Abstract
Thymoma is a rare neoplasm of the anterior mediastinum, which originates from the epithelium of the thymic gland; it occurs mainly in middle-aged adults and is much less common in children. The tumor has slow growth and is asymptomatic in most pediatric cases, thus resulting in an accidental discovery; one-third of the young patient presents symptoms related to the compression of the tumor mass on the surrounding anatomic structures and/or related to paraneoplastic syndromes. Surgery is the treatment of choice and complete resection of the thymoma achieves excellent long-term results in terms of disease-free survival. In this article, we report the clinical case of a 21-month-old girl who came to our observation for persistent cough for over a month investigated with a chest X-ray, performed in another hospital. The X-ray showed an extensive opacification of the left hemithorax with contralateral dislocation of the mediastinum. The instrumental investigations carried out in our hospital (ultrasound, computed tomography, and magnetic resonance of the chest) showed a voluminous expansive mass of the left antero-superior mediastinum, which occupied the entire ipsilateral hemithorax and not dissociable from the thymus. At the histologic examination, the mass resulted to be a B1 thymoma with a low degree of malignancy according to the histologic classification of thymic tumors of the World Health Organization.
Keyword: Pediatrics, Thymoma, Mediastinal mass, True thymic hyperplasia, Ultrasound, CT, MRI
Introduction
In pediatric age, thoracic tumors are mainly localized in the mediastinum, and lymphomas and leukemias are the most commonly found neoplasms in the anterior compartment, followed by germ cell tumors [1], [2], [3]. Thymoma is a rare epithelial neoplasm of the anterior mediastinum that mainly affects adults over 40 years of age and much less frequently children, constituting, respectively, approximately 20%-30% and 1%-4% of mediastinal masses, with no significant differences in frequency between the 2 sexes [[1], [2],[4], [5], [6]]. Thymoma is a slow-growing tumor that tends to invade surrounding anatomic structures but rarely develops distant metastases [5], [6], [7]. Most children are asymptomatic, and the diagnosis is most often after the occasional finding of a mediastinal enlargement evidenced by a routine chest X-ray taken for other clinical reasons; one-third of pediatric patients presents