Abstract
Purpose
To investigate the prevalence, incidence and characteristics of bacterial infections and their impact on outcome in critically ill patients infected with COVID-19.
Methods
We conducted a prospective observational study in eight Italian ICUs from February to May 2020; data were collected through an interactive electronic database. Kaplan–Meier analysis (limit product method) was used to identify the occurrence of infections and risk of acquisition.
Results
During the study period 248 patients were recruited in the eight participating ICUs. Ninety (36.3%) patients developed at least one episode of secondary infection. An ICU length of stay between 7 and 14 days was characterized by a higher occurrence of infectious complications, with ventilator-associated pneumonia being the most frequent. At least one course of antibiotic therapy was given to 161 (64.9%) patients. Overall ICU and hospital mortality were 33.9% and 42.9%, respectively. Patients developing bacteremia had a higher risk of ICU mortality [45.9% vs. 31.6%, odds ratio 1.8 (95% CI 0.9–3.7), p = 0.069] and hospital mortality [56.8% vs. 40.3%, odds ratio 1.9 (95% CI 1.1–3.9), p = 0.04].
Conclusion
In critically ill patients infected with COVID-19 the incidence of bacterial infections is high and associated with worse outcomes. Regular microbiological surveillance and strict infection control measures are mandated.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-021-01661-2.
Keywords: COVID-19, Critically ill patients, Secondary bacterial infection, Co-infection
Introduction
Respiratory viral infections may predispose to severe secondary bacterial infections leading to increased mortality [1]. Although most of the deaths in the 1918 Spanish flu epidemic were reportedly due to Pneumococcal pneumonia [2], only a few publications attempted to assess secondary bacterial infections during the 2009 H1N1 influenza pandemic [3]. Despite rigorous global containment and quarantine efforts, the incidence of COVID-19 has continued to rise since its first appearance in Wuhan in December 2020. For hospitalized patients, the associated mortality ranges from 10 to 13.5% [4], and from 19 to 61.5% in patients requiring intensive care [5–8]. Despite a reported overall bacterial infection rate of 7.1% in hospitalized COVID-19 patients, with 3.5% infected at hospital presentation and 15.5% developing nosocomial secondary bacterial infections, broad-spectrum antibiotics were administered to 74.5% of patients [4, 5, 9, 10].
The prevalence, incidence and characteristics of bacterial infections in critically ill patients infected with COVID-19 are limited to single center retrospective studies [11–17]. More detailed data on bacterial infections in intensive care unit (ICU) patients with COVID-2019 disease are urgently needed, both for epidemiology and to assess their possible impact on outcomes related to COVID-19 infection. Timely characterization of co- or secondary-infections (both bacterial and fungal) is essential for optimal management of more severe COVID-19 cases by both improving and streamlining antimicrobial management.
We thus performed a prospective observational study of critically ill patients with COVID-19 admitted to eight Italian ICUs from February to May 2020 to determine the prevalence of bacterial co-infections and secondary infections, and to assess their impact on outcome.
Methods
This multi-center prospective observational study was performed in eight Italian ICUs over a 6 months period commencing on 21 February 2020. The following variables (dates, categorical and discrete) were collected through an interactive electronic database:
demographic variables: age, sex, therapeutic diagnostic path before admission to ICU (date of admission to hospital and subsequent hospitalizations before admission to ICU);
co-morbidities: (i.e., hypertension, heart disease, chronic obstructive airways disease, neoplasm, insulin dependent/independent diabetes mellitus, chronic renal failure, chronic vascular disease, chronic pathological or iatrogenic immunosuppression);
date of onset of the first SARS COV-2 related symptoms, and their characteristics;
interstitial pulmonary alveolar involvement;
start date of non-invasive ventilation (NIV); date of hospitalization in ICU and date of intubation
SAPS 2 (or other score used to derive standardized mortality rate); SOFA score on admission and highest reached during hospitalization;
duration of mechanical ventilation, use of pronation, and performance of tracheostomy;
development of ARDS;
identification of major pulmonary embolism;
use of renal replacement therapy and duration;
echocardiographic signs of cardiomyopathy and myocardial failure;
hepatic dysfunction (alteration of liver enzyme and cholestatic indices);
other alterations in blood tests (ferritin, lactate dehydrogenase, creatine kinase);
specific anti-COVID-19 related therapies;
ICU and hospital outcomes.
