Abstract
Type 2 diabetes mellitus (T2DM) is one of the major chronic diseases, whose prevalence is increasing dramatically worldwide and can lead to a range of serious complications. Wnt ligands (Wnts) and their activating Wnt signalling pathways are closely involved in the regulation of various processes that are important for the occurrence and progression of T2DM and related complications. However, our understanding of their roles in these diseases is quite rudimentary due to the numerous family members of Wnts and conflicting effects via activating the canonical and/or non‐canonical Wnt signalling pathways. In this review, we summarize the current findings on the expression pattern and exact role of each human Wnt in T2DM and related complications, including Wnt1, Wnt2, Wnt2b, Wnt3, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, Wnt7b, Wnt8a, Wnt8b, Wnt9a, Wnt9b, Wnt10a, Wnt10b, Wnt11 and Wnt16. Moreover, the role of main antagonists (sFRPs and WIF‐1) and coreceptor (LRP6) of Wnts in T2DM and related complications and main challenges in designing Wnt‐based therapeutic approaches for these diseases are discussed. We hope a deep understanding of the mechanistic links between Wnt signalling pathways and diabetic‐related diseases will ultimately result in a better management of these diseases.
Keywords: canonical Wnt signalling pathway, complications, diabetes, non‐canonical Wnt signalling pathway, Wnt‐based therapeutics
1. INTRODUCTION
Diabetes, caused by the deficiency of insulin secretion and/or insulin resistance and characterized by chronic hyperglycaemia, is currently one of the most important metabolic diseases worldwide. Diabetic patients have a higher risk of developing a series of acute metabolic complications, such as diabetic ketoacidosis, and chronic vascular complications (angiopathy) including microvascular diseases such as diabetic retinopathy (DR), diabetic peripheral neuropathy (DPN), diabetic nephropathy (DN) and diabetic foot, and macrovascular diseases including cardiovascular disease manifesting as myocardial infarction and cerebrovascular disease resulting in strokes. 1 , 2 The prevalence and incidence rate are increasing rapidly in most countries, according to the latest data from International Diabetes Federation, more than 463 million adults are suffering from diabetes and the number is expected to rise to 700 million by 2045. Diabetes caused 4.2 million deaths and 10% of total health expenditure on adults in 2019. More severely, about 50% people with diabetes have not been diagnosed and about 79% of adults with diabetes are living in developing countries. Diabetes is mainly divided into type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM) and gestational diabetes mellitus (GDM); among them T2DM is the most common type and accounts for above 90% of all diabetes cases. 3 It is commonly believed that insulin resistance is the initial factor for the occurrence of T2DM, whereas dysfunction of pancreatic β‐cells is the determinant factor. 4 , 5 There is no cure for T2DM currently, the cornerstone of the treatment is reducing insulin resistance and stimulating pancreas to secret more insulin. Therefore, it is urgent to reveal the underlying pathogenic mechanisms of T2DM for a better therapeutic management.
The aetiology of T2DM has not been fully elucidated, and it is considered to be a complex polygenetic disease attributed to the interaction between hereditary predisposition and multiple acquired disposition; the latter of which includes the risk factors such as overweight, unhealthy diet, physical inactivity, increasing age and hypertension. 6 , 7 The abnormalities in many important signalling transduction pathways are critically involved in the occurrence and progression of T2DM and related complications. 8 , 9 , 10 , 11 Among them Wnt signalling pathways attract more attention due to the essential role in the embryogenesis and tissue homeostasis, and notorious role in the pathogenesis of multiple human diseases, especially in cancers. 12 , 13 , 14 The relationship between Wnt signalling pathways and T2DM was firstly documented by Grant et al in 2006; they found genetic polymorphism of TCF7L2 gene, which encodes an important transcription factor TCF4 in Wnt signalling pathways, contributed to the risk of T2DM through regulation of the expression of proglucagon gene. 15 Subsequently, emerging studies proved that dysregulation of Wnt signalling pathways participate in the occurrence and progression of T2DM through directly influencing the differentiation and proliferation of pancreatic β‐cells and the secretion and action of insulin. 16 , 17 , 18 However, due to the numerous components and resulting intricate networks, the role of Wnt pathways in the pathogenesis of T2DM and related complications seems to be contradictory, sometimes they function as protectors, while their activation is simultaneously required for the development of these disorders, and our understanding on their relationship is still quite rudimentary. Therefore, a more comprehensive understanding of their relationship will be helpful for a better therapeutic effect.
2. THE CANONICAL AND NON‐CANONICAL WNT SIGNALLING PATHWAYS
The Wnt signalling pathway is roughly divided into β‐catenin‐dependent (canonical) and β‐catenin‐independent (non‐canonical) signalling pathways, which activates distinct intracellular signalling pathways (Figure 1). Among them the canonical Wnt signalling gets more attention and is well understood. 19 The most crucial event in this signalling is the regulation in the turnover of β‐catenin, a pivotal component that acts as a transcriptional co‐activator in this cascade. In resting cells, the production of Wnts is suppressed and the protein level of cytoplasmic β‐catenin is low due to the activity of destruction complex composed of Axin, adenomatosis polyposis coli (APC), casein kinase 1 (CK1α) and glycogen synthase kinase (GSK‐3β). β‐catenin is captured and phosphorylated by the destruction complex, 20 followed by ubiquitinated by β‐transducin repeat‐containing protein (β‐TRCP) and dispatched to the proteasome for complete degradation, 21 without enough β‐catenin in nucleus, the bidirectional T cell factor/lymphoid enhancer–binding factors (TCF/LEF) begin to recruit transducin‐like enhancer protein (Groucho/TLE) and histone deacetylases (HDACs) to form a repressive complex, thus to inhibit the transcription of Wnt target genes. Conversely, the activation of canonical Wnt signalling is initiated by the formation of complex among Wnts, their cognate receptor Frizzled (Fzd) and coreceptor low‐density lipoprotein receptor–related protein 5/6 (LRP5/6) on the cell membrane. Consequently, the effector protein dishevelled (DVL) is recruited and polymerized to inactivate the destruction complex, which leads to the stabilization and accumulation of β‐catenin in the cytoplasm and the subsequent translocation into the nucleus to form an active complex with TCF/LEF by removing TLE/Groucho complexes and recruiting transcriptional co‐activators such as B cell CLL/lymphoma 9 (BCL9), Brahma‐related gene 1 (BRG1), CBP/p300 and Pygo. Finally, the transcription of Wnt target genes is driven and results in the changes of series of cellular processes. Collectively, the activation of canonical Wnt signalling mainly includes the following biological processes: the production and secretion of Wnts, the recognition of Wnts by their receptors, the inactivation of destruction complex, the accumulation of β‐catenin and translocation into nucleus and the activation of transcriptional complex of target genes.
FIGURE 1.
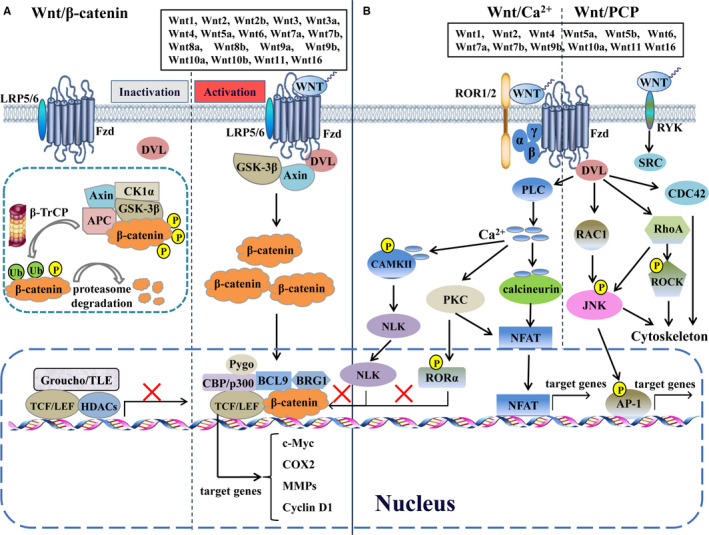
Multiple Wnt signalling pathways. Various Wnts regulate the canonical Wnt/β‐catenin pathway, the non‐canonical Wnt/planar cell polarity (PCP) pathway and Wnt/Ca2+ pathway. AP‐1, activator protein 1; APC, adenomatosis polyposis coli; BCL9, B cell CLL/lymphoma 9; BRG1, Brahma‐related gene 1; β‐TRCP, β‐transducin repeat‐containing protein; CAMKII, calmodulin‐dependent protein kinase II; CDC42, cell division cycle 42; CK1α, casein kinase 1; COX2, cytochrome c oxidase subunit 2; DVL, dishevelled; Fzd, frizzled; GSK‐3β, glycogen synthase kinase; HDACs, histone deacetylases; JNK, JUN N‐terminal kinase; LEF, lymphoid enhancer–binding factor; LRP5/6, low‐density lipoprotein receptor–related protein 5/6; MMPs, matrix metalloproteinases; NFAT, nuclear factor of activated T cells; NLK, nemo‐like kinase; PKC, protein kinase C; PLC, phospholipase C; RAC1, Rac family small GTPase 1; ROCK, Rho kinase; ROR1/2, receptor tyrosine kinase–like orphan receptor 1/2; RORα, retinoic acid–related orphan nuclear receptor α; RYK, receptor like tyrosine kinase; TCF, T cell factor; TLE, transducin‐like enhancer protein
The non‐canonical Wnt signalling is mainly subdivided into Wnt/Ca2+ and Wnt/planar cell polarity (PCP) signalling pathways, which are activated by some Wnts proteins, such as Wnt5a, Wnt5b and Wnt11, and eventually regulate the cellular polarity and migration‐related signalling pathways that have important roles in cell orientation during development and cell migration in metastasis formation. 22 The Wnt/Ca2+ signalling is activated by the complex formation among Wnt, Fzd, DVL and G proteins and the resulting activation of phospholipase C (PLC) activity and subsequent calcium influx, increased intracellular Ca2+ concentration activates various signalling pathways, including protein kinase C (PKC), Ca2+/calmodulin‐dependent protein kinase II (CAMKII) and Ca2+/calcineurin, leading to the phosphorylation of retinoic acid–related orphan nuclear receptor α (RORα) and/or the translocation of transcription factors, such as nuclear factor activated in T cells (NFAT) and Nemo‐like kinase (NLK). 23 Intriguingly, RORα and NLK function as inhibitors of canonical Wnt signalling via reducing the binding of β‐catenin to TCF/LEF transcription factors. 24 , 25 In the Wnt/PCP signalling, Wnts bind to the receptor like tyrosine kinase (RYK) to active SRC, or to the receptor tyrosine kinase–like orphan receptor 1/2 (ROR1/2)‐Fzd complex to activate DVL and further activate small Rho GTPases, including Rac family small GTPase 1 (RAC1), RhoA and cell division cycle 42 (CDC42), in a DVL‐dependent way. RhoA triggers ROCK and c‐Jun N‐terminal kinase (JNK) but RAC1 only activates JNK, thereby regulating rearrangements of cytoskeleton and/or activating related transcription factors, such as activator protein 1 (AP‐1) and NFAT. Moreover, some new non‐canonical Wnt signalling pathways, including Wnt/STOP signalling, Wnt/TOR signalling, Wnt‐YAP/TAZ signalling, Wnt/LRP5/mTOR/Akt signalling and Wnt/Hippo signalling have been discovered gradually and their roles in physiology and pathology has been well explored and discussed. 26 , 27 , 28 , 29 Theoretically, changes in any component involving in Wnt signalling pathways may result in the abnormalities of these pathways, and several studies have elaborately summarized the impact of Wnt pathways on T2DM and related complications. 16 , 17 , 18 In this review, we only focus on the current insights and recent advances in the role of each human Wnt and main related antagonists (sFRPs and WIF‐1) and coreceptor (LRP6) in the pathogenesis of T2DM and related complications.