symptoms due to the compression of the tumor mass on adjacent mediastinal structures, such as cough, dyspnea, chest pain, dysphagia or superior vena cava syndrome [2,[5], [6], [7]]. Paraneoplastic syndromes such as myasthenia gravis, pure red blood cell aplasia, acquired hypogammaglobulinemia, and connective tissue disorders rarely occur in children with thymoma [6], [7]. The sole surgery results to be curative in early-stage cancer, and it guarantees an excellent long-term prognosis [2,[5], [6], [7]]. In this article, we present the case of a 21-month-old girl with a voluminous mass occupying the left hemithorax, which at histologic examination resulted in a mediastinal thymoma type B1.
Case report
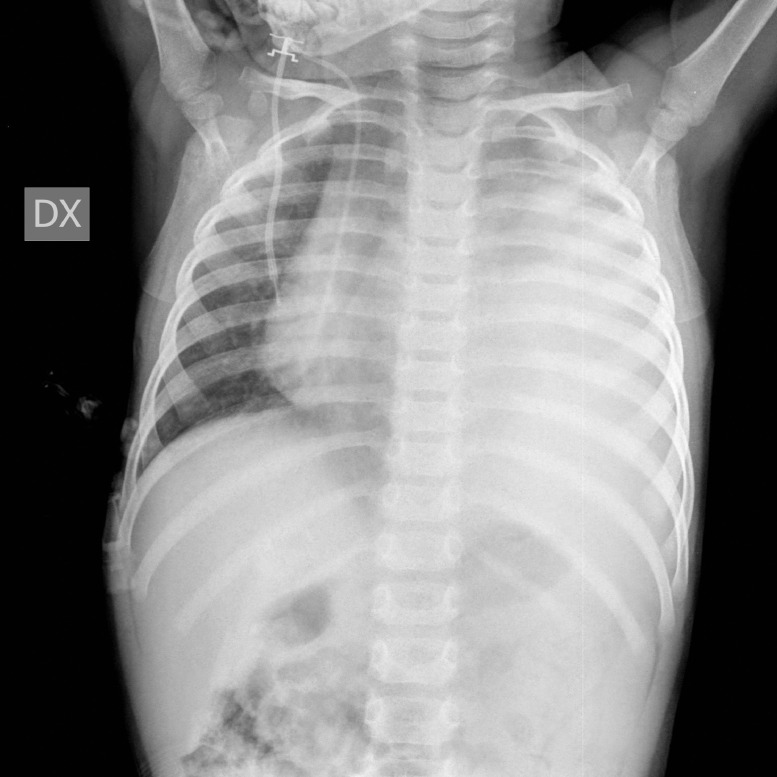
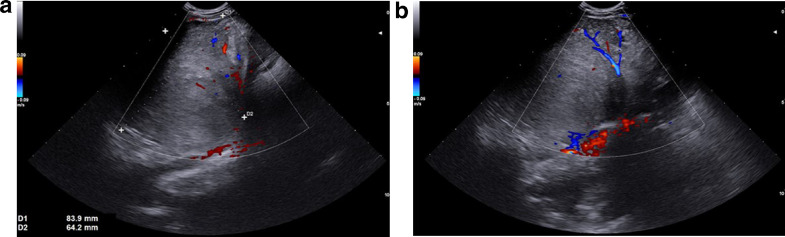
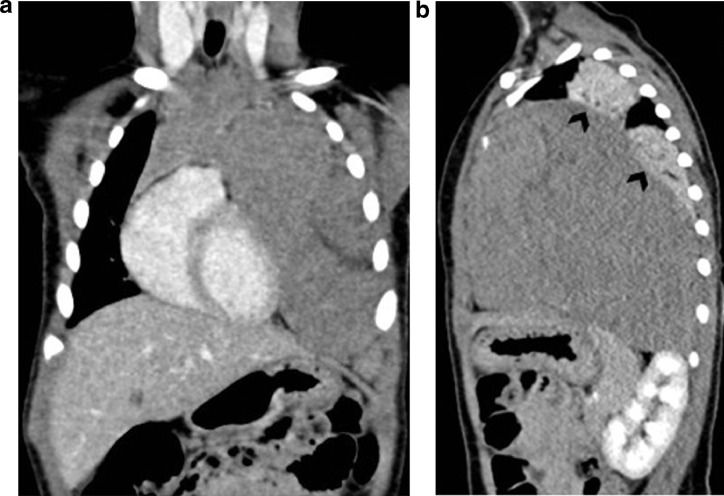
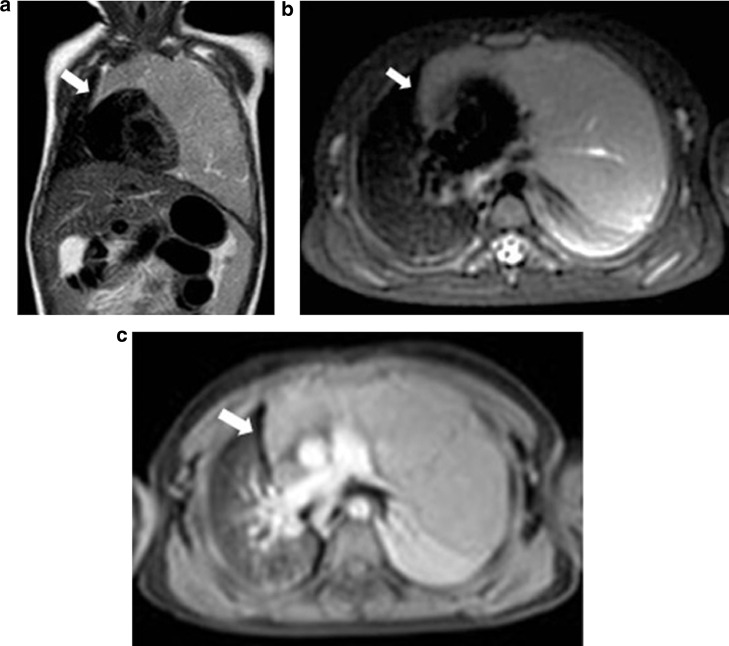
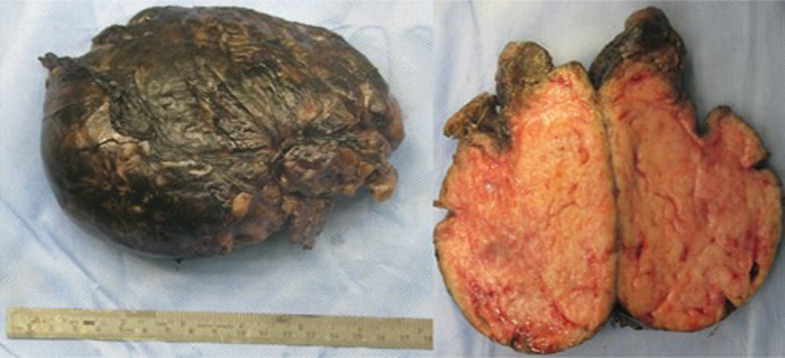
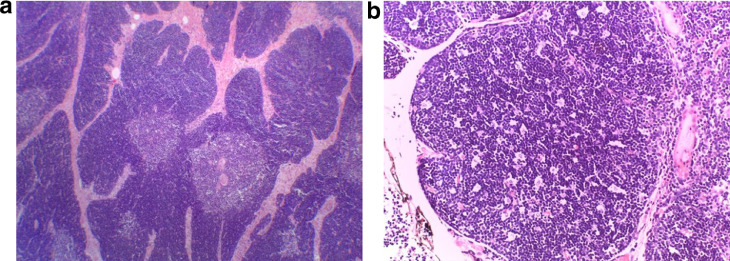
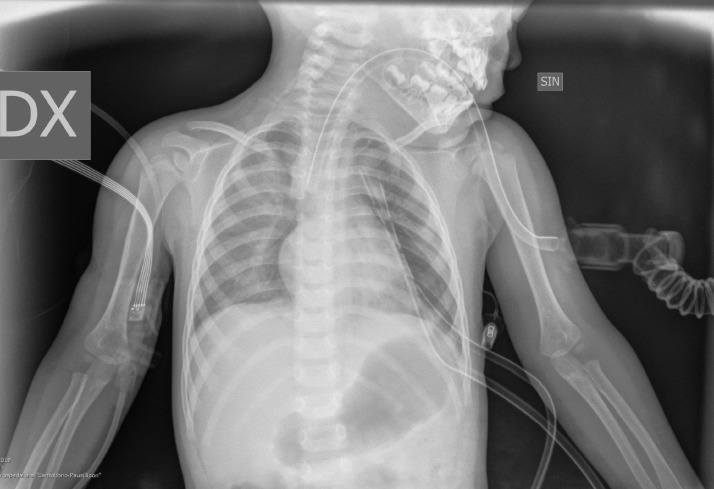
In September 2019, a 21-month-old girl came to our attention, sent by her pediatrician, for a cough persisting for over a month, which had not regressed after antibiotic, bronchodilator, and cortisone therapy; the chest X-ray, previously taken at another hospital, showed a massive clouding of the left hemithorax with contralateral displacement of the mediastinum (Fig. 1). The child was thus admitted to our hospital for further analysis and subjected to laboratory and instrumental investigations. The blood count with leukocyte formula, the peripheral blood smear, and blood chemistry tests, including the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and lactate dehydrogenase (LDH) values, were normal; urinalysis showed no alterations, and in particular vanylmandelic acid (VMA) and homovanillic acid (OMV) were not detectable; furthermore, the tumor markers alpha-fetoprotein (AFP) and human chorionic gonadotropin (Beta HCG) were negative. Thoraco-abdominal ultrasound showed a large localized neoformation in the anterior mediastinum, with mildly inhomogeneous echostructure and vascular signal at color Doppler; the mass did not displace the ipsilateral splanchnic organs and there were no abdominal lesions and no free fluid in the peritoneum (Fig. 2). Chest-abdomen-pelvis computed tomography (CT) and chest magnetic resonance (MR) were then performed, both with intravenous (i.v.) administration of contrast medium (cm). The CT showed a