Introduction
Although hypotheses have been proposed, the exact pathophysiological mechanisms of osteoarthritis (OA) still remain unknown. Evidence suggests that immunological events, immune-neuroendocrine dysregulation and the presence of low-grade local and systemic inflammation play a key role in the pathogenesis and progression of this disease (Scanzello et al., 2015; Galvez et al., 2017; Chow et al., 2020; Woodell-May et al., 2020). Traditional therapies for OA focus on minimizing the symptoms, but not cure the arthritis. If none of these measures are effective, surgery is the next option. However, any medical or surgical treatment can have severe side effects. Balneotherapy is a common practice for the treatment and rehabilitation of OA patients whose role in modern medicine needs to be better defined. Studies have demonstrated that the beneficial effects of balneotherapy are mediated by regulation of inflammatory cells and mediators (Gálvez et al., 2018). This article aims to provide a standpoint on the possible involvement of immune system in these processes, and why it should be considered a target for therapy in such instances, based on published literature. Furthermore, we propose that the balneotherapy effectiveness in this context be better examined in future studies, in order to expand its employment alone or as a complement to other treatments in the OA management.
Immunological Events in OA
It is increasingly recognized that immune cells and their molecular mediators play a part in OA development. Enhanced leukocyte infiltration in the synovium and the presence of activate macrophages (M1) in synovial fluid (SF) have been identified in OA patients (Deligne et al., 2015; Kraus et al., 2016; Liu et al., 2018). Concomitantly, pro-inflammatory cytokines are produced locally by infiltrating and resident cells in early and end-stage of OA, independently or on collaboration with other mediators (Goldring et al., 2011; Punzi et al., 2016).
The main triggering of these events seems to be the activation of innate immunity by damage-associated molecular patterns, including extracellular matrix fragments, high mobility box 1, uric acid, complement system, S100 proteins, and heat shock proteins (HSPs) that are released into the joint after trauma or age-related processes (Gobezie et al., 2007; Scanzello et al., 2008; Ke et al., 2015). These molecules, generated in part by oxidative stress, are able to bind in synovial cells pathogen-recognition receptors, such as toll-like receptors, the receptor for advanced glycation end products and the NLRP3 inflammasome, and induce pro-inflammatory mediators production (Kim et al., 2006; Steenvoorden et al., 2006; McAllister et al., 2018). Indeed, the levels of several inflammatory cytokines, such as IL-1β and IL-6, are higher in serum from OA compared to healthy subjects (Sohn et al., 2012); whereas elevated IL-6, IL-8 and CCL2 were found in OA SF (Kaneko et al., 2000; Li et al., 2015; Oliviero et al., 2020). Increased IL-1β, IL-6, TNF-α and IL-8 concentrations are also detected in synovial tissues and articular cartilage of OA patients (Ma et al., 2015; Ni et al., 2015; Böhm et al., 2016).
Moreover, high concentrations of cytokines could be secreted from senescent cells, which accumulate in the synovium and in cartilage surface, thus predisposing the joint to OA development (Jeon et al., 2017).
In turn, these inflammatory factors, carried by the SF, can activate chondrocytes to produce metalloproteinases (MMPs) which result in further cartilage damage (Nefla et al., 2016). The activation of NF-kB, PI3K/AKT/mTOR, and Wnt/β-Catenin signaling pathways seems to play a key role in these processes (Rigoglou et al., 2013; Zhou et al., 2017; Sun et al., 2020).
Concomitantly, the upregulation of other factors, such as inducible nitric oxide synthase, nitric oxide (NO), cyclooxygenase-2, prostaglandin E2 (PGE2), A Disintegrin And Metalloproteinase with Thrombospondin motif (ADAMTS)-5, ADAMTS-4, VEGF, TGF-β and Nerve Growth factor (NGF) exert their effect influencing the OA progression (Chow et al., 2020).
Finally, microRNA can be involved in OA processes by activating different signaling pathways, and thus promoting inflammatory factor release (Wu et al., 2019).
Current and Future Pharmaceutical Therapy for OA
Current pharmacological treatments for OA are focused on relieving symptoms and improving functional status. Paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) are the first line medication choices for pain management, but their long-term use is associated with side effects.
Other common options include intra-articular corticosteroid or hyaluronic acid (HA) injections. Both treatments are effective at reducing pain in OA patients. However, intra-articular corticosteroid injections have shown a short duration of action, resulting in the need for repeated administration, which can lead to local and systemic side effects (Raynauld et al., 2003). The durability of pain reduction has been demonstrated longer after intra-articular HA injection when compared to corticosteroids (Colen et al., 2010). Nevertheless, due to the heterogeneity of approach, further studies should be conducted to confirm this hypothesis.
Therefore, new treatments are required to prevent OA structural changes and progression. Molecules involved in the OA pathophysiological processes, especially in immunological events, could be an interesting candidate as therapeutic target. In this context, several drugs have demonstrated disease-modifying OA effects in preclinical and clinical studies (Figure 1).