Collected clinical, rheological and therapeutic markers of infection included:
C-reactive protein (CRP), procalcitonin (PCT) and white blood count (WBC) (maximum and minimum);
surveillance cultures (bronchial aspirate, skin and rectal swabs), and susceptibility patterns; diagnostic cultures (blood, bronchoalveolar lavage, urine, cerebrospinal fluid and any from other sites), and susceptibility patterns. Each isolate was reviewed by two members of the antimicrobial team to determine clinical significance and need for treatment.
Antibiotic therapy and duration.
Definitions
An episode of bacteremia was defined when one or more microorganisms were isolated from one or more blood cultures, and clinical evidence suggested this had arisen from a common source and were part of the same episode. If the source was unknown, all positive blood cultures occurring within 48 h of each other are considered as a single bacteremic episode [18–20].
Polymicrobial bacteremia either growth of ≥ 2 different species of microorganisms in the same blood culture, or growth of different species in ≥ 2 separate blood cultures within the same episode (< 48 h) and with clinical or microbiological evidence of the same source [21].
Break-through bacteremia bacteremia due to the same microorganism and occurring in patients treated with appropriate therapy for more than 24 h [22].
Ventilator-associated pneumonia (VAP) was defined as the presence of the following definition criteria [23, 24]:
Radiology ≥ 2 serial chest radiographs with ≥ 1 of the following: new or progressive infiltrate, consolidation, cavitation.
Signs/symptoms ≥ 1 of the following: fever (> 38 °C); leukopenia (< 4000 white blood cells/mL); leukocytosis (> 12,000 white blood cells/mL); altered mental status if age > 70 year.
PLUS
≥ 2 of the following: new purulent sputum (> 25 neutrophils and < 10 squamous epithelial cells per low power field × 100); change in sputum characteristics or amount; new or worsening cough; dyspnea; tachypnea; rales; worsening gas exchange.
Microbiology ≥ 1 of the following: positive quantitative culture from minimally contaminated lower respiratory tract specimen; (n.b. a specimen obtained via endotracheal suctioning is not a minimally contaminated specimen, and therefore, does not meet laboratory criteria); positive culture of pleural fluid; positive growth in blood culture not related to another source of infection.
Endpoints
The primary endpoint was the occurrence of VAP, bloodstream and urinary tract infections together with any other types of clinically significant infection and their attributable onset risk. Secondary endpoints were the impact of bacterial or fungal infection on ICU and hospital length of stay and outcomes.
Statistical analysis
Student’s t test was used for quantitative discrete variables, and Mann–Whitney non-parametric U test for non-normally distributed data. For categorical variables the Chi-squared test was applied to assess differences in proportions with Fisher’s exact test correction for expected values < 5. Under the conditional independence assumption, Cochran’s statistic was applied to assess the Mantel–Haenszel common odds ratio estimate (OR).
A Kaplan Meier analysis (limit product method) was used to identify occurrence of infections and risk of acquisition, and freedom from infection during the ICU stay of the patient. The log rank method was used to assess differences in stratification groups for the Kaplan Meier analyses.
Results
During the study period 248 patients were recruited in the eight participating ICUs. Table 1a, b shows the main characteristics of these patients, and after stratification by occurrence/non-occurrence of a secondary infectious complication. Patients developing an infectious complication experienced longer durations of mechanical ventilation, ICU and hospital stay, and a higher percentage of recorded severe acute respiratory distress syndrome [ARDS] (PaO2:FiO2 ratio < 100 mmHg/13.3 kPa). Differences in the proportion of microbiological cultures in the two groups likely relate to cultures being generally performed for clinical indications.