3. ROLES OF WNTS IN T2DM AND RELATED COMPLICATIONS
As initiators of Wnt signalling pathways, Wnts are a cluster of conserved secreted glycoproteins and widely expressing in all metazoan species; humans carry 19 members of Wnts with 40%‐90% amino acid sequence identity. Among them Wnt2b, Wnt3, Wnt3a, Wnt8a, Wnt8b, Wnt9a and Wnt10b mainly activate the canonical pathway, Wnt5b and Wnt11 activate the non‐canonical pathway, and Wnt1, Wnt2, Wnt4 Wnt5a, Wnt6, Wnt7a, Wnt7b, Wnt9b, Wnt10a and Wnt16 function as initiators for both signalling pathways. The structural characteristics and the maturation of all human Wnts have been elaborately summarized by our group and Kikuchi et al. 19 , 30 However, the detailed mechanism by which single Wnt is chosen to produce and activate specific Wnt pathway has not been fully clarified. In the following parts, the exact role of each Wnt in the development of T2DM and related complications will be discussed separately (Table 1).
TABLE 1.
Wnt activating the canonical and non‐canonical Wnt signalling pathways in the pathogenesis of diabetic‐related diseases
| Wnts | Expression Pattern in diabetic samples | Effect on diabetic‐related diseases | Type of Wnt signalling | Reference | |
|---|---|---|---|---|---|
| Wnt1 | No data | Deleterious | Diabetic nephropathy | Canonical | 31 |
| Protective | Diabetic microvascular disease | Canonical | 32 | ||
| Wnt2 | Increased in myocardial tissues | Deleterious | Diabetic cardiomyopathy | Canonical | 33 |
| Increased in retinal endothelial cells | Deleterious | Diabetic retinopathy | No data | 34 | |
| Wnt2b | Increased in islets | Deleterious | Dysfunction of islet | Canonical | 36 |
| Increased in serum | Deleterious | Diabetic nephropathy | Canonical | 37, 38 | |
| Wnt3 | Decreased in olfactory bulbs and hippocampus | Protective | Diabetic central nervous system complication | Canonical | 41, 42 |
| Wnt3a | Decreased in cardiomyocytes | Protective | Diabetic cardiomyopathy | Canonical | 43 |
| Increased in gastrocnemius | Deleterious | Insulin resistance | Canonical | 44 | |
| No data | Protective | Impaired proliferation of β‐cells and insulin secretion | Canonical | 47, 48 | |
| No data | Protective | Impaired bone formation | Canonical | 49 | |
| Increased in kidney | Deleterious | Diabetic nephropathy | Canonical | 50 | |
| Increased in vitreous fluid | Deleterious | Diabetic retinopathy | No data | 51 | |
| Increased in aortic tissues | Deleterious | Diabetic macrovascular disease | No data | 52 | |
| Decreased in adipose tissues | Protective | Adipogenesis and obesity | Canonical | 53, 54 | |
| Wnt4 | Decreased in pre‐diabetic but increased in diabetic in islets | Deleterious | Impaired proliferation of β‐cells and insulin secretion | Non‐canonical | 59, 60 |
| Decreased in islets | Protective | Apoptosis and dysfunction of islet | Canonical | 61 | |
| Decreased in kidney | Protective | Diabetic nephropathy | Canonical | 62 | |
| Increased in kidney | Deleterious | Diabetic nephropathy | Canonical | 67, 68 | |
| Increased in carotid arteries | Deleterious | Diabetic macrovascular disease | Canonical | 69 | |
| Wnt5a | Increased in islets | Deleterious | Apoptosis and dysfunction of islet | Canonical/ Non‐canonical | 61, 82 |
| Decreased in islet stellate cells | Protective | Impaired insulin secretion | Canonical | 70, 84 | |
| Increased in adipose tissues | Deleterious | Impaired insulin secretion | Non‐canonical | 78 | |
| Decreased in kidney | Protective | Diabetic nephropathy | Canonical | 62 | |
| Increased in plasma | Deleterious | Insulin resistance | Non‐canonical | 75, 76 | |
| Increased in peripheral blood macrophages | Deleterious | Diabetic macrovascular disease | Non‐canonical | 87, 88 | |
| Increased in ischaemic muscles and endothelial cells | Deleterious | Diabetic foot ulcers | Non‐canonical | 92 | |
| Increased in plasma, decreased in glomerular mesangium | Protective | Diabetic nephropathy | Canonical | 95, 96 | |
| Wnt5b | Increased in adipose tissues | Deleterious | Adipogenesis and obesity | Non‐canonical | 99, 100 |
| Wnt6 | Decreased in adipose tissues | Protective | Adipogenesis and obesity | Canonical | 101, 102 |
| Decreased in kidney | Protective | Diabetic nephropathy | Canonical | 103 | |
| Wnt7a | Increased in aortic tissues | Deleterious | Diabetic macrovascular disease | Canonical | 52, 108, 111 |
| Decreased in wounded skin tissues | Protective | Diabetic wounds | Canonical | 108 | |
| Decreased in hippocampus | Protective | Diabetic central nervous system complication | No data | 109 | |
| Wnt7b | No data | Protective | Diabetic arteriosclerosis | No data | 110 |
| Wnt8a | No data | ||||
| Wnt8b | No data | ||||
| Wnt9a | No data | Deleterious | Impaired differentiation of β‐cells | Canonical | 113 |
| No data | Protective | Impaired Insulin secretion | No data | 114 | |
| Wnt9b | No data | Protective | Diabetic foot ulcers | Canonical | 115 |
| Wnt10a | Increased in brown pre‐adipocyte cells | Protective | Adipogenesis and obesity | Canonical | 117, 118 |
| Increased in spinal cord tissues | Deleterious | Diabetic neuropathy | Canonical | 119 | |
| Wnt10b | Decreased in white adipose and skeletal muscle tissues | Protective | Adipogenesis and obesity, insulin resistance | Canonical | 120, 121, 122, 123, 124, 125 |
| Decreased in bone | Protective | Diabetic bone loss | Canonical | 127 | |
| Wnt11 | Increased in adipose tissues | Deleterious | Adipogenesis and obesity | Non‐canonical | 129, 130 |
| Wnt16 | Decreased in cortical bone | Protective | Diabetic osteopenia | Canonical/Non‐canonical | 132, 133 |
| Decreased in corpus cavernosum tissues | Protective | Diabetic erectile dysfunction | No data | 134 | |
3.1. Wnt1, Wnt2 and Wnt2b
At present, no direct evidence is available for the link between Wnt1 and T2DM; few studies on its role in different diabetic complications is also controversial. In T1DM‐induced rats, the inhibition of Wnt1/β‐catenin signalling by salidroside, a hypoglycaemic and antioxidant glycoside, was found to protect against DN, indicating a deleterious role of Wnt1 in diabetes. 31 However, Chong et al showed that erythropoietin (EPO) could protect endothelial cells (ECs) against elevated glucose exposure through activating Wnt1/β‐catenin pathway, suggesting a protective role of Wnt1 against diabetic microvascular disease. 32 Similarly, there are few studies concerning the role of Wnt2 in T2DM. An in vivo study shows that the mRNA and protein levels of Wnt2, β‐catenin and Wnt target genes are all increased progressively in myocardial tissues from rat following streptozotocin (STZ)‐induced diabetes, which is accompanied by the cardiac dysfunction and progressive cardiomyocyte apoptosis, suggesting that the activation of Wnt2/β‐catenin pathway may facilitate the development of diabetic cardiomyopathy. 33 Furthermore, a recent study shows that circular RNA circHIPK3 promotes DR by increasing the expression of Wnt2 in retinal ECs. 34 These data suggest that Wnt2 may be a potential target to control these diabetic complications. Wnt2b shares 70% amino acid sequence identity with Wnt2. Zhou et al found the epistasis between Wnt2b and TCF7L2 genes was associated with the susceptibility of T2DM in Chinese Han population. 35 The expression of Wnt2b and mediated canonical Wnt pathway is induced and inversely correlated with insulin expression in islets of T2DM patients, indicating inhibition of this pathway might be a new route to prevent the failure of β‐cells. 36 Moreover, Wnt2b is up‐regulated in serum of patients with DN and mesangial cells cultured in high glucose environment, and high glucose treatment could enhance the inflammation and extracellular matrix via activating the Wnt2b/β‐catenin pathway induced by two different long non‐coding RNAs, providing new mechanisms for understanding the development of DN and promising target for its treatment. 37 , 38
3.2. Wnt3 and Wnt3a
Wnt3 and Wnt3a share 85% amino acid sequence identity. At present, studies on Wnt3 are mainly focused on its role in malignancies rather than in diabetes; we have revealed the carcinogenic role of Wnt3 in the development of gastric and colorectal cancers, 39 , 40 and the current few studies on Wnt3 are mainly about its role in diabetic complication of central nervous system. A recent study revealed that the impaired cognitive function and loss of neurogenesis was associate with the inhibition of Wnt3/β‐catenin pathway in diabetic rat, and insulin treatment promoted neurogenesis via increasing the expression of Wnt3 in astrocytes. 41 Similarly, the canonical Wnt3 pathway is inhibited in the hippocampus of diabetic rat, and treadmill exercise alleviated Alzheimer disease–associated memory loss in diabetic rats by increasing neurogenesis through activating this signalling. 42 These results shed lights on the implementation of Wnt3 as a therapeutic candidate for targeting diabetic complication of central nervous system. Compared to its homologue, Wnt3a is known to regulate the pathogenesis of T2DM from several processes but its role is controversial. Under the similar hyperglycaemia condition in STZ‐induced diabetic rat, Wnt3a/β‐catenin pathway was found to be inhibited in cardiomyocytes, 43 but swimming exercise was proved to alleviate insulin resistance through inhibiting the overactivated Wnt3a/β‐catenin pathway in skeletal muscles. 44 Wnt3a is an important modulator of the differentiation and maturation of β‐cells, overexpressing Wnt3a promotes the proliferation of porcine pancreatic stem cells (PSCs), which are valuable in transplantation application of T2DM. 45 Similarly, activation of the canonical Wnt pathway by treating exogenous Wnt3a enhances the differentiation of human embryonic stem cells (hESCs) to pancreatic lineage cells and induces the proliferation of β‐cells and insulin secretion in vitro. 46 , 47 However, in a latest study on human‐induced pluripotent stem cell (hiPSC)–derived S7 cells, activating the canonical Wnt3a/β‐catenin and non‐canonical Wnt4/5a/5b signalling pathways simultaneously did not alter or improve glucose‐simulated insulin secretion (GSIS); instead, inhibition of these endogenous Wnts even modestly promoted the maturation of β‐cells. 48 In another study, activation of Wnt3a/β‐catenin pathway was found to improve the impaired osteointegration under diabetic condition. 49 Wnt3a/β‐catenin signalling is also overactivated in the kidneys of diabetic patients and animal models, hence inhibiting this signalling by PPARα displays a protective effect on DN. 50 Consistently, a significantly higher level of Wnt3a is detected in vitreous fluid samples from patients with proliferative DR compared with that in healthy subjects, 51 and the levels of Wnt3a and Wnt7a are up‐regulated in aortic tissues from mice with diabetic macrovascular disease. 52 Of note, Wnt3a also participates in the occurrence of T2DM by impacting adipocyte differentiation. Wnt3a and Wnt10b inhibits adipogenesis and related obesity via activating the canonical Wnt pathway. 53 , 54 Therefore, activation of canonical Wnt3a pathway has the effect of bidirectional regulation on T2DM and complications, depending on context, and further studies are needed to elucidate detailed mechanism of Wnt3a action in these disorders.