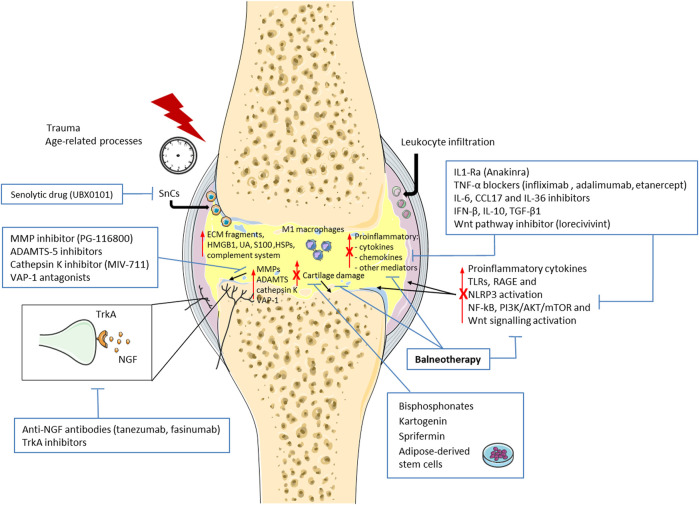
FIGURE 1.
Schematic representation of the new emerging therapies targeting immune system and immunomodulatory properties of balneotherapy in osteoarthritis (OA). Immunological events and low-grade inflammation play a key role in the pathogenesis and progression of OA. Damage-associated molecular patterns (DAMPs), including extracellular matrix (ECM) fragments, high mobility box 1 (HMGB1), uric acid (UA), complement system, S100 proteins, and heat shock proteins (HSPs) are released into the joint after trauma or age-related processes. These molecules, bind Toll-like receptors (TLRs), the receptor for advanced glycation end products (RAGE) and the intracellular NLRP3 inflammasome, and induce the production of pro-inflammatory mediators, such as cytokines and chemokines. Concomitantly, leukocyte infiltration, presence of activate macrophages (M1) and senescent cells (SnCs), levels metalloproteinases (MMPs), A Disintegrin And Metalloproteinase with Thrombospondin motif (ADAMTS), vascular adhesion protein-1 (VAP-1) and Nerve Growth factor (NGF) are enhanced, thus promoting cartilage damage and pain. Finally, NF-kB, PI3K/AKT/mTOR, and Wnt/β-Catenin signaling pathways are activated in these processes. New emerging treatment and balneotherapy have demonstrated a decrease in OA progression through immunomodulatory properties. ↑ increase; T inhibition; X reduction by balneotherapy.
Cytokine inhibitors represent a putative class of these agents. As IL-1β is thought to play a key role in OA development, and intra-articular injection of IL-1 receptor antagonist (IL1-Ra) has demonstrated to improve KOOS (Knee Injury and Osteoarthritis Outcome Score) in patients with anterior cruciate ligament (ACL) tear (Krauss et al., 2012), particular attention has focused on this cytokine. However, a randomized, controlled study evaluating the clinical response, safety, and tolerability of a single intra-articular injection of IL-1Ra in patients with knee OA, revealed no improvements in symptoms when compared with placebo (Chevalier et al., 2009). Results from a phase I trial investigating the adenovirus-mediated IL-1Ra gene transfer in knee OA are pending (Latourte et al., 2020).
TNF inhibition has also been investigated using IgG monoclonal antibodies (infliximab or adalimumab) or circulating receptor fusion protein (etanercept). Treatment with anti-TNF-α blockers has demonstrated a decrease in disease progression but not in symptoms in patients with hand OA (Verbruggen et al., 2012; Kloppenburg et al., 2018; Loef et al., 2018).
Other ongoing studies are evaluating inhibition of pro-inflammatory cytokines (IL-6, CCL17 and IL-36), or intra-articular effects of anti-inflammatory cytokines (IFN-β and IL-10) (Latourte et al., 2020).
Intra-articular release of TGF-β1 by retrovirally transduced human chondrocytes has also demonstrated good results, with improvement in cartilage damage and symptoms in patients with knee OA (Ha et al., 2012; Guermazi et al., 2017; Kim et al., 2018; Lee et al., 2019).
Matrix-degrading enzyme inhibition may be another attractive approach to attenuate cartilage damage, even though musculoskeletal toxicity has been observed after PG-116800 administration to patients with knee OA (Krzeski et al., 2007). Promising results may be obtained through aggrecanase inhibition. Indeed, ADAMTS-5 small molecules inhibitors or neutralizing antibodies have shown protective effects on cartilage and safety profile in vivo and in clinical studies (Malfait et al., 2019).
Similar effects were observed after intra-articular treatment with UBX0101, a senolytic drug capable of removing senescent cells accumulated in the joint (Jeon et al., 2017). In addition, less cartilage loss accompanied by bone remodeling reduction was found after administration of MIV-711, a cathepsin K inhibitor (Lindstrom et al., 2018; Conaghan et al., 2019). Analogous could be confirmed for bisphosphonates in early OA (Lane, 2018).
A chondroprotective activity, with signs of cartilage repair after injury has been also reported after intra-articular injection of adipose-derived stem cells in experimental OA (ter Huurne et al., 2012), but results obtained from clinical trials were not convincing (Pers et al., 2016; Emadedin et al., 2018; Freitag et al., 2019; Kim et al., 2019; Lee et al., 2019). Evaluations on emerging drugs promoting chondrogenesis, such as kartogenin and sprifermin, are ongoing (Eckstein et al., 2020; Johnson et al., 2020).
Studies on new therapies targeting signaling have identified a small-molecule Wnt pathway inhibitor, lorecivivint, as a potential disease-modifying OA drug (DMOAD). Administration of this agent facilitated cartilage regeneration in a rodent acute OA model (Deshmukh et al., 2018), and improved pain and function, with good safety and tolerability, in subjects with unilateral symptomatic knee OA (Yazici et al., 2017; Yazici et al., 2020).