Table 1.
Main characteristics of all patients, stratified according to occurrence or not of an infectious complication: (a) quantitative and (b) categorical variables
| Variables | All patients 248 |
Patients with infection(s) 101 |
Patients without infection(s) 147 |
p-value |
|---|---|---|---|---|
| a: Quantitative variables1 | ||||
| Age | 66 (58–72) | 58(65–71) | 66 (58–72) | 0.866 |
| BMI | 27 (25–31) | 27.5 (25–31) | 27 (26–31) | 0.832 |
| SAPS-2 | 37 (31–49) | 37 (31–43) | 37 (31–51) | 0.237 |
| SOFA | ||||
| Admission | 7 (5–9) | 7 (5–8) | 7 (5–9) | 0.655 |
| Worst recorded | 8 (6–10) | 7.5 (6–10) | 8 (6–10) | 0.252 |
| COVID-2019 | ||||
| Symptom onset days | 6 (5–8) | 6 (4–8) | 6 (6–8) | 0.525 |
| Therapy-days | 1 (0–2) | 7 (4–11.5) | 7 (4–12.5) | 0.624 |
| Length of stay | ||||
| Pre-ICU | 3 (2–6) | 3 (2–6) | 3 (2–6) | 0.639 |
| ICU | 16 (9–25) | 19.5 (11–31) | 14 (6–20.5) | 0.001 |
| Post-ICU | 15 (9–23) | 17.5 (10–22.5) | 13 (8–23.5) | 0.345 |
| Hospital | 33 (22–43) | 35 (26–6) | 29 (20–42) | 0.123 |
| Variables | All patients 248 |
Patients with infection(s) 101 |
Patients without infection(s) 147 |
OR 95% (CI) | p-values |
|---|---|---|---|---|---|
| b: categorical variables2 | |||||
| M:F (ratio) | 193:55 (3.5:1) | 77:24 (3.2:1) | 116:31 (3.75:1) | – | – |
| Past medical history | |||||
| Smoking | 49 (19.80%) | 21 (21.2%) | 28 (20.9%) | 1.1 (0.5–1.9) | 0.539 |
| Diabetes | 54(23.2%) | 27 (27.3%) | 27 (20.1%) | 1.5 (0.8–2.7) | 0.213 |
| Hypertension | 117 (47.20%) | 54 (54.5%) | 63 (47%) | 1.3 (0.8–2.3) | 0.29 |
| Coronary artery disease | 26 (11.2%) | 14 (12.1%) | 14 (10.4%) | 1.2 (0.5–2.7) | 0.421 |
| Vasculopathy | 34 (14.5%) | 14 (14.1%) | 20 (14%) | 0.9 (0.5–2) | 0.529 |
| COPD | 29 (12.5%) | 10 (10.1%) | 19 (14.3%) | 0.7 (0.3–1.5) | 0.227 |
| Neoplasm | 14 (6%) | 9 (9.2%) | 5 (3.7%) | 2.6 (0.8–8.1) | 0.099 |
| Chronic renal failure | 13 (5.6%) | 5 (5.1%) | 8 (6%) | 0.8 (0.3–2.7) | 0.502 |
| Immunosuppression | 9 (3.6%) | 4 (4%) | 5 (3.7%) | 0.9 (0.5–2) | 0.581 |
| SARS-COV-2 therapy | 181 (73.1%) | 79 (78.4%) | 102 (69.2%) | 1.6 (0.9–3) | 0.153 |
| Lopinavir–ritonavir | 201 (81.3%) | 85 (84.5%) | 116 (81%) | 0.9 (0.8–1.2) | 0.954 |
| Tocilizumab | 71 (28.6%) | 30 (29.3%) | 41 (27.7%) | 1.1 (0.6–2) | 0.572 |
| Hydroxychloroquine | 205 (82.8%) | 86 (84.8%) | 119 (81.2%) | 1.3 (0.6 -2.6) | 0.488 |
| SARS-COV-2 symptoms | |||||
| Fever | 216 (90%) | 86 (86%) | 130 (92.9%) | 0.5 (0.2–1.1) | 0.086 |
| Respiratory | 242 (97.9%) | 96 (96%) | 146 (99.3%) | 0.2 (0.1–1.6) | 0.164 |
| Gut symptoms | 29 (11.7%) | 11 (11%) | 18 12.1%) | 0.9 (0.4–2) | 0.841 |
