.
Predicted source proportion of metagenomic reads by SourceTracker2.
| MUM2 (species) | Azer2 (species) | Khor1 (species) | MUM2 (genus) | Azer2 (genus) | Khor1 (genus) | |
|---|---|---|---|---|---|---|
| tissue decomposers | 0.0212 | 0.1458 | 0.0386 | 0.0426 | 0.3524 | 0.3727 |
| salt-rich | 0.4725 | 0.0026 | 0.0036 | 0.7458 | 0.0145 | 0.0151 |
| laboratory reagents | 0.0003 | 0.0002 | 0.0002 | N/A | N/A | N/A |
| sheep skin | 0.0285 | 0.0850 | 0.0463 | 0.0829 | 0.3294 | 0.2244 |
| soil | 0.0009 | 0.0007 | 0.0046 | 0 | 0 | 0 |
| unknown | 0.4766 | 0.7657 | 0.9067 | 0.1287 | 0.3037 | 0.3878 |
(b) . Population genomics
We investigated how the Chehrābād sheep MUM2 relates to modern populations using a mitochondrial and autosomal variation. A 664 X mitochondrial genome of MUM2 falls within the C haplotype cluster in a maximum-likelihood phylogeny of modern sheep mitochondria (electronic supplementary material, figure S7). This clade is found at its highest frequency in southwest and east Asia [56,57], has been reported in ancient samples from Bronze Age Turkey [58] and is consistent with past and present-day patterns of mitochondrial diversity.
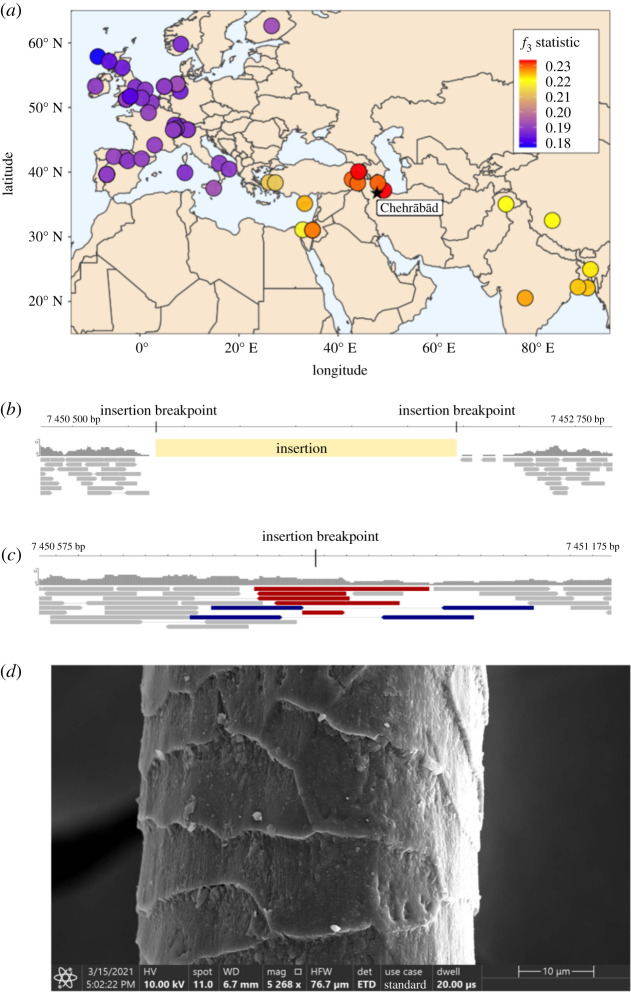
PCA from autosomal variation clusters MUM2 with modern southwest Asian breeds, using both global and Asian reference panels (electronic supplementary material, figure S8). f3 outgroup statistics show that MUM2 shares the most genetic drift with southwest Asian breeds, particularly those from Iran (figure 2a). ADMIXTURE and TreeMix analysis also confirmed the affinity of MUM2 with modern sheep breeds from southwest Asia (electronic supplementary material). Overall, there is genetic continuity between west Iranian sheep populations in Sassanid and modern time periods, although PCA using Ovine SNP50 genotypes of Asian breeds places MUM2 apart from sampled breeds (electronic supplementary material, figure S9), suggesting a degree of genetic flux during the past 1500–1600 years in Iranian sheep. This is consistent with evidence for genetic exchange across Asia prior to the development of modern breeds [59–61].
