Abstract
Parkinson’s disease (PD) is the most prevalent neurodegenerative motor disorder. While PD has been attributed to dopaminergic neuronal death in substantia nigra pars compacta (SNpc), accumulating lines of evidence have suggested that reactive astrogliosis is critically involved in PD pathology. These pathological changes are associated with α-synuclein aggregation, which is more prone to be induced by an A53T mutation. Therefore, the overexpression of A53T-mutated α-synuclein (A53T-α-syn) has been utilized as a popular animal model of PD. However, this animal model only shows marginal-to-moderate extents of reactive astrogliosis and astrocytic α-synuclein accumulation, while these phenomena are prominent in human PD brains. Here we show that Adeno-GFAP-GFP virus injection into SNpc causes severe reactive astrogliosis and exacerbates the A53T-α-syn-mediated PD pathology. In particular, we demonstrate that AAV-CMV-A53T-α-syn injection, when combined with Adeno-GFAP-GFP, causes more significant loss of dopaminergic neuronal tyrosine hydroxylase level and gain of astrocytic GFAP and GABA levels. Moreover, the combination of AAV-CMV-A53T-α-syn and Adeno-GFAP-GFP causes an extensive astrocytic α-syn expression, just as in human PD brains. These results are in marked contrast to previous reports that AAV-CMV-A53T-α-syn alone causes α-syn expression mostly in neurons but rarely in astrocytes. Furthermore, the combination causes a severe PD-like motor dysfunction as assessed by rotarod and cylinder tests within three weeks from the virus injection, whereas Adeno-GFAP-GFP alone or AAV-CMV-A53T-α-syn alone does not. Our findings implicate that inducing reactive astrogliosis exacerbates PD-like pathologies and propose the virus combination as an advanced strategy for developing a new animal model of PD.
Keywords: Adenovirus infections, Parkinson’s disease, Alpha-synuclein, Reactive astrogliosis, Mouse model
INTRODUCTION
Parkinson’s disease (PD), the most prevalent neurodegenerative movement disorder, is known for its characteristic symptoms, including resting tremor, bradykinesia, rigidity, and postural instability. These motor symptoms, called parkinsonism, have been mainly attributed to the deficiency of nigrostriatal dopamine caused by the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) [1]. However, the exact cause of neurodegeneration is largely unknown. Therefore, current treatments for PD are mostly focused on symptomatic relief through augmentation of dopaminergic signaling by supplying the dopamine precursor, levodopa. However, no disease-modifying therapy has been developed yet [2].
Establishing appropriate animal models that resemble human pathology is one of the most important prerequisites of preclinical research for understanding the pathology of PD and screening for potential therapeutic candidates. However, currently available PD mouse or rat models either do not show the progressive α-synuclein (α-syn) aggregation characteristics of PD (i.e., toxin-induced models) [3] or need a long time (over nine months) to be established (i.e., transgenic mice) [4, 5]. One of the most frequently utilized transgenic mouse models of PD is the A53T-mutated α-syn (A53T-α-syn) overexpression model, based on previous reports demonstrating that A53T mutation in the SNCA gene increases the pathological aggregation to cause PD [5]. However, the drawbacks of the current animal models necessitate an advanced mouse model which reflects the human PD pathology in a relatively short time.
Recently, accumulating lines of evidence have suggested the critical role of astrocytes in the pathology of PD [6-12]. Reactive astrocytes, which are observed in the SNpc of both PD patients and animal models, aberrantly synthesize and release GABA, leading to dopaminergic neuronal dysfunction [11, 12]. Moreover, reactive astrocytes have been reported to directly cause neuronal death via H2O2 [13] and lipocalin-2 [14]. Blocking reactive astrogliosis by pharmacologic inhibition of monoamine oxidase-B, which is the key enzyme for astrocytic GABA and H2O2 synthesis and astrogliosis [11, 15-17], significantly reverses or prevents the PD pathology and parkinsonian motor symptoms [11, 18]. These previous reports together suggest an idea that inducing reactive astrogliosis can exacerbate PD pathology and motor dysfunction.
We have previously demonstrated that intracerebral injection of adenovirus causes severe neuroinflammation, including reactive astrogliosis [13, 19, 20]. Particularly, intra-hippocampal injection of Adeno-GFAP-GFP virus exacerbates the Alzheimer’s disease (AD) pathology in the APP/PS1 transgenic mouse model of AD through inflaming the reactive astrogliosis and neurodegeneration [13]. The exacerbated AD pathology led to more severe memory deficits in the animals. Therefore, here we tested the hypothesis that adenovirus injection into the SNpc could further stimulate the PD pathology and worsen the parkinsonian motor deficits in the viral A53T-α-syn overexpression model of PD. We further propose this mouse PD model induced by the combination of adenovirus infection and A53T-α-syn overexpression as an advanced PD model, which shows an accelerated pathology of PD with severe reactive astrogliosis.