| Severe ARDS | 239 (96.2%) | 99 (99%) | 140 (94.6%) | 5.9 (3.1–8.5) | 0.022 |
| Acute renal failure | 34 (16.6%) | 22 (25.3%) | 2 (10.2%) | 3 (1.4–6.5) | 0.007 |
| Acute cardiac failure | 12 (5.7%) | 7 (8%) | 5 (4%) | 2.1 (0.7–6.7) | 0.242 |
| Acute liver failure | 47 (18.9%) | 22 (21.7%) | 25(16.9%) | 1.4 (0.7–2.8) | 0.465 |
| Vasoactive drugs | 151 (60.8%) | 68 (67.3%) | 83 (56.5%) | 1.6 (0.9–2.7) | 0.088 |
| Culture percentage | |||||
| Total population | 157 (63.3%) | 101 (100%) | 56 (38.1%) | 4.8 (2.6–9) | 0.001 |
| 0–72 h | 64 (25.8%) | 48 (47.5%) | 16 (10.9%) | 7.4 (3.9–14.2) | 0.001 |
| 72 h–7 days | 65 (26.2%) | 47 (46.5%) | 18 (12.2%) | 6.2 (3.3–11.7) | 0.001 |
| 8–14 days | 79 (31.9%) | 49 (48.5%) | 30 (20.4%) | 3.7 (2.1–6.4) | 0.001 |
| 15–21 days | 70 (28.2%) | 37 (36.6%) | 33 (22.4%) | 2 (1.1–3.5) | 0.021 |
| Severe infections | |||||
| N (%) | 101 (40.7%) | 101 (100%) | – | – | – |
| Bacteremia (%) | 37 (14.9%) | 37 (36.6%) (§) | – | – | – |
| VAP | 62 (25%) | 62 (61.4%) (§) | – | – | – |
| Urinary tract | 21 (8.5%) | 21 (20.8%) (§) | – | – | – |
| > 1 infection type (*) | 9 (3.6%) | 9 (9.9%) (§) | – | – | – |
| Outcome (alive) | |||||
| ICU | 154 (62.1%) | 62 (61.4%) | 92 (62.6%) | 1.2 (0.7–2.1) | 0.577 |
| Hospital | 133 (53.6%) | 53 (52.5%) | 80 (54.4%) | 1.2 (0.7–2) | 0.592 |
1Statistical comparisons are performed between group of patients with and without infections; p values are referring to that. BMI body mass index, SAPS-2 simplified acute physiology score, SOFA sequential organ failure assessment
2Statistical comparisons are performed between group of patients with and without infections; p values are referring to that. (*) patients developing more than 1 type of severe infection. (§) the sum up of percentage is > than 100% since a few patients developed more than 1 infection and 1 patient developed 3 type of infections. COPD chronic obstructive pulmonary disease, VAP ventilator-associated pneumonia
IQR inter-quartile range, COVID-2019 coronavirus disease-2019, ICU intensive care unit, M:F male/female ratio
Infections
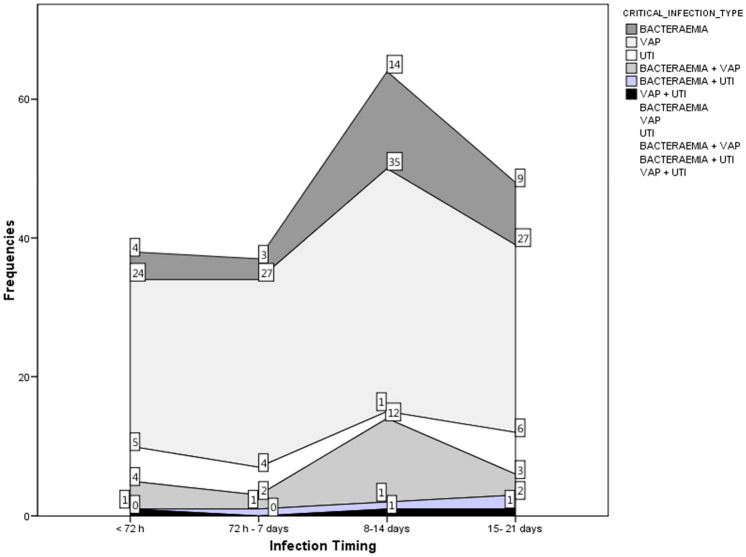
One hundred and one (40.7%) patients developed at least one episode of secondary infection. In total, there were 120 episodes (1.19 episodes per secondarily infected patient, or 0.49 episodes per patient within the whole cohort. Figure 1 shows bacteremia, ventilator-associated pneumonia (VAP) and urinary tract infection (UTI) rates, stratified according to length of stay. A length of stay between 7 and 14 days was characterized by a higher occurrence of infectious complications, with VAP being the most frequent. One patient developed a bacteremia, a VAP and a UTI.