voluminous solid expansive formation - which entirely occupied the left hemithorax - with smooth margins and mild and almost homogeneous enhancement. The mass originated from the anterosuperior mediastinum, had no cleavage plane with the thymus, and, due to its posterior development, it compressed the lung parenchyma, which was almost completely atelectatic. The lesion did not infiltrate the surrounding structures but caused a compressive effect on the mediastinum, which was displaced to the right. No other pathologic findings in the thoracoabdominal area were appreciated (Fig. 3). MRI showed a neoformation with the same characteristics of the signal of the right thymic lobe, which appeared hypointense in T1-weighted images and hyperintense in T2-weighted sequences, with and without fat signal suppression. The lesion did not present restricted diffusion and showed regular contours and homogeneous contrast enhancement, in absence of signs of the infiltration of the contiguous structures (Fig. 4). In order to establish a correct therapeutic approach, an ultrasound-guided biopsy with tru-cut was performed, and it was repeated a second time after 15 days. In both cases, the histologic samples consisted of thymic parenchyma with preserved immuno-architecture. Subsequently, it was also performed a bone marrow aspirate which showed the absence of atypical cells. On the basis of clinical and radiological findings, the little patient underwent surgery by a median sternotomy with complete removal of the mass and the entire thymus. Macroscopically the neoformation, apparently capsulated, measured 16 cm in maximum diameter, showed a smooth external surface with solution of continuity of about 9 cm, and, when cut, it was vaguely lobulated and white-pink in color (Fig. 5). On microscopic analysis the mass was provided with a thin fibrous capsule and showed an organoid structure, divided into lobules, which resembled the normal thymus; the lobules, of variable size, were irregular and separated by large hypocellular fibrous septa and were mainly made up of cortical areas and, to a lesser extent, of lighter areas of medullary differentiation. In the cortical areas, there were few epithelial cells (panCK +) dispersed in an abundant proliferation of immature T lymphocytes (CD1a +, CD99 +, TdT +), and sometimes macrophages phagocitating apoptotic bodies which gave the characteristic "starry shy" appearance. The areas of medullary