MATERIALS AND METHODS
Animals
Animal care and handling were performed according to the directives of the Animal Care and Use Committee and institutional guidelines of KIST (Seoul, Korea). Experiments were performed on 46 adult male C57B/6J mice including AAV-ctrl group (N=10), Adeno-only group (N=11), A53T-only group (N=12), and A53T+Adeno group (N=13). All animals were housed three to four per cage in a controlled animal facility with ad libitum access to food and water. The animal care unit was maintained on a 12 h light-dark cycle (07:00~19:00) with controlled temperature (21±1℃) and humidity (50%). (1) AAV-ctrl group mice were injected with AAV-CMV-EGFP virus into SNpc as a control group. (2) Adeno-only group mice were injected with Adeno-GFAP-GFP into SNpc to induce neuroinflammation, including reactive astrogliosis. (3) A53T-only group mice were injected with AAV-CMV-A53T-α-syn virus into SNpc as an existing PD mouse model. (4) A53T+Adeno group mice were injected with the combination of Adeno-GFAP-GFP and AAV-CMV-A53T-α-syn viruses into SNpc as an advanced PD mouse model.
Virus preparation and injection
We used the AAV-CMV-A53T-α-syn virus, which overexpresses A53T-α-syn to induce PD. To induce PD with reactive astrocytosis, we used the Adeno-GFAP-GFP virus. As a GFAP promoter, we used the gfaABC1D promoter, which is reported to highly specific to astrocytes [21]. AAV-CMV-EGFP virus was used as a control. All viruses were injected to SNpc (AP=-3.2, ML=+1.25, DV=-4.85, right hemisphere). (1) 1.5 µl of AAV-CMV-EGFP virus (5.5×1013 GC/ml; diluted 1:1 in saline), (2) 1.5 µl of Adeno-GFAP-GFP virus (diluted 1:1 in saline), (3) 1.5 µl AAV-CMV-A53T-a-syn (4.5×1013 GC/ml; diluted 1:1 in saline), or (4) the virus cocktail of 0.75 µl of Adeno-GFAP-GFP and 0.75 µl of AAV-CMV-A53T-α-syn (not diluted) was injected for the AAV-ctrl group, Adeno-only group, A53T-only group, or A53T+Adeno group. All viruses were manufactured by the KIST Virus Facility (http://virus.kist.re.kr/).
Behavior test
Two types of behavioral tests were used in this study: the forelimb-use asymmetry test (cylinder test) was used to assess motor deficit and asymmetry behavior, rotarod test was used to assess motor deficit. To perform the cylinder test, animals were placed in a transparent Plexiglas cylinder (20 cm in diameter and 30 cm in height) for two minutes to assess the frequency of usage of ipsilesional and contralesional forelimb to support an upright body posture against the wall of the cylinder. We counted the number of ipsilateral and contralateral forepaw usages for each 2-min session. The test score was calculated as the percentage of ipsilateral usages to the total number of ipsilateral and contralateral usages. Rotarod test was performed as previously described [11]. Each mouse was habituated for 2 days, and the actual test was performed at a fixed speed of 15 rpm on day 21 after virus injection. The cut-off time of the latency-to-fall was 3 min.
Immunohistochemistry
For DAB staining, the 30-μm-thick coronal sections for striatum and SNpc were immunostained with a DAB staining kit (TL-060-QHD, Thermo, MA, USA). The sections were incubated in Hydrogen Peroxide Block (TA-060-HP, Thermo, MA, USA) for 10 min, washed in phosphate-buffered saline (PBS) three times, incubated for 5 min in Ultravision Block (TA-060-UB, Thermo, MA, USA), and washed in PBS three times again. Then the samples were immunostained with a primary antibody (Rabbit anti-TH, p40101-0, Pel-freez, AR, USA; 1:500) in a blocking solution [0.3% Triton-X, 2% ready-to-use donkey serum (GTX30972, Genetex, CA, USA) in 0.1 M PBS] at 4°C on a shaker overnight. After washing in PBS 3 times, sections were incubated in Primary Antibody Amplifier Quanto (TA-060-QPB, Thermo, MA, USA) for 5 min and washed in PBS again. The sections were incubated in HRP Polymer Quanto for 1 h and washed four times in PBS. DAB-positive chromogen and DAB-positive substrate buffer (K3468, Dako, Denmark) were mixed at 1:10 ratio, and the sections were dipped in the mixture for 30 seconds and then washed. Finally, sections were mounted with a mounting solution (S0323, Dako, Denmark) and dried. A series of bright field images were obtained with an Olympus microscope.
For slice immunostaining, sections were first incubated for 1 h in a blocking solution (0.3% Triton-X, 2% normal serum in 0.1 M PBS) and then immunostained with a mixture of primary antibodies (Rabbit anti-TH, p40101-0, Pel-freez, AR, USA, 1:500; Chicken anti-GFAP, Millipore, MA, USA, AB5541, 1:500; Guinea pig anti-GABA, AB175, Millipore, MA, USA, 1:300; Mouse anti-a-synuclein, AB1903, Abcam, MA, USA, 1:200) in a blocking solution at 4℃. After extensive washing, sections were incubated with corresponding fluorescent secondary antibodies for 2 h and then washed with PBS 3 times. If needed, DAPI (Thermo Fisher Scientific, MA, USA; 1:3,000) staining was performed. Finally, sections were mounted with a fluorescent mounting medium (S3023, Dako, Denmark) and dried. A series of fluorescent images were obtained with an A1 Nikon confocal microscope, and Z-stack images in 2-μm steps were processed for further analysis using NIS-Elements (Nikon, Japan) software and ImageJ program (NIH, MD, USA). Any alterations in brightness or contrast were equally applied to the entire image set. Specificity of each primary antibody and immunoreaction was confirmed by omitting the primary antibody or changing fluorescent probes of the secondary antibodies. The secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (PA, USA).