Fig. 1.
Severe infection type (bacteremia, VAP, UTI, and combinations) stratified by days following ICU admission. Numbers represent occurrence of each variable
Micro-organisms
Figure S1a–c show the characteristics of the organisms identified, stratified by length of stay and types of infection. For both bacteremia and VAP, an initially higher occurrence of Gram-positive isolates was followed by a balanced mix with Gram-negative organisms. Polymicrobial and urinary tract infections were more frequently caused by Gram-negative microorganisms, though an increasing incidence of likely yeast-related urinary tract infection was seen in the latter period of a patient’s stay. Table 2a–c show isolates stratified by Gram stain and length of stay.
Table 2.
Positive culture results, stratified by days following ICU admission: (a) Isolates from blood cultures (b) Isolates from bronchoalveolar lavage and (c) Isolates from urine
| Variables | Days post-ICU admission | ||||
|---|---|---|---|---|---|
| < 72 h | 72 h–7 days | 8–14 days | 15–21 days | Total | |
| a: blood cultures | |||||
| Gram-positive | |||||
| Coagulase-negative Staphylococcus | 1 | 6 | 6 | 1 | 14 |
| Staphylococcus aureus | 2 | 3 | 3 | 8 | |
| Streptococcus spp. | 1 | 1 | 1 | 3 | |
| Streptococcus pneumoniae | 1 | 7 | 8 | ||
| Enterococcus faecalis | 5 | 5 | 2 | 12 | |
| Enterococcus faecium | 1 | 1 | |||
| Gram-negative | |||||
| Klebsiella pneumoniae | 7 | 7 | |||
| Pseudomonas aeruginosa | 3 | 3 | 2 | 8 | |
| Acinetobacter spp. | 8 | 2 | 3 | 13 | |
| Escherichia coli | 1 | 1 | 1 | 3 | |
| Bacillus spp. | 1 | 1 | |||
| Enterobacter aerogenes | |||||
| Yeasts | |||||
| Candida albicans | 1 | 3 | 3 | 1 | 8 |
| Candida parapsilosis | 1 | 1 | 1 | 3 | |
| b: Broncho-alveolar lavage culture | |||||
| Gram-positive | |||||
| Staphylococcus aureus | 17 | 6 | 7 | 4 | 34 |
| Streptococcus spp. | 1 | 2 | 1 | 4 | |
| Streptococcus pneumoniae | 4 | 1 | 5 | ||
| Enterococcus faecalis | 1 | 3 | 1 | 5 | |
| Enterococcus faecium | 3 | 1 | 1 | 5 | |
| Gram-negative | |||||
| Klebsiella pneumoniae | 9 | 10 | 6 | 25 | |
| Klebsiella oxythoca | 1 | 1 | 2 | ||
| Pseudomonas aeruginosa | 1 | 7 | 16 | 21 | 45 |
| Acinetobacter spp. | 1 | 3 | 6 | 10 | |
| Escherichia coli | 2 | 4 | 13 | 8 | 27 |
| Morganella morganii | 1 | 1 | |||
| Bacillus spp. | |||||
| Enterobacter aerogenes | 1 | 3 | 2 | 6 | |
| Enterobacter cloacae | 1 | 6 | 4 | 11 | |
| Hafnia alvei | 1 | 1 | |||
| Haemophylus influentiae | 7 | 2 | 9 | ||
| Proteus mirabilis | 2 | 1 | 3 | ||