differentiation presented mature T and B lymphocytes and Hassal corpuscles. In addition, some perivascular spaces were observed (Fig. 6). The lesion did not infiltrate the capsule or the surgical resection margin and was compatible with low-grade thymoma B1, according to the histologic classification of thymic tumors of the World Health Organization (WHO), pathologic stage pT1, and stage I according to Masaoka-Koga. After chest radiology post-operative (Fig. 7) the child was discharged after a post-operative course without substantial complications, was not subjected to any adjuvant treatment, pneumomediastin disappeared 1 week after surgery, and is currently disease-free.
Fig. 1.
Massive opacity of almost the entire left hemithorax, with a clear and regular upper margin, with postero-superior convexity, associated with displacement of the mediastinum toward the right. The right lung is normally ventilated. Diaphragm in place. The cardiac image cannot be judged. Presence of venous catheter with approach to the right and extremity in the right atrium.
Fig. 2.
(A-B). Thoracic abdominal ultrasound. The left intercostal oblique scans detect a large solid mass, located in the left anterior mediastinum, which shows intralesional flow signals on color-Doppler (A,B).
Fig. 3.
(A-B). Coronal (A) and sagittal (B) post-contrast CT images show a huge homogeneous soft tissue mass in the left hemithorax, arising in the upper part of the anterior mediastinum, and it is indissociable from the thymus. Note atelectasis of the left lung parenchyma (black arrowheads in B).
Fig. 4.
(A-B-C). Coronal T2-weighted TSE (A), axial T2-weighted SPAIR (B) and axial post-contrast T1-weighted THRIVE (C) MR images demonstrate a voluminous left thoracic mass which is indistinguishable from the right thymic lobe (white arrows).
Fig. 5.
Macroscopic appearance of the mass.
Fig. 6.
(A-B). Microscopic histologic examination. (A) Organoid architecture of B1 thymoma containing a light-staining medullary island, dark-staining cortical area and hypocellular, collagenous septa. E/E 40x. (B) Starry sky appearance in cortex. E/E 200x.
Fig. 7.
Examination performed in the RIA room: Signs of bilateral pneumomediastinum mainly in the right basal paracardiac. Signs of basal retrocardiac parenchymal bundling left. Presence of pleural drainage tubes (2) with apexes at the 4th intercostal space left. Presence of TET with apex at the soma of D4 mediastinum in axis.