Other interesting strategies to reduce pain have been observed using anti-NGF antibodies, such as tanezumab and fasinumab, even though increased risk of rapidly progressive OA was observed after patient treatment (Lane et al., 2010; Hochberg et al., 2016; Dakin et al., 2019; Berenbaum et al., 2020). The use of high-affinity NGF receptor (TrkA) inhibitors could be a viable alternative to avoid side effects (Krupka et al., 2019). New treatments targeting pain include inhibition of vascular adhesion protein-1 (VAP-1), an amine oxidase that increases in OA cartilage. The results of a Phase II clinical trial on a VAP-1 antagonist have not yet been published (Vakal et al., 2020).
Finally, platelet-rich plasma has been recently considered as innate immune response modulator, even though administration protocols and OA phenotypes target have to be refined (Andia et al., 2021).
Despite a number of potential DMOAD molecules have been identified, currently there are no approved drugs, and further studies are needed in this area.
Balneotherapy On OA
Besides pharmacological treatment, non-pharmacological interventions play a significant role in the OA prevention and treatment. Balneotherapy is one of the most common non-pharmacological approach for musculoskeletal complaints and rheumatic diseases that has demonstrated benefit for disease symptoms, and has been recently recommended by OARSI guidelines as a strategy for patients with multi-joint OA and comorbidities (Masiero, 2008; McAlindon et al., 2014; Cozzi et al., 2018; Masiero et al., 2018). Although the biological mechanisms underlying balneotherapy are not completely understood, it has been reported that it exerts some beneficial effects on the immune system due likely to its chemical, thermal and mechanical properties (Fioravanti et al., 2011; Tenti et al., 2015) (Figure 1).
In vitro researches have highlighted the immunomodulatory properties of mineral waters using animal or human OA cell cultures, or experimental models able to reproduce OA conditions in articular tissues. Treatment with H2S donors have showed anti-inflammatory and anti-oxidant activities limiting the MAPK/ERK and NF-kB pathway activation and reducing the production of several factors implicated in OA, including IL-6, IL-8, NO, PGE2 and MMP-13 (Kloesch et al., 2011; Burguera et al., 2014; Cheleschi et al., 2020). However, the applicability of cell culture models in this context remains a subject of debate since, under non-experimental conditions, the joint cells are never in direct contact with thermal water. The use of animal models may be more appropriate to identify the mechanisms involved, but so far only four studies have been conducted. In experimental OA murine models, balneotherapy decreased the levels of pro-inflammatory mediators in articular tissues, and systemic IL-1β and NO (Caraglia et al., 2005; Tékus et al., 2018; Kim et al., 2020; Vaamonde-García et al., 2020).
Also the number of studies in patients is limited: currently only three active projects are listed in clinical trial registry (ClinicalTrials.gov, 2020). However these confirm preclinical evaluations. Indeed, decreased serum levels of pro-inflammatory mediators (PGE2, leukotriene B4, IL-1β, TNF-α, IL-8, IL-6, and eHsp72) and expression of microRNA related to cartilage degradation were observed after mud pack treatments in OA patients (Bellometti et al., 1998; Giannitti et al., 2017; Ortega et al., 2017; Maccarone et al., 2020). Interestingly, balneotherapy increased circulating cortisol concentration that enhanced monocyte chemotaxis to damaged tissue and their switch to an anti-inflammatory phenotype (Galvez et al., 2020).
Of note, the association of mud-bath and glucosamine sulfate or ultrasound therapy has indicated improvement in pain, function and life quality in OA patients; thus suggesting combined treatment as an important therapeutic approach (Peluso et al., 2016; Király et al., 2021).
Conclusion
On these bases, we believe that therapeutic intervention to modulate immunological events could be an innovative strategy to contain OA progression, and balneotherapy may represent an interesting candidate to support pharmacological therapy. Strategies to improve research in this field need yet to be further refined and implemented. In this context, to identify the molecules and mechanisms associated with this pathological condition and spa treatment, alone or combined with other therapies, with in vivo and clinical studies, is crucial to validate the effectiveness and importance of pharmacological and non-pharmacological approaches.