Image quantification
Confocal microscopic images were analyzed using the ImageJ program (NIH, MD, USA) or Imaris 9 (Bitplane, UK). For measurement of GFAP-positive volume and GABA immunoreactivity in astrocytes, we made the surface for each GFAP-positive cell with GFAP-positive images using Imaris 9, and then we collected the volume value of each ROI or the integrated density value of GABA intensity in each ROI, respectively. For measurement of α-syn immunoreactivity in TH-positive neurons or astrocytes, we first threshold the binary TH-positive or GFAP-positive image to define single dopaminergic neuron or astrocyte as a region-of-interest (ROI) using ImageJ. Then we measured the intensity of α-syn in every ROI from 8-bit α-syn-positive images.
Statistical analysis
Statistical analyses were performed using Prism 9 (GraphPad Software, CA, USA). For comparison of multiple groups, One-way ANOVA with Tukey’s multiple comparison test was used. Data from multiple independent experiments were checked for normality before performing a parametric test. When the data are not normally distributed, we performed Kruskal-Wallis test with Duun’s multiple comparison test instead of One-way ANOVA with Tukey’s. A p-value less than 0.05 was considered to be statistically significant throughout the study. The significance level was represented as asterisks (*p<0.05, **p<0.01, ***p<0.001; ns, not significant). All data were represented as mean±SEM. No statistical method was used to predetermine sample size. Sample sizes were determined empirically based on similar experiments in the previous studies. Prior to administration of virus injection, animals were randomly allocated to each experimental group. The investigators were not blinded to the outcome assessments.
RESULTS
Adenovirus injection exacerbates A53T-mediated parkinsonian motor deficits
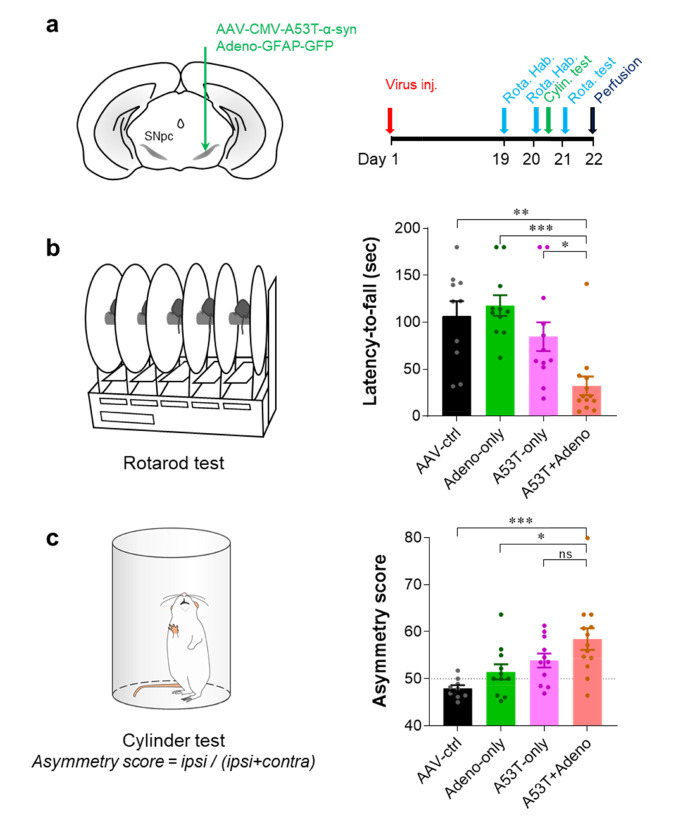
To test if adenovirus injection exacerbates the PD pathology and motor symptoms in the viral A53T PD model mice (A53T-only model), we unilaterally injected Adeno-GFAP-GFP and/or AAV-CMV-A53T-α-syn into the right SNpc (Fig. 1a) and performed two behavioral assays: rotarod test and cylinder test (Fig. 1a). To marginally induce the PD pathology of the A53T-only model, we used AAV-CMV-A53T-α-syn virus at 0.5-fold lower titer than what was used in the previous report [11] and performed the behavioral assays only 21 days (3 weeks) after virus injection (Fig. 1a). As expected, the A53T-only model showed only a marginal but not significant decrease in latency-to-fall in the rotarod test, compared to the control or Adeno-only group (Fig. 1b). In contrast, when the Adeno-GFAP-GFP was injected together with the AAV-CMV-A53T-α-syn (A53T+Adeno group), the latency-to-fall was significantly reduced, compared to all other groups (Fig. 1b). Similarly, we found a significant asymmetric usage of the forelimb in the A53T+Adeno group, compared to AAV-ctrl and Adeno-only groups. These results together indicate that the Adeno-GFAP-GFP injection accelerates the manifestation of A53T-α-syn-mediated parkinsonian motor deficits within only three weeks.