3.3. Wnt4
Wnt4 is the most abundantly expressed Wnt protein in β‐cells, whereas its role in diabetes is also contradicting because it functions as a biphasic initiator for canonical and non‐canonical Wnt pathways. Wnt4 is enriched in pancreatic islets of health mice and more abundant in insulin‐resistant obese mice, Wnt4 alone does not affect GSIS in primary murine β‐cells, but can antagonize the activation of canonical Wnt pathway mediated by Wnt3a and the resulting increase in cellular proliferation as well as GSIS in islets and β‐cell line INS1, 55 , 56 and depletion of Wnt4 results in a decrease in proliferation of INS1 cells. 57 Similarly, cellular proliferation and GSIS of β‐cell line MIN6 are not affected when exposed to Wnt4 protein, but suppressed significantly when knockdown of Wnt4. 58 Recently, Kozinski et al revealed the dynamic equilibrium of canonical and non‐canonical Wnt signalling pathways activated by Wnt3a and Wnt4 separately in β‐cells during the development of T2DM; they believed that in pre‐diabetic state, the increased secretion of Wnt3a and decreased secretion of Wnt4 by insulin‐resistant tissues were responsible for the increase in insulin secretion and β‐cell proliferation to adapt the systemic insulin resistance, whereas the expression profiles of Wnt3a and Wnt4 were reversed in a severe diabetic state, which correlated with the down‐regulated β‐cell proliferation and deficiency in insulin production. 59 Wnt4 also induces the expression of transcriptional factors that are indispensable for islet differentiation such as NKX6.1 and PDX1 in human islets by activating the non‐canonical Wnt pathway. 60 However, in another study, Wnt4 was found to reduce the protein levels of PDX1 and MAFA, and inhibiting Wnt4‐mediated non‐canonical signalling promoted the maturation of β‐cells. 48 These opposite results might be attributed to the heterogeneity of islet, a mixture of different cell types that may dilute the effect of different transcriptional factors during the differentiation and maturation processes. In addition, Wnt4 was found to activate the canonical Wnt pathway in islet endothelium, and mesenchymal stromal cell (MSC)–based therapies ameliorated oxidative stress–induced apoptosis and functional impairment of islet endothelium via activating the canonical Wnt4 signalling. 61 Moreover, Wnt4 is closely linked to the development of diabetic complications, especially in DN. High glucose condition induces transforming growth factor‐β1 (TGF‐β1)–mediated fibrosis in glomerular mesangial cells by inhibiting Wnt4‐ and Wnt5a‐mediated canonical pathways; therefore, restoring Wnt4 or Wnt5a significantly alleviates TGF‐β1‐mediated fibrosis in diabetic kidneys. 62 Similarly, melatonin, simvastatin, Salvia miltiorrhiza extracts and liraglutide treatment block the apoptosis of mesangial cells and improve the renal injury of diabetic rat by restoring the canonical Wnt signalling mediated by Wnt4 and Wnt5a. 63 , 64 , 65 , 66 However, sitagliptin or soybean isoflavones treatment was reported to alleviate the renal tubulointerstitial fibrosis in rat with DN by inhibiting the canonical Wnt4 pathway. 67 , 68 Moreover, the canonical Wnt4 pathway is up‐regulated in carotid arteries of diabetic rats with carotid artery injury, and silencing this cascade by microRNA‐24 is sufficient to attenuate proliferation of vascular smooth muscle cells (VSMC) and neointimal hyperplasia. 69 Overall, the role of Wnt4 varies depending on the tissue type; it mainly activates the non‐canonical Wnt pathway and acts as a negative regulator of canonical Wnt pathway in the context of islet, but mainly activates the canonical Wnt pathway in other tissues. In short, the general effect of Wnt4 seems to be deleterious, and further studies are required for a deeper understating of precise role of Wnt4 in diabetic‐related diseases.
3.4. Wnt5a and Wnt5b
Wnt5a, another initiator that activates the canonical and non‐canonical Wnt pathways, is closely related to a variety of metabolic disorders such as obesity and T2DM. Compared with that in healthy subject, plasma Wnt5a level is significantly decreased in patients with the onset T2DM, and a negative correlation is found between the Wnt5a level and fast blood glucose (FBG)/HbA1c levels. However, Wnt5a level is gradually increased in patients with long‐term T2DM or after 3 months of treatment, 70 , 71 and more studies demonstrate that the protein and mRNA levels of Wnt5a in circulation are elevated in obese individuals and patients with T2DM and positively correlated with IL‐6 concentration and metabolic disorders, such as increased triglyceride and FBG levels as well as insulin resistance. 72 , 73 Moreover, a mutual stimulation relationship between Wnt5a and inflammation cytokines is detected in cultured human adipocytes. 74 Wnt5a is widely expressed in adipose tissues, and the deleterious role of Wnt5a through activating the non‐canonical Wnt pathway in the development of inflammation and insulin resistance has been clearly revealed. 18 , 75 , 76 Recent studies showed that increased secretion of Wnt5a by enlarged adipocytes in the obese state could block insulin signalling and cause glucose intolerance via activating the non‐canonical Wnt5a/PCP pathway and systemic inflammation, 75 , 77 and an obviously positive correlation between the up‐regulated Wnt5a/PCP pathway and profound vascular insulin resistance were observed in visceral adipose tissue arterioles of obese individuals. 78 Therefore, treatment of anti‐inflammatory cytokine IL‐10 in 3T3‐L1 pre‐adipocytes or celecoxib in diabetic rat could suppress the adipogenesis and reverse the non‐alcoholic steatohepatitis (NASH) via targeting this non‐canonical Wnt5a pathway. 79 , 80
Wnt5a is another regulator with dual function regarding the effect on islet function, induction of Wnt5a expression blocks glucose‐induced β‐cell proliferation, 81 and treating β‐cells with exendin‐4, a glucagon‐like peptide‐1 receptor agonist regarded as a therapeutic reagent for T2DM, was found to promote β‐cell proliferation via inhibiting the expression of Wnt5a and mediated canonical Wnt pathway. 82 However, in diabetic mice, the levels of Wnt5a and its receptor Fzd5 are significantly decreased in islet stellate cells (ISCs), a type of stellate cell located in islet and activated in T2DM, and down‐regulation of Wnt5a expression in ISCs could decrease insulin secretion, 83 demonstrating a protective role of Wnt5a in maintaining the insulin secretion homeostasis of β‐cells. Similarly, Kuljanin et al found that the glucose‐lowering and islet regenerative capacities of bone marrow (BM)–derived multipotent stromal cells (MSCs) after transplantation into diabetic mice were mainly attributed to the activation of canonical Wnt5a pathway. 84
Wnt5a is essential for normal development of heart, and increased oxidative stress in diabetic pregnancies causes heart defects in foetuses by inhibiting the expression of Wnt5a and its induced canonical and non‐canonical Wnt pathways; hence, overexpressing superoxide dismutase 1 (SOD1) in vivo could ameliorate heart defect through restoration of non‐canonical Wnt5a/Ca2+ pathway. 85 However, the concentration of Wnt5a is elevated in the plasma and epicardial adipose tissue of patients with coronary artery disease (CAD) and independently associated with the presence of CAD and progression of calcified coronary plaque, 86 and adipose tissue–derived Wnt5a in obesity induces arterial oxidative stress and migration of vascular smooth muscle cells (VSMCs) via activating a new Wnt5a/USP17/RAC1/NADPH oxidases axis. 87 Moreover, oxidized low‐density lipoprotein (oxLDL)–induced activation of non‐canonical Wnt5a pathway and inhibition of canonical Wnt3a pathway could promote foam cell formation in human aortic VSMCs but inhibit their migration and proliferation. 88 Overactivation of Wnt5a/PCP pathway is also detected in ECs from patients with T2DM, thus inhibiting this signalling could ameliorate insulin resistance and dysfunction of ECs from T2DM patients. 78 , 89 Wnt5a is also highly expressed in circulating monocytes and macrophages in patients with peripheral artery disease and in ischaemic muscle of ob/ob mice or mice fed with high‐fat and high‐sucrose diet. Myeloid‐specific Wnt5a overexpression blunts regenerative angiogenesis in ischaemic hind limbs by activating the non‐canonical Wnt5a/JNK pathway and mediated elevation of VEGF‐A165b, an antiangiogenic VEGF‐A splice isoform 90 ; therefore, treatment with recombinant sFRP5, an extracellular inhibitor of non‐canonical Wnt signalling, could alleviate cardiac inflammation and protect the heart from ischaemia/reperfusion injury through inhibiting the non‐canonical Wnt5a/JNK signalling and the expression of inflammatory cytokine/chemokine in macrophages and ischaemic myocardium. 91 Likewise, Wnt5a is highly expressed in ischaemic muscles and ECs from mice overexpressing glutaredoxin‐1, an oxidation‐promoting enzyme which is increased in T2DM patients, and exogenous Wnt5a treatment could inhibit the revascularization in hind limb ischaemia via activating the non‐canonical Wnt pathway, 92 indicating a deleterious role of Wnt5a in diabetic foot. Although the increase in serum concentration of Wnt5a and a negative correlation between serum Wnt5a and glomerular filtration rate are detected in patients with DP, 93 more studies tend to prove the protective role of Wnt5a in the development of DP. It has been shown that a weak Wnt5a expression is detected in glomerular mesangium of diabetic rat and glomerular cells cultured in high glucose condition. Therefore, curcumin and exogenous superoxide dismutase administration as well as nitric oxide (NO) donor treatment could significantly alleviate the apoptosis of glomerular cells and diabetic renal fibrosis by restoring the canonical Wnt5a pathway. 94 , 95 , 96 Ando et al also showed that Wnt5a induced renal AQP2 expression via the activation of the non‐canonical Wnt5a/Ca2+ pathway could increase the urine concentration of mice with heritable nephrogenic diabetes insipidus (NDI), a hereditary disease characterized by defective urine concentration ability in kidney. 97 Taken together, these findings suggest the complicated roles of Wnt5a in the development of T2DM and related complications in a tissue‐specific manner.
Wnt5b shares 78% amino acid sequence identity with Wnt5a, and its expression is detectable in pancreas, adipose and liver. Wnt5b expression is up‐regulated in islets of mice fed with high‐fat diet (HFD), and UK Caucasian individuals carrying IVS3C>G variant (rs2270031) in the Wnt5b gene are predispose to T2DM. 58 , 98 Although the exact role of Wnt5b in the regulation of β‐cell proliferation and functions has not been elucidated, silencing the non‐canonical Wnt pathways induced by Wnt4/Wnt5a/Wnt5b and canonical Wnt3a/β‐catenin signalling together could drive the immature hiPSC‐derived S7 cells towards a mature phenotype, indicating the potential inhibitory effect of Wnt5b on the maturation of β‐cells. 48 Subsequent in vitro experiments showed that compared with the inhibitory effect on adipogenesis via activation of canonical Wnt signalling mediated by Wnt1 and Wnt10b, Wnt5b was overexpressed in 3T3‐L1 cells at an early phase of adipogenesis and could stimulate adipogenesis by antagonizing the canonical Wnt pathway, 99 , 100 suggesting that Wnt5b may contribute to the susceptibility to T2DM; therefore, down‐regulation of non‐canonical Wnt5b pathway could therefore decrease adipogenesis and increase β‐cell functions of T2DM subjects.
3.5. Wnt6
Wnt6 is highly homologous to Wnt1 but these two Wnts only share 43% amino acid sequence identity. The expression level of Wnt6 is decreased during adipogenesis and ectopic expression of Wnt6 suppresses the differentiation of 3T3‐L1 pre‐adipocytes through a β‐catenin‐dependent pathway, 101 and activation of Hedgehog signalling has been shown to prevent HFD‐induced obesity in mice by inducing the canonical Wnt6 pathway in adipose tissues. 102 Thus, induction of Wnt6 could ameliorate metabolic abnormalities such as obesity and T2DM. Moreover, Wnt6 is expressed in mesonephros of the developing mouse embryo, and loss of Wnt6 is obvious in tubulointerstitium of patients with DN and animal models with renal fibrosis; therefore, activating the canonical Wnt6 pathway suppresses renal fibrosis through inhibiting TGF‐β1‐induced activation of NF‐κB pathway. 103 The activation of ATF3/NFAT axis causes podocyte injury during the development of DN, and ATF3 is increased in glomeruli from proteinuric patients with DP, and Wnt6 is up‐regulated in ATF3‐overexpressed podocytes and identified as a target of this axis, indicating Wnt6 may aggravate podocyte injury and loss. 104 Furthermore, the activation of Wnt6/β‐catenin pathway in diabetic context establishes a pathological link between T2DM and cancer due to the inducing effect of this signalling on the amplification of centrosome, a symbolic event associated with high‐grade tumours and poor prognosis. 105 Thus, further studies are needed to explore the role of Wnt6 in the development of T2DM and related complications.