Figure 2.
(a) Shared genetic drift between MUM2 and modern sheep populations. Higher f3 values, in red, indicate higher shared drift, relative to the outgroup Asiatic mouflon. Visualization of read coverage of filtered bam files at woolly locus, in assemblies with (b) and without (c) the insertion. Reads highlighted in red overlap the insertion breakpoint, blue indicates an inferred overlap of the insertion point by the straddling read pair. Highlighted reads map only to one assembly and do not align to the other. (d) SEM image of MUM2 hair fibre, displaying the mosaic scales typical of a sheep hair shaft. Image by A. Steiger-Thirsfeld and G. Ruß-Popa.
(c) . Fibre genotype and phenotype and fat-tail genotype
The derived ‘woolly’ coat phenotype is thought to be influenced by an approximately 1.5 kbp insertion of a EIF2S2 retrogene into the IRF2BP2 3′ UTR, recessive to the ancestral allele associated with ‘hairy’ coat [19]. We exploited the length of the MUM2 DNA fragments to investigate this ‘woolly’ locus by searching for read pairs that either encompassed or overlapped the insertion breakpoint, indicative of a copy of the ‘hairy’ allele. No reads were found to overlap the diagnostic insertion breakpoints of the ‘woolly’ insert, which would indicate a copy of the ‘woolly’ allele (figure 2b). Five reads were found to uniquely map to the ‘hairy’ allele diagnostic position, with a further two read pairs inferred to overlap this breakpoint (figure 2c). We therefore infer this animal to be either homozygous or heterozygous for the dominant ‘hairy’ allele. In addition, SEM imaging of the mostly unpigmented mummified hair fibres revealed mosaic scales typical of sheep [62], with fine lines on the scale surface (figure 2d; electronic supplementary material), a characteristic of sheep hair fibres and particularly for mouflon and medium-wool breeds [63]. This may reflect MUM2 coming from a herd maintained for meat or milk production rather than wool, consistent with suggestions that ovicaprids were used as food for workers, and that sections of the mine were used as stables [1].
We also find evidence of a fat-tail associated allele (48/51 SNPs) (electronic supplementary material, table S6) at PDGFD, a gene likely controlling tail phenotype [18,44]. This observation, along with MUM2 sharing mitochondrial haplotype C with the majority of modern fat-tailed breeds [61], and the genomic affinity of MUM2 to modern fat-tailed breeds, although based on SNP-chip data, is intriguing. While we cannot determine the MUM2 tail phenotype directly, its genotype is similar to a medium-wool or hairy-coated fat-tail breed [1]. Hairy-coated sheep may have lower mortality rates, have higher birth weights, and be more robust than woolly coated [64], while fat-tail breeds are thought to be better adapted to arid environments [18]. If phenotypically similar to these sheep breeds, the flock represented by MUM2 could have provided a reliable meat and fat source for Chehrābād's miners. The faunal assemblage of the mine and paleoparasitological studies, although not very abundant, support the fact that sheep/goats were the most consumed animals by the miners [6,7,16,65].
Both woolly and fat-tailed sheep are depicted in the Early Bronze Age Mesopotamia but the spread of these phenotypes may have been uncoupled, and occurred via distinct processes [66,67]. Fat-tailed breeds were likely introduced from Southwest to East Asia in a period (700 BCE–1000 CE) broadly coinciding with the age of MUM2 [60]; the observed PDGFD genotype supports an ancient origin of this economically important trait. Wider aDNA analysis may elucidate when woolly and fat-tailed associated genotypes arose and how they may have influenced sheep breed development, which have their origins in fourth millennium BCE Mesopotamia [66,67]. Although the archaeozoological assemblages in the Iranian Plateau from the Antiquity and later Mediaeval periods are still limited, the diversity of the size of sheep bones is already an indication of the diversification of breeds in these periods [65,68]. Our results are consistent with MUM2 deriving from a herd used for meat and/or milk rather than wool production, and reflect sophisticated Sasanian-period husbandry practices and specialized sheep breeding.
Acknowledgements
We thank Daniel Bradley for his mentorship, discussions and reading of the paper; Andreas Steiger-Thirsfeld for SEM images and N. Tehrani for sample photography.
Data accessibility
Sequencing reads and mitochondrial sequences are available under European Nucleotide Archive accession PRJEB43881.