Fig. 1.
Adenovirus injection exacerbates A53T-mediated parkinsonian motor deficits. (a) Schematic diagram of virus injection (left) and experimental schedule (right). (b) Quantification of latency-to-fall assessed by rotarod test. (c) Quantification of asymmetry score of forelimb usage assessed by cylinder test. For all figures, mean±SEM; ns, non-significance; *p<0.05, **p<0.01, ***p<0.001 assessed by Kruskal-Wallis test with Dunn's multiple comparison test (b) or One-way ANOVA with Tukey’s multiple comparison test (c).
Adenovirus injection exacerbates A53T-mediated TH loss in dopaminergic neurons
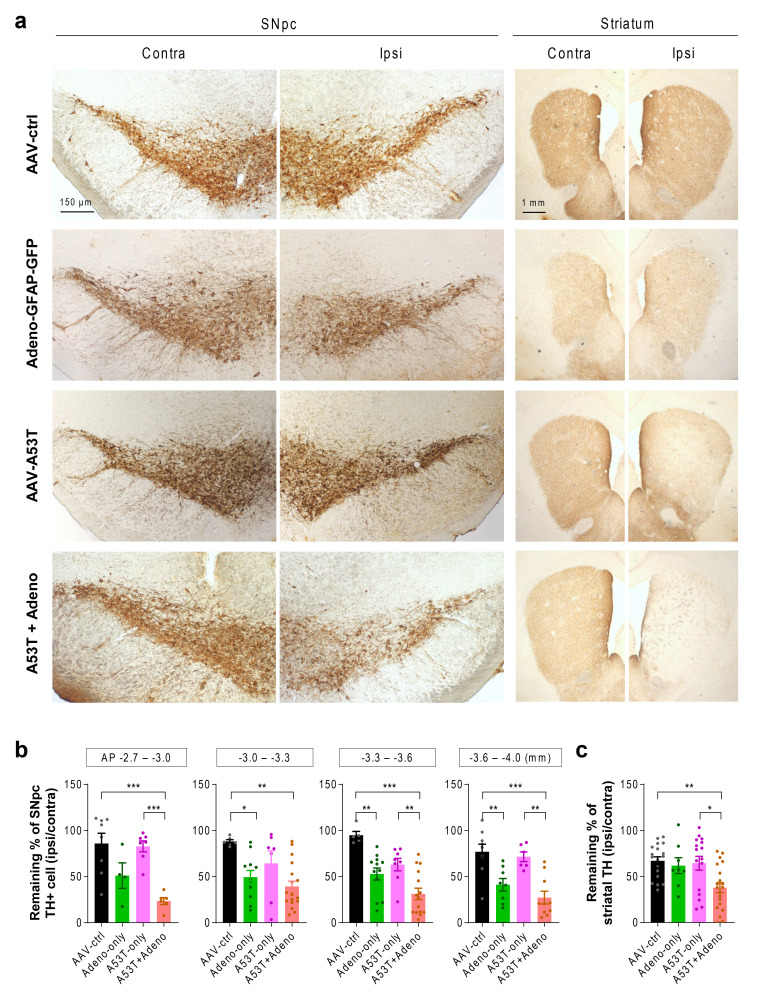
Next, we investigated whether the exacerbated motor deficits in the A53T+Adeno model are associated with the loss of nigrostriatal tyrosine hydroxylase (TH), the key dopamine-synthetic enzyme, by performing immunohistochemistry. Consistent with the findings from behavioral assays, we could not find a significant loss of TH-positive neurons in the A53T-only model. There was a partial but significant loss of TH-positive neurons in the SNpc of the Adeno-only group. However, the striatal TH level was not significantly reduced in the Adeno-only group. These results suggest that the TH loss in the SNpc of the Adeno-only group is less likely attributed to extensive neuronal death but more likely attributed to astrogliosis-mediated TH reduction in the SNpc, as we previously demonstrated [11]. The intact motor function of the Adeno-only group could be ascribed to the spared TH expression in the striatum. On the other hand, we found that the A53T+Adeno model mice showed a dramatic and significant loss of TH in the ipsilateral SNpc (Fig. 2a, b; 23% to 39% TH-positive neurons remaining). We also found that striatal TH level was significantly reduced in the A53T+Adeno model (38% remaining), whereas other groups did not show any significant reduction (Fig. 2a, c). These findings indicate that Adeno-GFAP-GFP injection exacerbates A53T-α-syn-mediated TH loss in dopaminergic neurons, which is associated with parkinsonian motor dysfunction.
Fig. 2.
Adenovirus injection exacerbates A53T-mediated TH loss. (a) Representative images of TH-stained SNpc tissues (left) and striatal tissues (right). (b) Quantification of TH-positive (TH+) neurons in the SNpc (at the AP coordinates of -2.7~3.0, -3.0~-3.3, -3.3~-3.6, -3.6~-4.0 mm). (c) Quantification of TH optical density in the striatum. For all figures, mean±SEM; ns, non-significance; *p<0.05, **p<0.01, ***p<0.001 assessed by Kruskal-Wallis test with Dunn’s multiple comparison test (b; AP -3.0~-3.3) or One-way ANOVA with Tukey’s multiple comparison test.