| Stenotrophomonas maltophilia | 1 | 1 | 2 | ||
| Serratia marcescens | 1 | 1 | 1 | 3 | |
| Rautella Ornithynolytica | 1 | 1 | |||
| Citrobacter freundii | 1 | 1 | |||
| Yeasts | |||||
| Candida albicans | 1 | 3 | 2 | 6 | |
| Candida glabrata | 1 | 1 | |||
| Aspergillus fumigatus | 1 | 2 | 3 | ||
| Aspergillus niger | 1 | 1 | |||
| c: urine culture | |||||
| Gram-positive | |||||
| Enterococcus faecalis | 2 | 2 | |||
| Enterococcus faecium | 1 | 1 | 1 | 3 | |
| Gram-negative | |||||
| Klebsiella pneumoniae | 1 | 1 | 1 | 3 | |
| Pseudomonas aeruginosa | |||||
| Escherichia coli | 3 | 1 | 4 | ||
| Enterobacter cloacae | 1 | 1 | |||
| Proteus mirabilis | 1 | 1 | 2 | ||
| Yeasts | |||||
| Candida albicans | 1 | 1 | 1 | 3 | |
Antibiotic therapy
At least one course of antibiotic therapy was given to 164 (66.1%) patients. All of the 101 patients with proven infection received targeted antibiotic therapy, whereas empirical antibiotics were given to 63 (42.9%) of 147 patients without proven infection. The percentage of patients on antibiotics—both targeted and empirical—increased progressively in relation to their ICU length of stay.
The use of antifungal agents was low, although also increasing in relation to ICU stay.
Mortality
Overall ICU and hospital mortality were 33.9% and 42.9%, respectively. Patients developing bacteremia had a higher risk of ICU mortality [45.9% vs. 31.6%], OR = 1.8 (95% CI 0.9–3.7); p = 0.07] and hospital mortality [56.8% vs. 40.3%, OR = 1.9 (95% CI 1.1–3.9); p = 0.04]. Patients developing VAP had a similar risk of ICU mortality [34.5% vs. 33.7%], OR = 1.1 (95% CI 0.5–1.9); p = 0.52] and hospital mortality [43.1% vs. 42.9%, OR = 1.1 (95% CI 0.9–1.8); p = 0.55]. Both ICU mortality [66.7% vs. 32.6%], OR = 4.2 (95% CI 1.1–17.1); p = 0.03] and hospital mortality [66.7% vs. 42%], OR = 2.8 (95% CI 0.7–11.3), p = 0.12] were higher in the nine patients developing both bacteremia and VAP.
In terms of illness severity, 59 patients developed septic shock with an ICU mortality rate of 46.7%. By comparison 31 patients developed sepsis with an ICU mortality rate of 40% (p = 0.572).
Figure 2 shows Kaplan Meier curves representing, respectively, the actuarial survival of patients in relation to developing bacteremia (Fig. 2a) or VAP (Fig. 2b). Survival was lower in patients developing bacteremia though the log rank test was not significantly different. A bacteremic episode occurred a median (IQR) time of 7 (3.5–9) days before the patient’s death. Kaplan Meier curves of patients experiencing more than one type of infection are not shown due to low numbers.
Fig. 2.