The data are provided in the electronic supplementary material [69].
Authors' contributions
C.R., K.G.D. and M.M. designed study; C.R., K.G.D., V.M., A.J.H. and F.M. performed laboratory work; C.R. and M.D.T. performed bioinformatic work; G.R.-P. was responsible for SEM analysis; H.D., H.L., Z.L., R.K., H.F., A.A., T.S. and M.M. worked directly with and provided the archaeological samples. All authors contributed to writing the manuscript, approve this study, and are accountable for all aspects of the work.
Competing interests
We declare we have no competing interests.
Funding
Study supported by ERC Investigator grant no. 295729-CodeX. M.D.T. was additionally supported by ERC investigator grant no. 787282-B2C.
References
- 1.Aali A, Stöllner T, Abar A, Rühli F. 2012. The salt men of Iran: the salt mine of Douzlākh. Chehrābād. 42, 61-81. [Google Scholar]
- 2.Aali A, Stöllner T. (eds) 2015. The archaeology of the Salt Miners. Interdisciplinary Research 2010–2014. Bochum, Germany: Metalla. [Google Scholar]
- 3.Stöllner T, Aali A, Bagherpour Kashani N. 2020. Tod im Salz. Eine archäologische Ermittlung in Persien. Begleitbuch, Katalog und graphic novel. Bochum, Germany: Nünnerich-Asmus Verlag Media. [Google Scholar]
- 4.Öhrström LM, Seiler R, Böni T, Aali A, Stöllner T, Rühli FJ. 2016. Erratum to: Radiological findings in an ancient Iranian salt mummy (Chehrābād ca. 410–350 BC). Skeletal Radiol. 45, 433. ( 10.1007/s00256-015-2316-0) [DOI] [PubMed] [Google Scholar]
- 5.Nasab H V, Aali A, Kazzazi M, Pollard M, Stöllner T. 2019. Reappraisal of the number of salt mummies identified in Chehrābād Salt Mine, Zanjan, Iran. Bioarchaeol. Near East 13, 23-47. [Google Scholar]
- 6.Mashkour M. 2014. Faunal report of the 2010 and 2011 excavation campaigns. In The archaoelogy of the salt miners (eds Aali A, Stöllner T), pp. 112-117. Bochum, Germany: Metatta. [Google Scholar]
- 7.Mashkour M, Fathi H, Davoudi H. 2020. Interaktionen zwischen Mensch und Tier im Salzbergwerk Douzlākh, Chehrābād. In Tod im salz. Eine archäologische Ermittlung in Persien. Begleitbuch, Katalog und graphic novel (eds Stöllner T, Aali A, Bagherpour Kashani N), pp. 219-224. Veröffentlichungen aus dem Deutschen Bergbau-Museum Bochum 246, Bochum/Oppenheim, Germany: Nünnerich-Asmus Verlag Media. [Google Scholar]
- 8.Warinner C, Bouwman A. 2015. Ancient DNA investigation of the Chehrābād Mummies: Interim Report. In The archaeology of the salt miners: interdisciplinary research 2010–2014 (eds Aali A, Stöllner T), pp. 90-96. Bochum, Germany: Deutsches Bergbau-Museum. [Google Scholar]
- 9.David R. 2008. Egyptian mummies and modern science. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Dabney J, Meyer M, Pääbo S. 2013. Ancient DNA damage. Cold Spring Harb. Perspect. Biol. 5, a012567. ( 10.1101/cshperspect.a012567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly KG, et al. 2018. Ancient goat genomes reveal mosaic domestication in the Fertile Crescent. Science 361, 85-88. ( 10.1126/science.aas9411) [DOI] [PubMed] [Google Scholar]
- 12.Kistler L, Ware R, Smith O, Collins M, Allaby RG. 2017. A new model for ancient DNA decay based on paleogenomic meta-analysis. Nucleic Acids Res. 45, 6310-6320. ( 10.1093/nar/gkx361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassidy LM, Teasdale MD, Carolan S, Enright R, Werner R, Bradley DG, Finlay EK, Mattiangeli V. 2017. Capturing goats: documenting two hundred years of mitochondrial DNA diversity among goat populations from Britain and Ireland. Biol. Lett. 13, 20160876. ( 10.1098/rsbl.2016.0876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Sullivan NJ, Teasdale MD, Mattiangeli V, Maixner F, Pinhasi R, Bradley DG, Zink A. 2016. A whole mitochondria analysis of the Tyrolean Iceman's leather provides insights into the animal sources of Copper Age clothing. Sci. Rep. 6, 31279. ( 10.1038/srep31279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teasdale MD, et al. 2017. The York Gospels: a 1000-year biological palimpsest. R. Soc. Open Sci. 4, 170988. ( 10.1098/rsos.170988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nezamabadi M, Aali A, Stöllner T, Mashkour M, Le Bailly M. 2013. Paleoparasitological analysis of samples from the Chehrabad salt mine (Northwestern Iran). Int. J. Paleopathol. 3, 229-233. ( 10.1016/j.ijpp.2013.03.003) [DOI] [PubMed] [Google Scholar]