3.6. Wnt7a and Wnt7b
Wnt7a and Wnt7b are also secreted proteins with 78% amino acid sequence identity, and both can activate the canonical and non‐canonical Wnt pathways. Although their expression levels have not been determined in islets of diabetic patients, some studies showed that Wnt7a and Wnt7b are required for pancreatic development through autocrine and paracrine mechanisms 106 , 107 ; therefore, stably expressing Wnt7a or Wnt7b alone could enhance the proliferation of human pancreatic progenitor cells (PPCs) through activating the non‐canonical Wnt/PKC signalling, suggesting their promising application in developing cell therapies for diabetes. 107 Moreover, the expression level of Wnt7a is decreased significantly in cultured human umbilical vein endothelial cells (HUVECs) treated with high glucose and in wounded skin tissues from diabetic rat; thus, localized injection of Wnt7a could reverse the overwhelmed inflammation in wounded skins and accelerate the wound healing rate of diabetic rat. 108 Similarly, dietary supplementation with resveratrol enhances the expression of hippocampal Wnt7a and neurogenesis of diabetic mice, indicating a potential neuroprotective role of Wnt7a in diabetes. 109 Moreover, Wnt7b has dual effect during diabetic arteriosclerosis. Wnt7b is detected in aortic tissues and essential for stabilizing normal phenotype and integrity of aortic endothelial cells (AoECs); therefore, specific deletion of Wnt7b in AoECs induces the arteriosclerotic injury in LDLR knockout mice fed diabetogenic diets. 110 However, the expression of Wnt7a and induced canonical pathway is also induced in aortic tissues from diabetic mice exhibiting cardiovascular calcification; therefore, suppressing vascular calcification could inhibit the aortic mRNA level of Wnt7a. 52 , 111 In general, most studies tend to prove that Wnt7a and Wnt7b are potential therapeutic candidates to ameliorate T2DM and related complications.
3.7. Wnt8a, Wnt8b, Wnt9a and Wnt9b
Wnt8a and Wnt8b share 63% amino acid sequence identity. At present, little is known about their role in the development of diabetes, and only one study indirectly showed that chimeras between Xenopus Wnt8 and mouse Fzd1 or Fzd2 could inhibit adipogenesis through the canonical and non‐canonical Wnt pathways. 112 Wnt9, formerly named Wnt14, shares 63% amino acid sequence identity with its analogue Wnt9b. Wnt9a is expressed in embryonic pancreas but not necessary for the formation and growth of pancreas; Wnt9a ablation inhibits the canonical Wnt pathway and leads to the up‐regulation of some genes in endocrine differentiation programme but increase in pancreatic endocrine cell number, including α‐cells, β‐cells and δ‐cells, supporting its negative regulation on endocrine differentiation. 113 However, there are few studies regarding the role of Wnt9b in the development of T2DM and related complications. Rundqvist et al found a positive correlation between Wnt9a expression and insulin sensitivity, and sprint exercise markedly induced the expression of Wnt9a in human skeletal muscles and up‐regulated the secretion of plasma insulin. 114 Wnt9b may play a deleterious role in the development of diabetic foot ulcers, because inhibiting the canonical Wnt9b pathway by circulating exosomal miR‐20b‐5p from T2DM patients is found to suppress the angiogenenic effect of HUVECs in vitro and delay the wound healing in vivo. 115
3.8. Wnt10a and Wnt10b
Wnt10a is another biphasic Wnt ligand that shares 62% amino acid sequence identity with Wnt10b. Although the exact role of Wnt10a in diabetes remains unclear, it is indeed important for adipogenesis and several studies have revealed their potential connection. 116 Wnt10a inhibits the pre‐adipocyte‐adipocyte transition, and the expression of Wnt10a and mediated canonical Wnt pathway is induced in brown pre‐adipocyte cells that show decreased ability to differentiate. 117 In another study, disruption of circadian clocks in mice results in increased adipogenesis and obesity through silencing the canonical Wnt10a pathway, indicating the protective role of Wnt10a in the development of obesity. 118 However, Wnt10a and mediated canonical Wnt signalling are also induced in spinal cord of diabetic rat, and dexmedetomidine treatment could alleviate diabetic neuropathy pain by inhibiting the canonical Wnt10a pathway. 119 The role of Wnt10b in the development of T2DM is comparatively well understood. Among all Wnts, Wnt10b is important negative regulator of adipocyte differentiation via activating the canonical Wnt pathway; lower Wnt10b expression and down‐regulation of Wnt10b/β‐catenin pathway are detected in white adipose and skeletal muscle tissues from men with overweight and prediabetes, 120 and inactivation of Wnt10b/β‐catenin pathway promotes the adipogenesis and diet‐induced obesity in mice. 121 On the contrary, transgenic mice in which Wnt10b is overexpressed in adipose resist body fat accumulation and glucose intolerance when fed with HFD or on ob/ob background. 122 , 123 Similarly, ectopic expression of Wnt10b in skeletal muscles decreases adipose deposits, hyperinsulinaemia and triglyceride plasma levels and improves glucose homeostasis in adult diet‐induced obese rats. 124 Activation of Wnt10b/β‐catenin pathway also increases the insulin sensitivity of skeletal muscle cells by decreasing lipid deposition in myoblasts through down‐regulation of SREBP‐1c 125 and participates in curcumin‐induced suppression of adipocyte differentiation. 126 Moreover, the expression of Wnt10b is inhibited in bone of mice with T1DM, and elevation of TNF‐α is found to be a critical factor leading to its down‐regulation and bone loss in diabetic environment; thus, overexpression of Wnt10b in bone has a potential utility for the treatment of bone loss in diabetic patients. 127 These findings demonstrate that Wnt10b blocks the development and function of adipose tissues and improves the glucose homeostasis and insulin sensitivity of whole body.
3.9. Wnt11
Wnt11 shows no homology to other Wnts and only shares 41% amino acid sequence identity with Wnt4. Wnt11 is expressed at low level throughout the development; it is mainly expressed in mesenchymal cells of the pancreas but weakly in the areas of endocrine cells. 128 Studies on adiponectin transgenic mice showing higher sensitivity to insulin revealed that hyperadiponectinaemia resulted in the down‐regulation of Wnt11 expression and chronic inflammation in adipose tissue, but the increase in the number of small adipocyte, a type of ‘good’ adipocyte. 129 Consistently, high levels of glucose selectively enhanced the expression of Wnt11 in mesenchymal progenitor cells (MPCs) to stimulate adipogenesis through the non‐canonical Wnt/PCP pathway. 130 These findings suggest that Wnt11 in adipose tissue contributes to the development of obesity‐linked disorders including T2DM.
3.10. Wnt16
Wnt16 is another Wnt ligand having two distinct mRNA isoforms, Wnt16a and Wnt16b, which only differ in the composition of 5’‐untranslational region and one exon. Wnt16b is expressed ubiquitously at significant levels in most adult tissues, whereas Wnt16a is barely expressed with high levels in pancreas. 131 Therefore, most studies on Wnt16 mainly refer to Wnt16b, and it is known to be a major determinant of bone homeostasis and fracture susceptibility in humans. Interestingly, osteoblast‐derived Wnt16 only activates the non‐canonical Wnt pathway in osteoclast progenitors to inhibit osteoclastogenesis, but activates both canonical and non‐canonical Wnt pathways in osteoblasts to increase osteoprotegerin expression and decrease osteoclastogenesis; mice with targeted deletion of Wnt16 in osteoblasts will develop spontaneous fractures due to low cortical bone thickness. 132 Diabetic patients have higher risk of fracture; the reduction in Wnt16 expression and mediated canonical Wnt signalling activity in cortical bone in diabetic environment might be responsible for the osteopenia, and activating Wnt16/β‐catenin pathway improves the bone strength and provides a therapeutic opportunity for the treatment of osteopenia in diabetic patients. 133 Moreover, the expression of Wnt16 is decreased in the penises of mice with diabetic erectile dysfunction and overexpression of Wnt16 accelerated the tube formation in cultured mouse cavernous ECs, suggesting the up‐regulation of Wnt16 might contribute to the treatment of diabetic‐related erectile dysfunction. 134 However, further studies are still required to clarify the role of Wnt16 in the development of diabetes.
4. ROLES OF MAIN ANTAGONISTS AND CORECEPTOR OF WNTS IN T2DM AND RELATED COMPLICATIONS
The signalling transduction of Wnt pathways will be blocked if their receptors are bound by competitive antagonists. Secreted frizzled‐related proteins (sFRPs) and Wnt inhibitory factor‐1 (WIF‐1) are classical Wnt antagonists that block all Wnt signalling pathways. As a family of soluble glycoproteins with five members containing cysteine‐rich domain (CRD) homologous to Fzd, sFRPs inhibit all Wnt signalling pathways by competing with Wnt ligands for binding Fzd and play important roles in the pathogenesis of T2DM and related complications. Human foetal aorta‐derived CD133+ progenitor cells accelerate the wound healing of diabetic ischaemic ulcers by activating Wnt pathways, whereas the presence of sFRP‐1 could abolish the reparative process by reducing CD133 expression in progenitor cells. Moreover, the expression of sFRP1, sFRP3 and sFRP4 is up‐regulated when foetal CD133(+) cell differentiation into CD133(−) cells, 135 indicating the deleterious role of sFRPs in diabetic wounds. However, inhibiting the expression of sFRP‐1 by miR‐27a could aggravate diabetic nephropathy by activating the canonical Wnt signalling. 136 In human subjects, circulatory sFRP2 is increased in patients with impaired glucose tolerance (IGT) and promotes the adipose angiogenesis through enhancing VEGF expression. 137 sFRP3 levels in serum and skeletal muscles are significantly reduced in T2DM patients and positively correlated with insulin sensitivity; thus, the treatment of myotubes with recombinant sFRP3 could significantly restore the inhibited insulin signalling induced by cytokine. 138 The gene expression of sFRP4 is increased in adipose tissues from obese individuals and T2DM patients and in islets from T2DM patients. Circulatory sFRP4 levels are also increased in patients with IGT and T2DM. Moreover, sFRP4 expression is positively correlated with the NAFLD activity score, elevated HbA1c level and insulin resistance, and reduced GSIS, suggesting that elevated SFRP4 is a valuable biomarker of β‐cell dysfunction, insulin resistance and T2DM. 139 , 140 , 141 Therefore, systemically elevated sFRP4 not only inhibits insulin secretion and GSIS in pancreatic β‐cells, 139 but induces the insulin resistance and lipogenesis in the liver. 141 sFRP5 is an anti‐inflammatory adipokine that exerts a promising therapeutic effect on inflammatory diseases, including obesity and T2DM, via antagonizing the non‐canonical Wnt5a signalling pathway, and the exact roles of sFRP5 in the pathogenesis of these diseases have been systematically outlined by Shen et al. 142
The inhibitory effect of WIF‐1 on Wnt pathways is exerted by its directly binding to Wnt ligands. However, there is little evidence on the role of WIF‐1 in the pathogenesis of T2DM and related complications. It is reported that the circulatory WIF‐1 and sFRP‐1 levels were significantly higher in non‐diabetic subjects who developed cardiovascular disease during the follow‐up period, suggesting the elevation in WIF‐1 may be a valuable predictor for future cardiovascular events. 143 The expression of WIF‐1 is also much higher in diabetic rat induced by STZ treatment. Jinmaitong (JMT), a compound prescription of traditional Chinese medicine, was found to ameliorate DPN in rat via relieving the inhibitory effect of WIF‐1 on the canonical Wnt pathway. 144 In short, the general effect of WIF‐1 seems to be deleterious diabetic environment.
LRP5/6 are essential coreceptors for the signalling transduction of the canonical Wnt signalling, whereas LRP5 is less effective than LRP6 for the activation of the Wnt pathway. Here, we only discuss the role of LRP6 in the pathogenesis of T2DM and related complications due to the limited space. LRP6 is required for the normal expression of insulin receptor and the stability of IGF receptor in humans, and a rare missense mutation in Wnt coreceptor LRP6 (R611C) is the aetiology of the autosomal dominant early‐onset coronary artery disease, T2DM and metabolic syndrome through blocking the canonical Wnt signalling. 145 , 146 , 147 The homozygote LRP6 knockout is lethal for mice, whereas the heterozygote LRP6 knockdown mice are resistant to glucose intolerance and obesity on HFD; this is associated with the inhibition in the canonical Wnt pathway and resultant increase in mitochondrial biogenesis and reduce in endogenous hepatic glucose output. 148 Moreover, the concentration of LRP6 is higher in the vitreous samples from patients with proliferative DR, 149 indicating the deleterious role of LRP6 in diabetes. However, reduced LRP6 expression and inactivation of canonical Wnt pathway are found to promote the progression of DN via enhancing GSK3β‐p53 interaction and the apoptosis of podocyte, and the deleterious effect can be prevented by green tea. 150 Interestingly, Towler and colleagues found that vascular smooth muscle LRP6 could inhibit the progression of diabetic arteriosclerosis in mice by restraining a novel non‐canonical Wnt/USF1 signalling, indicating a protective role of LRP6 in diabetes. 151 Therefore, the exact role of LRP6 in the pathogenesis of T2DM and related complications remains an intriguing question and needs in‐depth study.