Discussion
Thymoma is a very rare anterior mediastinal neoplasm in children; just over 50 cases have been reported in the literature, almost always older than 5 years [7], [8]. Therefore, the finding of thymoma in a 21-month-old girl is truly exceptional. Usually, the tumor is asymptomatic until it becomes large enough to cause local compression symptoms, such as persistent cough in our little patient [2,[5], [6], [7], [8]]. The rarity of the neoplasm often makes the diagnosis difficult. The first choice instrumental investigation in children with respiratory symptoms is a chest X-ray; however, an opaque hemithorax is not always precisely attributed to a pathology of the lung parenchyma, pleura, or mediastinum, and ultrasound is an effective imaging method, since it does not expose to ionizing radiation and allows an immediate diagnostic orientation, being more sensitive than the traditional radiography in patients with mediastinal masses [9], [10], [11]. In addition, in children, the incomplete ossification of the rib cage allows an optimal ultrasound evaluation of the anterior chest and mediastinal portions since the cartilage component is strongly represented [10]. Consistently with what happened in our case, thoracic ultrasound identifies with high diagnostic accuracy the solid nature of the lesion and its origin in the anterior mediastinum, assesses the echogenicity, homogeneity, and vascularity of the expansive formation, verifies the absence or the presence of pleural effusion, and acts as a guide for biopsy sampling [9], [10]. As in adults, even in pediatric patients, the diagnostic process continues with the execution of the CT scan, which represents the examination of choice for the evaluation of the masses of the anterior mediastinum, able to offer high spatial and temporal resolutions with very short acquisition time [[1], [2], [3], [4], [5],12]. The CT defines site, morphology, size, and extension of the neoformation evaluates its densitometric characteristics and allows to detect the possible presence of local invasion and metastasis. The CT findings we observed were represented by a solid mass in the left hemithorax, originating from the anterosuperior mediastinum, indissociable from the thymus, with regular margins and homogeneous enhancement, with no evidence of infiltration of adjacent structures, pleural and/or pericardial dissemination, lymphadenopathy, pleural effusion, and pulmonary and/or extrathoracic metastases. These findings oriented the diagnosis toward low-grade thymoma, excluding high-grade thymoma and lymphoma [[4], [5],7,[12], [13]]. Chest MRI is almost always a complementary method to CT for the study of the masses of the anterior mediastinum in pediatric age. Anyway, although it provides images with high contrast resolution, without using ionizing radiation, the long-lasting examination, the presence of respiratory compromise and/or compression of the superior vena cava aggravated by anesthesia and supine position, and the limited spatial resolution for the lesions of the lung parenchyma limit its execution [[4], [5],12,[14], [15]]. Furthermore, in children and adolescents up to the age of 16, MRI may not help, using in-phase and out-of-phase sequences, for the differential diagnosis between true thymic hyperplasia and thymoma; in fact, the low presence of adipose tissue in the normal thymus does not make appreciable the drop in the signal of the microscopic fat in the out-of-phase sequences in cases of hyperplasia. However, even before performing the MRI, we had rejected the hypothesis of true thymic hyperplasia, in consideration of both the anamnesis of the little patient, negative for stressful events (for example infections, pharmacologic and/or radiation therapies, surgery, trauma, burns, etc.), and of the asymmetrical enlargement of the thymus [[4], [5], [6],[12], [13], [14], [15]]. The MRI we performed was, however, very accurate in the diagnosis and pre-surgical staging of the thymoma; it confirmed the thymic origin of the neoformation, and through the use of the signal suppression sequences of the adipose tissue without and with contrast medium i.v., it proved the presence of the cleavage planes between the mass and the contiguous mediastinal structures, excluded the infiltration of large vessels, of the heart, of the diaphragm, and showed the absence of pleural and/or pericardial infiltration [1,[4], [5],12,[14], [15]]. Furthermore, the lesion had no restricted diffusion and could be compatible with a low-risk thymoma [12]. In our case, the confirmation of the presence of a low-grade of malignancy thymoma, and specifically of type B1 according to the WHO classification, was made possible by the histopathologic examination on the surgical piece, which highlighted the characteristics of this tumor. Thymoma B1 (also called predominantly cortical, organoid, or rich in lymphocytes) was distinguishable from true thymic hyperplasia by the presence of lobules and irregular fibrous septa, some cortical areas with a “starry shy” appearance, and perivascular spaces. In addition, the preserved immune architecture of the cortical and medullary areas allowed the differential diagnosis between the thymoma B1, rich in T lymphocytes, and the lymphoblastic lymphoma with T precursors cells [[5], [6],8]. The neoplasm did not infiltrate the capsule or the resection margin, and survival at 5 years from complete surgical removal of the thymoma, identified at stage I according to Masaoka, reaches 100% [16].
Conclusion
Thymoma is a very rare childhood cancer and its diagnosis is difficult. However, the clinical, and radiological findings appropriately integrated with the histologic ones allow a correct diagnosis in many cases. Finally, a good prognosis is not only closely linked to the stage of the neoplasm but is also directly related to surgical radicality.
Patient consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki declaration of 1975, and its late amendments. Additional informed consented was obtained from all patients for which identifying information is not included in this article.