Adenovirus injection exacerbates reactive astrogliosis
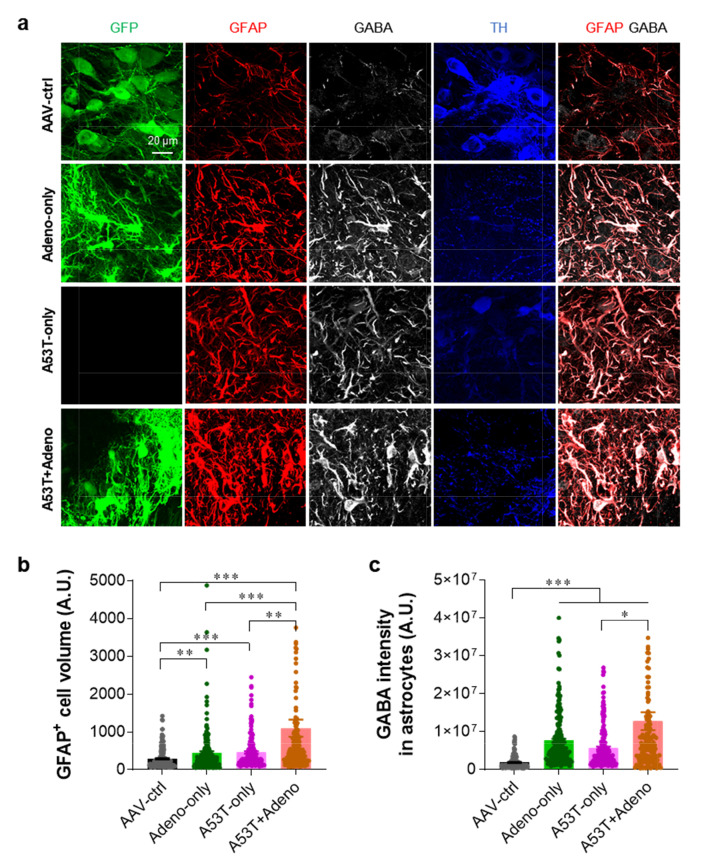
We previously reported that adenovirus injection causes severe reactive astrogliosis [19, 20, 22]. We also previously demonstrated that reactive astrocytes excessively express both GFAP and GABA in the SNpc of PD animal models, while normal astrocytes do not [11]. GFAP and GABA have also been well documented as prominent markers for reactive astrocytes in various brain regions [12, 15, 16, 20, 23-25]. Therefore, we investigated if the severe PD-like symptoms of the A53T+Adeno group are associated with reactive astrogliosis by performing immunofluorescence staining with antibodies against TH, GFAP, and GABA. We found that the A53T+Adeno group showed the highest astrocytic volume (Fig. 3a, b) and astrocytic GABA level (Fig. 3a, c), compared to all the other groups. These findings indicate that adenovirus injection exacerbates A53T-mediated reactive astrogliosis.
Fig. 3.
Adenovirus injection exacerbates reactive astrogliosis. (a) Representative confocal images of SNpc tissues stained with TH, GFAP, and GABA. (b) Quantification of the volume of GFAP-positive (GFAP+) astrocytes in the SNpc. (c) Quantification of the integrated density of GABA in the GFAP-positive astrocytes. For all figures, mean±SEM; ns, non-significance, ***p<0.001 assessed by Kruskal-Wallis test with Dunn's multiple comparison test.
Adenovirus injection exacerbates astrocytic expression of α-syn
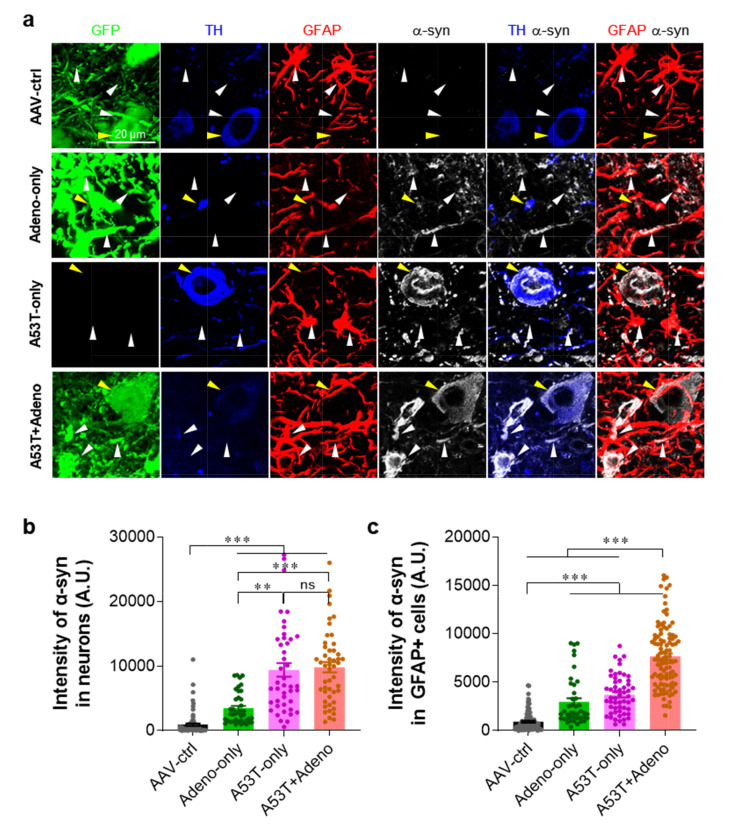
Based on a previous report demonstrating that the astrocytic expression of A53T-α-syn causes neurodegeneration in mice [26], we investigated whether adenovirus-mediated reactive astrogliosis is associated with the astrocytic α-syn expression by performing immunostaining with antibodies against GFAP and α-syn. We found that the A53T-only group showed a significantly increased α-syn expression in both TH-positive neurons and astrocytes in the SNpc, compared to the AAV-ctrl group (Fig. 4). More importantly, the A53T+Adeno group showed a further increase in the astrocytic α-syn expression compared to the A53T-only group, whereas the neuronal α-syn expression was not increased (Fig. 4). These findings indicate that Adeno-GFAP-GFP injection exacerbates A53T-mediated astrocytic expression of α-syn. Taken together, our findings suggest that adenovirus-induced reactive astrogliosis and the aberrant expression of astrocytic α-syn aggravate and accelerate the A53T-α-syn-mediated nigrostriatal dopaminergic neuronal loss and parkinsonian motor deficits.