Author Contributions
AS, LT, MM, and SM wrote and revised this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Andia I., Atilano L., Maffulli N. (2021). Moving toward Targeting the Right Phenotype with the Right Platelet-Rich Plasma (PRP) Formulation for Knee Osteoarthritis. Ther. Adv. Musculoskelet. Dis. 13, 1–9. 10.1177/1759720X211004336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellometti S., Galzigna L. (1998). Serum Levels of a Prostaglandin and a Leukotriene after thermal Mud Pack Therapy. J. Investig. Med. 46, 140–145. [PubMed] [Google Scholar]
- Berenbaum F., Blanco F. J., Guermazi A., Miki K., Yamabe T., Viktrup L., et al. (2020). Subcutaneous Tanezumab for Osteoarthritis of the Hip or Knee: Efficacy and Safety Results from a 24-week Randomised Phase III Study with a 24-week Follow-Up Period. Ann. Rheum. Dis. 79, 800–810. 10.1136/annrheumdis-2019-216296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm M., Apel M., Lowin T., Lorenz J., Jenei-Lanzl Z., Capellino S., et al. (2016). α-MSH Modulates Cell Adhesion and Inflammatory Responses of Synovial Fibroblasts from Osteoarthritis Patients. Biochem. Pharmacol. 116, 89–99. 10.1016/j.bcp.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Burguera E. F., Vela-Anero A., Magalhães J., Meijide-Faílde R., Blanco F. J. (2014). Effect of Hydrogen Sulfide Sources on Inflammation and Catabolic Markers on Interleukin 1β-Stimulated Human Articular Chondrocytes. Osteoarthritis Cartilage 22, 1026–1035. 10.1016/j.joca.2014.04.031 [DOI] [PubMed] [Google Scholar]
- Caraglia M., Beninati S., Giuberti G., D'Alessandro A. M., Lentini A., Abbruzzese A., et al. (2005). Alternative Therapy of Earth Elements Increases the Chondroprotective Effects of Chondroitin Sulfate in Mice. Exp. Mol. Med. 37, 476–481. 10.1038/emm.2005.58 [DOI] [PubMed] [Google Scholar]
- Cheleschi S., Gallo I., Tenti S. (2020). A Comprehensive Analysis to Understand the Mechanism of Action of Balneotherapy: Why, How, and where They Can Be Used? Evidence from In Vitro Studies Performed on Human and Animal Samples. Int. J. Biometeorol. 64, 1247–1261. 10.1007/s00484-020-01890-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier X., Goupille P., Beaulieu A. D., Burch F. X., Bensen W. G., Conrozier T., et al. (2009). Intraarticular Injection of Anakinra in Osteoarthritis of the Knee: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Rheum. 61, 344–352. 10.1002/art.24096 [DOI] [PubMed] [Google Scholar]
- Chow Y. Y., Chin K. Y. (2020). The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediators Inflamm. 2020, 8293921. 10.1155/2020/8293921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov. (2020). Balneotherapy & Osteoarthritis. Available at: https://clinicaltrials.gov/ct2/results?cond=Osteoarthritis&term=balneotherapy [Accessed April 29, 2021].
- Colen S., van den Bekerom M. P. J., Bellemans J., Mulier M. (2010). Comparison of Intra-articular Injections of Hyaluronic Acid and Corticosteroid in the Treatment of Osteoarthritis of the Hip in Comparison with Intra-articular Injections of Bupivacaine. Design of a Prospective, Randomized, Controlled Study with Blinding of the Patients and Outcome Assessors. BMC Musculoskelet. Disord. 11, 264. 10.1186/1471-2474-11-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaghan P., Bowes M. A., Kingsbury S. R., Brett A., Guillard G., Rizoska B., et al. (2019). Disease-Modifying Effects of a Novel Cathepsin K Inhibitor in Osteoarthritis: A Randomized Controlled Trial. Ann. Intern. Med. 172, 86–95. 10.7326/M19-0675 [DOI] [PubMed] [Google Scholar]
- Cozzi F., Ciprian L., Carrara M., Galozzi P., Zanatta E., Scanu A., et al. (2018). Balneotherapy in Chronic Inflammatory Rheumatic Diseases-A Narrative Review. Int. J. Biometeorol. 62, 2065–2071. 10.1007/s00484-018-1618-z [DOI] [PubMed] [Google Scholar]
- Dakin P., DiMartino S. J., Gao H., Maloney J., Kivitz A. J., Schnitzer T. J., et al. (2019). The Efficacy, Tolerability, and Joint Safety of Fasinumab in Osteoarthritis Pain: A Phase IIb/III Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Arthritis Rheumatol. 71, 1824–1834. 10.1002/art.41012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligne C., Casulli S., Pigenet A., Bougault C., Campillo-Gimenez L., Nourissat G., et al. (2015). Differential Expression of Interleukin-17 and Interleukin-22 in Inflamed and Non-inflamed Synovium from Osteoarthritis Patients. Osteoarthritis Cartilage 23, 1843–1852. 10.1016/j.joca.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Deshmukh V., Hu H., Barroga C., Bossard C., Kc S., Dellamary L., et al. (2018). A Small-Molecule Inhibitor of the Wnt Pathway (SM04690) as a Potential Disease Modifying Agent for the Treatment of Osteoarthritis of the Knee. Osteoarthritis Cartilage 26, 18–27. 10.1016/j.joca.2017.08.015 [DOI] [PubMed] [Google Scholar]