Kaplan–Meier curves for hospital survival stratified by development (or not) of a bacteremia and b ventilator-associated pneumonia. Numbers represent occurrence of each variable
Discussion
Our observational multicenter study reports that over a third of patients admitted to ICU for COVID-19 pneumonia developed at least one episode of severe bacterial infection. The peak incidence of infection was registered between 8 and 14 days following ICU admission. The most frequently reported infections were, in order, VAP, bacteremia and urinary tract infection. Unlike VAP alone, bacteremia was associated with a worse outcome. There was a tendency towards a shift from Gram-positive to Gram-negative species as the length of ICU stay increased.
Few studies have examined the frequency of bacterial and fungal infections and their impact on outcome in COVID-19 patients [5, 9]. Likewise, few studies have focused on an intensive care population with a marked variability (8.1–60%) in the reported incidence of infection [11–17]. A recent review reported that the incidence of bacterial infection was higher in fatal cases of patients with COVID-19 disease [4]. Secondary infection risk increased in those receiving invasive ventilation and intravascular devices, with a higher mortality rate [25]. Our study confirms these findings in a larger cohort of ICU patients. Of note, the US National Institutes of Health COVID-19 treatment guidelines state that there are no reliable estimates of the incidence or prevalence of co-pathogens in patients with COVID-19 and severe or critical illness to recommend empiric broad-spectrum antimicrobial therapy [26]. The reported incidence of invasive pulmonary aspergillosis in COVID-19 ranged from 19.6% to 33.3% [27, 28]. In our study we found only four cases though this may be an underestimate relating to the frequency of galactomannan testing which increased only after several months into the pandemic.
Interesting comparisons can be drawn against secondary infections following other primary viral chest infections. Rice and colleagues found that among critically ill patients with 2009 influenza A illness, bacterial co-infection diagnosed within 72 h of admission, especially with Staphylococcus aureus, was associated with significantly higher morbidity and mortality [29]. A high prevalence (20/101 [19.8%]) of early bacterial coinfection has also been reported during severe COVID-19 pneumonia, also with a high proportion of S aureus [30]. Our study also identified Staphylococcus aureus as the main pathogen, accounting for the majority of the early-onset bacterial coinfection.
Shafran et al. compared outcomes of patients with COVID-19 disease and influenza [31]. Bacterial infection was more common in the COVID-19 patients (12.6% vs. 8.7%), appeared later after admission (4 [1–8] vs. 1 [1–3] days), with Gram-positive bacteria being more frequently identified (28% vs. 9.5%). Secondary infection was associated with an approximate threefold increased risk of death in both COVID-19 disease and influenza groups but, after adjustment for age and illness severity, this only remained significant for COVID-19 infection. A recently published report from the United Kingdom, however, found no association in patients admitted to critical care who were identified to have a respiratory or bloodstream infection and subsequent mortality (unadjusted odds ratio 1.02, 95% CI 0.86–1.22; p = 0.81) [32]. S. aureus (17.8%), H. influenzae (12.7%) and P. aeruginosa (9.3%) were most frequently identified in positive sputum samples, S. aureus in positive deep respiratory samples (31.1%), and E Coli (26.7%) and S aureus (13.3%) in positive blood cultures as the causative organism of co-infections within two days of hospital admission. For secondary infections appearing after two days of hospital admission, K. pneumoniae, P aeruginosa, S aureus and E coli predominated in both respiratory samples and blood cultures (each below 15%).
The number of patients who received antibiotic therapy was high. For good stewardship, antibiotic selection should be based on local epidemiology, patient factors and clinical concern. Early discontinuation should be considered if no evidence of bacterial infection becomes apparent in terms of clinical signs and symptoms, laboratory and imaging findings.
Our study has several strengths. To our knowledge, it is the first prospective multicenter observational study addressing the specific topic of bacterial infection in COVID-19 patients admitted to ICU, and the first to provide data on the impact on mortality in this subset of patients. Since our study was limited to patients admitted to ICU the associations found between bacterial infection and mortality may not be generalizable to non-critically ill populations.