5. OPPORTUNITIES AND CHALLENGES IN DEVELOPING THERAPEUTICS FOR T2DM AND COMPLICATIONS BASED ON WNTS
As mentioned above, there is a close relationship between the dysregulation of Wnt signalling pathways and development of T2DM and related complications. Therefore, appropriate therapeutic approaches targeting Wnt signalling pathways are attractive for the treatment of these disorders, and some reagents that activate or inhibit Wnt signalling pathways have been developed and are undergoing pre‐clinical and clinical trials. For related information described in detail, please refer to review by Aamir et al 152 ; herein, we only discuss main challenges in developing therapeutics for T2DM and related complications based on Wnts.
It remains elusive concerning mechanisms that control the generation of distinct Wnts from a single Wnt gene. It may be attributed to different promoters regulating the expression of Wnt gene and regulators controlling the post‐translational modification, and both of which are simultaneously regulated by a wider range of other signalling pathways. Recently, sFRPs are found to act as the molecular switch to repress or promote specific non‐canonical Wnt signalling branches. Kaufmann and colleagues revealed that the presence of sFRP2 in the extracellular space could activate the non‐canonical Wnt5a/Ror2 signalling by stabilizing Wnt5a‐Ror2 complexes, but reduce the non‐canonical Wnt5a signalling mediated by Fzd7, a main receptor that activates the non‐canonical Wnt/PCP signalling, by preventing Fz7 endocytosis. 153 Moreover, Wnt signalling pathways are complicated networks in which different Wnts bind to various receptors and activate different effector pathways in a highly dose‐specific and tissue‐specific manner; thus, canonical and non‐canonical Wnt pathways activated by different Wnts may counteract with each other by different concentrations or in different tissues. Interestingly, the interaction of different Wnts could even change the activity of different Wnt signalling pathways. For instant, despite being the biphasic Wnt ligands, Wnt5a and Wnt11 mainly activate the non‐canonical Wnt signalling pathway when secreted alone. However, the post‐translational O‐sulphation of specific tyrosine residues in Wnt11 and Wnt5a will facilitate their interaction and enhance the canonical signalling activity that is required for the initiation of embryonic development. 154 Therefore, it is not surprising that we observe contradiction in the role of Wnts during the development of T2DM and complications, or even opposite effect of a specific Wnt on different tissues in similar diabetic environment. However, there is increasing evidence that Wnt signalling pathways have certain function in common. In brief, canonical Wnt pathway mediated by most Wnts plays a protective role in alleviating β‐cell dysfunction, excessive adipogenesis in obesity, insulin resistance and T2DM. Thus, activation of canonical Wnt pathway has potential therapeutic effect on obesity and T2DM, and several Wnt activators, such as genistein, kirenol, curcumin and isoquercitrin, have shown beneficial effects on obesity and T2DM at pre‐clinical level via activating the canonical Wnt pathway. 152 However, most of these activators are targeting components in canonical Wnt pathway rather than Wnts, and their efficacy and safety are only evaluated in cells or diseased animal models rather than in clinical trials. Furthermore, activation of canonical Wnt pathway may accelerate the progression of some diabetic complications, and diabetic patients have an increased risk of cancers due to the direct effect of hyperglycaemia and indirect effects of insulin resistance, hyperinsulinaemia and chronic inflammation, 155 , 156 and overactivation of canonical Wnt pathway serves as primary determinant for most human malignancies; hence, systemic application of Wnt activators on diabetic patients may aggravate certain diabetic complications or increase the risk of cancer incidence.
Compared with the conflicting role of canonical Wnt pathway, non‐canonical Wnt pathways are consistently overactivated in diabetes and related complications, and the expression of related Wnts, including Wnt4, Wnt5a, Wnt5b and Wnt11, is increased in diseased tissues in diabetic environment. Therefore, inhibition of the non‐canonical Wnt pathway is a promising therapeutic approach for the treatment obesity, T2DM and related complications. Post‐translational lipidation of Wnts is indispensable for their secretion and activity; almost all Wnts are modified with palmitic acid at conserved serine residue by porcupine (Porcn), a membrane‐bound O‐acyltransferase, 157 and several small molecule inhibitors targeting Porcn have been developed to prevent Wnts‐driven diseases. LGK974, a Porcn inhibitor, has neuroprotective potential and could ameliorate DPN in rats. 158 However, our unpublished data showed that treating diabetic mice with another Porcn inhibitor, C59, could exacerbate DPN by activating some crucial inflammatory pathways induced by the silence of canonical Wnt pathway. Consistently, C59 blocks the progression of mammary cancer in mice via down‐regulating the canonical Wnt1/β‐catenin pathway. 159 We predict the conflicting results might be attributed to the heterogenous function and balance of canonical and non‐canonical pathways during different time windows. Importantly, the normal signalling transduction of Wnt pathways is essential for the maintenance of tissue homeostasis and regeneration; therefore, systemic application of Wnt inhibitors may inhibit some protective Wnts and has unwanted side effects or even toxic on normal tissues when treating diabetic‐related diseases. Therefore, overexpressing Wnts that are aberrantly decreased or inhibiting overexpressed Wnts in determined tissues by using tissue‐specific delivery systems may be viable for treating these disorders. Indeed, several Wnt‐neutralizing antibodies and decoy receptors for Wnts have been developed and promising pre‐clinical trail results have been obtained in treating some human malignancies, 160 and there is an urgent need to evaluate their efficacy and safety in treating diabetic‐related diseases at clinical level.
6. CONCLUSIONS
The dysregulation of Wnt signalling pathways is important pathogenetic basis of series of human diseases, including T2DM and related complications. In recent decades, we have witnessed the remarkable progress in understanding of Wnt signalling pathways in these diseases on a mechanistic level. The function of non‐canonical Wnt pathways activated by few Wnts is consistent in diabetic‐related diseases, while the canonical Wnt pathway activated by most Wnts is either protective or deleterious in a context‐dependent manner, and it is impossible to produce therapeutic effect on these diseases via silencing or activating Wnt pathways alone. Therefore, it is required to have a comprehensive understanding of activation mechanisms and interactions of canonical and non‐canonical Wnt pathways in specific tissues and time windows during the occurrence and progression of diabetic‐related diseases. In this review, we systematically summarize the existing findings on the role of all human Wnts, their main antagonists (sFRPs and WIF‐1) and coreceptor (LRP6) in the development of T2DM and related complications and discus current main challenges in designing novel therapeutic strategies targeting Wnts for the treatment of these disorders. We believe a comprehensive understanding of their functions may pave the way for Wnts to serve as promising therapeutic targets for the prevention and treatment of diabetic‐related diseases.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose in relation to this review.
AUTHOR CONTRIBUTIONS
xiaobo Nie: Conceptualization (lead); Funding acquisition (equal); Project administration (lead); Supervision (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Xiaoyun Wei: Formal analysis (supporting); Visualization (supporting); Writing‐original draft (supporting). Han Ma: Validation (equal); Writing‐review & editing (equal). Lili Fan: Validation (equal); Writing‐review & editing (equal). Wei‐Dong Chen: Conceptualization (equal); Funding acquisition (equal); Supervision (equal).
Nie X, Wei X, Ma H, Fan L, Chen W‐D. The complex role of Wnt ligands in type 2 diabetes mellitus and related complications. J Cell Mol Med. 2021;25:6479–6495. 10.1111/jcmm.16663
Funding information
This work is supported by the National Natural Science Foundation of China [Grant No. 81700731], Key Program for Science and Technology of Henan Province [Grant No. 202102310043], Henan Provincial Natural Science Foundation [Grant No. 2015YBZR051], Innovation and Entrepreneurship Training Program for Undergraduate in Henan University [Grant No. 2020101902] and Medical Incubation Program for National Natural Science Foundation in Henan University to XN. The National Natural Science Foundation of China [Grant No. 81970726, 81472232], Henan Provincial Natural Science Foundation [Grant No. 182300410323], First Class Discipline Cultivation Project of Henan University [Grant No. 2019YLZDYJ19], Key Program for Science & Technology from Henan Education Department [Grant No. 21A310001] and Plan for Scientific Innovation Talent of Henan Province [Grant No. 154100510004] to W‐DC.
Contributor Information
Xiaobo Nie, Email: xbniemed@126.com.
Xiaoyun Wei, Email: wxy1607522379@163.com.
Han Ma, Email: HAN66881@126.com.
Lili Fan, Email: fan66962020@163.com.
Wei‐Dong Chen, Email: wdchenmed@163.com.