- 17.Arbuckle BS, Hammer EL. 2019. The rise of pastoralism in the Ancient Near East. J. Archaeol. Res. 27, 391-449. ( 10.1007/s10814-018-9124-8) [DOI] [Google Scholar]
- 18.Dong K, et al. 2020. Genomic analysis of worldwide sheep breeds reveals PDGFD as a major target of fat-tail selection in sheep. BMC Genomics 21, 800. ( 10.1186/s12864-020-07210-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demars J, et al. 2017. Genome-wide identification of the mutation underlying fleece variation and discriminating Ancestral Hairy Species from Modern Woolly Sheep. Mol. Biol. Evol. 34, 1722-1729. ( 10.1093/molbev/msx114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsey CB. 2009. Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337-360. ( 10.1017/S0033822200033865) [DOI] [Google Scholar]
- 21.Reimer PJ, et al. 2013. IntCal13 and marine13 radiocarbon age calibration curves 0–50,000 Years cal BP. Radiocarbon 55, 1869-1887. ( 10.2458/azu_js_rc.55.16947) [DOI] [Google Scholar]
- 22.Jónsson H, Ginolhac A, Schubert M, Johnson PLF, Orlando L. 2013. mapDamage2.0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29, 1682-1684. ( 10.1093/bioinformatics/btt193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood DE, Lu J, Langmead B. 2019. Improved metagenomic analysis with Kraken 2. Genome Biol. 20, 257. ( 10.1186/s13059-019-1891-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knights D, Kuczynski J, Charlson ES, Zaneveld J, Mozer MC, Collman RG, Bushman FD, Knight R, Kelley ST. 2011. Bayesian community-wide culture-independent microbial source tracking. Nat. Methods 8, 761-763. ( 10.1038/nmeth.1650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emmons AL, Mundorff AZ, Keenan SW, Davoren J, Andronowski J, Carter DO, Debruyn JM. 2020. Characterizing the postmortem human bone microbiome from surface-decomposed remains. PLoS ONE 15, e0218636. ( 10.1371/journal.pone.0218636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson CK, et al. 2015. Microbial diversity and the presence of algae in halite endolithic communities are correlated to atmospheric moisture in the hyper-arid zone of the Atacama Desert. Environ. Microbiol. 17, 299-315. ( 10.1111/1462-2920.12364) [DOI] [PubMed] [Google Scholar]
- 27.Ross AA, Müller KM, Scott Weese J, Neufeld JD. 2018. Comprehensive skin microbiome analysis reveals the uniqueness of human skin and evidence for phylosymbiosis within the class Mammalia. Proc. Natl Acad. Sci. USA 115, E5786-E5795. ( 10.1073/pnas.1801302115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreira-Grez B, Tam K, Cross AT, Yong JWH, Kumaresan D, Nevill P, Farrell M, Whiteley AS. 2019. the bacterial microbiome associated with arid biocrusts and the biogeochemical influence of biocrusts upon the underlying soil. Front. Microbiol. 10, 2143. ( 10.3389/fmicb.2019.02143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salter SJ, et al. 2014. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 12, 1-12. ( 10.1186/s12915-014-0087-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nayfach S, Rodriguez-Mueller B, Garud N, Pollard KS. 2016. An integrated metagenomics pipeline for strain profiling reveals novel patterns of bacterial transmission and biogeography. Genome Res. 26, 1612-1625. ( 10.1101/gr.201863.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korneliussen TS, Albrechtsen A, Nielsen R. 2014. ANGSD: analysis of next generation sequencing data. BMC Bioinf. 15, 356. ( 10.1186/s12859-014-0356-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792-1797. ( 10.1093/nar/gkh340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gouy M, Guindon S, Gascuel O. 2010. SeaView Version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221-224. ( 10.1093/molbev/msp259) [DOI] [PubMed] [Google Scholar]