In conclusion, given the higher incidence of bacterial infections in COVID-19 patients admitted to ICU and its potential impact on mortality, there should be regular microbiological surveillance and strict infection control measures.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Concept and design: VS, AC. Acquisition, analysis, or interpretation of data: VS, AC, VS, MS. Drafting of the manuscript: VS, AC, CN, MS. Critical revision of the manuscript for important intellectual content: MS, CN, DV. Statistical analysis: AC. Administrative, technical, or material support: All authors.
Funding
None.
Availability of data and materials
Data are available from the corresponding author, [V.D.S.], upon reasonable request.
Declarations
Conflict of interest
No conflicts of interest to declare.
Ethics approval
Local Institutional Review Boards approved the study.
Consent to participate
Informed consent was obtained from all individual participants enrolled in the study.
References
- 1.Cox MJ, Loman N, Bogaert D, O'Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020;1:11. doi: 10.1016/S2666-5247(20)30009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacIntyre CR, Chughtai AA, Barnes M, et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza A (H1N1) pdm09. BMC Infect Dis. 2018;18:637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically Ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Zangrillo A, Zanella A, COVID-19 Lombardy ICU Network et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes S, Troise O, Donaldson H, et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreitmann L, Monard C, Dauwalder O, et al. Early bacterial co-infection in ARDS related to COVID-19. Intensive Care Med. 2020;46:1787–1789. doi: 10.1007/s00134-020-06165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42:84–88. doi: 10.1017/ice.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koehler P, Cornely OA, Böttiger BW, et al. COVID-19-associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrasa H, Rello J, Tejada S, et al. COVID-19 study investigators SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020;39:553–561. doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling L, So C, Shum HP, et al. Critically ill patients with COVID-19 in Hong Kong: a multicentre retrospective observational cohort study. Crit Care Resusc. 2020;22:119–125. doi: 10.51893/2020.2.oa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittet D. Nosocomial bloodstream infections. In: Wenzel RP, editor. Prevention and control of nosocomial infections. 3. Baltimore: Williams & Wilkins; 1997. pp. 712–769. [Google Scholar]
- 19.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein MP, Towns ML, Quartey SM. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 21.Roberts FJ. Definition of polymicrobial bacteremia. Rev of Infect Dis. 1989;11:1029–1030. doi: 10.1093/clinids/11.6.1029. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein MP, Reller LB. Clinical importance of breakthrough bacteremia. Am J Med. 1984;76:175–180. doi: 10.1016/0002-9343(84)90770-8. [DOI] [PubMed] [Google Scholar]
- 23.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Mietto C, Pinciroli R, Patel N, Berra L. Ventilator associated pneumonia: evolving definitions and preventive strategies. Respir Care. 2013;58:990–1007. doi: 10.4187/respcare.02380. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Zhang Y, Wu J, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020;9:1958–1964. doi: 10.1080/22221751.2020.1812437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Dec 2020. [PubMed]
- 27.Lai CC, Yu WL. COVID-19 associated with pulmonary aspergillosis: a literature review. J Microbiol Immunol Infect. 2021;54:46–53. doi: 10.1016/j.jmii.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koehler P, Cornely OA, Böttiger BW, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice TW, Rubinson L, Uyeki TM, NHLBI ARDS Network et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40:1487–1498. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elabbadi A, Turpin M, Gerotziafas GT, Teulier M, Voiriot G, Fartoukh M. Bacterial coinfection in critically ill COVID-19 patients with severe pneumonia. Infection. 2021;49:559–562. doi: 10.1007/s15010-020-01553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shafran N, Shafran I, Ben-Zvi H, et al. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci Rep. 2021;11:12703. doi: 10.1038/s41598-021-92220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell CD, Fairfield CJ, Drake TM, ISARIC4C investigators et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021 doi: 10.1016/S2666-5247(21)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author, [V.D.S.], upon reasonable request.