Footnotes
Competing Interests: All authors must disclose any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
References
- 1.Gun F, Erginel B, Unüvar A, Kebudi R, Salman T, Celik A. Mediastinal masses in children: experience with 120 cases. Pediatr Hematol Oncol. 2012;29(2):141–147. doi: 10.3109/08880018.2011.646385. [DOI] [PubMed] [Google Scholar]
- 2.Wright CD. Mediastinal tumors and cysts in the pediatric population. Thorac Surg Clin. 2009;19(1):47–61. doi: 10.1016/j.thorsurg.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 3.McCarville MB. Malignant pulmonary and mediastinal tumors in children: differential diagnoses. Cancer Imaging. 2010;10(1A):S35–S41. doi: 10.1102/1470-7330.2010.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter BW, Benveniste MF, Madan R, Godoy MC, de Groot PM, Truong MT. ITMIG classification of mediastinal compartments and multidisciplinary approach to mediastinal masses. Radiographics. 2017;37(2):413–436. doi: 10.1148/rg.2017160095. [DOI] [PubMed] [Google Scholar]
- 5.Benveniste MF, Rosado-de-Christenson ML, Sabloff BS, Moran CA, Swisher SG, Marom EM. Role of imaging in the diagnosis, staging, and treatment of thymoma. Radiographics. 2011;31(7):1847–1861. doi: 10.1148/rg.317115505. [DOI] [PubMed] [Google Scholar]
- 6.Liang X, Lovell MA, Capocelli KE, Albano EA, Birch S, Keating AK. Thymoma in children: report of 2 cases and review of the literature. Pediatr Dev Pathol. 2010;13(3):202–208. doi: 10.2350/09-07-0672-OA.1. [DOI] [PubMed] [Google Scholar]
- 7.Yalçin B, Demir HA, Ciftçi AO, Orhan D, Varan A, Akyüz C. Thymomas in childhood: 11 cases from a single Institution. J Pediatr Hematol Oncol. 2012;34(8):601–605. doi: 10.1097/MPH.0b013e31825808e9. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca AL, Ozgediz DE, Christison-Lagay ER, Detterbeck FC, Caty MG. Pediatric thymomas: report of two cases and comprehensive review of the literature. Pediatr Surg Int. 2014;30(3):275–286. doi: 10.1007/s00383-013-3438-x. [DOI] [PubMed] [Google Scholar]
- 9.Caremani M, Benci A, Tacconi D, Occhini U, Lapini L, Caremani A. Sonographic management of mediastinal syndrome. J Ultrasound. 2009;12(2):61–68. doi: 10.1016/j.jus.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rea G, Sperandeo M, Di Serafino M, Vallone G, Tomà P. Neonatal and pediatric thoracic ultrasonography. J Ultrasound. 2019;22(2):121–130. doi: 10.1007/s40477-019-00357-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merli L, Nanni L, Curatola A, Pellegrino M, De Santis M, Silvaroli S. Congenital lung malformations: a novel application for lung ultrasound? J Ultrasound. 2019 doi: 10.1007/s40477-019-00406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li HR, Gao J, Jin C, Jiang JH, Ding JY. Comparison between CT and MRI in the diagnostic accuracy of thymic masses. J Cancer. 2019;10(14):3208–3213. doi: 10.7150/jca.30240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackman JB, Verzosa S, Kovach AE, Louissaint A, Jr, Lanuti M, Wright CD. High rate of unnecessary thymectomy and its cause. Can computed tomography distinguish thymoma, lymphoma, thymic hyperplasia, and thymic cysts? Eur J Radiol. 2015;84(3):524–533. doi: 10.1016/j.ejrad.2014.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Manson DE. Magnetic resonance imaging of the mediastinum, chest wall and pleura in children. Pediatr Radiol. 2016;46(6):902–915. doi: 10.1007/s00247-016-3598-7. [DOI] [PubMed] [Google Scholar]
- 15.Marom EM. Imaging thymoma. J Thorac Oncol. 2010;5(10):S296–S303. doi: 10.1097/JTO.0b013e3181f209ca. Suppl 4. [DOI] [PubMed] [Google Scholar]
- 16.Nolasco-de la Rosa AL, Mosiñoz-Montes R, Nuñez-Trenado LA, Román- Guzmán E, Chávez-Villicaña CE, Naranjo-Hernández G. Thymoma in childhood. A case report and review of literature. Cir Cir. 2016;84(4):324–328. doi: 10.1016/j.circen.2016.06.010. [DOI] [PubMed] [Google Scholar]