REFERENCES
- 1. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88‐98. [DOI] [PubMed] [Google Scholar]
- 2. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137‐188. [DOI] [PubMed] [Google Scholar]
- 3. Galicia‐Garcia U, Benito‐Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hudish LI, Reusch JE, Sussel L. beta Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129(10):4001‐4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Virally M, Blickle JF, Girard J, Halimi S, Simon D, Guillausseau PJ. Type 2 diabetes mellitus: epidemiology, pathophysiology, unmet needs and therapeutical perspectives. Diabetes Metab. 2007;33(4):231‐244. [DOI] [PubMed] [Google Scholar]
- 6. Himanshu D, Ali W, Wamique M. Type 2 diabetes mellitus: pathogenesis and genetic diagnosis. J Diabetes Metab Disord. 2020;19(2):1959‐1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: an exposure‐wide umbrella review of meta‐analyses. PLoS One. 2018;13(3):e0194127. 10.1371/journal.pone.0194127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu J, Sun X, Jiang Z, et al. Protective role of NRF2 in macrovascular complications of diabetes. J Cell Mol Med. 2020;24(16):8903‐8917. 10.1111/jcmm.15583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillen C, Benito M. mTORC1 overactivation as a key aging factor in the progression to type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2018;9:621. 10.3389/fendo.2018.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nandipati KC, Subramanian S, Agrawal DK. Protein kinases: mechanisms and downstream targets in inflammation‐mediated obesity and insulin resistance. Mol Cell Biochem. 2017;426(1–2):27‐45. 10.1007/s11010-016-2878-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antonioli L, Blandizzi C, Csoka B, Pacher P, Hasko G. Adenosine signalling in diabetes mellitus–pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2015;11(4):228‐241. 10.1038/nrendo.2015.10 [DOI] [PubMed] [Google Scholar]
- 12. Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11‐26. 10.1038/nrc3419 [DOI] [PubMed] [Google Scholar]
- 13. Nie X, Liu Y, Chen WD, Wang YD. Interplay of miRNAs and canonical Wnt signaling pathway in hepatocellular carcinoma. Front Pharmacol. 2018;9:657. 10.3389/fphar.2018.00657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ram Makena M, Gatla H, Verlekar D, Sukhavasi S, K Pandey M, C Pramanik K. Wnt/beta‐catenin signaling: the culprit in pancreatic carcinogenesis and therapeutic resistance. Int J Mol Sci. 2019;20(17):4242. 10.3390/ijms20174242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grant SFA, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7‐like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38(3):320‐323. 10.1038/ng1732 [DOI] [PubMed] [Google Scholar]
- 16. Welters HJ, Kulkarni RN. Wnt signaling: relevance to beta‐cell biology and diabetes. Trends Endocrinol Metab. 2008;19(10):349‐355. 10.1016/j.tem.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 17. Cohen H, Barash H, Meivar‐Levy I, et al. The Wnt/beta‐catenin pathway determines the predisposition and efficiency of liver‐to‐pancreas reprogramming. Hepatology. 2018;68(4):1589‐1603. 10.1002/hep.29827 [DOI] [PubMed] [Google Scholar]
- 18. Fuster JJ, Zuriaga MA, Ngo D‐M, et al. Noncanonical Wnt signaling promotes obesity‐induced adipose tissue inflammation and metabolic dysfunction independent of adipose tissue expansion. Diabetes. 2015;64(4):1235‐1248. 10.2337/db14-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kikuchi A, Yamamoto H, Sato A, Matsumoto S. New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol. 2011;291:21‐71. 10.1016/B978-0-12-386035-4.00002-1 [DOI] [PubMed] [Google Scholar]
- 20. Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis‐inducing activity, stability, and subcellular distribution of beta‐catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10(12):1443‐1454. [DOI] [PubMed] [Google Scholar]
- 21. Latres E, Chiaur DS, Pagano M. The human F box protein beta‐Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta‐catenin. Oncogene. 1999;18(4):849‐854. 10.1038/sj.onc.1202653 [DOI] [PubMed] [Google Scholar]
- 22. Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: Molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59(5):1107‐1117. 10.1016/j.jhep.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 23. Burn SF, Webb A, Berry RL, et al. Calcium/NFAT signalling promotes early nephrogenesis. Dev Biol. 2011;352(2):288‐298. 10.1016/j.ydbio.2011.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee JM, Kim IS, Kim H, et al. RORalpha attenuates Wnt/beta‐catenin signaling by PKCalpha‐dependent phosphorylation in colon cancer. Mol Cell. 2010;37(2):183‐195. 10.1016/j.molcel.2009.12.022 [DOI] [PubMed] [Google Scholar]
- 25. Xu D, Zhao W, Pan G, et al. Expression of Nemo‐like kinase after spinal cord injury in rats. J Mol Neurosci. 2014;52(3):410‐418. 10.1007/s12031-013-0191-5 [DOI] [PubMed] [Google Scholar]
- 26. Acebron SP, Niehrs C. beta‐catenin‐independent roles of Wnt/LRP6 Signaling. Trends Cell Biol. 2016;26(12):956‐967. [DOI] [PubMed] [Google Scholar]
- 27. Park H, Kim Y, Yu BO, et al. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162(4):780‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT‐LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17(5):745‐755. 10.1016/j.cmet.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heallen T, Zhang M, Wang J, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458‐461. 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nie X, Liu H, Liu L, Wang YD, Chen WD. Emerging roles of Wnt ligands in human colorectal cancer. Front Oncol. 2020;10:1341. 10.3389/fonc.2020.01341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shati AA, Alfaifi MY. Salidroside protects against diabetes mellitus‐induced kidney injury and renal fibrosis by attenuating TGF‐beta1 and Wnt1/3a/beta‐catenin signalling. Clin Exp Pharmacol Physiol. 2020;47(10):1692‐1704. 10.1111/1440-1681.13355 [DOI] [PubMed] [Google Scholar]
- 32. Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK‐3beta, and beta‐catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8(2):103‐120. 10.2174/156720211795495402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xi XH, Wang Y, Li J, et al. Activation of Wnt/beta‐catenin/GSK3beta signaling during the development of diabetic cardiomyopathy. Cardiovasc Pathol. 2015;24(3):179‐186. 10.1016/j.carpath.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 34. Shan K, Liu C, Liu B‐H, et al. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation. 2017;136(17):1629‐1642. 10.1161/CIRCULATIONAHA.117.029004 [DOI] [PubMed] [Google Scholar]
- 35. Zhou JB, Yang JK, Zhang BH, Lu J. Interaction of Wnt pathway related variants with type 2 diabetes in a Chinese Han population. PeerJ. 2015;3:e1304. 10.7717/peerj.1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee SH, Demeterco C, Geron I, Abrahamsson A, Levine F, Itkin‐Ansari P. Islet specific Wnt activation in human type II diabetes. Exp Diabetes Res. 2008;2008:728763. 10.1155/2008/728763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu XJ, Gong Z, Li SJ, Jia HP, Li DL. Long non‐coding RNA Hottip modulates high‐glucose‐induced inflammation and ECM accumulation through miR‐455‐3p/WNT2B in mouse mesangial cells. Int J Clin Exp Pathol. 2019;12(7):2435‐2445. [PMC free article] [PubMed] [Google Scholar]
- 38. Chang J, Yu Y, Fang Z, et al. Long non‐coding RNA CDKN2B‐AS1 regulates high glucose‐induced human mesangial cell injury via regulating the miR‐15b‐5p/WNT2B axis. Diabetol Metab Syndr. 2020;12(1):109. 10.1186/s13098-020-00618-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang HS, Nie X, Wu RB, et al. Downregulation of human Wnt3 in gastric cancer suppresses cell proliferation and induces apoptosis. Onco Targets Ther. 2016;9:3849‐3860. 10.2147/OTT.S101782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nie X, Xia F, Liu Y, et al. Downregulation of Wnt3 suppresses colorectal cancer development through inhibiting cell proliferation and migration. Front Pharmacol. 2019;10:1110. 10.3389/fphar.2019.01110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wakabayashi T, Hidaka R, Fujimaki S, Asashima M, Kuwabara T. Diabetes impairs Wnt3 protein‐induced neurogenesis in Olfactory bulbs via glutamate transporter 1 inhibition. J Biol Chem. 2016;291(29):15196‐15211. 10.1074/jbc.M115.672857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim DY, Jung SY, Kim K, Kim CJ. Treadmill exercise ameliorates Alzheimer disease‐associated memory loss through the Wnt signaling pathway in the streptozotocin‐induced diabetic rats. J Exerc Rehabil. 2016;12(4):276‐283. 10.12965/jer.1632678.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang BW, Fang WJ, Shyu KG. MicroRNA‐145 regulates disabled‐2 and Wnt3a expression in cardiomyocytes under hyperglycaemia. Eur J Clin Invest. 2018;48(1):e12867. 10.1111/eci.12867 [DOI] [PubMed] [Google Scholar]
- 44. Yang Q, Wang WW, Ma P, et al. Swimming training alleviated insulin resistance through Wnt3a/beta‐catenin signaling in type 2 diabetic rats. Iran J Basic Med Sci. 2017;20(11):1220‐1226. 10.22038/IJBMS.2017.9473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han W, He X, Zhang M, et al. Establishment of a porcine pancreatic stem cell line using T‐REx system‐inducible Wnt3a expression. Cell Prolif. 2015;48(3):301‐310. 10.1111/cpr.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nostro MC, Sarangi F, Ogawa S, et al. Stage‐specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138(5):861‐871. 10.1242/dev.055236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schinner S, Ülgen F, Papewalis C, et al. Regulation of insulin secretion, glucokinase gene transcription and beta cell proliferation by adipocyte‐derived Wnt signalling molecules. Diabetologia. 2008;51(1):147‐154. 10.1007/s00125-007-0848-0 [DOI] [PubMed] [Google Scholar]
- 48. Vethe H, Ghila L, Berle M, et al. The effect of Wnt pathway modulators on human iPSC‐derived pancreatic beta cell maturation. Front Endocrinol (Lausanne). 2019;10:293. 10.3389/fendo.2019.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ma X‐Y, Feng Y‐F, Ma Z‐S, et al. The promotion of osteointegration under diabetic conditions using chitosan/hydroxyapatite composite coating on porous titanium surfaces. Biomaterials. 2014;35(26):7259‐7270. 10.1016/j.biomaterials.2014.05.028 [DOI] [PubMed] [Google Scholar]
- 50. Cheng R, Ding L, He X, Takahashi Y, Ma JX. Interaction of PPARalpha with the canonic Wnt pathway in the regulation of renal fibrosis. Diabetes. 2016;65(12):3730‐3743. 10.2337/db16-0426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gao X, Ma K, Lu N, Hong T, Xu Y, Peng X. Correlation of increased intravitreous wnt3a with vascular endothelial growth factor in proliferative diabetic retinopathy. Retina. 2016;36(4):812‐818. 10.1097/IAE.0000000000000784 [DOI] [PubMed] [Google Scholar]
- 52. Al‐Aly Z, Shao JS, Lai CF, et al. Aortic Msx2‐Wnt calcification cascade is regulated by TNF‐alpha‐dependent signals in diabetic Ldlr‐/‐ mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2589‐2596. 10.1161/ATVBAHA.107.153668 [DOI] [PubMed] [Google Scholar]
- 53. Adi N, Perriotte‐Olson C, Desouza CV, Ramalingam R, Saraswathi V. Hematopoietic cyclooxygenase‐2 deficiency increases adipose tissue inflammation and adiposity in obesity. Obesity (Silver Spring). 2015;23(10):2037‐2045. 10.1002/oby.21184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950‐953. 10.1126/science.289.5481.950 [DOI] [PubMed] [Google Scholar]
- 55. Krutzfeldt J, Stoffel M. Regulation of wingless‐type MMTV integration site family (WNT) signalling in pancreatic islets from wild‐type and obese mice. Diabetologia. 2010;53(1):123‐127. 10.1007/s00125-009-1578-2 [DOI] [PubMed] [Google Scholar]
- 56. Bowen A, Kos K, Whatmore J, Richardson S, Welters HJ. Wnt4 antagonises Wnt3a mediated increases in growth and glucose stimulated insulin secretion in the pancreatic beta‐cell line, INS‐1. Biochem Biophys Res Commun. 2016;479(4):793‐799. 10.1016/j.bbrc.2016.09.130 [DOI] [PubMed] [Google Scholar]
- 57. Heller C, Kuhn MC, Mulders‐Opgenoorth B, et al. Exendin‐4 upregulates the expression of Wnt‐4, a novel regulator of pancreatic beta‐cell proliferation. Am J Physiol Endocrinol Metab. 2011;301(5):E864‐E872. 10.1152/ajpendo.00144.2011 [DOI] [PubMed] [Google Scholar]
- 58. Kurita Y, Ohki T, Soejima E, et al. A high‐fat/high‐sucrose diet induces WNT4 expression in mouse pancreatic beta‐cells. Kurume Med J. 2019;65(2):55‐62. 10.2739/kurumemedj.MS652008 [DOI] [PubMed] [Google Scholar]