- 34.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307-321. ( 10.1093/sysbio/syq010) [DOI] [PubMed] [Google Scholar]
- 35.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772-772. ( 10.1038/nmeth.2109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696-704. ( 10.1080/10635150390235520) [DOI] [PubMed] [Google Scholar]
- 37.Kijas JW, et al. 2012. Genome-wide analysis of the World's sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 10, e1001258. ( 10.1371/journal.pbio.1001258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Zhan X, Liang L, Abecasis GR, Lin X. 2015. Improved ancestry estimation for both genotyping and sequencing data using projection Procrustes analysis and genotype imputation. Am. J. Hum. Genet. 96, 926-937. ( 10.1016/j.ajhg.2015.04.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson N, Moorjani P, Luo Y, Mallick S, Rohland N, Zhan Y, Genschoreck T, Webster T, Reich D. 2012. Ancient admixture in human history. Genetics 192, 1065-1093. ( 10.1534/genetics.112.145037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickrell JK, Pritchard JK. 2012. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet. 8, e1002967. ( 10.1371/journal.pgen.1002967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander DH, Lange K. 2011. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinf. 12, 1-6. ( 10.1186/1471-2105-12-246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. 2011. Integrative genomics viewer. Nat. Biotechnol. 29, 24-26. ( 10.1038/nbt.1754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor WTT, et al. 2021. Evidence for early dispersal of domestic sheep into Central Asia. Nat. Hum. Behav. ( 10.1038/s41562-021-01083-y) [DOI] [PubMed] [Google Scholar]
- 44.Li X, et al. 2020. Whole-genome resequencing of wild and domestic sheep identifies genes associated with morphological and agronomic traits. Nat. Commun. 11, 2815. ( 10.1038/s41467-020-16485-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller W, et al. 2012. Polar and brown bear genomes reveal ancient admixture and demographic footprints of past climate change. Proc. Natl Acad. Sci. USA 109, E2382-E2390. ( 10.1073/pnas.1210506109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orlando L, et al. 2013. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 499, 74-78. ( 10.1038/nature12323) [DOI] [PubMed] [Google Scholar]
- 47.Librado P, et al. 2015. Tracking the origins of Yakutian horses and the genetic basis for their fast adaptation to subarctic environments. Proc. Natl Acad. Sci. USA 112, E6889-E6897. ( 10.1073/pnas.1513696112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sikora M, et al. 2019. The population history of northeastern Siberia since the Pleistocene. Nature 570, 182-188. ( 10.1038/s41586-019-1279-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broushaki F, et al. 2016. Early Neolithic genomes from the eastern Fertile Crescent. Science 353, 499-503. ( 10.1126/science.aaf7943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allentoft ME, et al. 2012. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils. Proc. R. Soc. B 279, 4724-4733. ( 10.1098/rspb.2012.1745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Briggs AW, Stenzel U, Meyer M, Krause J, Kircher M, Pääbo S. 2010. Removal of deaminated cytosines and detection of in vivo methylation in ancient DNA. Nucleic Acids Res. 38, e87. ( 10.1093/nar/gkp1163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawyer S, Krause J, Guschanski K, Savolainen V, Pääbo S. 2012. Temporal patterns of nucleotide misincorporations and DNA fragmentation in ancient DNA. PLoS ONE 7, e34131. ( 10.1371/journal.pone.0034131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwarz C, Debruyne R, Kuch M, Mcnally E, Schwarcz H, Aubrey AD, Bada J, Poinar H. 2009. New insights from old bones: DNA preservation and degradation in permafrost preserved mammoth remains. Nucleic Acids Res. 37, 3215-3229. ( 10.1093/nar/gkp159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert MTP, et al. 2004. Ancient mitochondrial DNA from hair. Curr. Biol. 14, R463-R464. ( 10.1016/j.cub.2004.06.008) [DOI] [PubMed] [Google Scholar]