Fig. 4.
Adenovirus injection exacerbates the astrocytic expression of α-syn. (a) Representative confocal images of SNpc tissues stained with TH, GFAP, and α-syn. Yellow arrowheads indicate the neuronal α-syn expression, while white arrowheads indicate the astrocytic α-syn expression. (b) Quantification of α-syn immunoreactivity in neurons. (c) Quantification of α-syn immunoreactivity in the GFAP-positive (GFAP+) astrocytes. For all figures, mean±SEM; ns, non-significance; *p<0.05, **p<0.01, ***p<0.001 assessed by Kruskal-Wallis test with Dunn's multiple comparison test.
DISCUSSION
In this study, we have demonstrated that adenovirus-mediated reactive astrogliosis exacerbated the A53T-α-syn-mediated pathology of PD. Particularly, the proposed A53T+Adeno model showed dramatic reactive astrogliosis, astrocytic α-syn expression, nigrostriatal TH loss, and PD-like motor deficits, which are more severe than those observed in the existing A53T-only model. Noticeably, all these pathological processes and severe motor symptoms were manifested only within 3 weeks after virus injection.
The highlight of our study is that adenovirus-mediated reactive astrogliosis can accelerate the PD pathology and stimulate astrocytic α-syn inclusion. As previously reported, the enhanced level of astrocytic α-syn could further stimulate astrocytic reactivity and neurodegeneration [26]. Moreover, as we have previously demonstrated, Adeno-GFAP-GFP exacerbates the expression level of astrocytic GABA [20]. The astrocytic GABA has been reported to tonically inhibit neighboring dopaminergic neurons in SNpc, leading to TH loss, dopamine deficiency, and parkinsonian motor deficits [11]. The excessive GABA from reactive astrocytes has been implicated in various brain diseases accompanying neuroinflammation, including Alzheimer’s disease, stroke, epilepsy, and inflammatory cytokine-induced anxiety [12, 15, 16, 20, 23-25]. In all these disease conditions, the excessive astrocytic GABA tonically inhibits the neighboring neurons leading to the relevant functional deficits. Therefore, it is possible that the aberrant astrocytic α-syn inclusion can boost the astrocytic GABA synthesis, leading to tonic inhibition of neighboring dopaminergic neurons in SNpc. This exciting possibility awaits future investigation.
We propose our newly developed A53T+Adeno model as a simple and advanced mouse model of PD, which shows severe and accelerated pathology and motor deficits within three weeks. Currently, available PD animal models can be divided into two categories [27]: toxin-induced and genetic models. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA), both of which specifically damage dopaminergic neurons, are the most widely used toxins for PD animal models [27]. The toxin-induced models have a strong advantage in that these models are relatively easy to construct. However, these models do not reflect the human PD pathology of progressive α-syn aggregation. On the other hand, genetic models include transgenic animal models [4, 5, 28-30], α-syn preformed fibril model [31], and viral vector-mediated gene modification-induced models [32]. Among several genes of interest, including SNCA [5], LRRK2 [28], PINK1 [29], and DJ-1 [30], SNCA encoding α-syn protein has been most frequently targeted to construct the animal model [33]. Given the fact that the A53T mutation of SNCA facilitates the aggregation of α-syn, A53T-α-syn transgenic (A53T-TG) mice have been widely used [5]. However, this model has serious drawbacks that no parkinsonian motor symptom is observed in A53T-TG mice until 9-16 months [5]. On the other hand, a mouse model of viral A53T-α-syn overexpression (viral A53T model) shows parkinsonian motor symptoms within 3 to 5 weeks depending on the virus titer and infection efficiency [11, 32]. However, the α-syn inclusion is barely seen in the SNpc astrocytes of this model in contrast to human PD patients [34]. These drawbacks of current PD mouse models have necessitated an advanced mouse model with the accelerated pathology and astrocytic α-syn inclusion. Our newly developed A53T+Adeno model overcomes these drawbacks of current PD mouse models by shortening the time for model construction within 3 weeks and distinct expression of astrocytic α-syn inclusion which is known to exacerbate the PD pathology. Moreover, the A53T+Adeno model shows more severe and less variable nigrostriatal TH loss, reactive astrogliosis, and motor symptoms, compared to the viral A53T-only model.