- 59. Kozinski K, Jazurek M, Dobrzyn P, et al. Adipose‐ and muscle‐derived Wnts trigger pancreatic beta‐cell adaptation to systemic insulin resistance. Sci Rep. 2016;6:31553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bader E, Migliorini A, Gegg M, et al. Identification of proliferative and mature beta‐cells in the islets of Langerhans. Nature. 2016;535(7612):430‐434. 10.1038/nature18624 [DOI] [PubMed] [Google Scholar]
- 61. Wang L, Qing L, Liu H, et al. Mesenchymal stromal cells ameliorate oxidative stress‐induced islet endothelium apoptosis and functional impairment via Wnt4‐beta‐catenin signaling. Stem Cell Res Ther. 2017;8(1):188. 10.1186/s13287-017-0640-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL. Sustained Wnt/beta‐catenin signaling rescues high glucose induction of transforming growth factor‐beta1‐mediated renal fibrosis. Am J Med Sci. 2012;344(5):374‐382. 10.1097/MAJ.0b013e31824369c5 [DOI] [PubMed] [Google Scholar]
- 63. Lin C‐L, Cheng HO, Tung C‐W, et al. Simvastatin reverses high glucose‐induced apoptosis of mesangial cells via modulation of Wnt signaling pathway. Am J Nephrol. 2008;28(2):290‐297. 10.1159/000111142 [DOI] [PubMed] [Google Scholar]
- 64. Huang L, Lin T, Shi M, Chen X, Wu P. Liraglutide suppresses production of extracellular matrix proteins and ameliorates renal injury of diabetic nephropathy by enhancing Wnt/beta‐catenin signaling. Am J Physiol Renal Physiol. 2020;319(3):F458‐F468. 10.1152/ajprenal.00128.2020 [DOI] [PubMed] [Google Scholar]
- 65. Wang W, Zhang J, Wang X, Wang H, Ren Q, Li Y. Effects of melatonin on diabetic nephropathy rats via Wnt/beta‐catenin signaling pathway and TGF‐beta‐Smad signaling pathway. Int J Clin Exp Pathol. 2018;11(5):2488‐2496. [PMC free article] [PubMed] [Google Scholar]
- 66. Xiang X, Cai HD, Su SL, et al. Salvia miltiorrhiza protects against diabetic nephropathy through metabolome regulation and wnt/beta‐catenin and TGF‐beta signaling inhibition. Pharmacol Res. 2019;139:26‐40. 10.1016/j.phrs.2018.10.030 [DOI] [PubMed] [Google Scholar]
- 67. Ren X, Zhu R, Liu G, et al. Effect of sitagliptin on tubulointerstitial Wnt/beta‐catenin signalling in diabetic nephropathy. Nephrology. 2019;24(11):1189‐1197. 10.1111/nep.13641 [DOI] [PubMed] [Google Scholar]
- 68. Liu CL, Yan L, Cai KR, et al. Effects of soybean isoflavones on Wnt/beta‐catenin and the TGF‐beta1 signaling pathway in renal tissue of type 2 diabetic rats. J Biol Regul Homeost Agents. 2018;32(3):455‐464. [PubMed] [Google Scholar]
- 69. Yang J, Fan Z, Yang J, Ding J, Yang C, Chen L. MicroRNA‐24 attenuates neointimal hyperplasia in the diabetic rat carotid artery injury model by inhibiting Wnt4 signaling pathway. Int J Mol Sci. 2016;17(6):765‐ 10.3390/ijms17060765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xu W, Geng H, Liu X, et al. Wingless‐type MMTV integration site family member 5a: a novel biomarker regulated in type 2 diabetes mellitus and diabetic kidney disease. J Diabetes Metab Disord. 2019;18(2):525‐532. 10.1007/s40200-019-00461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lu YC, Wang CP, Hsu CC, et al. Circulating secreted frizzled‐related protein 5 (Sfrp5) and wingless‐type MMTV integration site family member 5a (Wnt5a) levels in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2013;29(7):551‐556. 10.1002/dmrr.2426 [DOI] [PubMed] [Google Scholar]
- 72. Relling I, Akcay G, Fangmann D, et al. Role of wnt5a in metabolic inflammation in humans. J Clin Endocrinol Metab. 2018;103(11):4253‐4264. 10.1210/jc.2018-01007 [DOI] [PubMed] [Google Scholar]
- 73. Ya L, Jie Y, Mengxue Y, Pan W, Changwei Y, Jie X. Identification of nonylphenol and glucolipid metabolism‐related proteins in the serum of type 2 diabetes patients. Iran J Public Health. 2019;48(12):2210‐2215. [PMC free article] [PubMed] [Google Scholar]
- 74. Catalán V, Gómez‐Ambrosi J, Rodríguez A, et al. Activation of noncanonical Wnt signaling through WNT5A in visceral adipose tissue of obese subjects is related to inflammation. J Clin Endocrinol Metab. 2014;99(8):E1407‐E1417. 10.1210/jc.2014-1191 [DOI] [PubMed] [Google Scholar]
- 75. Zuriaga MA, Fuster JJ, Farb MG, et al. Activation of non‐canonical WNT signaling in human visceral adipose tissue contributes to local and systemic inflammation. Sci Rep. 2017;7(1):17326. 10.1038/s41598-017-17509-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pashirzad M, Shafiee M, Rahmani F, et al. Role of Wnt5a in the pathogenesis of inflammatory diseases. J Cell Physiol. 2017;232(7):1611‐1616. 10.1002/jcp.25687 [DOI] [PubMed] [Google Scholar]
- 77. Ouchi N, Higuchi A, Ohashi K, et al. Sfrp5 is an anti‐inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329(5990):454‐457. 10.1126/science.1188280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Farb MG, Karki S, Park S‐Y, et al. WNT5A‐JNK regulation of vascular insulin resistance in human obesity. Vasc Med. 2016;21(6):489‐496. 10.1177/1358863X16666693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim YH, Pyo S. Interleukin‐10 suppresses adipogenesis via Wnt5a signaling pathway in 3T3‐L1 preadipocytes. Biochem Biophys Res Commun. 2019;509(4):877‐885. 10.1016/j.bbrc.2019.01.033 [DOI] [PubMed] [Google Scholar]
- 80. Tian F, Zhang YJ, Li Y, Xie Y. Celecoxib ameliorates non‐alcoholic steatohepatitis in type 2 diabetic rats via suppression of the non‐canonical Wnt signaling pathway expression. PLoS One. 2014;9(1):e83819. 10.1371/journal.pone.0083819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wu X, Li Z, Chen K, et al. Egr‐1 transactivates WNT5A gene expression to inhibit glucose‐induced beta‐cell proliferation. Biochim Biophys Acta Gene Regul Mech. 2018;1861(9):803–814. 10.1016/j.bbagrm.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 82. Wu X, Liang W, Guan H, et al. Exendin‐4 promotes pancreatic beta‐cell proliferation via inhibiting the expression of Wnt5a. Endocrine. 2017;55(2):398‐409. 10.1007/s12020-016-1160-x [DOI] [PubMed] [Google Scholar]
- 83. Xu W, Liang J, Geng HF, et al. Wingless‐type MMTV integration site family member 5a is a key secreted islet stellate cell‐derived product that regulates islet function. Int J Endocrinol. 2019;2019:7870109. 10.1155/2019/7870109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kuljanin M, Bell GI, Sherman SE, Lajoie GA, Hess DA. Proteomic characterisation reveals active Wnt‐signalling by human multipotent stromal cells as a key regulator of beta cell survival and proliferation. Diabetologia. 2017;60(10):1987‐1998. 10.1007/s00125-017-4355-7 [DOI] [PubMed] [Google Scholar]
- 85. Wang F, Fisher SA, Zhong J, Wu Y, Yang P. Superoxide dismutase 1 in vivo ameliorates maternal diabetes mellitus‐induced apoptosis and heart defects through restoration of impaired Wnt signaling. Circ Cardiovasc Genet. 2015;8(5):665‐676. 10.1161/CIRCGENETICS.115.001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tong S, Du Y, Ji Q, et al. Expression of Sfrp5/Wnt5a in human epicardial adipose tissue and their relationship with coronary artery disease. Life Sci. 2020;245:117338. 10.1016/j.lfs.2020.117338 [DOI] [PubMed] [Google Scholar]
- 87. Akoumianakis I, Sanna F, Margaritis M, et al. Adipose tissue–derived WNT5A regulates vascular redox signaling in obesity via USP17/RAC1‐mediated activation of NADPH oxidases. Sci Transl Med. 2019;11(510):eaav5055. 10.1126/scitranslmed.aav5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ackers I, Szymanski C, Silver MJ, Malgor R. Oxidized low‐density lipoprotein induces WNT5A signaling activation in THP‐1 derived macrophages and a human aortic vascular smooth muscle cell line. Front Cardiovasc Med. 2020;7:567837. 10.3389/fcvm.2020.567837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bretón‐Romero R, Feng B, Holbrook M, et al. Endothelial dysfunction in human diabetes is mediated by Wnt5a‐JNK signaling. Arterioscler Thromb Vasc Biol. 2016;36(3):561‐569. 10.1161/ATVBAHA.115.306578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kikuchi R, Nakamura K, MacLauchlan S, et al. An antiangiogenic isoform of VEGF‐A contributes to impaired vascularization in peripheral artery disease. Nat Med. 2014;20(12):1464‐1471. 10.1038/nm.3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nakamura K, Sano S, Fuster JJ, et al. Secreted frizzled‐related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J Biol Chem. 2016;291(6):2566‐2575. 10.1074/jbc.M115.693937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Murdoch CE, Shuler M, Haeussler DJF, et al. Glutaredoxin‐1 up‐regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post‐ischemia limb revascularization. J Biol Chem. 2014;289(12):8633‐8644. 10.1074/jbc.M113.517219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Báez IR, Castro DOR, Gutiérrez SS, et al. Association of serum levels of secreted frizzled‐related protein 5 and Wnt member 5a with glomerular filtration rate in patients with type 2 diabetes mellitus and chronic renal disease: a cross‐sectional study. Sao Paulo Med J. 2020;138(2):133‐139. 10.1590/1516-3180.2019.0304.r2.09122019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ho C, Hsu YC, Lei CC, Mau SC, Shih YH, Lin CL. Curcumin rescues diabetic renal fibrosis by targeting superoxide‐mediated Wnt signaling pathways. Am J Med Sci. 2016;351(3):286‐295. 10.1016/j.amjms.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 95. Lin CL, Wang JY, Ko JY, et al. Superoxide destabilization of beta‐catenin augments apoptosis of high‐glucose‐stressed mesangial cells. Endocrinology. 2008;149(6):2934‐2942. 10.1210/en.2007-1372 [DOI] [PubMed] [Google Scholar]
- 96. Hsu YC, Lee PH, Lei CC, Ho C, Shih YH, Lin CL. Nitric oxide donors rescue diabetic nephropathy through oxidative‐stress‐and nitrosative‐stress‐mediated Wnt signaling pathways. J Diabetes Investig. 2015;6(1):24‐34. 10.1111/jdi.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ando F, Sohara E, Morimoto T, et al. Wnt5a induces renal AQP2 expression by activating calcineurin signalling pathway. Nat Commun. 2016;7:13636. 10.1038/ncomms13636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Salpea KD, Gable DR, Cooper JA, et al. The effect of WNT5B IVS3C>G on the susceptibility to type 2 diabetes in UK Caucasian subjects. Nutr Metab Cardiovasc Dis. 2009;19(2):140‐145. 10.1016/j.numecd.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 99. Kanazawa A, Tsukada S, Kamiyama M, Yanagimoto T, Nakajima M, Maeda S. Wnt5b partially inhibits canonical Wnt/beta‐catenin signaling pathway and promotes adipogenesis in 3T3‐L1 preadipocytes. Biochem Biophys Res Commun. 2005;330(2):505‐510. 10.1016/j.bbrc.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 100. van Tienen FH, Laeremans H, van der Kallen CJ, Smeets HJ. Wnt5b stimulates adipogenesis by activating PPARgamma, and inhibiting the beta‐catenin dependent Wnt signaling pathway together with Wnt5a. Biochem Biophys Res Commun. 2009;387(1):207‐211. 10.1016/j.bbrc.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 101. Cawthorn WP, Bree AJ, Yao Y, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta‐catenin‐dependent mechanism. Bone. 2012;50(2):477‐489. 10.1016/j.bone.2011.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shi Y, Long F. Hedgehog signaling via Gli2 prevents obesity induced by high‐fat diet in adult mice. Elife. 2017;6:e31649. 10.7554/eLife.31649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Beaton H, Andrews D, Parsons M, et al. Wnt6 regulates epithelial cell differentiation and is dysregulated in renal fibrosis. Am J Physiol Renal Physiol. 2016;311(1):F35‐F45. 10.1152/ajprenal.00136.2016 [DOI] [PubMed] [Google Scholar]
- 104. Zhang H, Liang S, Du Y, et al. Inducible ATF3‐NFAT axis aggravates podocyte injury. J Mol Med (Berl). 2018;96(1):53‐64. 10.1007/s00109-017-1601-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. He QJ, Wang PU, Liu QQ, et al. Secreted Wnt6 mediates diabetes‐associated centrosome amplification via its receptor FZD4. Am J Physiol Cell Physiol. 2020;318(1):C48‐C62. 10.1152/ajpcell.00091.2019 [DOI] [PubMed] [Google Scholar]