- 55.Metcalf JL, et al. 2016. Microbial community assembly and metabolic function during mammalian corpse decomposition. Science 351, 158-162. ( 10.1126/science.aad2646) [DOI] [PubMed] [Google Scholar]
- 56.Pereira F, Davis SJM, Pereira L, Mcevoy B, Bradley DG, Amorim A. 2006. Genetic signatures of a Mediterranean influence in Iberian Peninsula sheep husbandry. Mol. Biol. Evol. 23, 1420-1426. ( 10.1093/molbev/msl007) [DOI] [PubMed] [Google Scholar]
- 57.Chen S-Y, Duan Z-Y, Sha T, Xiangyu J, Wu S-F, Zhang Y-P. 2006. Origin, genetic diversity, and population structure of Chinese domestic sheep. Gene 376, 216-223. ( 10.1016/j.gene.2006.03.009) [DOI] [PubMed] [Google Scholar]
- 58.Demirci S, Baştanlar EK, Dağtaş ND, Pişkin E, Engin A, Özer F, Yüncü E, Doğan ŞA, Togan İ. 2013. Mitochondrial DNA diversity of modern, ancient and wild sheep (Ovis gmelinii anatolica) from Turkey: new insights on the evolutionary history of sheep. PLoS ONE 8, e81952. ( 10.1371/journal.pone.0081952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chessa B, et al. 2009. Revealing the history of sheep domestication using retrovirus integrations. Science 324, 532-536. ( 10.1126/science.1170587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Y-X, et al. 2017. Genomic reconstruction of the history of native sheep reveals the peopling patterns of nomads and the expansion of early pastoralism in East Asia. Mol. Biol. Evol. 34, 2380-2395. ( 10.1093/molbev/msx181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lv F-H, et al. 2015. Mitogenomic meta-analysis identifies two phases of migration in the history of Eastern Eurasian Sheep. Mol. Biol. Evol. 32, 2515-2533. ( 10.1093/molbev/msv139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rast-Eicher A. 2016. Fibres: microscopy of archaeological textiles and furs. Budapest, Hungary: Archaeolingua Alapítvány. [Google Scholar]
- 63.Rast-Eicher A. 2010. Fell- und Lederreste aus den Gräbern 2008. In Spiez-Einigen, Holleeweg 3, Naturwissenschaftliche Untersuchungen zu den bronzezeitlichen Bestattungen (eds Cooper C, Harbeck M, Kühn M, Rast-Eicher A, Schweissing M, Ulrich-Bochsler S, Vandorpe P), pp. 175-198. Bern, Switzerland: Service Archéologique du Canton de Berne. [Google Scholar]
- 64.Allain D, Pena-Arnaud B, Foulquie D, Bourdillon Y, François D. 2014. Introgression of wool-shedding genes into the Romane breed sheep. In 10. World congress of genetics applied to livestock production, pp. 17-22. Vancouver, Canada: American Society of Animal Science. [Google Scholar]
- 65.Mashkour M. 2013. Animal exploitation during the Iron Age to Achaemenid, Saasanian and early Islamic Periods. In Persia's imperial power in the late antiquity (eds Sauer EW, Rekavandi HO, Wilkinson TJ, Nokan-Den J), pp. 548-580. Oxford, UK: Oxbow Books. [Google Scholar]
- 66.Pöllath N, Schafberg R, Peters J. 2019. Astragalar morphology: approaching the cultural trajectories of wild and domestic sheep applying geometric morphometrics. J. Archaeol. Sci. 23, 810-821. ( 10.1016/j.jasrep.2018.12.004) [DOI] [Google Scholar]
- 67.Vila E, et al. 2021. EVOSHEEP: the makeup of sheep breeds in the ancient Near East. Antiquity 95(379), E2. ( 10.15184/aqy.2020.247) [DOI] [Google Scholar]
- 68.Khazaeli R. 2013. The subsistence economy of the Old Nishapur from the formation of the city up to the Mongol Period. Tehran, Iran: University of Tehran. [Google Scholar]
- 69.Rossi C, et al. 2021. Exceptional ancient DNA preservation and fibre remains of a Sasanian saltmine sheep mummy in Chehrābād, Iran. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Rossi C, et al. 2021. Exceptional ancient DNA preservation and fibre remains of a Sasanian saltmine sheep mummy in Chehrābād, Iran. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Sequencing reads and mitochondrial sequences are available under European Nucleotide Archive accession PRJEB43881.
The data are provided in the electronic supplementary material [69].