- Eckstein F., Kraines J. L., Aydemir A., Wirth W., Maschek S., Hochberg M. C. (2020). Intra-articular Sprifermin Reduces Cartilage Loss in Addition to Increasing Cartilage Gain Independent of Location in the Femorotibial Joint: post-hoc Analysis of a Randomised, Placebo-Controlled Phase II Clinical Trial. Ann. Rheum. Dis. 79, 525–528. 10.1136/annrheumdis-2019-216453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadedin M., Labibzadeh N., Liastani M. G., Karimi A., Jaroughi N., Bolurieh T., et al. (2018). Intra-articular Implantation of Autologous Bone Marrow-Derived Mesenchymal Stromal Cells to Treat Knee Osteoarthritis: a Randomized, Triple-Blind, Placebo-Controlled Phase 1/2 Clinical Trial. Cytotherapy 20, 1238–1246. 10.1016/j.jcyt.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Fioravanti A., Cantarini L., Guidelli G. M., Galeazzi M. (2011). Mechanisms of Action of Spa Therapies in Rheumatic Diseases: what Scientific Evidence Is There? Rheumatol. Int. 31, 1–8. 10.1007/s00296-010-1628-6 [DOI] [PubMed] [Google Scholar]
- Freitag J., Bates D., Wickham J., Shah K., Huguenin L., Tenen A., et al. (2019). Adipose-derived Mesenchymal Stem Cell Therapy in the Treatment of Knee Osteoarthritis: a Randomized Controlled Trial. Regen. Med. 14, 213–230. 10.2217/rme-2018-0161 [DOI] [PubMed] [Google Scholar]
- Galvez I., Torres-Piles S., Hinchado M. D., Alvarez-Barrientos A., Torralbo-Jimenez P., Guerrero J. (2017). Immune-neuroendocrine Dysregulation in Patients with Osteoarthritis: a Revision and a Pilot Study. Endocr. Metab. Immune Disord. Drug Targets 17, 78–85. 10.2174/1871530317666170320113613 [DOI] [PubMed] [Google Scholar]
- Galvez I., Torres-Piles S., Ortega E. (2020). Effect of Mud-bath Therapy on the Innate/inflammatory Responses in Elderly Patients with Osteoarthritis: a Discussion of Recent Results and a Pilot Study on the Role of the Innate Function of Monocytes. Int. J. Biometeorol. 64, 927–935. 10.1007/s00484-019-01748-4 [DOI] [PubMed] [Google Scholar]
- Gálvez I., Torres-Piles S., Ortega-Rincón E. (2018). Balneotherapy, Immune System, and Stress Response: A Hormetic Strategy? Int. J. Mol. Sci. 19, 1687. 10.3390/ijms19061687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannitti C., De Palma A., Pascarelli N. A., Cheleschi S., Giordano N., Galeazzi M., et al. (2017). Can Balneotherapy Modify microRNA Expression Levels in Osteoarthritis? A Comparative Study in Patients with Knee Osteoarthritis. Int. J. Biometeorol. 61, 2153–2158. 10.1007/s00484-017-1420-3 [DOI] [PubMed] [Google Scholar]
- Gobezie R., Kho A., Krastins B., Sarracino D. A., Thornhill T. S., Chase M., et al. (2007). High Abundance Synovial Fluid Proteome: Distinct Profiles in Health and Osteoarthritis. Arthritis Res. Ther. 9, R36. 10.1186/ar2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B., Otero M. (2011). Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 23 (5), 471–478. 10.1097/BOR.0b013e328349c2b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermazi A., Kalsi G., Niu J., Crema M. D., Copeland R. O., Orlando A., et al. (2017). Structural Effects of Intra-articular TGF-Β1 in Moderate to Advanced Knee Osteoarthritis: MRI-Based Assessment in a Randomized Controlled Trial. BMC Musculoskelet. Disord. 18, 461. 10.1186/s12891-017-1830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C., Noh M. J., Choi K. B., Lee K. H. (2012). Initial Phase I Safety of Retrovirally Transduced Human Chondrocytes Expressing Transforming Growth Factor-Beta-1 in Degenerative Arthritis Patients. Cytotherapy 14, 247–256. 10.3109/14653249.2011.629645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg M. C., Tive L. A., Abramson S. B., Vignon E., Verburg K. M., West C. R., et al. (2016). When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol. 68, 382–391. 10.1002/art.39492 [DOI] [PubMed] [Google Scholar]
- Jeon O. H., Kim C., Laberge R. M., Demaria M., Rathod S., Vasserot A. P., et al. (2017). Local Clearance of Senescent Cells Attenuates the Development of post-traumatic Osteoarthritis and Creates a Pro-regenerative Environment. Nat. Med. 23, 775–781. 10.1038/nm.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A., Woods A. K., Joseph S. B., Chatterjee A. K., Zhu S., Wisler J., et al. (2020). Development of KA34 as a Cartilage Regenerative Therapy for Osteoarthritis. Osteoarthritis Cartilage 28 (Suppl. 1), S518. 10.1016/j.joca.2020.02.814 [DOI] [Google Scholar]
- Kaneko S., Satoh T., Chiba J., Ju C., Inoue K., Kagawaet J., et al. (2000). Interleukin-6 and Interleukin-8 Levels in Serum and Synovial Fluid of Patients with Osteoarthritis. Cytokines Cel. Mol. Ther. 6, 71–79. 10.1080/13684730050515796 [DOI] [PubMed] [Google Scholar]
- Ke X., Jin G., Yang Y., Cao X., Fang R., Feng X., et al. (2015). Synovial Fluid HMGB-1 Levels Are Associated with Osteoarthritis Severity. Clin. Lab. 61, 809–818. 10.7754/clin.lab.2015.141205 [DOI] [PubMed] [Google Scholar]