- 106. Afelik S, Pool B, Schmerr M, Penton C, Jensen J. Wnt7b is required for epithelial progenitor growth and operates during epithelial‐to‐mesenchymal signaling in pancreatic development. Dev Biol. 2015;399(2):204‐217. 10.1016/j.ydbio.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 107. Kimura A, Toyoda T, Iwasaki M, Hirama R, Osafune K. Combined omics approaches reveal the roles of non‐canonical WNT7B signaling and YY1 in the proliferation of human pancreatic progenitor cells. Cell Chem Biol. 2020;27(12):1561‐1572.e7. 10.1016/j.chembiol.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 108. Wang W, Yan X, Lin Y, Ge H, Tan Q. Wnt7a promotes wound healing by regulation of angiogenesis and inflammation: Issues on diabetes and obesity. J Dermatol Sci. 2018;91(2):124‐133. 10.1016/j.jdermsci.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 109. Thomas J, Garg ML, Smith DW. Dietary resveratrol supplementation normalizes gene expression in the hippocampus of streptozotocin‐induced diabetic C57Bl/6 mice. J Nutr Biochem. 2014;25(3):313‐318. 10.1016/j.jnutbio.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 110. Cheng SL, Shao JS, Behrmann A, Krchma K, Towler DA. Dkk1 and MSX2‐Wnt7b signaling reciprocally regulate the endothelial‐mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1679‐1689. 10.1161/ATVBAHA.113.300647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shao JS, Cheng SL, Pingsterhaus JM, Charlton‐Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115(5):1210‐1220. 10.1172/JCI24140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kennell JA, MacDougald OA. Wnt signaling inhibits adipogenesis through beta‐catenin‐dependent and ‐independent mechanisms. J Biol Chem. 2005;280(25):24004‐24010. 10.1074/jbc.M501080200 [DOI] [PubMed] [Google Scholar]
- 113. Pujadas G, Cervantes S, Tutusaus A, et al. Wnt9a deficiency discloses a repressive role of Tcf7l2 on endocrine differentiation in the embryonic pancreas. Sci Rep. 2016;6(1):19223. 10.1038/srep19223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rundqvist HC, Montelius A, Osterlund T, Norman B, Esbjornsson M, Jansson E. Acute sprint exercise transcriptome in human skeletal muscle. PLoS One. 2019;14(10):e0223024. 10.1371/journal.pone.0223024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Xiong Y, Chen L, Yan C, et al. Circulating exosomal miR‐20b‐5p inhibition restores Wnt9b signaling and reverses diabetes‐associated impaired wound healing. Small. 2020;16(3):1904044. 10.1002/smll.201904044 [DOI] [PubMed] [Google Scholar]
- 116. Wang L, Xu S, Lee JE, et al. Histone H3K9 methyltransferase G9a represses PPARgamma expression and adipogenesis. EMBO J. 2013;32(1):45‐59. 10.1038/emboj.2012.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tseng Y‐H, Butte AJ, Kokkotou E, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol. 2005;7(6):601‐611. 10.1038/ncb1259 [DOI] [PubMed] [Google Scholar]
- 118. Guo B, Chatterjee S, Li L, et al. The clock gene, brain and muscle Arnt‐like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 2012;26(8):3453‐3463. 10.1096/fj.12-205781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhong JM, Lu YC, Zhang J. Dexmedetomidine reduces diabetic neuropathy pain in rats through the Wnt 10a/beta‐catenin signaling pathway. Biomed Res Int. 2018;2018:9043628. 10.1155/2018/9043628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Janssen LGM, van Dam AD, Hanssen MJW, et al. Higher plasma sclerostin and lower Wnt signaling gene expression in white adipose tissue of prediabetic south asian men compared with white caucasian men. Diabetes Metab J. 2020;44(2):326‐335. 10.4093/dmj.2019.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Park M, Yi J‐W, Kim E‐M, et al. Triggering receptor expressed on myeloid cells 2 (TREM2) promotes adipogenesis and diet‐induced obesity. Diabetes. 2015;64(1):117‐127. 10.2337/db13-1869 [DOI] [PubMed] [Google Scholar]
- 122. Longo KA, Wright WS, Kang S, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem. 2004;279(34):35503‐35509. 10.1074/jbc.M402937200 [DOI] [PubMed] [Google Scholar]
- 123. Wright WS, Longo KA, Dolinsky VW, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56(2):295‐303. 10.2337/db06-1339 [DOI] [PubMed] [Google Scholar]
- 124. Aslanidi G, Kroutov V, Philipsberg G, et al. Ectopic expression of Wnt10b decreases adiposity and improves glucose homeostasis in obese rats. Am J Physiol Endocrinol Metab. 2007;293(3):E726‐E736. 10.1152/ajpendo.00248.2007 [DOI] [PubMed] [Google Scholar]
- 125. Abiola M, Favier M, Christodoulou‐Vafeiadou E, Pichard AL, Martelly I, Guillet‐Deniau I. Activation of Wnt/beta‐catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP‐1c in skeletal muscle cells. PLoS One. 2009;4(12):e8509. 10.1371/journal.pone.0008509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ahn J, Lee H, Kim S, Ha T. Curcumin‐induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta‐catenin signaling. Am J Physiol Cell Physiol. 2010;298(6):C1510‐C1516. 10.1152/ajpcell.00369.2009 [DOI] [PubMed] [Google Scholar]
- 127. Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of bone and Wnt10b expression in male Type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015;156(9):3169‐3182. 10.1210/EN.2015-1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Heller RS, Dichmann DS, Jensen J, et al. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev Dyn. 2002;225(3):260‐270. 10.1002/dvdy.10157 [DOI] [PubMed] [Google Scholar]
- 129. Wada N, Hashinaga T, Otabe S, et al. Selective modulation of Wnt ligands and their receptors in adipose tissue by chronic hyperadiponectinemia. PLoS One. 2013;8(7):e67712. 10.1371/journal.pone.0067712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Keats EC, Dominguez JM 2nd, Grant MB, Khan ZA. Switch from canonical to noncanonical Wnt signaling mediates high glucose‐induced adipogenesis. Stem Cells. 2014;32(6):1649‐1660. 10.1002/stem.1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Fear MW, Kelsell DP, Spurr NK, Barnes MR. Wnt‐16a, a novel Wnt‐16 isoform, which shows differential expression in adult human tissues. Biochem Biophys Res Commun. 2000;278(3):814‐820. 10.1006/bbrc.2000.3852 [DOI] [PubMed] [Google Scholar]
- 132. Movérare‐Skrtic S, Henning P, Liu X, et al. Osteoblast‐derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med. 2014;20(11):1279‐1288. 10.1038/nm.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chen S, Liu D, He S, et al. Differential effects of type 1 diabetes mellitus and subsequent osteoblastic beta‐catenin activation on trabecular and cortical bone in a mouse model. Exp Mol Med. 2018;50(12):1‐14. 10.1038/s12276-018-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Shin SH, Kim WJ, Choi MJ, et al. Aberrant expression of Wnt family contributes to the pathogenesis of diabetes‐induced erectile dysfunction. Andrology. 2014;2(1):107‐116. 10.1111/j.2047-2927.2013.00162.x [DOI] [PubMed] [Google Scholar]
- 135. Barcelos LS, Duplaa C, Krankel N, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104(9):1095‐1102. 10.1161/CIRCRESAHA.108.192138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Shi M, Tian P, Liu Z, et al. MicroRNA‐27a targets Sfrp1 to induce renal fibrosis in diabetic nephropathy by activating Wnt/beta‐Catenin signalling. Biosci Rep. 2020;40(6):BSR20192794. 10.1042/BSR20192794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Crowley RK, O’Reilly MW, Bujalska IJ, et al. SFRP2 is associated with increased adiposity and VEGF expression. PLoS One. 2016;11(9):e0163777. 10.1371/journal.pone.0163777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pachori AS, Madan M, Nunez Lopez YO, Yi F, Meyer C, Seyhan AA. Reduced skeletal muscle secreted frizzled‐related protein 3 is associated with inflammation and insulin resistance. Obesity (Silver Spring). 2017;25(4):697‐703. 10.1002/oby.21787 [DOI] [PubMed] [Google Scholar]
- 139. Mahdi T, Hänzelmann S, Salehi A, et al. Secreted frizzled‐related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes. Cell Metab. 2012;16(5):625‐633. 10.1016/j.cmet.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 140. Anand K, Vidyasagar S, Lasrado I, et al. Secreted Frizzled‐Related Protein 4 (SFRP4): a novel biomarker of beta‐cell dysfunction and insulin resistance in individuals with prediabetes and type 2 diabetes. Diabetes Care. 2016;39(9):e147‐e148. 10.2337/dc16-0756 [DOI] [PubMed] [Google Scholar]
- 141. Hörbelt T, Knebel B, Fahlbusch P, et al. The adipokine sFRP4 induces insulin resistance and lipogenesis in the liver. Biochim Biophys Acta Mol Basis Dis. 2019;1865(10):2671‐2684. 10.1016/j.bbadis.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 142. Wang D, Zhang Y, Shen C. Research update on the association between SFRP5, an anti‐inflammatory adipokine, with obesity, type 2 diabetes mellitus and coronary heart disease. J Cell Mol Med. 2020;24(5):2730‐2735. 10.1111/jcmm.15023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ress C, Paulweber M, Goebel G, et al. Circulating Wnt inhibitory factor 1 levels are associated with development of cardiovascular disease. Atherosclerosis. 2018;273:1‐7. 10.1016/j.atherosclerosis.2018.03.045 [DOI] [PubMed] [Google Scholar]
- 144. Song W, Sun Y, Liang XC, et al. Jinmaitong ameliorates diabetes‐induced peripheral neuropathy in rats through Wnt/beta‐catenin signaling pathway. J Ethnopharmacol. 2021;266:113461. 10.1016/j.jep.2020.113461 [DOI] [PubMed] [Google Scholar]
- 145. Mani A, Radhakrishnan J, Wang H, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315(5816):1278‐1282. 10.1126/science.1136370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Singh R, De Aguiar R, Naik S, et al. LRP6 enhances glucose metabolism by promoting TCF7L2‐dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013;17(2):197‐209. 10.1016/j.cmet.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Srivastava R, Zhang J, Go GW, Narayanan A, Nottoli TP, Mani A. Impaired LRP6‐TCF7L2 activity enhances smooth muscle cell plasticity and causes coronary artery disease. Cell Rep. 2015;13(4):746‐759. 10.1016/j.celrep.2015.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liu W, Singh R, Choi CS, et al. Low density lipoprotein (LDL) receptor‐related protein 6 (LRP6) regulates body fat and glucose homeostasis by modulating nutrient sensing pathways and mitochondrial energy expenditure. J Biol Chem. 2012;287(10):7213‐7223. 10.1074/jbc.M111.286724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gao X, Ma K, Lu N, Xu Y, Hong T, Peng X. Elevated LRP6 levels correlate with vascular endothelial growth factor in the vitreous of proliferative diabetic retinopathy. Mol Vis. 2015;21:665‐672. [PMC free article] [PubMed] [Google Scholar]
- 150. Peixoto EB, Papadimitriou A, Teixeira DA, et al. Reduced LRP6 expression and increase in the interaction of GSK3beta with p53 contribute to podocyte apoptosis in diabetes mellitus and are prevented by green tea. J Nutr Biochem. 2015;26(4):416‐430. 10.1016/j.jnutbio.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 151. Cheng SL, Ramachandran B, Behrmann A, et al. Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR‐/‐ mice by restraining noncanonical Wnt signals. Circ Res. 2015;117(2):142‐156. 10.1161/CIRCRESAHA.117.306712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Aamir K, Khan HU, Sethi G, Hossain MA, Arya A. Wnt signaling mediates TLR pathway and promote unrestrained adipogenesis and metaflammation: therapeutic targets for obesity and type 2 diabetes. Pharmacol Res. 2020;152:104602. 10.1016/j.phrs.2019.104602 [DOI] [PubMed] [Google Scholar]
- 153. Brinkmann E‐M, Mattes B, Kumar R, et al. Secreted Frizzled‐related Protein 2 (sFRP2) redirects non‐canonical Wnt signaling from Fz7 to Ror2 during vertebrate gastrulation. J Biol Chem. 2016;291(26):13730‐13742. 10.1074/jbc.M116.733766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cha S‐W, Tadjuidje E, White J, et al. Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol. 2009;19(18):1573‐1580. 10.1016/j.cub.2009.07.062 [DOI] [PubMed] [Google Scholar]
- 155. Quoc Lam B, Shrivastava SK, Shrivastava A, Shankar S, Srivastava RK. The Impact of obesity and diabetes mellitus on pancreatic cancer: molecular mechanisms and clinical perspectives. J Cell Mol Med. 2020;24(14):7706‐7716. 10.1111/jcmm.15413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Ling S, Brown K, Miksza JK, et al. Association of type 2 diabetes with cancer: a meta‐analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care. 2020;43(9):2313‐2322. 10.2337/dc20-0204 [DOI] [PubMed] [Google Scholar]
- 157. Takada R, Satomi Y, Kurata T, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11(6):791‐801. 10.1016/j.devcel.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 158. Resham K, Sharma SS. Pharmacologic inhibition of porcupine, disheveled, and beta‐catenin in wnt signaling pathway ameliorates diabetic peripheral neuropathy in rats. J Pain. 2019;20(11):1338‐1352. 10.1016/j.jpain.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 159. Proffitt KD, Madan B, Ke Z, et al. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT‐driven mammary cancer. Cancer Res. 2013;73(2):502‐507. 10.1158/0008-5472.CAN-12-2258 [DOI] [PubMed] [Google Scholar]
- 160. Le PN, McDermott JD, Jimeno A. Targeting the Wnt pathway in human cancers: therapeutic targeting with a focus on OMP‐54F28. Pharmacol Ther. 2015;146:1‐11. 10.1016/j.pharmthera.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