In summary, we have established an advanced model of PD by combining viral A53T overexpression and adenovirus-induced reactive astrogliosis. For better application of the A53T+Adeno model in the research for the development disease-modifying drug of PD, this model should be further tested if the pathological features and motor symptoms last after three weeks from the viral injection. The novel concepts and tools that we have developed should be broadly utilized for investigating the etiology of PD as well as for developing novel therapeutic strategies to fight against PD.
ACKNOWLEDGEMENTS
This work was supported by Korea Institute of Science and Technology Grants (2G11310) to M.H.N.; and Institute for Basic Science (IBS), Center for Cognition and Sociality (IBS-R001-D2) to C.J.L.
References
- 1.Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 2.Lang AE, Espay AJ. Disease modification in Parkinson's disease: current approaches, challenges, and future considerations. Mov Disord. 2018;33:660–677. doi: 10.1002/mds.27360. [DOI] [PubMed] [Google Scholar]
- 3.Jagmag SA, Tripathi N, Shukla SD, Maiti S, Khurana S. Evaluation of models of Parkinson's disease. Front Neurosci. 2016;9:503. doi: 10.3389/fnins.2015.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken HH, Becker D, Voos W, Leuner K, Müller WE, Kudin AP, Kunz WS, Zimmermann A, Roeper J, Wenzel D, Jendrach M, García-Arencíbia M, Fernández-Ruiz J, Huber L, Rohrer H, Barrera M, Reichert AS, Rüb U, Chen A, Nussbaum RL, Auburger G. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 7.Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 8.L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Cossetti C, D'Adamo P, Zardini E, Andreoni L, Ihekwaba AE, Serra PA, Franciotta D, Martino G, Pluchino S, Marchetti B. Reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Neurobiol Dis. 2011;41:508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 10.Joe EH, Choi DJ, An J, Eun JH, Jou I, Park S. Astrocytes, microglia, and Parkinson's disease. Exp Neurobiol. 2018;27:77–87. doi: 10.5607/en.2018.27.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo JY, Nam MH, Yoon HH, Kim J, Hwang YJ, Won W, Woo DH, Lee JA, Park HJ, Jo S, Lee MJ, Kim S, Shim JE, Jang DP, Kim KI, Huh SH, Jeong JY, Kowall NW, Lee J, Im H, Park JH, Jang BK, Park KD, Lee HJ, Shin H, Cho IJ, Hwang EM, Kim Y, Kim HY, Oh SJ, Lee SE, Paek SH, Yoon JH, Jin BK, Kweon GR, Shim I, Hwang O, Ryu H, Jeon SR, Lee CJ. Aberrant tonic inhibition of dopaminergic neuronal activity causes motor symptoms in animal models of Parkinson's disease. Curr Biol. 2020;30:276–291.e9. doi: 10.1016/j.cub.2019.11.079. [DOI] [PubMed] [Google Scholar]
- 12.An H, Heo JY, Lee CJ, Nam MH. The pathological role of astrocytic MAOB in parkinsonism revealed by genetic ablation and over-expression of MAOB. Exp Neurobiol. 2021;30:113–119. doi: 10.5607/en21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun H, Im H, Kang YJ, Kim Y, Shin JH, Won W, Lim J, Ju Y, Park YM, Kim S, Lee SE, Lee J, Woo J, Hwang Y, Cho H, Jo S, Park JH, Kim D, Kim DY, Seo JS, Gwag BJ, Kim YS, Park KD, Kaang BK, Cho H, Ryu H, Lee CJ. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer's disease via H2O2- production. Nat Neurosci. 2020;23:1555–1566. doi: 10.1038/s41593-020-00735-y. [DOI] [PubMed] [Google Scholar]
- 14.Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia XG, Zhou H. Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci U S A. 2013;110:4069–4074. doi: 10.1073/pnas.1218497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JH, Ju YH, Choi JW, Song HJ, Jang BK, Woo J, Chun H, Kim HJ, Shin SJ, Yarishkin O, Jo S, Park M, Yeon SK, Kim S, Kim J, Nam MH, Londhe AM, Kim J, Cho SJ, Cho S, Lee C, Hwang SY, Kim SW, Oh SJ, Cho J, Pae AN, Lee CJ, Park KD. Newly developed reversible MAO-B inhibitor circumvents the shortcomings of irreversible inhibitors in Alzheimer's disease. Sci Adv. 2019;5:eaav0316. doi: 10.1126/sciadv.aav0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, Park HJ, Lee DY, Hong J, Kim HY, Oh SJ, Park SJ, Lee H, Yoon BE, Kim Y, Jeong Y, Shim I, Bae YC, Cho J, Kowall NW, Ryu H, Hwang E, Kim D, Lee CJ. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon BE, Woo J, Chun YE, Chun H, Jo S, Bae JY, An H, Min JO, Oh SJ, Han KS, Kim HY, Kim T, Kim YS, Bae YC, Lee CJ. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol. 2014;592:4951–4968. doi: 10.1113/jphysiol.2014.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nam MH, Park JH, Song HJ, Choi JW, Kim S, Jang BK, Yoon HH, Heo JY, Cho DW, Yang YS, Han SC, Kim S, Oh SJ, Jeon SR, Park KD, Lee CJ. KDS2010, a newly developed reversible MAO-B inhibitor, as an effective therapeutic candidate for Parkinson's disease. bioRxiv. 2020 doi: 10.1101/2020.07.06.190579. doi: 10.1101/2020.07.06.190579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo J, Im SK, Chun H, Jung SY, Oh SJ, Choi N, Lee CJ, Hur EM. Functional characterization of resting and adenovirus-induced reactive astrocytes in three-dimensional culture. Exp Neurobiol. 2017;26:158–167. doi: 10.5607/en.2017.26.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam MH, Cho J, Kwon DH, Park JY, Woo J, Lee JM, Lee S, Ko HY, Won W, Kim RG, Song H, Oh SJ, Choi JW, Park KD, Park EK, Jung H, Kim HS, Lee MC, Yun M, Lee CJ, Kim HI. Excessive astrocytic GABA causes cortical hypometabolism and impedes functional recovery after subcortical stroke. Cell Rep. 2020;32:107861. doi: 10.1016/j.celrep.2020.107861. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Messing A, Su M, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- 22.Nam MH, Ko HY, Lee S, Park YM, Hyeon SJ, Won W, Kim SY, Jo HH, Chung JI, Han YE, Lee GH, Ju Y, Stein TD, Kong M, Lee H, Lee SE, Oh SJ, Chun JH, Park KD, Ryu H, Yun M, Lee CJ. Visualization of reactive astrocytes in living brain of Alzheimer's disease patient. bioRxiv. 2021 doi: 10.1101/2021.04.13.439744. doi: 10.1101/2021.04.13.439744. [DOI] [Google Scholar]
- 23.Pandit S, Neupane C, Woo J, Sharma R, Nam MH, Lee GS, Yi MH, Shin N, Kim DW, Cho H, Jeon BH, Kim HW, Lee CJ, Park JB. Bestrophin1-mediated tonic GABA release from reactive astrocytes prevents the development of seizure-prone network in kainate-injected hippocampi. Glia. 2020;68:1065–1080. doi: 10.1002/glia.23762. [DOI] [PubMed] [Google Scholar]
- 24.Chun H, An H, Lim J, Woo J, Lee J, Ryu H, Lee CJ. Astrocytic proBDNF and tonic GABA distinguish active versus reactive astrocytes in hippocampus. Exp Neurobiol. 2018;27:155–170. doi: 10.5607/en.2018.27.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim HS, Park HJ, Woo J, Lee CJ, Shim I. Role of astrocytic GABAergic system on inflammatory cytokine-induced anxiety-like behavior. Neuropharmacology. 2019;160:107776. doi: 10.1016/j.neuropharm.2019.107776. [DOI] [PubMed] [Google Scholar]
- 26.Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson's disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kin K, Yasuhara T, Kameda M, Date I. Animal models for Parkinson's disease research: trends in the 2000s. Int J Mol Sci. 2019;20:5402. doi: 10.3390/ijms20215402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y, Dawson TM, Dawson VL. Models of LRRK2-associated Parkinson's disease. Adv Neurobiol. 2017;14:163–191. doi: 10.1007/978-3-319-49969-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandran JS, Lin X, Zapata A, Höke A, Shimoji M, Moore SO, Galloway MP, Laird FM, Wong PC, Price DL, Bailey KR, Crawley JN, Shippenberg T, Cai H. Progressive behavioral deficits in DJ-1-deficient mice are associated with normal nigrostriatal function. Neurobiol Dis. 2008;29:505–514. doi: 10.1016/j.nbd.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung HK, Ho HA, Pérez-Acuña D, Lee SJ. Modeling α-synuclein propagation with preformed fibril injections. J Mov Disord. 2019;12:139–151. doi: 10.14802/jmd.19046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ip CW, Klaus LC, Karikari AA, Visanji NP, Brotchie JM, Lang AE, Volkmann J, Koprich JB. AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease. Acta Neuropathol Commun. 2017;5:11. doi: 10.1186/s40478-017-0416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng H, Yuan L. Genetic variants and animal models in SNCA and Parkinson disease. Ageing Res Rev. 2014;15:161–176. doi: 10.1016/j.arr.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Sorrentino ZA, Giasson BI, Chakrabarty P. α-Synuclein and astrocytes: tracing the pathways from homeostasis to neurodegeneration in Lewy body disease. Acta Neuropathol. 2019;138:1–21. doi: 10.1007/s00401-019-01977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]