- Kim C. G., Lee D. G., Oh J., Lee Y. H., Lee Y. J., Song P. H., et al. (2020). Effects of Balneotherapy in Jeju Magma-Seawater on Knee Osteoarthritis Model. Sci. Rep. 10, 6620. 10.1038/s41598-020-62867-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. A., Cho M. L., Choi H. Y., Yoon C. S., Jhun J. Y., Oh H. J., et al. (2006). The Catabolic Pathway Mediated by Toll-like Receptors in Human Osteoarthritic Chondrocytes. Arthritis Rheum. 54, 2152–2163. 10.1002/art.21951 [DOI] [PubMed] [Google Scholar]
- Kim M. K., Ha C. W., In Y., Cho S. D., Choi E. S., Ha J. K., et al. (2018). A Multicenter, Double-Blind, Phase III Clinical Trial to Evaluate the Efficacy and Safety of a Cell and Gene Therapy in Knee Osteoarthritis Patients. Hum. Gene Ther. Clin. Dev. 29, 48–59. 10.1089/humc.2017.249 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Ha C. W., Park Y. B., Nam E., Lee J. E., Lee H. J. (2019). Intra-articular Injection of Mesenchymal Stem Cells for Clinical Outcomes and Cartilage Repair in Osteoarthritis of the Knee: a Meta-Analysis of Randomized Controlled Trials. Arch. Orthop. Trauma Surg. 139, 971–980. 10.1007/s00402-019-03140-8 [DOI] [PubMed] [Google Scholar]
- Király M., Gömöri E., Kiss R., Nógrádi N., Nusser N., Hodosi K., et al. (2021). Effects of Various Types of Ultrasound Therapy in Hip Osteoarthritis - a Double-Blind, Randomized, Controlled, Follow-Up Study. Physiother. Theor. Pract. 10, 1–11. 10.1080/09593985.2021.1895386 [DOI] [PubMed] [Google Scholar]
- Kloesch B., Liszt M., Broell J., Steiner G. (2011). Dimethyl Sulphoxide and Dimethyl Sulphone Are Potent Inhibitors of IL-6 and IL-8 Expression in the Human Chondrocyte Cell Line C-28/I2. Life Sci. 89, 473–478. 10.1016/j.lfs.2011.07.015 [DOI] [PubMed] [Google Scholar]
- Kloppenburg M., Ramonda R., Bobacz K., Kwok W. Y., Elewaut D., Huizinga T. W. J., et al. (2018). Etanercept in Patients with Inflammatory Hand Osteoarthritis (EHOA): a Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Ann. Rheum. Dis. 77, 1757–1764. 10.1136/annrheumdis-2018-213202 [DOI] [PubMed] [Google Scholar]
- Kraus V. B., McDaniel G., Huebner J. L., Stabler T. V., Pieper C. F., Shipes S. W., et al. (2016). Direct In Vivo Evidence of Activated Macrophages in Human Osteoarthritis. Osteoarthritis Cartilage 24, 1613–1621. 10.1016/j.joca.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss V. B., Birmingham J., Stabler T. V., Feng S., Taylor D. C., Moorman C. T., et al. (2012). Effects of Intraarticular IL1-Ra for Acute Anterior Cruciate Ligament Knee Injury: a Randomized Controlled Pilot Trial (NCT00332254). Osteoarthritis Cartilage 20, 271–278. 10.1016/j.joca.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Krupka E., Jiang G. L., Jan C. (2019). Efficacy and Safety of Intra-articular Injection of Tropomyosin Receptor Kinase A Inhibitor in Painful Knee Osteoarthritis: a Randomized, Double-Blind and Placebo-Controlled Study. Osteoarthritis Cartilage 27, 1599–1607. 10.1016/j.joca.2019.05.028 [DOI] [PubMed] [Google Scholar]
- Krzeski P., Buckland-Wright C., Bálint G., Cline G. A., Stoner K., Lyon R., et al. (2007). Development of Musculoskeletal Toxicity without clear Benefit after Administration of PG-116800, a Matrix Metalloproteinase Inhibitor, to Patients with Knee Osteoarthritis: a Randomized, 12-month, Double-Blind, Placebo-Controlled Study. Arthritis Res. Ther. 9, R109. 10.1186/ar2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N. E. (2018). Osteoarthritis: Bisphosphonates and OA - Is There a Bone and Joint Connection? Nat. Rev. Rheumatol. 14, 185–186. 10.1038/nrrheum.2018.18 [DOI] [PubMed] [Google Scholar]
- Lane N. E., Schnitzer T. J., Birbara C. A., Mokhtarani M., Shelton D. L., Smith M. D., et al. (2010). Tanezumab for the Treatment of Pain from Osteoarthritis of the Knee. N. Engl. J. Med. 363, 1521–1531. 10.1056/NEJMoa0901510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latourte A., Kloppenburg M., Richette P. (2020). Emerging Pharmaceutical Therapies for Osteoarthritis. Nat. Rev. Rheumatol. 16, 673–688. 10.1038/s41584-020-00518-6 [DOI] [PubMed] [Google Scholar]
- Lee B., Parvizi J., Bramlet D., Romness D. W., Guermazi A., Noh M., et al. (2019a). Results of a Phase II Study to Determine the Efficacy and Safety of Genetically Engineered Allogeneic Human Chondrocytes Expressing TGF-Β1. J. Knee Surg. 33, 167–172. 10.1055/s-0038-1676803 [DOI] [PubMed] [Google Scholar]
- Lee W. S., Kim H. J., Kim K. I., Kim G. B., Jin W. (2019b). Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cel Transl. Med. 8, 504–511. 10.1002/sctm.18-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Jiang B. E. (2015). Serum and Synovial Fluid Chemokine Ligand 2/monocyte Chemoattractant Protein 1 Concentrations Correlates with Symptomatic Severity in Patients with Knee Osteoarthritis. Ann. Clin. Biochem. 52, 276–282. 10.1177/0004563214545117 [DOI] [PubMed] [Google Scholar]
- Lindstrom E., Rizoska B., Tunblad K., Edenius C., Bendele A. M., Maul D., et al. (2018). The Selective Cathepsin K Inhibitor MIV-711 Attenuates Joint Pathology in Experimental Animal Models of Osteoarthritis. J. Transl. Med. 16, 56. 10.1186/s12967-018-1425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Zhang M., Zhao J., Zheng M., Yang H. (2018). Imbalance of M1/M2 Macrophages Is Linked to Severity Level of Knee Osteoarthritis. Exp. Ther. Med. 16, 5009–5014. 10.3892/etm.2018.6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef M., Kroon F. P. B., Bergstra S. A., van der Pol J. A., Lems W. F., Kerstens P. J. S. M., et al. (2018). TNF Inhibitor Treatment Is Associated with a Lower Risk of Hand Osteoarthritis Progression in Rheumatoid Arthritis Patients after 10 Years. Rheumatology 57, 1917–1924. 10.1093/rheumatology/key016 [DOI] [PubMed] [Google Scholar]
- Ma C., Zhang Y., Li Y. Q., Chen C., Cai W., Zeng Y. L. (2015). The Role of PPARγ in Advanced Glycation End Products-Induced Inflammatory Response in Human Chondrocytes. PLoS One 10, e0125776. 10.1371/journal.pone.0125776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarone M. C., Magro G., Solimene U., ScanuMasiero A. S. (2020). From In Vitro Research to Real Life Studies: an Extensive Narrative Review of the Effects of Balneotherapy on Human Immune Response. Sport Sci. Health 20, 1–19. 10.1007/s11332-021-00778-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait A. M., Tortorella M. D. (2019). The "elusive DMOAD": Aggrecanase Inhibition from Laboratory to Clinic. Clin. Exp. Rheumatol. 37 (Suppl. 120), 130–134. [PubMed] [Google Scholar]
- Masiero S. (2008). Thermal Rehabilitation and Osteoarticular Diseases of the Elderly. Aging Clin. Exp. Res. 20, 189–194. 10.1007/BF03324772 [DOI] [PubMed] [Google Scholar]
- Masiero S., Vittadini F., Ferroni C., Bosco A., Serra R., Frigo A. C., et al. (2018). The Role of thermal Balneotherapy in the Treatment of Obese Patient with Knee Osteoarthritis. Int. J. Biometereol. 62, 243–252. 10.1007/s00484-017-1445-7 [DOI] [PubMed] [Google Scholar]
- McAlindon T. E., Bannuru R. R., Sullivan M. C., Arden N. K., Berenbaum F., Bierma-Zeinstra S. M., et al. (2014). OARSI Guidelines for the Non-surgical Management of Knee Osteoarthritis. Osteoarthritis Cartilage 22, 363–388. 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- McAllister M. J., Chemaly M., Eakin A. J., Gibson D. S., McGilligan V. E. (2018). NLRP3 as a Potentially Novel Biomarker for the Management of Osteoarthritis. Osteoarthritis Cartilage 26, 612–619. 10.1016/j.joca.2018.02.901 [DOI] [PubMed] [Google Scholar]
- Nefla M., Holzinger D., Berenbaum F., Jacques C. (2016). The Danger from within: Alarmins in Arthritis. Nat. Rev. Rheumatol. 12, 669–683. 10.1038/nrrheum.2016.162 [DOI] [PubMed] [Google Scholar]
- Ni S., Miao K., Zhou X., Xu N., Li C., Zhu R., et al. (2015). The Involvement of Follistatin-like Protein 1 in Osteoarthritis by Elevating NF-Κb-Mediated Inflammatory Cytokines and Enhancing Fibroblast like Synoviocyte Proliferation. Arthritis Res. Ther. 17, 91. 10.1186/s13075-015-0605-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviero F., Ramonda R., Scanu A., Galozzi P., Favero M., Punzi L. (2020). Levels of Inflammatory Cytokines and Metalloproteinases Are Increased in Knee Synovial Fluid of Patients with Concomitant Erosive Hand Osteoarthritis. Clin. Exp. Rheumatol. 38, 800. [PubMed] [Google Scholar]
- Ortega E., Gálvez I., Hinchado M. D., Guerrero J., Martín-Cordero L., Torres-Piles S. (2017). Anti-inflammatory Effect as a Mechanism of Effectiveness Underlying the Clinical Benefits of Pelotherapy in Osteoarthritis Patients: Regulation of the Altered Inflammatory and Stress Feedback Response. Int. J. Biometeorol. 61, 1777–1785. 10.1007/s00484-017-1361-x [DOI] [PubMed] [Google Scholar]
- Peluso R., Caso F., Costa L., Sorbo D., Carraturo N., Di Minno M. N., et al. (2021). Mud-bath Therapy and Oral Glucosamine Sulfate in Patients with Knee Osteoarthritis: a Randomized, Controlled, Crossover Study. Clin. Exp. Rheumatol. 34, 618–624. [PubMed] [Google Scholar]
- Pers Y. M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F., et al. (2016). Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cell Transl. Med. 5, 847–856. 10.5966/sctm.2015-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzi L., Galozzi P., Luisetto R., Favero M., Ramonda R., Oliviero F., et al. (2016). Post-traumatic Arthritis: Overview on Pathogenic Mechanisms and Role of Inflammation. RMD Open 2, e000279. 10.1136/rmdopen-2016-000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynauld J. P., Buckland-Wright C., Ward R., Choquette D., Haraoui B., Martel-Pelletier J., et al. (2003). Safety and Efficacy of Long-Term Intraarticular Steroid Injections in Osteoarthritis of the Knee: a Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheum. 48, 370–377. 10.1002/art.10777 [DOI] [PubMed] [Google Scholar]
- Rigoglou S., Papavassiliou A. G. (2013). The NF-Κb Signalling Pathway in Osteoarthritis. Int. J. Biochem. Cel. Biol. 45, 2580–2584. 10.1016/j.biocel.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Scanzello C. R., Loeser R. F. (2015). Editorial: Inflammatory Activity in Symptomatic Knee Osteoarthritis: Not All Inflammation Is Local. Arthritis Rheumatol. 67, 2797–2800. 10.1002/art.39304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello C. R., Plaas A., Crow M. K. (2008). Innate Immune System Activation in Osteoarthritis: Is Osteoarthritis a Chronic Wound? Curr. Opin. Rheumatol. 20, 565–572. 10.1097/BOR.0b013e32830aba34 [DOI] [PubMed] [Google Scholar]
- Sohn D. H., Sokolove J., Sharpe O., Erhart J. C., Chandra P. E., Lahey L. J., et al. (2012). Plasma Proteins Present in Osteoarthritic Synovial Fluid Can Stimulate Cytokine Production via Toll-like Receptor 4. Arthritis Res. Ther. 14, R7. 10.1186/ar3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenvoorden M. M. C., Huizinga T. W. J., Verzijl N., Bank R. A., Ronday H. K., Luning H. A. F., et al. (2006). Activation of Receptor for Advanced Glycation End Products in Osteoarthritis Leads to Increased Stimulation of Chondrocytes and Synoviocytes. Arthritis Rheum. 54, 253–263. 10.1002/art.21523 [DOI] [PubMed] [Google Scholar]
- Sun K., Luo J., Guo J., Yao X., Jing X., Guo F. (2020). The PI3K/AKT/mTOR Signaling Pathway in Osteoarthritis: a Narrative Review. Osteoarthritis Cartilage 28, 400–409. 10.1016/j.joca.2020.02.027 [DOI] [PubMed] [Google Scholar]
- Tékus V., Borbély É., Kiss T., Perkecz A., Kemény Á., Horváth J., et al. (2018). Investigation of Lake Hévíz mineral Water Balneotherapy and Hévíz Mud Treatment in Murine Osteoarthritis and Rheumatoid Arthritis Models. Evid. Based Complement. Alternat. Med. 2018, 4816905. 10.1155/2018/4816905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenti S., Cheleschi S., Galeazzi M., Fioravanti A. (2015). Spa Therapy: Can Be a Valid Option for Treating Knee Osteoarthritis? Int. J. Biometeorol. 59, 1133–1143. 10.1007/s00484-014-0913-6 [DOI] [PubMed] [Google Scholar]
- ter Huurne M., Schelbergen R., Blattes R., Blom A., de Munter W., Grevers L. C., et al. (2012). Antiinflammatory and Chondroprotective Effects of Intraarticular Injection of Adipose-Derived Stem Cells in Experimental Osteoarthritis. Arthritis Rheum. 64, 3604–3613. 10.1002/art.34626 [DOI] [PubMed] [Google Scholar]
- Vaamonde-García C., Vela-Anero Á., Hermida-Gómez T., Fernández-Burguera E., Filgueira-Fernández P., Goyanes N., et al. (2020). Effect of Balneotherapy in Sulfurous Water on an In Vivo Murine Model of Osteoarthritis. Int. J. Biometeorol. 64, 307–318. 10.1007/s00484-019-01807-w [DOI] [PubMed] [Google Scholar]
- Vakal S., Jalkanen S., Dahlström K. M., Salminen T. A. (2020). Human Copper-Containing Amine Oxidases in Drug Design and Development. Molecules 25, 1293. 10.3390/molecules25061293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen G., Wittoek R., Vander Cruyssen B., Elewaut D. (2012). Tumour Necrosis Factor Blockade for the Treatment of Erosive Osteoarthritis of the Interphalangeal finger Joints: a Double Blind, Randomised Trial on Structure Modification. Ann. Rheum. Dis. 71, 891–898. 10.1136/ard.2011.149849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodell-May J. E., Sommerfeld S. D. (2020). Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 38, 253–257. 10.1002/jor.24457 [DOI] [PubMed] [Google Scholar]
- Wu Y., Lu X., Shen B., Zeng Y. (2019). The Therapeutic Potential and Role of miRNA, lncRNA, and circRNA in Osteoarthritis. Curr. Gene Ther. 19, 255–263. 10.2174/1566523219666190716092203 [DOI] [PubMed] [Google Scholar]
- Yazici Y., McAlindon T. E., Fleischmann R., Gibofsky A., Lane N. E., Kivitz A. J., et al. (2017). A Novel Wnt Pathway Inhibitor, SM04690, for the Treatment of Moderate to Severe Osteoarthritis of the Knee: Results of a 24-week, Randomized, Controlled, Phase 1 Study. Osteoarthritis Cartilage 25, 1598–1606. 10.1016/j.joca.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Yazici Y., McAlindon T. E., Gibofsky A., Lane N. E., Clauw D., Jones M., et al. (2020). Lorecivivint, a Novel Intraarticular CDC-like Kinase 2 and Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase 1A Inhibitor and Wnt Pathway Modulator for the Treatment of Knee Osteoarthritis: A Phase II Randomized Trial. Arthritis Rheumatol. 72, 1694–1706. 10.1002/art.41315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang T., Hamilton J. L., Chen D. (2017). Wnt/β-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis. Curr. Rheumatol. Rep. 19, 53. 10.1007/s11926-017-0679-z [DOI] [PMC free article] [PubMed] [Google Scholar]